Abstract
In recent years, the intestinal mucosa has proven to be an intriguing organ to study tissue oxygenation. The highly vascularized lamina propria juxtaposed to an anaerobic lumen containing trillions of metabolically active microbes results in one of the most austere tissue microenvironments in the body. Studies to date have determined that a healthy mucosa contains a steep oxygen gradient along the length of the intestine and from the lumen to the serosa. Advances in technology have allowed multiple independent measures and indicate that, in the healthy mucosa of the small and large intestine, the lumen-apposed epithelia experience Po2 conditions of <10 mmHg, so-called physiologic hypoxia. This unique physiology results from a combination of factors, including countercurrent exchange blood flow, fluctuating oxygen demands, epithelial metabolism, and oxygen diffusion into the lumen. Such conditions result in the activation of a number of hypoxia-related signaling processes, including stabilization of the transcription factor hypoxia-inducible factor. Here, we review the principles of mucosal oxygen delivery, metabolism, and end-point functional responses that result from this unique oxygenation profile.
Keywords: barrier function, colon, hypoxia, intestine, metabolism
studies of the mucosa have provided important insight into metabolic demands associated with normal tissue function. Central to all metabolic processes is the availability and, in some cases, the unavailability of molecular oxygen. The gastrointestinal (GI) tract, for example, is characterized by a particularly unique oxygenation profile, experiencing profound fluctuations in blood perfusion on regular intervals throughout the day (26). Even at baseline, epithelial cells lining the mucosa exist in a relatively low-oxygen-tension (Po2) environment, herein described as “physiologic hypoxia.” Countercurrent oxygen exchange mechanisms in the small intestine have revealed that oxygen from the arterial blood supply diffuses to adjacent venules, along the crypt villus axis, resulting in graded levels of low oxygen (124). A steep oxygen gradient has also been documented in more distal, colonic regions of the GI tract, from the anaerobic lumen, across the epithelium, to the richly vascularized subepithelial mucosa (1). Given the high-energy requirement of the gut and the integral role of the epithelium in maintaining intestinal homeostasis, it is not surprising that these cells have evolved a number of coping mechanisms for this relatively austere metabolic environment. Here, we discuss how such localized differences in oxygenation contribute fundamentally to the function of the healthy mammalian intestine.
Oxygen Landscape of the Intestine
A steep oxygen gradient exists within the human intestinal tract. Breathable air at sea level has a Po2 of ∼145 mmHg (∼21% O2). Measurements of the healthy lung alveolus have revealed a Po2 of 100–110 mmHg (119). By stark contrast, the most luminal aspect of the healthy colon exists at a Po2 below 10 mmHg (1, 68, 69). Such differences reflect a combination of oxygen sources, local metabolism, and the anatomy of blood flow (Fig. 1). The Po2 drops precipitously along the radial axis from the intestinal submucosa to the lumen, which is home to trillions of anaerobic microbes. Over the last 50 years, significant progress has been made toward describing oxygenation at this interface; there are now numerous methods and tools to measure Po2 in the gut of mammals and in cell culture systems. Results from these experiments provide direct support for the oxygen gradient along the radial axis of the gut and are summarized in Table 1.
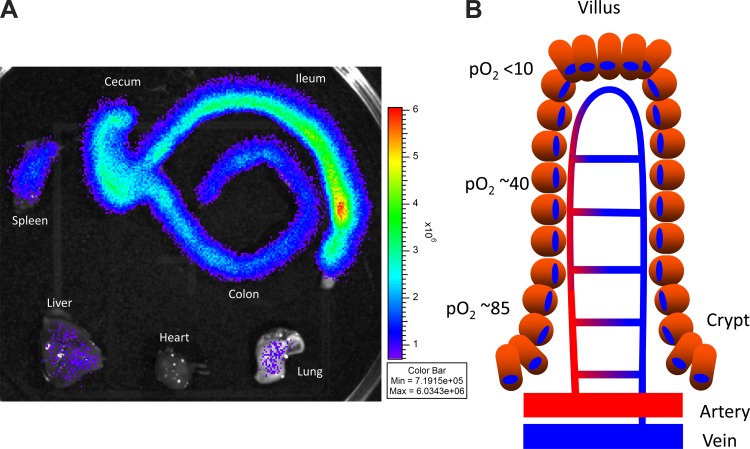
Fig. 1.
Comparison of basal hypoxia in intestinal tissue and other organs (A) and countercurrent blood flow in the healthy intestinal mucosa (B). A: organs from healthy hypoxia-inducible factor-luciferase reporter mice enable visualization of basal tissue hypoxia compared with other organs (113). B: a model of blood flow dynamics in the healthy intestinal mucosa. Countercurrent blood flow reduces local Po2 along the crypt-villus axis and results in low Po2 at the villus tip.
Table 1.
Measurements of intestinal mucosa oxygenation
| Location | Organ | Method | Po2, mmHg | Species | Reference |
|---|---|---|---|---|---|
| Serosa | Ileum | Electrode | 52 | Pig | 95 |
| Serosa | Terminal ileum | Electrode | 34 | Human | 125 |
| Serosa | Cecum | Electrode | 30 | Human | 125 |
| Serosa | Sigmoid colon | Electrode | 39 | Human | 125 |
| Mucosa | Colon | Pimonidazole | <10 | Mouse | 68 |
| Tissue | Cecum | OxyphorMicro probe | 40 | Mouse | 2 |
| Lumen | Rectum | Electrode | <1 | Human | 81 |
| Lumen | Duodenum | EPR | 32 | Mouse | 56 |
| Lumen | Small intestine | Electrode | <1 | Duck | 29 |
| Lumen | Ileum | EPR | 55.5 | Rat | 40 |
| Lumen | Cecum | OxyphorMicro probe | <1 | Mouse | 2 |
| Lumen | Ascending colon | EPR | 11 | Mouse | 56 |
| Lumen | Sigmoid colon | EPR | 3 | Mouse | 56 |
| Carcinoma | Colon | Electrode | 1–30 | Human | 142 |
EPR, electron paramagnetic resonance.
The Clark-type electrode was one of the initial tools that enabled measurement of tissue Po2. Placement of the probe onto tissue, such as the mucosal surface, generates a signal proportional to the flux of oxygen molecules to the cathode. In 1965, the Po2 in the lumen of the small intestine of the domestic duck was measured using this method. Single-point oxygenation measurements found the Po2 to be <0.5 mmHg (29). Subsequent adopters of this technology have characterized the oxygen concentration in the small intestine and colon to be heterogeneous with Po2 that ranges from <1 to >30 mmHg, respectively (125). Despite the probe's sensitivity and ability to provide real-time oxygen ionization information, it was invasive, sampled a limited area, and had a low signal-to-noise ratio due to oxygen leakage.
A more recent method, termed electron paramagnetic resonance (EPR) oximetry, is an imaging technique that enables oxygen to be quantified across larger areas in the colonic lumen repeatedly and noninvasively. It requires ingestion of activated charcoal, which acts as a spin probe, although any paramagnetic material that interacts with oxygen, such as nitroxides, lithium phthalocyanine, or India ink, can be utilized (47). Oxygen concentrations within the intestinal lumen are proportional to the decay of the spin polarization when subjected to an external magnetic field. Using this approach, luminal Po2 was shown to decrease along the longitudinal gut axis: 32, 11, and 3 mmHg in the duodenum, ascending colon, and sigmoid colon, respectively (56). One concern with this method is its limited spatial resolution. Another concern is the discrepancy in results between EPR and the Clark-type electrode. For example, EPR measurements of the distal colon found Po2 of 3 mmHg, while Po2 measured with the Clark-type electrode was <0.5 mmHg (56, 81). Nevertheless, despite their individual limitations, the Clark-type electrode and EPR have enabled insight into intestinal oxygen concentrations along the longitudinal axis of the mammalian gut.
More recently, a specialized intraluminal probe that uses phosphorescence quenching has enabled very accurate intraluminal Po2 measurement (1). Tissue oxygenation is quantified by exciting the probe with a pulse of light from an optical fiber and measuring the phosphorescence decay as molecular oxygen quenches phosphorescence. This probe has advantages over those used for EPR oximetry, because it cannot be endocytosed and, therefore, remains within the lumen and is minimally impacted by the viscous luminal contents. This surface Po2 of mouse cecal tissue was determined to be ∼40 mmHg with this technique and that of the cecal lumen to be <1 mmHg. Furthermore, microbiome analysis demonstrated that this radial oxygen gradient facilitates oxygen-tolerant organisms near the mucosa. The microbes inhabiting the gut reflect the local Po2; hence, it is not surprising that strict anaerobes thrive in this environment, given the exceedingly low Po2 measured within the lumen.
The discovery that 2-nitroimidazoles form adducts in hypoxic cells has also enabled the study of oxygen gradients in mammalian intestinal tissues both in vitro and in vivo (138). In their oxidized form, these compounds are taken up by living cells and readily excreted. However, when the Po2 is <10 mmHg, these compounds are reduced and, therefore, able to form adducts with thiol groups in proteins, leading to retention in hypoxic cells. Furthermore, pimonidazole HCl, a derivative of 2-nitroimidazole, has aided in validating EPR Po2 measurements (40). Pimonidazole staining could even be used to predict Po2 electrode measurements (100). It is neither dependent on redox enzymes nor changed by the NADH and NADPH levels (5). This technology, coupled with immunostaining, has been used to visually reflect the oxygenation of mouse tumors (6). Furthermore, it has been used to visualize the steep oxygen gradient between the gut lumen and submucosa (Fig. 2), a phenomenon sometimes referred to as “physiologic hypoxia” (26). In fact, it is a more stable marker than staining for hypoxia-inducible factor (HIF)-1α, since it is retained in chronically hypoxic cells (48), and such physiologic hypoxia can be reversed by oxygenation of the colonic lumen (e.g., using oxygenated perfluorodecalin) (57). These nitroimidazole dyes have also been used to image inflammatory lesions and revealed that mucosal lesions are profoundly hypoxic or even anoxic, similar to some large tumors, and penetrate deep into the mucosal tissue. It is likely that there are multiple contributing factors (i.e., vasculitis, vasoconstriction, edema, and increased O2 consumption) that predispose the inflamed intestinal epithelia to decreased oxygen delivery and hypoxia (68). These 2-nitroimidazole compounds have shown significant clinical utility, for example, in tumor imaging and in the identification of stroke regions within the brain (132). As opposed to other mucosal imaging techniques, these compounds are superior: they only image viable tissue and are inactive within apoptotic or necrotic regions (77). Studies are underway to use these compounds as adjunct radiosensitizers for enhancing chemotherapy targeting (50). Pimonidazole is now used widely as a hypoxia marker in both research and clinical studies of both normal and disease tissues (64, 120, 144).
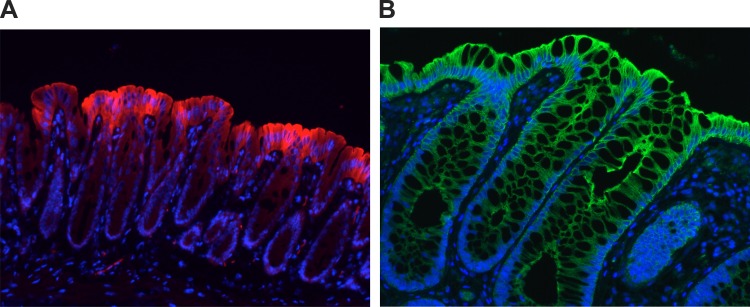
Fig. 2.
Physiologic hypoxia in the colonic epithelium mirrors localization of human β-defensin-1 (hBD1) in human colonic biopsies. A: “physiologic hypoxia.” Colonic mucosa of healthy mice shows small amounts of pimonidazole and nitroimidazole adduct along the luminal aspect of the colon (red), suggestive of physiologic hypoxia in the normal colon. B: immunofluorescence staining of hBD1 (green) in human colonic biopsies reveals localization within epithelium. The staining pattern is similar to that of pimonidazole, with the greatest intensity along the luminal aspect. DAPI (blue) was used to visualize nuclei.
Technologies to accurately measure oxygen consumption in cultured cells have also developed rapidly over the past several years. These technologies have enabled studies that monitor local oxygen concentrations in real time and during modeled conditions that mimic the mammalian microenvironment (e.g., leukocyte interactions with intestinal epithelial cells) (16). The SDR OxoDish system and the Seahorse Bioscience XF extracellular flux analyzer have been recently developed to precisely monitor oxygen consumption and are valid alternatives to the Clark-type electrodes, which were tedious and required significant expertise to operate (16, 110). The OxoDish uses a fluorescent dye embedded in a sensor spot at the bottom of a plate that is dependent on local Po2. This technology enables real-time data acquisition and continuous monitoring, but it samples only a small area near the sensor and requires indirect calculation of oxygen consumption rates.
The XF extracellular flux analyzer uses optical sensors to measure proton and oxygen in an isolated volume of media. The XF technology combines a sophisticated electro-optical instrument with “smart plastic” cartridges, which allows for real-time measurements of cellular bioenergetics in a noninvasive and multiwell microplate format. Mitochondrial respiration, indicated by oxygen consumption rates, and lactic acid production, from glycolysis, are indicated by changes in the extracellular acidification rate and can be measured in real time. This instrument enables characterization of in vitro metabolic activity and determination of oxygen consumption rates, glycolysis, ATP production, and respiratory capacity (38).
These investigations into intestinal epithelial physiology, such as measuring oxygen consumption in response to microbial-derived substrates (74). The ever-growing number of tools available to quantify intestinal Po2 has painted a much more complete picture of oxygen homeostasis in the epithelial interface. No doubt, further studies and technologic improvements will lead to a better understanding of intestinal physiology and cross talk with the microbiota.
Perfusion of the Intestinal Mucosa
Oxygenation of the intestinal epithelium depends on the balance of oxygen delivery, consumption, and diffusion into the lumen. Oxygen reaches the epithelium by way of three vessels, the celiac, superior, and inferior mesenteric arteries, which supply the digestive organs, including the small intestine and colon. These three vessels, in the unfed state, receive 20–25% of cardiac output (93). Small arterial branches penetrate the intestinal muscularis and coalesce into a submucosal arterial plexus that forms a vascular layer throughout the length of the intestines. Despite its mass, the muscularis receives only a fraction of the blood flow, with up to 80% being directed to the mucosal layer (21, 24). In the small intestine, 60% of mucosal blood flow is distributed to the villi and 40% to the crypts (93). Under fasting conditions, only a fraction of mucosal capillaries are utilized (124). However, following a meal, capillaries are recruited, as intestinal blood flow can increase up to 200% (93).
The small and large intestine differ in structure and function. The small intestine is optimized for digestion and absorption and has villi that amplify surface area. Villi necessitate a unique microcirculation to maintain perfusion. Each villus contains one or two arterioles, 10–15 μm in diameter, which travel toward the villous tip, where they form a hairpin capillary structure. The blood travels up these vessels and then back, in the opposite direction, toward a single venule. Within the villi, afferent and efferent vessels are separated by a distance of only 20 μm (86). Interestingly, this arrangement allows for a countercurrent shunt in which oxygen carried into the villus is able to diffuse to the venule without being transported through the vascular circuit bound to red blood cells; this reduces the oxygen content of blood delivered to the villous tip (52) (Fig. 1). Direct evidence for the existence of a countercurrent oxygen shunt was obtained when Kampp et al. (67) placed an oximeter in the venous outflow of an intestinal segment. They obtained upstream precapillary access, which enabled injection of oxygen-saturated blood and blood with impaired oxygen-carrying capacity (methemoglobin), which, in turn, allowed red cell transit time to be measured. After injection, a peak in postcapillary oxygen was detected earlier than the time it took for red blood cells to circulate, indicating the presence of an extravascular oxygen shunt in the villous circulation. This countercurrent shunt has also been demonstrated in humans. In consenting surgical patients, intraoperative arterial injection of a noble gas was observed to enter venous blood more quickly than would be possible had it remained within the vasculature, implying that diffusible gasses such as oxygen are able to short-cut the vasculature at the villous tip (52). Countercurrent shunt explains why partial occlusion of the superior mesenteric artery causes animals to develop mucosal lesions that could be prevented with application of oxygenated saline to the lumen. Despite autoregulatory responses that preserve overall blood flow in this model, red cell transit time is prolonged three- to fivefold, which is thought to increase the fraction of oxygen diffused through the countercurrent shunt and result in lower oxygen delivery to the villus tip (51, 86).
Neural regulation of intestinal microvasculature is coordinated by extrinsic and enteric innervation. Sympathetic input controls vasoconstriction, which originates from the celiac and mesenteric ganglia, and acts primarily on mucosal arterioles (137). This arrangement is part of the coordinated acute stress response that diverts blood to the brain and skeletal muscle during physical activity or periods of stress (104). The primary stimulus for neural-mediated vasodilation is mechanical input, mediated by intrinsic enteric neurons. This has the effect of increasing blood flow in response to luminal signals. However, during periods of low sympathetic activity, metabolic regulation of mucosal vasodilation appears to play a greater role in regulating mucosal blood flow than does neural input (137).
Metabolic regulation of intestinal blood flow occurs even during the fasting state. The small intestine is presented with 8.5 liters of fluid per day, including its own secretions. It absorbs 6 liters, and the remainder is presented to the colon, which absorbs all but ∼100 ml, which is lost in stool (13). Much of this fluid transport is mediated by sodium absorption and is driven by the basolateral Na-K-ATPase (11). Continuous ion and fluid transport has a profound impact on intestinal blood flow. This was illustrated in a rat model where intestinal blood flow of fasting animals was reduced by >40% following replacement of intraluminal sodium chloride solution with isotonic mannitol, which impaired ion transport (11). This function was attributed to nitric oxide (NO)-mediated vasodilation, as pharmacologic inhibition of NO synthase (NOS) produced results of similar magnitude (11). In support of this mechanism, it has been shown that an increase in the osmolarity of submucosal lymph caused a dose-dependent increase in arteriolar dilation that was dependent on NO (128). NO is a gaseous signaling molecule with autocrine and paracrine actions. It acts by diffusing into the vascular smooth muscle and induces vasodilation by activating soluble guanylate cyclase, leading to the formation of cGMP, although recent evidence indicates that NO metabolites are also active (136). This ultimately promotes calcium reuptake, loss of myosin phosphorylation, and relaxation of smooth muscle. In noninflamed states, most NO in the intestinal mucosa is synthesized in endothelial cells by endothelial NOS (eNOS) using oxygen and l-arginine as substrates. Although it requires oxygen, eNOS functions well in a low-Po2 environment, with a Km of 6.3 μmol/l for oxygen (Po2 <1 mmHg) (108). The lifespan of NO is inversely correlated to the local Po2; therefore, NO persists much longer in low-oxygen environments (134). NO is normally inactivated by oxidized cytochrome c, but this activity is impaired in low-oxygen conditions (102) and may explain why NO formed in the low-Po2 environment of the intestinal mucosa could have an exaggerated lifespan and action. Cellular Po2 influences eNOS expression in endothelial cells, thereby linking local Po2 with NO-mediated perfusion (28, 58, 99). Intraluminal bacteria may also contribute to NO production near the intestinal epithelium (37). In contrast with eukaryotic production of NO, which relies on arginine, bacteria are able to reduce nitrogen oxides, which are used as electron acceptors in anaerobic environments. However, there is no evidence that NO derived from the microbiota influences intestinal blood flow (126).
Postprandial hyperemia is the marked increase in blood flow to the small intestine stimulated by intraluminal nutrients, particularly lipids and carbohydrates (25). Mesenteric artery flow increases by 28–132% (15, 41, 42, 139, 140) and corresponds with sequential perfusion of the duodenum, jejunum, and then ileum (93). In healthy subjects, splanchnic oxygen uptake increases by 40–64% after consumption of a meal of 860–1000 kcal (53, 87). Blood flow associated with postprandial hyperemia is not distributed equally; it is preferentially directed to the mucosa in response to multiple signals. NO plays an important role in hyperemia associated with intraluminal glucose (10), and postprandial hyperemia can be prevented by inhibition of NOS (94). Interestingly, the role of NO in postprandial hyperemia is dependent on adenosine signaling. Adenosine is a purine nucleoside that mediates vasoconstriction or vasodilation, depending on concentration, tissue, and receptor subtype (91). Adenosine is a potent vasodilator in the intestine, and this response is mediated by A1 or A2B receptors (91, 92). ATP-intensive epithelial processes, such as glucose absorption, are believed to stimulate flux of adenosine into the circulation. Infusion of adenosine into the canine superior mesenteric artery resulted in a 2.5-fold increase in intestinal blood flow (49), and adenosine receptor antagonism prevented food-induced hyperemia (118) and the appearance of NO metabolites in the portal circulation (92). Use of intravital microscopy to observe the influence of NOS inhibition and adenosine receptor inhibition on postprandial hyperemia demonstrated the linkage between these pathways in responding to acute perfusion demands (91). Additional mediators such as prostaglandins likely also contribute to postprandial hyperemia, but their role is less well defined (23).
In contrast to the small intestine, where absorption of ingested nutrients is a major determinant of blood flow, postprandial hyperemia has not been observed in the colon (12, 44). Signals for changes in colonic blood flow are derived from short-chain fatty acids (SCFAs). Physiologic concentrations of these compounds can exceed 100 mM in the proximal colon (14), and it is estimated that they provide as much as 10% of energy requirements in humans and up to 70% in ruminant animals (8). In the colon, SCFAs are an important stimulus for perfusion. The best human evidence for this was provided by Mortensen et al. (98), who utilized resected colonic segments to determine the response of resistance vessels to varying concentrations of SCFA. Treatment with SCFAs, individually or in combination, but not glutamine, produced dose-dependent vasodilation starting at concentrations as low as 3 mM (98). The concentration of SCFA to which the resistance vessels are exposed in vivo is unclear. SCFAs, comprising primarily butyrate, acetate, and propionate, are present at high concentrations within the lumen. Butyrate is the only SCFA extensively metabolized at the epithelium, while significant amounts of acetate and propionate enter the portal circulation. SCFA concentrations in portal blood have been measured at autopsy to be 0.375 mM (30). However, this has been diluted by blood returning from the proximal GI tract, small intestine, spleen, and pancreas, which are not sites of SCFA production, so the actual concentration in the colonic vasculature would most certainly be higher. Support for the homeostatic role of SCFA-stimulated colonic perfusion is provided Mortensen et al. (97), who instilled SCFAs into the human rectum to promote postsurgical healing. This intervention demonstrated a marked increase in mucosal blood flow following 10–14 days of SCFA instillation into the human rectum, although it is not clear if this is the result of resistance vessel dilation or increased metabolic activity. The mechanism for SCFA-induced vasodilation appears to be independent of NO (31). Recent studies reveal a role for the SCFA receptor GPR41/FFAR3 in systemic blood pressure regulation (97, 106). While colonic blood flow in response to extracellular SCFA receptor stimulation has not been studied, it represents a possible mechanism to explain this phenomenon.
Epithelial Oxygen Consumption
The intestinal epithelium has diverse roles, including secretion, absorption, and immunity. An incredible amount of energy is invested to harvest energy from food. The thermic effect of food (i.e., the energy expended to digest and absorb food) has been quantified to be ∼10% of the total daily energy expenditure in humans (107). To support these activities, gastrointestinal oxygen consumption increases disproportionally to gastrointestinal blood flow (22). One study reported that 79% of ATP consumed by the Na-K-ATPase pump was derived from oxidative phosphorylation (34), and inhibition of basal sodium absorption in the ileum of fasting rats reduced oxygen consumption by nearly half (11). Moreover, the rate of oxygen consumption by the human colon, ∼8 μM·h−1·cm−2, is even greater than values reported for the rat (17, 114, 115). In one study, notable for its use of full-thickness healthy colonic tissue obtained during surgery, ex vivo treatment with ouabain, an inhibitor of the Na-K-ATPase pump, decreased whole tissue oxygen consumption by 26% (17). The small intestine and colon are dependent on ATP derived from oxidative phosphorylation, and epithelial oxygen consumption is an important determinant of oxygen balance at the interface between host and environment.
It is well established that butyrate, an end product of anaerobic bacterial metabolism, is the preferred energy source of the colonic epithelium, even over circulating energy sources (109), to the extent that very little of it escapes into the portal circulation (30). Butyrate metabolism has a direct bearing on epithelial oxygen consumption. Intestinal epithelial cell lines stimulated with butyrate exhibit an increased and sustained oxygen consumption rate that results in depletion of environmental oxygen relative to glucose control (74). Antibiotic depletion of the microbiota was shown to increase the Po2 of the colonic epithelium, as indicated by an oxygen-sensitive probe. This treatment mirrored germ-free mice, which also have higher Po2 of the colonic epithelium compared with controls (74). Restoration of luminal butyrate in antibiotic-treated mice reconstituted the physiologic low Po2 of the colonic epithelium and hypoxia-dependent signaling (74). Interestingly, Donohoe et al. (35) showed that, compared with conventionalized mice, the colonocytes of germ-free mice are ATP-deficient and the provision of butyrate can reverse this energy deficit by restoring oxidative respiration. Thus, given the voracious epithelial oxygen consumption in the presence of butyrate, it is possible that the prolific production of SCFAs in the cecum could, at least in part, explain the proximal-to-distal Po2 gradient along the longitudinal gut axis (56).
Luminal Oxygen Diffusion
The intestinal epithelium is a single cell layer with a surface area that approximates 300 m2. This cell layer is positioned between the low-Po2 lumen and the highly vascular lamina propria. Electron microscopy indicates that the average distance separating the base of the epithelial cells from the fenestrae of the capillaries is narrower in the colon than in the ileum (1.04 vs. 1.94 μm) (79). This arrangement permits oxygen to freely diffuse into the lumen. Establishment of the oxygen gradient depends on prior microbial colonization, which has been noted to occur sequentially, with oxygen-tolerant organisms being established before strict anaerobes (18, 117). Mucosal samples from human ileostomy sites show that facultative anaerobes dominate locations that are normally colonized by strict anaerobes in the native bowel (55). Beyond this, there is direct evidence that oxygen diffusion from the vasculature influences the mucosal microbiota. Albenberg et al. (1) showed that exposure of mice to hyperbaric oxygen (100% O2 at 2 atmospheres of pressure) for 4 days reduced the frequency of Anaerostipes, an obligate anaerobe, and caused complex alterations of 28 other species. Importantly, intraluminal oxygen returned toward baseline quickly after the animals returned to room air. This suggests that microbial utilization of oxygen by aero-tolerant organisms near the mucosa was active in driving the gradient and in facilitating anaerobic organisms deeper within the lumen. This arrangement of nonanaerobic organisms at the outer radial axis of the intestinal lumen was observed when the microbiome of rectal biopsies and paired stool samples from healthy individuals were analyzed (1). Taken together, these studies indicate that mucosal-associated organisms in the lumen actively consume host-derived oxygen and simultaneously contribute to the steep gradient across the epithelium and enable anaerobic organisms to thrive within the deep luminal space.
Hypoxia-Inducible Factor
HIF is a global regulator of oxygen homeostasis and facilitates both oxygen delivery and adaptation of oxygen deprivation in numerous cell types, including intestinal epithelial cells (122). HIF is a member of the Per-ARNT-Sim (PAS) domain family of basic helix-loop-helix transcription factors (143). HIFα is degraded in the presence of oxygen and is stabilized when oxygen is limited; HIF-1β is expressed in abundance and forms a heterodimer with HIFα to regulate gene transcription (62, 123). HIF-2α is an isoform of HIF-1α that is regulated and functions in a similar manner, with some notable differences in target gene specificity (83).
Under adequate-oxygen conditions, prolyl hydroxylase enzymes (PHDs) hydroxylate the α-subunits of HIF, which enables binding to the von Hippel-Lindau protein (65). PHDs use oxygen as a substrate for hydroxylation of HIF and are inhibited under hypoxic conditions. In this reaction, oxygen is inserted into the prolyl residue and into the co-substrate α-ketoglutarate, which splits it into CO2 and succinate. All three PHD isoforms are expressed on intestinal epithelium, and loss of PHD domains has been implicated in detrimental phenotypes. These include loss of exercise performance with PHD1 homozygous knockout, enhanced tumor angiogenesis with PHD2 heterozygous knockout, and decreased neuronal apoptosis, abnormal sympathoadrenal system development, and reduced blood pressure with PHD3 homozygous knockout (26).
Binding of hydroxylated HIFα to von Hippel-Lindau protein leads to HIFα ubiquitination, which targets the protein for subsequent degradation by the proteasome. There are physiologic and chemical mechanisms to inhibit HIF activation. Factor-inhibiting HIF-1 blocks HIF transactivation by hydroxylating an asparaginyl residue and blocking association of HIFα with the p300 coactivator protein (88). HIFα is also stabilized by inhibitors of hydroxylases, including dimethyloxalylglycine, a competitive antagonist of α-ketoglutarate. Other classes of HIF stabilizers include iron chelators, PHD active site inhibitors, cullin-2 deneddylators, and Fe2+ substitutes (36).
Original studies indicated that HIF is stabilized in a graded fashion with decreasing oxygen concentrations (63). Hundreds of genes are positively and negatively regulated in response to hypoxia in a HIF-dependent manner. Binding of HIF to target gene promoters, as determined by EMSA or chromatin immunoprecipitation, has been observed for a large number of these genes. Many genes respond to hypoxia in a HIF-independent manner. This could reflect indirect regulation by HIF, for example, by transcriptional repressors and microRNAs, or regulation by other pathways (78, 96, 146). Interestingly, only 40% of HIF-1-binding sites are within 2.5 kb of the transcriptional start site (120).
Epithelial Responses to HIF
An expanding body of literature points to HIF as the key mediator of intestinal epithelial adaptation to its low-Po2 microenvironment. HIF coordinates transcriptional responses that directly influence the determinants of oxygen homeostasis, including perfusion, metabolism, and barrier maintenance (Fig. f3). HIF is a fundamental regulator of whole body oxygen delivery through regulation of critical genes, such as erythropoietin and vascular endothelial growth factor, that support production and distribution of red blood cells (61). The intestinal epithelium also contributes to whole body oxygen distribution through its role in iron absorption, which supports erythropoiesis. Here too, HIF has emerged as a key regulator. Intestinal epithelial HIF, specifically HIF-2, targets include the gene-encoding divalent metal transporter 1, which mediates uptake of Fe2+ from the lumen (90), and ferroportin, which mediates basolateral iron efflux from epithelial cells (133). Hepcidin is a circulating protein produced in the liver that prevents iron efflux by binding ferroportin, inducing internalization and degradation. Hepcidin has recently been shown to be negatively regulated by hypoxia in a process mediated by both HIF-1 (105) and platelet-derived growth factor BB (127). HIF is also involved in production of adenosine, which, as discussed above, plays a fundamental role in regulating perfusion of the intestinal mucosa. This is achieved by the membrane-bound proteins CD39 and CD73, which enzymatically convert ATP/ADP to AMP and AMP to adenosine, respectively (4). Importantly, CD39 and CD73 expression is regulated by HIF-1α, providing yet another link between HIF and oxygen delivery (116, 131).
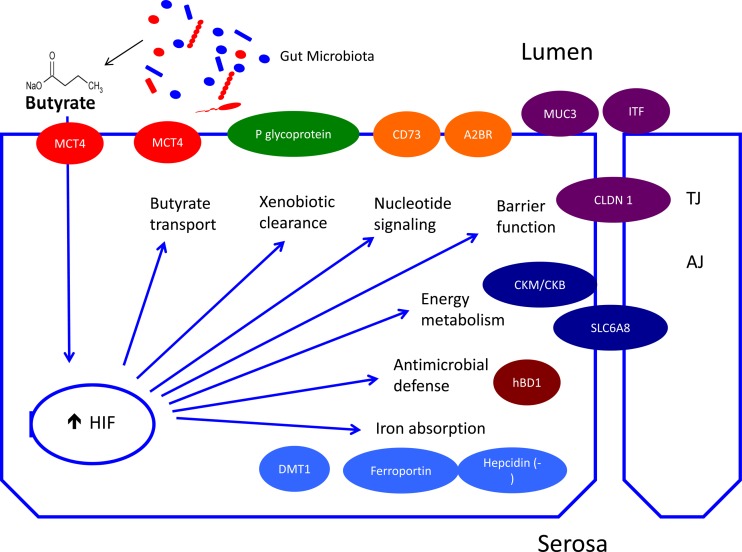
Fig. 3.
Oxygen homeostasis and physiologic regulation of intestinal epithelial function. In addition to the influence of countercurrent blood flow (see Fig. 1), microbial-derived short-chain fatty acids (e.g., butyrate) stimulate epithelial metabolism and deplete intracellular oxygen to the extent that hypoxia-inducible factor (HIF) 1 is stabilized. Transcriptional HIF responses in the normal colon include the physiologic regulation of genes important for butyrate transport [monocarboxylate transporter 1 (MCT1)], xenobiotic clearance (P-glycoprotein), adenosine metabolism (CD39 and CD73), epithelial barrier function [MUC3, intestinal trefoil factor (ITF), and claudin (CLDN1)], energy metabolism [creatine kinase enzymes (CKM/CKB) and SLC6A8], antimicrobial defense (hBD1), and iron absorption [divalent metal transporter 1 (DMT1), ferroportin, and hepcidin]. TJ, tight junction; AJ, adherens junction.
HIF also influences metabolic adaptation of the intestinal epithelium to its low-Po2 environment. The small intestine is subject to pronounced Po2 fluctuations associated with periodic ingestion of nutrients and appears flexible in its use of metabolic substrates, utilizing both glucose and glutamine to varying degrees (75, 141). In contrast, the colonic epithelium consistently favors oxidative metabolism of butyrate (17, 34). Notably, in the colon, the insatiable metabolism of butyrate depletes local oxygen, resulting in HIF stabilization and transcriptional activation of HIF target genes (74). HIF target genes that influence metabolism classically include those involved in glycolysis (33, 89), as well as pyruvate dehydrogenase kinase (76). This kinase inactivates pyruvate dehydrogenase, thereby preventing glucose-derived pyruvate from entering the tricarboxylic acid cycle as acetyl-CoA. Butyrate, in its role as a histone deacetylase inhibitor, also stimulates pyruvate dehydrogenase kinase 1 expression (9). This enables acetyl-CoA derived from β-oxidation of butyrate to enter the tricarboxylic acid cycle. In addition, monocarboxylate transporter 4, a butyrate transporter, is induced by HIF-1α, facilitating further butyrate uptake from the lumen (71, 135). These observations help explain how >70% of oxygen consumed by the human colonic epithelium is attributed to butyrate oxidation (109).
The contribution of HIF to the intestinal barrier is multifaceted and includes classical and nonclassical components of the epithelial barrier (72). Through cytoskeletal anchoring, tight junctions form the backbone of the epithelial barrier and help maintain polarity of epithelial cells by preventing lipid diffusion between apical and basolateral membranes (59). Claudins are integral membrane proteins responsible for selective permeability of tight junctions. Recently, claudin-1 (CLDN1) was identified to explain an aberrant junctional morphology of HIF-1β-deficient intestinal epithelial cell lines (112). This work showed that HIF maintains CLDN1 expression through binding hypoxia response element sequences in the gene promoter. The reintroduction of CLDN1 into HIF-1β knockdown cells restored barrier function and morphologic abnormalities (112). Adherens junctions are just basal to tight junctions. These structures are critical components of the apical junction complex and anchor to the perijunctional cytoskeleton, which includes a circumferential ring of actin and myosin. Notably, creatine kinase enzymes (CKM and CKB), as well as the creatine transporter (SLC6A8), were shown to be positively regulated by HIF-2α through interactions with hypoxia response elements in their promoter (46). Moreover, cytosolic CKB co-localizes with adherens junctions and plays an important role in supplying energy at junctional sites for tasks such as tight junction assembly, maintenance, and restitution (46).
Given its location between the vasculature and lumen, xenobiotic clearance is an important function of the intestinal epithelium. P-glycoprotein, also called multidrug resistance protein 1, has broad substrate specificity and is a primary effector of xenobiotic transport into the lumen. P-glycoprotein is transcriptionally regulated by HIF-1, thereby providing a distinct example of HIF-mediated barrier augmentation (27). In conjunction with mucus-secreting goblet cells, the intestinal epithelium extends its barrier apically through formation of the mucus layer. Mucus is a complex mixture of glycoproteins that allows delivery of nutrients to the epithelium while preventing exposure to potentially damaging substances and organisms. At least 10 distinct gel-forming and surface mucins are secreted by the intestinal epithelium (82). The mucus layer consists of an adherent layer, which is normally devoid of bacteria, and a thicker superficial layer, which is many times the diameter of the epithelium. Diameter of the intestinal mucus layer has been reported to range from 123–480 μm in the small intestine (15–29 μm firmly adherent) to 642–830 μm in the colon (101–116 μm firmly adherent) in thickness (7, 130). HIF regulates several components of the mucus layer that are secreted by intestinal epithelial cells. First, MUC3 is a HIF-1α target whose product, mucin-3, co-localizes with intestinal trefoil factor, another barrier-protective molecule characterized by robust trefoil domains (84, 85). Interestingly, intestinal trefoil factor itself is positively regulated by HIF-1α (43). One reason the mucus layer is such an effective microbial barrier is that it functions as a reservoir for secreted antimicrobial peptides (3). Defensins are a prominent class of antimicrobial peptides that are cationic, cysteine-rich, and possess broad antimicrobial activity (45, 103). Human β-defensin-1 (hBD1) is notable within the intestinal epithelium because it is constitutively secreted, whereas other defensins are only induced by inflammatory mediators (54, 101). Constitutive expression of hBD1 was shown to depend on basal HIF-1α signaling in multiple intestinal epithelial cell lines, and hBD1 expression correlated with other HIF target genes in human tissues (73). Another distinguishing feature of hBD1 is that the full spectrum of its antimicrobial activity is only revealed when its disulfide bonds are reduced (121). Reduction of the hBD1 disulfide bonds is accomplished by thioredoxin, which co-localizes with hBD1 in the colonic mucus; oxidation of hBD1 is prevented by the low-Po2 environment of the lumen (60). Considered as a whole, HIF signaling coordinates the transcription of manifold barrier protective genes that maintain the structure and function of the intestinal epithelium in low-Po2 environments (Figs. 2 and 3).
HIF is known to interact with other oxygen-responsive signaling pathways that are critical for epithelial homeostasis. For example, hypoxia is known to influence pathways such as the AMP-activated protein kinase pathway, which is activated when PHD activity is limited during hypoxia (145). Furthermore, AMP-activated protein kinase was shown to be necessary, although not sufficient, for the transcriptional regulation of HIF-1 (80). The X-box binding protein (XBP1) transcription factor provides another example. XBP1 is a regulator of the unfolded protein response (70). Intestinal epithelial XBP1 is critical for maintaining Paneth and goblet cell numbers and preventing colitis (70). XBP1 has been shown to protect cells from hypoxia-induced apoptosis (111), possibly by facilitating expression of HIF-1α targets through recruitment of RNA polymerase II (19). Another factor with a homeostatic role in the intestinal epithelium is NF-κB. The physiologic role for NF-κB is illustrated by intestinal epithelial-specific deletion, which reveals its part in immune homeostasis (147) and expression of antimicrobial peptides and antiapoptotic genes (129). Here too, the low-Po2 epithelial environment is critical. IKKβ, which mediates NF-κB repression, is itself regulated by oxygen-dependent PHD1, such that basal NF-κB activity is maintained in oxygen-limiting environments (32). Finally, the Wnt/β-catenin pathway is linked to oxygenation. The homeostatic role of β-catenin in the intestinal epithelium was exemplified when inducible loss of this signal caused terminal differentiation of intestinal stem cells, resulting in loss of crypt structure and impaired intestinal epithelial cell proliferation (39). HIF acts in a yin-yang manner to balance β-catenin signaling by exerting opposing pressures, with HIF-1α negatively (66) and HIF-2α positively regulating this pathway (20).
Conclusions
Differences in baseline Po2 in mucosal tissues and the profound shifts in energy demand during normal physiologic functions of the intestine provide a unique opportunity to understand tissue metabolism in health and disease. Results from in vitro and in vivo model systems have provided keen insight toward a better understanding of homeostatic physiology. Of particular recent interest is the interplay between tissue oxygenation and the microbiota, many of which culminate on HIF target pathways that are strongly associated with tissue barrier function and metabolic pathways fundamental to normal intestinal function. Ongoing studies to better define localized metabolomic signatures hold promise in elucidating the interplay of multiple pathways relevant to health and disease.
GRANTS
This work was supported by National Institutes of Health Grants DK-50189, DK-104713, DK-095491, F30 DK-096709, and TL1 TR-001081 and Veterans Affairs Merit Award I01BX002182.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.Z., C.J.K., and S.P.C. prepared the figures; L.Z., C.J.K., and S.P.C. drafted the manuscript; L.Z., C.J.K., and S.P.C. edited and revised the manuscript; L.Z., C.J.K., and S.P.C. approved the final version of the manuscript.
REFERENCES
- 1.Albenberg L, Esipova TV, Judge CP, Bittinger K, Chen J, Laughlin A, Grunberg S, Baldassano RN, Lewis JD, Li H, Thom SR, Bushman FD, Vinogradov SA, Wu GD. Correlation between intraluminal oxygen gradient and radial partitioning of intestinal microbiota. Gastroenterology 18: 1055–1063, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antoni L, Nuding S, Weller D, Gersemann M, Ott G, Wehkamp J, Stange EF. Human colonic mucus is a reservoir for antimicrobial peptides. J Crohns Colitis 7: e652–e664, 2013. [DOI] [PubMed] [Google Scholar]
- 4.Antonioli L, Pacher P, Vizi ES, Hasko G. CD39 and CD73 in immunity and inflammation. Trends Mol Med 19: 355–367, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arteel GE, Thurman RG, Raleigh JA. Reductive metabolism of the hypoxia marker pimonidazole is regulated by oxygen tension independent of the pyridine nucleotide redox state. Eur J Biochem 253: 743–750, 1998. [DOI] [PubMed] [Google Scholar]
- 6.Arteel GE, Thurman RG, Yates JM, Raleigh JA. Evidence that hypoxia markers detect oxygen gradients in liver: pimonidazole and retrograde perfusion of rat liver. Br J Cancer 72: 889–895, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atuma C, Strugala V, Allen A, Holm L. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am J Physiol Gastrointest Liver Physiol 280: G922–G929, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Bergman EN. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol Rev 70: 567–590, 1990. [DOI] [PubMed] [Google Scholar]
- 9.Blouin JM, Penot G, Collinet M, Nacfer M, Forest C, Laurent-Puig P, Coumoul X, Barouki R, Benelli C, Bortoli S. Butyrate elicits a metabolic switch in human colon cancer cells by targeting the pyruvate dehydrogenase complex. Int J Cancer 128: 2591–2601, 2011. [DOI] [PubMed] [Google Scholar]
- 10.Bohlen HG. Mechanism of increased vessel wall nitric oxide concentrations during intestinal absorption. Am J Physiol Heart Circ Physiol 275: H542–H550, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Bohlen HG, Lash JM. Intestinal absorption of sodium and nitric oxide-dependent vasodilation interact to dominate resting vascular resistance. Circ Res 78: 231–237, 1996. [DOI] [PubMed] [Google Scholar]
- 12.Bond JH, Prentiss RA, Levitt MD. The effects of feeding on blood flow to the stomach, small bowel, and colon of the conscious dog. J Lab Clin Med 93: 594–599, 1979. [PubMed] [Google Scholar]
- 13.Boron WF, Boulpaep EL. Medical Physiology: A Cellular and Molecular Approach. Philadelphia, PA: Saunders/Elsevier, 2009, p. xii. [Google Scholar]
- 14.Bugaut M. Occurrence, absorption and metabolism of short chain fatty acids in the digestive tract of mammals. Comp Biochem Physiol B 86: 439–472, 1987. [DOI] [PubMed] [Google Scholar]
- 15.Burns GP, Schenk WG Jr.. Effect of digestion and exercise on intestinal blood flow and cardiac output. An experimental study in the conscious dog. Arch Surg 98: 790–794, 1969. [DOI] [PubMed] [Google Scholar]
- 16.Campbell EL, Bruyninckx WJ, Kelly CJ, Glover LE, McNamee EN, Bowers BE, Bayless AJ, Scully M, Saeedi BJ, Golden-Mason L, Ehrentraut SF, Curtis VF, Burgess A, Garvey JF, Sorensen A, Nemenoff R, Jedlicka P, Taylor CT, Kominsky DJ, Colgan SP. Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity 40: 66–77, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carra GE, Ibanez JE, Saravi FD. Electrogenic transport, oxygen consumption, and sensitivity to acute hypoxia of human colonic epithelium. Int J Colorectal Dis 26: 1205–1210, 2011. [DOI] [PubMed] [Google Scholar]
- 18.Celesk RA, Asano T, Wagner M. The size pH, and redox potential of the cecum in mice associated with various microbial floras. Proc Soc Exp Biol Med 151: 260–263, 1976. [DOI] [PubMed] [Google Scholar]
- 19.Chen X, Iliopoulos D, Zhang Q, Tang Q, Greenblatt MB, Hatziapostolou M, Lim E, Tam WL, Ni M, Chen Y, Mai J, Shen H, Hu DZ, Adoro S, Hu B, Song M, Tan C, Landis MD, Ferrari M, Shin SJ, Brown M, Chang JC, Liu XS, Glimcher LH. XBP1 promotes triple-negative breast cancer by controlling the HIF1α pathway. Nature 508: 103–107, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi H, Chun YS, Kim TY, Park JW. HIF-2α enhances β-catenin/TCF-driven transcription by interacting with β-catenin. Cancer Res 70: 10101–10111, 2010. [DOI] [PubMed] [Google Scholar]
- 21.Chou CC. Relationship between intestinal blood flow and motility. Annu Rev Physiol 44: 29–42, 1982. [DOI] [PubMed] [Google Scholar]
- 22.Chou CC. Splanchnic and overall cardiovascular hemodynamics during eating and digestion. Fed Proc 42: 1658–1661, 1983. [PubMed] [Google Scholar]
- 23.Chou CC, Alemayehu A, Mangino MJ. Prostanoids in regulation of postprandial jejunal hyperemia and oxygen uptake. Am J Physiol Gastrointest Liver Physiol 257: G798–G808, 1989. [DOI] [PubMed] [Google Scholar]
- 24.Chou CC, Grassmick B. Motility and blood flow distribution within the wall of the gastrointestinal tract. Am J Physiol Heart Circ Physiol 235: H34–H39, 1978. [DOI] [PubMed] [Google Scholar]
- 25.Chou CC, Kvietys P, Post J, Sit SP. Constituents of chyme responsible for postprandial intestinal hyperemia. Am J Physiol Heart Circ Physiol 235: H677–H682, 1978. [DOI] [PubMed] [Google Scholar]
- 26.Colgan SP, Taylor CT. Hypoxia: an alarm signal during intestinal inflammation. Nat Rev Gastroenterol Hepatol 7: 281–287, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Comerford KM, Wallace TJ, Karhausen J, Louis NA, Montalto MC, Colgan SP. Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res 62: 3387–3394, 2002. [PubMed] [Google Scholar]
- 28.Coulet F, Nadaud S, Agrapart M, Soubrier F. Identification of hypoxia-response element in the human endothelial nitric-oxide synthase gene promoter. J Biol Chem 278: 46230–46240, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Crompton DW, Shrimpton DH, Silver IA. Measurements of the oxygen tension in the lumen of the small intestine of the domestic duck. J Exp Biol 43: 473–478, 1965. [DOI] [PubMed] [Google Scholar]
- 30.Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 28: 1221–1227, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cummings JH, Rombeau JL, Sakata T. Physiological and Clinical Aspects of Short-Chain Fatty Acids. New York: Cambridge University Press, 2004, p. xx. [Google Scholar]
- 32.Cummins EP, Berra E, Comerford KM, Ginouves A, Fitzgerald KT, Seeballuck F, Godson C, Nielsen JE, Moynagh P, Pouyssegur J, Taylor CT. Prolyl hydroxylase-1 negatively regulates IκB kinase-β, giving insight into hypoxia-induced NFκB activity. Proc Natl Acad Sci USA 103: 18154–18159, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Miguel MP, Alcaina Y, de la Maza DS, Lopez-Iglesias P. Cell metabolism under microenvironmental low oxygen tension levels in stemness, proliferation and pluripotency. Curr Mol Med 15: 343–359, 2015. [DOI] [PubMed] [Google Scholar]
- 34.Del Castillo JR, Ricabarra B, Sulbaran-Carrasco MC. Intermediary metabolism and its relationship with ion transport in isolated guinea pig colonic epithelial cells. Am J Physiol Cell Physiol 260: C626–C634, 1991. [DOI] [PubMed] [Google Scholar]
- 35.Donohoe DR, Garge N, Zhang X, Sun W, O'Connell TM, Bunger MK, Bultman SJ. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab 13: 517–526, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eltzschig HK, Bratton DL, Colgan SP. Targeting hypoxia signalling for the treatment of ischaemic and inflammatory diseases. Nat Rev Drug Discov 13: 852–869, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Espey MG. Role of oxygen gradients in shaping redox relationships between the human intestine and its microbiota. Free Radic Biol Med 55: 130–140, 2013. [DOI] [PubMed] [Google Scholar]
- 38.Ferrick DA, Neilson A, Beeson C. Advances in measuring cellular bioenergetics using extracellular flux. Drug Discov Today 13: 268–274, 2008. [DOI] [PubMed] [Google Scholar]
- 39.Fevr T, Robine S, Louvard D, Huelsken J. Wnt/β-catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol Cell Biol 27: 7551–7559, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fisher EM, Khan M, Salisbury R, Kuppusamy P. Noninvasive monitoring of small intestinal oxygen in a rat model of chronic mesenteric ischemia. Cell Biochem Biophys 67: 451–459, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fronek K, Fronek A. Combined effect of exercise and digestion on hemodynamics in conscious dogs. Am J Physiol 218: 555–559, 1970. [DOI] [PubMed] [Google Scholar]
- 42.Fronek K, Stahlgren LH. Systemic and regional hemodynamic changes during food intake and digestion in nonanesthetized dogs. Circ Res 23: 687–692, 1968. [DOI] [PubMed] [Google Scholar]
- 43.Furuta GT, Turner JR, Taylor CT, Hershberg RM, Comerford K, Narravula S, Podolsky DK, Colgan SP. Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J Exp Med 193: 1027–1034, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gallavan RH Jr, Chou CC, Kvietys PR, Sit SP. Regional blood flow during digestion in the conscious dog. Am J Physiol Heart Circ Physiol 238: H220–H225, 1980. [DOI] [PubMed] [Google Scholar]
- 45.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol 3: 710–720, 2003. [DOI] [PubMed] [Google Scholar]
- 46.Glover LE, Bowers BE, Saeedi B, Ehrentraut SF, Campbell EL, Bayless AJ, Dobrinskikh E, Kendrick AA, Kelly CJ, Burgess A, Miller L, Kominsky DJ, Jedlicka P, Colgan SP. Control of creatine metabolism by HIF is an endogenous mechanism of barrier regulation in colitis. Proc Natl Acad Sci USA 110: 19820–19825, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goda F, Liu KJ, Walczak T, O'Hara JA, Jiang J, Swartz HM. In vivo oximetry using EPR and India ink. Magn Reson Med 33: 237–245, 1995. [DOI] [PubMed] [Google Scholar]
- 48.Goethals L, Debucquoy A, Perneel C, Geboes K, Ectors N, De Schutter H, Penninckx F, McBride WH, Begg AC, Haustermans KM. Hypoxia in human colorectal adenocarcinoma: comparison between extrinsic and potential intrinsic hypoxia markers. Int J Radiat Oncol Biol Phys 65: 246–254, 2006. [DOI] [PubMed] [Google Scholar]
- 49.Granger HJ, Norris CP. Role of adenosine in local control of intestinal circulation in the dog. Circ Res 46: 764–770, 1980. [DOI] [PubMed] [Google Scholar]
- 50.Guise CP, Mowday AM, Ashoorzadeh A, Yuan R, Lin WH, Wu DH, Smaill JB, Patterson AV, Ding K. Bioreductive prodrugs as cancer therapeutics: targeting tumor hypoxia. Chin J Cancer 33: 80–86, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haglund U, Lundgren O. The small intestine in hemorrhagic shock (Letter). Gastroenterology 66: 625–627, 1974. [PubMed] [Google Scholar]
- 52.Hallback DA, Hulten L, Jodal M, Lindhagen J, Lundgren O. Evidence for the existence of a countercurrent exchanger in the small intestine in man. Gastroenterology 74: 683–690, 1978. [PubMed] [Google Scholar]
- 53.Hansen HJ, Engell HC, Ring-Larsen H, Ranek L. Splanchnic blood flow in patients with abdominal angina before and after arterial reconstruction. A proposal for a diagnostic test. Ann Surg 186: 216–220, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harder J, Bartels J, Christophers E, Schroder JM. Isolation and characterization of human β-defensin-3, a novel human inducible peptide antibiotic. J Biol Chem 276: 5707–5713, 2001. [DOI] [PubMed] [Google Scholar]
- 55.Hartman AL, Lough DM, Barupal DK, Fiehn O, Fishbein T, Zasloff M, Eisen JA. Human gut microbiome adopts an alternative state following small bowel transplantation. Proc Natl Acad Sci USA 106: 17187–17192, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.He G, Shankar RA, Chzhan M, Samouilov A, Kuppusamy P, Zweier JL. Noninvasive measurement of anatomic structure and intraluminal oxygenation in the gastrointestinal tract of living mice with spatial and spectral EPR imaging. Proc Natl Acad Sci USA 96: 4586–4591, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hindryckx P, Devisscher L, Laukens D, Venken K, Peeters H, De Vos M. Intrarectal administration of oxygenated perfluorodecalin promotes healing of murine colitis by targeting inflammatory hypoxia. Lab Invest 91: 1266–1276, 2011. [DOI] [PubMed] [Google Scholar]
- 58.Hoffmann A, Gloe T, Pohl U. Hypoxia-induced upregulation of eNOS gene expression is redox-sensitive: a comparison between hypoxia and inhibitors of cell metabolism. J Cell Physiol 188: 33–44, 2001. [DOI] [PubMed] [Google Scholar]
- 59.Ivanov AI, Parkos CA, Nusrat A. Cytoskeletal regulation of epithelial barrier function during inflammation. Am J Pathol 177: 512–524, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jaeger SU, Schroeder BO, Meyer-Hoffert U, Courth L, Fehr SN, Gersemann M, Stange EF, Wehkamp J. Cell-mediated reduction of human β-defensin 1: a major role for mucosal thioredoxin. Mucosal Immunol 6: 1179–1190, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jelkmann W, Hellwig-Burgel T. Biology of erythropoietin. Adv Exp Med Biol 502: 169–187, 2001. [DOI] [PubMed] [Google Scholar]
- 62.Jiang BH, Rue E, Wang GL, Roe R, Semenza GL. Dimerization, DNA binding, and transactivation properties of hypoxia-inducible factor 1. J Biol Chem 271: 17771–17778, 1996. [DOI] [PubMed] [Google Scholar]
- 63.Jiang BH, Semenza GL, Bauer C, Marti HH. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am J Physiol Cell Physiol 271: C1172–C1180, 1996. [DOI] [PubMed] [Google Scholar]
- 64.Kaanders JH, Wijffels KI, Marres HA, Ljungkvist AS, Pop LA, van den Hoogen FJ, de Wilde PC, Bussink J, Raleigh JA, van der Kogel AJ. Pimonidazole binding and tumor vascularity predict for treatment outcome in head and neck cancer. Cancer Res 62: 7066–7074, 2002. [PubMed] [Google Scholar]
- 65.Kaelin WG Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell 30: 393–402, 2008. [DOI] [PubMed] [Google Scholar]
- 66.Kaidi A, Williams AC, Paraskeva C. Interaction between β-catenin and HIF-1 promotes cellular adaptation to hypoxia. Nat Cell Biol 9: 210–217, 2007. [DOI] [PubMed] [Google Scholar]
- 67.Kampp M, Lundgren O, Nilsson NJ. Extravascular shunting of oxygen in the small intestine of the cat. Acta Physiol Scand 72: 396–403, 1968. [DOI] [PubMed] [Google Scholar]
- 68.Karhausen J, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP, Haase VH. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest 114: 1098–1106, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Karhausen J, Ibla JC, Colgan SP. Implications of hypoxia on mucosal barrier function. Cell Mol Biol (Noisy-le-grand) 49: 77–87, 2003. [PubMed] [Google Scholar]
- 70.Kaser A, Lee AH, Franke A, Glickman JN, Zeissig S, Tilg H, Nieuwenhuis EE, Higgins DE, Schreiber S, Glimcher LH, Blumberg RS. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell 134: 743–756, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kekuda R, Manoharan P, Baseler W, Sundaram U. Monocarboxylate 4 mediated butyrate transport in a rat intestinal epithelial cell line. Dig Dis Sci 58: 660–667, 2013. [DOI] [PubMed] [Google Scholar]
- 72.Kelly CJ, Colgan SP. Targeting hypoxia to augment mucosal barrier function. J Epith Biol Pharm 5: 67–76, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kelly CJ, Glover LE, Campbell EL, Kominsky DJ, Ehrentraut SF, Bowers BE, Bayless AJ, Saeedi BJ, Colgan SP. Fundamental role for HIF-1α in constitutive expression of human β-defensin-1. Mucosal Immunol 6: 1110–1118, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, Bayless AJ, Wilson KE, Glover LE, Kominsky DJ, Magnuson A, Weir TL, Ehrentraut SF, Pickel C, Kuhn KA, Lanis JM, Nguyen V, Taylor CT, Colgan SP. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe 17: 662–671, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kight CE, Fleming SE. Oxidation of glucose carbon entering the TCA cycle is reduced by glutamine in small intestine epithelial cells. Am J Physiol Gastrointest Liver Physiol 268: G879–G888, 1995. [DOI] [PubMed] [Google Scholar]
- 76.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab 3: 177–185, 2006. [DOI] [PubMed] [Google Scholar]
- 77.Kizaka-Kondoh S, Konse-Nagasawa H. Significance of nitroimidazole compounds and hypoxia-inducible factor-1 for imaging tumor hypoxia. Cancer Sci 100: 1366–1373, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kulshreshtha R, Ferracin M, Wojcik SE, Garzon R, Alder H, Agosto-Perez FJ, Davuluri R, Liu CG, Croce CM, Negrini M, Calin GA, Ivan M. A microRNA signature of hypoxia. Mol Cell Biol 27: 1859–1867, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kvietys PR, Wilborn WH, Granger DN. Effects of net transmucosal volume flux on lymph flow in the canine colon. Structural-functional relationship. Gastroenterology 81: 1080–1090, 1981. [PubMed] [Google Scholar]
- 80.Lee M, Hwang JT, Lee HJ, Jung SN, Kang I, Chi SG, Kim SS, Ha J. AMP-activated protein kinase activity is critical for hypoxia-inducible factor-1 transcriptional activity and its target gene expression under hypoxic conditions in DU145 cells. J Biol Chem 278: 39653–39661, 2003. [DOI] [PubMed] [Google Scholar]
- 81.Lind Due V, Bonde J, Kann T, Perner A. Extremely low oxygen tension in the rectal lumen of human subjects. Acta Anaesthesiol Scand 47: 372, 2003. [DOI] [PubMed] [Google Scholar]
- 82.Linden SK, Sutton P, Karlsson NG, Korolik V, McGuckin MA. Mucins in the mucosal barrier to infection. Mucosal Immunol 1: 183–197, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Loboda A, Jozkowicz A, Dulak J. HIF-1 and HIF-2 transcription factors—similar but not identical. Mol Cell 29: 435–442, 2010. [DOI] [PubMed] [Google Scholar]
- 84.Longman RJ, Douthwaite J, Sylvester PA, Poulsom R, Corfield AP, Thomas MG, Wright NA. Coordinated localisation of mucins and trefoil peptides in the ulcer associated cell lineage and the gastrointestinal mucosa. Gut 47: 792–800, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Louis NA, Hamilton KE, Canny G, Shekels LL, Ho SB, Colgan SP. Selective induction of mucin-3 by hypoxia in intestinal epithelia. J Cell Biochem 99: 1616–1627, 2006. [DOI] [PubMed] [Google Scholar]
- 86.Lundgren O, Svanvik J. Mucosal hemodynamics in the small intestine of the cat during reduced perfusion pressure. Acta Physiol Scand 88: 551–563, 1973. [DOI] [PubMed] [Google Scholar]
- 87.Madsen JL, Sondergaard SB, Moller S. Meal-induced changes in splanchnic blood flow and oxygen uptake in middle-aged healthy humans. Scand J Gastroenterol 41: 87–92, 2006. [DOI] [PubMed] [Google Scholar]
- 88.Mahon PC, Hirota K, Semenza GL. FIH-1: a novel protein that interacts with HIF-1α and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev 15: 2675–2686, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Marin-Hernandez A, Gallardo-Perez JC, Ralph SJ, Rodriguez-Enriquez S, Moreno-Sanchez R. HIF-1α modulates energy metabolism in cancer cells by inducing over-expression of specific glycolytic isoforms. Mini Rev Med Chem 9: 1084–1101, 2009. [DOI] [PubMed] [Google Scholar]
- 90.Mastrogiannaki M, Matak P, Keith B, Simon MC, Vaulont S, Peyssonnaux C. HIF-2α, but not HIF-1α, promotes iron absorption in mice. J Clin Invest 119: 1159–1166, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Matheson PJ, Li N, Harris PD, Zakaria el R, Garrison RN. Glucose-induced intestinal vasodilation via adenosine A1 receptors requires nitric oxide but not KATP+ channels. J Surg Res 168: 179–187, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Matheson PJ, Spain DA, Harris PD, Garrison RN, Wilson MA. Glucose and glutamine gavage increase portal vein nitric oxide metabolite levels via adenosine A2b activation. J Surg Res 84: 57–63, 1999. [DOI] [PubMed] [Google Scholar]
- 93.Matheson PJ, Wilson MA, Garrison RN. Regulation of intestinal blood flow. J Surg Res 93: 182–196, 2000. [DOI] [PubMed] [Google Scholar]
- 94.Matheson PJ, Wilson MA, Spain DA, Harris PD, Anderson GL, Garrison RN. Glucose-induced intestinal hyperemia is mediated by nitric oxide. J Surg Res 72: 146–154, 1997. [DOI] [PubMed] [Google Scholar]
- 95.Mellstrom A, Mansson P, Jonsson K, Hartmann M. Measurements of subcutaneous tissue Po2 reflect oxygen metabolism of the small intestinal mucosa during hemorrhage and resuscitation. An experimental study in pigs. Eur Surg Res 42: 122–129, 2009. [DOI] [PubMed] [Google Scholar]
- 96.Mole DR, Blancher C, Copley RR, Pollard PJ, Gleadle JM, Ragoussis J, Ratcliffe PJ. Genome-wide association of hypoxia-inducible factor (HIF)-1α and HIF-2α DNA binding with expression profiling of hypoxia-inducible transcripts. J Biol Chem 284: 16767–16775, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mortensen FV, Hessov I, Birke H, Korsgaard N, Nielsen H. Microcirculatory and trophic effects of short chain fatty acids in the human rectum after Hartmann's procedure. Br J Surg 78: 1208–1211, 1991. [DOI] [PubMed] [Google Scholar]
- 98.Mortensen FV, Nielsen H, Mulvany MJ, Hessov I. Short chain fatty acids dilate isolated human colonic resistance arteries. Gut 31: 1391–1394, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nase GP, Tuttle J, Bohlen HG. Reduced perivascular Po2 increases nitric oxide release from endothelial cells. Am J Physiol Heart Circ Physiol 285: H507–H515, 2003. [DOI] [PubMed] [Google Scholar]
- 100.Nordsmark M, Loncaster J, Aquino-Parsons C, Chou SC, Ladekarl M, Havsteen H, Lindegaard JC, Davidson SE, Varia M, West C, Hunter R, Overgaard J, Raleigh JA. Measurements of hypoxia using pimonidazole and polarographic oxygen-sensitive electrodes in human cervix carcinomas. Radiother Oncol 67: 35–44, 2003. [DOI] [PubMed] [Google Scholar]
- 101.O'Neil DA, Porter EM, Elewaut D, Anderson GM, Eckmann L, Ganz T, Kagnoff MF. Expression and regulation of the human β-defensins hBD-1 and hBD-2 in intestinal epithelium. J Immunol 163: 6718–6724, 1999. [PubMed] [Google Scholar]
- 102.Palacios-Callender M, Hollis V, Mitchison M, Frakich N, Unitt D, Moncada S. Cytochrome c oxidase regulates endogenous nitric oxide availability in respiring cells: a possible explanation for hypoxic vasodilation. Proc Natl Acad Sci USA 104: 18508–18513, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pazgier M, Hoover DM, Yang D, Lu W, Lubkowski J. Human β-defensins. Cell Mol Life Sci 63: 1294–1313, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Perko MJ, Nielsen HB, Skak C, Clemmesen JO, Schroeder TV, Secher NH. Mesenteric, coeliac and splanchnic blood flow in humans during exercise. J Physiol 513: 907–913, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Peyssonnaux C, Zinkernagel AS, Schuepbach RA, Rankin E, Vaulont S, Haase VH, Nizet V, Johnson RS. Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs). J Clin Invest 117: 1926–1932, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pluznick J. A novel SCFA receptor, the microbiota, and blood pressure regulation. Gut Microbes 5: 202–207, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Reed GW, Hill JO. Measuring the thermic effect of food. Am J Clin Nutr 63: 164–169, 1996. [DOI] [PubMed] [Google Scholar]
- 108.Rengasamy A, Johns RA. Determination of Km for oxygen of nitric oxide synthase isoforms. J Pharmacol Exp Ther 276: 30–33, 1996. [PubMed] [Google Scholar]
- 109.Roediger WE. Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut 21: 793–798, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rogers GW, Brand MD, Petrosyan S, Ashok D, Elorza AA, Ferrick DA, Murphy AN. High throughput microplate respiratory measurements using minimal quantities of isolated mitochondria. PLos One 6: e21746, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Romero-Ramirez L, Cao H, Nelson D, Hammond E, Lee AH, Yoshida H, Mori K, Glimcher LH, Denko NC, Giaccia AJ, Le QT, Koong AC. XBP1 is essential for survival under hypoxic conditions and is required for tumor growth. Cancer Res 64: 5943–5947, 2004. [DOI] [PubMed] [Google Scholar]
- 112.Saeedi BJ, Kao DJ, Kitzenberg DA, Dobrinskikh E, Schwisow KD, Masterson JC, Kendrick AA, Kelly CJ, Bayless AJ, Kominsky DJ, Campbell EL, Kuhn KA, Furuta GT, Colgan SP, Glover LE. HIF-dependent regulation of claudin-1 is central to intestinal epithelial tight junction integrity. Mol Biol Cell 26: 2252–2262, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Safran M, Kim WY, O'Connell F, Flippin L, Gunzler V, Horner JW, Depinho RA, Kaelin WG Jr. Mouse model for noninvasive imaging of HIF prolyl hydroxylase activity: assessment of an oral agent that stimulates erythropoietin production. Proc Natl Acad Sci USA 103: 105–110, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Saravi FD, Cincunegui LM, Saldena TA, Carra GE, Ibanez JE. Increased oxygen consumption caused by cAMP- and Ca2+-mediated chloride secretion in rat distal colon. Acta Gastroenterol Latinoam 35: 13–18, 2005. [PubMed] [Google Scholar]
- 115.Saravi FD, Saldena TA, Carrera CA, Ibanez JE, Cincunegui LM, Carra GE. Oxygen consumption and chloride secretion in rat distal colon isolated mucosa. Dig Dis Sci 48: 1767–1773, 2003. [DOI] [PubMed] [Google Scholar]
- 116.Sarkar K, Cai Z, Gupta R, Parajuli N, Fox-Talbot K, Darshan MS, Gonzalez FJ, Semenza GL. Hypoxia-inducible factor 1 transcriptional activity in endothelial cells is required for acute phase cardioprotection induced by ischemic preconditioning. Proc Natl Acad Sci USA 109: 10504–10509, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Savage DC. Factors involved in colonization of the gut epithelial surface. Am J Clin Nutr 31: S131–S135, 1978. [DOI] [PubMed] [Google Scholar]
- 118.Sawmiller DR, Chou CC. Adenosine plays a role in food-induced jejunal hyperemia. Am J Physiol Gastrointest Liver Physiol 255: G168–G174, 1988. [DOI] [PubMed] [Google Scholar]
- 119.Schaible B, Schaffer K, Taylor CT. Hypoxia, innate immunity and infection in the lung. Respir Physiol Neurobiol 174: 235–243, 2010. [DOI] [PubMed] [Google Scholar]
- 120.Schodel J, Oikonomopoulos S, Ragoussis J, Pugh CW, Ratcliffe PJ, Mole DR. High-resolution genome-wide mapping of HIF-binding sites by ChIP-seq. Blood 117: e207–e217, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schroeder BO, Wu Z, Nuding S, Groscurth S, Marcinowski M, Beisner J, Buchner J, Schaller M, Stange EF, Wehkamp J. Reduction of disulphide bonds unmasks potent antimicrobial activity of human β-defensin 1. Nature 469: 419–423, 2011. [DOI] [PubMed] [Google Scholar]
- 122.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell 148: 399–408, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Semenza GL, Jiang BH, Leung SW, Passantino R, Concordet JP, Maire P, Giallongo A. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J Biol Chem 271: 32529–32537, 1996. [DOI] [PubMed] [Google Scholar]
- 124.Shepherd AP. Metabolic control of intestinal oxygenation and blood flow. Fed Proc 41: 2084–2089, 1982. [PubMed] [Google Scholar]
- 125.Sheridan WG, Lowndes RH, Young HL. Intraoperative tissue oximetry in the human gastrointestinal tract. Am J Surg 159: 314–319, 1990. [DOI] [PubMed] [Google Scholar]
- 126.Sobko T, Reinders C, Norin E, Midtvedt T, Gustafsson LE, Lundberg JO. Gastrointestinal nitric oxide generation in germ-free and conventional rats. Am J Physiol Gastrointest Liver Physiol 287: G993–G997, 2004. [DOI] [PubMed] [Google Scholar]
- 127.Sonnweber T, Nachbaur D, Schroll A, Nairz M, Seifert M, Demetz E, Haschka D, Mitterstiller AM, Kleinsasser A, Burtscher M, Trubsbach S, Murphy AT, Wroblewski V, Witcher DR, Mleczko-Sanecka K, Vecchi C, Muckenthaler MU, Pietrangelo A, Theurl I, Weiss G. Hypoxia induced downregulation of hepcidin is mediated by platelet derived growth factor BB. Gut 63: 1951–1959, 2014. [DOI] [PubMed] [Google Scholar]
- 128.Steenbergen JM, Bohlen HG. Sodium hyperosmolarity of intestinal lymph causes arteriolar vasodilation in part mediated by EDRF. Am J Physiol Heart Circ Physiol 265: H323–H328, 1993. [DOI] [PubMed] [Google Scholar]
- 129.Steinbrecher KA, Harmel-Laws E, Sitcheran R, Baldwin AS. Loss of epithelial RelA results in deregulated intestinal proliferative/apoptotic homeostasis and susceptibility to inflammation. J Immunol 180: 2588–2599, 2008. [DOI] [PubMed] [Google Scholar]
- 130.Strugala V, Allen A, Dettmar PW, Pearson JP. Colonic mucin: methods of measuring mucus thickness. Proc Nutr Soc 62: 237–243, 2003. [DOI] [PubMed] [Google Scholar]
- 131.Synnestvedt K, Furuta GT, Comerford KM, Louis N, Karhausen J, Eltzschig HK, Hansen KR, Thompson LF, Colgan SP. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest 110: 993–1002, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Takasawa M, Moustafa RR, Baron JC. Applications of nitroimidazole in vivo hypoxia imaging in ischemic stroke. Stroke 39: 1629–1637, 2008. [DOI] [PubMed] [Google Scholar]
- 133.Taylor M, Qu A, Anderson ER, Matsubara T, Martin A, Gonzalez FJ, Shah YM. Hypoxia-inducible factor-2α mediates the adaptive increase of intestinal ferroportin during iron deficiency in mice. Gastroenterology 140: 2044–2055, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Thomas DD, Liu X, Kantrow SP, Lancaster JR Jr. The biological lifetime of nitric oxide: implications for the perivascular dynamics of NO and O2. Proc Natl Acad Sci USA 98: 355–360, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ullah MS, Davies AJ, Halestrap AP. The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1α-dependent mechanism. J Biol Chem 281: 9030–9037, 2006. [DOI] [PubMed] [Google Scholar]
- 136.Umbrello M, Dyson A, Pinto BB, Fernandez BO, Simon V, Feelisch M, Singer M. Short-term hypoxic vasodilation in vivo is mediated by bioactive nitric oxide metabolites, rather than free nitric oxide derived from haemoglobin-mediated nitrite reduction. J Physiol 592: 1061–1075, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Vanner S, Surprenant A. Neural reflexes controlling intestinal microcirculation. Am J Physiol Gastrointest Liver Physiol 271: G223–G230, 1996. [DOI] [PubMed] [Google Scholar]
- 138.Varghese AJ, Gulyas S, Mohindra JK. Hypoxia-dependent reduction of 1-(2-nitro-1-imidazolyl)-3-methoxy-2-propanol by Chinese hamster ovary cells and KHT tumor cells in vitro and in vivo. Cancer Res 36: 3761–3765, 1976. [PubMed] [Google Scholar]
- 139.Vatner SF, Franklin D, Van Citters RL. Coronary and visceral vasoactivity associated with eating and digestion in the conscious dog. Am J Physiol 219: 1380–1385, 1970. [DOI] [PubMed] [Google Scholar]
- 140.Vatner SF, Patrick TA, Higgins CB, Franklin D. Regional circulatory adjustments to eating and digestion in conscious unrestrained primates. J Appl Physiol 36: 524–529, 1974. [DOI] [PubMed] [Google Scholar]
- 141.Vaugelade P, Posho L, Darcy-Vrillon B, Bernard F, Morel MT, Duee PH. Intestinal oxygen uptake and glucose metabolism during nutrient absorption in the pig. Proc Soc Exp Biol Med 207: 309–316, 1994. [DOI] [PubMed] [Google Scholar]
- 142.Vaupel P, Kelleher DK. Blood flow and oxygenation status of gastrointestinal tumors. Adv Exp Med Biol 737: 133–138, 2012. [DOI] [PubMed] [Google Scholar]
- 143.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA 92: 5510–5514, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Westbury CB, Pearson A, Nerurkar A, Reis-Filho JS, Steele D, Peckitt C, Sharp G, Yarnold JR. Hypoxia can be detected in irradiated normal human tissue: a study using the hypoxic marker pimonidazole hydrochloride. Br J Radiol 80: 934–938, 2007. [DOI] [PubMed] [Google Scholar]
- 145.Yan H, Zhang DX, Shi X, Zhang Q, Huang YS. Activation of the prolyl-hydroxylase oxygen-sensing signal cascade leads to AMPK activation in cardiomyocytes. J Cell Mol Med 16: 2049–2059, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yun Z, Maecker HL, Johnson RS, Giaccia AJ. Inhibition of PPARγ2 gene expression by the HIF-1-regulated gene DEC1/Stra13: a mechanism for regulation of adipogenesis by hypoxia. Dev Cell 2: 331–341, 2002. [DOI] [PubMed] [Google Scholar]
- 147.Zaph C, Troy AE, Taylor BC, Berman-Booty LD, Guild KJ, Du Y, Yost EA, Gruber AD, May MJ, Greten FR, Eckmann L, Karin M, Artis D. Epithelial-cell-intrinsic IKK-β expression regulates intestinal immune homeostasis. Nature 446: 552–556, 2007. [DOI] [PubMed] [Google Scholar]