Abstract
Although renin is a critical regulatory enzyme of the cardiovascular system, its roles in organogenesis and the establishment of cardiovascular homeostasis remain unclear. Mammalian renin-expressing cells are widespread in embryonic kidneys but are highly restricted, specialized endocrine cells in adults. With a functional pronephros, embryonic zebrafish are ideal for delineating the developmental functions of renin-expressing cells and the mechanisms governing renin transcription. Larval zebrafish renin expression originates in the mural cells of the juxtaglomerular anterior mesenteric artery and subsequently at extrarenal sites. The role of renin was determined by assessing responses to renin-angiotensin system blockade, salinity variation, and renal perfusion ablation. Renin expression did not respond to renal flow ablation but was modulated by inhibition of angiotensin-converting enzyme and altered salinity. Our data in larval fish are consistent with conservation of renin's physiological functions. Using transgenic renin reporter fish, with mindbomb and cloche mutants, we show that Notch signaling and the endothelium are essential for developmental renin expression. After inhibition of angiogenesis, renin-expressing cells precede angiogenic sprouts. Arising from separate lineages, but relying on mutual interplay with endothelial cells, renin-expressing cells are among the earliest mural cells observed in larval fish, performing both endocrine and paracrine functions.
Keywords: renin, zebrafish, notch, endothelium, angiogenesis
renin is the rate-limiting enzyme of the renin-angiotensin system (RAS). Mammalian ANG II, the effector of the RAS, is principally involved in blood pressure homeostasis by regulation of vasomotor tone and Na+ retention. ANG II also regulates cell proliferation and angiogenesis (12, 22). Pathological activation of the RAS is associated with cardiovascular diseases, including hypertension and heart failure, and is consequently a major target of therapeutic agents (11).
Renin and its cognate cells are required for normal renal development (2), including angiogenesis of the renal vascular tree (59). In mice, ablation of the renin gene or renin-expressing cells leads to renal developmental abnormalities (55, 71, 79). The vasculature of mammalian embryonic kidneys have a transient but extensive coverage of mural renin-expressing cells (17, 31, 32, 47, 63); however, their function is unclear. Adult renin-expressing cells are restricted to the juxtaglomerular apparatus and play a vital role in ion homeostasis by secreting activated renin enzyme via cytoplasmic granules (17, 31, 36, 63). Upon physiological challenge by RAS inhibition or low Na+, prearteriolar vascular smooth muscle cells (VSMC) reestablish their embryonic renin phenotype (67), a process dependent on Notch (61) and endothelial nitric oxide (51).
The piscine renin gene (ren) was characterized in zebrafish and fugu by Liang et al. (43). Teleosts represent the taxon with the most primitive renin and RAS known (14). If functionally conserved, the fish RAS may also function in ion homeostasis and development. The zebrafish pronephros is functional from 76 hours postfertilization (hpf) (13), and recent studies in fish have suggested that larval renin (24) and the RAS (35) may be involved in ion homeostasis. Canonical ANG II-mediated Na+ uptake via aldosterone activation of the epithelial Na+ channel in the distal nephron of mammals may differ in zebrafish, which lack aldosterone synthase.
The aims of the present study were to 1) create a ren reporter transgene to characterize spatial and temporal renin expression; 2) measure transgene expression in response to RAS blockade, salinity challenge, and ablation of renal perfusion; 3) assess the positioning of renin-expressing cells before angiogenesis; and 4) address the mechanisms underlying developmental renin expression in zebrafish by determining the requirement for the endothelium and Notch signaling.
Using transgenic zebrafish and in situ mRNA analysis, we report both juxtaglomerular and extrarenal mural renin-expressing cells during development. Modulation of the ren transgene by RAS blockade confirmed the conserved role of renin within this ancient enzyme cascade. Although not affected by ablated renal perfusion, the response of ren expression to salinity variation was consistent with an endocrine function of renin-expressing cells in ion homeostasis of larval fish. After restriction of angiogenesis, ren-expressing cells were observed preceding angiogenic sprout tips. Notch signaling and an intact endothelium are essential for developmental ren expression.
METHODS
Fish lines and husbandry.
Experiments were approved by the local ethics committee and conducted in accordance with the Animals (Scientific Procedures) Act 1986 in a United Kingdom Home Office-approved establishment. Fish (Danio rerio) were maintained at 28.5°C, as previously described by Westerfield (75). Established lines used included WIK, casper (76), cloche (clom39) (70), mindbomb (mibta52b) (30), Tg(kdrl:GFP) (10), Tg(kdrl:mCherry) (58), and Tg(wt1b:GFP) (56), where GFP is green fluorescent protein. Embryos were staged according to Kimmel et al. (33) and anesthetized with 40 μg/ml tricaine methanesulfonate. Mutants were phenotypically selected as previously described (30, 70).
Generation of Tg(ren:RFP) fish.
A 6.46-kb region from the ren translational initiation site (43) to the neighboring upstream zgc:162200 gene was isolated from BAC CH73-270F23 (CHORI, Oakland, CA) using the following primer sequences with attB sites for gateway recombination into pDONR4-PIR (Invitrogen): ren forward 5′- GGGGACAACTTTGTATAGAAAAGTTGCTCGCCACACGGTTGTGAAA-3′ and ren reverse 3′-GGGGACTGCTTTTTTGTACAAACTTGCTCTCACTGATGGATCTAT-5′. The fragment was recombined upstream of red fluorescent protein (RFP) and simian virus 40 polyA by three-way gateway cloning into pDestTol2CG2 (containing minimal tol2 ends and cardiac myosin light chain:GFP) of the tol2 system (38). Plasmid DNA was coinjected with transposase mRNA. Fish with visible ren-RFP fluorescence displayed similar expression patterns.
Patent vasculature visualization.
Patent vessels were visualized in live Tg(ren:RFP) fish by injection, via the sinus venosus, of a 1% solution of FITC-dextran [relative molecular mass (Mr): 500,000, Molecular Probes] in 1× PBS. After injection and recovery, fish were imaged live within 2 h under anesthesia.
Physiological challenges.
Salinity solutions were prepared as previously described (24, 60). Captopril (C4042, Sigma-Aldrich) was administered at 0.1 mM. A dose response in conditioned water (CW) determined that a 0.1 mM captopril exposure between 24 and 96 hpf was the maximum dose for 100% viability with no overt phenotypes (data not shown). In all treatments, embryos were maintained in CW until 24 hpf, after which they were dechorinated and transferred into respective solutions in triplicate.
In situ hybridization.
In situ hybridizations (ISHs) were conducted on 0.003% phenylthiourea-treated fish using standard protocols (73). Briefly, a 500-bp digoxigenin (DIG)-labeled RNA probe was synthesised from ren cDNA. Embryos were rehydrated, permeablized, and incubated at 65°C for 16 h in hybridization buffer. After hybridization, DIG-labeled RNA probes were detected with an alkaline phosphatase-conjugated anti-DIG antibody (Roche) visualized by reaction with 5-bromo-4-chloro-3-indolylphosphate and nitroblue tetrazolium.
Hemodynamic blockade.
Blood flow was stopped at 26–28 hpf by injection of molten 1% low-melting (LMP) agarose in 1× PBS containing 0.1% FITC-dextran (Mr: 500,000) via the sinus venosus. Sham embryos were injected with the same omitting LMP agarose. Embryos were selected at 30 hpf for a confirmed lack of flow and monitored daily for flow absence until use.
VEGF receptor inhibition.
Anterior mesenteric artery (AMA) angiogenesis was inhibited with 0.2 μM axitinib (PZ0193, Sigma) in CW with 0.002% DMSO as similarly performed by Chimote et al. (8) Dechorinated embryos were axitinib treated from 24 to 60 hpf and then maintained in CW to 76 hpf. The vasculature was normal in DMSO-treated controls.
Imaging.
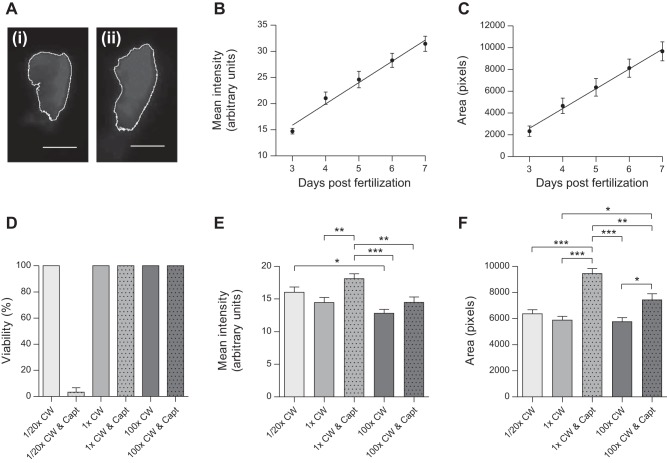
Whole mount ISHs were embedded in 3% methylcellulose and imaged using a Leica MZ16F stereomicroscope with top lighting. For confocal (×20 objective, ×2 averaging) and multiphoton (×15 objective) microscopy, anesthetized embryos were mounted in LMP agarose and imaged with a Zeiss LSM510, Leica SP5, or Olympus FV1000MPE. Optical thickness ranged between 1 and 2 μm. Huygens Professional was used for deconvolution. Maximal intensity projections were created with Fiji. For ren-RFP analysis, anesthetized embryos were imaged live in respective treatments using a Nikon Eclipse inverted compound microscope (×20 objective). Fluorescence intensity, exposure, and gain were kept constant for each experiment. Mean RFP intensity and RFP area were determined with Fiji based on a defined color threshold (see Fig. 3A).
Fig. 3.
Evaluation of ren transgene expression at the AMA during development and after physiological challenge. A: representative grayscale ren-RFP images of Tg(ren:RFP, casper) used for mean RFP intensity and RFP area analysis. The white outline was based on the set color threshold for 1× conditioned water (CW; i) and 1× CW and captopril (Capt; ii). Scale bar = 20 μm. B and C: regression analysis (n = 9) showing linear increases in both mean ren-RFP intensity (R2 = 0.7096, P < 0.0001; B) and ren-RFP area (R2 = 0.5911, P < 0.0001; C) at the AMA from 3 to 7 dpf. D: 100% viability was seen across all salinity and captopril treatments except for dilute medium (1:20× CW) with the angiotensin-converting enzyme inhibitor captopril (0.1 mM), in which viability was just 3% by 96 hpf (n = 3, P <0.0001 by ANOVA). E: by 96 hpf, mean ren-RFP intensity was significantly affected by ambient salinity and captopril treatments (n = 31–35, P < 0.0001 by ANOVA). Post hoc analysis showed that 0.1 mM captopril in the control medium (1× CW) increased mean ren-RFP intensity; this effect was not significant in high salinity. Mean ren-RFP intensity was modulated by salinity alone, resulting in significantly lower RFP expression between low and high salinities. F: the area of ren-RFP expression was also significantly affected by captopril treatment (n = 31–35, P < 0.0001 by ANOVA) in both control medium and high salinity by 96 hpf. Salinity alone did not change the area of ren-RFP expression at the AMA.
Statistics.
Statistical analyses were performed with GraphPad Prism 5 (La Jolla, CA). Linear regression was used to analyze developmental ren-RFP. Differences in mean ren-RFP intensity or ren-RFP area across salinity and captopril treatments were subjected to ANOVA and post hoc Tukey honestly significant difference tests. Values are reported as means ± SE. P values of <0.05 were considered significant.
RESULTS
Larval fish renin characterization.
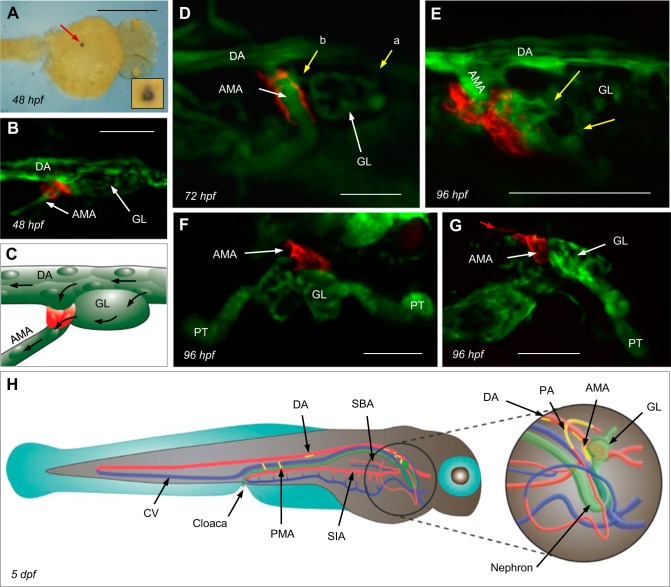
A ren:RFP transgene was created to label ren-expressing cells with RFP. Zebrafish ren mRNA is detectable by ISH at 24 hpf (43). In the same location as ren mRNA (Fig. 1A), ren-RFP was detectable at the dorsal aorta (DA) from ∼48 hpf (Fig. 1, B and C). Injected FITC-dextrans too large to pass the glomerular filtration barrier (34) delineated patent glomerular arterioles draining into the renin-expressing AMA (Fig. 1D). The AMA supplies the swim bladder arteries and the vascular plexus of the gastrointestinal tract (33) and also drains the interrenal vessel (9). The closely associated expression of endothelial kdrl-GFP (10) and ren-RFP is consistent with mural cell ren expression (Fig. 1E), as previously reported in mammals (63). Renin-expressing cells are also in contact with Wilms tumor 1b-expressing glomerular primordial cells (Fig. 1, F and G) (3, 56), as also shown by Laing et al. (43) and Hoshijima and Hirose (24) with podocin and ren ISHs.
Fig. 1.
Localization of the principal site of renin gene (ren) expression. A: dorsal view of ren mRNA within the location of the anterior mesenteric artery (AMA). The lumen is visible in the inset. Scale bar = 500 μm. hpf, hours postfertilization. B: ren-red fluorescent protein (RFP) in Tg(ren:RFP, kdrl:GFP, casper; where GFP is green fluorescent protein) becomes first visible at ∼48 hpf at the origin of the AMA, where it branches off the ventral dorsal artery (DA) immediately caudal to the glomerular primordium (GL). The exact timing of ren-RFP expression varies between individuals. Scale bar = 25 μm. C: schematic of the image in B showing renin expression (red), the AMA, DA, and GL endothelium (green), and blood flow (arrows). D: Tg(ren:RFP, casper) and FITC-dextrans showing mural ren-RFP and a patent vasculature. Afferent arterioles entering the GL (a) and an efferent arteriole draining into the AMA at 72 hpf (b) are shown. Scale bar = 50 μm. E: multiphoton projection showing details of mural cell ren-RFP and kdrl-GFP at the AMA in Tg(ren:RFP, kdrl:GFP, casper) at 96 hpf. Yellow arrows show two glomerular arterioles draining into the AMA. Scale bar = 50 μm. F and G: dorsal (F) and sagittal (G) views of Tg(ren:RFP, wt1b:GFP, casper) at 96 hpf showing juxtaglomerular ren-RFP at the AMA. At 96 hpf, faint ren-RFP expression was visible on the ventral DA immediately caudal to the AMA (red arrow). Developing proximal tubules (PTs) also express Wilms' tumor 1b (wt1b)-GFP. Scale bars = 50 μm. H: schematic showing sites of renin expression at 5 days postfertization (dpf). Mural renin cells are shown in yellow, arterial vessels are red, venous vessels are blue, and the GL, nephron, and cloaca are in green. SIA, supraintestinal artery.
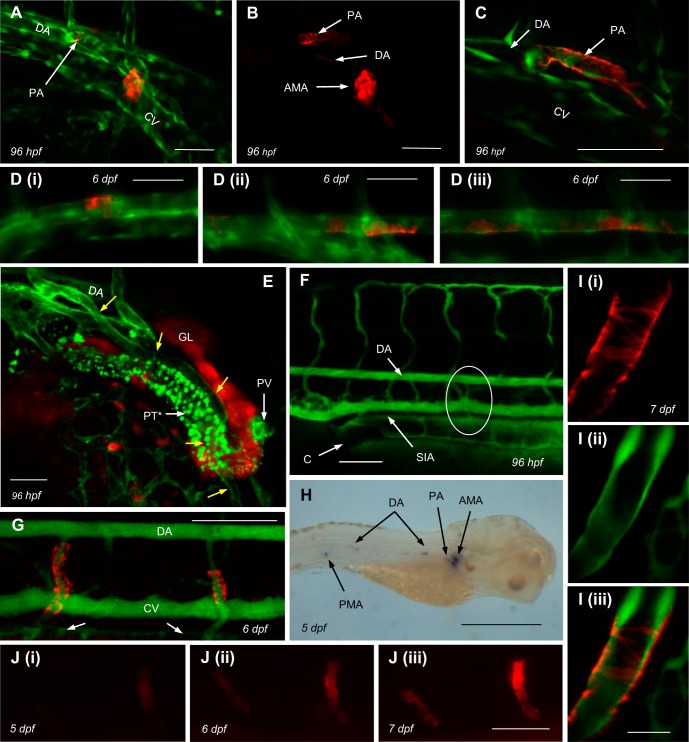
Albeit less marked than at the principal site of ren expression, the AMA, mural ren was also identified at extrarenal locations. At ∼96 hpf, RFP extended from the origins of the peritubular (Fig. 2E) pectoral arteries (PAs; Figs. 1H and 2, A–C). From ∼96 hpf, the anterior DA faintly expressed discontinuous ren (Fig. 2, A and B). From ∼5 days postfertilization (dpf), particularly on the ventral surface, intermittent RFP was also faintly visible in the mid and posterior DA (Figs. 1H and 2D). From ∼5 dpf, increasingly intense mural renin expression appeared at the posterior mesenteric arteries (PMAs), which also supply the gastrointestinal tract (Fig. 2, G–J). Expression of ren appeared at the PAs and PMAs after patency was established (Fig. 2F). ISH for ren mRNA at 48 hpf and 5 dpf confirmed the faithful recapitulation of renin expression by the ren-RFP transgene (Figs. 1A and 2H). At all locations, the onset of renin expression observed by both ren-RFP and ren ISH varied between individuals.
Fig. 2.
Extrarenal ren expression in larval fish. A: in addition to AMA renin, Tg(ren:RFP, kdrl:GFP, capser) displayed fainter ren-RFP expression at pectoral artery (PA) origins and the anterior ventral DA. B: ren-RFP channel only of the image shown in A. Scale bars = 50 μm. C: detail of mural ren-RFP extending down the PA from its branch point with the DA in Tg(ren:RFP, kdrl:GFP, capser). Scale bar = 50 μm. D: Tg(ren:RFP, kdrl:GFP, capser) showing faint ren-RFP along the anterior (i), mid (ii), and posterior (iii) DA. E: false color image [kdrl (green) wt1b (red)] of Tg(kdrl:mCherry, wt1b:GFP, capser) at 96 hpf showing the association of the PA (yellow arrows) with the PT. *mCherry channel auto-fluorescence in PT cells. The returning pectoral vein (PV) is also immediately adjacent to the PT. Scale bar = 50 μm. F: Tg(ren:RFP, capser) and injected FITC-dextrans showing the patent posterior mesenteric artery (PMA; circled) supplying blood from the DA to the SIA at 96 hpf; ren-RFP is not expressed in PMA at this age. Scale bar = 100 μm. G: Tg(ren:RFP, casper) injected with FITC-dextrans at 6 dpf showing patent PMAs with mural ren-RFP draining into the SIA (arrows pointing to SIA). Scale bar = 100 μm. H: in situ image of ren mRNA at 5 dpf showing expression at locations consistent with the positions of the AMA, PA, ventral DA, and PMA. Scale bar = 500 μm. I: 2-μm confocal section showing detail of mural ren-RFP at the PMA at 7dpf. Scale bar = 25 μm. Mural ren-RFP displayed the characteristic banding pattern (i), but no such pattern was seen in endothelial kdrl:GFP expression (ii). Merged images are shown in iii. J: consecutive images (i–iii) of increasing ren-RFP expression at the PMAs of Tg(ren:RFP, casper) between 5 and 7 dpf. Scale bar = 50 μm.
To test if ren expression in larval zebrafish increases during development, as observed in mammals (19, 49, 63), mean ren-RFP intensity (a surrogate of ren expression) and RFP area over the AMA were quantified using image analysis in Tg(ren:RFP) fish (Fig. 3A) from 3 to 7 dpf. Both RFP intensity and RFP area increased linearly (Fig. 3, B and C), demonstrating the increasing level of ren transcription and extent of mural renin expression during larval fish development.
Physiological challenge and renin expression.
In adult mammals, low Na+ or RAS inhibition by angiotensin-converting enzyme (ACE) inhibitors, such as captopril, upregulate ren mRNA and renin protein (21, 80). In combination, low salinity and captopril result in juxtaglomerular cell hypertrophy and renin cell recruitment along renal arterioles (7, 18, 36, 67). Blockade of the RAS cascade arrests the ANG II-mediated negative feedback loop regulating ren transcription and renin cell recruitment (36). Responses of the ren-RFP transgene to physiological challenge were determined at the AMA by RFP analysis. Combined captopril and dilute medium resulted in a 97% loss of viability (Fig. 3D), revealing an increased requirement for a functional RAS in low salinity. In normal salinity, consistent with RAS-mediated negative feedback on ren expression, RAS blockade increased mural cell ren-RFP expression and ren-RFP coverage at the AMA. In high salinity, the effect of captopril on ren-RFP coverage was attenuated and RFP expression was not significantly modulated (Fig. 3, E and F). The decreased ren-RFP expression observed between low and high salinities was compatible with a role for renin in ion homeostasis in larval fish (Fig. 3E). Salinity alone did not affect mural ren-RFP coverage at the AMA (Fig. 3F).
Renin expression and hemodynamic blockade.
In mammals, decreased renal perfusion increases renin expression via putative baroceptor-mediated upregulation (20, 36). In fish, hemodynamic force is required for normal organ development, including the heart (1, 25) and glomerulus (68). The effect of hemodynamic flow on renin expression was tested in larval fish using injected agar to globally arrest circulation. Despite the ability to survive up to 7 dpf without circulatory flow, assays for blood flow ablation are limited in zebrafish (25, 68). Cardiovascular flow begins at ∼24 hpf (33) and may be completely ablated by agar from 26 to 28 hpf. Fish without flow display evasive swim behavior and maintain a heartbeat up to 5 dpf. Ablation of hemodynamic flow and associated renal perfusion did not result in clear observable differences in ren-RFP expression at the AMA (Fig. 4, A and B); image analysis of RFP was not possible due to edema (Fig. 4D).
Fig. 4.
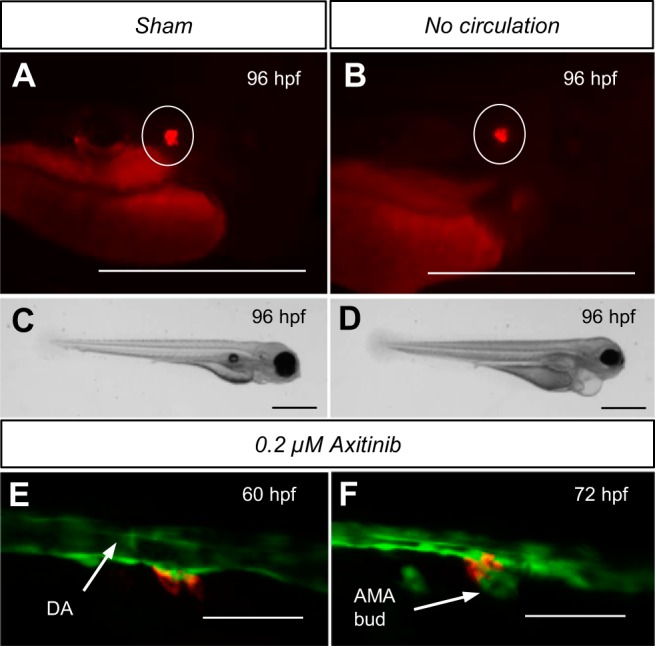
Effect of hemodynamic flow on developmental ren expression and ren association with angiogenesis. The effect of renal perfusion blockade was tested. AMA ren-RFP expression (circled) was visible in both sham-injected embryos (A) and embryos lacking hemodynamic flow (B) throughout development. RFP was also visible in no-flow embryos at 5 dpf (data not shown). Areas other than those circled are autofluorescence from the yolk sac and the gastrointestinal tract. C: sham-injected embroys appeared normal and displayed no gross phenotype. D: at 96 hpf, no-flow embryos exhibited significant pericardial and peritoneal edema. VEGF receptor inhibition by 0.2 μM axitinib from 24 to 60 hpf was used to assess renin cell location before AMA angiogenesis in Tg(ren:RFP, kdrl:GFPcasper). E: with AMA angiogenesis inhibited, ren-RFP cells were present on the ventral DA at the initiation site of AMA formation. F: after washout of axitinib at 60 hpf, AMA angiogenesis proceeded, and, by 72 hpf, mural renin cells were positioned at the AMA origin. The decreased DA diameter at 72 hpf was due to diminished hemodynamic flow. Scale bars = 50 μm.
Angiogenesis and renin-expressing cells.
Renin-expressing cells observed at branch points of the developing murine renal vasculature suggest an involvement in angiogenesis (59). Indeed, embryonic ablation of several RAS components in rodents results in renal developmental pathologies indicating the requirement of the RAS during renal development (23, 55, 66, 71). Axitinib, a highly selective inhibitor of VEGF receptors 1–3, inhibits angiogenesis in both mammals (26) and zebrafish (8, 81). Inhibition of the formation of the AMA by angiogenesis allowed detection of ren-RFP before its formation, which otherwise forms too early for detectable transgene expression. With angiogenesis inhibited, ren-expressing cells were observed at the ventral DA marking the initiation point of the preceding AMA angiogenic sprout (Fig. 4E). After axitinib washout (Fig. 4F), AMA angiogenesis proceeded and ren-expressing cells were located at the AMA origin as normal. Expression of both ren-RFP and VEGF receptor (kdrl-GFP) were strongest at the interface between the two cell types.
Notch signaling and renin expression.
Mammalian renin cells contain high levels of Notch3 and express Jagged1 and Hey1, which regulate gene transcription via the transcriptional regulator RBP-J, which binds to the renin promoter (4). Murine RPB-J knockout results in reduced renin mRNA, plasma renin, juxtaglomerular cell number, and ability to recruit renin cells after physiological challenge (61). In mice, RBP-J deletion in FOXD1 cells (mural and renin cell progenitors) causes renal developmental defects and a severe reduction in renin expression (46). The requirement of Notch signaling for ren expression in developing fish was assessed in Notch signaling-impaired mibta52b mutants, which are phenotypically the strongest of mutant mib alleles (82). The ubiquitin ligase mib is essential for the Delta-mediated activation of the transmembrane Notch receptor in target cells (29). At the 30-somite stage (∼24 hpf), Notch3 is strongly expressed in the DA but is not detectable in mib mutants (40). Neither ren-RFP nor ren mRNA were expressed in mib mutants at any stage (Fig. 5). Thus, Notch signaling is essential for embryonic and larval ren expression.
Fig. 5.
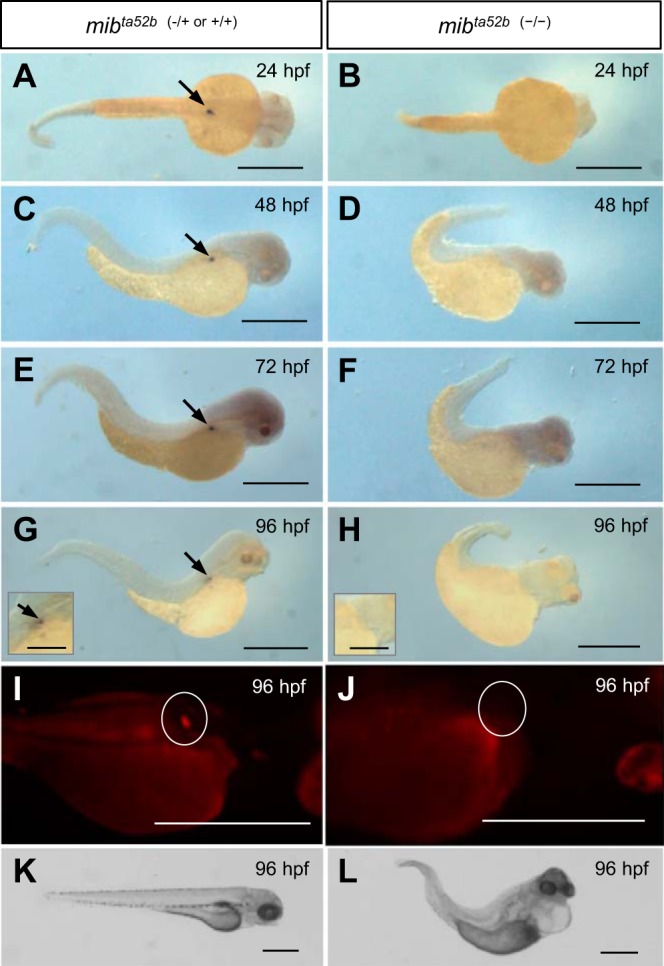
Requirement of the Notch pathway for developmental ren expression. The requirement of functional notch signaling for ren expression was tested between 24 and 96 hpf in notch-impaired mib mutants. A, C, E, and G: in situ images of “wild-type” mibta52b (−/+ or +/+) fish confirming ren expression at the AMA between 24 and 96 hpf (arrows). B, D, F, and H: ren was not detectable in mibta52b (−/−) mutants between 24 and 96 hpf. I: wild-type Tg[ren:RFP, mibta52b (−/+ or +/+)] larvae express ren-RFP (circled). J: at 96 hpf, ren-RFP was not visible in the area of the AMA (circled) in Tg[ren:RFP, mibta52b (−/−)] mutants. Areas other than those circled are autofluorescence. K: mibta52b (−/+ or +/+) larvae lacked any gross phenotype. L: at 96 hpf, mibta52b (−/−) mutants displayed pericardial edema, cerebral hemorrhage, and a malformed and pigment-free tail, and lacked blood flow. Scale bars = 500 μm (250 μm in insets).
Renin expression and the endothelium.
In mammals, renin cell positioning and control of renin expression rely on endothelial NO (51) and connexin gap junctions between the two cell types (6, 16, 37, 72), and may explain why isolated renin cells lose the ability to secrete renin (64). The effect of a lack of endothelium was tested in vivo with endothelium-deficient clom39 mutants (69). The clom39 allele includes deletion of lyscardiolipin acyltransferase, which is required for hemangioblast differentiation to hematopoietic and endothelial lineages (42, 44, 45, 70). Although ren mRNA was expressed in early stage clo mutants (Fig. 6, A–D), by 96 hpf neither ren mRNA nor ren-RFP were detectable (Fig. 6). This is consistent with an endothelial requirement by mural ren-expressing cells for maintained ren expression and confirms that the lineage of ren-expressing cells is distinct from that of the hemangioblast.
Fig. 6.
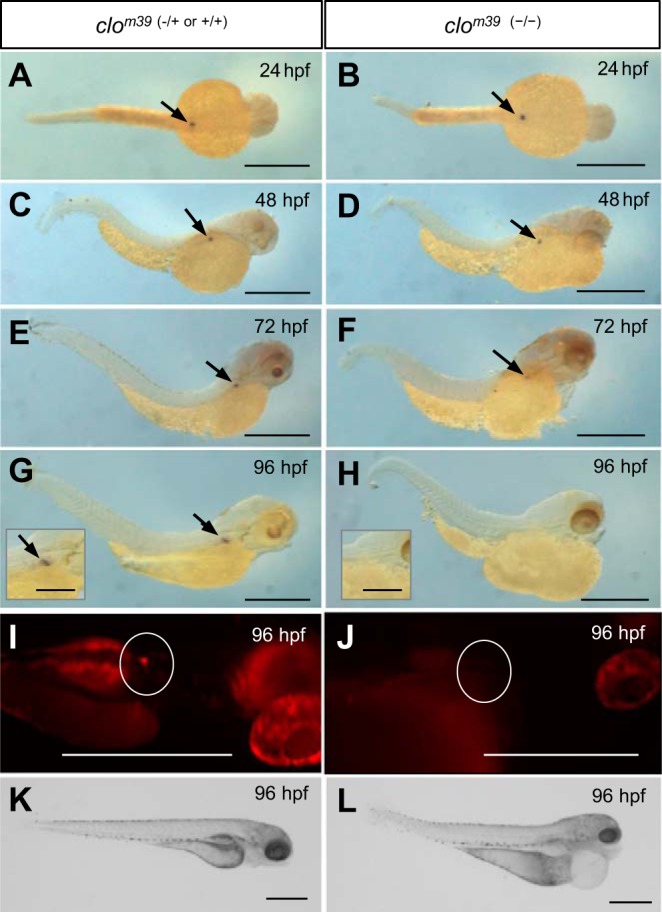
Necessity of the endothelium for maintained ren expression. The requirement of the endothelium for ren expression was tested in avascular clo mutants. A, C, E, and G: in situ images of wild-type clom39 (−/+ or +/+) fish confirming ren expression between 24 and 96 hpf (arrows). B and D: all clom39 (−/−) mutants expressed ren mRNA between 24 and 48 hpf. F: at 72 hpf, only 54% (n = 15) of clom39 (−/−) mutants expressed ren mRNA; the remaining individuals expressed varying degrees ren mRNA. H: by 96 hpf, ren mRNA was not detectable in clom39 (−/−) mutants. I: at 96 hpf, ren-RFP (circled) was visible in Tg[ren:RFP, clom39 (−/+ or +/+)] larvae. J: by 96 hpf, ren-RFP was not visible in the area of the AMA (circled) in Tg[ren:RFP, clom39 (−/−)] mutants. Areas other than those circled are autofluorescence. K: clom39 (−/+ or +/+) larvae lacked any gross phenotype. L: at 96 hpf, clom39 (−/−) mutants displayed severe peritoneal and pericardial edemas, and lacked blood flow. Scale bars = 500 μm (250 μm in insets).
DISCUSSION
The present study provides novel in vivo evidence of the distinct lineage and essential relationship between endothelial and mural ren-expressing cells. The 300-bp mammalian proximal renin promoter shows strong sequence conservation between mammalian genomes (53). Conversely, that of the zebrafish has poor sequence identity (40.8%) when aligned with mouse Ren (Vector NTI, Invitrogen). The mammalian renin promoter contains transcription factor-binding sites necessary for renin expression, including a cAMP-responsive element (52), a HOX·PBX site, and a recognition sequence for RBP-J (53); these sequences are also present in the 6.4-kb zebrafish promoter/enhancer sequence sufficient for bona fide transgene expression.
In common with mammals, (49, 63) larval zebrafish ren is expressed in mural cells of the developing vasculature. Tubuloglomerular feedback (TGF) in mammals controls glomerular flow via afferent arteriolar smooth muscle cell vasoconstriction (57). The pronephric renin-expressing cells of zebrafish are postglomerular, which suggests that tubuloglomerular feedback may not exist in larval fish. Mural cells of the AMA and DA express early VSMC markers (62, 65, 77) and the pericyte marker PDGF receptor b (pdgfrb) in the DA (74). However, these cells lack myofilaments and cytoskeletal plaques, which is consistent with undifferentiated and noncontractile cells (39, 48, 62). Due to low circulatory pressures in fish (62, 77), AMA mural cells may not directly impact on vascular tone but rather perform angiogenic and endocrine functions. It is currently unknown if the tubuloglomerular feedback mechanism is acquired in the adult mesonephric kidney.
The mammalian extrarenal RAS is characterized by low levels of renin or prorenin involving either local renin synthesis or tissue uptake from plasma (5, 54). In the murine embryo, renin is expressed in the submandibular (in males only) and adrenal glands, but its role is not clear (31). Mural ren expression at the mesenteric arteries, in conjunction with strong gastrointestinal ACE expression (Rauch et al.; direct data submission to zfin.org), suggests a requirement for the RAS by the developing gastrointestinal tract. In mammals, a local RAS in the digestive organ may play a role in Na+ and water absorption (15, 54).
The classic functions of the mammalian RAS, including VSMC contraction, aldosterone release, and renal tubular Na+ reabsorption, are mediated by the ANG II type 1 (AT1) receptor (12). A second receptor [the ANG II type 2 (AT2) receptor], proposed to modulate the actions of the AT1 receptor, is found in abundance in early fetal development, but expression falls after birth. Similarly, expression of the zebrafish AT2 receptor (agtr2), seen principally in the DA at 24 hpf, begins to diminish by 36 hpf (78). As expression of renin continues to increase after 36 hpf, the principal roles of renin/RAS in developing zebrafish, be it for ion homeostasis or other functions, may be via the AT1 rather than AT2 receptor.
The limited response of ren expression to salinity challenge is compatible with its conserved role for ion homeostasis in larval fish. Before the acquisition of a functional pronephros at 72 hpf, the RAS may be of greater importance for developmental purposes, as regulated ion uptake occurs at gill and integument ionocytes (7). Although fish lack aldosterone, acute exposure to ion-poor water or ANG II results in increased whole body Na+ and Na+/Cl− cotransporter mRNA (35). However, orthologues of the mammalian epithelial Na+ channel have yet to be identified teleosts (27, 28). Decreased renal perfusion increases renin expression via a putative baroceptor-mediated upregulation in mammals (20, 36). The lack of a ren response to flow suggests that this regulation may not occur in larval zebrafish, although it may be acquired in the mesonephric kidney.
The presence of ren-expressing cells preceding the AMA angiogenic sprout suggests a role in angiogenesis of vessels destined to harbor mural renin-expressing cells. This may explain the presence of ren at the zebrafish PA branch and the predominance of renin-expressing cells at vessel branch points in embryonic rat kidneys (59). Pericytes at angiogenic sprout tips are also observed in developing rat mesenteries (50), but their role is not understood. Renin cells may not just be involved in branching, as zebrafish PMA ren-expressing cells originate midvessel, as do many murine embryonic renin cells (63).
Our data using ISH and a ren-RFP transgene show that both the Notch pathway and the presence of endothelial cells are indispensable for developmental ren expression. As in mammals, zebrafish mural cells are thought to originate from the lateral plate mesoderm (62). Although displaying a disorganized and incompletely patent vasculature, mib mutants still generate kdrl-expressing endothelial cells (40, 41).
Zebrafish mural ren-expressing cells perform an endocrine role in cardiovascular homeostasis and may also participate in angiogenesis. The ren-RFP transgene is currently the earliest transgenic marker of mural cells in zebrafish, appearing at least as early as pdgfrb-expressing pericytes (74). During development, the RAS may not only be required by the developing kidney and its vasculature but also other organs, including the gastrointestinal tract. Arising from distinct lineages, the mutual interplay, including Notch, between ren-expressing cells and the endothelium is essential. The response of zebrafish ren to salinity variation and an ACE inhibitor widely used in human antihypertensive treatment reveals the functional conservation of the RAS. Transgenic ren reporter fish will be valuable for modeling mammalian renal physiology using drug and genetic screens relating to the renin cell phenotype, renal organogenesis, and the cardiorenal axis.
GRANTS
This work was financially supported by a British Heart Foundation Centre of Research Excellence award. The authors also acknowledge financial support from the Wellcome Trust for the zebrafish facility.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.A.R., L.J.M., R.F.V., C.A.M., and J.J.M. conception and design of research; S.A.R., L.J.M., and R.F.V. performed experiments; S.A.R., L.J.M., R.F.V., C.A.M., and J.J.M. analyzed data; S.A.R., L.J.M., R.F.V., C.A.M., and J.J.M. interpreted results of experiments; S.A.R. prepared figures; S.A.R., L.J.M., C.A.M., and J.J.M. drafted manuscript; S.A.R., L.J.M., R.F.V., C.A.M., and J.J.M. edited and revised manuscript; S.A.R., L.J.M., R.F.V., C.A.M., and J.J.M. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Prof. K. Kawakami, Dr. D. Lyons, and Dr. T. Czopka for Tol2 kit plasmids; Dr. J. Koth and C. Wilson for mutant lines; Dr. T. Gillespie and Dr. R. Wiegand for microscopy advice; Dr. C. Kenyon, Dr. M. Bailey, and Dr. C. Tucker for helpful discussions; R. Grant for assistance with illustrations; and facility staff for fish husbandry.
REFERENCES
- 1.Banjo T, Grajcarek J, Yoshino D, Osada H, Miyasaka KY, Kida YS, Ueki Y, Nagayama K, Kawakami K, Matsumoto T, Sato M, Ogura T. Haemodynamically dependent valvulogenesis of zebrafish heart is mediated by flow-dependent expression of miR-21. Nat Commun 4: 1–11, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berg AC, Chernavvsky-Sequeira C, Lindsey J, Gomez RA, Sequeira-Lopez ML. Pericytes synthesize renin. World J Nephrol 2: 11–16, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bollig F, Mehringer R, Perner B, Hartung C, Schafer M, Schartl M, Volff JN, Winkler C, Englert C. Identification and comparative expression analysis of a second wt1 gene in zebrafish. Dev Dyn 235: 554–561, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Brunskill EW, Sequeira-Lopez MLS, Pentz ES, Lin E, Yu J, Aronow BJ, Potter SS, Gomez RA. Genes that confer the identity of the renin cell. J Am Soc Nephrol 22: 2213–2225, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell DJ. Clinical relevance of local renin angiotensin systems. Front Endocrinol 5: 113–113, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castrop H, Hoecherl K, Kurtz A, Schweda F, Todorov V, Wagner C. Physiology of kidney renin. Physiol Rev 90: 607–673, 2010. [DOI] [PubMed] [Google Scholar]
- 7.Chang WJ, Wang YF, Hu HJ, Wang JH, Lee TH, Hwang PP. Compensatory regulation of Na+ absorption by Na+/H+ exchanger and Na+-Cl− cotransporter in zebrafish (Danio rerio). Front Zool 10: 1–12, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chimote G, Sreenivasan J, Pawar N, Subramanian J, Sivaramakrishnan H, Sharma S. Comparison of effects of anti-angiogenic agents in the zebrafish efficacy-toxicity model for translational anti-angiogenic drug discovery. Drug Design Dev Ther 8: 1107–1123, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiu CH, Liu YW, Takada S, Chou CW. Development and fibronectin signaling requirements of the zebrafish interrenal vessel. PLoS One 7: 1–16, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi J, Dong L, Ahn J, Dao D, Hammerschmidt M, Chen JN. FoxH1 negatively modulates flk1 gene expression and vascular formation in zebrafish. Dev Biol 304: 735–744, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohn JN. Role of the renin-angiotensin system in cardiovascular disease. Cardiovasc Drugs Ther 24: 341–344, 2010. [DOI] [PubMed] [Google Scholar]
- 12.Crowley SD, Coffman TM. Recent advances involving the renin-angiotensin system. Exp Cell Res 318: 1049–1056, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drummond I. Making a zebrafish kidney: a tale of two tubes. Trends Cell Biol 13: 357–365, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Fournier D, Luft FC, Bader M, Ganten D, Andrade-Navarro MA. Emergence and evolution of the renin-angiotensin-aldosterone system. J Mol Med 90: 495–508, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garg M, Angus PW, Burrell LM, Herath C, Gibson PR, Lubel JS. Review article: the pathophysiological roles of the renin-angiotensin system in the gastrointestinal tract. Aliment Pharmacol Ther 35: 414–428, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerl M, Voeckl J, Kurt B, van Veen TAB, Kurtz A, Wagner C. Inducible deletion of connexin 40 in adult mice causes hypertension and disrupts pressure control of renin secretion. Kidney Int 87: 557–563, 2015. [DOI] [PubMed] [Google Scholar]
- 17.Gomez RA, Belyea B, Medrano S, Pentz ES, Sequeira-Lopez ML. Fate and plasticity of renin precursors in development and disease. Pediatr Nephrol 29: 721–726, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomez RA, Lynch KR, Chevalier RL, Everett AD, Johns DW, Wilfong N, Peach MJ, Carey RM. Renin and Angiotensinogen gene-expression and intrarenal renin distribution during ACE inhibition. Am J Physiol Renal Fluid Electrolyte Physiol 254: F900–F906, 1988. [DOI] [PubMed] [Google Scholar]
- 19.Gomez RA, Pupilli C, Everett AD. Molecular and cellular aspects of renin during kidney ontogeny. Pediatr Nephrol 5: 80–87, 1991. [DOI] [PubMed] [Google Scholar]
- 20.Hackenthal E, Paul M, Ganten D, Taugner R. Morphology, physiology, and molecular-biology of renin secretion. Physiol Rev 70: 1067–1116, 1990. [DOI] [PubMed] [Google Scholar]
- 21.Harding P, Sigmon DH, Alfie ME, Huang PL, Fishman MC, Beierwaltes WH, Carretero OA. Cyclooxygenase-2 mediates increased renal renin content induced by low-sodium diet. Hypertension 29: 297–302, 1997. [DOI] [PubMed] [Google Scholar]
- 22.Herichova I, Szantoova K. Renin-angiotensin system: upgrade of recent knowledge and perspectives. Endocr Regul 47: 39–52, 2013. [DOI] [PubMed] [Google Scholar]
- 23.Hilgers KF, Reddi V, Krege JH, Smithies O, Gomez RA. Aberrant renal vascular morphology and renin expression in mutant mice lacking angiotensin-converting enzyme. Hypertension 29: 216–221, 1997. [DOI] [PubMed] [Google Scholar]
- 24.Hoshijima K, Hirose S. Expression of endocrine genes in zebrafish larvae in response to environmental salinity. J Endocrinol 193: 481–491, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Hove JR, Koster RW, Forouhar AS, Acevedo-Bolton G, Fraser SE, Gharib M. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature 421: 172–177, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Hu-Lowe DD, Zou HY, Grazzini ML, Hallin ME, Wickman GR, Amundson K, Chen JH, Rewolinski DA, Yamazaki S, Wu EY, McTigue MA, Murray BW, Kania RS, O'Connor P, Shalinsky DR, Bender SL. Nonclinical antiangiogenesis and antitumor activities of axitinib (AG-013736), an oral, potent, and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1, 2, 3. Clin Cancer Res 14: 7272–7283, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Hwang PP, Chou MY. Zebrafish as an animal model to study ion homeostasis. Pflügers Arch 465: 1233–1247, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hwang PP, Lee TH, Lin LY. Ion regulation in fish gills: recent progress in the cellular and molecular mechanisms. Am J Physiol Regul Integr Comp Physiol 301: R28–R47, 2011. [DOI] [PubMed] [Google Scholar]
- 29.Itoh M, Chitnis A. Mind bomb is a ubiquitin ligase that is essential for efficient activation of Notch signaling by Delta. FASEB J 17: A995–A995, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Jiang YJ, Brand M, Heisenberg CP, Beuchle D, Furutani-Seiki M, Kelsh RN, Warga RM, Granato M, Haffter P, Hammerschmidt M, Kane DA, Mullins MC, Odenthal J, van Eeden FJ, Nusslein-Volhard C. Mutations affecting neurogenesis and brain morphology in the zebrafish, Danio rerio. Development 123: 205–216, 1996. [DOI] [PubMed] [Google Scholar]
- 31.Jones CA, Hurley MI, Black TA, Kane CM, Pan L, Pruitt SC, Gross KW. Expression of a renin/GFP transgene in mouse embryonic, extra-embryonic, and adult tissues. Physiol Genomics 4: 75–81, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Jones CA, Sigmund CD, McGowan RA, Kanehaas CM, Gross KW. Expression of murine renin genes during fetal development. Mol Endocrinol 4: 375–383, 1990. [DOI] [PubMed] [Google Scholar]
- 33.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn 203: 253–310, 1995. [DOI] [PubMed] [Google Scholar]
- 34.Kramer-Zucker AG, Wiessner S, Jensen AM, Drummond IA. Organization of the pronephric filtration apparatus in zebrafish requires Nephrin, Podocin and the FERM domain protein Mosaic eyes. Dev Biol 285: 316–329, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumai Y, Bernier NJ, Perry SF. Angiotensin-II promotes Na+ uptake in larval zebrafish, Danio rerio, in acidic and ion-poor water. J Endocrinol 220: 195–205, 2014. [DOI] [PubMed] [Google Scholar]
- 36.Kurtz A. Renin release: sites, mechanisms, and control. Annu Rev Physiol 73: 377–399, 2011. [DOI] [PubMed] [Google Scholar]
- 37.Kurtz L, Schweda F, de Wit C, Kriz W, Witzgall R, Warth R, Sauter A, Kurtz A, Wagner C. Lack of connexin 40 causes displacement of renin-producing cells from afferent arterioles to the extraglomerular mesangium. J Am Soc Nephrol 18: 1103–1111, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Kwan KM, Fujimoto E, Grabher C, Mangum BD, Hardy ME, Campbell DS, Parant JM, Yost HJ, Kanki JP, Chien CB. The Tol2kit: A multisite Gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev Dyn 236: 3088–3099, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Lamont RE, Vu W, Carter AD, Serluca FC, MacRae CA, Childs SJ. Hedgehog signaling via angiopoietin1 is required for developmental vascular stability. Mech Dev 127: 159–168, 2010. [DOI] [PubMed] [Google Scholar]
- 40.Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, Campos-Ortega JA, Weinstein BM. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development 128: 3675–3683, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Lawson ND, Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol 248: 307–318, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Leshchiner I, Alexa K, Kelsey P, Adzhubei I, Austin-Tse CA, Cooney JD, Anderson H, King MJ, Stottmann RW, Garnaas MK, Ha S, Drummond IA, Paw BH, North TE, Beier DR, Goessling W, Sunyaev SR. Mutation mapping and identification by whole-genome sequencing. Genome Res 22: 1541–1548, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liang P, Jones CA, Bisgrove BW, Song L, Glenn ST, Yost HJ, Gross KW. Genomic characterization and expression analysis of the first nonmammalian renin genes from zebrafish and pufferfish. Physiol Genomics 16: 314–322, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Liao EC, Paw BH, Oates AC, Pratt SJ, Postlethwait JH, Zon LI. SCL/Tal-1 transcription factor acts downstream of cloche to specify hematopoietic and vascular progenitors in zebrafish. Genes Dev 12: 621–626, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liao W, Bisgrove BW, Sawyer H, Hug B, Bell B, Peters K, Grunwald DJ, Stainier DYR. The zebrafish gene cloche acts upstream of a flk-1 homologue to regulate endothelial cell differentiation. Development 124: 381–389, 1997. [DOI] [PubMed] [Google Scholar]
- 46.Lin EE, Sequeira-Lopez MLS, Gomez RA. RBP-J in FOXD1+ renal stromal progenitors is crucial for the proper development and assembly of the kidney vasculature and glomerular mesangial cells. Am J Physiol Renal Physiol 306: F249–F258, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lopez M, Pentz ES, Robert B, Abrahamson DR, Gomez RA. Embryonic origin and lineage of juxtaglomerular cells. Am J Physiol Renal Physiol 281: F345–F356, 2001. [DOI] [PubMed] [Google Scholar]
- 48.Miano JM, Georger MA, Rich A, Bentley KLDM. Ultrastructure of zebrafish dorsal aortic cells. Zebrafish 3: 455–463, 2006. [DOI] [PubMed] [Google Scholar]
- 49.Minuth M, Hackenthal E, Poulsen K, Rix E, Taugner R. Renin immunocytochemistry of the differentiating juxtaglomerular apparatus. Anat Embryol 162: 173–181, 1981. [DOI] [PubMed] [Google Scholar]
- 50.Nehls V, Denzer K, Drenckhahn D. Pericyte involvement in capillary sprouting during angiogenesis in-situ. Cell Tissue Res 270: 469–474, 1992. [DOI] [PubMed] [Google Scholar]
- 51.Neubauer B, Machura K, Kettl R, Lopez MLSS, Friebe A, Kurtz A. Endothelium-derived nitric oxide supports renin cell recruitment through the nitric oxide-sensitive guanylate cyclase pathway. Hypertension 61: 400–407, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pan L, Black TA, Shi Q, Jones CA, Petrovic N, Loudon J, Kane C, Sigmund CD, Gross KW. Critical roles of a cyclic AMP responsive element and an E-box in regulation of mouse renin gene expression. J Biol Chem 276: 45530–45538, 2001. [DOI] [PubMed] [Google Scholar]
- 53.Pan L, Gross KW. Transcriptional regulation of renin–an update. Hypertension 45: 3–8, 2005. [DOI] [PubMed] [Google Scholar]
- 54.Paul M, Mehr AP, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev 86: 747–803, 2006. [DOI] [PubMed] [Google Scholar]
- 55.Pentz ES, Moyano MA, Thornhill BA, Luisa M, Lopez SS, Gomez RA. Ablation of renin-expressing juxtaglomerular cells results in a distinct kidney phenotype. Am J Physiol Regul Integr Comp Physiol 286: R474–R483, 2004. [DOI] [PubMed] [Google Scholar]
- 56.Perner B, Englert C, Bollig F. The Wilms tumor genes wt1a and wt1b control different steps during formation of the zebrafish pronephros. Dev Biol 309: 87–96, 2007. [DOI] [PubMed] [Google Scholar]
- 57.Peti-Peterdi J. Calcium wave of tubuloglomerular feedback. Am J Physiol Renal Physiol 291: F473–F480, 2006. [DOI] [PubMed] [Google Scholar]
- 58.Proulx K, Lu A, Sumanas S. Cranial vasculature in zebrafish forms by angioblast cluster-derived angiogenesis. Dev Biol 348: 34–46, 2010. [DOI] [PubMed] [Google Scholar]
- 59.Reddi V, Zaglul A, Pentz ES, Gomez RA. Renin-expressing cells are associated with branching of the developing kidney vasculature. J Am Soc Nephrol 9: 63–71, 1998. [DOI] [PubMed] [Google Scholar]
- 60.Rider SA, Tucker CS, del-Pozo J, Rose KN, MacRae CA, Bailey MA, Mullins JJ. Techniques for the in vivo assessment of cardio-renal function in zebrafish (Danio rerio) larvae. J Physiol 590: 1803–1809, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rivera RMC, Monteagudo MC, Pentz ES, Glenn ST, Gross KW, Carretero O, Sequeira-Lopez MLS, Gomez RA. Transcriptional regulator RBP-J regulates the number and plasticity of renin cells. Physiol Genomics 43: 1021–1028, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Santoro MM, Pesce G, Stainier DY. Characterization of vascular mural cells during zebrafish development. Mech Dev 126: 638–649, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sauter A, Machura K, Neubauer B, Kurtz A, Wagner C. Development of renin expression in the mouse kidney. Kidney Int 73: 43–51, 2008. [DOI] [PubMed] [Google Scholar]
- 64.Schweda F, Friis U, Wagner C, Skott O, Kurtz A. Renin release. Physiology 22: 310–319, 2007. [DOI] [PubMed] [Google Scholar]
- 65.Seiler C, Abrams J, Pack M. Characterization of zebrafish intestinal smooth muscle development using a novel sm22 α-b promoter. Dev Dyn 239: 2806–2812, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sequeira-Lopez ML, Gomez RA. Development of the renal arterioles. J Am Soc Nephrol 22: 2156–2165, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sequeira-Lopez ML, Pentz ES, Nomasa T, Smithies O, Gomez RA. Renin cells are precursors for multiple cell types that switch to the renin phenotype when homeostasis is threatened. Dev Cell 6: 719–728, 2004. [DOI] [PubMed] [Google Scholar]
- 68.Serluca FC, Drummond IA, Fishman MC. Endothelial signaling in kidney morphogenesis: a role for hemodynamic forces. Curr Biol 12: 492–497, 2002. [DOI] [PubMed] [Google Scholar]
- 69.Stainier DY, Fouquet B, Chen JN, Warren KS, Weinstein BM, Meiler SE, Mohideen M, Neuhauss SC, SolnicaKrezel L, Schier AF, Zwartkruis F, Stemple DL, Malicki J, Driever W, Fishman MC. Mutations affecting the formation and function of the cardiovascular system in the zebrafish embryo. Development 123: 285–292, 1996. [DOI] [PubMed] [Google Scholar]
- 70.Stainier DY, Weinstein BM, Detrich HW, Zon LI, Fishman MC. Cloche, an early acting zebrafish gene, is required by both the endothelial and hematopoietic lineages. Development 121: 3141–3150, 1995. [DOI] [PubMed] [Google Scholar]
- 71.Takahashi N, Lopez M, Cowhig JE, Taylor MA, Hatada T, Riggs E, Lee G, Gomez RA, Kim HS, Smithies O. Ren1c homozygous null mice are hypotensive and polyuric, but heterozygotes are indistinguishable from wild-type. J Am Soc Nephrol 16: 125–132, 2005. [DOI] [PubMed] [Google Scholar]
- 72.Taugner R, Kirchheim H, Forssmann WG. Myoendothelial contacts in glomerular arterioles and in renal interlobular arteries of rat, mouse and Tupaia-belangeri. Cell Tissue Res 235: 319–325, 1984. [DOI] [PubMed] [Google Scholar]
- 73.Thisse C, Thisse B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protoc 3: 59–69, 2008. [DOI] [PubMed] [Google Scholar]
- 74.Wang Y, Pan L, Moens CB, Appel B. Notch3 establishes brain vascular integrity by regulating pericyte number. Development 141: 307–317, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Westerfield M. The Zebrafish Book: a Guide for the Laboratory Use of Zebrafish (Brachydanio rerio). Eugene, OR: Univ. of Oregon Press, 1995. [Google Scholar]
- 76.White RM, Sessa A, Burke C, Bowman T, LeBlanc J, Ceol C, Bourque C, Dovey M, Goessling W, Burns CE, Zon LI. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell 2: 183–189, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Whitesell TR, Kennedy RM, Carter AD, Rollins EL, Georgijevic S, Santoro MM, Childs SJ. An α-smooth muscle actin (acta2/α sma) zebrafish transgenic line marking vascular mural cells and visceral smooth muscle cells. PLos One 9: 1–10, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wong KS, Proulx K, Rost MS, Sumanas S. Identification of vasculature-specific genes by microarray analysis of Etsrp/Etv2 overexpressing zebrafish embryos. Dev Dyn 238: 1836–1850, 2009. [DOI] [PubMed] [Google Scholar]
- 79.Yanai K, Saito T, Kakinuma Y, Kon Y, Hirota K, Taniguchi-Yanai K, Nishijo N, Shigematsu Y, Horiguchi H, Kasuya Y, Sugiyama F, Yagami K, Murakami K, Fukamizu A. Renin-dependent cardiovascular functions and renin-independent blood-brain barrier functions revealed by renin-deficient mice. J Biol Chem 275: 5–8, 2000. [DOI] [PubMed] [Google Scholar]
- 80.Yang TX, Endo Y, Huang YG, Smart A, Briggs JP, Schnermann J. Renin expression in COX-2-knockout mice on normal or low-salt diets. Am J Physiol Renal Physiol 279: F819–F825, 2000. [DOI] [PubMed] [Google Scholar]
- 81.Yang X, Cui W, Yu S, Xu C, Chen G, Gu A, Li T, Cui Y, Zhang X, Bian X. A synthetic dl-nordihydroguaiaretic acid (Nordy), inhibits angiogenesis, invasion and proliferation of glioma stem cells within a zebrafish xenotransplantation model. PLos One 9: 1–9, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang C, Li Q, Lim CH, Qiu X, Jiang YJ. The characterization of zebrafish antimorphic mib alleles reveals that Mib and Mind bomb-2 (Mib2) function redundantly. Dev Biol 305: 14–27, 2007. [DOI] [PubMed] [Google Scholar]