Abstract
cAMP is a highly regulated secondary messenger involved in many biological processes. Chronic activation of the cAMP pathway by catecholamines results in cardiac hypertrophy and fibrosis; however, the mechanism by which elevated cAMP leads to cardiomyopathy is not fully understood. To address this issue, we increased intracellular cAMP levels in HL-1 cardiomyocytes, a cell line derived from adult mouse atrium, using either the stable cAMP analog N6,2′-O-dibutyryladenosine 3′,5′-cyclic monophosphate (DBcAMP) or phosphodiesterase (PDE) inhibitors caffeine and theophylline. Elevated cAMP levels increased cell size and altered expression levels of cardiac genes and micro-RNAs associated with hypertrophic cardiomyopathy (HCM), including Myh6, Myh7, Myh7b, Tnni3, Anp, Bnp, Gata4, Mef2c, Mef2d, Nfatc1, miR208a, and miR208b. In addition, DBcAMP altered the expression of DNA methyltransferases (Dnmts) and Tet methylcytosine dioxygenases (Tets), enzymes that regulate genomic DNA methylation levels. Changes in expression of DNA methylation genes induced by elevated cAMP led to increased global DNA methylation in HL-1 cells. In contrast, inhibition of DNMT activity with 5-azacytidine treatment decreased global DNA methylation levels and blocked the increased expression of several HCM genes (Myh7, Gata4, Mef2c, Nfatc1, Myh7b, Tnni3, and Bnp) observed with DBcAMP treatment. These results demonstrate that cAMP induces cardiomyocyte hypertrophy and altered HCM gene expression in vitro and that DNA methylation patterns mediate the upregulation of HCM genes induced by cAMP. These data identify a previously unknown mechanism by which elevated levels of cAMP lead to increased expression of genes associated with cardiomyocyte hypertrophy.
Keywords: adenosine 3′,5′-cyclic monophosphate; deoxyribonucleic acid methylation; cardiomyocyte hypertrophy
adenosine 3′,5′-cyclic monophosphate (cAMP) is a highly regulated secondary messenger involved in various intracellular processes (12). With sympathetic stimulation in the heart, cAMP produces a series of positive chronotropic, inotropic, and lusitropic effects that involve the activation of the cAMP-dependent protein kinase (PKA) and phosphorylation of key proteins (12). Activated PKA phosphorylates many transcription factors, including the cAMP response element-binding protein (CREB) (46). CREB binds to cAMP response elements (CREs) on gene promoters and activates gene expression (46). The main physiological process for lowering cAMP level in cardiac cells is through catalytic hydrolysis by cyclic nucleotide phosphodiesterases (PDEs) (2).
Some forms of chronic heart diseases in humans are associated with elevated levels of catecholamines such as epinephrine and norepinephrine, which have an inverse correlation with survival (33). Chronic activation of β-adrenergic receptors (β-ARs) or downstream cAMP targets, including PKA, results in cardiac hypertrophy and fibrosis, which can lead to ventricular dysfunction and heart failure (38, 41). The exact mechanism as to how elevated cAMP levels lead to cardiomyopathy are not fully understood.
Epigenetic processes that affect cardiomyocyte function and gene expression include DNA methylation, histone modification, and micro-RNA (miRNA) regulation (5, 49). DNA methylation patterns are established and maintained by DNA methyltransferases (DNMTs) (20, 47). DNMT1 is associated mainly with maintenance of methylation patterns, whereas DNMT3a and DNMT3b are more involved with de novo DNA methylation during early embryogenesis (13). Tet methylcytosine dioxygenases (Tets) facilitate the demethylation of the genome by catalyzing the conversion of 5-methylcytosine (5mC) to 5-hydroxymethylcytosine (5hmC) (27, 30). Methylation of normally unmethylated cytosine-phosphate-guanine (CpG) sites that are located in the promoter region are associated with transcriptional repression of many genes (28). DNA methylation inhibits gene expression through suppressing transcription factor binding or recruiting histone deacetylases that cause chromatin condensation and gene inactivation (50). Methylation of CpGs within the gene body also influences gene regulation and alternative splicing and can lead to increased gene expression in mammalian cells and tissues (21, 48, 53, 57).
Emerging data show that aberrant DNA methylation patterns play an important role in cardiovascular disease, including atherogenesis (54), coronary artery disease (44, 45), dilated cardiomyopathy (24), and heart failure (29, 36, 37). Furthermore, DNA methylation regulates the expression of myosin heavy chain 7 (Myh7), a sarcomere protein important for cardiac contractility (8). However, the effects of altered DNA methylation patterns on the expression of other cardiac genes remain unexplored. Additionally, it is unknown if activation of the cAMP signaling pathway modulates DNA methylation status and, if so, how this may lead to the development of cardiac disease.
Hypertrophic cardiomyopathy (HCM) is associated with thickening of the heart muscle and is governed by a network of specific genes (26, 52). HCM is associated with enhanced expression of cardiac structural genes, hormones, and transcription factors (26). These HCM genes include myosin heavy chain 6 (Myh6) and Myh7, atrial natriuretic peptide (Anp), B-type natriuretic peptide (Bnp), GATA-binding protein 4 (Gata4), myocyte enhancer factor 2c (Mef2c) and 2d (Mef2d), and nuclear factor of activated T cells, cytoplasmic, calcineurin-dependent 1 (Nfatc1). Increased expression of Myh7 is a common feature of HCM and heart failure (6, 22). Troponin is involved in cardiac muscle contraction, and elevated serum levels of troponin I and T are markers of cardiac muscle injury (1, 39).
miRNAs miR208a, miR208b, and miR499 are located within the Myh6, Myh7, and myosin heavy chain 7b (Myh7b) gene regions, respectively, and are coexpressed with their corresponding myosin genes (40). Increased expression of miR208a is often found in patients with cardiomyopathy, cardiac fibrosis, and heart failure (40). miR208a stimulates the expression of two slow-twitch myosins and their intronic miRNAs, Myh7/miR208b and Myh7b/miR499 (40). miR208b and miR499 are functionally redundant and are involved in the specification of muscle fiber identity by activating slow and repressing fast-twitch myofiber genes (51). The circulating levels of miR208b and miR499 are highly elevated after myocardial damage and are correlated with a poor prognosis following myocardial infarction (10, 23).
We propose that DNA methylation plays a role in the detrimental effects of long-term sustained activation of the cAMP signaling pathway. In this study, HL-1 cells, a cell line derived from adult mouse cardiomyocytes (7), were used to investigate the interaction between cAMP, DNA methylation modification, and cardiomyocyte hypertrophy. These cells are uniquely suited for this study because of their homogeneity, rapid growth, ease of manipulation, and differentiated cardiomyocyte traits (7). In addition, HL-1 cells have been used previously to study cardiomyocyte hypertrophy (31). Herein, we report that the cell size, expression of HCM genes, and global DNA methylation level in HL-1 cells are markedly induced by elevated cAMP levels. We also discover that DNA methylation plays a role in mediating the cAMP-induced alteration in HCM gene expression.
MATERIALS AND METHODS
Materials.
N6,2′-O-dibutyryladenosine 3′,5′-cyclic monophosphate sodium salt (DBcAMP), Rp-adenosine 3′,5′-cyclic monophosphorothioate triethylammonium salt (Rp-cAMP), caffeine, theophylline, 5-azacytidine (5-aza), Claycomb medium, FBS, norepinephrine, l-glutamine, penicillin/streptomycin, and β-actin antibody were purchased from Sigma-Aldrich (St. Louis, MO). Antibodies MYH6 (K-13, sc-168676), MYH7 (A4.951, sc-53090), goat antimouse IgG-HRP (sc-2005), goat antirabbit IgG-HRP (sc-2030), and donkey antigoat IgG-HRP (sc-2020) were purchased from Santa Cruz Biotechnology (Dallas, TX). DNMT1 antibody (no. 5119) was purchased from Cell Signaling Technology (Danvers, MA). DNMT3a (ab13888) and DNMT3b (ab16049) antibodies were from Abcam (Cambridge, MA). Alexa Fluor 488 phalloidin and 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) were ordered from Life Technologies (Grand Island, NY).
HL-1 cell culture and treatment.
HL-1 cardiomyocytes were obtained from Dr. William C. Claycomb (7) and cultured with Claycomb medium supplemented with 10% FBS, 0.1 mM norepinephrine, 2 mM l-glutamine, and 100 U/ml penicillin/streptomycin. Cells were treated with drugs [DBcAMP, caffeine, theophylline, 5-aza, Rp-cAMP, DBcAMP + Rp-cAMP, or DBcAMP + 5-aza] or vehicle (dimethyl sulfoxide or PBS) for 2–48 h, with daily changes of medium. Cells were imaged for morphological changes with an Axio Vert.A1 inverted microscope (Zeiss, Jena, Germany). The area of attached cells was quantitated by the Zen Lite 2012 software (Zeiss). Three to five pictures were randomly taken of each biological replicate, and five random cells in each picture were measured.
Flow cytometry analysis.
After 48 h of drug treatment, trypsinized cardiomyocytes were fixed in 2% paraformaldehyde (PFA) for 10 min and washed in PBS three times. Samples were read on a LSRFortessa cell analyzer (BD Biosciences, Franklin Lakes, NJ), and 200,000 events/sample (n = 3) were acquired. The relative change in forward scatter is a proxy for cell size alteration (42). Data were analyzed with FlowJo Data Analysis software (FlowJo, Ashland, OR).
Immunofluorescence.
Cardiomyocytes cultured on cover slips were fixed in 4% PFA for 10 min and washed in PBS three times. Cells were permeabilized with 0.5% Triton X-100 in PBS, blocked with 2% bovine serum albumin/2% goat serum, and incubated with Alexa Fluor 488 Phalloidin and DAPI for 1 h (25). After being washed with PBS, cover slips were mounted on glass slides with Fluoromount G (Electron Microscopy Sciences, Hatfield, PA). Cells were imaged with an Axio Vert.A1 inverted microscope (Zeiss). Their attached area was measured by the Zen Lite 2012 software (Zeiss). Three to five pictures were randomly taken of each biological replicate, and five random cells in each picture were measured.
Quantitative real-time PCR analysis.
Total RNA was isolated with the RNeasy Plus Mini Kit (Qiagen, Valencia, CA) or the RNAqueous-Micro Total RNA Isolation Kit (Life Technologies). cDNA was synthesized using the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). Published quantitative real-time PCR (qPCR) primer pairs were used (18). Myh6 (PPM04500A) and Myh7 (PPM67019A) primers were proprietary sequences designed by Qiagen SABiosciences. β-Actin primers were used as an internal control. SYBRGreen PCR Master Mix (Life Technologies Applied Biosystems) was used to perform qPCR analysis in a GeneAmp 7300 Real Time PCR System (Life Technologies Applied Biosystems) (18). The 2−ΔΔCT method was used for relative quantification (17).
qPCR analysis of miRNA expression.
Total RNA including small RNA was isolated with the mirVana miRNA Isolation Kit (Life Technologies) and reverse transcribed to cDNA with the miScript II RT Kit (Qiagen). Primers for the mature miRNAs, miR208a, miR208b, miR499, and RNU6-2, were designed and purchased from Qiagen. snRNA RNU6-2 was used as an internal miRNA control. qPCR was performed with the miScript SYBR Green PCR Kit (Qiagen) in a GeneAmp 7300 Real Time PCR System (Life Technologies Applied Biosystems) (18, 19). The 2−ΔΔCT method was used for relative quantification (17).
Western blotting.
Cells were homogenized in RIPA buffer (Thermo Scientific, Rockford, IL) supplemented with Complete Protease Inhibitor Cocktail Tablets (Roche, Basel, Switzerland) by a Sonic Dismembrator (Thermo Scientific). Protein was quantitated with the Pierce BCA Protein Assay kit (Thermo Scientific), separated on a Criterion Tris·HCl Precast Gel (7.5% polyacrylamide; Bio-Rad), and transferred to a nitrocellulose membrane (Bio-Rad) using the Bio-Rad electrotransfer system (18, 56). Blots were probed with antisera to target proteins and against β-actin, as a control for sample loading. After detection with the Pierce ECL Plus Western Blotting Substrate (Thermo Scientific) in a ChemiDoc XRS+ Imaging System (Bio-Rad), band densitometry was quantitated with the Image Lab software (Bio-Rad).
Intracellular cAMP measurement.
cAMP levels in the HL-1 cells were measured after treatment with caffeine, theophylline, or vehicle for 48 h by using the cAMP Enzyme Immunoassay Kit (Direct; Sigma-Aldrich). Briefly, cells were trypsinized from the culture flasks, counted, homogenized in 0.1 M hydrochloric acid, and centrifuged. The supernatants were collected and measured for cAMP levels by using the manufacturer's protocol. The concentrations of cAMP were calculated based on the cAMP standard curve and then correlated to cell counts.
Measurement of 5mC and 5hmC levels.
Genomic DNA was isolated with the DNeasy Blood & Tissue Kit (Qiagen) and treated with RNase to remove RNA contaminants. The MethylFlash Methylated DNA Quantification Kit (Colorimetric; Epigentek, Farmingdale, NY) and MethylFlash Hydroxymethylated DNA Quantification Kit (Colorimetric; Epigentek) were used to quantitate the percentage of 5mC and 5hmC in genomic DNA samples, respectively (4, 16). After colorimetric reaction, the methylated cytosine was measured with a Synergy HT Multi-Mode Microplate Reader (BioTek, Winooski, VT).
Search for functional CREs in gene promoter.
Cardiac genes and DNA methylation genes were searched in the CREB Target Gene Database (http://natural.salk.edu/CREB/) (55). The results indicated if CREs are present in the promoters of the genes of interest.
Statistical analysis.
All experiments were performed at least three times. Results were analyzed using GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA). Data are presented as means ± SE. Statistical differences between treatment groups were determined using Student's t-test or one-way ANOVA followed by Neuman-Keulls post hoc test. A P value <0.05 is considered statistically significant. For qPCR analysis, statistical differences between treatments were determined on the linearized 2−ΔCT values (9). Fold change of gene and miRNA expression was calculated with the 2−ΔΔCT method (9).
RESULTS
DBcAMP induces cardiomyocyte hypertrophy.
HL-1 cells were treated with increasing concentrations of DBcAMP for 48 h. Cardiomyocyte enlargement was observed by phase-contrast imaging (Fig. 1, A-C) and by Alexa Fluor 488 phalloidin staining of the actin cytoskeleton, which outlines the cell bodies (Fig. 1, D–F). Cell size quantification indicated that the area of attached cells was increased by DBcAMP treatment by 4.96 ± 0.36- and 4.23 ± 0.31-fold at 1 and 1.5 mM, respectively (Fig. 1G). The flow cytometry results also demonstrated that DBcAMP significantly increased the cell size of HL-1 cardiomyocytes by 18.5 ± 4.0, 44.3 ± 3.3, and 20.8 ± 1.5% at 0.5, 1, and 1.5 mM, respectively (Fig. 1H). It is worth noting that flow cytometer uses forward scatter as a proxy of the size of floating cells, whereas immunostaining measures the surface area of attached cells; thus, the fold changes in cell size are smaller based on the flow cytometry data compared with those from immunostaining.
Fig. 1.
N6,2′-O-dibutyryladenosine 3′,5′-cyclic monophosphate (DBcAMP) induces HL-1 cell hypertrophy. HL-1 cardiomyocytes were treated with vehicle or DBcAMP for 48 h before phase-contrast images were taken (A, B, and C). Cells were stained with phalloidin (actin cytoskeleton, green) and 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI, nucleus, blue) to illustrate cell morphology (D, E, and F). Scale bar represents 50 μM. Increased area of attached cells was observed in HL-1 cells after DBcAMP treatments at 1 and 1.5 mM (G; n = 3, ***P < 0.001 vs. vehicle). In addition, HL-1 cells treated with vehicle or DBcAMP for 48 h were examined by a flow cytometer for relative change in forward scatter, a proxy of cell size, compared with vehicle. The average percent changes in size relative to vehicle are shown in H (n = 3, ***P < 0.001 vs. vehicle; ###P < 0.001 vs. 1 mM DBcAMP).
DBcAMP increases the expression of genes and miRNAs associated with HCM.
Next, the expressions of HCM-related genes and miRNAs were examined in HL-1 cells treated with DBcAMP for 2, 4, and 48 h. After acute exposure to DBcAMP for 2 and 4 h, mRNA expressions of HCM genes were unchanged (data not shown). However, at 48 h, mRNA expressions of cardiac structural genes [Myh6, Myh7, Myh7b, troponin I type 3 (Tnni3)], hormonal genes (Anp and Bnp), and transcription factors (Gata4, Mef2c, Mef2d, Nfatc1) were upregulated by DBcAMP (Fig. 2, A and B). In addition, expressions of miR208a and miR208b were increased following 48 h of DBcAMP treatment (Fig. 2C). Western blotting analysis demonstrated that increased mRNA expression of Myh6 and Myh7 induced by DBcAMP led to increased protein expression as well (Fig. 2, D and E).
Fig. 2.
DBcAMP increases expression of cardiac genes and micro-RNAs (miRNAs) associated with hypertrophic cardiomyopathy in HL-1 cardiomyocytes. Quantitative real-time PCR (qPCR) results revealed that DBcAMP treatment for 48 h significantly altered the mRNA expression of cardiac functional and hormonal genes (A) as well as cardiac transcription factors (B) in HL-1 cells. DBcAMP also increased the expression of cardiac-specific miRNAs (C). Western blotting analysis showed that DBcAMP increased the protein expression of myosin heavy chain 6 (MYH6) and myosin heavy chain 7 (MYH7) (D and E). Bars ± SE represent fold changes that are normalized to β-actin or snRNA RNU6-2 and relative to individual controls (n = 3, *P < 0.05, **P < 0.01, and ***P < 0.001 vs. vehicle).
The prediction of functional CREs on gene promoters was conducted with the CREB Target Gene Database (55). HCM genes, including Myh7, Bnp, Gata4, Mef2c, Mef2d, and Nfatc1, contain a CRE within −3 kb to 300 bp from the transcription start site (Table 1). However, other HCM genes Myh6 and Tnni3 do not have promoter CREs.
Table 1.
Computational prediction of functional cAMP-response elements on promoters of mouse genes
| Gene Symbol | Gene Name | Accession No. | CRE Predictiona |
|---|---|---|---|
| Myh6 | Myosin, heavy polypeptide 6, cardiac muscle, α | NM_010856 | − |
| Myh7 | Myosin, heavy polypeptide 7, cardiac muscle, β | NM_080728 | +; CRE_TATA |
| Myh7b | Myosin, heavy chain 7B, cardiac muscle, β | NM_001085378.2 | N/A |
| Tnni3 | Troponin I, cardiac | NM_009406 | − |
| Anp | Natriuretic peptide type A | NM_008725.2 | N/A |
| Bnp | Natriuretic peptide precursor type B | NM_008726 | +; Half-site CRE |
| Gata4 | GATA-binding protein 4 | NM_008092 | +; CRE_TATA |
| Mef2c | Myocyte enhancer factor 2C | NM_025282 | +; CRE_TATA |
| Mef2d | Myocyte enhancer factor 2D | NM_133665 | +; CRE_TATA |
| Nfatc1 | Nuclear factor of activated T cells, cytoplasmic 1 | NM_016791 | +; CRE_NoTATA |
| Nfatc1 | Nuclear factor of activated T cells, cytoplasmic 1, transcript variant 2 | NM_198429 | +; CRE_TATA |
| Dnmt1 | DNA methyltransferase 1 | NM_010066 | − |
| Dnmt3a | DNA methyltransferase 3A | NM_153743 | +; Half-site CRE |
| Dnmt3b | DNA methyltransferase 3B | NM_010068 | − |
| Tet1 | Tet methylcytosine dioxygenase 1 | NM_001253857.1 | N/A |
| Tet2 | Tet methylcytosine dioxygenase 2 | NM_145989 | − |
| Tet3 | Tet methylcytosine dioxygenase 3 | NM_183138.2 | − |
Presence of cAMP-response elements (CRE) in gene promoters is predicted by the CREB Target Gene Database http://natural.salk.edu/CREB/; −, functional CRE is not predicted in the promoter. +, functional CRE is predicted in the promoter in the distant promoter (−3 kb to 300 bp from transcription start site).
N/A, the gene of interest is not included in the CREB Target Gene Database; CRE_TATA, the gene of interest is predicted to have a functional CRE and TATA box; half-site CRE, half-CRE is predicted to be present in the promoter; CRE_NoTATA, the gene of interest is predicted to have a functional CRE and no TATA box.
PDE inhibitors upregulate the expression of HCM-related genes.
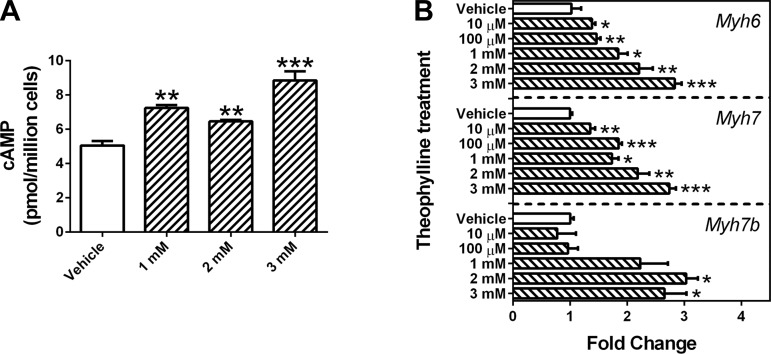
In addition to DBcAMP, HL-1 cells were treated with nonselective PDE inhibitors (caffeine or theophylline), which increase intracellular cAMP levels by blocking PDE-mediated degradation of cAMP (3). Results from the intracellular cAMP assay indicate that high concentrations of caffeine (>3 mM) significantly increased cellular cAMP levels (Fig. 3A). In addition, flow cytometry analysis showed that caffeine at 5 mM significantly increased the size of cardiomyocytes similar to 0.5 mM DBcAMP treatment (Fig. 3B). Moreover, caffeine markedly increased the expression of miRNAs and HCM genes at the mRNA level (Fig. 3, C–E). At the protein level, caffeine also increased MYH6 expression (Fig. 3, F and G).
Fig. 3.
Phosphodiesterase inhibitor caffeine increases the expression of cardiac genes and miRNAs associated with hypertrophic cardiomyopathy in HL-1 cells. Caffeine treatment for 48 h significantly increased intracellular cAMP levels (A), which were correlated to cell number. Flow cytometry data showed that cell size was increased after caffeine treatment (B). qPCR results revealed that the mRNA expression of cardiac functional and hormonal genes (C) and cardiac transcription factors (D) were altered in HL-1 cells treated with caffeine for 48 h. In addition, cardiac-specific miRNA expression was increased with caffeine treatments (E). Western blotting analysis showed that DBcAMP increased the protein expression of MYH6 (F and G). Bars ± SE represent fold changes that are normalized to β-actin or snRNA RNU6-2 and relative to individual controls (n = 3, *P < 0.05, **P < 0.01, and ***P < 0.001 vs. vehicle).
Similar to caffeine, theophylline increased the intracellular cAMP level (Fig. 4A). It also increased the mRNA expression of cardiac myosin genes Myh6, Myh7, and Myh7b in a concentration-dependent manner (Fig. 4B). However, the flow cytometry result showed that the cell size was unchanged after treatment with theophylline at 1–3 mM for 48 h (data not shown).
Fig. 4.
Phosphodiesterase inhibitor theophylline increases the expression of cardiac genes associated with hypertrophic cardiomyopathy in HL-1 cells. Theophylline treatment for 48 h significantly increased intracellular cAMP levels (A), which were correlated to cell number. Theophylline also increased the mRNA expression of Myh6, Myh7, and Myh7b (B). Bars ± SE represent fold changes that are normalized to β-actin and relative to individual controls (n = 3, *P < 0.05, **P < 0.01, and ***P < 0.001 vs. vehicle).
DBcAMP induces global DNA hypermethylation.
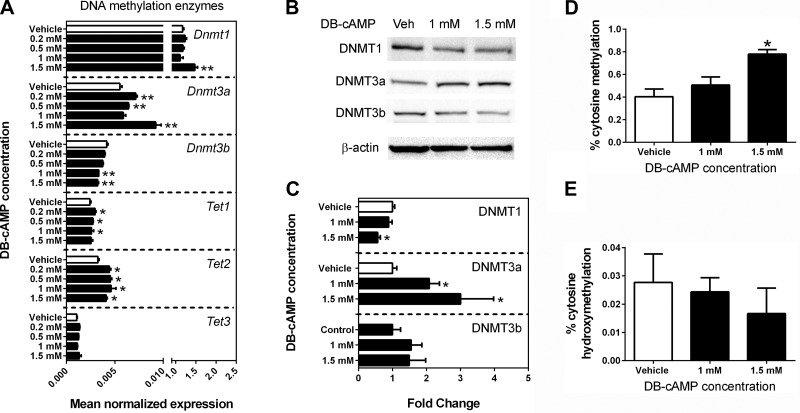
To investigate if DNA methylation mediates the hypertrophic effects of elevated cAMP, the expression of DNA methylation enzymes as well as global DNA methylation and hydroxymethylation levels were examined following DBcAMP treatment. DBcAMP treatment caused an upregulation of Dnmt1, Dnmt3a, Tet1, and Tet2 mRNAs and a downregulation of Dnmt3b mRNA (Fig. 5A). At the protein level, DBcAMP decreased DNMT1 and increased DNMT3a expression (Fig. 5, B and C). Consistent with upregulation of the de novo methyltransferase Dnmt3a, an increase in genomewide cytosine methylation was observed at the highest level of DBcAMP treatment (Fig. 5D). However, percent cytosine hydroxymethylation remained unaffected by DBcAMP treatment (Fig. 5E).
Fig. 5.
DBcAMP alters the expression of DNA methylation enzymes and increases global DNA methylation levels in HL-1 cells. qPCR results revealed that DBcAMP treatment of HL-1 cells for 48 h significantly altered the mRNA expression of DNA methylation enzymes (A). Bars ± SE represent fold changes normalized to β-actin and relative to the DNA methyltransferase (Dnmt1) expression in the vehicle group (n = 3, *P < 0.05 and **P < 0.01 vs. vehicle). Protein expressions of DNMT1 and DNMT3a were also affected by DBcAMP (B and C). The fold change was normalized to β-actin. DBcAMP treatment for 48 h significantly increased the level of global cytosine methylation (D) but did not change % cytosine hydroxymethylation (E).
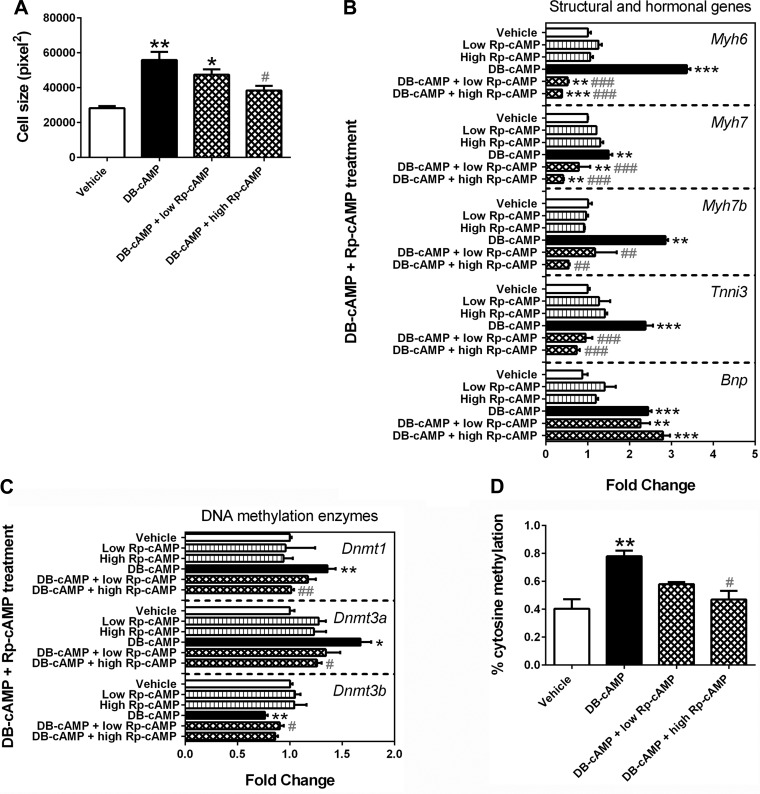
Rp-cAMP inhibits the DBcAMP-induced actions in HL-1 cells.
Rp-cAMP, a competitive inhibitor of cAMP-induced PKA activation, was used to investigate if the above observed DBcAMP effects were mediated by PKA. Cell size analysis on phase-contrast images showed that Rp-cAMP blocked the DBcAMP-induced cardiomyocyte enlargement (Fig. 6A). Rp-cAMP also inhibited the upregulation of Myh6, Myh7, Myh7b, and Tnni3 mRNA expression following DBcAMP treatment (Fig. 6B). Furthermore, Rp-cAMP normalized the expression of Dnmt mRNAs and global DNA methylation levels in DBcAMP-treated cells (Fig. 6, C and D). However, Rp-cAMP was unable to normalize the mRNA expression of Bnp (Fig. 6B), indicating that DBcAMP-induced Bnp expression may not be mediated by PKA activation.
Fig. 6.
Rp-adenosine 3′,5′-cyclic monophosphorothioate triethylammonium salt (Rp-cAMP) blocks the DBcAMP-induced actions in HL-1 cells. HL-1 cells were treated with vehicle [dimethyl sulfoxide (DMSO)], 100 μM Rp-cAMP (low), 250 μM Rp-cAMP (high), 1.5 mM DBcAMP, 1.5 mM DBcAMP + 100 μM Rp-cAMP (low), or 1 mM cAMP + 250 μM Rp-cAMP (high) for 48 h. Cell size analysis on phase-contrast images showed that Rp-cAMP blocked the DBcAMP-induced cardiomyocyte enlargement (A). Rp-cAMP also inhibited the upregulation of Myh6, Myh7, Myh7b, and troponin I type 3 (Tnni3) mRNA expression following DBcAMP treatment (B). Furthermore, Rp-cAMP normalized the expression of Dnmts mRNAs (C) as well as global DNA methylation levels in DBcAMP-treated cells (D). However, Rp-cAMP was unable to normalize the mRNA expression of B-type natriuretic peptide (Bnp) (B). Bars ± SE represent fold changes that are normalized to β-actin and relative to individual controls (n = 3, *P < 0.05, **P < 0.01, and ***P < 0.001 vs. vehicle; #P < 0.05, ##P < 0.01, and ###P < 0.001 vs. 1.5 mM DBcAMP group).
5-aza alters cardiac gene expression in HL-1 cells.
To determine if the expressions of HCM genes are sensitive to changes in the DNA methylation level, HL-1 cells were treated with the nonselective DNMT inhibitor 5-aza for 48 h, which at 0.5 and 1 μM concentrations significantly reduced global DNA methylation levels (Fig. 7A). Meanwhile, mRNA expressions of Myh7, Myh7b, Bnp, Gata4, and Nfatc1-a were upregulated by 5-aza (Fig. 7, B and C). In particular, Bnp was increased 10-fold with 1 μM 5-aza treatment, indicating that Bnp mRNA expression is highly sensitive to DNA methylation changes. In contrast, Myh6 and Anp mRNAs were downregulated in response to DNMT inhibition (Fig. 7B), whereas mRNA expression levels of Tnni3, Mef2c, Mef2d, Nfatc1-b, and Nfatc1-c were unaffected by 5-aza treatment (Fig. 7, B and C). At the protein level, 5-aza treatment repressed the expression of MYH6 and MYH7 (Fig. 7, D and E).
Fig. 7.
5-Azacytidine (5-aza) induces global DNA hypomethylation and changes cardiac gene expression in HL-1 cardiomyocytes. 5-aza Treatment for 48 h decreased the level of global cytosine methylation (A). qPCR results revealed that 5-aza treatment for 48 h significantly increased the mRNA expression of Myh7, Myh7b, Bnp, GATA-binding protein 4 (Gata4), and nuclear factor of activated T cells, cytoplasmic, calcineurin-dependent 1 (Nfatc1)-a and decreased the mRNA expression of Myh6 and Anp (B and C). Western blotting analysis showed that 5-aza decreased the protein expression of MYH6 and MYH7 (D and E). Bars ± SE represent fold changes that are normalized to β-actin and relative to individual controls (n = 3, *P < 0.05, **P < 0.01, and ***P < 0.001 vs. vehicle).
5-aza normalizes cardiac gene expression altered by DBcAMP treatment.
Because DBcAMP increases global DNA methylation levels in HL-1 cells, we tested whether inhibition of DNMT activities by 5-aza would reverse the mRNA expression changes induced by DBcAMP. HL-1 cells were treated with 1 mM DBcAMP, which caused an increase in cell size, along with 0.5 μM 5-aza for 48 h, which was shown to reduce global DNA methylation levels in HL-1 cells (Fig. 7A). Compared with the vehicle, 1 mM DBcAMP increased the mRNA expression of cardiac genes (Mhy7, Gata4, Mef2c, Nfatc1-b, Nfatc1-c, Myh7b, Tnni3, Bnp, and Anp; Fig. 8). However, DBcAMP-induced upregulation of many of these genes, including Myh7, Gata4, Mef2c, Nfatc1-b, and Nfatc1-c, was normalized back down to baseline (vehicle) levels when also treated with 5-aza (Fig. 8A). Some genes, including Myh7b, Tnni3, and Bnp, were reduced with 5-aza treatment compared with DBcAMP treatment alone but stayed significantly higher than control in HL-1 cells when cotreated with DBcAMP and 5-aza (Fig. 8B). In addition, 5-aza increased the expression of Nfatc1-a in DBcAMP-treated cells, even though its expression was unaffected by 1 mM DBcAMP treatment alone (Fig. 8B). The expression of Anp was increased by DBcAMP but was not affected by 5-aza in the presence of DBcAMP (Fig. 8B).
Fig. 8.
5-aza normalizes the changes in gene expression induced by DBcAMP. HL-1 cells were treated with vehicle (DMSO), 1 mM DBcAMP, or 1 mM DBcAMP + 0.5 μM 5-aza for 48 h. qPCR analysis detected abnormal mRNA expression following treatments when compared with vehicle (n = 3, *P < 0.05, **P < 0.01, and ***P < 0.001 vs. vehicle). Notably, 5-aza reversed the DBcAMP-induced upregulation of Myh7, Gata4, Mef2c, Nfatc1-b, and Nfatc1-c back to baseline (A; n = 3, #P < 0.05, ##P < 0.01, and ###P < 0.001 vs. 1 mM DBcAMP group). The expression levels of Myh7b, Tnni3, and Bnp were reduced in the DBcAMP + 5-aza groups compared with DBcAMP treatment alone but remained elevated compared with control (B). Furthermore, 5-aza increased the expression of Nfatc1-a in DBcAMP-treated cells, even though it was not inducible by DBcAMP alone (B). The mRNA expression of Anp was increased by DBcAMP but was not affected by 5-aza in the presence of DBcAMP (B). Bars ± SE represent fold changes that are normalized to β-actin and relative to individual controls.
However, 5-aza treatment did not alter cell size and was unable to restore normal cell size following DBcAMP treatment (Fig. 9).
Fig. 9.
5-aza Does not inhibit cell hypertrophy induced by DBcAMP. HL-1 cells were treated with vehicle (DMSO), 0.5 μM 5-aza, 1 μM 5-aza, 1 mM DBcAMP, 1 mM DBcAMP + 0.5 μM 5-aza, or 1 mM cAMP + 1 μM 5-aza for 48 h and examined by a flow cytometer for relative change in size compared with vehicle. The average % change in size relative to vehicle are shown (n = 3, **P < 0.01 and ***P < 0.001 vs. vehicle).
DISCUSSION
It has been known that chronic activation of β-ARs or downstream cAMP targets results in cardiac hypertrophy and fibrosis (38, 41). However, the direct effects of sustained elevation of cAMP levels on HCM gene expression and DNA methylation have not been investigated in cardiomyocytes. In this study, intracellular cAMP levels in HL-1 cardiomyocytes were increased by adding DBcAMP, a cell-permeable and stable cAMP analog, or PDE inhibitors to the medium. Elevation of cAMP levels triggered substantial changes in cell size and HCM gene expression. In addition, DBcAMP altered the expression of genes involved in epigenetic mechanisms, including DNA methylation enzymes and miRNAs. These epigenetic effects may represent a previously unknown mechanism by which elevated levels of cAMP lead to cardiac muscle disease.
During development, cardiac-specific myosin genes Myh6 and Myh7 have distinctive expression patterns, with Myh7 as the predominant fetal isoform and Myh6 as the dominant adult isoform in mice (22, 34). Increased Myh7 expression is a common feature of cardiac hypertrophy in mice (6, 22). We found that DBcAMP increased the expression of Myh6, Myh7, and Myh7b. In addition, caffeine treatment at concentrations that inhibit PDEs led to increased intracellular cAMP level and altered expression of genes related to HCM, including upregulation of Myh6, Myh7, and Myh7b. Similarly, theophylline, which is also a nonspecific PDE inhibitor, increased the mRNA expression of these same cardiac myosin genes (Myh6, Myh7, and Myh7b) as well. These results indicate that the expression of these important cardiac myosin heavy chain genes is sensitive to cAMP levels. We cannot rule out completely that some of the effects of caffeine exposure are mediated by inhibition of adenosine receptors. However, we observe a doubling of cAMP levels with caffeine treatment, and we are observing the same effects on gene expression as elevated cAMP. In addition, we have reported that lower concentrations of caffeine (<2 mM) have no effect on many of these genes, indicating that the effects of caffeine are due to inhibition of PDE and not inhibition of adenosine receptors (18).
Expression of Myh6 and Myh7 is regulated by cardiac transcription factors, including Gata4, Mef2c, Mef2d, and Nfatc1 (11, 32, 35). Furthermore, miR208a stimulates Myh7 and Myh7b expression (40). Our results revealed that DBcAMP increased the expression of Gata4, Mef2c, Mef2d, Nfatc1, and miR208a. This could be a potential mechanism that leads to the upregulation of Myh6, Myh7, and Myh7b expression. The transcription factors Gata4, Mef2c, Mef2d, and Nfatc1 also stimulate transcription of Tnni3, Anp, and Bnp (14), and we observed upregulation of these genes following DBcAMP treatment as well. Increased expression of Tnni3 and miR208b indicates myocardial injury (10, 39), and upregulation of Anp and Bnp is a protective response to cardiac hypertrophy and heart failure that leads to dilation of blood vessels (15). Elevated levels of Anp and Bnp after DBcAMP exposure may therefore be a protective response to elevated cAMP levels.
The DBcAMP effects on cell size and mRNA expression of HCM genes can be blocked by Rp-cAMP, a competitive antagonist of cAMP-induced activation of PKA. This result suggests that the cAMP-induced cardiomyocyte hypertrophy is mediated by PKA activation. Activated PKA phosphorylates the transcription factor CREB, which binds to CREs in gene promoters and activates gene expression (46). However, there was no immediate response to DBcAMP treatment on gene expression after 2 or 4 h of exposure. Furthermore, DBcAMP is a weak cAMP analog to phosphorylate CREB (43). Thus, this may indicate that another mechanism is mediating the cAMP effects on HCM gene expression. Further studies are needed to determine the roles of CREB and CREs in regulating HCM gene expression.
Our results showed that, in addition to miRNA, another epigenetic mechanism, DNA methylation, is influenced by elevated cAMP levels. We found that DBcAMP increased the mRNA and protein expression of Dnmt3a, the DNA methylation enzyme involved in de novo methylation. Upregulation of Dnmt3a may be responsible for global cytosine hypermethylation of the HL-1 cardiomyocyte genome. The effects of DBcAMP on hypermethylation and expression changes in Dnmts can be blocked by Rp-cAMP, indicating that these effects are mediated by PKA activation. Although we observed an overall increase in DNA methylation in HL-1 cells exposed to elevated levels of cAMP, the gene-specific effects induced by the changes in expression of all these DNA methylation enzymes could be either hypermethylation or hypomethylation.
Although Tet1 and Tet2 were upregulated by DBcAMP, global cytosine hydroxymethylation was unchanged. It is possible that Tet1 and Tet2 first catalyze 5mc to 5hmc, and further transform 5hmC to 5-formylcytosine and 5-carboxycytosine (30), thus keeping the 5hmc levels unchanged.
Because DNA methylation is involved in regulation of gene transcription, altered DNA methylation patterns may play a role in the cAMP-induced changes in HCM gene expression that we observed. The effect that altered DNA methylation patterns have on gene expression is influenced by the location of the changes (promoter, gene body, or repetitive regions), and thus DNA hypermethylation may have different effects on gene expression depending on where in the genome it occurs (28, 53). It is known that promoter methylation represses Myh7 (8), but the relationship between DNA methylation and expression of other HCM genes remains unclear. To investigate this relationship, gene expression was examined after treating HL-1 cells with the nonspecific DNMT inhibitor 5-aza, which reduced the genomewide level of DNA methylation. The results showed that 5-aza increased the mRNA expression of Myh7, Myh7b, Bnp, Gata4, and Nfatc1-a, indicating that these genes are activated by DNA demethylation induced by 5-aza. In contrast, expression of Myh6 and Anp was reduced by 5-aza. It is possible that their gene body was hypomethylated after 5-aza treatment, which has been reported to be associated with reduced gene expression (53). Thus, our studies demonstrated that there are a set of HCM genes that are sensitive to altered DNA methylation patterns at the gene expression level. Further studies are needed to identify the specific CpG sites that regulate the transcription of these HCM genes.
To test if the changes in gene expression observed with elevated cAMP levels are mediated by alterations in the DNA methylation pattern, cardiomyocytes were treated with both 5-aza to inhibit DNMT activity and DBcAMP to increase intracellular cAMP levels. This analysis revealed that the HCM genes that we examined could be broken down into four groups. The first group of genes identified were upregulated by DBcAMP, but their mRNA expression levels returned to baseline when DNMT activity was inhibited, including Myh7, Gata4, Mef2c, Nfatc1-b, and Nfatc1-c. The second group of genes was reduced with 5-aza treatment when compared with DBcAMP treatment alone but stayed significantly higher than control in HL-1 cells when cotreated with DBcAMP and 5-aza, including Myh7b, Tnni3, and Bnp. The third group of genes was not influenced by 1 mM DBcAMP treatment alone, but their mRNA expression was increased in response to 5-aza treatment, including Nfatc1-a. The forth group of genes was not affected in mRNA expression by 5-aza in the presence of DBcAMP. Identification of the first and second sets of genes indicates that DNA methylation plays a role in mediating cAMP-induced alterations in HCM gene expression. It is known that mRNA expression is not always correlated to protein expression; however, because DNA methylation directly regulates mRNA expression, we mainly examined the mRNA transcripts of hypertrophic genes in this experiment. In addition, we demonstrated that both DBcAMP and 5-aza treatment influences protein expression as well.
Although cAMP induced hypertrophy of the HL-1 cardiomyocytes, inhibition of DNMT activity by 5-aza did not restore normal cell size following DBcAMP treatment. These results indicate that cAMP-induced cell size enlargement may not be dominantly mediated by changes in global DNA methylation levels. It is also possible that 5-aza is unable to modify the methylation of specific genes or gene regions that lead to cell hypertrophy, which is evidenced by the elevated mRNA expressions of Myh7b, Tnni3, Bnp, and Anp even after DBcAMP and 5-aza cotreatment. Modulation of methylation in specific genes instead of globally decreasing DNA methylation levels with a DNMT inhibitor may be more effective in protecting cardiomyocytes against DBcAMP-induced hypertrophy.
In conclusion, overall, these data demonstrate that many genes associated with HCM are sensitive to elevated cAMP levels and PKA activation at the gene and protein expression levels in HL-1 cardiomyocytes. In addition, the altered expression of some of these HCM genes in response to increased cAMP levels appears to be mediated by changes in DNA methylation. However, cAMP-induced cardiomyocyte hypertrophy is not reversed by the nonspecific DNMT inhibitor 5-aza-induced reduction in global DNA methylation levels. Further studies are needed to investigate the effects of cAMP on the methylation state of specific CpGs in individual HCM gene promoters to identify the genes and regulatory elements that mediate cardiomyocyte hypertrophy.
GRANTS
This work was supported by National Institute of Child Health and Human Development Grant R01-HD-58086 to S. A. Rivkees and C. C. Wendler.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: X.F., S.A.R., and C.C.W. conception and design of research; X.F., J.W.-H., and L.J. performed experiments; X.F. and J.W.-H. analyzed data; X.F. and C.C.W. interpreted results of experiments; X.F. prepared figures; X.F. drafted manuscript; X.F., J.R., D.A.F., S.A.R., and C.C.W. edited and revised manuscript; X.F., S.A.R., and C.C.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. William C. Claycomb for providing the HL-1 cardiomyocytes. We thank Ryan Poulsen and Olivia Shi for technical assistance.
REFERENCES
- 1.Babuin L, Jaffe AS. Troponin: the biomarker of choice for the detection of cardiac injury. Can Med Assoc J 173: 1191–1202, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev 58: 488–520, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Boswell-Smith V, Spina D, Page CP. Phosphodiesterase inhibitors. Br J Pharmacol 147, Suppl 1: S252–S257, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buscariollo DL, Fang X, Greenwood V, Xue H, Rivkees SA, Wendler CC. Embryonic caffeine exposure acts via A1 adenosine receptors to alter adult cardiac function and DNA methylation in mice. PloS one 9: e87547, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang CP, Bruneau BG. Epigenetics and cardiovascular development. Ann Rev Physiol 74: 41–68, 2012. [DOI] [PubMed] [Google Scholar]
- 6.Chien KR. Myocyte survival pathways and cardiomyopathy: implications for trastuzumab cardiotoxicity. Semin Oncol 27: 9–100, 2000. [PubMed] [Google Scholar]
- 7.Claycomb WC, Lanson NA Jr, Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, and Izzo NJ Jr. HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci USA 95: 2979–2984, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clifford CP, Nunez DJ. Human beta-myosin heavy chain mRNA prevalence is inversely related to the degree of methylation of regulatory elements. Cardiovasc Res 38: 736–743, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Corrales J, Fang X, Thornton C, Mei W, Barbazuk WB, Duke M, Scheffler BE, Willett KL. Effects on specific promoter DNA methylation in zebrafish embryos and larvae following benzo[a]pyrene exposure. Comp Biochem Physiol Toxicol Pharmacol 163: 37–46, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corsten MF, Dennert R, Jochems S, Kuznetsova T, Devaux Y, Hofstra L, Wagner DR, Staessen JA, Heymans S, Schroen B. Circulating MicroRNA-208b and MicroRNA-499 reflect myocardial damage in cardiovascular disease. Circ Cardiovasc Genetics 3: 499–506, 2010. [DOI] [PubMed] [Google Scholar]
- 11.Dai YS, Cserjesi P, Markham BE, Molkentin JD. The transcription factors GATA4 and dHAND physically interact to synergistically activate cardiac gene expression through a p300-dependent mechanism. J Biol Chem 277: 24390–24398, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Daniel PB, Walker WH, Habener JF. Cyclic AMP signaling and gene regulation. Ann Rev Nutr 18: 353–383, 1998. [DOI] [PubMed] [Google Scholar]
- 13.Denis H, Ndlovu MN, Fuks F. Regulation of mammalian DNA methyltransferases: a route to new mechanisms. EMBO Rep 12: 647–656, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dirkx E, da Costa Martins PA, De Windt LJ. Regulation of fetal gene expression in heart failure. Biochim Biophys Acta 1832: 2414–2424, 2013. [DOI] [PubMed] [Google Scholar]
- 15.Eindhoven JA, van den Bosch AE, Boersma E, Roos-Hesselink JW. The usefulness of brain natriuretic peptide in simple congenital heart disease - a systematic review. Cardiol Young 23: 315–324, 2013. [DOI] [PubMed] [Google Scholar]
- 16.Fang X, Corrales J, Thornton C, Scheffler BE, Willett KL. Global and gene specific DNA methylation changes during zebrafish development. Comp Biochem Physiol B Biochem Mol Biol 166: 99–108, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang X, Dong W, Thornton C, Scheffler B, Willett KL. Benzo(a)pyrene induced glycine N-methyltransferase messenger RNA expression in Fundulus heteroclitus embryos. Marine Environ Res Suppl 69: S74–S76, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang X, Mei W, Barbazuk WB, Rivkees SA, Wendler CC. Caffeine exposure alters cardiac gene expression in embryonic cardiomyocytes. Am J Physiol Regul Integr Comp Physiol 307: R1471–R1487, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang X, Thornton C, Scheffler BE, Willett KL. Benzo[a]pyrene decreases global and gene specific DNA methylation during zebrafish development. Environ Toxicol Pharmacol 36: 40–50, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faulk C, Dolinoy DC. Timing is everything: the when and how of environmentally induced changes in the epigenome of animals. Epigenetics 6: 791–797, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flores K, Wolschin F, Corneveaux JJ, Allen AN, Huentelman MJ, Amdam GV. Genome-wide association between DNA methylation and alternative splicing in an invertebrate (Abstract). BMC Genomics 13: 480, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frey N, Katus HA, Olson EN, Hill JA. Hypertrophy of the heart: a new therapeutic target? Circulation 109: 1580–1589, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Gidlof O, Smith JG, Miyazu K, Gilje P, Spencer A, Blomquist S, Erlinge D. Circulating cardio-enriched microRNAs are associated with long-term prognosis following myocardial infarction (Abstract). BMC Cardiovasc Dis 13: 12, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haas J, Frese KS, Park YJ, Keller A, Vogel B, Lindroth AM, Weichenhan D, Franke J, Fischer S, Bauer A, Marquart S, Sedaghat-Hamedani F, Kayvanpour E, Kohler D, Wolf NM, Hassel S, Nietsch R, Wieland T, Ehlermann P, Schultz JH, Dosch A, Mereles D, Hardt S, Backs J, Hoheisel JD, Plass C, Katus HA, Meder B. Alterations in cardiac DNA methylation in human dilated cardiomyopathy. EMBO Mol Med 5: 413–429, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He YW, Wang HS, Zeng J, Fang X, Chen HY, Du J, Yang XY. Sodium butyrate inhibits interferon-gamma induced indoleamine 2,3-dioxygenase expression via STAT1 in nasopharyngeal carcinoma cells. Life Sci 93: 509–515, 2013. [DOI] [PubMed] [Google Scholar]
- 26.Hershberger RE, Hedges DJ, Morales A. Dilated cardiomyopathy: the complexity of a diverse genetic architecture. Nat Rev Cardiol 10: 531–547, 2013. [DOI] [PubMed] [Google Scholar]
- 27.Inoue A, Zhang Y. Replication-dependent loss of 5-hydroxymethylcytosine in mouse preimplantation embryos (Abstract). Science 334: 194, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kass SU, Pruss D, Wolffe AP. How does DNA methylation repress transcription? Trends Genet 13: 444–449, 1997. [DOI] [PubMed] [Google Scholar]
- 29.Koczor CA, Lee EK, Torres RA, Boyd A, Vega JD, Uppal K, Yuan F, Fields EJ, Samarel AM, Lewis W. Detection of differentially methylated gene promoters in failing and nonfailing human left ventricle myocardium using computation analysis. Physiol Genomics 45: 597–605, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kohli RM, Zhang Y. TET enzymes, TDG and the dynamics of DNA demethylation. Nature 502: 472–479, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Landstrom AP, Kellen CA, Dixit SS, van Oort RJ, Garbino A, Weisleder N, Ma J, Wehrens XH, Ackerman MJ. Junctophilin-2 expression silencing causes cardiocyte hypertrophy and abnormal intracellular calcium-handling. Circ Heart Fail 4: 214–223, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee Y, Nadal-Ginard B, Mahdavi V, Izumo S. Myocyte-specific enhancer factor 2 and thyroid hormone receptor associate and synergistically activate the alpha-cardiac myosin heavy-chain gene. Mol Cell Biol 17: 2745–2755, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lohse MJ, Engelhardt S, Eschenhagen T. What is the role of beta-adrenergic signaling in heart failure? Circ Res 93: 896–906, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Lompre AM, Nadal-Ginard B, Mahdavi V. Expression of the cardiac ventricular alpha- and beta-myosin heavy chain genes is developmentally and hormonally regulated. J Biol Chem 259: 6437–6446, 1984. [PubMed] [Google Scholar]
- 35.Meissner JD, Umeda PK, Chang KC, Gros G, Scheibe RJ. Activation of the beta myosin heavy chain promoter by MEF-2D, MyoD, p300, and the calcineurin/NFATc1 pathway. J Cell Physiol 211: 138–148, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Movassagh M, Choy MK, Goddard M, Bennett MR, Down TA, Foo RS. Differential DNA methylation correlates with differential expression of angiogenic factors in human heart failure. PLoS One 5: e8564, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Movassagh M, Choy MK, Knowles DA, Cordeddu L, Haider S, Down T, Siggens L, Vujic A, Simeoni I, Penkett C, Goddard M, Lio P, Bennett MR, Foo RS. Distinct epigenomic features in end-stage failing human hearts. Circulation 124: 2411–2422, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Movsesian MA, Bristow MR. Alterations in cAMP-mediated signaling and their role in the pathophysiology of dilated cardiomyopathy. Curr Topics Dev Biol 68: 25–48, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Newby LK, Jesse RL, Babb JD, Christenson RH, De Fer TM, Diamond GA, Fesmire FM, Geraci SA, Gersh BJ, Larsen GC, Kaul S, McKay CR, Philippides GJ, Weintraub WS. ACCF 2012 expert consensus document on practical clinical considerations in the interpretation of troponin elevations: a report of the American College of Cardiology Foundation task force on Clinical Expert Consensus Documents. J Am Coll Cardiol 60: 2427–2463, 2012. [DOI] [PubMed] [Google Scholar]
- 40.Oliveira-Carvalho V, Carvalho VO, Bocchi EA. The emerging role of miR-208a in the heart. DNA Cell Biol 32: 8–12, 2013. [DOI] [PubMed] [Google Scholar]
- 41.Osadchii OE. Cardiac hypertrophy induced by sustained beta-adrenoreceptor activation: pathophysiological aspects. Heart Fail Rev 12: 66–86, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Petersen CA, Krumholz KA, Burleigh BA. Toll-like receptor 2 regulates interleukin-1beta-dependent cardiomyocyte hypertrophy triggered by Trypanosoma cruzi. Infect Immun 73: 6974–6980, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seternes OM, Sorensen R, Johansen B, Moens U. Activation of protein kinase A by dibutyryl cAMP treatment of NIH 3T3 cells inhibits proliferation but fails to induce Ser-133 phosphorylation and transcriptional activation of CREB. Cell Signal 11: 211–219, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Sharma P, Garg G, Kumar A, Mohammad F, Kumar SR, Tanwar VS, Sati S, Sharma A, Karthikeyan G, Brahmachari V, Sengupta S. Genome wide DNA methylation profiling for epigenetic alteration in coronary artery disease patients. Gene 541: 31–40, 2014. [DOI] [PubMed] [Google Scholar]
- 45.Sharma P, Kumar J, Garg G, Kumar A, Patowary A, Karthikeyan G, Ramakrishnan L, Brahmachari V, Sengupta S. Detection of altered global DNA methylation in coronary artery disease patients. DNA Cell Biol 27: 357–365, 2008. [DOI] [PubMed] [Google Scholar]
- 46.Shaywitz AJ, Greenberg ME. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Ann Rev Biochem 68: 821–861, 1999. [DOI] [PubMed] [Google Scholar]
- 47.Shirodkar AV, Marsden PA. Epigenetics in cardiovascular disease. Curr Opin Cardiol 26: 209–215, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shukla S, Kavak E, Gregory M, Imashimizu M, Shutinoski B, Kashlev M, Oberdoerffer P, Sandberg R, Oberdoerffer S. CTCF-promoted RNA polymerase II pausing links DNA methylation to splicing. Nature 479: 74–79, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sim CB, Ziemann M, Kaspi A, Harikrishnan KN, Ooi J, Khurana I, Chang L, Hudson JE, El-Osta A, Porrello ER. Dynamic changes in the cardiac methylome during postnatal development. FASEB J 29: 1329–1343, 2015. [DOI] [PubMed] [Google Scholar]
- 50.Szyf M. Epigenetics, DNA methylation, and chromatin modifying drugs. Ann Rev Pharmacol Toxicol 49: 243–263, 2009. [DOI] [PubMed] [Google Scholar]
- 51.van Rooij E, Quiat D, Johnson BA, Sutherland LB, Qi X, Richardson JA, Kelm RJ Jr, Olson EN. A family of microRNAs encoded by myosin genes governs myosin expression and muscle performance. Dev Cell 17: 662–673, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu H, Baldini A. Genetic pathways to mammalian heart development: recent progress from manipulation of the mouse genome. Semin Cell Dev Biol 18: 77–83, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang X, Han H, De Carvalho DD, Lay FD, Jones PA, Liang G. Gene body methylation can alter gene expression and is a therapeutic target in cancer. Cancer Cell 26: 577–590, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ying AK, Hassanain HH, Roos CM, Smiraglia DJ, Issa JJ, Michler RE, Caligiuri M, Plass C, Goldschmidt-Clermont PJ. Methylation of the estrogen receptor-alpha gene promoter is selectively increased in proliferating human aortic smooth muscle cells. Cardiovasc Res 46: 172–179, 2000. [DOI] [PubMed] [Google Scholar]
- 55.Zhang X, Odom DT, Koo SH, Conkright MD, Canettieri G, Best J, Chen H, Jenner R, Herbolsheimer E, Jacobsen E, Kadam S, Ecker JR, Emerson B, Hogenesch JB, Unterman T, Young RA, Montminy M. Genome-wide analysis of cAMP-response element binding protein occupancy, phosphorylation, and target gene activation in human tissues. Proc Natl Acad Sci USA 102: 4459–4464, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao L, Kang I, Fang X, Wang W, Lee MA, Hollins RR, Marshall MR, Chung S. Gamma-tocotrienol attenuates high-fat diet-induced obesity and insulin resistance by inhibiting adipose inflammation and M1 macrophage recruitment. Int J Obes (Lond) 39: 438–446, 2015. [DOI] [PubMed] [Google Scholar]
- 57.Zhou Y, Lu Y, Tian W. Epigenetic features are significantly associated with alternative splicing (Abstract). BMC Genomics 13: 123, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]