Abstract
Proximal tubular injury and apoptosis are key mediators of the development of kidney fibrosis, a hallmark of chronic kidney disease. However, the molecular mechanism by which tubular apoptotic cell death leads to kidney fibrosis is poorly understood. In the present study, we tested the roles of Bcl-2-associated X (Bax) and Bcl-2 antagonist/killer (Bak), two crucial proteins involved in intrinsic apoptotic cell death, in the progression of kidney fibrosis. Mice with proximal tubule-specific Bax deletion, systemic deletion of Bak, and dual deletion of Bax and Bak were subjected to unilateral ureteral obstruction (UUO). Dual deficiency of Bax and Bak inhibited tubular apoptosis and atrophy. Consistent with decreased tubular injury, dual ablation of Bax and Bak suppressed UUO-induced inflammation and kidney fibrosis with decreased tubular cell cycle arrest, expression of fibrogenic and inflammatory cytokines, and oxidative stress in the kidney. Bax or Bak deficiency was insufficient to prevent apoptosis and all other aforementioned malevolent effects, suggesting compensatory mediation by each other in the respective signaling pathways. These data suggest that dual ablation of Bax and Bak in the kidney is required to prevent UUO-induced tubular apoptosis and the consequent kidney inflammation and fibrosis.
Keywords: apoptosis, chronic kidney disease, fibrosis, inflammation, oxidative stress, Bcl-2-associated X, Bcl-2 antagonist/killer
chronic kidney disease (CKD) remains as a life-threatening problem (69). The incidence of CKD along with its main causes, such as diabetes and hypertension, is increasing, resulting in mounting financial burden to the families and societies of the world (1, 64). Kidney fibrosis is a significant characteristic of CKD, leading to the loss of kidney function (64). The cellular mechanisms that lead to renal fibrosis are complex and include inflammation, oxidative stress, and proximal tubule cell apoptosis and cell cycle arrest. Although several molecules, including various cytokines [IL-13, IL-21, and transforming growth factor (TGF)-β1], as well as the renin-angiotensin-aldosterone system have been implicated in renal fibrosis (64), an effective strategy to treat kidney fibrosis remains as a major unmet medical need. Current efforts to optimize renin-angiotensin-aldosterone system blockade and control blood pressure may reduce proteinuria, a surrogate marker of renal disease, but only partially reduce the progression of CKD.
Kidney tubular injury has been recognized to be a primary cause of kidney fibrosis (32, 57, 67). In the kidney, apoptosis is a major cause of tubular cell loss, resulting in tubular atrophy and functional loss (3, 43, 47, 57). Tubular atrophy is associated with the progression of CKD, including kidney fibrosis (57). Reversible injury results in a normal repair process by intrinsic cellular mechanism and/or paracrine effect of extrarenal sources, whereas irreversible injury of tubular epithelial cell, such as tubular atrophy, triggers fibrogenic signaling (4). An abnormal repair process in injured tubular cell has been implicated in G2/M cell cycle arrest, resulting in the secretion of profibrotic cytokines by the arrested cell and the activation of a profibrotic signaling pathway (67). In tubular injury-mediated fibrosis, the inflammatory response is a critical step that is required for the initiation and progression of kidney fibrosis. Activation of the innate immune system by injured epithelial cell-secreted proinflammatory cytokines, such as IL-1, IL-6, and TNF-α, not only trigger kidney inflammation but also result in fibroblast activation (66). A number of studies have demonstrated that blockage of proinflammatory cytokines or deletion of immune cells, including macrophage, could prevent kidney fibrosis (9, 66).
Bcl-2-associated X (Bax) and Bcl-2 antagonist/killer (Bak) are proapoptotic members of the Bcl-2 family that govern mitochondrial outer membrane permeabilization to elicit apoptotic cell death (62). Several reports have demonstrated that proapoptotic proteins, including Bax and Bak, are associated with apoptotic and necrotic cell death in kidney diseases and that blockade of their functions could blunt disease progression (8, 36, 43, 63, 70). However, a direct role of Bax and Bak in kidney fibrosis, in isolation or in combination, has not been studied. In the present study, we investigated the role of Bax and Bak in kidney fibrosis and whether dual inhibition of their functions synergistically prevents unilateral ureteral obstruction (UUO)-induced tubular apoptosis and interstitial fibrosis.
MATERIALS AND METHODS
Mice and surgical preparation.
Mice were cared before and during the experimental procedures in accordance with the policies of the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center and the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. All protocols received prior approval from the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center. Baxfl/fl and Bak−/− mice (52) (Jackson Lab, Bar Harbor, ME) were crossed to generate Baxfl/flBak;−/− mice. These mice were then bred with Pepck Cre recombinase-expressing mice (a kind gift from Dr. Volker Haase, Vanderbilt University) to generate mice with proximal tubule-specific knockout (KO) of Bax and Bak (Bax-null and Bak-mice). C57BL/6J, Baxfl/fl with Pepck Cre recombinase, Bak-null, and double-KO Bax and Bak mice were anesthetized by intraperitoneal injection of a cocktail containing ketamine (200 mg/kg body wt) and xylazine (16 mg/kg body wt). The right ureter was obstructed completely near the renal pelvis using 5-0 silk, as previously described (19). Sham-operated mice underwent the same surgical procedure except for the ureter ligation. After 7 days of UUO or sham surgery, kidneys were either fixed in 4% formaldehyde for histological experiments or snap frozen in liquid nitrogen for biochemical experiments. Periodic acid-Schiff-stained sections were used to determine histological damage scores as previously described (17).
Collagen deposition.
Collagen deposition was assessed by Sirius red staining as previously described (28). Sirius red-positive areas were expressed as the ratio of the Sirius red-positive area to total area in five randomly chosen fields per kidney.
Histology and evaluation of tubular injury.
Periodic acid-Schiff-stained sections were used to evaluate tubular injury. Tubular atrophy and dilatation were expressed as the ratio of numbers of atrophied or dilated tubules to numbers of total tubules in five randomly chosen fields per kidney.
Apoptotic cell death.
A TUNEL assay on kidney sections to evaluate apoptotic cells was carried out using the In Situ Cell Death Detection kit with fluorescein (Roche, Mannheim, Germany) as previously described (16).
Immunohistochemistry and immunofluorescent staining.
Immunohistochemical staining of the kidneys was performed on paraffin-embedded sections as previously described (18, 21). Briefly, 4% paraformaldehyde-fixed kidney sections were rehydrated and labeled with antibodies against α-smooth muscle actin (α-SMA; Sigma, St. Louis, MO), polymorphonuclear neutrophil (PMN; Accurate, Westbury, NY), F4/80 (Proteintech, Chicago, IL), phosphorylated (p-)histone H3 (Santa Cruz Biotechnology, Santa Cruz, CA), p-Smad3 (Abcam, Cambridge, MA), and Ki67 (Novus Biologicals, Littleton, CO). Sections were then incubated with peroxidase- or FITC-conjugated secondary antibodies (Vector Laboratories, Burlingame, CA). 4′,6-Diamidino-2-phenylindole dihydrochloride (Sigma) or Meyer's hematoxylin (Electron Microscopy Sciences, Hatfield, PA) were used to stain nuclei. The α-SMA-positive area was measured in five randomly chosen fields per kidney using ImageJ software (NIH, Baltimore, MD). Respective numbers of PMN-, F4/80-, p-histone H3-, and Ki67-positive cells were counted in five randomly chosen fields per kidney.
Western blot analysis.
Western blot analysis was conducted as previously described (20) using various antibodies against the following proteins: connective tissue growth factor (CTGF), TGF-β1, ICAM-1, p53, and GAPDH (Santa Cruz Biotechnology), p-EGF receptor (EGFR), p-Smad3, and p-JNK (Cell Signaling), fibronectin (Cedarlane, Burlington, NC), α-SMA (Sigma), TNF-α, IL-1β, and IL-6 (Abcam), and cyclin B1, cyclin D1, and cleaved caspase-3 (Cell Signaling). GAPDH immunoblot analysis was used as a loading control on stripped membranes. Band intensities were analyzed by ImageJ software (NIH).
Measurement of lipid peroxidation.
The level of lipid hydroperoxide in the kidneys was measured using a lipid hydroperoxide assay kit (Cayman) as previously described (28).
Statistical analyses.
ANOVA was used to compare data among groups. Differences between two groups were assessed by two-tailed Student's t-test. P values of <0.05 were considered statistically significant.
RESULTS
Effect of deletion of Bax and Bak in tubular apoptosis and atrophy after UUO.
To investigate the role of the proapoptotic proteins Bax and Bak in UUO-induced kidney fibrosis, we generated mice with proximal tubule-specific deletion of Bax and/or Bak. Mice were subjected to UUO, a well-established kidney fibrosis model, for the induction of kidney fibrosis. As in our previous studies (19, 21, 24, 26), UUO induced severe tubular cell damage and death, including tubular dilatation, atrophy, apoptosis, and necrosis in the kidney (Figs. 1A and 2A). Dual deletion of Bax and Bak resulted in less TUNEL-positive apoptotic tubular epithelial cell death and expression of cleaved caspase-3 in the kidney during UUO compared with contralateral kidneys derived from wild-type and single deletion mutants of Bax or Bak (Fig. 1). Bax or Bak KO mice showed a decrease in apoptotic cell death in UUO-kidneys, but this was not significantly different compared with that in WT kidneys (Fig. 1). Similar to apoptotic cell death, dual ablation of Bax and Bak showed a decrease in atrophied tubules (Fig. 2B). Tubular necrosis, including dilation, was similar in all groups after UUO (Fig. 2C). These results indicate that dual ablation of Bax and Bak is required to prevent UUO-induced apoptotic cell death and the subsequent tubular atrophy.
Fig. 1.
Inhibition of unilateral ureteral obstruction (UUO)-induced kidney tubular apoptosis by dual ablation of Bax and Bak. Four groups of mice {wild-type (WT), Bcl-2-associated X (Bax)fl/fl;Pepck-Cre [Bax proximal tubule (PT)-specific knockout (KO)], Bcl-2 antagonist/killer (Bak)-null (Bak KO), and Baxfl/fl;Pepck Cre; Bak-null [double KO (DKO)] mice} were subjected to UUO or sham operation for 7 days. A: tubular apoptosis was evaluated by TUNEL assay. B: TUNEL-positive cells were measured in five randomly chosen fields per kidney. C: expression of cleaved caspase-3 was examined by Western blot analysis. Anti-GAPDH antibody was used as a loading control. D: Western blot band densities were evaluated using ImageJ software. Green fluorescence indicates TUNEL-positive cells. Arrow, tubular TUNEL-positive cells. CLK, contralateral kidney. Data are expressed as means ± SE; n = 3. *P < 0.05 vs. respective sham-operated mice; #P < 0.05 vs. UUO in WT mice.
Fig. 2.
Dual ablation of Bax and Bak decreases kidney tubular atrophy during UUO. Four groups of mice (WT, Bax PT-KO, Bak KO, and DKO mice) were subjected to UUO or sham operation for 7 days. A: tubular necrosis was evaluated by Periodic acid-Schiff staining. *Atrophied tubules in Periodic acid-Schiff-stained kidney sections. B and C: tubular atrophy (B) and dilatation (C) were measured in five randomly chosen fields per kidney. Data are expressed as means ± SE; n = 3. *P < 0.05 vs. respective sham-operated mice; #P < 0.05 vs. UUO in WT mice.
Dual loss of Bax and Bak inhibits UUO-induced kidney inflammation.
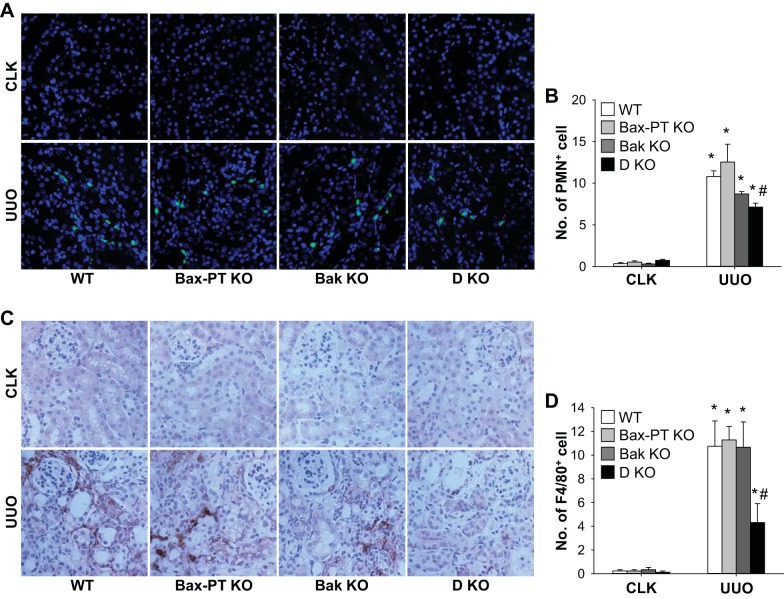
To determine the relationship between apoptotic cell death and consequent atrophy as well as the inflammation level in UUO kidneys, we examined neutrophil and macrophage recruitment into UUO kidneys. Infiltration of cells into the kidney was evaluated by counting the numbers of PMN-positive cells for neutrophils and F4/80-positive cells for macrophages. Numbers of both neutrophils and macrophages were significantly increased in the kidneys after UUO (Fig. 3). Bax or Bak ablation did not result in significant differences in the number of inflammatory cells compared with those of WT kidneys (Fig. 3). However, unlike Bax or Bak single deletion, dual ablation of Bax and Bak markedly blocked neutrophil and macrophage infiltration into UUO kidneys (Fig. 3).
Fig. 3.
Dual ablation of Bax and Bak reduces UUO-induced recruitment of neutrophils and macrophages into the kidney. Mice were subjected to UUO or sham operation for 7 days. Paraffin-embedded kidney sections were used for immunohistochemistry staining with anti-polymorphonuclear neutrophil (PMN; A) or F4/80 (C) antibodies. B and D: PMN-positive (B) and F4/80-positive (D) cells were quantified in five randomly chosen fields per kidney. Data are expressed as means ± SE; n = 3. *P < 0.05 vs. respective sham-operated mice; #P < 0.05 vs. UUO in WT mice.
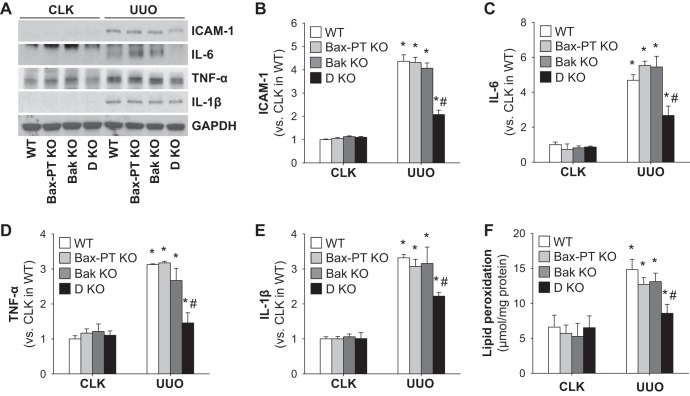
The inflammatory response was further examined by analyzing the expression of ICAM-1, a critical factor involved in the recruitment of leukocytes to injured tissue. The expression level of ICAM-1 increased proportionally to the number of neutrophils and macrophages recruited into UUO kidneys (Fig. 4, A and B). Proinflammatory cytokines (IL-6, TNF-α, and IL-1β), which could be secreted by inflammatory immune and injured tubule cells, was also upregulated in UUO kidneys of WT and Bax or Bak single deletion mice but suppressed in those of double-KO mice (Fig. 4, A and C–E). These data suggest that lowered tubular apoptotic cell death and atrophy observed in double-KO mouse kidneys after UUO may lead to decreased inflammation levels.
Fig. 4.
Dual ablation of Bax and Bak alleviates inflammatory signals and oxidative stress during UUO. Mice were subjected to UUO or sham operation for 7 days. A: expression of ICAM-1, IL-6, TNF-α, and IL-1β was examined by Western blot analysis. Anti-GAPDH antibody was used as a loading control. B–E: Western blot band densities of ICAM-1 (B), IL-6 (C), TNF-α (D), and IL-1β (E) were evaluated using ImageJ software. F: kidney oxidative stress was examined by lipid peroxidation using a lipid hydroperoxide assay. Data are expressed as means ± SE; n = 3. *P < 0.05 vs. respective sham-operated mice; #P < 0.05 vs. UUO in WT mice.
Next, to determine whether an attenuated oxidative stress level is involved in preventing the injury in dual KO of Bax and Bak, oxidative stress levels were analyzed in the different mouse groups. In line with our previous studies, UUO significantly elevated oxidative stress levels, as determined by lipid peroxidation levels (Fig. 4F). Dual KO of Bax and Bak reduced UUO-induced oxidative stress in the kidney compared with those of WT, Bax KO, or Bak KO kidneys (Fig. 4F). These data suggest that double deletion of Bax and Bak prevents increased oxidative stress from inducing kidney fibrosis, but single deletion of Bax may be compensated by Bak or vice versa and is ineffective in protecting the kidneys.
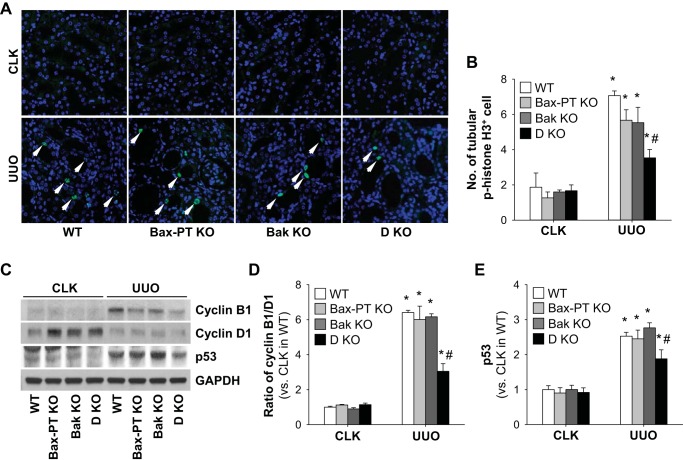
Dual deficiency of Bax and Bak alleviates UUO-induced tubular cell cycle arrest.
Proximal tubule injury in UUO kidneys is associated with loss of tubular function and tubular cell cycle arrest leading to kidney fibrosis (67). To evaluate cell cycle arrest as a function of Bax and Bak, we evaluated the number of p-histone H3-positive cells, a marker for the G2/M phase, in UUO kidneys. The number of p-histone H3-positive cells was significantly increased in WT kidneys with UUO compared with sham-operated control mouse kidneys (Fig. 5, A and B). The ratio of cyclin B1 to cyclin D1, a marker of G2/M arrest, showed a similar pattern with expression of p-histone H3 (Fig. 5, C and D). However, dual deletion of Bax and Bak prevented cell cycle arrest, as shown by fewer p-histone H3-positive cells and a decreased ratio of cyclin B1 to cyclin D1 in the kidney compared with all other groups after UUO (Fig. 5, A–D). Bax or Bak single deletion did not result in decreases of p-histone H3-positive cells and the ratio of cyclin B1 to cyclin D1 compared with those of WT kidneys (Fig. 5, A–D). Furthermore, to define the relationship between Bax and Bak as well as cell cycle arrest-related factors in UUO kidneys, we examined the level of p53 in UUO kidneys. It is known that p53, the earliest responder of DNA damage, is a key molecule regulating the cell cycle, including cell cycle arrest, as well as apoptosis (10, 33). UUO kidneys revealed an upregulation of p53 compared with control kidneys (Fig. 5, C and E). In line with G2/M arrest, the expression level of p53 decreased in dual Bax and Bak-deleted kidneys but not in Bax or Bak single-deleted UUO kidneys (Fig. 5, C and E). These data suggest that dual deletion of Bax and Bak may prevent UUO-induced tubular cell cycle arrest by regulating p53 expression.
Fig. 5.
Dual ablation of Bax and Bak blocks UUO-induced tubular cell cycle arrest. Mice were subjected to UUO or sham operation for 7 days. A: paraffin-embedded kidney sections were used for immunofluorescent staining with anti-phosporylated (p-)histone H3 antibody. B: tubular p-histone H3-positive cells were measured in five randomly chosen fields per kidney. C: expression of cyclin B1, cyclin D1, and p53 were examined by Western blot analysis. Anti-GAPDH antibody was used as a loading control. D and E: Western blot band densities of the cyclin B1-to-cyclin D1 ratio (D) and p53 (E) were evaluated using ImageJ software. Arrow, p-histone H3-positive cells. Data are expressed as means ± SE; n = 3. *P < 0.05 vs. respective sham-operated mice; #P < 0.05 vs. UUO in WT mice.
Deletion of Bax and Bak inhibits UUO-induced kidney fibrosis.
To determine whether dual ablation of Bax and Bak and the consequential decreased tubular apoptosis and inflammation indeed prevent kidney fibrosis after UUO, we evaluated the levels of extracellular matrix protein (ECM) deposition and fibrotic markers. UUO kidneys showed a significant increase of collagen deposition, as measured by Sirius red-positive areas (Fig. 6, A and B). Similarly, another ECM protein, fibronectin, was markedly enhanced in the kidney after UUO (Fig. 6, E and F). Expression of α-SMA, a marker of fibroblast activation and myofibroblast formation, was markedly increased (Fig. 6, C and D). Dual ablation of Bax and Bak significantly inhibited UUO-induced collagen and fibronectin deposition as well as the expression of α-SMA, whereas expression of these proteins in Bax or Bak KO mice did not differ from those of WT mice (Fig. 6). These data demonstrate that the reduced apoptotic cell death and inflammation in Bax- and Bak-deficient mice attenuated the expression of ECM and fibrotic proteins, resulting in inhibition of kidney fibrosis.
Fig. 6.
Ablations of both Bax and Bak prevent kidney fibrosis. Mice were subjected to UUO or sham operation for 7 days. A and C: collagen deposition (A) and α-smooth muscle actin (α-SMA)-positive areas (C) were evaluated by Sirius red and immunofluorescent staining with anti-α-SMA antibody in paraffin-embedded kidneys, respectively. B and D: Sirius red-positive (B) and α-SMA-positive (D) areas were measured in five randomly chosen fields per kidney using ImageJ software. E: fibronectin expression was examined by Western blot analysis. Anti-GAPDH antibody was used as a loading control. F: Western blot band densities were evaluated using ImageJ software. Data are expressed as means ± SE; n = 3. *P < 0.05 vs. respective sham-operated mice; #P < 0.05 vs. UUO in WT mice.
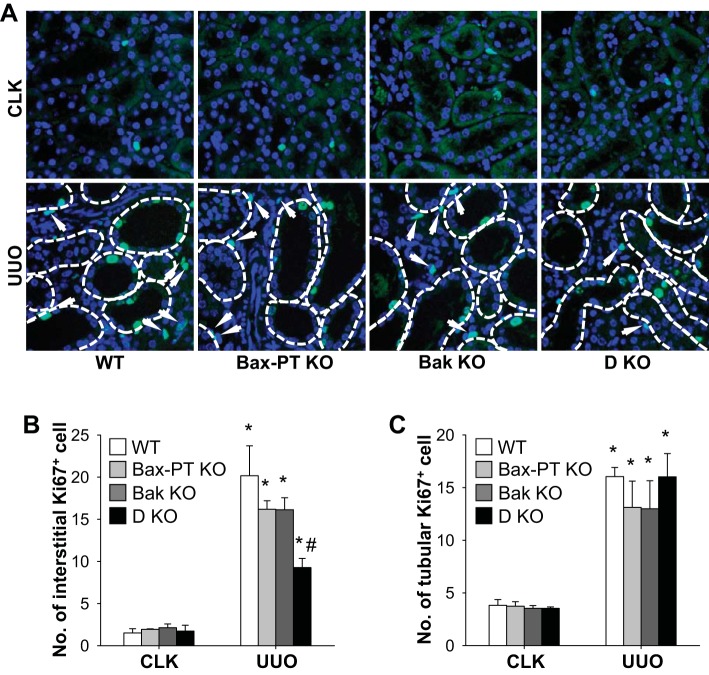
Double KO of Bax and Bak reduces fibrogenic signaling and interstitial cell proliferation in the UUO kidney.
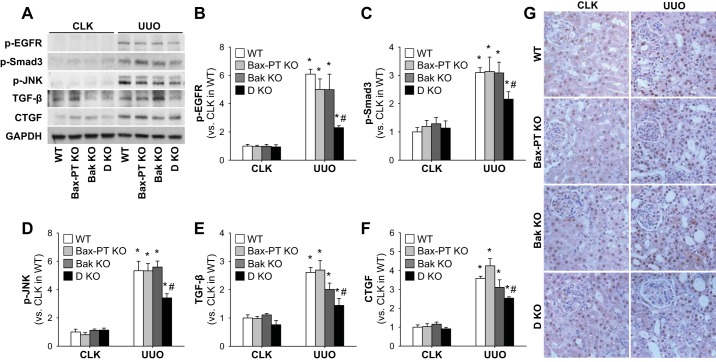
To delve into the molecular mechanism by which dual deletion of Bax and Bak prevents kidney fibrosis after UUO, we analyzed the expression and activation of key fibrogenic signals that may trigger kidney fibrosis. Since it has been recognized that activation of EGFR, Smad3, and JNK is associated with TGF-β-dependent kidney fibrosis (5, 34, 45, 67), we first examined the expression of these molecules in the mouse kidney after UUO. Expression levels of all these proteins were upregulated in UUO WT kidneys but were suppressed in dual-KO Bax and Bak kidneys (Fig. 7, A–D). Increased expression of TGF-β, a downstream signal of EGFR, coincided with the increased fibrosis in UUO kidneys. Dual ablation of Bax and Bak prevented the augmentation of TGF-β compared with other groups (Fig. 7, A and E). CTGF, which triggers kidney fibrosis in a TGF-β-independent manner (44), was also increased in UUO kidneys, but its expression level was attenuated in dual-KO Bax and Bak mice (Fig. 7, A and F). In line with the results from Western blot analysis, immunohistochemistry for p-Smad3 showed that UUO elevated the number of p-Smad3-positive cells in WT kidneys but not those of double-KO Bax and Bak kidneys (Fig. 7G). These findings indicate that dual KO of Bax and Bak may attenuate fibrosis by blocking both TGF-β-dependent and -independent mechanisms. Next, we determined the effect of double KO of Bax and Bak in interstitial and tubular cell proliferation. UUO increased the proliferation of interstitial cells (Fig. 8, A–B), which include resident fibroblasts, bone marrow-derived cells, and pericytes (31). Interstitial cell proliferation was suppressed in kidneys of mice with dual deletion of Bax and Bak compared with those of WT, Bax KO, and Bak KO kidneys after UUO (Fig. 8B). Tubular cell proliferation was not altered between WT or single-KO or dual-KO UUO kidneys (Fig. 8C). These results demonstrate that dual deficiency of Bax and Bak inhibits kidney fibrosis by suppressing fibrogenic signaling and interstitial cell proliferation.
Fig. 7.
Ablations of both Bax and Bak inhibit the expression of fibrogenic signals in UUO-induced kidney fibrosis. Mice were subjected to UUO or sham operation for 7 days. A: expression of p-EGF receptor (EGFR), p-Smad3, p-JNK, transforming growth factor (TGF)-β, and connective tissue growth factor (CTGF) was examined by Western blot analysis. Anti-GAPDH antibody was used as a loading control. B–F: Western blot band densities of p-EGFR (B), p-Smad3 (C), p-JNK (D), TGF-β (E), and CTGF (F) were evaluated using ImageJ software. G: paraffin-embedded kidney sections were used for immunohistochemistry staining with anti-p-Smad3 antibody. Data are expressed as means ± SE; n = 3. *P < 0.05 vs. respective sham-operated mice; #P < 0.05 vs. UUO in WT mice.
Fig. 8.
Dual ablation of Bax and Bak reduces UUO-induced interstitial cell proliferation in the kidney. Mice were subjected to UUO or sham operation for 7 days. A: paraffin-embedded kidney sections were used for immunofluorescent staining with anti-Ki67 antibody. B and C: interstitial (B) and tubular (C) Ki67-positive cells were measured in five randomly chosen fields per kidney. Arrow, interstitial Ki67-positive cells. Data are expressed as means ± SE; n = 3. *P < 0.05 vs. respective sham-operated mice; #P < 0.05 vs. UUO in WT mice.
DISCUSSION
The major forms of cell death in the injured tubule are apoptosis and necrosis (3, 43). During the past several decades, tubular apoptosis has been established as an important contributor to kidney fibrosis (3, 57). Although apoptosis was initially considered to be inconsequential to the development of fibrogenesis, a recent report (23) has suggested that apoptosis-related proteins are directly related with disease progression. However, the molecular mechanisms by which proapoptotic proteins and apoptotic cells may trigger fibrogenesis have not been established.
In the present study, we defined the role of apoptosis in kidney fibrosis using mice with proximal tubule-targeted deletion of Bax and systemic deletion of Bak. We showed that prevention of tubular apoptosis by dual inhibition of Bax and Bak resulted in the suppression of kidney fibrosis and inflammation in the UUO model of CKD (Fig. 9). This protective effect seems to be associated with inhibition of apoptosis-induced tubular injury and inflammation as well as cell cycle arrest. Intriguingly, single KO of Bax or Bak did not result in the prevention of apoptosis or kidney fibrosis, suggesting there is a synergy between Bax and Bak in inducing apoptotic cell death and that loss of one may be compensated by the other. Our results agree with a previous report (60) showing that mouse embryonic fibroblasts with dual deletion of Bax and Bak, but not Bax or Bak, are resistant to apoptosis when exposed to drugs inducing mitochondrial dysfunction but not to extrinsic death signals such as TNF-α plus actinomycin D. Moreover, global double KO of Bax and Bak showed less hepatocyte apoptosis and delayed mortality in mice administered agonistic antibody to Fas, by preserving mitochondrial function (60).
Fig. 9.

A scheme for the role of Bax/Bak in UUO-induced kidney fibrosis. PTC, proximal tubule cells; ECM, extracellular matrix.
Proximal tubule injury plays a major role in the initiation and progression of kidney fibrosis (4, 14, 41). Proximal tubule cell recovers completely or incompletely after injury, depending on the injury burden and duration (12, 67). Injured proximal tubule cells that fail to fully recover, due to irreversible damage, can trigger the release or activation of fibrogenic and inflammatory signals (4, 57). Recent reports (12, 48) have demonstrated that selective tubular cell injury, using transgenic mice with diphtheria toxin receptor, resulted in chronic kidney injury, including kidney fibrosis and inflammation. Grgic et al. (12) showed that single injection of diphtheria toxin allowed the injured kidney to fully recover but that repetitive administration led to kidney fibrosis, indicating that irreversible and sustained tubular damage can induce kidney fibrosis.
Tightly regulated apoptosis is beneficial in normal and mildly injured cells and organs to preserve their functions (13, 23). Moreover, proper apoptosis in the kidney could prevent tubular necrosis, thereby suppressing inflammation and subsequent fibrosis (13). However, dysregulated and excessive apoptosis could result in functional loss, leading to chronic kidney failure (25, 39, 40, 51, 57). Although it is possible that Bax/Bak may also contribute to anti-inflammatory pathways, such signaling pathways remain to be defined. In the kidney, prolonged and severe injury induce massive tubular apoptosis and subsequent tubular atrophy (47, 57). Failure of adjacent epithelial cells to replace the denuded region by dedifferentiation and proliferation leads to functional loss of the kidney (3, 57). Apoptotic cells could stimulate fibrogenesis by both direct and indirect pathways (23). Damaged tubule-secreted fibrogenic factors, such as TGF-β and CTGF, trigger the activation of fibroblasts and recruitment of immune cells into the damaged site. A recent report (30) has suggested that apoptotic endothelial cells generate CTGF production, leading to skin fibrosis. The apoptotic cell body is phagocytosed to resolve inflammation by macrophage, but it secretes profibrotic cytokines and growth factors to stimulate tissue restoration, ultimately leading to tissue fibrosis (37, 42, 50). Failed phagocytosis of the apoptotic cell body by macrophages could directly result in tissue inflammation and fibrosis (11). Indeed, Wang et al. (58) demonstrated that administered apoptotic cells labeled with fluorescent beads into the lung were phagocytosed by macrophages but still remained in the lung during several weeks and elicited lung inflammation and fibrosis via upregulation of TNF-α and TGF-β, showing direct evidence of tissue inflammation by massive apoptotic cell bodies. Furthermore, inactivation of caspase-3, which is a final cascade of both death receptor- and mitochondria-mediated apoptosis, prevents nonalcoholic steatohepatitis-associated liver fibrosis through suppression of the inflammatory response by inhibition of apoptotic cell death (55). Particularly, several cytokines seems critical factors to progress tissue fibrosis. It has been reported that IL-6 or TNF-α neutralization results in the suppression of tissue fibrosis (7, 39). Our present findings show that inhibition of apoptotic epithelial cell death by dual tubular ablation of Bax and Bak results in the suppression of UUO-induced kidney inflammation and inhibition of cell cycle arrest and fibrogenic signals to prevent kidney fibrosis.
It is well known that Bcl-2 family genes regulate cell cycle progression in tumor cells. In general, proapoptotic factors promote cell cycle progression, whereas antiapoptotic factors suppress its progression, although their roles are cell dependent (52, 71). Tubular epithelial cell cycle arrest is a critical factor associated with the progression of kidney diseases and could be triggered by both acute and chronic kidney injury and inflammation (2, 22, 65). Several markers have been used for the detection of cell cycle arrest in kidney fibrosis: of these, the levels of cell cycle regulator proteins, p53 and p21, and G2/M checkpoint proteins, p-histone H3 and checkpoint kinase, have been investigated for the identification of cell cycle-arrested cells (15, 27, 38, 68). The data presented in this study show that p-histone H3 and the ratio of cyclin B1 to cyclin D1, a marker of G2/M phase and arrest, are increased in tubular epithelial cells of UUO kidneys. Furthermore, the expression of p53 protein, which is involved in cell cycle arrest, is upregulated (56). Deletion of Bax and Bak prevented the increase in the expression of the aforementioned cell cycle proteins, suggesting that inhibition of tubular apoptosis and the consequent decreased inflammation and oxidative stress could prevent cell cycle arrest in kidney fibrosis.
Activation of JNK is another mechanism that can elicit epithelial cell cycle arrest, inflammation, and fibrosis (6, 29, 54, 67). Pharmacological inhibition of JNK blocks the secretion of fibrogenic molecules in kidney tubular cells with G2/M arrest (67). Tubular JNK activation has also been correlated with the expression of inflammatory cytokines in TGF-β- and IL-1β-treated rat tubular cells, ischemia-reperfusion injury-induced rat kidney fibrosis, and in kidney tubular epithelial cells in patients with diverse kidney diseases (6). Moreover, activation of JNK stimulates fibrogenic signals, such as TGF-β and CTGF (29). These reports support our current findings that Bax and Bak deficiency-mediated decreases of JNK activation may lead to the prevention of cell cycle arrest and inflammatory molecules, including TNF-α, IL-6, and IL-1β.
EGFR can trigger kidney fibrosis in CKD models of ischemia-reperfusion injury, UUO, and ANG II infusion (5, 34, 53). Chen et al. (5) reported that chronic ANG II infusion activates EGFR-mediated fibrogenic signaling, resulting kidney fibrosis. Consistent with these data, sustained EGFR activation in the kidney leads to tubular cell cycle arrest and tubular apoptosis, resulting in kidney fibrosis (53). Genetic or pharmacological intervention of EGFR prevented UUO-induced kidney fibrosis (34). EGFR signaling can activate TGF-β/Smad3 signaling, a well-established driving force for kidney fibrosis that potentiates fibroblast activation and ECM secretion from tubular and interstitial cells (35, 44). Furthermore, Yang and colleagues (67) showed that TGF-β is elevated in in vivo and in vitro kidney tubular cell with G2/M arrest. Our present data reveal that EGFR activation is suppressed in UUO kidneys with dual KO of Bax and Bak, along with downregulation of TGF-β. CTGF can trigger kidney fibrosis in a TGF-β-independent pathway as well as in cooperation with TGF-β (44, 59). We observed that CTGF also showed the same pattern as that of TGF-β in UUO kidneys with dual KO of Bax and Bak. Samarakoon et al. (46) reported that TGF-β can reciprocally activate EGFR resulting in the expression of profibrogenic genes, such as CTGF. Collectively, these results suggest that Bax/Bak deletion may indirectly regulate the EGFR-TGF-β-CTGF axis to induce kidney fibrogenesis.
A previous report (49) has suggested that Bax or Bak KO is sufficient to prevent apoptotic cell death in in vitro and in vivo models of injury. Wei and colleagues (61) reported that single KO of Bak or Bax protects the kidney from ischemia-reperfusion injury via inhibition of tubular apoptosis and mitochondrial fragmentation. However, mice with dual deletion of Bax and Bak in proximal tubules have not been studied in any disease models. Our current results, for the first time, show that deletion of both Bax and Bak is required to prevent kidney fibrosis through the suppression of tubular apoptotic cell death and the subsequent inflammation and cell cycle arrest. The discrepancy in apoptosis may be caused by a distinct mechanism between experimental models of severe ischemia-reperfusion injury versus UUO.
Collectively, our findings suggest that tubular epithelial apoptosis and the subsequent downstream activation of inflammatory signaling and oxidative stress can induce kidney fibrosis. Although Bax and Bak deletion did not prevent necrotic cell death in kidney fibrosis, our data show that their combined deletion prevents kidney fibrosis via the suppression of apoptosis, inflammatory, and fibrogenic signaling. The development of pharmacological agents targeting both Bax and Bak is required to prevent apoptosis, which might represent a novel strategy for the treatment of kidney fibrosis or slowing its progression.
GRANTS
B. J. Padanilam is supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-083291.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: H.-S.J. and B.J.P. conception and design of research; H.-S.J. performed experiments; H.-S.J. and B.J.P. analyzed data; H.-S.J. and B.J.P. interpreted results of experiments; H.-S.J. prepared figures; H.-S.J. drafted manuscript; H.-S.J. and B.J.P. edited and revised manuscript; H.-S.J. and B.J.P. approved final version of manuscript.
ACKNOWLEDGMENTS
Authors thank Dr. Volker H. Haase (Vanderbilt University) for providing the Pepck Cre transgenic mouse. The authors also thank Kelly E. Long and Sherry N. Westphal for mouse care.
REFERENCES
- 1.Bakris G, Vassalotti J, Ritz E, Wanner C, Stergiou G, Molitch M, Nesto R, Kaysen GA, Sowers JR; CKD Consensus Working Group. National Kidney Foundation consensus conference on cardiovascular and kidney diseases and diabetes risk: an integrated therapeutic approach to reduce events. Kidney Int 78: 726–736, 2010. [DOI] [PubMed] [Google Scholar]
- 2.Bonventre JV. Maladaptive proximal tubule repair: cell cycle arrest. Nephron Clin Pract 127: 61–64, 2014. [DOI] [PubMed] [Google Scholar]
- 3.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121: 4210–4221, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canaud G, Bonventre JV. Cell cycle arrest and the evolution of chronic kidney disease from acute kidney injury. Nephrol Dial Transplant 30: 575–583, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J, Chen JK, Nagai K, Plieth D, Tan M, Lee TC, Threadgill DW, Neilson EG, Harris RC. EGFR signaling promotes TGFβ-dependent renal fibrosis. J Am Soc Nephrol 23: 215–224, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Borst MH, Prakash J, Sandovici M, Klok PA, Hamming I, Kok RJ, Navis G, van Goor H. c-Jun NH2-terminal kinase is crucially involved in renal tubulo-interstitial inflammation. J Pharmacol Exp Ther 331: 896–905, 2009. [DOI] [PubMed] [Google Scholar]
- 7.Diaz JA, Booth AJ, Lu G, Wood SC, Pinsky DJ, Bishop DK. Critical role for IL-6 in hypertrophy and fibrosis in chronic cardiac allograft rejection. Am J Transplant 9: 1773–1783, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Docherty NG, O'Sullivan OE, Healy DA, Fitzpatrick JM, Watson RW. Evidence that inhibition of tubular cell apoptosis protects against renal damage and development of fibrosis following ureteric obstruction. Am J Physiol Renal Physiol 290: F4–F13, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Duffield JS. Macrophages and immunologic inflammation of the kidney. Semin Nephrol 30: 234–254, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elkholi R, Chipuk JE. How do I kill thee? Let me count the ways: p53 regulates PARP-1 dependent necrosis. Bioessays 36: 46–51, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elliott MR, Ravichandran KS. Clearance of apoptotic cells: implications in health and disease. J Cell Biol 189: 1059–1070, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grgic I, Campanholle G, Bijol V, Wang C, Sabbisetti VS, Ichimura T, Humphreys BD, Bonventre JV. Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int 82: 172–183, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Havasi A, Borkan SC. Apoptosis and acute kidney injury. Kidney Int 80: 29–40, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Humphreys BD, Xu F, Sabbisetti V, Grgic I, Naini SM, Wang N, Chen G, Xiao S, Patel D, Henderson JM, Ichimura T, Mou S, Soeung S, McMahon AP, Kuchroo VK, Bonventre JV. Chronic epithelial kidney injury molecule-1 expression causes murine kidney fibrosis. J Clin Invest 123: 4023–4035, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jang HS, Han SJ, Kim JI, Lee S, Lipschutz JH, Park KM. Activation of ERK accelerates repair of renal tubular epithelial cells, whereas it inhibits progression of fibrosis following ischemia/reperfusion injury. Biochim Biophys Acta 1832: 1998–2008, 2013. [DOI] [PubMed] [Google Scholar]
- 16.Jang HS, Kim J, Kim KY, Kim JI, Cho MH, Park KM. Previous ischemia and reperfusion injury results in resistance of the kidney against subsequent ischemia and reperfusion insult in mice; a role for the Akt signal pathway. Nephrol Dial Transplant 27: 3762–3770, 2012. [DOI] [PubMed] [Google Scholar]
- 17.Jang HS, Kim J, Park YK, Park KM. Infiltrated macrophages contribute to recovery after ischemic injury but not to ischemic preconditioning in kidneys. Transplantation 85: 447–455, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Jang HS, Kim JI, Han SJ, Park KM. Recruitment and subsequent proliferation of bone marrow-derived cells in the postischemic kidney are important to the progression of fibrosis. Am J Physiol Renal Physiol 306: F1451–F1461, 2014. [DOI] [PubMed] [Google Scholar]
- 19.Jang HS, Kim JI, Jung KJ, Kim J, Han KH, Park KM. Bone marrow-derived cells play a major role in kidney fibrosis via proliferation and differentiation in the infiltrated site. Biochim Biophys Acta 1832: 817–825, 2013. [DOI] [PubMed] [Google Scholar]
- 20.Jang HS, Kim JI, Kim J, Park JW, Park KM. Angiotensin II removes kidney resistance conferred by ischemic preconditioning. Biomed Res Int 2014: 602149, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jang HS, Kim JI, Noh M, Rhee MH, Park KM. Regulator of G protein signaling 2 (RGS2) deficiency accelerates the progression of kidney fibrosis. Biochim Biophys Acta 1842: 1733–1741, 2014. [DOI] [PubMed] [Google Scholar]
- 22.Jenkins RH, Davies LC, Taylor PR, Akiyama H, Cumbes B, Beltrami C, Carrington CP, Phillips AO, Bowen T, Fraser DJ. miR-192 induces G2/M growth arrest in aristolochic acid nephropathy. Am J Pathol 184: 996–1009, 2014. [DOI] [PubMed] [Google Scholar]
- 23.Johnson A, DiPietro LA. Apoptosis and angiogenesis: an evolving mechanism for fibrosis. FASEB J 27: 3893–3901, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jung KJ, Jang HS, Kim JI, Han SJ, Park JW, Park KM. Involvement of hydrogen sulfide and homocysteine transsulfuration pathway in the progression of kidney fibrosis after ureteral obstruction. Biochim Biophys Acta 1832: 1989–1997, 2013. [DOI] [PubMed] [Google Scholar]
- 25.Kiley SC, Thornhill BA, Tang SS, Ingelfinger JR, Chevalier RL. Growth factor-mediated phosphorylation of proapoptotic BAD reduces tubule cell death in vitro and in vivo. Kidney Int 63: 33–42, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Kim J, Padanilam BJ. Loss of poly(ADP-ribose) polymerase 1 attenuates renal fibrosis and inflammation during unilateral ureteral obstruction. Am J Physiol Renal Physiol 301: F450–F459, 2011. [DOI] [PubMed] [Google Scholar]
- 27.Kim J, Padanilam BJ. Renal denervation prevents long-term sequelae of ischemic renal injury. Kidney Int 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim J, Padanilam BJ. Renal nerves drive interstitial fibrogenesis in obstructive nephropathy. J Am Soc Nephrol 24: 229–242, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lan R, Geng H, Polichnowski AJ, Singha PK, Saikumar P, McEwen DG, Griffin KA, Koesters R, Weinberg JM, Bidani AK, Kriz W, Venkatachalam MA. PTEN loss defines a TGF-β-induced tubule phenotype of failed differentiation and JNK signaling during renal fibrosis. Am J Physiol Renal Physiol 302: F1210–F1223, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laplante P, Sirois I, Raymond MA, Kokta V, Beliveau A, Prat A, Pshezhetsky AV, Hebert MJ. Caspase-3-mediated secretion of connective tissue growth factor by apoptotic endothelial cells promotes fibrosis. Cell Death Differ 17: 291–303, 2010. [DOI] [PubMed] [Google Scholar]
- 31.LeBleu VS, Taduri G, O'Connell J, Teng Y, Cooke VG, Woda C, Sugimoto H, Kalluri R. Origin and function of myofibroblasts in kidney fibrosis. Nat Med 19: 1047–1053, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leung KC, Tonelli M, James MT. Chronic kidney disease following acute kidney injury-risk and outcomes. Nat Rev Nephrol 9: 77–85, 2013. [DOI] [PubMed] [Google Scholar]
- 33.Liu B, Bhatt D, Oltvai ZN, Greenberger JS, Bahar I. Significance of p53 dynamics in regulating apoptosis in response to ionizing radiation, and polypharmacological strategies. Sci Rep 4: 6245, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu N, Guo JK, Pang M, Tolbert E, Ponnusamy M, Gong R, Bayliss G, Dworkin LD, Yan H, Zhuang S. Genetic or pharmacologic blockade of EGFR inhibits renal fibrosis. J Am Soc Nephrol 23: 854–867, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loeffler I, Wolf G. Transforming growth factor-β and the progression of renal disease. Nephrol Dial Transplant 29, Suppl 1: i37–i45, 2014. [DOI] [PubMed] [Google Scholar]
- 36.Mao H, Li Z, Zhou Y, Li Z, Zhuang S, An X, Zhang B, Chen W, Nie J, Wang Z, Borkan SC, Wang Y, Yu X. HSP72 attenuates renal tubular cell apoptosis and interstitial fibrosis in obstructive nephropathy. Am J Physiol Renal Physiol 295: F202–F214, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mathai SK, Gulati M, Peng X, Russell TR, Shaw AC, Rubinowitz AN, Murray LA, Siner JM, Antin-Ozerkis DE, Montgomery RR, Reilkoff RA, Bucala RJ, Herzog EL. Circulating monocytes from systemic sclerosis patients with interstitial lung disease show an enhanced profibrotic phenotype. Lab Invest 90: 812–823, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Megyesi J, Tarcsafalvi A, Li S, Hodeify R, Seng NS, Portilla D, Price PM. Increased expression of p21WAF1/CIP1 in kidney proximal tubules mediates fibrosis. Am J Physiol Renal Physiol 308: F122–F130, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meldrum KK, Misseri R, Metcalfe P, Dinarello CA, Hile KL, Meldrum DR. TNF-α neutralization ameliorates obstruction-induced renal fibrosis and dysfunction. Am J Physiol Regul Integr Comp Physiol 292: R1456–R1464, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Miyajima A, Chen J, Lawrence C, Ledbetter S, Soslow RA, Stern J, Jha S, Pigato J, Lemer ML, Poppas DP, Vaughan ED, Felsen D. Antibody to transforming growth factor-β ameliorates tubular apoptosis in unilateral ureteral obstruction. Kidney Int 58: 2301–2313, 2000. [DOI] [PubMed] [Google Scholar]
- 41.Moll S, Ebeling M, Weibel F, Farina A, Araujo Del Rosario A, Hoflack JC, Pomposiello S, Prunotto M. Epithelial cells as active player in fibrosis: findings from an in vitro model. PLoS One 8: e56575, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murray LA, Chen Q, Kramer MS, Hesson DP, Argentieri RL, Peng X, Gulati M, Homer RJ, Russell T, van Rooijen N, Elias JA, Hogaboam CM, Herzog EL. TGF-β driven lung fibrosis is macrophage dependent and blocked by serum amyloid P. Int J Biochem Cell Biol 43: 154–162, 2011. [DOI] [PubMed] [Google Scholar]
- 43.Padanilam BJ. Cell death induced by acute renal injury: a perspective on the contributions of apoptosis and necrosis. Am J Physiol Renal Physiol 284: F608–F627, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Qi W, Chen X, Poronnik P, Pollock CA. Transforming growth factor-β/connective tissue growth factor axis in the kidney. Int J Biochem Cell Biol 40: 9–13, 2008. [DOI] [PubMed] [Google Scholar]
- 45.Qin W, Chung AC, Huang XR, Meng XM, Hui DS, Yu CM, Sung JJ, Lan HY. TGF-β/Smad3 signaling promotes renal fibrosis by inhibiting miR-29. J Am Soc Nephrol 22: 1462–1474, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Samarakoon R, Overstreet JM, Higgins PJ. TGF-β signaling in tissue fibrosis: redox controls, target genes and therapeutic opportunities. Cell Signal 25: 264–268, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schelling JR, Cleveland RP. Involvement of Fas-dependent apoptosis in renal tubular epithelial cell deletion in chronic renal failure. Kidney Int 56: 1313–1316, 1999. [DOI] [PubMed] [Google Scholar]
- 48.Sekine M, Monkawa T, Morizane R, Matsuoka K, Taya C, Akita Y, Joh K, Itoh H, Hayashi M, Kikkawa Y, Kohno K, Suzuki A, Yonekawa H. Selective depletion of mouse kidney proximal straight tubule cells causes acute kidney injury. Transgenic Res 21: 51–62, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shamas-Din A, Kale J, Leber B, Andrews DW. Mechanisms of action of Bcl-2 family proteins. Cold Spring Harb Perspect Biol 5: a008714, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stout RD, Jiang C, Matta B, Tietzel I, Watkins SK, Suttles J. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J Immunol 175: 342–349, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Sunami R, Sugiyama H, Wang DH, Kobayashi M, Maeshima Y, Yamasaki Y, Masuoka N, Ogawa N, Kira S, Makino H. Acatalasemia sensitizes renal tubular epithelial cells to apoptosis and exacerbates renal fibrosis after unilateral ureteral obstruction. Am J Physiol Renal Physiol 286: F1030–F1038, 2004. [DOI] [PubMed] [Google Scholar]
- 52.Takeuchi O, Fisher J, Suh H, Harada H, Malynn BA, Korsmeyer SJ. Essential role of BAX,BAK in B cell homeostasis and prevention of autoimmune disease. Proc Natl Acad Sci USA 102: 11272–11277, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang J, Liu N, Tolbert E, Ponnusamy M, Ma L, Gong R, Bayliss G, Yan H, Zhuang S. Sustained activation of EGFR triggers renal fibrogenesis after acute kidney injury. Am J Pathol 183: 160–172, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Terzi F, Burtin M, Hekmati M, Federici P, Grimber G, Briand P, Friedlander G. Targeted expression of a dominant-negative EGF-R in the kidney reduces tubulo-interstitial lesions after renal injury. J Clin Invest 106: 225–234, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thapaliya S, Wree A, Povero D, Inzaugarat ME, Berk M, Dixon L, Papouchado BG, Feldstein AE. Caspase 3 inactivation protects against hepatic cell death and ameliorates fibrogenesis in a diet-induced NASH model. Dig Dis Sci 59: 1197–1206, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Valenzuela MT, Guerrero R, Nunez MI, Ruiz De Almodovar JM, Sarker M, de Murcia G, Oliver FJ. PARP-1 modifies the effectiveness of p53-mediated DNA damage response. Oncogene 21: 1108–1116, 2002. [DOI] [PubMed] [Google Scholar]
- 57.Venkatachalam MA, Griffin KA, Lan R, Geng H, Saikumar P, Bidani AK. Acute kidney injury: a springboard for progression in chronic kidney disease. Am J Physiol Renal Physiol 298: F1078–F1094, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang L, Scabilloni JF, Antonini JM, Rojanasakul Y, Castranova V, Mercer RR. Induction of secondary apoptosis, inflammation, and lung fibrosis after intratracheal instillation of apoptotic cells in rats. Am J Physiol Lung Cell Mol Physiol 290: L695–L702, 2006. [DOI] [PubMed] [Google Scholar]
- 59.Wang Q, Usinger W, Nichols B, Gray J, Xu L, Seeley TW, Brenner M, Guo G, Zhang W, Oliver N, Lin A, Yeowell D. Cooperative interaction of CTGF and TGF-β in animal models of fibrotic disease. Fibrogenesis Tissue Repair 4: 4, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292: 727–730, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wei Q, Dong G, Chen JK, Ramesh G, Dong Z. Bax and Bak have critical roles in ischemic acute kidney injury in global and proximal tubule-specific knockout mouse models. Kidney Int 84: 138–148, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Westphal D, Dewson G, Czabotar PE, Kluck RM. Molecular biology of Bax and Bak activation and action. Biochim Biophys Acta 1813: 521–531, 2011. [DOI] [PubMed] [Google Scholar]
- 63.Whelan RS, Konstantinidis K, Wei AC, Chen Y, Reyna DE, Jha S, Yang Y, Calvert JW, Lindsten T, Thompson CB, Crow MT, Gavathiotis E, Dorn GW 2nd, O'Rourke B, Kitsis RN. Bax regulates primary necrosis through mitochondrial dynamics. Proc Natl Acad Sci USA 109: 6566–6571, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol 214: 199–210, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wynn TA. Fibrosis under arrest. Nat Med 16: 523–525, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med 18: 1028–1040, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med 16: page after 143, 531, and 535–543, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ying Y, Kim J, Westphal SN, Long KE, Padanilam BJ. Targeted deletion of p53 in the proximal tubule prevents ischemic renal injury. J Am Soc Nephrol 25: 2707–2716, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zeisberg M, Neilson EG. Mechanisms of tubulointerstitial fibrosis. J Am Soc Nephrol 21: 1819–1834, 2010. [DOI] [PubMed] [Google Scholar]
- 70.Zhang G, Oldroyd SD, Huang LH, Yang B, Li Y, Ye R, El Nahas AM. Role of apoptosis and Bcl-2/Bax in the development of tubulointerstitial fibrosis during experimental obstructive nephropathy. Exp Nephrol 9: 71–80, 2001. [DOI] [PubMed] [Google Scholar]
- 71.Zinkel S, Gross A, Yang E. BCL2 family in DNA damage and cell cycle control. Cell Death Differ 13: 1351–1359, 2006. [DOI] [PubMed] [Google Scholar]