Abstract
We report an 80-year-old man who presented with sponta- neous regression of hepatocellular carcinoma (HCC). He complained of sudden right flank pain and low-grade fever. The level of protein induced by vitamin K antagonist (PIVKA)-II was 1137 mAU/mL. A computed tomography scan in November 2000 demonstrated a low-density mass located in liver S4 with marginal enhancement and a cystic mass of 68 mm × 55 mm in liver S6, with slightly high density content and without marginal enhancement. Angiography revealed that the tumor in S4 with a size of 25 mm × 20 mm was a typical hypervascular HCC, and transarterial chemoembolization was performed. However, the tumor in S6 was hypovascular and atypical of HCC, and thus no therapy was given. In December 2000, the cystic mass regressed spontaneously to 57 mm × 44 mm, and aspiration cytology revealed bloody fluid, and the mass was diagnosed cytologically as class I. The tumor in S4 was treated successfully with a 5 mm margin of safety around it. The PIVKA-II level normalized in February 2001. In July 2001, the tumor regressed further but presented with an enhanced area at the posterior margin. In November 2001, the enhanced area extended, and a biopsy revealed well-differentiated HCC, although the previous tumor in S4 disappeared. Angiography demonstrated two tumor stains, one was in S6, which was previously hypovascular, and the other was in S8. Subsequently, the PIVKA-II level started to rise with the doubling time of 2-3 wk, and the tumor grew rapidly despite repeated transarterial embolization with gel foam. In February 2003, the patient died of bleeding into the peritoneal cavity from the tumor that occupied almost the entire right lobe. Considering the acute onset of the symptoms, we speculate that local ischemia possibly due to rapid tumor growth, resulted in intratumoral bleeding and/or hemorrhagic necrosis, and finally spontaneous regression of the initial tumor in S6.
INTRODUCTION
According to previous reports, spontaneous regression (SR) of malignant tumors is estimated to occur once in 60000-100000 cancer patients[1]. Neuroblastomas and urinary bladder cancers are well-known to regress spontaneously. However, in hepatocellular carcinoma (HCC) only 30 cases of SR have been reported in the English-language literature[2-7]. Among them, there has been only 1 report of 2 cases of recurrent HCC after SR[4]. In both cases, however, the new lesion developed at a different site in the liver while the preexisting HCC was regressing, suggesting that multicentric hepatocarcinogenesis was involved in the recurrence. In this paper, we present a case of spontaneously regressing HCC, which recurred locally during the course of 8 mo. Four months later, a new lesion developed at a different site in the liver, possibly through intrahepatic metastasis from the original tumor, and progressed rapidly. We discussed the possible mechanisms of SR in HCC.
CASE REPORT
An 80-year-old man was admitted to our hospital on November 14, 2000 due to right flank pain and low-grade fever, which continued for a day and worsened gradually. He drank 350 mL of beer every day but had no history of the use of herbal medicines.
His blood pressure was 170/104 mmHg and body temperature was 37.2 °C. There was no remarkable physical finding. The laboratory data on admission revealed that his white blood cell count and C-reactive protein (CRP) were elevated to 10600/mm3 and 6.2 mg/dL, respectively. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) also were elevated to 201 IU/L and 234 IU/L, respectively. The level of alfa-fetoprotein (AFP) was within the normal limit, but protein induced by vitamin K antagonist (PIVKA)-II was 1137 mAU/mL. His serum was negative for hepatitis B surface antigen and positive for hepatitis C antibody.
Enhanced computed tomography (CT) on admission demonstrated 2 hepatic tumors (Figure 1A). One was a low-density mass located in liver S4 with marginal enhancement. The other was a cystic mass of 68 mm × 55 mm in S6 with slightly high density content but without marginal enhancement. Angiography demonstrated that the right hepatic lobe was supplied by the replaced right hepatic artery. A celiac arteriogram showed tumor stain in S4 with a size of 25 mm × 20 mm, which was diagnosed as typical HCC (Figure 2A). Transarterial chemoembolization was performed using an emulsion of 3.2 mL Lipiodol (iodized oil manufactured by Andre Guerbert, Aulnay-sous-Bois, France) and 32 mg epirubicin[8]. The super mesenteric arteriogram, on the other hand, demonstrated that the tumor in S6 was hypovascular (Figure 2B). Because this image was atypical of HCC, no therapy was given.
Figure 1.
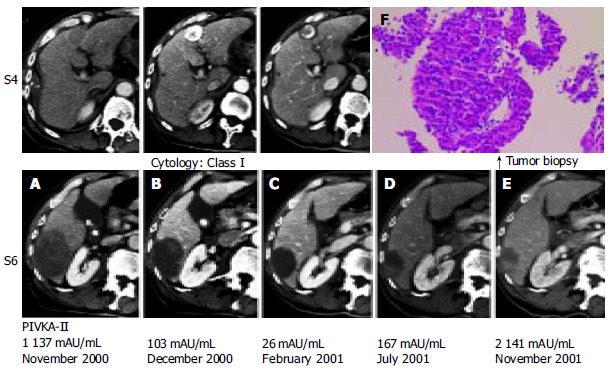
Enhanced computed tomography (CT). A: A low-density mass located in liver S4, with marginal enhancement and a cystic mass of 68 mm × 55 mm in S6. B: Lipiodol accumulation in the tumor in S4 and the cystic mass in S6. C: Microwave coagulation performed for HCC in S4 during cholecystectomy. D: Further decrease in tumor size and enhanced area at the posterior cystic lumen of the tumor. E: Extension of the enhanced area to the surrounding area of cystic tumor. F: A well-differentiated HCC shown by histological evaluation.
Figure 2.
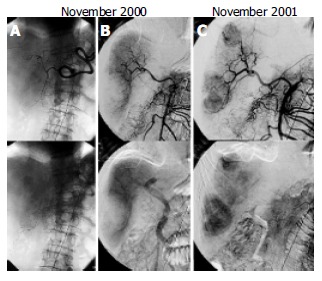
A: Celiac arteriogram showing tumor stain with a size of 25 mm × 20 mm in S4. B: Blood supply of right hepatic lobe by the replaced right hepatic artery. C: Super mesenteric arteriogram demonstrating two tumor stains.
Low-grade fever disappeared on the second day of admission and the white blood cell count, CRP and transaminases became almost normal on the eighth day. Because the patient wanted to undergo an operation for gallbladder stones, preoperative evaluation for cholecystectomy was carried out. In December 2000, just before the operation, a CT scan showed that Lipiodol was accumulated in the tumor in S4 and that the cystic mass in S6 was decreased in size spontaneously, down to 57 mm × 44 mm (Figure 1B). During the operation in December 2000, microwave coagulation was performed for HCC in S4. The surface of the cystic tumor in S6 was dark red and needle aspiration revealed bloody fluid, which was diagnosed cytologically as class I. Because there was no evidence of malignancy, we did not give any treatment for this lesion.
After the operation, the tumor in S6 continued to decrease in size (Figure 1C), whereas the tumor in S4 was treated successfully with a 5 mm margin of safety around it. The PIVKA-II level fell to the normal limit in February 2001. A CT scan in July 2001 revealed that the tumor was decreased further in size but demonstrated an enhanced area at the posterior margin of the tumor (Figure 1D). Furthermore, the PIVKA-II level increased slightly. In November 2001, a CT scan demonstrated that the enhanced area extended to the surrounding area of the cystic tumor (Figure 1E). Histological evaluation by tumor biopsy from the lesion in S6 revealed a well-differentiated HCC (Figure 1F). The celiac arteriogram in November 2001 showed that the previous tumor stain in S4 disappeared. The super mesenteric arteriogram demonstrated 2 tumor stains; one was in S6, which was previously hypovascular, and the other was in S8 (Figure 2D).
Subsequently, transarterial embolization with gel foam (TAE) was repeated but the tumor resisted therapy, with rapid invasion and intrahepatic metastasis. In February 2003, the patient died of bleeding into the peritoneal cavity from the tumor that occupied almost the entire right lobe (Figure 3).
Figure 3.
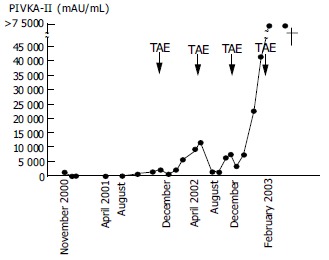
Clinical course of the patient. TAE: transarterial embolization with gel foam.
DISCUSSION
To our knowledge, there has been only 1 report of 2 cases of recurrence of HCCs after SR[4]. In both cases, the recurrent HCCs developed at different sites in the liver before the preexisting HCC regressed completely, suggesting that multicentric hepatocarcinogenesis, rather than intrahepatic metastasis, was likely to have been involved in the recurrence. However, in our case, evidence of local recurrence in the regressing tumor was observed by CT scan before the newly developed lesion in S8 was found by angiography. In addition, when local recurrence was detected by CT scan, the serum PIVKA-II level started to rise again. This clinical course suggests that the new lesion in S8 might be due to intrahepatic metastasis from the locally recurrent tumor in S6, rather than multicentric hepatocarcinogenesis.
In previous reports, some authors made a definitive diagnosis by histological study after tumor resection but others suspected SR based on the following up radiological findings and the tumor markers[2-7]. In this case, the PIVKA-II level was 1137 mAU/mL on the first admission and decreased dramatically to 103 in 1 mo. However, at that point we were not certain whether this was due to SR of the HCC in S6 or to transarterial chemoembolization for a typical HCC in S4. In addition, a diagnosis of class I by tumor aspiration cytology in S6 prevented us from performing S6 segmentectomy, in view of evidence-based medicine. We could not make a definitive diagnosis of the tumor in S6 until it showed radiological signs of recurrence and was proven histologically to be HCC.
The precise mechanism of SR of HCC remains unclear, but various factors have been speculated to play a role, such as alcohol withdrawal[9], androgen withdrawal[10], and intake of herbal medicine[2,11-12]. Secondary bacterial infection in the tumor has also been supposed to cause SR through the stimulation of cytokine production[13,14] and fever[15]. Because of the hypervascular nature of HCC, another important factor might be an insufficient blood supply to the tumor, possibly due to rapid natural tumor growth[16,17], spontaneous arterial thrombosis[18], or gastrointestinal bleeding[19,20]. In our case, there was no history of alcohol or androgen withdrawal or the use of herbal medicines. The initial symptoms of right flank pain and fever suddenly appeared and lasted for only a day. Laboratory data showed that the levels of transaminases were elevated on admission but almost normalized in about a week. In addition, angiography clarified that the tumor in S6 was hypovascular. Furthermore, this tumor was cystic with slightly high density content, from which bloody fluid was obtained. These clinical manifestations suggest that the acute onset of the symptoms was due to local ischemia, leading to intratumoral bleeding or hemorrhagic necrosis, and as a result, causing SR.
In this case, the PIVKA-II level after recurrence showed a logarithmic increase throughout the entire clinical course, except that it stabilized for one month after each TAE. The doubling time of the PIVKA-II level, which is thought to reflect the tumor doubling time, was calculated to be 2-3 wk. This indicates that the recurrent tumor grew very rapidly, compared with the average natural growth rate of HCC reported previously[21,22]. The PIVKA-II level was elevated to 1137 mAU/mL on the first admission, decreased along with SR of the tumor in S6, and rose again after recurrence. It has been reported that HCCs with high PIVKA-II levels during the initial stage show aggressive behaviors and a poor prognosis. Therefore, we speculate that the initial tumor in S6 grew as fast as the recurrent tumor and this rapid growth itself might be the cause of insufficient blood supply to the tumor, finally inducing SR, as suggested in previous reports[16,17].
Footnotes
Edited by Wang XL Proofread by Zhu LH and Xu FM
References
- 1.Cole WH. Efforts to explain spontaneous regression of cancer. J Surg Oncol. 1981;17:201–209. doi: 10.1002/jso.2930170302. [DOI] [PubMed] [Google Scholar]
- 2.Takeda Y, Togashi H, Shinzawa H, Miyano S, Ishii R, Karasawa T, Takeda Y, Saito T, Saito K, Haga H, et al. Spontaneous regression of hepatocellular carcinoma and review of literature. J Gastroenterol Hepatol. 2000;15:1079–1086. doi: 10.1046/j.1440-1746.2000.02202.x. [DOI] [PubMed] [Google Scholar]
- 3.Izuishi K, Ryu M, Hasebe T, Kinoshita T, Konishi M, Inoue K. Spontaneous total necrosis of hepatocellular carcinoma: report of a case. Hepatogastroenterology. 2000;47:1122–1124. [PubMed] [Google Scholar]
- 4.Lee HS, Lee JS, Woo GW, Yoon JH, Kim CY. Recurrent hepatocellular carcinoma after spontaneous regression. J Gastroenterol. 2000;35:552–556. doi: 10.1007/s005350070080. [DOI] [PubMed] [Google Scholar]
- 5.Matsuo R, Ogata H, Tsuji H, Kitazono T, Shimada M, Taguchi K, Fujishima M. Spontaneous regression of hepatocellular carcinoma--a case report. Hepatogastroenterology. 2001;48:1740–1742. [PubMed] [Google Scholar]
- 6.Ikeda M, Okada S, Ueno H, Okusaka T, Kuriyama H. Spontaneous regression of hepatocellular carcinoma with multiple lung metastases: a case report. Jpn J Clin Oncol. 2001;31:454–458. doi: 10.1093/jjco/hye092. [DOI] [PubMed] [Google Scholar]
- 7.Morimoto Y, Tanaka Y, Itoh T, Yamamoto S, Mizuno H, Fushimi H. Spontaneous necrosis of hepatocellular carcinoma: a case report. Dig Surg. 2002;19:413–418. doi: 10.1159/000065822. [DOI] [PubMed] [Google Scholar]
- 8.Fan J, Ten GJ, He SC, Guo JH, Yang DP, Wang GY. Arterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol. 1998;4:33–37. doi: 10.3748/wjg.v4.i1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gottfried EB, Steller R, Paronetto F, Lieber CS. Spontaneous regression of hepatocellular carcinoma. Gastroenterology. 1982;82:770–774. [PubMed] [Google Scholar]
- 10.McCaughan GW, Bilous MJ, Gallagher ND. Long-term survival with tumor regression in androgen-induced liver tumors. Cancer. 1985;56:2622–2626. doi: 10.1002/1097-0142(19851201)56:11<2622::aid-cncr2820561115>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 11.Chien RN, Chen TJ, Liaw YF. Spontaneous regression of hepatocellular carcinoma. Am J Gastroenterol. 1992;87:903–905. [PubMed] [Google Scholar]
- 12.Lam KC, Ho JC, Yeung RT. Spontaneous regression of hepatocellular carcinoma: a case study. Cancer. 1982;50:332–336. doi: 10.1002/1097-0142(19820715)50:2<332::aid-cncr2820500228>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe N, Yamauchi N, Maeda M, Neda H, Tsuji Y, Okamoto T, Tsuji N, Akiyama S, Sasaki H, Niitsu Y. Recombinant human tumor necrosis factor causes regression in patients with advanced malignancies. Oncology. 1994;51:360–365. doi: 10.1159/000227366. [DOI] [PubMed] [Google Scholar]
- 14.Nishimura T, Watanabe K, Yahata T, Ushaku L, Ando K, Kimura M, Saiki I, Uede T, Habu S. Application of interleukin 12 to antitumor cytokine and gene therapy. Cancer Chemother Pharmacol. 1996;38 Suppl:S27–S34. doi: 10.1007/s002800051033. [DOI] [PubMed] [Google Scholar]
- 15.Jansen PM, van der Pouw Kraan TC, de Jong IW, van Mierlo G, Wijdenes J, Chang AA, Aarden LA, Taylor FB, Hack CE. Release of interleukin-12 in experimental Escherichia coli septic shock in baboons: relation to plasma levels of interleukin-10 and interferon-gamma. Blood. 1996;87:5144–5151. [PubMed] [Google Scholar]
- 16.Suzuki M, Okazaki N, Yoshino M, Yoshida T. Spontaneous regression of a hepatocellular carcinoma--a case report. Hepatogastroenterology. 1989;36:160–163. [PubMed] [Google Scholar]
- 17.Iwasaki M, Furuse J, Yoshino M, Moriyama N, Kanemoto H, Okumura H. Spontaneous regression of hepatocellular carcinoma: a case report. Jpn J Clin Oncol. 1997;27:278–281. doi: 10.1093/jjco/27.4.278. [DOI] [PubMed] [Google Scholar]
- 18.Imaoka S, Sasaki Y, Masutani S, Ishikawa O, Furukawa H, Kabuto T, Kameyama M, Ishiguro S, Hasegawa Y, Koyama H. Necrosis of hepatocellular carcinoma caused by spontaneously arising arterial thrombus. Hepatogastroenterology. 1994;41:359–362. [PubMed] [Google Scholar]
- 19.Gaffey MJ, Joyce JP, Carlson GS, Esteban JM. Spontaneous regression of hepatocellular carcinoma. Cancer. 1990;65:2779–2783. doi: 10.1002/1097-0142(19900615)65:12<2779::aid-cncr2820651228>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 20.Tocci G, Conte A, Guarascio P, Visco G. Spontaneous remission of hepatocellular carcinoma after massive gastrointestinal haemorrhage. BMJ. 1990;300:641–642. doi: 10.1136/bmj.300.6725.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ebara M, Ohto M, Shinagawa T, Sugiura N, Kimura K, Matsutani S, Morita M, Saisho H, Tsuchiya Y, Okuda K. Natural history of minute hepatocellular carcinoma smaller than three centimeters complicating cirrhosis. A study in 22 patients. Gastroenterology. 1986;90:289–298. doi: 10.1016/0016-5085(86)90923-6. [DOI] [PubMed] [Google Scholar]
- 22.Nakajima T, Moriguchi M, Mitsumoto Y, Katagishi T, Kimura H, Shintani H, Deguchi T, Okanoue T, Kagawa K, Ashihara T. Simple tumor profile chart based on cell kinetic parameters and histologic grade is useful for estimating the natural growth rate of hepatocellular carcinoma. Hum Pathol. 2002;33:92–99. doi: 10.1053/hupa.2002.30194. [DOI] [PubMed] [Google Scholar]


