Abstract
Ictal bradyarrhythmias are rare episodes occurring in patients with or without a past cardiac history. These episodes go unnoticed unless the patient is monitored on simultaneous video-electroencephalogram and 1-lead electrocardiogram. Recognizing ictal bradyarrhythmias is important, since episodes may predispose patients to sudden, unexplained death in epilepsy. We present 2 cases of ictal asystole in patients with right temporal lobe epilepsy. The first patient had seizures refractory to medical therapy and received a pacemaker. The seizures in the second patient responded well to antiepileptic medication, and a pacemaker was deferred. These cases highlight the differing cardiovascular treatment options for ictal asystole.
Keywords: ictal asystole, pacemaker
Introduction
Ictal asystole defined as the absence of ventricular complexes for >3 seconds following an electrographic seizure onset is a rare and potentially life-threatening event that may occur in patients with epilepsy. It has been considered a predisposing factor for sudden, unexplained death in epilepsy (SUDEP).1,2 It may go unrecognized until patients are evaluated with video-electroencephalogram (vEEG) and 1-lead electrocardiogram (ECG). Indeed, a recent study has shown that ictal asystole occurred in approximately 1% of patients monitored with vEEG and ECG who did not have cardiac risk factors or underlying arrhythmia.3-5
Cardiac pacemaker implantation is a definitive treatment of ictal asystole. When to implant a cardiac pacemaker for ictal asystole is unclear. The National Institute for Health and Clinical Excellence has stated that a pacemaker is recommended for secondary prevention of out-of-hospital cardiac arrest.6 However, not everyone with ictal asystole may be a candidate. Additionally, pacemaker implantation does carry risks of infection, hematoma, pneumothorax, local pain, cardiac perforation, inadvertent arterial puncture, and lead failure or damage.6 It is our purpose to present 2 cases of patients with right temporal lobe epilepsy who had syncope-like episodes. Both were found to have ictal asystole when monitored with concomitant vEEG and ECG. Their cases provide the challenges behind reasoning for when to pace and when not to pace. The Institutional Review Board of the Cleveland Clinic deems their approval not required for this single case report.
Case 1
A 40-year-old, right-handed male with a 60-year history of multiple auras described as warm cephalic flushing followed by staring spells and occasional generalized tonic–clonic seizures was concomitantly monitored on vEEG and 1-lead ECG. His past medical history was significant for 2 simple febrile seizures, each lasting <1 minute, at the age of 8 and 10 months. He had no other epilepsy risk factors. He did not achieve adequate seizure control on levetiracetam, lamotrigine, oxcarbazepine, and valproic acid and is currently on topiramate. He was neurologically intact on examination. His brain magnetic resonance imaging (MRI) was normal.
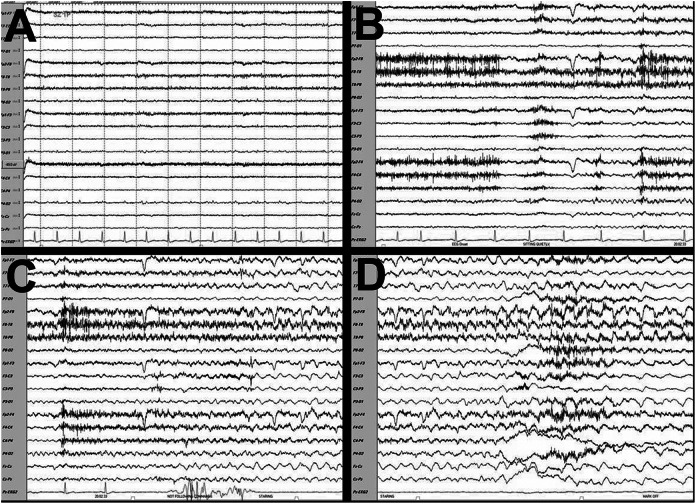
While being monitored on vEEG and ECG, his typical seizure was recorded (Figure 1A-D). His ictal EEG showed low amplitude rhythmic sharply contoured 6 to 7 Hz theta waves in the right temporal area (T8 max), evolving into moderate amplitude sharply contoured theta/delta waves in the right hemisphere with bilateral spread. Fourteen seconds after onset of electrographic seizure, his ECG lead developed asystole lasting 22 seconds (Figure 1C and D). There was no preceding bradycardia. On video monitoring, the ictal semiology consisted of facial flush and staring followed by loss of consciousness. During asystole, his head fell backward with deviation toward the left. The seizure duration was 72 seconds. His interictal ECG lead (Figure 1A) was normal sinus rhythm at 84 beats per minute (bpm). A 12-lead ECG obtained immediately postictally showed normal sinus rhythm at 83 bpm with normal QTc, PR interval, and QRS duration. He was discharged on topiramate. Given his multiple failed medications with recurring seizures, he underwent pacemaker implantation.
Figure 1.
Electroencephalogram (EEG). A, Interictal EEG showing a heart rate of 84 beats per minute. B, Ictal onset in right temporal lobe (T8 max) with a 6 to 7 Hz rhythm evolving into sharply contoured theta/delta rhythm. C and D, Asystole was noted 14 seconds after ictal onset. Total seizure duration was 72 seconds. The asystole lasted 22 seconds. Sensitivity 12 microvolts, low frequency at 1.6 Hz, and high frequency 70 Hz. Black bar represents 1 second.
Case 2
A 57-year-old, right-handed male with a history of seizures was monitored on vEEG and ECG. His seizures were described as an aura of lightheadedness followed by nausea, diaphoresis, and déjà-vu while staring blankly with lips quivering, typically lasting 1 minute and occurring once per month. All seizures have been similar in semiology and without tonic–clonic activity. He was started on levetiracetam 1500 mg twice daily.
His brain MRI showed a nonenhancing right temporal lobe lesion concerning for a low-grade astrocytoma. Brain biopsy of the lesion was consistent with World health Organization grade II astrocytoma. His only other possible significant epilepsy risk factor was head injury as a child without loss of consciousness. Physical examination revealed no neurological deficits.
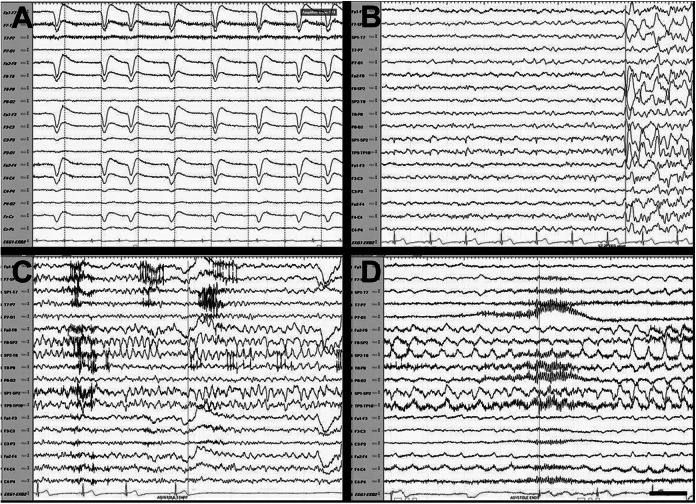
On vEEG monitoring, he had sharply contoured delta in the right anterior temporal region (SP2/FT10) evolving into sharply contoured theta and then delta waves (Figure 2A-D). Electrocardiogram lead showed bradycardia (54 bpm) followed by asystole 15 seconds after EEG ictal onset. Electroencephalogram then showed right temporal slowing to delta and spreading to bilateral temporal regions. The asystole lasted 29 seconds (Figure 2C and D). The seizure ended at 63 seconds. His second seizure was similar with asystole lasting 24 seconds. His interictal ECG was unremarkable with a heart rate of 72 bpm. His immediate postictal ECG showed a heart rate of 56 bpm with normal PR and QT intervals and normal QRS complex.
Figure 2.
Electroencephalogram (EEG). A, Interictal EEG showing a heart rate of 84 beats per minute. B, EEG immediately prior to seizure onset. C and D, Ictal onset in the right temporal lobe (SP2/FT10) showing sharply contoured, rhythmic delta then theta then delta waves. Asystole was seen 15 seconds after ictal onset with total duration of seizure lasting 63 seconds. The asystole lasted 29 seconds. Sensitivity 12 microvolts, low frequency at 1.6 Hz, and high frequency 70 Hz. Black bar represents 1 second.
A cardiac workup was unremarkable. He was restarted on his levetiracetam at an increased dose of 2000 mg twice daily, and oxcarbazepine 300 mg twice daily was added. He has had no further seizure activity or asystolic events. Since his seizures were medically controlled, the final recommendation from the cardiology consult was to continue treating the seizures medically and defer pacemaker implantation at this time.
Discussion
Bradycardia and asystole are rare cardiovascular phenomena with potential life-threatening consequences that may occur as a result of seizures.3-5 There are currently no guidelines for the care of patients with ictal arrhythmias from the epilepsy societies. These 2 cases describe ictal asystole occurring in patients with right temporal lobe epilepsy, resulting in different cardiac treatment plans. Interictally, both our patients demonstrated a baseline heart rate of 70 to 90 bpm without any underlying cardiac structural abnormalities or arrhythmias. Nevertheless, asystole developed 14 to 15 seconds after ictal onset. There were no ictal or postictal tachyarrhythmias. One patient received a pacemaker while the other did not. The patient who received the pacemaker was felt to have medically refractory epilepsy. The second patient was felt to have medically responsive epilepsy.
The indication for pacemaker implantation in patients with ictal arrhythmias has been a matter of debate. The risks must be weighed against its possible benefits. Case reports have been published advocating pacemaker implantation as a possible preventative measure for SUDEP. Rugg-Gunn et al evaluated 20 patients with refractory partial seizures with implanted loop recorders.4 In this study, 3377 seizures were recorded with ictal bradycardia (defined as heart rate <40 bpm) occurring 8 times. Of these patients, 3 had ictal asystole lasting 4 to 14 seconds. All 3 received a pacemaker. Their seizures arose from either the left (n = 2) or the right temporal lobes (n = 1). These events occurred 60 to 401 days after start of cardiac recording. Another patient had symptomatic bradycardia (30 bpm) and opted for pacemaker implantation. The group’s conclusion was that ictal bradyarrhythmias are underreported and that pacemaker implantation in those patients with greatest risk (ie, those with asystole) is indicated for possible prevention of SUDEP. However, pacemaker implantation is not without risks. Complications include infection, hematoma, pneumothorax, local pain, cardiac perforation, inadvertent arterial puncture, and lead failure or damage.6
Ictal bradyarrhythmias have been postulated to be secondary to vagal cardiac activation.7 However, it is not clear whether bradyarrhythmias are from vagal-mediated activation of cardiac function or uncoordinated autonomic effect.7,8 Prior case reports have demonstrated that ictal bradyarrhythmias begin between 5 and 20 seconds after ictal discharge in partial seizures and 60 and 100 seconds in generalized seizures.7
Left hemispheric seizures have been reported more likely to induce asystole or bradycardia.7,9,10 Localization for a cardiac depressor area has been proposed to be the anterior cingulate cortex of the frontal lobe, the insular region, or the nucleus ambiguus and may require bilateral mesial temporal region activation.7,11-13 Indeed, left insular stimulation has been shown to produce cardiac depressor effects.10 This finding led to the suggestion that cardiac parasympathetic function lateralizes to the left insular region, and cardiac sympathetic function lateralizes to the right insular region.10 However, localization and lateralization of ictal bradycardia have been a matter of controversy.9,11,12 Our cases further highlight this controversy with the seizures described as arising from the right temporal region with resulting ictal asystole.
When should one consider the possibility of ictal asystole in patients with known epilepsy? A case report from Zubair et al described a 31-year-old male who had a change in his baseline seizures where he would lose muscle tone.14 He was found to have ictal asystole lasting 8 to10 seconds. A dual-chamber pacemaker was implanted. A case report from Strzelczyk et al describes a 41-year-old male with a history of right temporal lobe epilepsy who had a basal skull fracture with subdural hematoma following a fall.12 During extended EEG monitoring, he was found to have ictal asystole (10 seconds). A pacemaker was implanted, and he has not fallen since. These cases of focal epilepsy illustrate that a change in ictal semiology (eg, unexplained falls) should alert providers to the possibility of ictal asystole. Once attributed to epileptic phenomenon itself, is responsiveness of seizures to medical therapy alone enough to warrant conservative treatment while medial refractory seizures get pacemakers? Does origin, duration, semiology, or seizures dictate initiation of pacemakers?
In summary, the diagnosis of ictal bradyarrhythmias begins with a high index of suspicion. Once identified (with vEEG, ECG, and/or loop recorder), a careful review of medications for proarrhythmic properties is recommended. A cardiac workup as deemed necessary by the consulting cardiology team for unrelated causes of arrhythmias may include a 12-lead ECG, 24-hour cardiac Holter monitor, 21-day cardiac event monitor, cardiac echocardiogram, and a stress test. If ictal origin to asystole is ascertained, a careful discussion of the risks and benefits of pacemaker implantation must occur with the patient and the cardiology team. In patients with medically refractory epilepsy who have ictal asystole, pacemaker implantation may be considered if the risk–benefit ratio favors implantation. If the patient is medically responsive to antiepileptics, and pacemaker implantation is deferred, a close follow-up is essential.
Acknowledgments
We thank Drs Andreas Alexopolous and Deborah Tepper for critical appraisal.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Baumgartner C, Lurger S, Leutmezer F. Autonomic symptoms during epileptic seizures. Epileptic Disord. 2001;3(3):103–116. [PubMed] [Google Scholar]
- 2. Nashef L. Sudden unexpected death in epilepsy: terminology and definitions. Epilepsia 1997;38(11 suppl):S6–S8. [DOI] [PubMed] [Google Scholar]
- 3. Schuele SU, Bermeo AC, Alexopoulos AV, et al. Video-electrographic and clinical features in patients with ictal asystole. Neurology. 2007;69(5):434–441. [DOI] [PubMed] [Google Scholar]
- 4. Rugg-Gunn FJ, Simister RJ, Squirrell M, Holdright DR, Duncan JS. Cardiac arrhythmias in focal epilepsy: a prospective long-term study. Lancet. 2004;364(9452):2212–2219. [DOI] [PubMed] [Google Scholar]
- 5. Leutmezer F, Schernthaner C, Lurger S, Pötzelberger K, Baumgartner C. Electrocardiographic changes at the onset of epileptic seizures. Epilepsia. 2003;44(3):348–354. [DOI] [PubMed] [Google Scholar]
- 6. Modi S, Rao A, Hughes S, Ramsdale D. Cardiac device therapy 1: theory, technology, and terminology. Br J Hosp Med (Lond). 2008;69(11):620–624. [DOI] [PubMed] [Google Scholar]
- 7. Rocamora R, Kurthen M, Lickfett L, Von Oertzen J, Elger CE. Cardiac asystole in epilepsy: clinical and neurophysiologic features. Epilepsia. 2003;44(2):179–185. [DOI] [PubMed] [Google Scholar]
- 8. Lathers CM, Schraeder PL. Autonomic dysfunction in epilepsy: characterization of autonomic cardiac neural discharge associated with pentylenetetrazol-induced epileptogenic activity. Epilepsia. 1982;23(6):633–647. [DOI] [PubMed] [Google Scholar]
- 9. Britton JW, Ghearing GR, Benarroch EE, Cascino GD. The ictal bradycardia syndrome: localization and lateralization. Epilepsia. 2006;47(4):737–744. [DOI] [PubMed] [Google Scholar]
- 10. Oppenheimer SM, Gelb A, Girvin JP, Hachinski VC. Cardiovascular effects of human insular cortex stimulation. Neurology. 1992;42(9):1727–1732. [DOI] [PubMed] [Google Scholar]
- 11. Britton JW, Benarroch E. Seizures and syncope: anatomic basis and diagnostic considerations. Clin Auton Res. 2006;16(1):18–28. [DOI] [PubMed] [Google Scholar]
- 12. Strzelczyk A, Bauer S, Knake S, Oertel WH, Hamer HM, Rosenow F. Ictal asystole in temporal lobe epilepsy before and after pacemaker implantation. Epileptic Disord. 2008;10(1):39–44. doi: 10.1684/epd.2008.0166. [DOI] [PubMed] [Google Scholar]
- 13. Rossetti AO, Dworetzky BA, Madsen JR, Golub O, Beckman JA, Bromfield EB. Ictal asystole with convulsive syncope mimicking secondary generalisation: a depth electrode study. J Neurol Neurosurg Psychiatry. 2005;76(6):885–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zubair S, Arshad AB, Saeed B, Luqman S, Oommen KJ. Ictal asystole--late manifestation of partial epilepsy and importance of cardiac pacemaker. Seizure. 2009;18(6):457–461. doi:10.1016/j.seizure.2009.03.004. [DOI] [PubMed] [Google Scholar]