Abstract
AIM: Acute hepatitis may seldom have a fulminant course. In the treatment of this medical emergency, potential liver support measure must provide immediate and sufficient assistance to the hepatic function. The goal of our study was to study the adequacy of hepatocyte transplantation (HCTx) in two different anatomical sites, splenic parenchyma and peritoneal cavity, in a rat model of reversible acute hepatitis induced by carbon tetrachloride (CCl4).
METHODS: After CCl4 intoxication, 84 male Wistar rats used as recipients were divided in to four experimental groups accordingly to their treatment: Group A (n = 24): intrasplenic transplantation of 10 × 106 isolated hepatocytes, Group B (n = 24): intraperitoneal transplantation of 20 × 106 isolated hepatocytes attached on plastic microcarriers, Group C (n = 18): intrasplenic injection of 1 mL normal saline (sham-operated controls), Group D (n = 18): intraperitoneal injection of 2.5 mL normal saline (sham-operated controls). Survival, liver function tests (LFT) and histology were studied in all four groups, on d 2, 5 and 10 post-HCTx.
RESULTS: The ten-day survival (and mean survival) in the 4 groups was 72.2% (8.1 ± 3.1), 33.3% (5.4 ± 3.4), 0% (3.1 ± 1.3) and 33.3% (5.4 ± 3.6) in groups A, B, C, D, respectively (PAB < 0.05, PAC < 0.05, PBD = NS). In the final survivors, LFT (except alkaline phosphatase) and hepatic histology returned to normal, independently of their previous therapy. Viable hepatocytes were identified within splenic parenchyma (in group A on d 2) and both in the native liver and the fatty tissue of abdominal wall (in group B on d 5).
CONCLUSION: A significantly better survival of the intrasplenically transplanted animals has been demonstrated. Intraperitoneal hepatocytes failed to promptly engraft. A different timing between liver injury and intraperitoneal HCTx may give better results and merits further investigation.
INTRODUCTION
Acute hepatitis may seldom have a fulminant course. In the treatment of this medical emergency, potential liver support measure must provide immediate and sufficient assistance to the hepatic function. Hepatocyte transplantation (HCTx) is a mainly experimental and minimally invasive method for liver support, offering the opportunity for native liver regeneration in cases of reversible failure or serving as a “bridge” to whole liver transplantation in cases of irreversible damage. However, for the clinical implementation of this newly developed technique both theoretical and practical aspects have to be further elucidated.
Various anatomical sites for cell implantation such as native liver, spleen, peritoneal cavity, kidney, lung, pancreas and fat pads have been investigated by different teams since the introduction of the method, but their results are not conclusive due to the great methodological diversity[1-7]. The spleen is considered as the most privileged anatomical site for HCTx. It is accessible by means of laparotomy. It can entrap a limited, but a sufficient number of hepatocytes within its sinusoids, providing conditions very similar to natural cell microenvironment. This process has been described by Mito in the early 1980’s, by the term “splenic hepatization”[8]. On the other hand, peritoneal cavity is very promising as it can be accessed by minimally invasive means and allows the injection of a greater number of cells. However, the major disadvantage of intraperitoneal route was the inability of injected cells to promptly engraft and thus to receive an adequate vascular supply. This led to cell death in three days following transplantation[9]. To overcome this limitation, Demetriou et al[10] introduced the technique of cell attachment on collagen-coated dextran-microcarriers, prior to transplantation. Since then, most experimental studies have relied on this concept. Microcarrier-attached hepatocytes can survive for a long time, forming neovascularized aggregates[11].
The aim of our study was to study the adequacy of HCTx in two different anatomical sites, splenic parenchyma and peritoneal cavity, in a rat model of reversible acute hepatitis induced by carbon tetrachloride (CCl4).
MATERIALS AND METHODS
Donors
Hepatocyte isolation Fifteen male Wistar rats (Institute Pasteur-Athens), weighing 300-450 g were used as donors of hepatocytes. For cell isolation, we used collagenase perfusion/incubation technique, which was previously described by our team[12]. Aliquots were taken for cell count (Neubauer hemocytometer) and determination of cellular viability (Trypan Blue Exclusion Test).
Recipients
Development of acute hepatitis model In preliminary experiments, we tested a wide range of CCl4 doses (0.5-4 mL/kg) given intraperitoneally in 35 controls. Total bilirubin, SGOT, SGPT and ALP levels were measured before and after CCl4 administration and correlated with the histological severity of liver damage. A single dose of 1 mL/kg CCl4, dissolved in olive oil in a ratio of 1:5 v/v, resulted in approximately 10% mortality of acute hepatic injury within 24 h. This dosage allowed liver support measures to be initiated before animal death and was used for all experiments reported herein.
Experimental groups
One hundred male Wistar rats (Institute Pasteur-Athens) weighing 300-450 g were used as candidate recipients. They were maintained in a 48-h dark/light cycle, had free access to food and water and were given CCl4 at the above-mentioned dose (d 0). Twenty-four hours later (d 1), 84 survivors were divided into 4 groups accordingly to their subsequent therapy: Group A (n = 24): intrasplenic transplantation of 10 × 106 isolated hepatocytes, Group B (n = 24): intraperitoneal transplantation of 20 × 106 isolated hepatocytes attached on plastic microcarriers, Group C (n = 18): intrasplenic injection of 1 mL normal saline (sham-operated controls), Group D (n = 18): intraperitoneal injection of 2.5 mL normal saline (sham-operated controls).
Transplantation technique
All rats were transplanted under ketamine and midazolame anesthesia. For intasplenic HCTx, cell suspension was diluted in 1 mL of Hank’s solution and injected after laparotomy using a 27G needle. Leakage was prevented with suture at the point of injection. Prior to intraperitoneal HCTx, cells were attached onto gelatin-coated plastic beads (Sigma, 1.5 g/10 g of liver tissue) by incubation at 37 °C for 45 min. Cell suspension was diluted in 2.5 mL of Hank’s solution and subsequently injected using an 18G needle
Survival, liver function tests and histological study
Eighteen rats in groups A and B and nine rats in control groups C and D were observed for a 10-day period, in order to determine survival (survival rate and mean survival). Three animals from each group were sacrificed on d 2 and 5 respectively for biochemical and histological study. Final survivors were also sacrificed on day 10 for both biochemical and histological study. Sacrifice was achieved under general anesthesia according to the guidelines of the Hellenic National Research Council. Blood samples were taken directly from the left ventricle. Total bilirubin, SGPT, SGOT and alkaline phosphatase were measured in all groups. Liver, spleen and cell aggregates (when macroscopically visible) were taken for anatomical study. After fixation in buffered formaldehyde 40 g/L, they were stained with hematoxylin/eosin. The prepared sections were assessed systematically under code by one hepatopathologist, who was unaware of protocol features.
Determination of normal and baseline values
Normal and baseline values (24 h after CCl4 administration) of liver function tests were measured in ten controls before and 24 h after CCl4-intoxication.
Statistical analysis
For statistical analysis of mean survival and liver function tests we used Student’s t-test. For statistical analysis of 10-day survival rate we used chi-square test. Statistical significance level was set at P < 0.05.
RESULTS
Number and viability of isolated hepatocytes
A mean of approximately 250 × 106 of liver cells was harvested from each donor, with a mean viability greater than 90%.
Survival rate
In all 4 groups, the 10-day survival rate was: 13/18 (72.2%), 6/18 (33.3%), 0/9 (0%) and 3/9/(33.3%) in groups A, B, C, D, respectively. Furthermore, the mean survival was 8.1 ± 3.1 d, 5.4 ± 3.4 d, 3.1 ± 1.3 d and 5.4 ± 3.6 d in groups A, B, C, D, respectively (Figure 1 and Figure 2). Statistically significant difference between transplanted groups (A and B) and their respective controls (C and D) was proven only for the intrasplenic HCTx (PAC < 0.05, PBD = NS). Differences in 10-day survival rate and mean survival between groups A and B were also statistically significant (PAB < 0.05). All animals with a decreased survival time died within six days after CCl4 poisoning.
Figure 1.
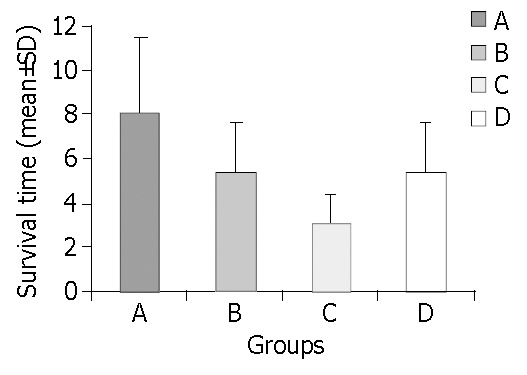
Mean survival in all four groups (PAC < 0.05, PBD = NS, PAB < 0.05).
Figure 2.
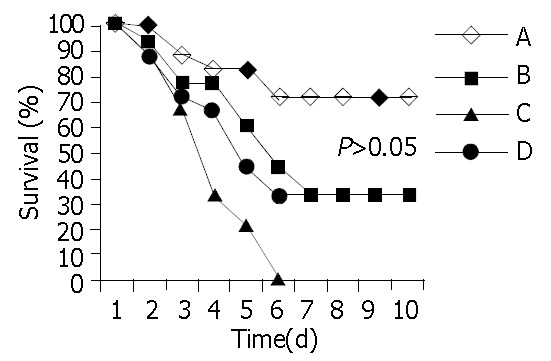
Survival curves in all four groups. Notice that all animal deaths occurred within the first 6 d after poisoning.
Laboratory findings
After CCl4 administration the rats responded with a marked increase in both serum aminotransferases, a moderate elevation in total bilirubin, while alkaline phosphatase was not affected. Table 1 summarizes the mean total bilirubin, SGPT, SGOT and alkaline phosphatase values during the whole period of the experimental observation.
Table 1.
Liver function tests during the 10-day observation period
| Day 2 | Day 5 | Day 10 | ||
| Total Bilirubin (mg/dL) | A | 0.53 ± 0.05 | 0.33 ± 0.05 | 0.35 ± 0.10 |
| B | 0.60 ± 0.10 | 0.46 ± 0.05 | 0.37 ± 0.14 | |
| C | 0.86 ± 0.10 | 0.76 ± 0.10 | 1 | |
| D | 0.86 ± 0.15 | 0.66 ± 0.15 | 0.20 ± 0.10 | |
| SGPT (IU/L) | A | 273 ± 76.2 | 67.5 ± 32.1 | 29.2 ± 11 |
| B | 400.3 ± 155 | 61.6 ± 27.7 | 54.8 ± 25.0 | |
| C | 710 ± 29.3 | 90.3 ± 23.1 | 1 | |
| D | 507.3 ± 91.2 | 66 ± 22.7 | 56.3 ± 14.9 | |
| SGOT (IU/L) | A | 380.6 ± 63.4 | 68.3 ± 24.1 | 84 ± 13.3 |
| B | 779.3 ± 235.2 | 287.6 ± 114 | 76.8 ± 30.9 | |
| C | 1088.3 ± 131.7 | 610.6 ± 148.6 | 1 | |
| D | 836.6 ± 171.2 | 250.6 ± 139.6 | 112 ± 63.2 | |
| Alkaline Phosphatase (IU/L) | A | 469.3 ± 157.5 | 427.6 ± 129.5 | 343.1 ± 180.5 |
| B | 88.6 ± 50 | 58.6 ± 39 | 564.3 ± 142.7 | |
| C | 123.6 ± 50.5 | 116.3 ± 37.4 | 1 | |
| D | 104 ± 31.1 | 129.3 ± 41.7 | 792.3 ± 246.5 | |
All differences between final and baseline values were statistically significant. 1All animals died before d 10. (Normal Values: Total Bilirubin: 0.18 ± 0.07 mg/dL, SGPT: 44.1 ± 24.8 IU/L, SGOT: 53.4 ± 23.6 IU/L, Alk. Phosphatase: 86.8 ± 49.6 IU/L -Baseline Value: Total Bilirubin: 0.6 ± 0.09 mg/dL, SGPT: 616.1 ± 117.5 IU/L, SGOT: 1296.7 ± 381.2 IU/L, Alk. Phosphatase: 91.4 ± 63 IU/L).
On d10, in all experimental groups total bilirubin and aminotransferase levels were significantly lower than their pre-transplantation levels. In contrast, all final survivors presented significantly higher levels of alkaline phosphatase, independently of their previous therapy.
Histological findings
Twenty-four hours following CCl4 intoxication, liver submassive necrosis affecting zone 3 of the hepatic lobule was the main histological finding. The type of cell injury leading to necrosis was more often ballooning degeneration and apoptosis. Normal hepatic histology was present in all final survivors. Viable hepatocytes were found within splenic parenchyma by d 2, forming structures similar to liver plates (Figure 3). Microcarrier-cell aggregates were macroscopically detected by d 5, within the native liver and the fatty tissue of the abdominal wall. The histological study of implants revealed presence of viable hepatocytes and formation of neo-vessels in the surrounding tissues (Figure 4).
Figure 3.
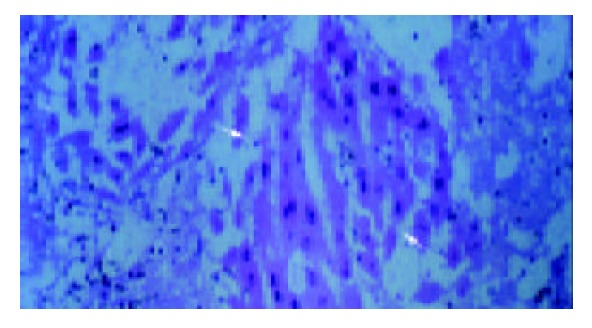
Viable hepatocytes within splenic parenchyma, forming structures similar to liver plates on d 2 (the so-called “splenic hepatization”).
Figure 4.
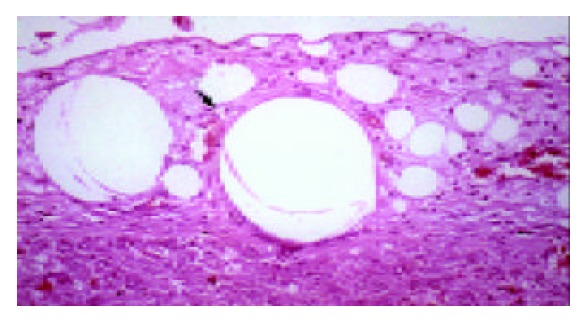
Microcarrier-cell aggregates within native liver parenchyma, Formation of neo-vessels in surrounding tissue on d 5.
DISCUSSION
To the best of our knowledge, this is the first experimental study, investigating two of the most popular anatomical sites for HCTx, in a model of chemically induced acute hepatic injury. In their original work, te Velde et al[13] compared and found equivalent intrasplenic and intraperitoneal HCTx, in a model of liver ischemia-induced failure. However, liver ischemia based on liver devascularization in rodents is a model that induces massive hepatic necrosis within a few hours, but in contrast to hepatic failure in humans, the damage is always irreversible. Thus, we have chosen to work on CCl4-induced acute hepatitis model, although it did not strictly meet the criteria of acute liver failure, because the induced liver damage was potentially reversible, mostly resembling the clinical course of the syndrome in humans and consequently was considered more realistic[14,15].
A significantly better survival of intrasplenically transplanted animals has been demonstrated, suggesting the superiority of the spleen as a site for cell implantation in this experimental model. Intraperitoneal hepatocytes failed to promptly engraft and provided a delayed and probably useless liver support, indicating that the right timing between induction of liver injury and HCTx seems to be of major importance. Actually, Demetriou et al[16] observed that when microcarrier-attached hepatocytes were transplanted 3 d prior to 90% hepatectomy in rats, they induced a marked improvement in long term survival, whereas when they were transplanted after hepatectomy they did not.
Furthermore, the rats succeeding to survive until d 6, remained alive for the whole 10-day period and their liver function tests and histology returned almost to normal, independently of their previous therapy. These survival data and laboratory findings are highly suggestive of the presence of a “crucial period” (extending from d 0 to d 6 in this experimental model), during which liver support measures must be initiated. Reversal of hepatic damage seems to be due initially to the direct action of engrafted hepatocytes during this “crucial period” and subsequently to the host liver recovery, taking place in all survivors beyond this period, independently of their initial treatment. Actually, the significant increase in alkaline phosphatase levels, occurring after d 5, could more possibly be attributed to the native liver regeneration than to a late cholestasis[17].
In conclusion, intrasplenic HCTx has been proved effective on providing liver support, after CCl4-intoxication. In contrast, a certain inability of intraperitoneally transplanted hepatocytes for immediate engraftment and vascularization, even after attachment onto microcarriers was observed in this experimental model. A different timing between liver injury and HCTx might give better results and merits further investigation. Theoretically, the intraperitoneal route has many advantages, such as easy accessibility and great capacity for cells implantation. To our opinion research should be encouraged towards this direction. It seems highly possible that the recent advances in various fields, such as the development of new biomaterials and especially the introduction of humoral factors accelerating the process of neovascularization, may overcome the current limitations of the intraperitoneal HCTx[18,19].
Footnotes
Edited by Wang XL Proofread by Xu FM
References
- 1.Matas AJ, Sutherland DE, Steffes MW, Mauer SM, Sowe A, Simmons RL, Najarian JS. Hepatocellular transplantation for metabolic deficiencies: decrease of plasms bilirubin in Gunn rats. Science. 1976;192:892–894. doi: 10.1126/science.818706. [DOI] [PubMed] [Google Scholar]
- 2.Mito M, Ebata H, Kusano M, Onishi T, Saito T, Sakamoto S. Morphology and function of isolated hepatocytes transplanted into rat spleen. Transplantation. 1979;28:499–505. doi: 10.1097/00007890-197912000-00013. [DOI] [PubMed] [Google Scholar]
- 3.Sutherland DE, Numata M, Matas AJ, Simmons RL, Najarian JS. Hepatocellular transplantation in acute liver failure. Surgery. 1977;82:124–132. [PubMed] [Google Scholar]
- 4.Ricordi C, Flye MW, Lacy PE. Renal subcapsular transplantation of clusters of hepatocytes in conjunction with pancreatic islets. Transplantation. 1988;45:1148–1151. doi: 10.1097/00007890-198806000-00034. [DOI] [PubMed] [Google Scholar]
- 5.Selden C, Gupta S, Johnstone R, Hodgson HJ. The pulmonary vascular bed as a site for implantation of isolated liver cells in inbred rats. Transplantation. 1984;38:81–83. [PubMed] [Google Scholar]
- 6.Vroemen JP, Buurman WA, van der Linden CJ, Visser R, Heirwegh KP, Kootstra G. Transplantation of isolated hepatocytes into the pancreas. Eur Surg Res. 1988;20:1–11. doi: 10.1159/000128734. [DOI] [PubMed] [Google Scholar]
- 7.Groth CG, Arborgh B, Björkén C, Sundberg B, Lundgren G. Correction of hyperbilirubinemia in the glucuronyltransferase-deficient rat by intraportal hepatocyte transplantation. Transplant Proc. 1977;9:313–316. [PubMed] [Google Scholar]
- 8.Mito M, Ebata H, Kusano M, Onishi T, Hiratsuka M, Saito T. Studies on ectopic liver utilizing hepatocyte transplantation into the rat spleen. Transplant Proc. 1979;11:585–591. [PubMed] [Google Scholar]
- 9.Henne-Bruns D, Krüger U, Sümpelmann D, Lierse W, Kremer B. Intraperitoneal hepatocyte transplantation: morphological results. Virchows Arch A Pathol Anat Histopathol. 1991;419:45–50. doi: 10.1007/BF01600151. [DOI] [PubMed] [Google Scholar]
- 10.Demetriou AA, Whiting JF, Feldman D, Levenson SM, Chowdhury NR, Moscioni AD, Kram M, Chowdhury JR. Replacement of liver function in rats by transplantation of microcarrier-attached hepatocytes. Science. 1986;233:1190–1192. doi: 10.1126/science.2426782. [DOI] [PubMed] [Google Scholar]
- 11.Arkadopoulos N, Rozga J, Petrovic L. Intraperitoneal transplantation of microcarrier-attached hepatocytes. In: Mito M, Sawa M editor. Hepatocyte transplantation. Basel Karger. 1997:98–106. [Google Scholar]
- 12.Fotiadis C, Pilichos H, Preza A, Papalois A, Demonakou M, Perrea D, Xekouki P, Poussios D, Karampela E, Papadopoulou A, et al. Transplantation of cultured allogeneic hepatocytes without immunosuppression. Transplant Proc. 2001;33:2400–2403. doi: 10.1016/s0041-1345(01)02037-1. [DOI] [PubMed] [Google Scholar]
- 13.te Velde AA, Bosman DK, Oldenburg J, Sala M, Maas MA, Chamuleau RA. Three different hepatocyte transplantation techniques for enzyme deficiency disease and acute hepatic failure. Artif Organs. 1992;16:522–526. doi: 10.1111/j.1525-1594.1992.tb00336.x. [DOI] [PubMed] [Google Scholar]
- 14.Sommer BG, Sutherland DE, Simmons RL, Najarian JS. Hepatocellular transplantation for experimental ischemic acute liver failure in dogs. J Surg Res. 1980;29:319–325. doi: 10.1016/0022-4804(80)90064-5. [DOI] [PubMed] [Google Scholar]
- 15.Chamuleau RAFM. Hepatocyte transplantation for acute liver failure. In: Mito M, Sawa M editor. Hepatocyte transplantation. Basel Karger. 1997:159–163. [Google Scholar]
- 16.Demetriou AA, Reisner A, Sanchez J, Levenson SM, Moscioni AD, Chowdhury JR. Transplantation of microcarrier-attached hepatocytes into 90% partially hepatectomized rats. Hepatology. 1988;8:1006–1009. doi: 10.1002/hep.1840080505. [DOI] [PubMed] [Google Scholar]
- 17.Pelton JJ, Hoffman JP, Eisenberg BL. Comparison of liver function tests after hepatic lobectomy and hepatic wedge resection. Am Surg. 1998;64:408–414. [PubMed] [Google Scholar]
- 18.Mooney DJ, Kaufmann PM, Sano K, McNamara KM, Vacanti JP, Langer R. Transplantation of hepatocytes using porous, biodegradable sponges. Transplant Proc. 1994;26:3425–3426. [PubMed] [Google Scholar]
- 19.de Roos WK, Borel Rinkes IH, Minnee P, Toet KH, Bouwman E, Kooistra T, Valerio D, Bruijn JA, Terpstra OT. Hepatocyte transplantation into solid supports in the rhesus monkey: the influence of acidic fibroblast growth factor on prevascularization and hepatocyte survival. Transplant Proc. 1995;27:633–634. [PubMed] [Google Scholar]


