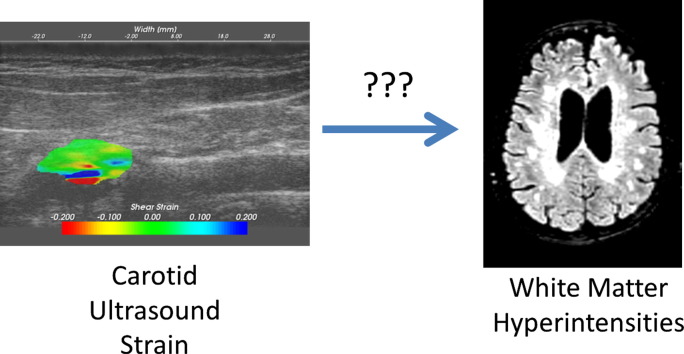
Abstract
Higher local carotid artery strain has previously been shown to be a characteristic of unstable carotid plaques. These plaques may be characterized by microvascular changes that predispose to intraplaque hemorrhage, increasing the likelihood of embolization. Little is known however, about how these strain indices correspond with imaging markers of brain health and metrics of brain structure. White matter hyperintensities (WMHs), which are bright regions seen on T2-weighted brain MRI imaging, are postulated to result from cumulative ischemic vascular injury. Consequently, we hypothesized that plaques that are more prone to microvascular changes and embolization, represented by higher strain indices on ultrasound, would be associated with an increased amount of WMH lesion volume. This relationship would suggest not only emboli as a cause for the brain degenerative changes, but more importantly, a common microvascular etiology for large and small vessel contributions to this process. Subjects scheduled to undergo a carotid endarterectomy were recruited from a neurosurgery clinic. Prior to surgery, participating subjects underwent both ultrasound strain imaging and brain MRI scans as part of a larger clinical study on vascular health and cognition. A linear regression found that maximum absolute strain and peak to peak strain in the surgical side carotid artery were predictive of WMH burden. Furthermore, the occurrence of microembolic signals monitored using transcranial Doppler (TCD) ultrasound examinations also correlated with increasing lesion burden. It is becoming increasingly recognized that cognitive decline is often multifactorial in nature. One contributing extra-brain factor may be changes in the microvasculature that produce unstable carotid artery plaques. In this study, we have shown that higher strain indices in carotid artery plaques are significantly associated with an increased WMH burden, a marker of vascular mediated brain damage.
Keywords: White matter hyperintensities, Carotid plaque, Ultrasound strain, MRI
Abbreviations: WMH, white matter hyperintensity; HITS, high intensity transient signals
Graphical abstract
Highlights
-
•
We examine how carotid artery plaque strain indices correspond with MRI metrics.
-
•
Strain in the ICA predicts increased white matter hyperintensity lesion burden.
-
•
Subjects with embolizing plaques have greater white matter lesion burden.
1. Introduction
Estimates of carotid stenosis (progressive occlusion of the carotid artery with atherosclerotic plaque) in the general population range from 0–7.5%, with the prevalence increasing drastically with age (de Weerd, 2010). Carotid atherosclerosis is a major precipitant of both symptomatic and asymptomatic (silent) strokes, with an estimated 7–18% attributable to this particular pathology (Petty, 1999; Rubin, 2014; White, 2005). The mechanism is commonly the release of emboli from plaque developing in the walls of the carotid artery (Rothwell, 1996). Some plaques have greater inherent instability, and are thus more likely to release emboli that enter into the brain vasculature (Kwee, 2008). One method to further characterize the stability of plaques is ultrasound elastography (Ophir, 1991; Varghese, 2009). This modality, although developed over 20 years ago, has only recently come to be utilized in clinical practice, specifically in vascular imaging (Varghese, 2009). Newly developed methods in our laboratory, using a hierarchical block motion tracking algorithm, have increased the efficiency and accuracy of strain analyses (McCormick, 2012). Normal and abnormal tissues display markedly different properties on ultrasound elastography, notably disparate strain parameters (Ophir, 1991; Varghese, 2009).
Higher carotid artery strain has been shown to be a characteristic of unstable carotid plaques that are more likely to embolize (Wang, 2014a). Atherosclerotic vessels with lipidic plaque tend to have higher strain than vessels with calcified plaque, with the degree of strain within individual parts of the plaque varying based on composition, which can be determined using ultrasonic methods (Ribbers, 2007; Shi, et al. 2007, Shi, et al. 2008a, Shi, et al. 2009). It has been suggested that plaques that are physically unstable during pulsation are more prone to releasing emboli during the cardiac cycle, and thus may be an important contributor to silent strokes (Dempsey, 2010). These unstable, lipidic plaques, as opposed to calcified plaques, undergo greater deformations during the cardiac cycle, which are manifested as higher strain measurements during ultrasound strain imaging (Dempsey, 2010). Non-calcified, hypoechoic plaques have been shown to present with more than 20% accumulated axial strain during a cardiac cycle, whereas more calcified, firmer plaques present with approximately 5% of accumulated axial strain (Shi, 2008b). In fact, this research has been extended by our laboratory to show that higher strain values, both absolute maximum strain and peak-to-peak strain, are associated with indices of decline in global cognitive performance (Wang, et al. 2014a, Wang, et al. 2014b; Rocque, 2012). Little is known, however, about how these strain measurements correspond with imaging markers of brain health.
White matter hyperintensities (WMHs), which are bright regions seen on T2-weighted brain MRI imaging, are postulated to result from cumulative ischemic vascular injury. Approximately 50% of individuals will have some degree of WMH by age 40, and by the mid-60s, the majority of adults have WMH observable on imaging (Wen, 2009; Wen and Sachdev, 2004). Vascular risk factors, such as hypertension, atrial fibrillation, and increased intima–media arterial thickness, positively correlate with increased WMH lesion burden (Casado Naranjo, 2015; Knopman, 2011; Alosco, 2013). WMHs have been shown to be positively associated with the degree of cognitive decline, specifically reduced cognitive speed and flexibility (Birdsill, 2014). A community based sample of over 700 healthy adults found that increased WMH lesion burden was associated with poorer performance on executive function tasks (Brickman, 2011). Thus, it is important to investigate the relationship between carotid artery disease, a prevalent public health issue, with that of imaging markers of vascular mediated brain damage.
The degree of carotid stenosis in a population with high grade occlusion (70–99%), with an average age of 69.1 years, appeared to be positively correlated with the total WMH lesion burden (Enzinger, 2010). Furthermore, a study of patients with recent acute lacunar infarcts (average age 56.2 years), found that the extent of carotid disease was associated with a particular increase in periventricular WMH (Kandiah, 2014). In fact, Kandiah and colleagues suggest that the presence of periventricular WMH on imaging should prompt clinicians to examine for the presence of significant carotid vascular disease, and not just attribute the increase in WMH to small vessel intracranial pathology. Other researchers have not found that stenosis correlates with the volume of WMH, but rather that other metrics have significant associations with lesion burden. It may be that it is not the stenosis per se but rather the instability of the plaque that predicts lesion burden. In a population with an average age of 70.2, who have a history of transient ischemic attack or amaurosis fugax (painless, monocular vision loss), and a stenosis of at least 30%, intraplaque hemorrhage, another metric of plaque instability, is significantly associated with WMH lesion burden (Altaf, 2008).
Arterial stiffness measurements have recently been shown to correlate with increased white matter changes in the brain. In a comparative study of patients (average age 53.2) with and without ischemic leukoaraiosis, patients with increased white matter disease had higher strain and pulse wave velocity measurements in the carotid artery (Turk, 2015). In that study, however, patients with unstable carotid plaques or greater than 50% stenosis were excluded (Turk, 2015). Since unstable plaques are believed to embolize at a higher rate than more stable plaques, we sought to determine how plaque stability is associated with WMH lesion volume in a sample of patients scheduled to undergo carotid endarterectomy for treatment of their carotid vascular disease. We hypothesized that plaques that are more unstable, represented by higher strain measurements on ultrasound and more prone to embolization, would be associated with an increased amount of white matter injury, represented by WMH lesion volume, on MRI.
2. Methods
2.1. Participants
Subjects scheduled to undergo clinically indicated carotid endarterectomy for carotid disease were recruited into an investigational multi-modal research protocol from neurosurgery clinics and inpatient units at a tertiary care medical center in Madison, WI. Clinical indications were based on those proposed by the ACAS or NASCET trial criteria (Biller, 1998). Patients provided informed consent using a protocol that received prior approval by the University of Wisconsin — Madison institutional review board. Before surgery, subjects (N = 26, 15 males, 11 females, average age 70.27 years) underwent brain MRI imaging, ultrasound strain imaging and medical examinations.
2.2. Ultrasound strain imaging
Ultrasound Radiofrequency (RF) data were acquired using a Siemens S2000 system using an 18L6 linear array transducer (Siemens Ultrasound, Mountain View, CA, USA). The transmit center frequency of the transducer was 11.4 MHz with a single transmit focus set at the depth of the plaque. The ultrasound transducer was positioned to scan the internal carotid artery, however, plaque might extend to the bifurcation in the ultrasound images. RF data over two cardiac cycles were obtained, and plaque regions were segmented at end-diastole using the Medical Imaging Interaction Toolkit (MITK). A hierarchical block-matching motion tracking algorithm was utilized to estimate the accumulated axial, lateral and shear strain distribution in plaques over two cardiac cycles. Maximum and peak-to-peak strain indices were calculated from the strain images.
Successful utilization and reproducibility of ultrasound based strain imaging depend on the estimation of deformation and thereby local strains within the carotid artery wall with high spatial resolution, signal-to-noise ratio (SNR) and over a large dynamic range, while minimizing signal decorrelation and noise artifacts (Varghese and Ophir, 1997; Varghese, 2001). Briefly, this study uses a robust and multi-level hierarchical block matching method for improved strain estimation that incorporates several advances in deformation tracking and displacement estimation (McCormick, 2012). For example, in multi-level methods, local displacements are estimated from a coarse to fine scale, by re-estimating and refining local displacements based on prior information obtained at coarser scales to improve spatial resolution (Shi and Varghese, 2007). A three-level image pyramid was utilized with the hierarchical algorithm to improve computational efficiency (McCormick, 2012; Shi and Varghese, 2007). Discontinuous motion of the artery wall was also addressed in these algorithms (Shi and Varghese, 2007). A dynamic frame skip concept was utilized to ensure that sufficient deformation was always present between the pre- and post-deformation frames processed over a cardiac cycle enabling a smaller frame skip during systole, and a larger frame-skip during end diastole (Daniels and Varghese, 2010). This step improved efficiency of the strain estimation and accumulation algorithm, while reducing registration and accumulation errors incurred if all frame pairs with smaller deformations were processed. A three-stage recursive Bayesian regularization that improves the strain SNR by approximately 11 dB for a 7% applied deformation (when compared to median filtering) was incorporated improving accuracy and precision of displacement estimation (McCormick et al., 2011).
2.3. Transcranial Doppler imaging
The transcranial Doppler (TCD) examination was performed with the SONARA Digital Bilateral Systems transcranial Doppler system (Natus, formerly CareFusion, Middleton, WI). A 2.0 MHz transducer was used to image the right and left middle cerebral arteries through the transtemporal window with pulse wave Doppler. Monitoring was done for 1 h. The total number of High Intensity Transient Signals (HITS) was recorded and reviewed after each monitoring session by two observers and a physician.
The Sonara Digital Bilateral Systems transcranial Doppler ultrasound machine utilizes an emboli detection algorithm to identify embolic signals. The following criteria were utilized to differentiate HITS from artifacts; 1) high intensity signal compared to the background blood flow signal detected by the system, 2) transient signal less than 300 ms in duration, 3) unidirectional signal within the Doppler velocity spectrum and 4) the presence of an audible chirp, click, thud or moan noise (Consensus Committee of the Ninth International Cerebral Hemodynamic Symposium, 1995) and 5) a change in complex mode. Two observers and the physician reviewed all HITS based on the criteria listed above to differentiate real HITS (suggesting microemboli) from artifacts. The examination was considered positive for the presence of microemboli if one or more HITS were detected at the time of the TCD examination.
2.4. MRI
The 3 Tesla MRI was conducted on a GE x750 scanner. A T1-weighted volume was acquired in the axial plane with a 3D fast spoiled gradient echo-sequence using the following parameters: inversion time (TI) = 450 ms; repetition time (TR) = 8.1 ms; echo time (TE) = 3.2 ms; flip angle = 12°; acquisition matrix = 256 × 256 mm; field of view (FOV) = 256 mm; and slice thickness = 1.0 mm. A 3D T2-weighted fluid attenuated inversion recovery (FLAIR) sequence was acquired in the sagittal plane using the following parameters: TI = 1868 ms, TR = 6000 ms; TE = 123 ms; flip angle = 90°; acquisition matrix = 256 × 256 mm; FOV = 256 mm; slice thickness = 2.0 mm, and no gap yielding a voxel resolution of 1 mm × 1 mm × 2 mm.
2.5. WMH segmentation
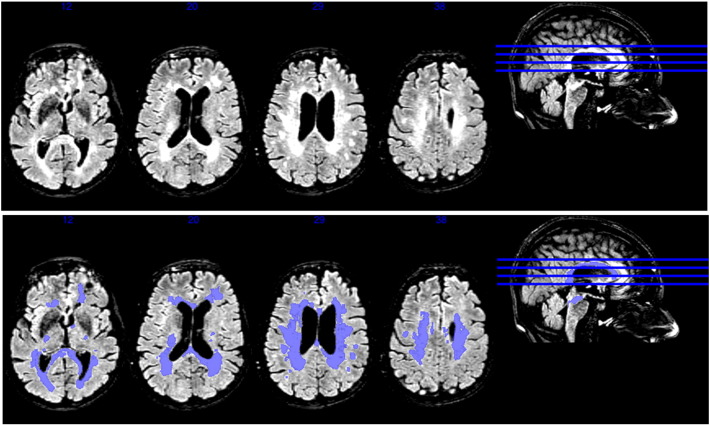
WMH total lesion volume was calculated using the Lesion Segmentation Toolbox (LST) version 1.2.2 add-on in the Statistical Parametric Mapping (SPM8) imaging software (Schmidt, 2012). Briefly, the LST uses T1-weighted and T2-FLAIR images for lesion segmentation. Gray matter, white matter, and cerebrospinal fluid tissue classes are determined using the information from the T1 scan. The T2-FLAIR scan is used to detect hyperintense voxels, which are designated as lesions (see example in Fig. 1). An analysis looking at the accuracy of this WMH segmentation tool box found high correspondence (R2 = 0.94) with manual tracing of WMH in multiple sclerosis patients (Schmidt, 2012). An advantage of this automated method is that it reduces the subjectivity and human error inherent in manual tracing methods. The automated method does not separate periventricular white matter hyperintensities from deep white matter hyperintensities, however, which have been shown to be associated with differing pathologies and cognitive outcomes (Shim, 2015).
Fig. 1.
MRI images of a subject with significant WMH lesion burden. The top image is the T2 FLAIR scan, while the bottom panel is the lesion segmentation calculated by the LST toolbox add-on to SPM8 as an overlay.
In order to determine the extent of WMH burden in our population relative to a control population, we calculated Z-scores based on the WMH lesion volume in N = 64 healthy subjects (37 women; 27 men; age 69.06 SD: 4.52) from a previously acquired cognitively normal cohort from the University of Wisconsin Alzheimer's Disease Research Center. This cohort was acquired on the same scanner using the same sequences as the study population and processed in an identical manner. An independent two-tailed t-test of age across the control and study populations revealed no significant difference (p = 0.562).
2.6. Statistical analysis
IBM SPSS software version 22 was used for statistical analysis. Linear regressions with carotid artery plaque strain as the independent variable and WMH total lesion volume as the dependent variable were performed. Age, gender, BMI, stenosis percentage, hypertension, and hyperlipidemia treatment covariates were controlled for because they relate to vascular health. Strain values were log transformed to obtain normal distributions for analyses. When examining the relationship between HITS (independent variable) and WMH (dependent variable), a WMH Z-score obtained using a comparably aged population was used, and consequently, age was not included as a covariate in this particular analysis. α for significance was set at p = 0.05.
3. Results
3.1. Ultrasound strain and WMH
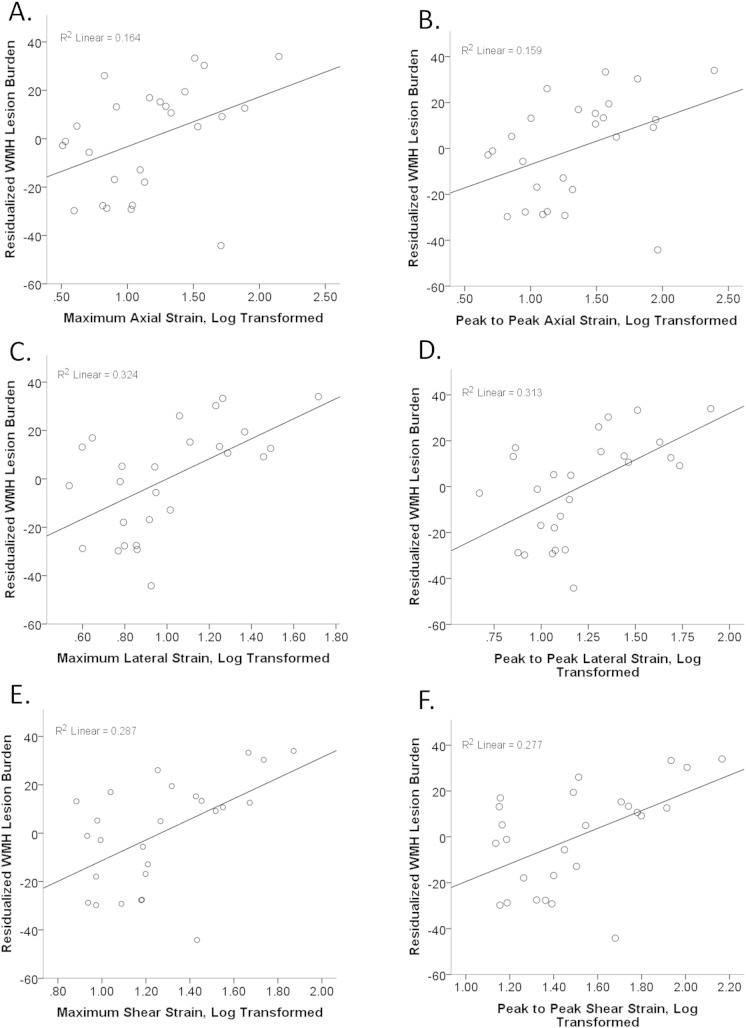
Detailed information regarding subject demographic and medical characteristics can be found in Table 1. Significant relationships were found between measurements of plaque strain and the total amount of WMH in the brain. Controlling for age, gender, BMI, hypertension, hyperlipidemia treatment, and stenosis percentage, higher strain predicted a greater amount of WMH lesion volume in the brain. Significant relationships were seen for strain measured in the axial and lateral directions, both absolute maximum and peak to peak, as well as shear strain, again for both absolute maximum and peak to peak strain (Fig. 2A–F). Specifically, maximum absolute strain, measured in the axial direction, was positively predictive of WMH burden with a beta value of 0.464 (t[DF = 18] = 2.319, p = .032) (Fig. 2A). For peak to peak strain measured in the axial direction, the beta was 0.483 (t[DF = 18] = 2.355, p = .030) (Fig. 2B). Maximum absolute strain and peak to peak strains measured in the lateral direction were predictive with beta values of 0.555 (t[DF = 18] = 3.365, p = 0.003) and 0.564 (t[DF = 18] = 3.361, p = 0.003, respectively) (Fig. 2C–D). When examining the relationship between shear strain and WMH lesion burden, maximum shear strain and peak to peak shear strain were positively associated with WMH, with beta values of 0.600 (t[DF = 18] 3.416, p = 0.003) and 0.610 (t[DF = 18] 3.412, p = 0.003) (Fig. 2E–F).
Table 1.
Subject characteristics (means unless otherwise noted).
| Age | 70.27 years (SD: 10.13) |
|---|---|
| Gender | 15 males, 11 females |
| WMH lesion volume | Mean: 51.45 ml (SD: 27.20); median: 38.00 ml Mean Z: 1.81; median Z: 0.88; Z range: −0.76–5.96 |
| BMI | 27.64 (SD: 3.31) |
| Stenosis percentage | 74.08 (SD: 16.48); range: 40%–99% |
| Subjects undergoing treatment for hyperlipidemia | 69.23% (18/26) |
| Subjects with hypertension | 84.62% (22/26) |
Fig. 2.
Series of scatter plots examining the relationship between varying metrics of plaque strain in the internal carotid artery and total WMH lesion burden. The strain measurement is on the x-axis; strain measurements were log transformed to achieve normal distributions appropriate for linear regression analyses. WMH total lesion volume was adjusted for age, gender, BMI, treatment for hyperlipidemia (yes/no), hypertension, and stenosis percentage. The residualized WMH values after adjusting for these covariates is plotted on the y-axis. A positive association is seen, with increasing strain corresponding with an increasing amount of WMH. Relationships between WMH and A. maximum absolute axial strain, B. peak to peak axial strain, C. maximum absolute lateral strain, D. peak to peak lateral strain, E. maximum absolute shear strain and F. peak to peak shear strain.
3.2. HITS and WMH
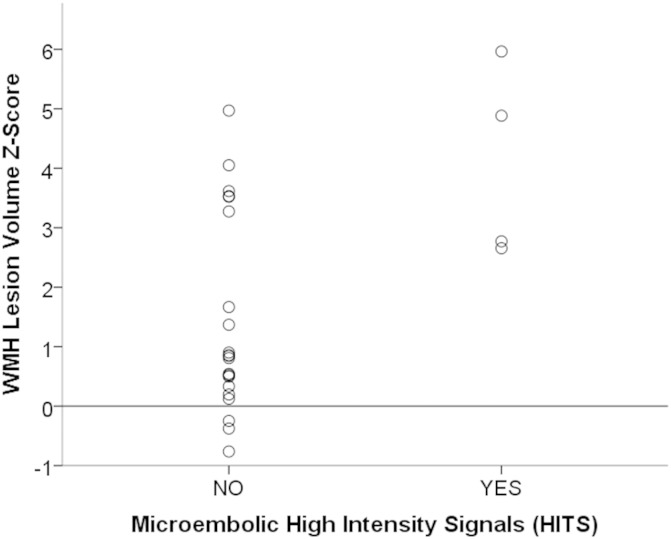
The WMH Z-scores of our patients ranged from −0.76 to 5.96, with a mean of 1.81. This means that on average, our subjects had a mean WMH lesion burden approaching two standard deviations greater than a normal, comparably aged population. Linear regression was performed to examine if microembolic HITS during transcranial Doppler ultrasound examinations predicted the extent of WMH lesion burden. Although the exact number of HITS was recorded, a binary paradigm of whether emboli occurred or did not occur was used for statistical analysis because the variable was not normally distributed. Controlling for gender, hypertension, hyperlipidemia treatment, stenosis percentage and BMI, the presence of a microembolic signal during the ultrasound was associated with an increase in the WMH lesion volume, with a beta of 0.436 (t[DF19] = 2.404, p = 0.027). Interestingly, all of the subjects presenting with embolic HITS had a WMH Z-score greater than 0, approaching or in some cases exceeding a value of 3 (Fig. 3). The range of WMH Z-scores for the patients who did not present with any emboli during the ultrasound was varied with no clustering of scores apparent.
Fig. 3.
Plot of WMH Z-score against whether HITS occurred during transcranial Doppler ultrasound. HITS is on the x-axis, WMH Z-score is on the y-axis. Horizontal line marks Z-score of 0, which represents the mean total lesion volume in a normal population. All patients with HITS during ultrasound are above this line.
4. Discussion
This study showed that the degree of stability of internal carotid artery atherosclerotic plaque is associated with increased vascular injury markers on brain MRI. Specifically, higher carotid artery plaque strain measured in the axial and lateral directions, along with shear strain, was positively related to total WMH lesion burden, the increase of which has been associated with decreased cognitive performance (Birdsill, 2014; Brickman, 2011). Additionally, presence of embolic hits during TCD ultrasound examination was related to the extent of the WMH lesion burden.
Although patients with cardiovascular disease commonly report subjective cognitive/memory decline, the basis for such complaints is currently not clear. A study of 47 patients aged 55–85 with cardiovascular disease showed that subjective cognitive complaints were associated with objective decline of cognitive performance on testing at 1 year follow-up (Haley, 2009). Thus, the present study is important in that it may suggest a potential link between vascular health and brain MRI metrics commonly associated with cognitive decline, and this link is in need of further study. It should be emphasized, however, that this is a correlational study, and that the present study design does not permit causal conclusions about the relationship between plaque strain, WMH, and cognitive decline to be made.
What is particularly unique about the current study is that it involves pre-carotid endarterectomy surgical patients, including those patients with unstable plaques. Given that arterial stiffness has previously been associated with an increased amount of white matter changes, we extended this examination to include patients with unstable plaques and significant degrees of stenosis (Turk, 2015). Turk et al. propose that stiffness of the carotid may be a better, more sensitive indicator of vascular mediated white matter disease than aortic stiffness, which at present, is considered the gold standard for large arterial stiffness measurements. The present study extends this to examine the strain within the plaque itself, rather than general carotid arterial stiffness, and consequently may signal future clinical utility for ultrasound elastography in determining whether a patient would derive significant clinical benefit from a carotid endarterectomy procedure.
The degree of carotid stenosis has previously been shown to be associated with degree of WMH lesion burden, particularly in periventricular areas (Kandiah, 2014). It is important to recognize, however, that there are other metrics of carotid disease besides percentage of occlusion that may be contributing to vascular mediated brain damage. In a study of 51 patients (average age 72), with symptomatic carotid disease, the presence of intraplaque hemorrhage on ultrasound was associated with a greater extent of ischemic brain damage (Altaf, 2011). However, in patients with only mild to moderate degrees of occlusion, neither intraplaque hemorrhage nor a thin fibrous cap (metrics of plaque stability) was associated with the occurrence of microembolic signals during TCD examination (Truijman, 2014). A reason that study may not have found a relationship between these metrics of plaque stability and embolic events is that Doppler imaging is time-limited, and thus may not capture all patients with embolic events. Furthermore, the occurrence of microembolic events does not account for previous infarcts or cumulative subclinical vascular injury. As our study examines the correspondence of HITS during ultrasound with the degree of WMH (as a metric of cumulative vascular injury) it helps to provide an inferential picture of how the stability of carotid plaque relates to cumulative lesion burden, examined via total WMH lesion burden, over a longer period of time.
The correlation between microembolic signal presence during TCD and increased WMH provides further credence to the idea that unstable plaques in carotid artery disease may mediate changes in imaging metrics of brain health. The etiology may have a common microvascular component, as we have previously shown the importance of vessel atherogenesis to plaque instability. Increased microvessel density in the plaque itself, as well as greater expression of pro-angiogenic, pro-metabolic and pro-neoplastic genes, was observed in plaques from symptomatic stroke patients compared to plaques from those who were asymptomatic (Türeyen, 2006; Vemuganti and Dempsey, 2006; Vemuganti and Dempsey, 2005). These mechanisms suggest that a similar small vessel process may be present in both carotid wall plaque and brain small vessel disease in these atherosclerotic patients. Future research should extend this work to see if WMH lesion burden is correlated with changes in brain gene expression, as we have previously shown that cerebral ischemia changes the expression profile of non-coding RNAs and angiogenic cytokines (Vemuganti, 2013; Wesley, 2013). Additionally, given that diabetes leads to exacerbated post-stroke pathology, it will be prudent to examine how diabetes may mediate the relationship between carotid plaque strain and WMH lesion burden (Tureyen, 2011).
As with any study, it is important to recognize its inherent limitations. This is a cross-sectional study, so causal relationships cannot be claimed, however, longitudinal follow-up analyses are being pursued. Sample size was relatively small; current efforts are underway to accrue more participants so these analyses can be replicated in a larger population. Furthermore, as these patients were recruited by neurosurgeons at a tertiary care medical center, there may be a referral bias to those participants with increased medical care access. However, as this medical center receives patients not just from its immediate urban area, but more rural areas around the state, this increases the generalizability of these results to the population at large. It should also be recognized that only analyzing patients scheduled to undergo carotid endarterectomy surgery in the near future may bias our sample to include subjects with more vulnerable plaques; ideally, the study would have benefitted from the inclusion of subjects with high grade carotid stenosis who were not undergoing surgery, and this is an important avenue to explore in future studies. Finally, there was a lack of a closely matched control population undergoing all study procedures. We attempted to rectify this by computing a WMH Z-score for the HITS analysis using a simultaneously recruited cognitively normal, healthy cohort from the Wisconsin Alzheimer's Disease Research Center that provided an appropriate comparison group for WMH burden.
5. Conclusion
This study extends upon previous data that shows an association between higher internal carotid artery strain and cognition, identifying a positive relationship between plaque instability and white matter changes on imaging. One can conclude that this provides further evidence for the increasing link between overall vascular health and cognitive decline, and that unstable atherosclerotic plaque may be cognitively pathological even if major ischemic stroke events do not occur. It also suggests that carotid plaque instability will be more clinically important than the process of stenosis alone. The cumulative vascular toll of internal carotid disease observed in this study merits replication in a larger sample size. As ultrasound elastography becomes more integrated into clinical practice, it may be that metrics of plaque stability can soon be used as factors in evaluating whether patients would benefit from intensive medical and selective surgical intervention.
Acknowledgments
The authors would like to acknowledge the funding sources for the work completed in this paper, specifically R01 NS064034 and P50-AG033514 (NIH). Furthermore, we would like to thank the Clinical Research Unit of the Department of Neurosurgery for the help in coordinating the study and the Alzheimer's Disease Research Center, specifically Jennifer Oh, for the assistance in MRI pre-processing. Lastly, the authors would like to extend their deepest gratitude to all individuals who participated in the study.
References
- Alosco M.L. Independent and interactive effects of blood pressure and cardiac function on brain volume and white matter hyperintensities in heart failure. J. Am. Soc. Hypertens. 2013;7(5):336–343. doi: 10.1016/j.jash.2013.04.011. 23735419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altaf N. Brain white matter hyperintensities are associated with carotid intraplaque hemorrhage. Radiology. 2008;248(1):202–209. doi: 10.1148/radiol.2481070300. 18566173 [DOI] [PubMed] [Google Scholar]
- Altaf N. Plaque hemorrhage is a marker of thromboembolic activity in patients with symptomatic carotid disease. Radiology. 2011;258(2):538–545. doi: 10.1148/radiol.10100198. 21163919 [DOI] [PubMed] [Google Scholar]
- Biller J. Guidelines for carotid endarterectomy: a statement for healthcare professionals from a Special Writing Group of the Stroke Council, American Heart Association. Circulation. 1998;97(5):501–509. doi: 10.1161/01.cir.97.5.501. 9490248 [DOI] [PubMed] [Google Scholar]
- Birdsill A.C. Regional white matter hyperintensities: aging, Alzheimer's disease risk, and cognitive function. Neurobiol. Aging. 2014;35(4):769–776. doi: 10.1016/j.neurobiolaging.2013.10.072. 24199958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman A.M. White matter hyperintensities and cognition: testing the reserve hypothesis. Neurobiol. Aging. 2011;32(9):1588–1598. doi: 10.1016/j.neurobiolaging.2009.10.013. 19926168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casado Naranjo I. Association of vascular factors and amnestic mild cognitive impairment: a comprehensive approach. J. Alzheimers Dis. 2015;44(2):695–704. doi: 10.3233/JAD-141770. 25362037 [DOI] [PubMed] [Google Scholar]
- Consensus Committee of the Ninth International Cerebral Hemodynamic Symposium Basic identification criteria of Doppler microembolic signals. Stroke. 1995;26(6):1123. [PubMed] [Google Scholar]
- Daniels M.J., Varghese T. Dynamic frame selection for in vivo ultrasound temperature estimation during radiofrequency ablation. Phys. Med. Biol. 2010;55(16):4735–4753. doi: 10.1088/0031-9155/55/16/008. 20671353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Weerd M. Prevalence of asymptomatic carotid artery stenosis in the general population: an individual participant data meta-analysis. Stroke. 2010;41(6):1294–1297. doi: 10.1161/STROKEAHA.110.581058. 20431077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey R.J. A review of carotid atherosclerosis and vascular cognitive decline: a new understanding of the keys to symptomology. Neurosurg. 2010;67(2):484–493. doi: 10.1227/01.NEU.0000371730.11404.36. 20644437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enzinger C. High-grade internal carotid artery stenosis and chronic brain damage: a volumetric magnetic resonance imaging study. Cerebrovasc. Dis. 2010;30(6):540–546. doi: 10.1159/000319025. 20948197 [DOI] [PubMed] [Google Scholar]
- Haley A.P. Subjective cognitive complaints relate to white matter hyperintensities and future cognitive decline in patients with cardiovascular disease. Am. J. Geriatr. Psychiatry. 2009;17(11):976–985. doi: 10.1097/JGP.0b013e3181b208ef. 20104055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandiah N. Carotid stenosis: a risk factor for cerebral white-matter disease. J. Stroke Cerebrovasc. Dis. 2014;23(1):136–139. doi: 10.1016/j.jstrokecerebrovasdis.2012.11.007. 23265783 [DOI] [PubMed] [Google Scholar]
- Knopman D.S. Vascular risk factors and longitudinal changes on brain MRI: the ARIC study. Neurology. 2011;76(22):1879–1885. doi: 10.1212/WNL.0b013e31821d753f. 21543737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwee R.M. Identifying vulnerable carotid plaques by noninvasive imaging. Neurol. 2008;70(24 Pt 2):2401–2409. doi: 10.1212/01.wnl.0000314697.76580.cb. 18541873 [DOI] [PubMed] [Google Scholar]
- McCormick M. Methods for robust in vivo strain estimation in the carotid artery. Phys. Med. Biol. 2012;57(22):7329–7353. doi: 10.1088/0031-9155/57/22/7329. 23079725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick M., Rubert N., Varghese T. Bayesian regularization applied to ultrasound strain imaging. I.E.E.E. Trans. Biomed. Eng. 2011;58(6):1612–1620. doi: 10.1109/TBME.2011.2106500. 21245002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ophir J. Elastography: a quantitative method for imaging the elasticity of biological tissues. Ultrason. Imaging. 1991;13(2):111–134. doi: 10.1177/016173469101300201. 1858217 [DOI] [PubMed] [Google Scholar]
- Petty G.W. Ischemic stroke subtypes: a population-based study of incidence and risk factors. Stroke. 1999;30(12):2513–2516. doi: 10.1161/01.str.30.12.2513. 10582970 [DOI] [PubMed] [Google Scholar]
- Ribbers H. Noninvasive two-dimensional strain imaging of arteries: validation in phantoms and preliminary experience in carotid arteries in vivo. Ultrasound Med. Biol. 2007;33(4):530–540. doi: 10.1016/j.ultrasmedbio.2006.09.009. 17280769 [DOI] [PubMed] [Google Scholar]
- Rocque B.G. Impaired cognitive function in patients with atherosclerotic carotid stenosis and correlation with ultrasound strain measurements. J. Neurol. Sci. 2012;322(1–2):20–24. doi: 10.1016/j.jns.2012.05.020. 22658531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell P.M. The role of carotid atherosclerosis in the aetiology of ischaemic stroke. Cerebrovasc Dis. 1996;6(Suppl 2):1. [Google Scholar]
- Rubin M.N. Asymptomatic carotid stenosis: what we can learn from the next generation of randomized clinical trials. JRSM Cardiovasc Dis. 2014;3 doi: 10.1177/2048004014529419. 25247072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt P. An automated tool for detection of FLAIR-hyperintense white-matter lesions in multiple sclerosis. Neuroimage. 2012;59(4):3774–3783. doi: 10.1016/j.neuroimage.2011.11.032. 22119648 [DOI] [PubMed] [Google Scholar]
- Shi H. Ultrasonic attenuation estimation in small plaque samples using a power difference method. Ultrason. Imaging. 2007;29(1):15–30. doi: 10.1177/016173460702900102. 17491296 [DOI] [PubMed] [Google Scholar]
- Shi H. Relationship between ultrasonic attenuation, size and axial strain parameters for ex vivo atherosclerotic carotid plaque. Ultrasound Med. Biol. 2008;34(10):1666–1677. doi: 10.1016/j.ultrasmedbio.2008.02.014. 18490099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H. Preliminary in vivo atherosclerotic carotid plaque characterization using the accumulated axial strain and relative lateral shift strain indices. Phys. Med. Biol. 2008;53(22):6377–6394. doi: 10.1088/0031-9155/53/22/008. 18941278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H. In vivo attenuation and equivalent scatterer size parameters for atherosclerotic carotid plaque: preliminary results. Ultrasonics. 2009;49(8):779–785. doi: 10.1016/j.ultras.2009.06.004. 19640556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Varghese T. Two-dimensional multi-level strain estimation for discontinuous tissue. Phys. Med. Biol. 2007;52(2):389–401. doi: 10.1088/0031-9155/52/2/006. 17202622 [DOI] [PubMed] [Google Scholar]
- Shim Y.S. Pathological correlates of white matter hyperintensities on magnetic resonance imaging. Dement. Geriatr. Cogn. Disord. 2015;39(1–2):92–104. doi: 10.1159/000366411. 25401390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truijman M.T. Intraplaque hemorrhage, fibrous cap status, and microembolic signals in symptomatic patients with mild to moderate carotid artery stenosis: the plaque at RISK study. Stroke. 2014;45(11):3423–3426. doi: 10.1161/STROKEAHA.114.006800. 25256179 [DOI] [PubMed] [Google Scholar]
- Türeyen K. Increased angiogenesis and angiogenic gene expression in carotid artery plaques from symptomatic stroke patients. Neurosurg. 2006;58(5):971–977. doi: 10.1227/01.NEU.0000210246.61817.FE. 16639334 [DOI] [PubMed] [Google Scholar]
- Tureyen K. Exacerbated brain damage, edema and inflammation in type-2 diabetic mice subjected to focal ischemia. J. Neurochem. 2011;116(4):499–507. doi: 10.1111/j.1471-4159.2010.07127.x. 21133923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk M. Ultrasound diagnosis of carotid artery stiffness in patients with ischemic leukoaraiosis. Ultrasound Med. Biol. 2015;41(1):64–71. doi: 10.1016/j.ultrasmedbio.2014.08.002. 25438859 [DOI] [PubMed] [Google Scholar]
- Varghese T. Tradeoffs in elastographic imaging. Ultrason. Imaging. 2001;23(4):216–248. doi: 10.1177/016173460102300402. 12051276 [DOI] [PubMed] [Google Scholar]
- Varghese T. Quasi-static ultrasound elastography. Ultrasound Clin. 2009;4(3):323–338. doi: 10.1016/j.cult.2009.10.009. 20798841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varghese T., Ophir J. A theoretical framework for performance characterization of elastography: the strain filter. I.E.E.E. Trans. Ultrason. Ferroelectr. Freq. Contr. 1997;44(1):164–172. doi: 10.1109/58.585212. 18244114 [DOI] [PubMed] [Google Scholar]
- Vemuganti R. All's well that transcribes well: non-coding RNAs and post-stroke brain damage. Neurochem. Int. 2013;63(5):438–449. doi: 10.1016/j.neuint.2013.07.014. 23954844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemuganti R., Dempsey R.J. Carotid atherosclerotic plaques from symptomatic stroke patients share the molecular fingerprints to develop in a neoplastic fashion: a microarray analysis study. Neuroscience. 2005;131(2):359–374. doi: 10.1016/j.neuroscience.2004.08.058. 15708479 [DOI] [PubMed] [Google Scholar]
- Vemuganti R., Dempsey R.J. Increased expression of genes that control ionic homeostasis, second messenger signaling and metabolism in the carotid plaques from patients with symptomatic stroke. J. Neurochem. 2006;97(Suppl. 1):92–96. doi: 10.1111/j.1471-4159.2005.03516.x. 16635256 [DOI] [PubMed] [Google Scholar]
- Wang X. Correlation of cognitive function with ultrasound strain indices in carotid plaque. Ultrasound Med. Biol. 2014;40(1):78–89. doi: 10.1016/j.ultrasmedbio.2013.08.001. 24120415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. Estimation of ultrasound strain indices in carotid plaque and correlation to cognitive dysfunction. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2014:5627–5630. doi: 10.1109/EMBC.2014.6944903. 25571271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen W. White matter hyperintensities in the forties: their prevalence and topography in an epidemiological sample aged 44–48. Hum. Brain Mapp. 2009;30(4):1155–1167. doi: 10.1002/hbm.20586. 18465744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen W., Sachdev P. The topography of white matter hyperintensities on brain MRI in healthy 60- to 64-year-old individuals. Neuroimage. 2004;22(1):144–154. doi: 10.1016/j.neuroimage.2003.12.027. 15110004 [DOI] [PubMed] [Google Scholar]
- Wesley U.V. Galectin-3 enhances angiogenic and migratory potential of microglial cells via modulation of integrin linked kinase signaling. Brain Res. 2013;1496:1–9. doi: 10.1016/j.brainres.2012.12.008. 23246924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White H. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation. 2005;111(10):1327–1331. doi: 10.1161/01.CIR.0000157736.19739.D0. 15769776 [DOI] [PubMed] [Google Scholar]