the role of Na-K-ATPase in the regulation of epithelial cell polarity, adhesion, and migration has been demonstrated. Some of these processes, such as tight junction (TJ) formation, require ion transport function of Na-K-ATPase by its catalytic subunit Na-K-α. Other functions, such as motility suppression, cell adhesion, and contact inhibition, are mediated by the regulatory subunit Na-K-β, typically in association with Na-K-α but independent of the pump activity. Our recent study (5) showed that Na-K-β controls cell migration and phosphatidylinositol 3-kinase (PI3K)/ERK signaling via Na/Ca exchanger 1 (NCX1)-mediated regulation of intracellular Ca2+ concentration ([Ca2+]i), highlighting the ion dependency of Na-K-β-mediated suppression of cell migration. Na-K-β also regulates membrane localization of other ion transporters, such as the large-conductance Ca2+-activated K+ (BKCa) channel and Na-K-2Cl cotransporter isoform 2 (NKCC2). This leads us to speculate that these ion transporters might also be involved in facilitating additional functions of Na-K-β. In summary, we have come full circle: we started with the notion that the sole function of Na-K-ATPase is regulation of ion homeostasis, progressed to acceptance of the view that its subunit components can mediate diverse cellular processes by activating specific signaling pathways, independent of Na-K-ATPase activity, and, finally, closed the loop with recent studies that indicate that Na-K-β not only regulates membrane localization and activity of Na-K-α but also of other ion transporters such as NCX1, BKCa, and NKCC2, thereby governing ion homeostasis in numerous ways. Here, we propose that ion homeostasis may be crucial for the multiple cell functions mediated by Na-K-β.
Na-K-ATPase Subunit Components and Their Function in Ion Homeostasis
Na-K-ATPase is a hetero-oligomeric protein with a catalytic α-subunit (Na-K-α), a regulatory β-subunit (Na-K-β), and an auxiliary γ-subunit (reviewed in Refs. 17 and 55). Na-K-α is a polytopic membrane protein that possesses binding sites for Na+, K+, and ATP. Using energy gained from ATP hydrolysis, Na-K-α mediates efflux of three Na+ and influx of two K+ against the electrochemical gradient. Na-K-α is also the receptor for cardiac glycosides, such as digitalis and ouabain, which inhibit Na-K-ATPase activity. Na-K-β is a single-transmembrane glycoprotein with a large extracellular domain and a short cytoplasmic tail. The conventional role of Na-K-β is enhancement of the rate of synthesis, half-life, trafficking, and membrane stabilization of Na-K-α and regulation of its enzymatic activity (1, 12, 18, 41). There are four isoforms of Na-K-α and three isoforms of Na-K-β, which are expressed in a tissue-specific manner. Na-K-α1 and Na-K-β1 are ubiquitously expressed and, in this Viewpoint, are referred to as Na-K-α and Na-K-β, respectively, unless otherwise specified. The γ-subunit, belonging to the FXYD family of proteins, which is named based on the presence of an invariant amino acid signature sequence containing the FXYD motif (53), regulates Na-K-ATPase pump activity by binding to Na-K-α (57).
The regulation of ion homeostasis by the Na+ pump and its physiological role have been well studied since its discovery in 1957 (51). The pump activity is pivotal in maintaining the osmotic equilibrium and membrane potential, providing gradient for other Na+-dependent ion transporters and ancillary solute transport. Na-K-ATPase is functionally coupled to other ion transporters, such as NCX1 (4, 45), ATP-sensitive K+ channels (32, 38), glutamate transporters (46), and the BKCa channel (54), to execute housekeeping and specialized functions related to ion homeostasis.
Na-K-ATPase Functions in Addition to Ion Homeostasis
Recognition of the adhesion molecule on glia as Na-K-β2, with 40% identity to Na-K-β1 (21), indicates that other isoforms of Na-K-β may function similarly and that Na-K-ATPase subunits may have additional functions unrelated to pumping ions. Several decades after its discovery, additional functions of Na-K-ATPase, such as regulation of TJ, epithelial polarity, cell attachment, adhesion, migration, and proliferation, and signal transduction, were identified (6, 10, 13, 40, 58, 61). Epithelial cells mediate contact between adjacent cells and basement membrane via membrane protein complexes that form TJ, adherens junctions (AJ), desmosomes, gap junctions, and hemidesmosomes (15). TJ and septate junctions (the TJ equivalent in invertebrates) regulate paracellular ion transport by forming zones of apparent membrane fusions, or the “kissing points” (60), between adjacent cells and polarize the epithelial cell membrane into apical and basolateral membrane domains (11, 22, 56). Na-K-ATPase, initially used as a marker for basolateral membrane domain in epithelial cells, was later established as a regulator of TJ and epithelial polarity (13, 24, 33, 42, 64). Complete inhibition of Na-K-ATPase pump activity with ouabain for shorter duration retained cell viability but prevented TJ formation, increased paracellular permeability, and disrupted existing TJ in epithelial cells (19, 42, 64). The molecular mechanism by which Na-K-ATPase pump inhibition disrupted TJ formation involved 1) inhibition of RhoA activity, mediating rearrangement of TJ proteins and desmosomes (42), 2) reduction of protein phosphatase 2A (PP2A) activity, inducing hyperphosphorylation of the TJ protein occludin and disassembly of TJ strands (39), 3) regulation of Src family kinases (20), and 4) activation of ERK, leading to upregulation of claudin 3 and disruption of the apical junctional complex (14). These findings demonstrate the role of Na-K-ATPase pump activity in TJ biogenesis.
Increasing evidence suggests the presence of Na-K-ATPase in apical junctions (31, 39, 65). In agreement with this localization, Na-K-ATPase and its enzymatic activity are required for apicobasal polarity and vectorial transport of ions to facilitate lumen formation during organogenesis, as demonstrated in three-dimensional cultures of Madin-Darby canine kidney (MDCK) cells in collagen, zebrafish gut, Drosophila trachea, and mouse blastocysts (3, 36, 64). From these studies, we gain a new appreciation for the role of Na-K-ATPase-mediated ion homeostasis in TJ formation and organogenesis.
Na-K-ATPase Functions Independent of Ion Transport
As these additional functions of Na-K-ATPase were uncovered, it was necessary to clarify whether these functions are dependent on the pump activity or on the protein-protein interactions mediated by its constituent subunits. Pharmacological inhibition of Na-K-ATPase pump activity by ouabain at higher concentrations was used to ascertain the involvement of the ion transport activity in mediating these functions. Another approach was utilized following the identification of specific amino acid residues on opposite faces of the transmembrane domain of Na-K-β that mediate 1) homo-oligomerization and cell adhesion via the glycine zipper motif on one face and 2) hetero-oligomerization with Na-K-α via the heptad repeat motif on the other face of the transmembrane domain, thereby regulating pump activity (7). Cells expressing mutant Na-K-β that fail to hetero-oligomerize with Na-K-α were used to determine the contribution of ion transport activity in additional cell functions governed by Na-K-ATPase.
AJ mediate the formation of adhesive contacts between adjacent cells and chiefly comprise E-cadherin, a classical cell adhesion molecule, coupled to the actin cytoskeleton via interacting proteins (63, 68). Earlier studies revealed that Na-K-β functions synergistically with E-cadherin in restoring the epithelial phenotype of virally transformed cells (43). Further reports show that Na-K-β by itself can mediate intercellular adhesion by cis-homo-oligomerization, i.e., interaction between the transmembrane regions of two Na-K-β molecules within the same cell via the glycine zipper motif, in addition to Na-K-β trans-homo-oligomerization between adjacent cells facilitated by N-glycans and the immunoglobulin-like fold (resembling cell adhesion molecules) within the extracellular domain (2, 7, 50, 59, 62). Cell adhesion was unaltered in the heptad repeat motif mutant of Na-K-β, which had reduced Na-K-ATPase pump activity, confirming that Na-K-β-induced cell adhesion was independent of Na-K-ATPase pump activity (7). This characteristic of Na-K-β was shown to be K+-dependent (28), consistent with the presence of two K+-binding sites in extracellular and cytoplasmic domains of Na-K-β (12).
Knockdown of Na-K-β activated PI3K/ERK signaling associated with increased proliferation, reduced contact inhibition, and disrupted polarized phenotype in epithelial cells (8). Na-K-ATPase pump activity was not involved in these functions, since the heptad repeat mutant of Na-K-β with compromised Na-K-ATPase function did not activate PI3K/ERK signaling or disrupt epithelial polarity. Na-K-ATPase also functions as a signaling scaffold for Src, p85 subunit of PI3K, PKC, PP2A, PLC, and inositol 1,4,5-trisphosphate receptor proteins (16, 23, 37, 67, 69). Activation of these signaling pathways in the presence of ligand (cardiac glycosides) at concentrations that do not inhibit Na-K-ATPase activity resulted in proliferation or differentiation in a cell-type-specific manner (27, 30, 34, 67). Thus, regulation of signal transduction downstream of Na-K-ATPase is independent of pump activity.
Overexpression of Na-K-β in invasive MSV-MDCK cells suppressed motility and invasion (6, 43), while knockdown of Na-K-β increased migration and reduced contact inhibition (5, 8, 25), confirming the role of Na-K-β in the regulation of cell migration. This migration was dependent on protein-protein interactions of Na-K-ATPase subunits with PI3K and annexin II but independent of pump function (6). Thus the role of Na-K-β as a motility suppressor independent of ion transport function was consolidated.
Our recent study showed that activation of PI3K/ERK signaling and increased migration of Na-K-β knockdown cells were Ca2+-dependent (5). The increase in [Ca2+]i was mediated by the reduction of functional NCX1, a major regulator of Ca2+ in renal epithelial cells. Although Na-K-β-mediated suppression of PI3K/ERK signaling and motility is independent of Na-K-ATPase activity, it requires NCX1 function and is Ca2+-dependent. Similarly, suppression of proliferation and contact inhibition may also be Ca2+-dependent.
Na-K-β Regulates Other Ion Transporters
We showed recently that Na-K-β interacts with NCX1 and regulates its membrane localization, ascertaining the role of Na-K-β as a molecular chaperone responsible for the membrane trafficking of proteins in addition to Na-K-α (5). Similarly, other studies have shown that Na-K-β interacts with and targets the transport of BKCa channels to specific regions of plasma membrane, thereby regulating [Ca2+]i (26). The reduction of BKCa channel protein in the absence of Na-K-β led to significantly reduced membrane potential (26). Changes in membrane potential due to altered BKCa channel activity increased cancer cell migration and invasion (66), indicating that the BKCa channel may function downstream of Na-K-β in regulation of migration. Na-K-β also controls exocytosis and membrane expression of the NKCC2 channel, which is an important player in the regulation of Na+ and Cl− reabsorption (9). We propose that Na-K-β acts as a molecular chaperone for multiple ion transporters, resulting in regulation of ion homeostasis circuitously.
Many studies have shown the involvement of specific ions and their transporters in the regulation of cell functions (29, 47, 49), for example, homeostasis of intracellular Na+ concentration in maintenance of the epithelial phenotype (44), [Ca2+]i in regulation of cellular migration machinery and biogenesis of TJ formation (35, 52), and K+ balance in control of cell volume, thereby regulating sperm motility (48). Our study identified NCX1 and [Ca2+]i as downstream mediators in suppression of motility by Na-K-β. It is tempting to speculate that, similar to NCX1, other ion transporters regulated by Na-K-β are also responsible for governing multiple and overlapping pathways in which Na-K-ATPase subunits regulate diverse cellular processes.
Conclusions
Na-K-ATPase, composed of evolutionarily conserved Na-K-α and Na-K-β subunits, performs numerous cell functions. Na-K-ATPase-regulating TJ biogenesis is dependent on pump activity, while Na-K-β-regulating cell migration independent of pump function was shown by Na-K-ATPase inhibition (6). Use of cells expressing heptad repeat motif mutants of Na-K-β (7) enabled the determination of a minimal contribution of Na-K-ATPase pump activity in cell adhesion and contact inhibition (8). Although Na-K-ATPase pump activity was not involved, our recent study showed that restoration of the function of another ion transporter, NCX1, in Na-K-β knockdown cells reverted [Ca2+]i dependent PI3K/ERK signaling and suppressed migration (5). Moreover, Na-K-β has been shown to regulate other ion transporters, such as BKCa and NKCC2, apart from Na-K-α. Based on these studies, we infer that Na-K-β facilitates its moonlighting functions by regulating cell surface expression of ion transporters and, thereby, controlling ion homeostasis (Fig. 1). Thus, in disease conditions associated with loss of Na-K-β, the membrane localization and function of Na-K-α, as well as other ion transporters, are likely to be affected, leading to synchronous deterioration of ion homeostasis.
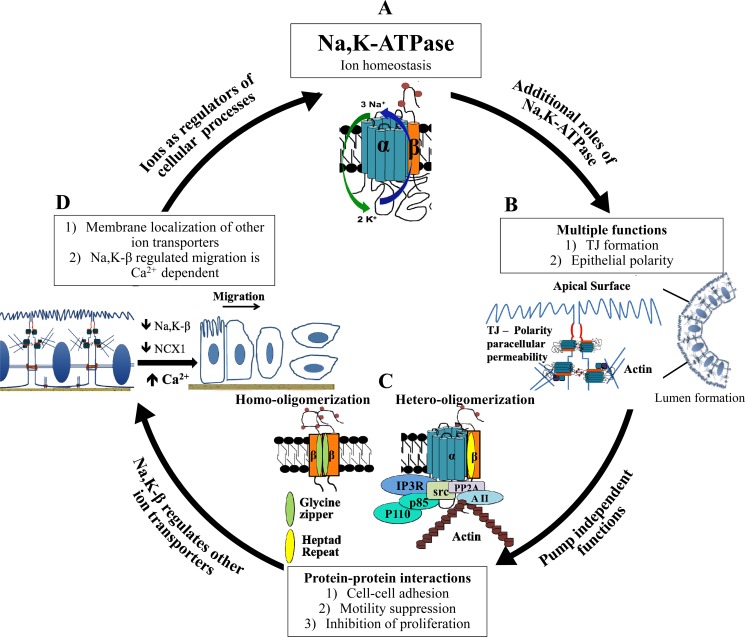
Fig. 1.
Role of Na-K-ATPase in ion-dependent regulation of epithelial cell functions. A: since its discovery by Jens Skou in 1957 (51), the role of Na-K-ATPase (composed of Na-K-α and Na-K-β subunits) in the regulation of ion homeostasis has been studied extensively. B: studies in the early 2000s elucidated other functions of Na-K-ATPase in the formation and maintenance of tight junctions (TJ) and preservation of epithelial polarity. A pictorial representation shows Na-K-ATPase localized to TJ regulating paracellular permeability and the formation of fluid-filled lumen. C: in the mid to late 2000s, various groups studied the pump-independent roles of Na-K-ATPase, specifically, the role of protein-protein interactions in the regulation of cell-cell adhesion and in suppression of motility and proliferation. The cartoon depicts protein-protein interactions mediated by Na-K-ATPase subunits. The glycine zipper motif (shown in green in the homodimerization cartoon) and N-glycan domains (shown as 3 red circles in the extracellular region) of Na-K-β mediate the interaction of Na-K-β with Na-K-β involved in cell adhesion. The heptad repeat motif of Na-K-β that mediates the interaction of Na-K-α with Na-K-β is shown in orange in the heterodimerization (Na-K-α/β) cartoon. Na-K-α interaction with Src, inositol 1,4,5-trisphosphate receptor (IP3R), and p85 subunit of phosphatidylinositol 3-kinase (PI3K) is shown, along with Na-K-β association with annexin II (A II) and protein phosphatase 2A (PP2A). D: additional functions of Na-K-β in the regulation of membrane localization of other ion transporters [such as Na/Ca exchanger 1 (NCX1), large-conductance Ca2+-activated K+ (BKCa) channel, and Na-K-2Cl cotransporter isoform 2 (NKCC2)], apart from its cognate partner Na-K-α, have come to light in recent years. Recently, we showed that Na-K-β-mediated migration is Ca2+-dependent and the loss of Na-K-β mediates reduction in NCX1, thereby increasing intracellular Ca2+ concentration ([Ca2+]i) and activating PI3K/ERK. In conclusion, ions are the mediators of Na-K-ATPase-dependent cellular processes governing formation and maintenance of the epithelial phenotype. The cartoon represents the increase in the rate of migration of epithelial cells with Na-K-β knockdown, mediated by a reduction of NCX1 and an increase in intracellular Ca2+.
GRANTS
This study was supported by American Heart Association Grant 10SDG260011, National Institute of General Medical Sciences Delaware CTR ACCEL Grant U54 GM-104941, National Institute of General Medical Sciences Delaware IDeA Networks of Biomedical Research Excellence Grant P20 GM-103446, and the Nemours Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.L.B. and S.P.B. prepared the figure; S.L.B. and S.P.B. drafted the manuscript; S.L.B., A.G., and S.P.B. edited and revised the manuscript; S.L.B., A.G., and S.P.B. approved the final version of the manuscript; A.G. and S.P.B. developed the concept and designed the research.
REFERENCES
- 1.Ackermann U, Geering K. Mutual dependence of Na,K-ATPase α- and β-subunits for correct posttranslational processing and intracellular transport. FEBS Lett 269: 105–108, 1990. [DOI] [PubMed] [Google Scholar]
- 2.Bab-Dinitz E, Albeck S, Peleg Y, Brumfeld V, Gottschalk KE, Karlish SJ. A C-terminal lobe of the β-subunit of Na,K-ATPase and H,K-ATPase resembles cell adhesion molecules. Biochemistry 48: 8684–8691, 2009. [DOI] [PubMed] [Google Scholar]
- 3.Bagnat M, Cheung ID, Mostov KE, Stainier DY. Genetic control of single lumen formation in the zebrafish gut. Nat Cell Biol 9: 954–960, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Baker PF, Blaustein MP, Hodgkin AL, Steinhardt RA. The influence of calcium on sodium efflux in squid axons. J Physiol 200: 431–458, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balasubramaniam SL, Gopalakrishnapillai A, Gangadharan V, Duncan RL, Barwe SP. Sodium-calcium exchanger 1 regulates epithelial cell migration via calcium-dependent extracellular signal-regulated kinase signaling. J Biol Chem 290: 12463–12473, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barwe SP, Anilkumar G, Moon SY, Zheng Y, Whitelegge JP, Rajasekaran SA, Rajasekaran AK. Novel role for Na,K-ATPase in phosphatidylinositol 3-kinase signaling and suppression of cell motility. Mol Biol Cell 16: 1082–1094, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barwe SP, Kim S, Rajasekaran SA, Bowie JU, Rajasekaran AK. Janus model of the Na,K-ATPase β-subunit transmembrane domain: distinct faces mediate α/β assembly and β-β homo-oligomerization. J Mol Biol 365: 706–714, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barwe SP, Skay A, McSpadden R, Huynh TP, Langhans SA, Inge LJ, Rajasekaran AK. Na,K-ATPase β-subunit cis homo-oligomerization is necessary for epithelial lumen formation in mammalian cells. J Cell Sci 125: 5711–5720, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carmosino M, Torretta S, Procino G, Timperio A, Zolla L, Svelto M. Na+/K+-ATPase β1-subunit is recruited in Na-K-2Cl co-transporter isoform 2 multiprotein complexes in rat kidneys: possible role in blood pressure regulation. J Hypertens 32: 1842–1853, 2014. [DOI] [PubMed] [Google Scholar]
- 10.Cereijido M, Contreras RG, Shoshani L, Larre I. The Na+-K+-ATPase as self-adhesion molecule and hormone receptor. Am J Physiol Cell Physiol 302: C473–C481, 2012. [DOI] [PubMed] [Google Scholar]
- 11.Cereijido M, Valdes J, Shoshani L, Contreras RG. Role of tight junctions in establishing and maintaining cell polarity. Annu Rev Physiol 60: 161–177, 1998. [DOI] [PubMed] [Google Scholar]
- 12.Chow DC, Forte JG. Functional significance of the β-subunit for heterodimeric P-type ATPases. J Exp Biol 198: 1–17, 1995. [DOI] [PubMed] [Google Scholar]
- 13.Contreras RG, Shoshani L, Flores-Maldonado C, Lazaro A, Cereijido M. Relationship between Na+,K+-ATPase and cell attachment. J Cell Sci 112: 4223–4232, 1999. [DOI] [PubMed] [Google Scholar]
- 14.de Souza WF, Barbosa LA, Liu L, de Araujo WM, de-Freitas-Junior JC, Fortunato-Miranda N, Fontes CF, Morgado-Diaz JA. Ouabain-induced alterations of the apical junctional complex involve α1- and β1-Na,K-ATPase downregulation and ERK1/2 activation independent of caveolae in colorectal cancer cells. J Membr Biol 247: 23–33, 2014. [DOI] [PubMed] [Google Scholar]
- 15.Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol 17: 375–412, 1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gable ME, Abdallah SL, Najjar SM, Liu L, Askari A. Digitalis-induced cell signaling by the sodium pump: on the relation of Src to Na+/K+-ATPase. Biochem Biophys Res Commun 446: 1151–1154, 2014. [DOI] [PubMed] [Google Scholar]
- 17.Geering K. Functional roles of Na,K-ATPase subunits. Curr Opin Nephrol Hypertens 17: 526–532, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Geering K. Subunit assembly and functional maturation of Na,K-ATPase. J Membr Biol 115: 109–121, 1990. [DOI] [PubMed] [Google Scholar]
- 19.Genova JL, Fehon RG. Neuroglian, gliotactin, and the Na+/K+ ATPase are essential for septate junction function in Drosophila. J Cell Biol 161: 979–989, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giannatselis H, Calder M, Watson AJ. Ouabain stimulates a Na+/K+-ATPase-mediated SFK-activated signalling pathway that regulates tight junction function in the mouse blastocyst. PLos One 6: e23704, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gloor S, Antonicek H, Sweadner KJ, Pagliusi S, Frank R, Moos M, Schachner M. The adhesion molecule on glia (AMOG) is a homologue of the β-subunit of the Na,K-ATPase. J Cell Biol 110: 165–174, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gumbiner B. Structure, biochemistry, and assembly of epithelial tight junctions. Am J Physiol Cell Physiol 253: C749–C758, 1987. [DOI] [PubMed] [Google Scholar]
- 23.Haas M, Wang H, Tian J, Xie Z. Src-mediated inter-receptor cross-talk between the Na+/K+-ATPase and the epidermal growth factor receptor relays the signal from ouabain to mitogen-activated protein kinases. J Biol Chem 277: 18694–18702, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Hammerton RW, Krzeminski KA, Mays RW, Ryan TA, Wollner DA, Nelson WJ. Mechanism for regulating cell surface distribution of Na+,K+-ATPase in polarized epithelial cells. Science 254: 847–850, 1991. [DOI] [PubMed] [Google Scholar]
- 25.Huynh TP, Barwe SP, Lee SJ, McSpadden R, Franco OE, Hayward SW, Damoiseaux R, Grubbs SS, Petrelli NJ, Rajasekaran AK. Glucocorticoids suppress renal cell carcinoma progression by enhancing Na,K-ATPase β1-subunit expression. PLos One 10: e0122442, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jha S, Dryer SE. The β1-subunit of Na+/K+-ATPase interacts with BKCa channels and affects their steady-state expression on the cell surface. FEBS Lett 583: 3109–3114, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaplan JG. Membrane cation transport and the control of proliferation of mammalian cells. Annu Rev Physiol 40: 19–41, 1978. [DOI] [PubMed] [Google Scholar]
- 28.Kitamura N, Ikekita M, Sato T, Akimoto Y, Hatanaka Y, Kawakami H, Inomata M, Furukawa K. Mouse Na+/K+-ATPase β1-subunit has a K+-dependent cell adhesion activity for β-GlcNAc-terminating glycans. Proc Natl Acad Sci USA 102: 2796–2801, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuo IY, Ehrlich BE. Ion channels in renal disease. Chem Rev 112: 6353–6372, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J, Tian J, Haas M, Shapiro JI, Askari A, Xie Z. Ouabain interaction with cardiac Na+/K+-ATPase initiates signal cascades independent of changes in intracellular Na+ and Ca2+ concentrations. J Biol Chem 275: 27838–27844, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Liu L, Ivanov AV, Gable ME, Jolivel F, Morrill GA, Askari A. Comparative properties of caveolar and noncaveolar preparations of kidney Na+/K+-ATPase. Biochemistry 50: 8664–8673, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mauerer UR, Boulpaep EL, Segal AS. Regulation of an inwardly rectifying ATP-sensitive K+ channel in the basolateral membrane of renal proximal tubule. J Gen Physiol 111: 161–180, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nelson WJ, Hammerton RW. A membrane-cytoskeletal complex containing Na+,K+-ATPase, ankyrin, and fodrin in Madin-Darby canine kidney (MDCK) cells: implications for the biogenesis of epithelial cell polarity. J Cell Biol 108: 893–902, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nguyen AN, Wallace DP, Blanco G. Ouabain binds with high affinity to the Na,K-ATPase in human polycystic kidney cells and induces extracellular signal-regulated kinase activation and cell proliferation. J Am Soc Nephrol 18: 46–57, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Nigam SK, Rodriguez-Boulan E, Silver RB. Changes in intracellular calcium during the development of epithelial polarity and junctions. Proc Natl Acad Sci USA 89: 6162–6166, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paul SM, Ternet M, Salvaterra PM, Beitel GJ. The Na+/K+ ATPase is required for septate junction function and epithelial tube-size control in the Drosophila tracheal system. Development 130: 4963–4974, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Pedemonte CH, Efendiev R, Bertorello AM. Inhibition of Na,K-ATPase by dopamine in proximal tubule epithelial cells. Semin Nephrol 25: 322–327, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Priebe L, Friedrich M, Benndorf K. Functional interaction between KATP channels and the Na+-K+ pump in metabolically inhibited heart cells of the guinea-pig. J Physiol 492: 405–417, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rajasekaran SA, Barwe SP, Gopal J, Ryazantsev S, Schneeberger EE, Rajasekaran AK. Na-K-ATPase regulates tight junction permeability through occludin phosphorylation in pancreatic epithelial cells. Am J Physiol Gastrointest Liver Physiol 292: G124–G133, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Rajasekaran SA, Barwe SP, Rajasekaran AK. Multiple functions of Na,K-ATPase in epithelial cells. Semin Nephrol 25: 328–334, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Rajasekaran SA, Gopal J, Willis D, Espineda C, Twiss JL, Rajasekaran AK. Na,K-ATPase β1-subunit increases the translation efficiency of the α1-subunit in MSV-MDCK cells. Mol Biol Cell 15: 3224–3232, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rajasekaran SA, Palmer LG, Moon SY, Peralta Soler A, Apodaca GL, Harper JF, Zheng Y, Rajasekaran AK. Na,K-ATPase activity is required for formation of tight junctions, desmosomes, and induction of polarity in epithelial cells. Mol Biol Cell 12: 3717–3732, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rajasekaran SA, Palmer LG, Quan K, Harper JF, Ball WJ Jr, Bander NH, Peralta Soler A, Rajasekaran AK. Na,K-ATPase β-subunit is required for epithelial polarization, suppression of invasion, and cell motility. Mol Biol Cell 12: 279–295, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rajasekaran SA, Rajasekaran AK. Na,K-ATPase and epithelial tight junctions. Front Biosci 14: 2130–2148, 2009. [DOI] [PubMed] [Google Scholar]
- 45.Reuter H, Henderson SA, Han T, Ross RS, Goldhaber JI, Philipson KD. The Na+-Ca2+ exchanger is essential for the action of cardiac glycosides. Circ Res 90: 305–308, 2002. [DOI] [PubMed] [Google Scholar]
- 46.Rose EM, Koo JC, Antflick JE, Ahmed SM, Angers S, Hampson DR. Glutamate transporter coupling to Na,K-ATPase. J Neurosci 29: 8143–8155, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwab A, Fabian A, Hanley PJ, Stock C. Role of ion channels and transporters in cell migration. Physiol Rev 92: 1865–1913, 2012. [DOI] [PubMed] [Google Scholar]
- 48.Schwab A, Hanley P, Fabian A, Stock C. Potassium channels keep mobile cells on the go. Physiology (Bethesda) 23: 212–220, 2008. [DOI] [PubMed] [Google Scholar]
- 49.Schwab A, Stock C. Ion channels and transporters in tumour cell migration and invasion. Philos Trans R Soc Lond B Biol Sci 369: 20130102, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shoshani L, Contreras RG, Roldan ML, Moreno J, Lazaro A, Balda MS, Matter K, Cereijido M. The polarized expression of Na+,K+-ATPase in epithelia depends on the association between β-subunits located in neighboring cells. Mol Biol Cell 16: 1071–1081, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skou JC. The influence of some cations on an adenosine triphosphatase from peripheral nerves. Biochim Biophys Acta 23: 394–401, 1957. [DOI] [PubMed] [Google Scholar]
- 52.Stuart RO, Sun A, Panichas M, Hebert SC, Brenner BM, Nigam SK. Critical role for intracellular calcium in tight junction biogenesis. J Cell Physiol 159: 423–433, 1994. [DOI] [PubMed] [Google Scholar]
- 53.Sweadner KJ, Rael E. The FXYD gene family of small ion transport regulators or channels: cDNA sequence, protein signature sequence, and expression. Genomics 68: 41–56, 2000. [DOI] [PubMed] [Google Scholar]
- 54.Tajima N, Itokazu Y, Korpi ER, Somerharju P, Kakela R. Activity of BKCa channel is modulated by membrane cholesterol content and association with Na+/K+-ATPase in human melanoma IGR39 cells. J Biol Chem 286: 5624–5638, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taniguchi K, Kaya S, Abe K, Mardh S. The oligomeric nature of Na/K-transport ATPase. J Biochem 129: 335–342, 2001. [DOI] [PubMed] [Google Scholar]
- 56.Tepass U, Tanentzapf G, Ward R, Fehon R. Epithelial cell polarity and cell junctions in Drosophila. Annu Rev Genet 35: 747–784, 2001. [DOI] [PubMed] [Google Scholar]
- 57.Therien AG, Pu HX, Karlish SJ, Blostein R. Molecular and functional studies of the γ-subunit of the sodium pump. J Bioenerg Biomembr 33: 407–414, 2001. [DOI] [PubMed] [Google Scholar]
- 58.Tian J, Xie ZJ. The Na-K-ATPase and calcium-signaling microdomains. Physiology (Bethesda) 23: 205–211, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tokhtaeva E, Sachs G, Souda P, Bassilian S, Whitelegge JP, Shoshani L, Vagin O. Epithelial junctions depend on intercellular trans-interactions between the Na,K-ATPase β1-subunits. J Biol Chem 286: 25801–25812, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol 2: 285–293, 2001. [DOI] [PubMed] [Google Scholar]
- 61.Vagin O, Dada LA, Tokhtaeva E, Sachs G. The Na-K-ATPase α1β1-heterodimer as a cell adhesion molecule in epithelia. Am J Physiol Cell Physiol 302: C1271–C1281, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vagin O, Tokhtaeva E, Sachs G. The role of the β1-subunit of the Na,K-ATPase and its glycosylation in cell-cell adhesion. J Biol Chem 281: 39573–39587, 2006. [DOI] [PubMed] [Google Scholar]
- 63.van Roy F, Berx G. The cell-cell adhesion molecule E-cadherin. Cell Mol Life Sci 65: 3756–3788, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Violette MI, Madan P, Watson AJ. Na+/K+-ATPase regulates tight junction formation and function during mouse preimplantation development. Dev Biol 289: 406–419, 2006. [DOI] [PubMed] [Google Scholar]
- 65.Vogelmann R, Nelson WJ. Fractionation of the epithelial apical junctional complex: reassessment of protein distributions in different substructures. Mol Biol Cell 16: 701–716, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wondergem R, Bartley JW. Menthol increases human glioblastoma intracellular Ca2+, BK channel activity and cell migration. J Biomed Sci 16: 90, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xie JX, Li X, Xie Z. Regulation of renal function and structure by the signaling Na/K-ATPase. IUBMB Life 65: 991–998, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yap AS, Brieher WM, Gumbiner BM. Molecular and functional analysis of cadherin-based adherens junctions. Annu Rev Cell Dev Biol 13: 119–146, 1997. [DOI] [PubMed] [Google Scholar]
- 69.Yuan Z, Cai T, Tian J, Ivanov AV, Giovannucci DR, Xie Z. Na/K-ATPase tethers phospholipase C and IP3 receptor into a calcium-regulatory complex. Mol Biol Cell 16: 4034–4045, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]