Abstract
Background
Laparoscopic surgery is being increasingly offered to the older person.
Objective
To systematically review the literature regarding laparoscopic colorectal cancer surgery in older people and compare to younger adult populations.
Study selection
We included randomized controlled trials that compared open to laparoscopic colorectal cancer surgery. Older people were defined as being 65 years and above.
Outcome measures
Overall survival and post-operative morbidity and mortality. Secondary endpoints were length of hospital stay, wound recurrence, disease-free survival and conversion rate.
Results
Seven trials included older people, average age of approximately 70 years. Two reported data specific to older patients (over 70 years): The ALCCaS study reported reduced length of stay and short-term complication rates in the laparoscopic group when compared to open surgery (8 versus 10 days, and 36.7% versus 50.6% respectively) and the CLASICC study reported equivalent 5 year survival between arms and a reduction of 2 days length of stay following laparoscopic surgery in older people. In trials which considered data on older and younger participants all five trials reported comparable overall survival and showed comparable or reduced complication rates; two demonstrated significantly shorter length of stay following laparoscopic surgery compared to open surgery.
Conclusion
Large numbers of older people have been included in well-conducted, multi-centre, randomized controlled trials for laparoscopic and open colorectal cancer surgery. This systematic review suggests that age itself should not be a factor when considering the best surgical option for older patients.
Keywords: Systematic review, Older people, Laparoscopic surgery
Highlights
-
•
Seven well conducted randomised controlled trials of open versus laparoscopic colorectal cancer surgery have included older people.
-
•
Age alone should not be a barrier to laparoscopic colorectal cancer surgery.
-
•
The effect of comorbidity in older people undergoing laparoscopic surgery is less clear and warrants further study.
1. Introduction
1.1. Background
As the population ages, a greater number of older people are presenting with colorectal cancer requiring surgical resection. Over the past 20 years laparoscopic surgery for colorectal cancer has become an increasingly common surgical option. This evidence base has been developed via a series of increasingly large and well-conducted studies, reinforced by numerous meta-analyses [1–3]. The majority of these studies were conducted in much younger populations [4]. Whether older people undergoing surgery present the same challenges as younger people is not known. Factors such as impaired wound healing, restrictions on mobility, frailty, sarcopenia, multi-morbidity and poly-pharmacy may influence the outcome of surgery. Whether laparoscopic surgery in the older person confers the same safety profile and benefit as the younger person has not been widely explored.
Our objectives were to determine the outcomes of laparoscopic surgery in comparison to open surgery and to systematically review the evidence base on which laparoscopic surgery is being offered to older people.
2. Methods
2.1. Systematic literature search
In December 2014, we systematically searched the literature and electronic databases (MEDLINE, Embase and Cochrane Library) using the following search terms as key words; colon, colorectal, rectum, sigmoid, laparoscopic, open, older person, elderly, neoplasm, cancer, tumour and malignant. We did not apply any language restrictions. We hand searched the reference lists of all selected trials and contacted trial authors. The full search strategy is given in appendix 1.
2.2. Study design and participants
All randomized controlled trials (RCT) that compared open versus laparoscopic surgery for colorectal cancer were included. Our focus was on people aged 65 years and above and we excluded any trial that specified an upper age limit. We excluded any trial that included less than 100 participants in either randomized arm and trials published before 2000. The study was registered at the research registry, UIN 305.
2.3. Primary endpoints
Overall survival, post-operative mortality and morbidity.
2.4. Secondary endpoints
Length of hospital stay, port (or wound) site recurrence and conversion from laparoscopic to open surgery rate.
2.5. Data extraction
Two authors performed the literature search (SM and JCB) and three authors independently reviewed the articles for suitability and extracted the preselected endpoints (JH, MS and KM). Two authors (SM and JH) independently reviewed the studies to assign a Jadad [5] score to help assess the quality of the selected studies. Disputes were settled by mutual consent between all authors.
3. Results
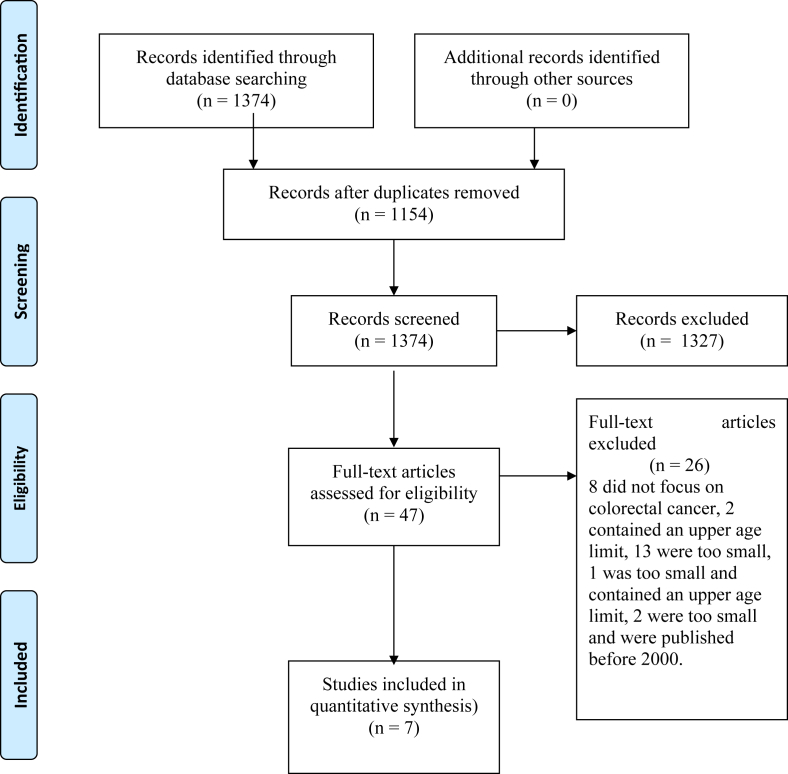
We identified 1374 studies, 210 duplicates were removed. The remaining reviewed and the abstracts assessed. Following this 47 full papers were obtained. Of these 26 were excluded (no focus on colorectal cancer, contained less than 100 per randomisation arm, were published before 2000 or included an upper age limit). This left a total of 21 papers, derived from 7 study groups. The details are shown in the PRISMA diagram, Fig. 1[6]. The included trials and baseline characteristics are listed in Table 1.
Fig. 1.
Flow diagram of systematic review of laparoscopic versus open surgery for colorectal cancer, including the reasons for exclusion.
Table 1.
Description of randomised controlled trials comparing laparoscopic versus open colorectal surgery.
| Date randomised | Date published | Number of centres | Largest participants number | |
|---|---|---|---|---|
| ALCCaS | January 1998 | 2000; 2012 | 31 | 592 |
| CLASICC | July 1996 | 2005; 2007; 2010; 2013 | 27 | 794 |
| Leung KL et al. | September 1993 | 2004 | 2 | 403 |
| Lacy AM et al. | November 1993 | 2002 | 1 | 219 |
| COST | August 1994 | 2002; 2004; 2007 | 48 | 872 |
| COLOR I | March 1997 | 2005, 2009 | 30 | 1076 |
| COLOR II | January 2004 | 2013 | 30 | 1044 |
| Braga M et al. | February 2000 | 2002; 2004; 2005 (a,b); 2007 (a,b); 2010 | 1 | 391 |
Summary: only 7 different trial group/s.
3.1. Description of the included studies
Each trial reported age differently with the average age of all included trial arms being approximately 70 years old (Table 2). Three included nonagenarians and one participant aged over 100 years old. Two studies published specific data on the older person [7,8].
Table 2.
Distribution of participants' age for the selected RCTs.
| Laparoscopic arm | Open arm | Sub-analysis on age | |
|---|---|---|---|
| ALCCaS | 71.1 (35.9–94.2) | 69.4 (34.3–100.1) | Yes |
| CLASICC | 69 (11) | 69 (11) | Yes |
| Leung KL et al. | 67.1 (11.7) | 66.5 (12.3) | No |
| Lacy AM et al. | 68 (12) | 71 (11) | No |
| COST | a70 (28–96) | a69 (29–94) | No |
| COLOR I | 71 (27–92) | 71 (31–95) | No |
| COLOR II | 66.8 (34–93) | 65.8 (31–90) | No |
| Braga M et al. | 63.7 (13.8) | 65.1 (12.6) | No |
All ages are mean ± SD or range as appropriate.
Median age quoted.
The outcomes from the included studies are discussed individually in detail below (short-term outcomes Table 3; long-term outcomes Table 4).
Table 3.
Short-term outcomes after surgery in selected RCTs.
| Laparoscopic arm | Open arm | P value | ||
|---|---|---|---|---|
| Conversion rate (%) | ALCCaS | 14.6% | ||
| CLASICC | 29% | |||
| Leung KL et al. | 23.2% | |||
| Lacy AM et al. | Not documented | |||
| COST | 21% | |||
| COLOR I | 17% | |||
| COLOR II | 16% | |||
| Braga M et al. | 5.1% | |||
| Length of hospital stay (days) | ALCCaS | 7 (1–55) | 8 (4–59) | 0.0001 |
| CLASICC | 9 (7–13)* | 11 (8–15)* | n/a | |
| Leung KL et al. | 8.2 (2–99) | 8.7 (3–39) | >0.05 | |
| Lacy AM et al. | 5.2 (2.1)* | 7.9 (9.3)* | 0.005 | |
| COST | 5.6 (0.26) | 6.4 (0.23) | <0.001 | |
| COLOR I | 8.2 (6.6)* | 9.3 (7.3)* | <0.0001 | |
| COLOR II | 8 (6–13) | 9 (7–14) | 0.036 | |
| Braga M et al. | 9.4 (4) | 12.7 (5–29) | 0.002 | |
| Post-operative morbidity (%)ˆ | ALCCaS | 37.8% | 45.3% | 0.062 |
| CLASICC | 29% | 31% | 0.78 | |
| Leung KL et al. | 24% | 26% | <0.001 | |
| Lacy AM et al. | 11% | 28.7% | 0.001 | |
| COST | 20% | 21% | 0.64 | |
| COLOR I | 21% | 20% | 0.90 | |
| COLOR II | 40% | 37% | 0.424 | |
| Braga M et al. | 20.6% | 38.3% | 0.003 | |
| 28 Day post-operative mortality (%)¶ | ALCCaS (in hospital death) | 1.4% | 0.7% | 0.448 |
| CLASICC | 4% | 5% | 0.57 | |
| Leung KL et al.** | 0.6% | 2.4% | 0.97 | |
| Lacy AM et al. | 1% | 3% | 0.19 | |
| COST | 1% | <1% | 0.40 | |
| COLOR I*** | 1% | 2% | 0.47 | |
| COLOR II*** | 1% | 2% | 0.409 | |
| Braga M et al. | <1% | 0 | n/a |
All lengths of hospital stay are median (range) unless *mean ± SD. ¶Actual percentages not documented so calculated from projected figures. ˆThis varied between studies but included infection, would or anastomotic failure, renal or liver failure, ileus and return to theatre **no data for time period ***30 day mortality. In study groups where different outcomes are published in different papers, the figures are taken from those papers that had short-term outcomes as their primary aim.
Table 4.
Long-term outcomes after surgery in selected RCTs (n = 6).
| Laparoscopic arm | Open arm | P value | ||
|---|---|---|---|---|
| Follow up (months) | ALCCaS | 62.4 (1–135) | ||
| CLASICC | 62.9 (22.0–92.8) | |||
| Leung KL et al. | 52.7 (38.9)¶ | 49.2 (35.4)¶ | >0.05 | |
| Lacy AM et al. | 44 (27–85) | 43 (27–85) | ||
| COST | 51 | |||
| COLOR I | 53 (0.03–60) | |||
| Braga M et al.§ | 73 (48–106) | |||
| Port site/wound recurrences Numbers (%) |
ALCCaS | |||
| CLASICC | 10 | 2 | >0.05 | |
| Leung KL et al. | 0 | 0 | ||
| Lacy AM et al. | 1 | 0 | ||
| COST | 2 (0.5%) | 1 (0.2%) | 0.58 | |
| COLOR I | 1.3% | 0.4% | 0.09 | |
| Braga M et al.§ | 0 | |||
| Overall survival | ALCCaS | 77.7% | 76.0% | 0.64 |
| CLASICC | 82.7 (69.1–94.8)♮ | 78.3 (65.8–106.6)♮ | 0.08 | |
| Leung KL et al.* | 76.1% (3.7%) | 72.9% (4.0) | 0.61 | |
| Lacy AM et al.¶ | 80% | 70% | 0.16 | |
| COST | 76/435♯ | 84/428♯ | 0.51 | |
| COLOR I⌃ | 81.8% (78.4–85.1) | 84.2% (81.1–87.3) | 0.45 | |
| Braga M et al.§ | 72% | 66% | 0.321 | |
COLOR II has been removed from these tables as no long-term follow up.
Missing values are due to missing values in the published work.
All follow up times are median (range) unless ¶median ± interquartile range.
Disease free and overall survivals are documented as 5 year survival (±Standard Error) unless ♮ median (range) or *mean ± SD or ♯number of patients with recurrence (or death)/total number of patients in that group. ¶Actual percentages not documented so calculated from figures – see text for discussion on cancer-related survival. ⌃3 year survival rates (95% Confidence intervals).
§Taken from Braga et al. 2010 as the only paper in this series to document long term outcomes – this paper only included left sided colonic resections.
3.2. The Australasian Laparoscopic Colon Cancer Study group (ALCCaS) study (2008, 2012)
Between 1998 and 2005, the ALCCaS study randomised 592 participants to laparoscopic or open colorectal cancer resection. Primary outcome measures included overall survival and post-operative mortality. Secondary outcome measures included post-operative complications and length of stay [9,10].
Patients in their laparoscopic arm were older (71 versus 69 years), 71.2% of the laparoscopic cases and 72% of the open cases were graded American Society of Anaesthesiology (ASA) 1 or II. Body Mass Index (BMI) was 25 and 26 in the two groups.
They demonstrated laparoscopic surgery in patients over 70 years was associated with a reduced length of stay (8 versus 10 days) and a reduced complication rate (36.7% versus 50.6%), when compared on open surgery. They found that patients under 70 years had a reduced length of stay when compared to over 70 years (7 versus 8 days laparoscopic surgery, 8 versus 10 days open surgery). Similarly the younger patient group experienced fewer complications with open and laparoscopic surgery (30% versus 36.7% laparoscopic surgery, 34% versus 50.6% open surgery) [7].
3.3. CLASICC study (2005, 2007, 2010, 2013)
This group involved 794 patients, between 1996 and 2002, in 27 UK centres [8,11–13]. Their 5 year outcome data included a subgroup analysis for older patients [8]. They randomised 526 patients into the laparoscopic arm and 268 patients into open surgery. Primary outcome measures included overall survival (OS) and wound site recurrence.
There were 410 patients aged over 70 (51.6%). They demonstrated no difference in overall survival at 5 years between older patients undergoing laparoscopic colorectal resection (67.7% versus 65%).
Length of stay was 2 days shorter for patients in the laparoscopic group (9 versus 11 days). There was no reported difference in number of patients with short-term complications in either arm (10% versus 10%). Similarly, there was no difference in the total number of complications (13% open surgery versus 14% laparoscopic surgery).
3.4. Leung et al. (2004)
Leung et al. began recruitment in 1993 [14] in two centres and published their findings in 2004 once 403 patients had completed the study. The mean age was 67.1 years in the laparoscopic group and 66.5 years in the open group.
They found a significant reduction in length of hospital stay, median 8.2 days for laparoscopic versus 8.7 days for open. Overall survival was similar in both groups, leading this group to conclude that laparoscopic surgery had short-term outcome advantages over open with similar long-term oncological outcomes.
3.5. Lacy et al. (2002)
Lacy et al. [15] recruited the smallest patient numbers in this systematic review, but does include a wider selection of patients: all elective curative colon cancer resections. This group had mean ages of 68 years in the laparoscopic arm and 71 in the open arm.
This group's primary aim was to assess cancer-related survival. Their median follow up was nearly 4 years in both of the randomised groups. They found a reduction in hospital stay (5.2 days versus 7.9 days) and no significant difference in overall survival.
3.6. The COST trial (2004, 2007)
This series of publications by the Clinical Outcomes of Surgical Therapy (COST) group started recruitment in 1994. In 2004 [16] and 2007 [17] published their outcomes concluding that laparoscopic colon surgery is an acceptable alternative to open surgery. Forty-eight centres participated in this study (66 surgeons) recruiting 872 patients. The mean age of patients was 70 years in the laparoscopic group and 69 in the open group. They identified a reduced hospital stay (5.6 days versus 6.4) with laparoscopic surgery, but no differences in morbidity and mortality and long-term survival.
3.7. COLOR I and COLOR II trials (2005, 2009, 2013)
These three publications analysed patients that were undergoing elective surgery for colon (COLOR I) [18,19] and rectal cancer (COLOR II) [20]. In COLOR I, recruited 1076 patients, in 30 sites, making it the largest trial performed in laparoscopic colon surgery. The median age of people in the laparoscopic arm was 71 years (range 54–84 years, 10th and 90th percentile) and 71 (54–83 years) in the open arm. COLOR II recruited 1044 patients, aged 66.8 years (10.5 SD) in the laparoscopic arm and 65.8 years (10.9) in the open arm, making it the largest publication on laparoscopic rectal cancer.
Both studies recorded a reduction in hospital stay with laparoscopic surgery, (however, only one day difference in the mean stay). Conversion rates post-operative morbidity and mortality were equivalent between laparoscopic and open groups.
The primary aim of COLOR I was disease-free survival at 3 years and these results were published in 2013. With a median follow up of 53 months for all patients, disease-free survival was 74.2% in the laparoscopic group versus 76.2% in the open group. Longer-term outcomes are awaited.
3.8. Braga et al. (2002–2010)
This group published 7 papers which met our inclusion criteria [21–27]. All are from one centre with varying patient numbers, differing inclusion criteria (e.g. right colon cancers only) and primary aims (e.g. short term outcomes or cost analysis), although none of these analyses focused on age. The largest study included 391 patients undergoing elective resection for colon and rectal cancers, with a mean age of 63.7 years in the laparoscopic group and 65.1 in the open group. Morbidity was significantly reduced in the laparoscopic group (20.6% versus 38.3% in the open arm), as was hospital stay (mean 9.4 days versus 12.7). At 48 months overall survival in both groups was similar.
Five-year survival was 72% in the laparoscopic group versus 66% in the open group, which did not reach statistical significance [24].
3.9. Assessment of the quality of the included studies
Six studies were determined to have a Jadad score of three and one (Lacy) [15] of 2. Due to the inherent difficulties involved in blinding surgical studies, all of the studies described lost a mark. The Lacy study lost a mark due to a weak description of their randomisation protocol. All of the studies performed their statistical analysis on an intention to treat basis. The results are summarised in Table 5.
Table 5.
Participant inclusion and exclusion criteria of the selected RCTs.
| Inclusion criteria | Exclusion criteria | Jadad score | |
|---|---|---|---|
| ALCCaS | Colon | Transverse colon cancers; rectal cancers; T4, Stage IV; intestinal obstruction; ASA 4/5; BMI > 35 | 3 |
| CLASICC | Colon and rectum | Transverse colon cancers: unsuitable for GA. | 3 |
| Leung KL et al. | Recto-sigmoid cancers only | Low rectal cancers (<5 cm); T4 disease; Stage IV disease; intestinal obstruction | 3 |
| Lacy AM et al. | Colon | Transverse colon cancers; rectal cancers; T4; Stage IV; previous colonic surgery; ASA 4/5; intestinal obstruction | 2 |
| COST | Colon | Transverse colon cancers; rectal cancers; T4; Stage IV; intestinal obstruction; adhesions | 3 |
| COLOR | I – colon II rectum |
Transverse colon cancers; T4; Stage IV; intestinal obstruction; BMI > 30 (COLOR I only).a | 3 |
| Braga M et al. | Colon and rectum | Transverse colon cancers; T4 stage IV; ASA 4/5b | 3 |
COLOR II excluded T3 rectal cancers within 2 mm from endopelvic fascia.
A total of 7 studies here analysing various types of surgical resection, but the exclusion criteria listed here is common to all.
The ALCCaS study was multisite and randomised participants via a centralised computer-generated system. They included anyone for surgery with ascending, descending or sigmoid cancers. Patients with a BMI over 35, advanced stage cancers, transverse colon and rectal cancers and those who were graded ASA IV or V were excluded. There was no attempt at blinding the surgical technique used and they reported a 14.6% ‘laparoscopic conversion to open’ rate [10].
In the CLASICC study randomisation occurred via a central service using the telephone [12]. Patients were excluded if they had transverse colon cancers or suffered significant cardiopulmonary disease that would not allow them to tolerate a pneumoperitoneum. Surgeons, clinicians and nurses were not blinded to the intervention in this study. Mean BMI was 26 and up to 85% of patients were classified as ASA I or II. The laparoscopic-converted-to-open surgery rate was 29% and in this group patients had a worse overall survival of 49.6%. Surgeons needed to have completed 20 resections to participate in the study.
Leung et al. randomised participants via computer and blinded staff but did not attempt to blind the surgical technique used. They did not state any anaesthetic or body habitus exclusion criteria. They converted 23.3% of laparoscopic procedures [14].
Lacy et al. randomised participants using computer-generated random numbers via a blinded investigator. There was no attempt at blinding the surgical method used. They excluded rectal and transverse colon cancers but did not report a conversion to open rate. They did not state any restriction criteria relating to fitness for anaesthesia but did exclude people with previous abdominal surgery [15].
The COST study was a large North American study that focused on colon cancer [16]. It randomised people via centralised minimisation algorithm. Surgeons must have completed at least 20 laparoscopic procedures to participate but did not attempt to blind the technique used. They included 121 (28%) open and 112 (26%) laparoscopic cases who were classed as ASA grade 3. They also included 26 people with ASA grade 4. They had no other significant exclusion criteria. Their conversion rate was 21%.
COLOR I and II randomised by blinded centralised staff using a computer-generated list [19,20]. COLOR I excluded people with BMI >30 Kg/M2 and people with “absolute contraindications to anaesthesia and a long pneumoperitoneum” but do not elaborate. COLOR II do not state BMI as a contraindication but did exclude people with an ASA grade of greater than three. Although, 7 people with ASA grade 4 were ultimately included. In the laparoscopic arm they included 131 (18%) people with ASA grade of 3 and 61 (19%) in the open arm. The age of this group of people was 44–90 years, median 71 years, mean 71 years (personal communication). Surgeons needed to have completed 20 resections to participate in the study.
Braga and colleagues [25], excluded people with New York Heart Association cardiovascular dysfunction of grade 3 or above and respiratory dysfunction, assessed according to a reduced partial pressure of oxygen (<70 mm/Hg). They randomised participants using computer-generated random numbers via a blinded investigator. There was no attempt at blinding the surgical method used.
4. Discussion
Our results show that a large number of older people were included in RCTs of laparoscopic versus open surgery for colon and rectal cancer. Perhaps more pertinently, these studies were large, well conducted and contemporary. Furthermore, in some trials they included people who would be considered very elderly, including people aged over 100 years old (ALCCaS, COST, COLOR I) [7,16,19].
Much of the current evidence base applied in Geriatric medicine and surgery is extrapolated from younger populations that did not necessarily include older people. Therefore our aims were not to test benefits or harms of laparoscopic and open surgery. They were to ensure that findings of other meta-analyses and current opinion, namely that laparoscopic surgery has short-term beneficial effects and an equivalent long-term safety profile, applies equally to the older person. We found that older people were included in randomised controlled studies and based on our selected outcome measures (overall survival, length of hospital stay, morbidity, mortality, recurrence, conversion rate and disease free survival) we found nothing in our results to suggest that older people behave significantly differently compared to younger people. Nonetheless, in order to ensure that our findings are truly representative and generalizable it is important to critique those studies in more detail.
The inclusion criteria used in our search warrant discussion. We limited RCTs to trials that contained a minimum of 100 patients. We considered that smaller trials were more prone to bias and less generalizable. Commonly these trials were older, ground breaking studies, which were establishing the baseline safety and the potential of laparoscopic surgery. Our decision to limit trials to only those which reported after 2000 also ensures that the surgical techniques involved, be they open or laparoscopic, are comparable with those undertaken today. Another limitation was our decision to only include RCTs. There have been a number of observational studies assessing the safety and practically of colorectal surgery in people aged over 80 years [28,29]. However, none of them have assessed laparoscopic outcomes directly with open procedures and we considered the level of epidemiological evidence they provided to be lower than that which was available from RCT data.
By excluding any trial which stipulated an upper age limit, whatever that may be, we potentially excluded the study of some older people who were enrolled in modern well conducted RCTs e.g. the COREAN trial [30]. However, the exclusion of any person from a RCT based on age, will have introduced a bias regarding the average age of the cohort. Further, by not operating on older people, the surgeons performing the procedures will be less experienced in operating on this cohort of people further reducing the generalizability of their findings.
The mean age of the participants in all of the studies was approximately 70 years old. It is likely, but not certain, that the studies quoting mean ages and standard deviation included people who would be considered very elderly. However, the actual numbers and breakdown of age were not given in any of the papers and it is possible that the actual number of older people was small. However, three of the studies did include people in their nineties and one included people aged over a hundred. We also used an arbitrary figure of 65 years as the definition of old. Geriatricians rarely use chronical age itself and are more comfortable with the concept of biological age. Factors such as frailty [31] or cognitive impairment [32] are likely to play important roles in surgical course and post-operative outcome. However, for the purposes of defining a population to study in this systematic review age was a useful starting point.
It is also important to consider the fitness of the people selected for surgery in our studies. In other words, were the people recruited into this trial, reflective of clinical practice. The two studies that published subgroup analyses on the older person (CLASICC and ALCCaS) only selected fit people, as did the Braga group. This is evidenced by their exclusion criteria, which were an ASA grade of 2 or less or moderate heart failure. The CLASICC study excluded patients with cardio-pulmonary disease who would be unlikely to tolerate a pneumoperitoneum and reported that 85% of their participants had an ASA grade of 2 or below. Further, four of our studies (COLOR I and II, Lacy and ALCCaS) excluded people with a high BMI and the CLASSIC study stated a mean BMI of 26 Kg/M2. These results suggest a patient cohort that may not represent clinical practice. A further consideration, is the site of the tumour. None of the trials not give enough detail to meaningfully differentiate between rectal or colon cancer on the basis of age. These two tumours behave differently. We would encourage future trials to publish no only more detailed breakdown of age distribution within their trial but also the site of the tumour by age distribution.
In contrast, the COST, COLOR I and II studies [16,19,20] did include more medically unfit participants. While the COST and COLOR I do not quote the ages of the people with a higher ASA grade, neither of the trials report that older people were excluded from this anaesthetic group. The COLOR II trial provided us with an age range for ASA grade 3 and randomised at least one person aged 90 years into their study. Therefore, it appears that unfit older people were included in at least some of the trials we have included in our review. Nonetheless, there does appear to be further scope for reporting of laparoscopic surgical outcomes in older people with higher preoperative morbidity.
The conversion rate of 14.6% in ALCCaS compares favourably to practice at the time [10]. The CLASICC study had previously come under criticism for its high conversion rate [12]. The authors had tried to address this issue by including surgeons who had performed at least 20 resections. It is now believed that the learning curve for this type of surgery is 50–100 cases [33]. It is likely, therefore, that the 29% conversion rate is higher than would be seen currently. The conversion rates seen in COLOR trials (17% and 16%) are more likely to be representative of modern conversion rates [19,20]. None of the trials commented on the conversion rate in older people. We would urge further reporting of this outcome in older people, especially those who may have received chemoradiation preoperatively, which may have further complicated the surgical procedure.
5. Conclusions
We identified seven large scale, well conducted and contempory studies that included older people, including the very elderly of which two reported sub-group analyses of their older patients. There was comparatively less evidence on the fitness of older people but some studies did include older people with significant preoperative co-morbidity, however we would encourage further reporting of outcomes in more frail older people undergoing laparoscopic surgery. In conclusion, it appears that fit older people of any age benefit from laparoscopic surgery when compared to open surgery, with no obvious increase in detrimental effects.
Ethical approval
This was a systematic review and did not require ethical approval.
Sources of funding
None.
Author contribution
-
•
Study conception and design. McCarthy and Hewitt.
-
•
Acquisition of data. Coode-Bate, Moug and Hewitt.
-
•
Analysis and interpretation of data. Moug, McCarthy and Hewitt.
-
•
Writing manuscript. Moug, McCarthy, Coode-Bate, Stechman and Hewitt.
Conflicts of interest
None.
Guarantor
Jonathan Hewitt.
Acknowledgement
‘COLOR II study group (principal investigator: Professor H.J. Bonjer, MD, PhD – coordinating investigator: C.L. Deijen, MD)’ who provided additional information regarding their trial.
Appendix 1. Search strategy
#10 #9 NOT animal
#9 #1 and #2 and #3 and #7 and #8
#8 (laparoscop*)
#7 Search (colectom*) or (surgery) or (resection)
#6 Search #1 and #2 and #3 and #4 and #5
#5 Search (older person) or (elderly) or (older)
#4 Search (laparoscop*) or (colectom*) or (surgery) or (resection) or (open) or (sigmoid)
#3 Search (rect*) or (colorect*) or (colon)
#2 Search neoplasm* or cancer or tumor or tumour or carcinom* or malignan* or laparoscopy
#1 Search random* or blind*
References
- 1.Kuhry E. Long-term results of laparoscopic colorectal cancer resection. Cochrane Database Syst. Rev. 2008;(2):CD003432. doi: 10.1002/14651858.CD003432.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reza M.M. Systematic review of laparoscopic versus open surgery for colorectal cancer. Br. J. Surg. 2006;93(8):921–928. doi: 10.1002/bjs.5430. [DOI] [PubMed] [Google Scholar]
- 3.Schwenk W. Short term benefits for laparoscopic colorectal resection. Cochrane Database Syst. Rev. 2005;(3):CD003145. doi: 10.1002/14651858.CD003145.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schiphorst A.H. Representation of the elderly in trials of laparoscopic surgery for colorectal cancer. Colorectal Dis. 2014;16(12):976–983. doi: 10.1111/codi.12806. [DOI] [PubMed] [Google Scholar]
- 5.Jadad A.R. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin. Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 6.Liberati A. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann. Intern. Med. 2009;151(4):W65–W94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 7.Allardyce R.A. Australasian Laparoscopic Colon Cancer Study shows that elderly patients may benefit from lower postoperative complication rates following laparoscopic versus open resection. Br. J. Surg. 2010;97(1):86–91. doi: 10.1002/bjs.6785. [DOI] [PubMed] [Google Scholar]
- 8.Jayne D.G. Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br. J. Surg. 2010;97(11):1638–1645. doi: 10.1002/bjs.7160. [DOI] [PubMed] [Google Scholar]
- 9.Bagshaw P.F. Long-term outcomes of the Australasian randomized clinical trial comparing laparoscopic and conventional open surgical treatments for colon cancer: the Australasian Laparoscopic Colon Cancer Study trial. Ann. Surg. 2012;256(6):915–919. doi: 10.1097/SLA.0b013e3182765ff8. [DOI] [PubMed] [Google Scholar]
- 10.Hewett P.J. Short-term outcomes of the Australasian randomized clinical study comparing laparoscopic and conventional open surgical treatments for colon cancer: the ALCCaS trial. Ann. Surg. 2008;248(5):728–738. doi: 10.1097/SLA.0b013e31818b7595. [DOI] [PubMed] [Google Scholar]
- 11.Green B.L. Long-term follow-up of the Medical Research Council CLASICC trial of conventional versus laparoscopically assisted resection in colorectal cancer. Br. J. Surg. 2013;100(1):75–82. doi: 10.1002/bjs.8945. [DOI] [PubMed] [Google Scholar]
- 12.Guillou P.J. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365(9472):1718–1726. doi: 10.1016/S0140-6736(05)66545-2. [DOI] [PubMed] [Google Scholar]
- 13.Jayne D.G. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. J. Clin. Oncol. 2007;25(21):3061–3068. doi: 10.1200/JCO.2006.09.7758. [DOI] [PubMed] [Google Scholar]
- 14.Leung K.L. Laparoscopic resection of rectosigmoid carcinoma: prospective randomised trial. Lancet. 2004;363(9416):1187–1192. doi: 10.1016/S0140-6736(04)15947-3. [DOI] [PubMed] [Google Scholar]
- 15.Lacy A.M. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet. 2002;359(9325):2224–2229. doi: 10.1016/S0140-6736(02)09290-5. [DOI] [PubMed] [Google Scholar]
- 16.A comparison of laparoscopically assisted and open colectomy for colon cancer. N. Engl. J. Med. 2004;350(20):2050–2059. doi: 10.1056/NEJMoa032651. [DOI] [PubMed] [Google Scholar]
- 17.Fleshman J. Laparoscopic colectomy for cancer is not inferior to open surgery based on 5-year data from the COST Study Group trial. Ann. Surg. 2007;246(4):655–662. doi: 10.1097/SLA.0b013e318155a762. discussion 662-4. [DOI] [PubMed] [Google Scholar]
- 18.Colon Cancer Laparoscopic or Open Resection Study, G Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol. 2009;10(1):44–52. doi: 10.1016/S1470-2045(08)70310-3. [DOI] [PubMed] [Google Scholar]
- 19.Veldkamp R. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol. 2005;6(7):477–484. doi: 10.1016/S1470-2045(05)70221-7. [DOI] [PubMed] [Google Scholar]
- 20.van der Pas M.H. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013;14(3):210–218. doi: 10.1016/S1470-2045(13)70016-0. [DOI] [PubMed] [Google Scholar]
- 21.Braga M. Laparoscopic resection in rectal cancer patients: outcome and cost-benefit analysis. Dis. Colon Rectum. 2007;50(4):464–471. doi: 10.1007/s10350-006-0798-5. [DOI] [PubMed] [Google Scholar]
- 22.Braga M. Laparoscopic vs. open colectomy in cancer patients: long-term complications, quality of life, and survival. Dis. Colon Rectum. 2005;48(12):2217–2223. doi: 10.1007/s10350-005-0185-7. [DOI] [PubMed] [Google Scholar]
- 23.Braga M. Open right colectomy is still effective compared to laparoscopy: results of a randomized trial. Ann. Surg. 2007;246(6):1010–1014. doi: 10.1097/SLA.0b013e31815c4065. discussion 1014-5. [DOI] [PubMed] [Google Scholar]
- 24.Braga M. Randomized clinical trial of laparoscopic versus open left colonic resection. Br. J. Surg. 2010;97(8):1180–1186. doi: 10.1002/bjs.7094. [DOI] [PubMed] [Google Scholar]
- 25.Braga M. Laparoscopic versus open colorectal surgery: a randomized trial on short-term outcome. Ann. Surg. 2002;236(6):759–766. doi: 10.1097/01.SLA.0000036269.60340.AE. disscussion 767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Braga M. Laparoscopic versus open colorectal surgery: cost-benefit analysis in a single-center randomized trial. Ann. Surg. 2005;242(6):890–895. doi: 10.1097/01.sla.0000189573.23744.59. discussion 895-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vignali A. Laparoscopic colorectal surgery modifies risk factors for postoperative morbidity. Dis. Colon Rectum. 2004;47(10):1686–1693. doi: 10.1007/s10350-004-0653-5. [DOI] [PubMed] [Google Scholar]
- 28.Chaudhary B.N. Short-term outcome following elective laparoscopic colorectal cancer resection in octogenarians and nonagenarians. Colorectal Dis. 2012;14(6):727–730. doi: 10.1111/j.1463-1318.2011.02735.x. [DOI] [PubMed] [Google Scholar]
- 29.Cheung H.Y. Laparoscopic resection for colorectal cancer in octogenarians: results in a decade. Dis. Colon Rectum. 2007;50(11):1905–1910. doi: 10.1007/s10350-007-9070-x. [DOI] [PubMed] [Google Scholar]
- 30.Kang S.B. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol. 2010;11(7):637–645. doi: 10.1016/S1470-2045(10)70131-5. [DOI] [PubMed] [Google Scholar]
- 31.Hubbard R.E., Story D.A. Patient frailty: the elephant in the operating room. Anaesthesia. 2014;69(Suppl. 1):26–34. doi: 10.1111/anae.12490. [DOI] [PubMed] [Google Scholar]
- 32.Saczynski J.S. Cognitive trajectories after postoperative delirium. N. Engl. J. Med. 2012;367(1):30–39. doi: 10.1056/NEJMoa1112923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li J.C. The learning curve for laparoscopic colectomy: experience of a surgical fellow in an university colorectal unit. Surg. Endosc. 2009;23(7):1603–1608. doi: 10.1007/s00464-009-0497-0. [DOI] [PubMed] [Google Scholar]