Abstract
Purines induce transient contraction and prolonged relaxation of detrusor muscles. Transient contraction is likely due to activation of inward currents in smooth muscle cells, and prolonged relaxation may be due to activation of small-conductance Ca2+-activated K+ (SK) channels via P2Y1 receptors expressed by detrusor PDGF receptor (PDGFR)α+ cells. We investigated whether other subtypes of P2Y receptors are involved in the activation of SK channels in PDGFRα+ cells of detrusor muscles. Quantitative analysis of transcripts revealed that P2ry2, P2ry4, and P2ry14 are expressed in PDGFRα+ cells of P2ry1-deficient/enhanced green fluorescent protein (P2ry1−/−/eGFP) mice at similar levels as in wild-type mice. UTP, a P2Y2/P2Y4 agonist, activated large outward currents in detrusor PDGFRα+ cells. SK channel blockers and an inhibitor of phospholipase C completely abolished currents activated by UTP. In contrast, UTP activated nonselective cation currents in smooth muscle cells. Under current-clamp (current = 0), UTP induced significant hyperpolarization of PDGFRα+ cells. MRS2500, a selective P2Y1 antagonist, did not affect UTP-activated outward currents in PDGFRα+ cells from wild-type mice, and activation of outward currents by UTP was retained in P2ry1−/−/eGFP mice. As a negative control, we tested the effect of MRS2693, a selective P2Y6 agonist. This compound did not activate outward currents in PDGFRα+ cells, and currents activated by UTP were unaffected by MRS2578, a selective P2Y6 antagonist. The nonselective P2Y receptor blocker suramin inhibited UTP-activated outward currents in PDGFRα+ cells. Our data demonstrate that P2Y2 and/or P2Y4 receptors function, in addition to P2Y1 receptors, in activating SK currents in PDGFRα+ cells and possibly in mediating purinergic relaxation responses in detrusor muscles.
Keywords: purinergic receptors, detrusor relaxation, interstitial cells, potassium channels
purinergic signaling is involved in a variety of physiological responses in the urinary bladders of mammals (1, 6, 13). Purines bind to P2X receptors, which are highly expressed by smooth muscle cells (SMC). Activation of P2X receptors in detrusor muscles induces contraction (20, 29). Purines and pyrimidines also activate responses via binding to P2Y receptors, and studies have suggested that binding of P2Y receptors in detrusor muscles induces relaxation (2, 19). Relaxation is mediated by stabilization of membrane excitability of detrusor muscles, and this response may limit contractions of the detrusor during bladder filling.
Small-conductance Ca2+-activated K+ (SK) channels constitute an important conductance that serves to stabilize membrane excitability in detrusor muscles. SK channel antagonists or deactivation of SK genes leads to detrusor overactivity (14, 15). This effect may be partially mediated by SK conductance in SMCs (14, 15, 24, 27); however, we recently found that Kcnn3 (which encodes SK3 channels) is highly expressed by PDGF receptor (PDGFR)α+ cells and that, in comparison, genes encoding SK channels are minimally expressed by SMCs (18). SMCs display very low current densities attributable to SK conductances, whereas robust SK currents are generated in PDGFRα+ cells at holding potentials mimicking physiological membrane potentials. P2ry1 (which encodes P2Y1 receptors) is also expressed predominantly by PDGFRα+ cells in bladder muscles, and binding of P2Y1 receptors by purine agonists couples to activation of SK channels (19).
There are eight subtypes of P2Y receptors in mammals, including P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11–P2Y14 (12). P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11 receptors are coupled to effectors via the Gq/11-phospholipase C (PLC) pathway, whereas P2Y12–P2Y14 receptors are coupled through Gi/o (12). SK channels are Ca2+-activated K+ channels, so generation of inositol 1,4,5-trisphosphate (IP3) through coupling via Gq/11 and activation of PLC and release of Ca2+ from IP3 receptor-operated stores is a potential mechanism for activation of SK channels in response to purine or pyrimidine stimulation (19).
We have found that detrusor PDGFRα+ cells, purified by fluorescence-activated cell sorting (FACS), display enriched expression of P2ry1, P2ry2, and P2ry4 relative to unsorted cells (19). ATP and a selective P2Y1 agonist activated SK currents in these cells and induced relaxation in intact detrusor muscles, but the outward currents activated by ATP were not abolished in detrusor PDGFRα+ cells from P2ry1-deficient/enhanced green fluorescent protein (P2ry1−/−/eGFP) mice (19). P2Y2 and P2Y4 receptors are activated preferentially by pyrimidines, such as UTP. Thus, we investigated whether other subtypes of P2Y receptors are possibly linked to activation of SK channels in PDGFRα+ cells of detrusor muscles.
MATERIALS AND METHODS
Preparation of detrusor PDGFRα+ cells and SMCs.
Animals were maintained and all experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Institutional Animal Use and Care Committee of the University of Nevada. C57BL/6J and Pdgfratm11(EGFP)Sor/J (both purchased from Jackson Laboratory, Bar Harbor, ME) mice (6–12 wk) were used for this study. Pdgfratm11(EGFP)Sor/J bred with P2ry1−/− (called P2ry1−/−/eGFP) mice at 6–12 wk of age were also used for this study. PDGFRα+ cells and SMCs were prepared similarly as previously described (18, 19). Briefly, detrusor muscles (with the urothelium removed) were incubated in Ca2+-free solution containing 0.5 mg/ml papain and 0.5 mg/ml l-DTT (both from Sigma, St. Louis, MO) at 37°C for 20–25 min, rinsed, and then further digested in Ca2+-free solution including 3–5 mg/ml collagenase type II (Sigma) and 100 μM CaCl2 at 37°C for 40–50 min. Single cells were dissociated by trituration. For PDGFRα+ cells, the resulting cell suspensions were plated onto glass coverslips previously coated with murine collagen (2.5 μg/ml, Falcon/BD). PDGFRα+ cells were maintained in a humidified atmosphere of 95% O2-5% CO2 at 37°C and used for recordings within 6–8 h of tissue digestion. For SMCs, aliquots of the resulting cell suspensions were transferred to a 0.5-ml chamber and allowed to adhere for 10–15 min; cells then were thoroughly rinsed in bath recording solutions.
Cell purification, RNA isolation, RT-PCR, and quantitative PCR.
PDGFRα+ cells were purified by FACS (Becton Dickinson FACSAria using the blue laser at 488 nm and the GFP emission detector at 530/30 nm). Total RNA was isolated from PDGFRα+ cells using an illustra RNAspin Mini RNA Isolation kit (GE Healthcare, Little Chalfont, UK), and first-strand cDNA was synthesized using SuperScript III (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. PCR was performed with specific primers using Go-Taq Green Master Mix (Promega, Madison, WI). The following PCR primers designed against murine sequences (with GenBank Accession Numbers given in parentheses for the reference nucleotide sequence) were used: Gapdh (NM_008084), P2ry1 (NM_008772), P2ry2 (NM_008773), P2ry4 (NM_020621), P2ry6 (NM_183168), P2ry12 (NM_027571), P2ry13 (NM_028808), and P2ry14 (NM_133200). PCR products were analyzed on 2% agarose gels and visualized by ethidium bromide. Quantitative PCR was performed with the same primers as PCR using Fast Syber green chemistry (Applied Biosystems, Foster City, CA) on a 7900HT Real Time PCR System (Applied Biosystems). Regression analysis of the mean values of three multiplex quantitative PCRs for the log10 diluted cDNA was used to generate standard curves. Unknown amounts of mRNA were plotted relative to the standard curve for each set of primers and graphically plotted using Microsoft Excel. This gave transcriptional quantification of each gene relative to the endogenous Gapdh standard after log transformation of the corresponding raw data.
Patch-clamp recordings.
Patch pipettes were pulled from borosilicate capillaries (Sutter instruments, Novato, CA). When filled with the pipette solution, pipette tip resistances were 3–4 MΩ. The whole cell configuration was achieved in Ca2+-containing physiological salt solution bath solution of the following composition (in mM): 135 NaCl, 5 KCl, 1.2 MgCl2, 2 CaCl2, 10 glucose, and 10 HEPES, pH 7.4 with Tris base. The pipette solution was of the following composition (in mM): 135 KCl (K+-rich)/135 CsCl (Cs+-rich), 0.012 CaCl2, 3 MgATP, 0.1 Na2GTP, 2.5 creatine phosphate disodium, 0.1 EGTA, 10 glucose, and 10 HEPES, pH 7.2 with Tris base. Cells were placed in a 0.5-ml chamber mounted on an inverted microscope (Nikon). PDGFRα+ cells were identified by the fluorescence of eGFP in nuclei. Whole cell recordings were made under voltage- and current-clamp conditions. An Axopatch 200B amplifier with a CV-203BU headstage (Molecular Devices, Sunnyvale, CA) was used. All data were analyzed using pCLAMP software (Axon Instruments) and Graphpad Prism (version 3.0, Graphpad Software, San Diego, CA). All recordings were made at ∼30°C.
Drugs.
All drugs and reagents including UTP, U-73122, U-73433, MRS2500, MRS2693, MRS2578, suramin, apamin, and UCL1684 were purchased from Sigma-Aldrich. UTP was freshly dissolved in Ca2+-containing physiological salt solution to the final concentration. Other drugs were made in stock and then diluted to their final concentrations in the bath solution for whole cell recordings.
Statistical analyses.
All data are expressed as means ± SE. All statistical analyses were performed using Graphpad Prism. Student's paired and unpaired t-tests were used to compare groups of data, and differences were considered to be significant at P < 0.05.
RESULTS
Transcriptional expression of P2rys in PDGFRα+ cells.
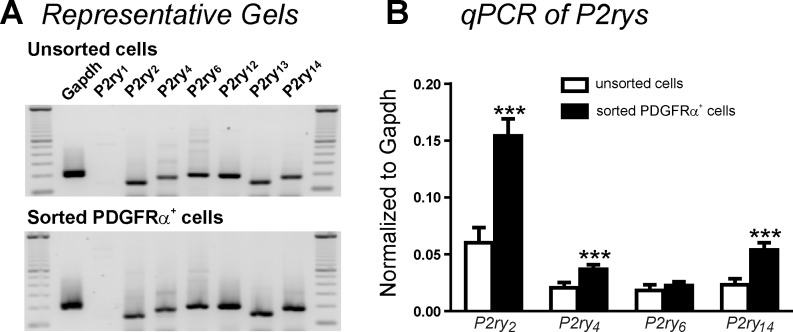
As shown in a previous study (19), P2ry2 and P2ry4 are expressed in detrusor PDGFRα+ cells. We determined P2ry1–P2ry14 in PDGFRα+ cells from P2ry1−/−/eGFP mice (see materials and methods) to allow FACS purification of cells. PDGFRα+ cells from these mice showed no transcripts of P2ry1, but P2ry2, P2ry4, and P2ry14 were enriched 2.6-fold, 1.8-fold, and 2.3-fold, respectively, relative to the unsorted population of cells dispersed from detrusor muscles (Fig. 1B). These values are not significantly different from our previous report (19) examining transcripts of P2Y receptors (e.g., P2ry2: 2.1-fold, P2ry4: 2.4-fold, and P2ry14: 2.1 fold compared with unsorted cells) in wild-type mice, suggesting that there is no compensation (i.e., up- or downregulation of expression) of P2ry2, P2ry4, or P2ry14 in P2ry1−/−/eGFP mice.
Fig. 1.
End-point gels and quantitative analysis of P2ry transcripts in unsorted cells and sorted detrusor PDGF receptor (PDGFR)α+ cells from P2ry1-deficient/enhanced green fluorescent protein (P2ry1−/−/eGFP) mice. A: all P2rys except P2ry1 were detected in PDGFRα+ cells purified by FACS or in unsorted cells dispersed from detrusor muscles of P2ry1−/−/eGFP mice. B: summary graph showing that P2ry2, P2ry4, and P2ry14 were enriched in detrusor PDGFRα+ cells from P2ry1−/−/eGFP mice compared with unsorted cells. P2ry12 and P2ry13 were minimally expressed and thus not displayed. Expression of all transcripts was normalized to Gapdh. qPCR, quantitative PCR. ***P < 0.001.
Responses of PDGFRα+ cells and SMCs to UTP.
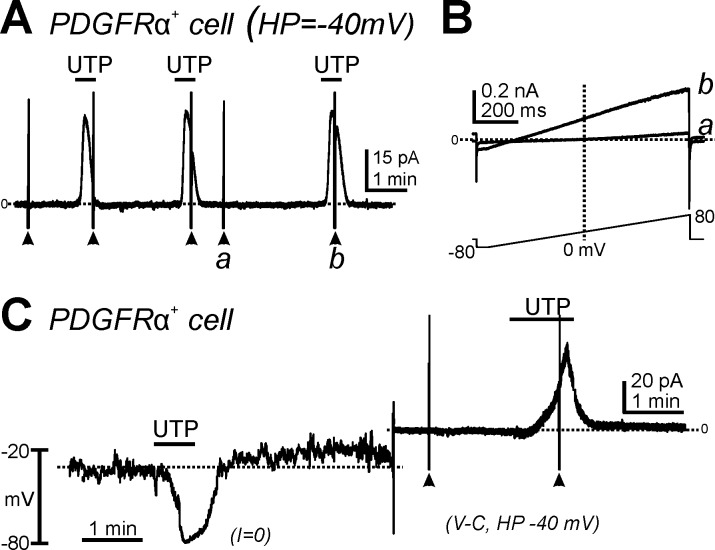
Patch-clamp experiments using the whole cell configuration were performed to determine the effects of UTP, a nonselective P2Y2/P2Y4 agonist, on membrane currents in PDGFRα+ cells isolated from wild-type PDGFRα/eGFP mice. When PDGFRα+ cells were held at −40 mV, UTP (10 μM) induced large outward currents (Fig. 2, A and C). These responses were repeatable without apparent desensitization (Fig. 2A). The outward current evoked by UTP averaged was 3.9 ± 0.8 pA/pF at a holding potential of −40 mV (n = 20). When cells were ramped from −80 to +80 mV (Fig. 2B, bottom), a small-amplitude outwardly rectifying K+ current was evoked under control conditions (Fig. 2B,a). UTP activated large-amplitude outward currents and shifted the reversal potential toward K+ equilibrium potential (EK) (Fig. 2B,b). Under current-clamp conditions (current = 0; Fig. 2C, left), UTP application induced hyperpolarization from −30.2 ± 4.3 to −76.3 ± 3.6 mV (n = 6, P < 0.001). In the same cells, UTP activated the outward current under voltage-clamp conditions at a holding potential of −40 mV (Fig. 2C, right).
Fig. 2.
Effects of UTP on the membrane currents and membrane potential in PDGFRα+ cells. A: cells were held at −40 mV. UTP (10 μM) activated outward currents in PDGFRα+ cells, and these responses were reproducible in response to multiple applications. B: expanded timescales at points designated as a and b in A showing current responses to ramp potentials before (a) and after (b) addition of UTP. C: UTP (10 μM) hyperpolarized PDGFRα+ cells under current-clamp mode [current (I) = 0] and activated outward currents in the same cells under voltage-clamp mode [V-C; holding potential (HP) = −40 mV].
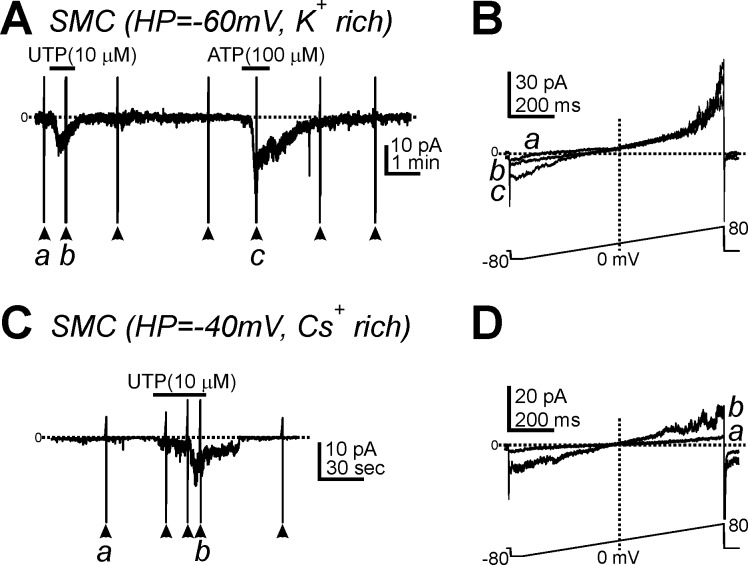
UTP (10 μM) activated small inward currents in SMCs (0.9 ± 0.4 pA/pF, n = 6) at −60 mV with an internal K+-rich solution (Fig. 3, A and B) and after replacement of K+ with a Cs+-rich solution (1.1 ± 0.2 pA/pF, n = 8; Fig. 3, C and D) at −40 mV. The inward currents activated by UTP in SMCs reversed close to 0 mV (Fig. 3D), suggesting that UTP activates a nonselective cation current in SMCs.
Fig. 3.
Effects of UTP on membrane currents in smooth muscle cells (SMCs). A: UTP (10 μM) and ATP (100 μM) induced inward currents in SMCs dialyzed with a K+-rich solution at a HP of −60 mV. B: expanded timescales from control conditios (a) and in the presence of UTP (b) in A. C: UTP activated inward currents in SMCs dialyzed with Cs+-rich pipette solution (HP = −40 mV). D: expanded timescales from before (a) and after UTP (b) in C. Horizontal dotted lines denote 0 pA. Arrowheads denote the applications of ramp potentials (protocol shown in bottom traces in A and C).
Activation of SK channels by UTP in PDGFRα+ cells.
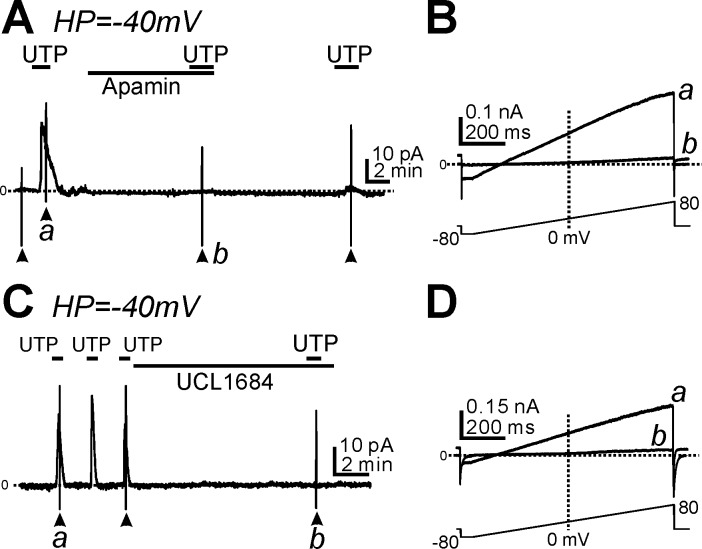
The outward current activated by UTP in PDGFRα+ cells displayed properties similar to SK currents (e.g., reversal potential close to EK, voltage independence, and rectification at positive potentials; see Fig. 2B,b). Therefore, we tested the effects of SK channel blockers on the current activated by UTP. Apamin (300 nM), a SK channel blocker, abolished the current activated by UTP at a holding potential of −40 mV (n = 6, P < 0.001; Fig. 4A) and the voltage-independent current in response to ramp potentials (Fig. 4B,b). We also tested the effects of UCL1684 (1 μM), another SK channel blocker, on the outward current activated by UTP. UCL1684 inhibited the UTP-activated current in a manner similar to the effects of apamin (n = 5, P < 0.001 for UTP-activated current at −40 mV before and after UCL1684; Fig. 4, C and D). These data suggest that current activated by UTP in PDGFRα+ cells is due to activation of SK channels.
Fig. 4.
Effect of small-conductance Ca2+-activated K+ (SK) channel blockers on the outward currents activated by UTP in PDGFRα+ cells. A and C: cells were held at −40 mV. UTP (10 μM) activated outward currents, and these responses were inhibited by apamin (0.3 μM; A) or UCL1684 (1 μM; C). B and D: expanded timescaless at points denoted by a and b in A and C showing the outward currents activated by UTP (B,a and D,a); these currents were blocked by apamin (B,b) or UCL1684 (D,b). Horizontal dotted lines in all A–D denote 0 pA. Arrowheads denote the application of ramp potentials (see protocols shown in bottom traces in B and D).
Activation of SK channels by UTP occurs through a Gq/11-PLC signaling pathway in PDGFRα+ cells.
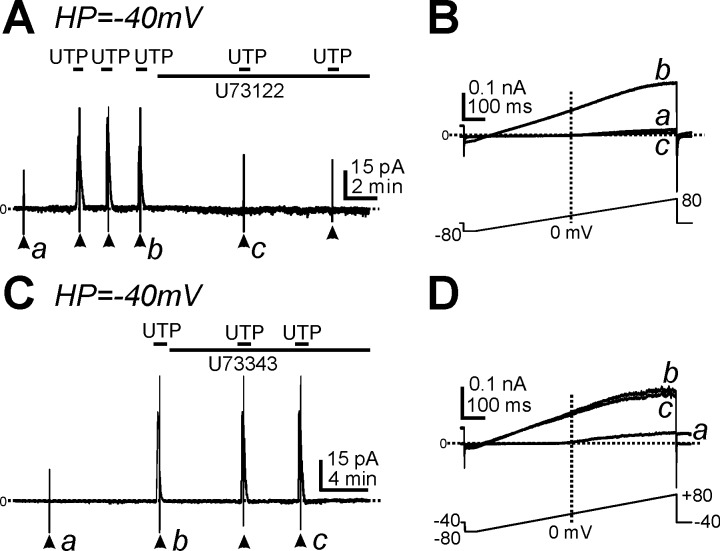
We investigated whether UTP activated outward currents in PDGFRα+ cells via a Gq/11-PLC pathway by testing the effects of U73122, a PLC inhibitor. U73122 (1 μM) blocked the current activated by UTP at a holding potential of −40 mV (n = 5, P < 0.001; Fig. 5A) and voltage-independent currents in response to ramp depolarization (n = 5; Fig. 5B). For comparison, we also tested the effects of U73343 (1 μM), an inactive analog of U73122. U73343 had no effect on the current evoked by UTP (n = 4; Fig. 5, C and D). These data suggest that UTP activated SK channel currents through a Gq/11-PLC pathway in PDGFRα+ cells.
Fig. 5.
Effect of a phospholipase C (PLC) inhibitor on outward currents activated by UTP in PDGFRα+ cells. A: cells were held at −40 mV. UTP induced outward currents, and these responses were blocked by U73122 (1 μM). B: expanded timescales from points denoted by a–c in A showing current responses to ramp potentials under control conditions (a), after the addition of UTP (b), and after the addition of U73122 and UTP (c). C: cells were held at −40 mV. UTP (10 μM) activated outward currents before and after the addition of U73343 (1 μM), an inactive analog of U73122. D: expanded timescales from points denoted by a--c in C. The bottom traces in B and D depict the ramp depolarization protocols. Horizontal dotted lines in A–D denote 0 pA. Arrowheads denote the application of ramp potentials.
Activation of SK currents by UTP through P2Y receptors in PDGFRα+ cells.
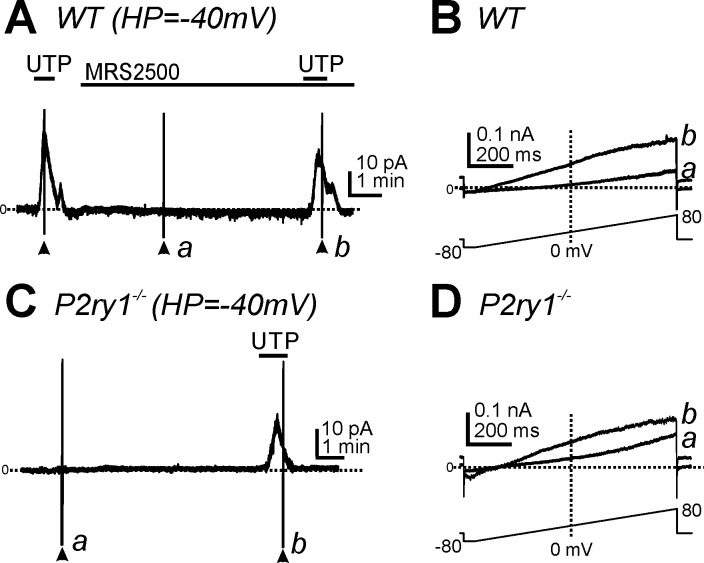
P2ry1, P2ry2, and P2ry4 transcripts are enriched in PDGFRα+ cells from wild-type mice in relation to transcripts of unsorted cells obtained by enzymatic dispersion (19). We investigated the effect of the P2Y1 antagonist MRS2500 to determine the role of P2Y1 receptors in UTP responses. MRS2500 (1 μM) did not decrease outward currents activated by UTP in PDGFRα+ cells at a holding potential of −40 mV (n = 7, P > 0.05; Fig. 6A) or the current-voltage relationship evoked by ramp depolarization (Fig. 6B,b). We also tested the effects of UTP on PDGFRα+ cells from P2ry1−/−/eGFP mice. UTP activated outward currents (2.5 ± 0.9 pA/pF, n = 6) at a holding potential of −40 mV in these cells (Fig. 6C), which was not significantly different from the responses of UTP from PDGFRα+ cells at the same holding potential in wild-type mice (n = 20, P > 0.05). The currents activated by UTP in cells of P2ry1−/−/eGFP mice had the same voltage independence as currents activated in wild-type cells (Fig. 6D,b). Thus, the findings from PDGFRα+ cells from P2ry1−/−/eGFP mice were consistent with pharmacological findings from wild-type mice and suggest that P2Y1 receptors are not necessary for the effects of UTP on PDGFRα+ cells.
Fig. 6.
Effects of P2Y1 antagonist and deletion on outward currents activated by UTP in PDGFRα+ cells. A: UTP (10 μM) activated outward currents in PDGFRα+ cells from wild-type mice before and after the addition of MRS2500 (1 μM; HP = −40 mV). B: expanded timescales showing responses to ramp potentials during only MRS2500 (a) and with MRS2500 and UTP (b) from the points denoted in A. C: UTP (10 μM) also activated outward currents at −40 mV in PDGFRα+ cells from P2ry1−/−/eGFP mice. D: current traces at expanded timescales during ramp potentials in control conditions (a) and after the addition of UTP (b) from points denoted in C. Horizontal dotted lines in A–D denote 0 pA. Arrowheads denote the applications of ramp potentials.
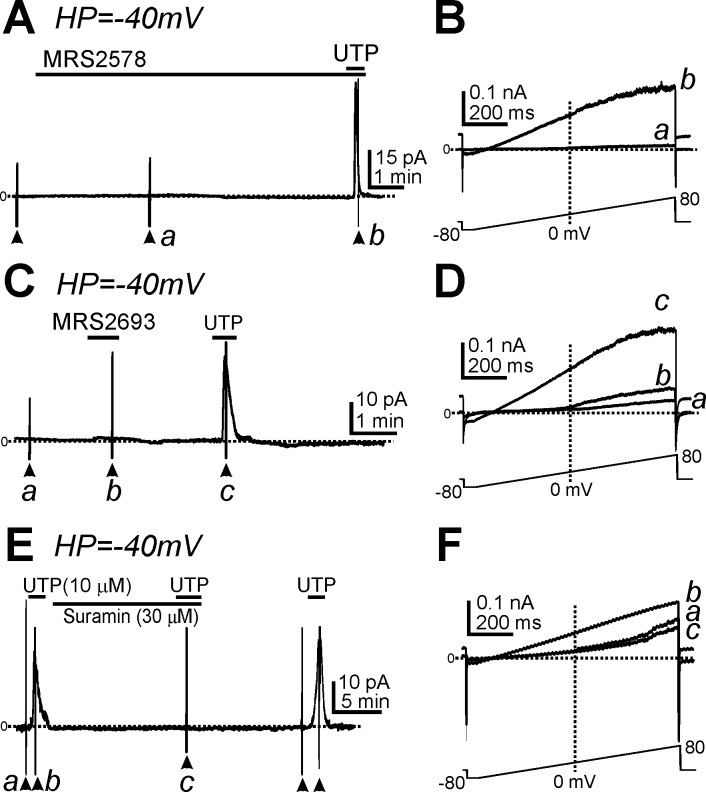
Although P2ry6 transcripts were not significantly enriched in PDGFRα+ cells (19), we tested a potential role for P2Y6 receptors in mediating UTP responses in wild-type PDGFRα+ cells. A selective P2Y6 antagonist, MRS2578, was tested on UTP responses in PDGFRα+ cells. MRS2578 (10 μM) had no effect on the outward currents activated by UTP (i.e., 4.1 ± 0.7 pA/pF, n = 6, P > 0.05; Fig. 7, A and B), and the P2Y6 agonist MRS2693 (2 μM) caused only a tiny increase in outward current in PDGFRα+ cells (Fig. 7, C and D,b) that was insignificant compared with the response to UTP (n = 5; Fig. 7, C and D,c). We also tested the effect of the nonselective P2Y receptor blocker suramin (30 μM) on UTP-activated outward currents. Suramin completely abolished the UTP response (n = 5; Fig. 7, E and F).
Fig. 7.
Effects of P2Y6 agonist and antagonist and nonselective P2Y receptor inhibitor on PDGFRα+ cells. A: UTP induced outward currents in the presence of MRS2578 (a selective P2Y6 antagonist). B: expanded timescales showing responses to ramp potentials before (a; pretreatment with MRS2578) and after the addition of UTP (b) from points denoted in A. C: MRS2693 (a selective P2Y6 agonist) activated only small-amplitude outward currents at a HP of −40 mV in PDGFRα+ cells. D: expanded timescales of ramp depolarization from control conditions (a), with MRS2693 (b), and with UTP (c) in C. The current-voltage curves in D show that the minor response to MRS2693 was not a voltage-independent SK-like current, as activated by UTP. E: suramin (a nonselective P2Y antagonist) inhibited UTP-activated outward currents at a HP of −40 mV in PDGFRα+ cells. F: expanded timescales of ramp depolarization from control conditions (a), with UTP (b), and UTP in the presence of suramin (c) in E. Horizontal dotted lines in A–F denote 0 pA. Arrowheads denote applications of ramp potentials.
DISCUSSION
Recently, we reported that purified detrusor PDGFRα+ cells display enriched expression of P2ry1, P2ry2, P2ry4, and P2ry14 relative to unsorted cells (19). In the present study, we confirmed the enrichment in P2ry2, P2ry4, and P2ry14 expression in detrusor PDGFRα+ cells and showed no apparent compensation by up- or downregulation of expression in P2ry1−/−/eGFP mice. Expression of P2ry2, P2ry4, and P2ry14 might indicate additional roles for PDGFRα+ cells in mediating purinergic responses or different receptors might be involved in activating SK currents in PDGFRα+ cells. UTP, an agonist with affinity for P2Y2 and P2Y4 receptors, activated large outward currents in detrusor PDGFRα+ cells of wild-type and P2ry1−/−/eGFP mice. The outward currents activated by UTP were completely inhibited by SK channel blockers and an inhibitor of PLC. These data suggest that activation of SK channels via P2Y2 and/or P2Y4 receptors, coupled through the Gq/11-PLC pathway, might be used to reduce detrusor muscle excitability, possibly during bladder filling.
P2Y receptors are G protein-coupled receptors that can be activated by adenosine and uridine nucleotides (e.g., ATP, ADP, UTP, UDP, etc.) (7). These nucleotides can be released from many types of cells in response to mechanical stress, O2 deprivation, and infection via opening of pannexin 1 channels (4, 5, 8, 26). ATP and UTP activate P2Y2 receptors equally, and P2Y4 receptors are activated mainly by UTP (12). Both receptors are coupled through Gq/11 to activate PLC and increase intracellular Ca2+ via IP3 receptors (9, 22). We have previously reported the expression and functional role of P2Y1 receptors in PDGFRα+ cells using ATP as the activating nucleotide (19). In the present study, we investigated a potential role for P2Y2 and P2Y4 receptors using UTP as an agonist. Activation of P2Y2 and P2Y4 receptors has been shown to regulate many ion channels, including Ca2+-activated Cl− channels, cystic fibrosis transmembrane conductance regulator, and G protein-coupled inward rectifying K+ channels (1, 17, 23, 25). P2Y14 receptors are also expressed by detrusor PDGFRα+ cells. P2Y14 receptors are activated mainly by UDP and coupling to effectors via Gi/o (12). We have not investigated the specific role of these receptors in PDGFRα+ cells.
We reported functional expression of SK channels in detrusor PDGFRα+ cells, but low expression of these channels in SMCs results in very low current densities in these cells (18). ATP activates SK currents in detrusor PDGFRα+ cells via P2Y1 receptors (19). A parallel pathway was detected in the present study, whereby UTP activated outward currents with properties of SK currents, and the outward current was blocked by SK channel-blocking drugs. We hypothesize that Gq/11-coupled receptors lead to an increase in intracellular Ca2+ through synthesis of IP3 and release of Ca2+ from IP3 receptor-operated stores, as appears to be the case in PDGFRα+ cells in the gastrointestinal tract (3). Enhanced intracellular Ca2+ appears to be the signal that activates SK currents in PDGFRα+ cells, and this may be the common link in purine signaling via P2Y1, P2Y2, and P2Y4 receptors and activation of outward currents in detrusor PDGFRα+ cells.
UTP is not an activator of P2Y1 receptors (1, 12), but we isolated detrusor PDGFRα+ cells from P2ry1−/−/eGFP mice to confirm that SK channel activation is not dependent on P2Y1 receptors and is likely to occur through binding to other receptors, possibly P2Y2 and P2Y4, which are expressed in PDGFRα+ cells. No specific or selective P2Y2/P2Y4 agents are currently available, so we could not determine which receptor (or both) is coupled to SK currents. We also excluded the possibility that P2Y6 are involved in activating SK currents, since P2Y6 receptors are also coupled through Gq/11 (12). P2Y6 antagonist had no effect on the currents activated by UTP, and a selective P2Y6 agonist activated only a fraction of the current activated by UTP. At present, we know little about the involvement of P2Y14 receptors, which can also bind pyrimidine nucleotides. P2Y14 receptors couple through Gi/o and inhibit adenylate cyclase (12). Decreasing cAMP and subsequently PKA activity might decrease mobilization of intracellular Ca2+ via release from stores (10, 21, 28). Thus, a contribution from P2Y14 in the UTP effects on PDGFRα+ cells seems unlikely. We also found that responses to UTP responses were blocked by a PLC inhibitor. Taken together, the availability of purine receptors and pharmacological tests suggest that UTP activates SK currents via P2Y2 and/or P2Y4 receptors.
In intact bladder muscles, ATP causes transient depolarization and contraction that is followed by relaxation (19, 20). The depolarization response is likely mediated by P2X receptors expressed by SMCs (29). P2X receptors facilitate Ca2+ entry into SMCs; however, depolarization amplifies Ca2+ entry by activation of L-type Ca2+ channels (16). The transient nature of the depolarization and contraction and the subsequent development of relaxation are due, in part, to deactivation of P2X receptors and to activation of P2Y1 receptors that are coupled to SK channels (19). We suggest that the hyperpolarizing effect of activating SK currents in PDGFRα+ cells, via electrical coupling to SMCs, exerts a net hyperpolarizing influence on SMCs, decreases activation of L-type Ca2+ channels, and reduces Ca2+ entry. It has been previously shown that UTP/UDP causes relaxation of rat detrusor muscle strips (2). It is possible that release of UTP and other purines during stretch of the bladder (4) activates outward current through binding of P2Y1, P2Y2, and P2Y4. This break on excitability might be an important means of stabilizing detrusor excitability to avoid premature, nonvoiding contractions during filling. A previous study (15) supported this idea by showing that blockade of SK currents or knockout of SK channels results in the development of nonvoiding contractions during bladder filling in animal models. By discovering new receptors and pathways that can increase the open probability of SK channels in PDGFRα+ cells, it might be possible to develop new approaches for therapies for overactive bladder.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-098388. COBRE Core B was used for the FACS (P30 GM110767).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: H.L., K.M.S., and S.D.K. conception and design of research; H.L., B.H.K., E.Y., and N.E.G. performed experiments; H.L., E.Y., and S.D.K. analyzed data; H.L., K.M.S., and S.D.K. interpreted results of experiments; H.L., B.H.K., N.E.G., and S.D.K. prepared figures; H.L. and S.D.K. drafted manuscript; H.L., B.H.K., E.Y., N.E.G., K.M.S., and S.D.K. approved final version of manuscript; K.M.S. and S.D.K. edited and revised manuscript.
REFERENCES
- 1.Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev 58: 281–341, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aronsson P, Andersson M, Ericsson T, Giglio D. Assessment and characterization of purinergic contractions and relaxations in the rat urinary bladder. Basic Clin Pharmacol Toxicol 107: 603–613, 2010. [DOI] [PubMed] [Google Scholar]
- 3.Baker SA, Hennig GW, Ward SM, Sanders KM. Temporal sequence of activation of cells involved in purinergic neurotransmission in the colon. J Physiol 593: 1945–1963, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett 572: 65–68, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Bargiotas P, Krenz A, Hormuzdi SG, Ridder DA, Herb A, Barakat W, Penuela S, von Engelhardt J, Monyer H, Schwaninger M. Pannexins in ischemia-induced neurodegeneration. Proc Natl Acad Sci USA 108: 20772–20777, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burnstock G. Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol Rev 58: 58–86, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Burnstock G. Purinergic signalling–an overview. Novartis Found Symp 276: 26–57 and 275–281, 2006. [PubMed] [Google Scholar]
- 8.Chekeni FB, Elliott MR, Sandilos JK, Walk SF, Kinchen JM, Lazarowski ER, Armstrong AJ, Penuela S, Laird DW, Salvesen GS, Isakson BE, Bayliss DA, Ravichandran KS. Pannexin 1 channels mediate “find-me” signal release and membrane permeability during apoptosis. Nature 467: 863–867, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Communi D, Motte S, Boeynaems JM, Pirotton S. Pharmacological characterization of the human P2Y4 receptor. Eur J Pharmacol 317: 383–389, 1996. [DOI] [PubMed] [Google Scholar]
- 10.Conti M, Mika D, Richter W. Cyclic AMP compartments and signaling specificity: role of cyclic nucleotide phosphodiesterases. J Gen Physiol 143: 29–38, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Endoh T. Modulation of voltage-dependent calcium channels by neurotransmitters and neuropeptides in parasympathetic submandibular ganglion neurons. Arch Oral Biol 49: 539–557, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Erb L, Weisman GA. Coupling of P2Y receptors to G proteins and other signaling pathways. WIREs Membr Transp Signal 1: 789–803, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gur S, Kadowitz PJ, Hellstrom WJ. Purinergic (P2) receptor control of lower genitourinary tract function and new avenues for drug action: an overview. Curr Pharm Des 13: 3236–3244, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Herrera GM, Heppner TJ, Nelson MT. Regulation of urinary bladder smooth muscle contractions by ryanodine receptors and BK and SK channels. Am J Physiol Regul Integr Comp Physiol 279: R60–R68, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Herrera GM, Pozo MJ, Zvara P, Petkov GV, Bond CT, Adelman JP, Nelson MT. Urinary bladder instability induced by selective suppression of the murine small conductance calcium-activated potassium (SK3) channel. J Physiol 551: 893–903, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hill-Eubanks DC, Werner ME, Heppner TJ, Nelson MT. Calcium signaling in smooth muscle. Cold Spring Harb Perspect Biol 3: a004549, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kellerman D, Evans R, Mathews D, Shaffer C. Inhaled P2Y2 receptor agonists as a treatment for patients with cystic fibrosis lung disease. Adv Drug Deliv Rev 54: 1463–1474, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Lee H, Koh BH, Peri LE, Sanders KM, Koh SD. Functional expression of SK channels in murine detrusor PDGFRα+ cells. J Physiol 591: 503–513, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee H, Koh BH, Peri LE, Sanders KM, Koh SD. Purinergic inhibitory regulation of murine detrusor muscles mediated by PDGFRα+ interstitial cells. J Physiol 592: 1283–1293, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee HY, Bardini M, Burnstock G. Distribution of P2X receptors in the urinary bladder and the ureter of the rat. J Urol 163: 2002–2007, 2000. [PubMed] [Google Scholar]
- 21.Marx SO, Reiken S, Hisamatsu Y, Gaburjakova M, Gaburjakova J, Yang YM, Rosemblit N, Marks AR. Phosphorylation-dependent regulation of ryanodine receptors: a novel role for leucine/isoleucine zippers. J Cell Biol 153: 699–708, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen T, Erb L, Weisman GA, Marchese A, Heng HH, Garrad RC, George SR, Turner JT, O'Dowd BF. Cloning, expression, and chromosomal localization of the human uridine nucleotide receptor gene. J Biol Chem 270: 30845–30848, 1995. [DOI] [PubMed] [Google Scholar]
- 23.Paradiso AM, Ribeiro CM, Boucher RC. Polarized signaling via purinoceptors in normal and cystic fibrosis airway epithelia. J Gen Physiol 117: 53–67, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parajuli SP, Soder RP, Hristov KL, Petkov GV. Pharmacological activation of small conductance calcium-activated potassium channels with naphtho[1,2-d]thiazol-2-ylamine decreases guinea pig detrusor smooth muscle excitability and contractility. J Pharmacol Exp Ther 340: 114–123, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parr CE, Sullivan DM, Paradiso AM, Lazarowski ER, Burch LH, Olsen JC, Erb L, Weisman GA, Boucher RC, Turner JT. Cloning and expression of a human P2U nucleotide receptor, a target for cystic fibrosis pharmacotherapy. Proc Natl Acad Sci USA 91: 13067, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seror C, Melki MT, Subra F, Raza SQ, Bras M, Saidi H, Nardacci R, Voisin L, Paoletti A, Law F, Martins I, Amendola A, Abdul-Sater AA, Ciccosanti F, Delelis O, Niedergang F, Thierry S, Said-Sadier N, Lamaze C, Metivier D, Estaquier J, Fimia GM, Falasca L, Casetti R, Modjtahedi N, Kanellopoulos J, Mouscadet JF, Ojcius DM, Piacentini M, Gougeon ML, Kroemer G, Perfettini JL. Extracellular ATP acts on P2Y2 purinergic receptors to facilitate HIV-1 infection. J Exp Med 208: 1823–1834, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thorneloe KS, Knorn AM, Doetsch PE, Lashinger ES, Liu AX, Bond CT, Adelman JP, Nelson MT. Small-conductance, Ca2+-activated K+ channel 2 is the key functional component of SK channels in mouse urinary bladder. Am J Physiol Regul Integr Comp Physiol 294: R1737–R1743, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tovey SC, Dedos SG, Rahman T, Taylor EJ, Pantazaka E, Taylor CW. Regulation of inositol 1,4,5-trisphosphate receptors by cAMP independent of cAMP-dependent protein kinase. J Biol Chem 285: 12979–12989, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vial C, Evans RJ. P2X receptor expression in mouse urinary bladder and the requirement of P2X1 receptors for functional P2X receptor responses in the mouse urinary bladder smooth muscle. Br J Pharmacol 131: 1489–1495, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]