Abstract
Recent studies have demonstrated that functionally discrete pools of phosphodiesterase (PDE) activity regulate distinct cellular functions. While the importance of localized pools of enzyme activity has become apparent, few studies have estimated enzyme activity within discrete subcellular compartments. Here we present an approach to estimate near-membrane PDE activity. First, total PDE activity is measured using traditional PDE activity assays. Second, known cAMP concentrations are dialyzed into single cells and the spatial spread of cAMP is monitored using cyclic nucleotide-gated channels. Third, mathematical models are used to estimate the spatial distribution of PDE activity within cells. Using this three-tiered approach, we observed two pharmacologically distinct pools of PDE activity, a rolipram-sensitive pool and an 8-methoxymethyl IBMX (8MM-IBMX)-sensitive pool. We observed that the rolipram-sensitive PDE (PDE4) was primarily responsible for cAMP hydrolysis near the plasma membrane. Finally, we observed that PDE4 was capable of blunting cAMP levels near the plasma membrane even when 100 μM cAMP were introduced into the cell via a patch pipette. Two compartment models predict that PDE activity near the plasma membrane, near cyclic nucleotide-gated channels, was significantly lower than total cellular PDE activity and that a slow spatial spread of cAMP allowed PDE activity to effectively hydrolyze near-membrane cAMP. These results imply that cAMP levels near the plasma membrane are distinct from those in other subcellular compartments; PDE activity is not uniform within cells; and localized pools of AC and PDE activities are responsible for controlling cAMP levels within distinct subcellular compartments.
Keywords: phosphodiesterase, protein kinase A, G protein-coupled receptor, CNG channel, cAMP compartmentalization
in the last 40 years a wide variety of studies have provided compelling evidence supporting the existence of compartmentalized cAMP signals (reviewed in Refs. 7, 11, 36, 37). The existence of compartmentalized signals infers that localized signaling complexes exist within subcellular compartments. However, to date few studies have attempted to estimate localized enzyme concentrations within subcellular compartments. For example, with the notable exception of sensory neurons (6, 8, 9, 19, 24, 25), we know little about the subcellular concentrations of critical enzymes, such as receptors, adenylyl cyclase, protein kinase A (PKA), and phosphodiesterase (PDE) (4, 10, 48). Here we propose a three-tiered approach to estimate PDE activity near the plasma membrane of living cells: 1) measurement of the total cellular PDE activity in cell lysates and pharmacological determination of distinct PDE subtypes; 2) estimation of the rate at which known concentrations of cAMP dialyze into cells using plasma membrane-localized cyclic nucleotide-gated (CNG) channels as the readout; and 3) utilization of mathematical models to describe the activity and spatial distribution of pharmacologically distinct PDEs based on both total cellular PDE activity and the time course of cAMP dialysis into the cell.
The utility of this approach was tested by estimating the level of PDE activity near the plasma membrane of HEK-293 cells. HEK-293 cells were chosen as a model system because they have become a standard model for the study of kinetics and subcellular distribution of cAMP signals in single cells (14, 26, 29–31, 33, 39, 42, 45, 46, 48). In addition, the work of several laboratories has demonstrated that PDE4 activity is primarily responsible for the hydrolysis of cAMP near the plasma membrane of these cells (31, 33, 39, 45, 46, 48). We observed two populations of PDE activity in cell lysates of HEK-293 cells, rolipram and 8-methoxymethyl IBMX (8MM-IBMX) sensitive PDE activities. We then monitored the ability of these PDEs to hydrolyze cAMP near the plasma membrane of HEK-293 cells. Known cAMP concentrations were introduced into single cells via the patch pipette, and their ability to activate adenovirus-expressed CNG channels was assessed. We observed that 10 μM rolipram, a selective PDE4 inhibitor, Ki ∼ 0.1 μM (2, 16), had marked effects on the magnitude of cAMP-induced currents through CNG channels, even in the presence of 100 μM cAMP (∼33·Km). Subsequent addition of 100 μM IBMX, a nonselective PDE inhibitor, Ki ∼5 and 10 μM for PDE4 and PDE1, had no additional effect on currents through CNG channels. We also observed that 100 μM 8MM-IBMX, a somewhat selective PDE1 inhibitor that does not inhibit PDE4, Ki ∼10 μM for PDE1, had little or no effect on the magnitude of cAMP-induced currents.
We next utilized a previously published mathematical model of cAMP compartmentalization to estimate the level of PDE4 activity near the plasma membrane (near CNG channels). This model consisted of two subcellular compartments - a near-membrane compartment and a cytosolic compartment. Fits of the model to experimental data indicate that 1) PDE activity in the near-membrane compartment is ∼20-fold lower than total cellular PDE activity; 2) the 8MM-IBMX-sensitive PDE activity is primarily in the cytosolic compartment; and 3) the flux coefficient between near-membrane and cytosolic compartments is also low, indicating a large diffusional hindrance to cAMP entering the near-membrane compartment. This model is highly sensitive to changes in the Vmax of PDE activity in either compartment, as well as the flux coefficient between compartments 1 and 2. Thus these results highlight the importance of the relative levels of PDE activity within each subcellular compartment. cAMP flux between compartments must be hindered to allow the different PDE activities within these compartments to be effective in shaping distinct cAMP signals within subcellular compartments.
MATERIALS AND METHODS
Cell culture and channel expression.
Culture and adenovirus infection of HEK-293 cells were performed as described previously (29). Briefly, HEK-293 cells were maintained in 10 ml of minimal essential medium (MEM; Life Technologies) containing 10% vol/vol fetal bovine serum (Gemini) and grown in 100-mm culture dishes at 37°C in a humidified atmosphere of 95% air-5% CO2. Cells were plated at ∼60% confluence in 100 mm dishes for infection with an adenovirus encoding the C460W/E583M CNG channel at a multiplicity of infection of ∼10 pfu/cell (31). Two hours postinfection, hydroxyurea was added to the cell media at a final concentration of 1 mM to inhibit viral replication. Twenty-four hours postinfection, cells were detached with phosphate-buffered saline containing 0.03% EDTA, resuspended in serum-containing media, and assayed within 12 h. All experiments were conducted at room temperature, 20–22°C. Unless otherwise stated, all reagents were purchased from Sigma.
Estimation of cell volume.
The cell volume was estimated based on the cross-sectional areas of a given cell segmented from sequential confocal image stacks. Briefly, HEK-293 cells were grown on coverslips and maintained in MEM before green fluorescent protein (GFP)-adenoviral infection as described previously (31). A plasma membrane stain (CellMask; Life Technologies) was used per manufacturer's instructions to define the cellular limits. Cells were imaged using either a PerkinElmer Ultraview RS-3 or a Nikon A1R confocal microscope system. Cross-sectional areas were estimated by visualizing soluble fluorophores, fluorescein, or GFP, as described previously (15). Volumes were rendered in the PerkinElmer Volocity or Nikon Elements image analysis software packages.
Measurement of PDE activity.
cAMP PDE activity was measured according to the method of Thompson and Appleman (40) as detailed previously (33, 40, 41, 49). Briefly, cells were harvested and homogenized in ice-cold hypotonic buffer containing the following (mM): 20 Tris·HCl, 5 MgCl2, and 0.5 EDTA, pH 7.4, with protease inhibitors: 10 μM TLCK, 2 μM leupeptin, 2 μM pepstatin A, 10 μM benzamidine, and 2,000 U aprotinin/ml. Cells were homogenized and aliquots were assayed for PDE activity using 0.01–20 μM [3H]cAMP as a substrate. Protein concentrations were determined using protein assays (Bio-Rad Laboratories) with BSA as a standard.
Measurement of cellular cAMP levels.
HEK-293 cells were plated at 33% confluence in sixwell plates and assayed 24–48 h later. Cells were washed and assayed in a solution containing the following (mM): 145 NaCl, 4 KCl, 10 HEPES, 10 d-glucose, 1 MgCl2, and 1 CaCl2, pH 7.4. Additions were made from 100× stock solutions. Reactions were terminated by addition of 1 N HCl (0.1 N HCl final) and plates were incubated on ice for 15 min, after which the cells were scraped from the dish. Cellular cAMP levels were measured using an enzyme immunoassay (Cayman Chemical). Sample cAMP concentrations were calculated from standard curves. Protein concentrations were determined using protein assays with BSA as a standard. Data are presented as means ± SE, performed in triplicate.
Single cell measurement of cAMP signals.
CNG channels were used to monitor cAMP levels in single HEK-293 cells. The whole cell patch-clamp technique was used to measure CNG channel activity, as described previously (30, 33). Briefly, recordings were made by an HEKA EPC10 patch-clamp amplifier system. To ensure adequate voltage control, pipette resistance was limited to 4 MΩ and averaged 2.1 ± 0.1 MΩ (n = 73); access resistance (Ra) was 4.3 ± 0.1 MΩ. Voltage offsets were zeroed with the pipette in the bath solution; no additional corrections were made for the liquid junction potential difference. Experiments with a series resistance-induced error in excess of 5 mV were discarded. Current records were typically sampled at 10 kHz and filtered at 2 kHz and stored on a PC. Currents were recorded during 400-ms steps to a membrane potential of +20 or −20 mV from a holding potential of 0 mV. The pipette solution contained the following (mM): 140 KCl, 0.5 MgCl2, 10 HEPES, 5 Na2ATP, 0.5 Na2GTP, pH 7.4; the bath solution contained the following (mM): 140 NaCl, 4 KCl, 10 d-glucose, and 10 HEPES, and either 0.1 or 10 MgCl2, pH 7.4. IBMX and rolipram (Calbiochem) were added to control solutions from concentrated DMSO stocks (final DMSO concentrations ≤0.2%). Extracellular solutions were applied using a SF-77B solution switcher (Warner Instruments) with a mechanical switch time of 1–2 ms. The time to exchange the extracellular solution was measured by applying a 140-mM KCl solution to a depolarized cell (+50 mV) and monitoring changes in current through endogenous voltage-gated K+ channels; for each experiment, it was <100 ms. The bulk solution within the bath chamber was changed within 20 s using a custom-built, gravity-driven perfusion system. Baseline current (non-CNG channel current) was monitored by measuring the residual current in the presence of 10 mM Mg2+ (a CNG channel blocker), approximately once per minute. No Mg2+-blockable currents were observed in control cells (cells not expressing CNG channels). Data were analyzed using custom scripts written in the MATLAB programming environment (v 7.4, MathWorks). Electrophysiological data were converted to formats compatible with MATLAB software using a custom script (Bruxton).
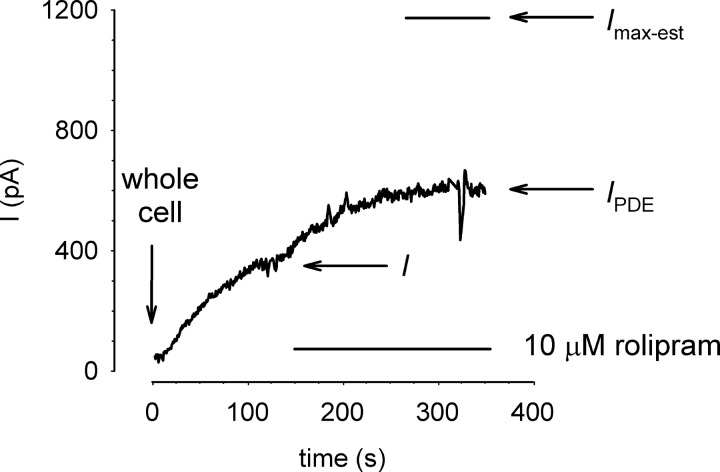
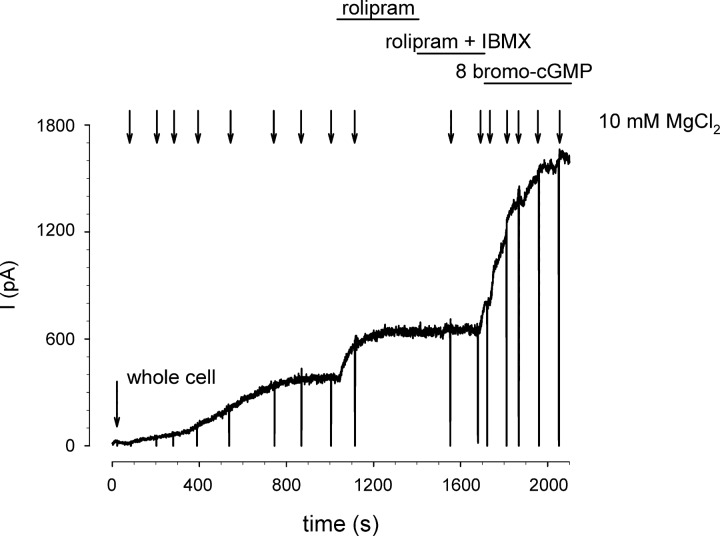
To assess the effects of PDE activity on cAMP-mediated activation of CNG channels we estimated the fractional steady-state activation of CNG channels as I/Imax-est, where I is the current through CNG channels in the absence of PDE inhibitors; Imax-est is the estimated maximal current defined as the current in the presence of PDE inhibitors (IPDE) divided by the expected fractional CNG channel current at the concentration of cAMP in the patch pipette (Ifrac). The expected fractional current is based on previously published cAMP concentration response curves for each construct (see Refs. 29, 31). For example, in the experiment depicted in Fig. 1, I = 320 pA, IPDE = 590 pA, and Ifrac = 0.5 for the wild-type (WT) CNG channel (K1/2 = 40 μM) with 40 μM cAMP in the patch pipette. Thus Imax-est = 1,180 pA, and I/Imax-est = 0.27. To confirm the validity of this approach for estimating the maximal current, we added the membrane permeant cGMP analog 8-bromo-cGMP (100 μM) to the bath solution in a subset of experiments (e.g., see Fig. 4). One hundred micromolar 8-bromo-cGMP was sufficient to maximally activate CNG channels. No significant difference between the two approaches for estimating Imax was observed.
Fig. 1.
Estimation of maximal current through cyclic nucleotide-gated (CNG) channels in the whole cell configuration. Currents were triggered by wash in of cAMP following “break in” to whole cell configuration. The rate of cAMP wash in was assessed by monitoring activation of wild-type (WT) CNG channels (K1/2 = 40 μM). Wash in of 40 μM cAMP triggered a steady rise in current through CNG channels, reaching a plateau of 320 pA within 200 s. Subsequent exposure of the cell to 10 μM rolipram triggered an additional increase in current to a plateau of 590 pA. Based on the plateau current in the presence of rolipram and 40 μM cAMP in the patch pipette, we estimate the maximal current to be 1,080 pA (indicated level) and I/Imax-est to be 0.27.
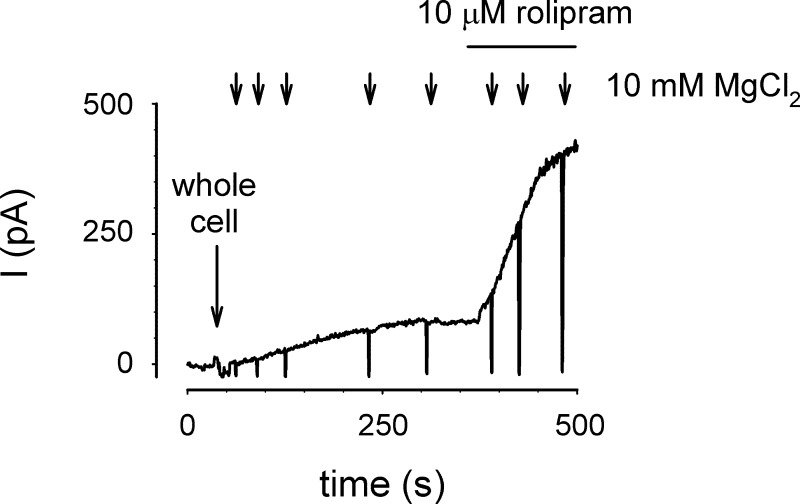
Fig. 4.
PDE4 activity effectively hydrolyzes the influx of 5 μM cAMP. The influx of cAMP from the patch pipette into a single HEK-293 cell was monitored using E583M/C460W channels. Following break in to whole cell configuration (at indicated time), the influx of 5 μM cAMP caused a steady increase in CNG channel current that reached a plateau in ∼300 s. Subsequent addition of 10 μM rolipram to the bath solution triggered a substantial increase in current through CNG channels, suggesting that cellular PDE4 activity is capable of hydrolyzing a steady influx of 5 μM cAMP before it reaches CNG channels. Brief exposure to buffer containing 10 mM MgCl2 (arrows) was used to ensure the stability of the pipette-cell interface.
Mathematical equations describing the spatial spread of cAMP signals.
We have utilized the compartmental model framework (29, 30) to model the spatial spread of cAMP from the patch pipette to the plasma membrane. This formulation has allowed us to estimate the concentration of PDE4 activity near the plasma membrane. The equations used to describe the system are as follows:
| (1) |
| (2) |
| (3) |
| (4) |
where V1, V2, and V3 are the volumes of the near-membrane, cytosolic, and patch pipette compartments, compartments 1, 2, and 3, respectively; [C1], [C2], and [C3] are the cAMP concentrations in each compartment; J12 and J23 are the flux coefficients between compartments 1 and 2, and 2 and 3; EPDE4:C1 and EPDE4:C2 are the maximal cAMP hydrolysis rates due to rolipram sensitive PDE activity (PDE4 activity) in compartments 1 and 2; EPDE8MM:C1 and EPDE8MM:C2 are the maximal cAMP hydrolysis rates due to 8MM-IBMX-sensitive PDE activity (non-PDE4 activity) in compartments 1 and 2; Km-PDE4 and Km-PDE8MM are the Michaelis constants for PDE4 and non-PDE4 activities, KI-PDE4 and KI-PDE8MM are the inhibition constants of rolipram and 8MM-IBMX for PDE4 and the 8MM-IBMX sensitive PDE; [Irol] and [I8MM] are the concentrations of rolipram and 8MM-IBMX; iCNG is the current through CNG channels at a given cAMP concentration; and iCNGmax is the current through CNG channels at saturating cAMP concentrations. Simulations were conducted using a fourth order Runge Kutta method as implemented within the MATLAB and SCoP (v 3.51, Simulation Resources) programming environments. Parameter optimization was performed manually.
Statistical analysis.
Data were analyzed using Student's t-test or one-way ANOVA followed by Newman-Kuels post hoc test, as appropriate. Data analysis was performed using GraphPad Prism software with a significance level of P < 0.05.
RESULTS
CNG channels have been used as cAMP biosensors in a variety of cell types (29, 31, 33–35, 43, 45, 46, 48). Several of these studies have led to quantitative descriptions of the cAMP signaling pathways (29, 30, 33, 48). A limitation of these studies has been the lack of information describing the subcellular distribution of PDE activity. Traditionally, biochemical assays have measured PDE activity in particulate and soluble cellular fractions; however, this segregation of cellular proteins into two fractions does not necessarily reflect the distribution of PDE activity within cells. Here we propose an alternative approach to estimate localized PDE activity: monitoring the ability of endogenous PDE activity to hydrolyze known concentrations of cAMP in both in vitro PDE assays and in localized regions of single, living cells.
Characterization of total PDE activity in HEK-293 cells.
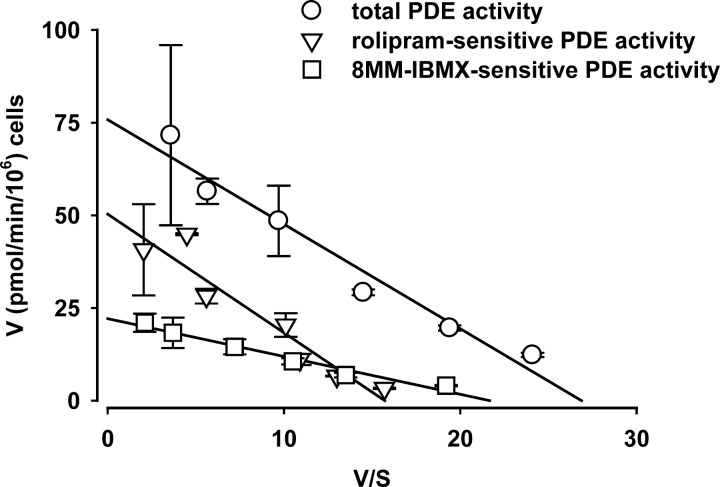
We next estimated the kinetic properties of cAMP PDE activity in HEK-293 cells using substrate concentrations ranging from 0.1 to 20 μM cAMP (Fig. 2). Total PDE activity had a Km of 2.8 ± 0.4 μM with a Vmax of 0.63 μM/s, assuming an accessible cell volume of 2.4 pl. We observed two subpopulations of PDE activity: a rolipram-sensitive population and an 8MM-IBMX-sensitive population. The rolipram-sensitive PDE (PDE4) had a Km of 3 μM and a Vmax of 0.42 μM/s; the 8MM-IBMX-sensitive PDE (PDE8MM) had a Km of 1.0 μM and a Vmax of 0.17 μM/s. Although 8MM-IBMX has been described as a selective PDE1 inhibitor it is not entirely selective (3, 21, 22). However, 8MM-IBMX does not inhibit PDE4 (23), and as such, these results indicate at least two pharmacologically distinct PDE populations in HEK-293 cells.
Fig. 2.
Estimated phosphodiesterase (PDE) activity in HEK-293 cells. Total cellular PDE activity was estimated as described in the materials and methods. Two primary pools of PDE activity were identified: a rolipram-sensitive PDE activity and an 8-methoxymethyl IBMX (8MM-IBMX)-sensitive PDE activity. Based on these data the rolipram-sensitive PDE activity had a Km of 3 μM and a Vmax of 0.42 μM/s; the 8MM-IBMX-sensitive PDE activity had a Km of 1 μM and a Vmax of 0.17 μM/s.
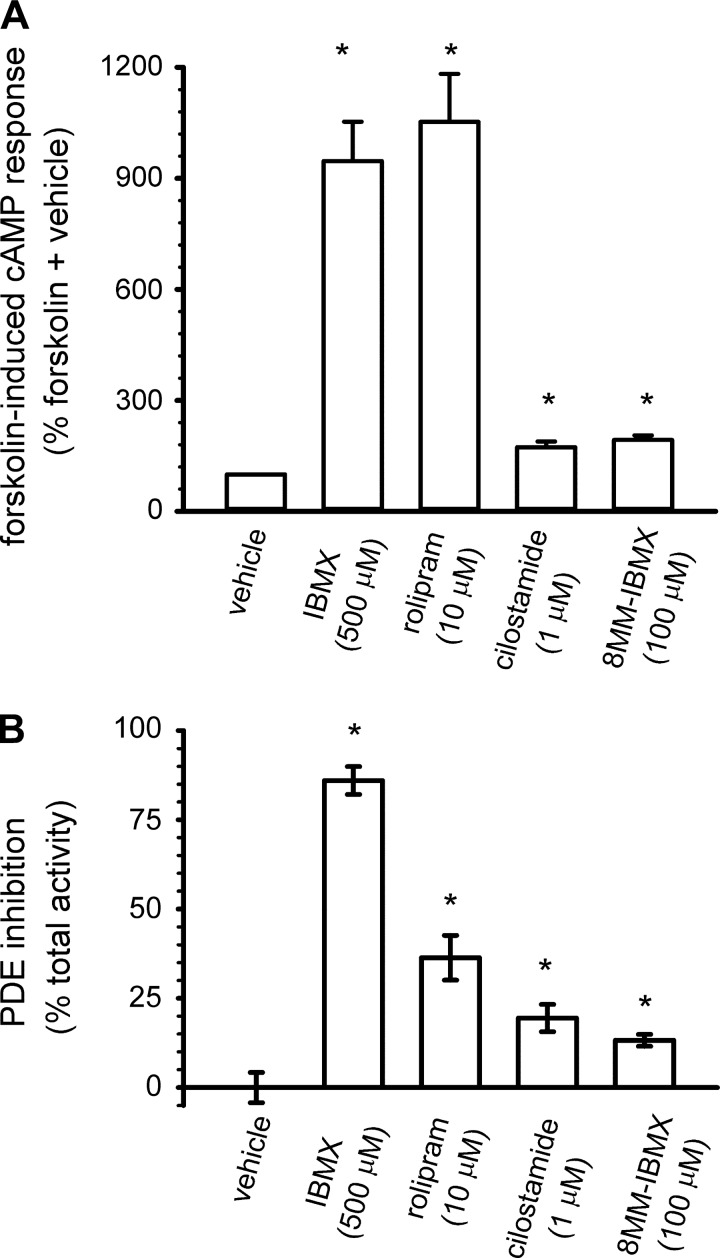
We next sought to determine whether other PDE subtypes were present in HEK-293 cells. Thus we examined the ability of several PDE inhibitors to potentiate 10 μM forskolin-induced cAMP levels. Both IBMX and rolipram triggered large increases in forskolin-induced cAMP accumulation (Fig. 3A). 8-MM-IBMX, a somewhat selective PDE1 inhibitor, and cilostamide, a selective PDE3 inhibitor, also potentiated forskolin-induced cAMP accumulation. Similarly, IBMX and rolipram inhibited significant levels of PDE activity assessed at 1 μM cAMP (Fig. 3B). 8-MM-IBMX and cilostamide inhibited small portions of total PDE activity. Thus, several IBMX sensitive PDEs may be present in HEK-293 cells.
Fig. 3.
PDE activity in HEK-293 cells. A: effect of nonselective (IBMX) and subtype selective PDE inhibitors on 10 μM forskolin-induced cAMP accumulation was assessed using enzyme immunoassays (as described in materials and methods). B: effects of nonselective and subtype selective inhibitors on total PDE activity, assessed at 1 μM cAMP. Data represent means ± SE; n ≥ 4. *Statistically different from vehicle control.
Monitoring PDE activity within single cells.
We utilized adenovirus-expressed CNG channels to monitor changes in intracellular cAMP levels. Two channel constructs were used for these experiments, channels composed of WT CNGA2 subunits (WT channels, K1/2 = 40 μM) and channels composed of CNGA2 subunits containing the C460W and E583M mutations (C460/E583M channels, K1/2 = 1.1 μM) (31). These channels offer high fidelity measurements of cAMP signals. Known concentrations of cAMP were dialyzed into the cell via the whole cell patch pipette. A typical experiment is depicted in Fig. 4. In this experiment, a HEK-293 cell that expressed C460W/E583M channels was dialyzed with 5 μM cAMP. After achieving whole cell configuration there was a delay followed by a slow increase in current. Rapid application of 10 mM MgCl2 blocked the observed currents, indicating that currents were through CNG channels (29, 30, 33, 48). The current reached a plateau in ∼3 min. Subsequent addition of 10 μM rolipram triggered a threefold increase in current, indicating a marked increase in cAMP near the plasma membrane. No MgCl2-blockable currents were observed in either uninfected or GFP-encoding-adenovirus infected HEK-293 cells, consistent with previous results (29, 30, 33, 48).
The experiments described above indicate that in HEK-293 cells endogenous PDE4 activity was capable of limiting the levels of cAMP that reached the plasma membrane when 5 μM cAMP were dialyzed from the patch pipette. We next sought to determine whether endogenous PDE activity could limit cAMP levels reaching CNG channels when 40 μM cAMP was dialyzed into the cell. For this experiment CNG channels comprised of WT CNGA2 subunits (K1/2 ∼40 μM) were used. Figure 5 depicts a typical experiment. Upon achieving the whole cell configuration there was an initial delay followed by an increase in current through CNG channels that reached a plateau within 10 min. Application of 10 μM rolipram triggered an additional 30% increase in current. Subsequent application of both 10 μM rolipram and 100 μM IBMX (a nonselective PDE inhibitor) did not further potentiate currents through CNG channels. These data strongly suggest that in HEK-293 cells rolipram-sensitive PDEs are uniquely localized to hydrolyze cAMP near CNG channels and that other IBMX-sensitive PDEs have little effect on cAMP levels in these near membrane domains. Addition of 8-bromo-cGMP (a membrane permeant cGMP analog) caused a twofold increase in current through CNG channels, indicating that in the presence of rolipram 40 μM cAMP caused half maximal activation of current through CNG channels.
Fig. 5.
PDE4 activity effectively hydrolyzes 40 μM cAMP washed in from the patch pipette. The influx of 40 μM cAMP from the patch pipette was monitored using WT CNG channels. Exposure to 10 μM rolipram caused an additional increase in current through CNG channels, indicating that PDE4 activity is sufficient to effectively hydrolyze cAMP washed in from the patch pipette. Subsequent exposure to 100 μM IBMX caused no additional increase in current. Exposure to the membrane permeant cGMP analog 8-bromo-cGMP (100 μM) triggered an additional increase in current through CNG channels. These data indicate that in the presence of rolipram, the wash in of 40 μM cAMP from the patch pipette half-maximally activates CNG channels, consistent with the 40 μM K1/2 for WT CNG channels.
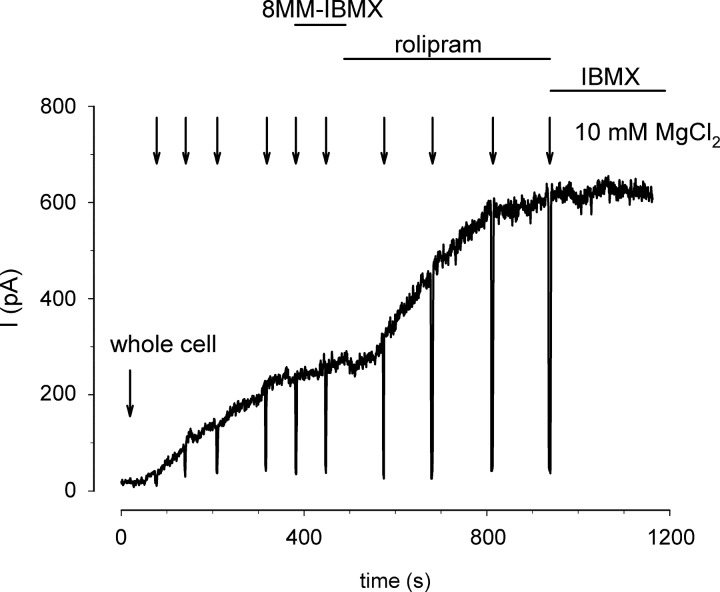
The data described above indicate that PDE4 activity has a profound effect on the ability of cAMP to traverse from the patch pipette to the plasma membrane. We next sought to determine whether 8MM-IBMX-sensitive PDE activity influenced the ability of exogenous cAMP to activate CNG channels. C460W/E583M channels were used to monitor the influx of 5 μM cAMP from the patch pipette. Upon achieving whole cell configuration we observed an initial delay followed by a steady increase in current through CNG channels (Fig. 6). The current reached a plateau within 6 min. Addition of 100 μM 8MM-IBMX caused little or no increase in current. Subsequent addition of 10 μM rolipram caused a twofold increase in current through CNG channels. This and similar experiments indicate that extracellular application of 8MM-IBMX was not able to potentiate cAMP-induced currents through CNG channels. We have demonstrated that 8MM-IBMX successfully crosses the plasma membrane and inhibits PDE activity in other cellular systems (not shown). In addition, we conducted experiments in which 5, 10, or 40 μM cAMP and 100 μM 8MM-IBMX were included in the patch pipette to ensure that 8MM-IBMX had access to the intracellular space of HEK-293 cells. Intracellular 8-MM-IBMX caused no discernable increase in cAMP-induced currents at the cAMP concentrations tested (Fig. 7).
Fig. 6.
8MM-IBMX sensitive PDE activity does not effectively hydrolyze 5 μM cAMP washed in from the patch pipette. The wash in of 5 μM cAMP was monitored with C460W/E583M channels. Wash in of 5 μM cAMP caused a steady increase in current through CNG channels. Current levels reached a plateau in ∼400 s. Exposure to 100 μM 8MM-IBMX did not trigger an additional increase in current through CNG channels. Addition of 10 μM rolipram caused a substantial increase in current. These data indicate the 8MM-IBMX-sensitive PDE activity in HEK-293 cells is not sufficient to alter the cAMP levels that reached CNG channels.
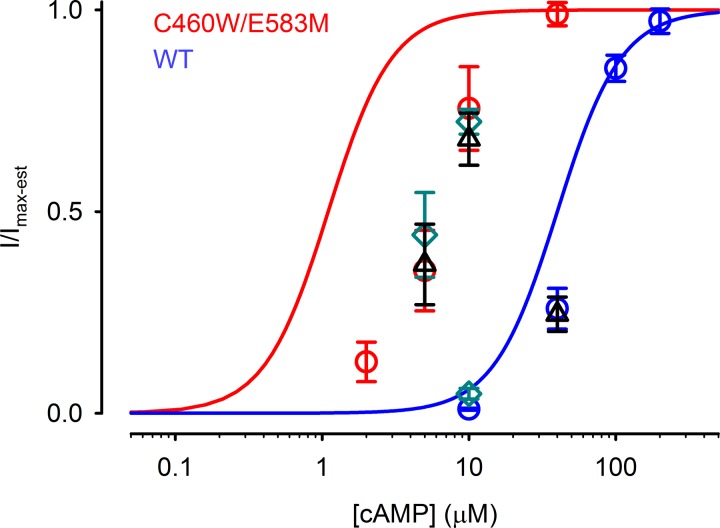
Fig. 7.
Summary of the steady-state cAMP dose response relations for CNG channel activity in the whole cell setting. Fractional currents through C460W/E583M (red circles) or WT (blues circles) CNG channels are plotted as a function of [cAMP] in the patch pipette. Solid lines represent the Hill equation for C460W/E583M (red) and WT (blue) CNG channels based on data obtained from excised patches: Hill coefficient of 2 and K1/2 of 1 and 40 μM, respectively (29–31). Fractional currents through C460W/E583M or WT channels are dramatically right shifted compared with the K1/2 of each construct. Inclusion of the PDE inhibitor 8MM-IBMX in the extracellular solution (green diamonds) or pipette solution (black triangles) did not significantly alter I/Imax-est. Imax-est is defined in the materials and methods (see Fig. 1).
Figure 7 depicts the cumulative data from experiments conducted at several cAMP concentrations. Solid lines represent the cAMP concentration dependence of WT (blue) and C460W/E583M (red) channels measured in excised patches (31). The dose dependence of CNG channel activity to cAMP introduced via the patch pipette was significantly right-shifted in cells expressing either WT (blue circles) or C460W/E583M (red circles) channels. 8MM-IBMX introduced in the extracellular solution (green diamonds) or via the patch pipette (black triangles) had little or no effect on the cAMP dose dependence of CNG channels. These data strongly suggest that right shift in cAMP dose dependence observed in the whole cell setting is primarily due to PDE4 activity.
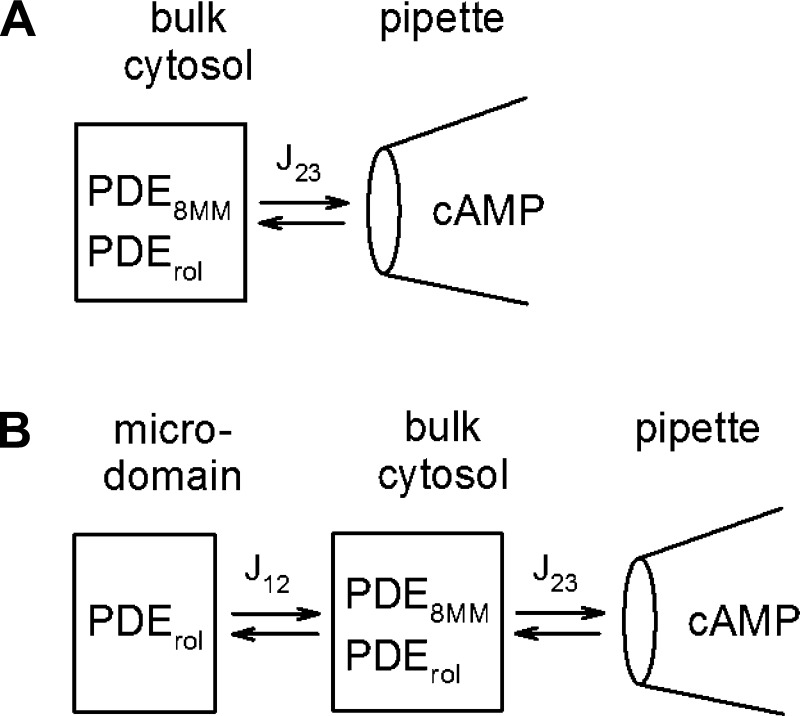
The data presented thus far suggest that the PDE4 inhibitor rolipram has a profound effect on the ability of cAMP introduced via the patch pipette to activate CNG channels in the plasma membrane; however, the PDE inhibitor 8MM-IBMX has little or no effect on CNG channel activation. To better understand this phenomenon we used previously published compartmental models to describe the scenario (27, 29–31). Two models were considered: the first model considered is a one compartment model in which cAMP is introduced into the cell via the patch pipette and freely (rapidly) diffuses throughout the cell, leading to activation of CNG channels (Fig. 8A). Rolipram- and 8MM-IBMX-sensitive PDEs are both present within this compartment. The second model considered is a two compartment model in which cAMP is introduced into the cell via the patch pipette and freely diffuses throughout the cytosolic space, but access to the near-membrane space (containing CNG channels) is slowed (Fig. 8B). In this model the rolipram-sensitive PDE activity is in the near-membrane space and the bulk cytosol, whereas the 8MM-sensitive PDE activity is only in the cytosolic space. Parameters used in each model are presented in Table 1.
Fig. 8.
Schematic representation of the 1 and 2 compartment models used to describe the wash in of cAMP from the patch pipette to CNG channels in the plasma membrane. A: 1 compartment model. B: 2 compartment model.
Table 1.
Parameters for simulations of 1 and 2 compartment models
| 2 Compartment Model |
||||
|---|---|---|---|---|
| Parameter | 1 Compartment Model | Compartment 1 | Compartment 2 | Parameter Definition |
| Vpipette, pl | 4 × 104 | 4 × 104 | Pipette volume (29, 30) | |
| V, pl | 2.048 | 0.048 | 2 | Compartment volume (29, 30) |
| J2/3, l/s | 1.6 × 10−13 | N/A | 1.6 × 10−13 | Flux coefficient from pipette to cytosol (29, 30) |
| J1/2, l/s | N/A | 1.6 × 10−16 | N/A | Flux coefficient from compartment 1 to compartment 2† (29, 30) |
| EPDE4:Ci, μM/s | 0.42 | 0.021 | 0.42 | Maximal hydrolysis rate for PDE4 in indicated compartment*† |
| Km-PDE4, μM | 3 | 3 | 3 | Michaelis constant for PDE4*† (10, 16) |
| Ki-PDE4, μM | 0.2 | 0.2 | 0.2 | Inhibition constant for PDE4 (10, 16) |
| EPDE8MM:Ci, μM/s | 0.17 | 0 | 0.17 | Maximal hydrolysis rate for 8MM-IBMX sensitive PDE in indicated compartment*† |
| Km-PDE8MM, μM | 1 | N/A | 1 | Michaelis constant of 8MM-IBMX sensitive PDE* |
| Ki-PDE8MM, μM | 5 | N/A | 5 | Inhibition constant of 8MM-IBMX-sensitive PDE (1, 31) |
| K1/2, μM | 1 or 40 (as indicated) | 1 or 40 (as indicated) | N/A | [cAMP] at which 50% of CNG channels are active (29–31) |
| N | 2 | 2 | N/A | Hill coefficient for CNG channel activation (29–31) |
PDE, phosphodiesterase; CNG, cyclic nucleotide gated; 8MM-IBMX, 8-methoxymethyl IBMX.
Parameters were measured experimentally.
Parameters were manually fit to data.
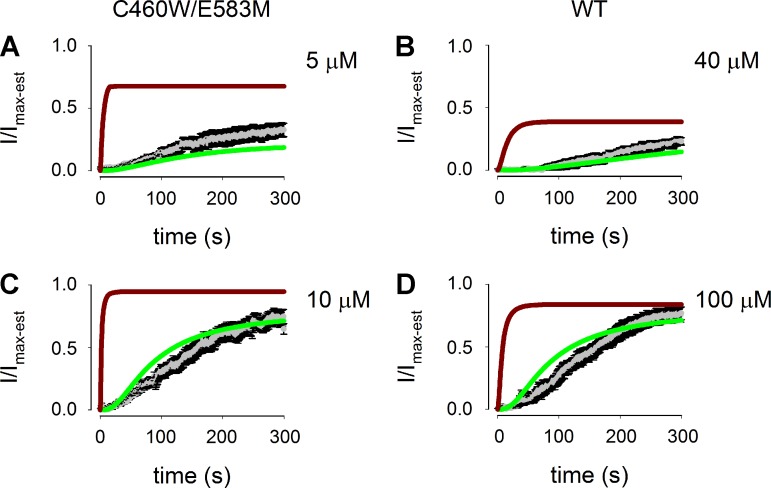
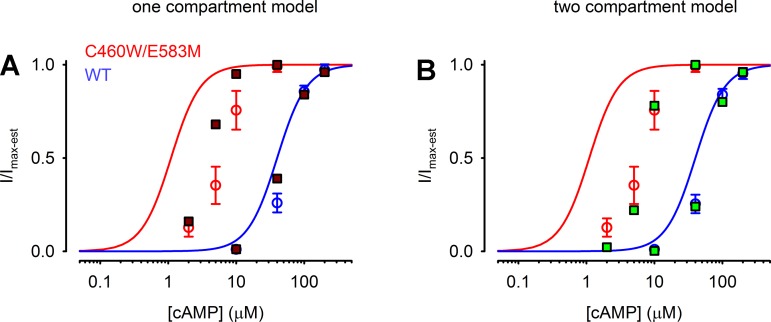
Fits of both the one compartment (brown lines) and two compartment (green lines) models to the wash in of 5 and 10 μM cAMP as measured with C460W/E583M channels as well as 40 and 100 μM cAMP as measured with WT CNG channels (gray symbols with black error bars) are shown in Fig. 9. The two compartment model clearly provides a better fit to the experimental data than the one compartment model. The primary reason for the better fit of the two compartment model is that the flux coefficient between compartments 1 and 2 provides a slow accumulation of cAMP in compartment 1; thus the relatively low PDE activity in compartment 1 is able to hydrolyze sufficient cAMP to maintain the experimentally observed levels. Similarly, the two compartment model provides a better fit to the stady-state cAMP concentration dependence of CNG channel activity (Fig. 10, A and B). These simulations clearly suggest that intracellular barriers to diffusion in conjunction with localized PDE4 activity are sufficient to describe the experimentally observed time course of cAMP wash in and accumulation in the near-membrane (near CNG-channel) space.
Fig. 9.
Fits of 1 and 2 compartment models to the time course of cAMP wash in from the patch pipette to CNG channels. Gray symbols represent average values of normalized currents through C460W/E583M channels or WT CNG channels elicited by cAMP wash in from the patch pipette, and the SE is depicted by black error bars. Brown lines represent fits of the 1 compartment model. Green lines represent fits of the 2 compartment model. A: activation of C460W/E583M channels elicited by wash in of 5 μM cAMP. B: activation of WT CNG channels elicited by wash in of 40 μM cAMP. C: activation of C460W/E583M channels elicited by wash in of 10 μM cAMP. D: activation of WT CNG channels elicited by wash in of 100 μM cAMP. Fits of the 2 compartment model adequately describe all data sets.
Fig. 10.
Fits of 1 and 2 compartment models to stady-state cAMP wash in data. Experimental data (red and blue circles) are as described in Fig. 7. A: simulation results for a 1 compartment model (brown squares) fit to experimental data. It is apparent from these results that a one compartment model does not adequately fit the data. B: steady-state results from a 2 compartment model (green squares) display a clear right shift from the excised patch concentration response (solid lines) and better fit the experimental data.
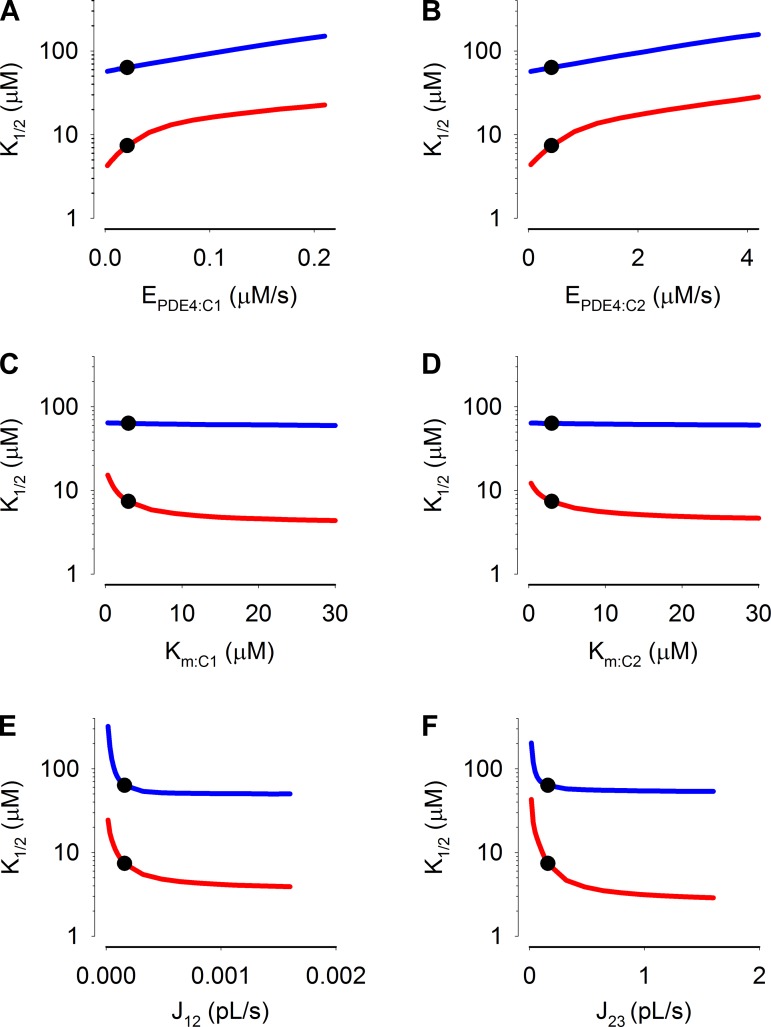
We next sought to assess the sensitivity of the model to critical parameters, specifically, the maximal PDE activities in compartments 1 and 2, the Michaelis constants of the PDE activities, and the flux coefficients between compartments 1 and 2 as well as between compartment 2 and the patch pipette (Fig. 11). Interestingly, we observed that near the optimized values the sensitivities of simulations of C460W/E583M channels (red lines) to these parameters were greater than those for simulations of WT channels (blue lines). This indicates that data obtained with the higher affinity construct better constrains the model. These simulations also indicate that inclusion of additional PDE activities in the cytosolic compartment would not substantially alter the apparent K1/2 of CNG channels (Fig. 11B, at the steepest point in the curve a twofold increase in EPDE:C2 would cause ∼0.3-fold increase in the apparent K1/2). Thus, with the exception of small effects of Km:PDE4 on steady-state WT channel response (which were nearly flat, blue lines Fig. 11, C and D), the sensitivities of the simulations to model parameters validate the use of multicompartment models to describe localized cAMP signaling, and indicate that the parameters presented in Table 1 represent a reasonable fit of the model to experimental data.
Fig. 11.
Estimated sensitivity of simulation results to changes in the maximal cAMP hydrolysis rates, Michaelis constants, and flux coefficients. Circles indicate values used in simulations. Lines indicate effect of changing indicated parameters on K1/2 of the simulated C460W/E583M (red) and WT CNG (blue) channel responses: EPDE4 in compartment 1 (A); EPDE4 in compartment 2 (B); Km of PDE4 in compartment 1 (C); Km of PDE4 in compartment 2 (D); the flux coefficient between compartments 1 and 2 (E); and the flux coefficients between compartments 2 and 3 (F).
DISCUSSION
We have used a three-tiered approach to estimate PDE activity near the plasma membrane. We first estimated total PDE activity in cell populations. We next measured the ability of a plasma membrane-localized sensor (adenovirus-expressed CNG channels) to detect known concentrations of cAMP introduced into the cell via the patch pipette. Finally, we used these data to constrain parameters in compartmental models describing the spatial distribution of cAMP within cells. We observed both rolipram- and 8-MM-IBMX-sensitive PDE activities in HEK-293 cells lysates. We next observed that 10 μM rolipram had a profound effect on the magnitude of cAMP-induced currents through CNG channels, even in the presence of 100 μM cAMP (∼33·Km of PDE4) in the patch pipette. In contrast, we observed that 100 μM 8MM-IBMX had little or no effect on the magnitude of cAMP-induced currents, even when introduced via the patch pipette. In addition, exposure to IBMX did not further potentiate rolipram-induced increases in current through CNG channels. Thus rolipram-sensitive PDE activity is uniquely positioned to regulate cAMP levels near CNG channels, regardless of whether cAMP is produced by endogenous adenylyl cyclase (31) or introduced via the patch pipette. Finally, we used the data described above to constrain both one and two compartment models describing the spatial spread of cAMP within cells. Simulations of the two compartment models well describe the observed time course of cAMP-induced CNG channel activation and steady-state CNG channel activation, whereas the one compartment model was not able to adequately describe the time course of CNG channel activation, and did not describe the steady-state CNG channel activation as well as the two compartment model.
In order for the two compartment model to fit the time course of cAMP-mediated activation of CNG channels, PDE activity in the near-membrane compartment had to be markedly less than the total cellular PDE activity, ∼20-fold less. In addition, the spatial spread of cAMP between the near-membrane compartment and the bulk cytosol needed to be lower than would be expected for diffusion in salt water. This slow spatial spread of cAMP is consistent with previous studies indicating that the spatial spread of cAMP within intact cells is hindered (see Refs. 15, 36 and references therein). Analogous studies have provided evidence of hindered diffusion of other small molecules, including Na+ (5, 13, 20). Taken together, these data indicate that under physiological conditions the slow spatial spread of cAMP signals allows low concentrations of PDE4 to effectively regulate cAMP signals in near-membrane compartments without significantly altering cAMP levels in the rest of the cell. Under less physiological conditions, e.g., sustained exposure to supersaturating hormone or forskolin concentrations or saturating concentrations of PDE inhibitors, cAMP compartmentalization may break down, leading to spurious activation of effector proteins throughout the cell.
Several studies have demonstrated the importance of specific pools of PDE4 activity in regulating localized cAMP signals and downstream effectors (4, 12, 34, 39). Studies have also implicated the importance of AKAPs in maintaining PKA-mediated regulation of PDE4 activity and that disruption of PKA-AKAP interactions dramatically alters the time course of agonist-induced cAMP signals (33, 35, 46, 48). Based on these studies it is interesting to speculate about the role(s) of scaffold proteins such as AKAPs in regulating local enzyme concentration. The dogma in the field is that scaffolds ensure high local enzyme concentration and thus facilitate rapid, high-fidelity signaling. This is undoubtedly true for many signaling pathways. For example, Tavalin (38) determined that expression of AKAP79 increased the local concentration of PKC ∼10-fold. In the study of Tavalin, calcium-calmodulin-dependent protein kinase II (CaMKII) or a catalytically active fragment of PKC (PKM) was introduced via the patch pipette into HEK-293 cells expressing either the GluR1 receptor alone or the GluR1 receptor and AKAP79. It was observed that the AKAP79-expressing cells had an ∼10-fold higher apparent affinity for PKM (based on changes in stady-state GluR1 currents), whereas the apparent affinity for CaMKII was similar to that observed in HEK-293 cells only expressing GluR1. These results indicated that AKAP79 has the potential to increase local enzyme concentrations ∼10-fold. In contrast, the data presented here indicate that there is a low level of endogenous PDE4 activity in near-membrane compartments of HEK-293 cells. Thus we propose a model in which precise concentrations of enzymes are constrained to specific subcellular compartments by protein scaffolds and other interactions such as protein localization within lipid rafts. The number of protein scaffolds within a subcellular compartment could be tuned to particular biological setpoints to ensure specific cellular responses. Such biological setpoints would not necessarily be rigid. Rather, they would likely be regulated, allowing the flexibility necessary for cells to adapt to changing environments by altering the number (or type) of scaffolds within specific subcellular compartments. Conversely, the effective diffusion coefficient could be regulated, perhaps by altering the local gelation state within a cell.
The experiments presented here utilized CNG channels to monitor localized cAMP levels. While the use of CNG channels to monitor cyclic nucleotide levels has provided a unique insight into cyclic nucleotide signaling, they cannot be readily used to probe cyclic nucleotide levels at different locations within individual cells. In the future, studies utilizing fluorescence and fluorescence resonance energy transfer (FRET) probes to determine the kinetics and spatial spread of second messengers will be required to better understand the relationship(s) among local enzyme concentration, effective diffusion coefficients, and the kinetics and spatial spread of agonist-induced signals. Recent improvements in fluorophores utilized in FRET probes and in approaches used to measure FRET and other multiplexed fluorescence signals should facilitate our understanding of the multifaceted signaling paradigms within complex cellular environments (18, 32).
In conclusion, the data presented here can be summarized as follows: PDE activity in the near-plasma membrane compartment, and more specifically in the same discrete domains as expressed CNG channels, is lower than in the bulk cytosol. We have estimated that the near-membrane PDE activity is ∼20-fold less. The relative sensitivity of the model to key parameters, including PDE concentrations in near-membrane and cytosolic compartments as well as the flux coefficients between compartments, suggests that the data have sufficient information content to constrain the model. In addition, the flux coefficient between the patch pipette and cytosolic compartment is well constrained (28, 29). However, two caveats to the model should be mentioned. First, the estimate of the flux coefficient between compartments 1 and 2 largely depends on the volume of compartment 1, which is unknown. Currently, we can only estimate the sensitivity of the simulations to the flux coefficient (Fig. 11E). Second, the two compartment model presented here assumes a single, discrete barrier to free, unfettered diffusion. While this model has been used successfully to describe a variety of observations about second messenger signaling (17, 27, 29, 30, 44, 46, 47), it is unlikely that there is only a single, discrete diffusional barrier within cells (15, 36). Thus, as we learn more about the nature of the intracellular environment, we will be able to make more quantitatively accurate estimates of local PDE activity. Even with these caveats, the data presented here strongly suggest that 1) a subset of PDE4 activity is localized within discrete signaling domains near the plasma membrane; 2) the exchange of cAMP between these domains and the bulk cytosol is hindered; and 3) PDE activity in the near-membrane signaling domains is lower than in the bulk cytosol. As such, these results are suggestive of an alternative perspective of the physiological roles of scaffold proteins in the cAMP signaling pathway: the concentration of scaffolds dictates the concentration of signaling enzymes within discrete subcellular domains, and therefore dictates basal signaling activity as well as the response to agonists.
GRANTS
This work was supported by National Institutes of Health Grants R01-HL-094455, P01-HL-06629, and S10-RR-027535 and the Abraham Mitchell Cancer Research Fund.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: W.X., W.P.F., A.L.B., C.D.O., B.Z., and W.R. performed experiments; W.X., W.P.F., A.L.B., C.D.O., B.Z., W.R., and T.C.R. analyzed data; W.X., W.P.F., C.D.O., B.Z., W.R., S.J.L., and T.C.R. interpreted results of experiments; W.X. and T.C.R. prepared figures; W.X. drafted manuscript; W.X., W.P.F., A.L.B., C.D.O., B.Z., W.R., S.J.L., and T.C.R. edited and revised manuscript; W.X., W.P.F., A.L.B., C.D.O., B.Z., W.R., S.J.L., and T.C.R. approved final version of manuscript; W.R., S.J.L., and T.C.R. conception and design of research.
ACKNOWLEDGMENTS
We thank Drs. M. Kiskowski-Byrne, J.W. Karpen, T. Stevens, and S. Sayner for valuable discussions about this work and helpful comments on the manuscript.
REFERENCES
- 1.Ahn HS, Crim W, Romano M, Sybertz E, Pitts B. Effects of selective inhibitors on cyclic nucleotide phosphodiesterases of rabbit aorta. Biochem Pharmacol 38: 3331–3339, 1989. [DOI] [PubMed] [Google Scholar]
- 2.Beavo JA. Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiol Rev 75: 725–748, 1995. [DOI] [PubMed] [Google Scholar]
- 3.Beavo JA. Multiple isozymes of cyclic nucleotide phosphodiesterase. Adv Second Messenger Phosphoprotein Res 22: 1–38, 1988. [PubMed] [Google Scholar]
- 4.Blackman BE, Heimann J, Horner K, Wang D, Richter W, Rich TC, Conti M. PDE4D and PDE4B function in distinct subcellular compartments in mouse embryonic fibroblasts. J Biol Chem 286: 12590–12601, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bridge JH, Smolley JR, Spitzer KW. The relationship between charge movements associated with ICa and INa-Ca in cardiac myocytes. Science 248: 376–378, 1990. [DOI] [PubMed] [Google Scholar]
- 6.Burns ME, Baylor DA. Activation, deactivation, and adaptation in vertebrate photoreceptor cells. Annu Rev Neurosci 24: 779–805, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Calebiro D, Maiellaro I. cAMP signaling microdomains and their observation by optical methods. Front Cell Neurosci 8: 350, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cameron DA, Pugh EN Jr. The magnitude, time course and spatial distribution of current induced in salamander rods by cyclic guanine nucleotides. J Physiol 430: 419–439, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen CH, Nakamura T, Koutalos Y. Cyclic AMP diffusion coefficient in frog olfactory cilia. Biophys J 76: 2861–2867, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conti M, Beavo J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem 76: 481–551, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Conti M, Mika D, Richter W. Cyclic AMP compartments and signaling specificity: role of cyclic nucleotide phosphodiesterases. J Gen Physiol 143: 29–38, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Creighton J, Zhu B, Alexeyev M, Stevens T. Spectrin-anchored phosphodiesterase 4D4 restricts cAMP from disrupting microtubules and inducing endothelial cell gap formation. J Cell Sci 121: 110–119, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Despa S, Bers DM. Na/K pump current and Nai in rabbit ventricular myocytes: local Nai depletion and Na buffering. Biophys J 84: 4157–4166, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DiPilato LM, Cheng X, Zhang J. Fluorescent indicators of cAMP and Epac activation reveal differential dynamics of cAMP signaling within discrete subcellular compartments. Proc Natl Acad Sci USA 101: 16513–16518, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feinstein WP, Zhu B, Leavesley SJ, Sayner SL, Rich TC. Assessment of cellular mechanisms contributing to cAMP compartmentalization in pulmonary microvascular endothelial cells. Am J Physiol Cell Physiol 302: C839–C852, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Houslay MD, Sullivan M, Bolger GB. The multienzyme PDE4 cyclic adenosine monophosphate-specific phosphodiesterase family: intracellular targeting, regulation, and selective inhibition by compounds exerting anti-inflammatory and antidepressant actions. Adv Pharmacol 44: 225–342, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Iancu RV, Jones SW, Harvey RD. Compartmentation of cAMP signaling in cardiac myocytes: a computational study. Biophys J 92: 3317–3331, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klarenbeek J, Goedhart J, van Batenburg A, Groenewald D, Jalink K. Fourth-generation epac-based FRET sensors for cAMP feature exceptional brightness, photostability and dynamic range: characterization of dedicated sensors for FLIM, for ratiometry and with high affinity. PLoS One 10: e0122513, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koutalos Y, Nakatani K, Yau KW. The cGMP-phosphodiesterase and its contribution to sensitivity regulation in retinal rods. J Gen Physiol 106: 891–921, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leblanc N, Hume JR. Sodium current-induced release of calcium from cardiac sarcoplasmic reticulum. Science 248: 372–376, 1990. [DOI] [PubMed] [Google Scholar]
- 21.Lorenz KL, Wells JN. Potentiation of the effects of sodium nitroprusside and of isoproterenol by selective phosphodiesterase inhibitors. Mol Pharmacol 23: 424–430, 1983. [PubMed] [Google Scholar]
- 22.Loughney K, Martins TJ, Harris EAS, Hicks JB, Sonnenburg WK, Beavo JA, Ferguson K. Isolation and characterization of cDNAs corresponding to two human calcium, calmodulin-regulated, 3′-5′-cyclic nucleotide phosphodiesterases. J Biol Chem 271: 796–806, 1996. [DOI] [PubMed] [Google Scholar]
- 23.Marley PD, Thomson KA. Regulation of cyclic AMP metabolism in bovine adrenal medullary cells. Biochem Pharmacol 44: 2105–2110, 1992. [DOI] [PubMed] [Google Scholar]
- 24.Molday RS. Photoreceptor membrane proteins, phototransduction, and retinal degenerative diseases: The Friedenwald Lecture. Invest Ophthalmol Vis Sci 39: 2493–2513, 1998. [PubMed] [Google Scholar]
- 25.Nikonov S, Lamb TD, Pugh EN Jr. The role of steady phosphodiesterase activity in the kinetics of the light-adapted salamander rod photoresponse. J Gen Physiol 116: 795–824, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oliveira RF, Terrin A, Di Benedetto G, Cannon RC, Koh W, Kim M, Zaccolo M, Blackwell KT. The role of type 4 phosphodiesterases in generating microdomains of cAMP: large scale stochastic simulations. PLoS One 22: e11725, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piggott LA, Hassell KA, Berkova Z, Morris AP, Silberbach M, Rich TC. Natriuretic peptides and nitric oxide stimulate cGMP synthesis in different cellular compartments. J Gen Physiol 128: 3–14, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pusch M, Neher E. Rates of diffusional exchange between small cells and a measuring patch pipette. Pflügers Arch 411: 204–211, 1988. [DOI] [PubMed] [Google Scholar]
- 29.Rich TC, Fagan KA, Nakata H, Schaack J, Cooper DM, Karpen JW. Cyclic nucleotide-gated channels colocalize with adenylyl cyclase in regions of restricted cAMP diffusion. J Gen Physiol 116: 147–161, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rich TC, Fagan KA, Tse TE, Schaack J, Cooper DM, Karpen JW. A uniform extracellular stimulus triggers distinct cAMP signals in different compartments of a simple cell. Proc Natl Acad Sci USA 98: 13049–13054, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rich TC, Tse TE, Rohan JG, Schaack J, Karpen JW. In vivo assessment of local phosphodiesterase activity using tailored cyclic nucleotide-gated channels as cAMP sensors. J Gen Physiol 118: 63–77, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rich TC, Webb KJ, Leavesley SJ. Perspectives on: Cyclic nucleotide microdomains and signaling specificity: Can we decipher the information content contained within cyclic nucleotide signals? J Gen Physiol 143: 17–27, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rich TC, Xin WCM, Hassell KA, Piggott LA, Le X, Karpen JW, Conti M. Cellular mechanisms underlying prostaglandin-induced transient cAMP signals near the plasma membrane of HEK-293 cells. Am J Physiol Cell Physiol 292: C319–C331, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rochais F, Abi-Gerges A, Horner K, Lefebvre F, Cooper DM, Conti M, Fischmeister R, Vandecasteele G. A specific pattern of phosphodiesterases controls the cAMP signals generated by different Gs-coupled receptors in adult rat ventricular myocytes. Circ Res 98: 1081–1088, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rochais F, Vandecasteele G, Lefebvre F, Lugnier C, Lum H, Mazet JL, Cooper DM, Fischmeister R. Negative feedback exerted by cAMP-dependent protein kinase and cAMP phosphodiesterase on subsarcolemmal cAMP signals in intact cardiac myocytes: an in vivo study using adenovirus-mediated expression of CNG channels. J Biol Chem 279: 52095–52105, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Saucerman JJ, Greenwald EC, Polanska-Grabowska R. Mechanisms of cyclic AMP compartmentalization revealed by computational models. J Gen Physiol 143: 39–48, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steinberg SF, Brunton LL. Compartmentation of G protein-coupled signaling pathways in cardiac myocytes. Annu Rev Pharmacol Toxicol 41: 751–773, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Tavalin SJ. AKAP79 selectively enhances protein kinase C regulation of GluR1 at a Ca2+-calmodulin-dependent protein kinase II/protein kinase C site. J Biol Chem 283: 11445–11452., 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Terrin A, Di Benedetto G, Pertegato V, Cheung YF, Baillie G, Lynch MJ, Elvassore N, Prinz A, Herberg FW, Houslay MD, Zaccolo M. PGE1 stimulation of HEK293 cells generates multiple contiguous domains with different [cAMP]: role of compartmentalized phosphodiesterases. J Cell Biol 175: 441–451, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thompson WJ, Appleman MM. Multiple cyclic nucleotide phosphodiesterase activities from rat brain. Biochemistry 10: 311–316, 1971. [PubMed] [Google Scholar]
- 41.Thompson WJ, Ashikaga T, Kelly JJ, Liu L, Zhu B, Vemavarapu L, Strada SJ. Regulation of cyclic AMP in rat pulmonary microvascular endothelial cells by rolipram-sensitive cyclic AMP phosphodiesterase (PDE4). Biochem Pharmacol 63: 797–807, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Violin JD, Dipilato LM, Yildirim N, Elston TC, Zhang J, Lefkowitz RJ. Beta2-adrenergic receptor signaling and desensitization elucidated by quantitative modeling of real time cAMP dynamics. J Biol Chem 283: 2949–2961, 2008. [DOI] [PubMed] [Google Scholar]
- 43.Walsh KB, Rich TC, Coffman Z. Development of a high throughput assay for monitoring cAMP levels in cardiac ventricular myocytes. J Cardiovasc Pharm 53: 223–230, 2009. [DOI] [PubMed] [Google Scholar]
- 44.Warrier S, Ramamurthy G, Eckert RL, Nikolaev VO, Lohse MJ, Harvey RD. cAMP microdomains and L-type Ca2+ channel regulation in guinea-pig ventricular myocytes. J Physiol 580: 765–776, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Willoughby D, Baillie GS, Lynch MJ, Ciruela A, Houslay MD, Cooper DM. Dynamic regulation, desensitization, and cross-talk in discrete subcellular microdomains during beta2-adrenoceptor and prostanoid receptor cAMP signaling. J Biol Chem 282: 34235–34249, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Willoughby D, Wong W, Schaack J, Scott JD, Cooper DM. An anchored PKA and PDE4 complex regulates subplasmalemmal cAMP dynamics. EMBO J 25: 2051–2061, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xie M, Rich TC, Scheitrum C, Conti M, Richter W. Inactivation of multidrug resistance proteins disrupts both cellular extrusion and intracellular degradation of cAMP. Mol Pharmacol 80: 281–293, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xin W, Tran TM, Richter W, Clark RB, Rich TC. Functional roles of GRK and PDE activities in the regulation of b2 adrenergic signaling. J Gen Physiol 134: 349–364, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu B, Kelly J, Vemavarapu L, Thompson WJ, Strada SJ. Activation and induction of cyclic AMP phosphodiesterase (PDE4) in rat pulmonary microvascular endothelial cells. Biochem Pharmacol 68: 479–491, 2004. [DOI] [PubMed] [Google Scholar]