Abstract
Postarrest myocardial dysfunction includes the development of low cardiac output or ventricular systolic or diastolic dysfunction after cardiac arrest. Impaired left ventricular systolic function is reported in nearly two-thirds of patients resuscitated after cardiac arrest. Hypotension and shock requiring vasopressor support are similarly common after cardiac arrest. Whereas shock requiring vasopressor support is consistently associated with an adverse outcome after cardiac arrest, the association between myocardial dysfunction and outcomes is less clear. Myocardial dysfunction and shock after cardiac arrest develop as the result of preexisting cardiac pathology with multiple superimposed insults from resuscitation. The pathophysiology involves cardiovascular ischemia/reperfusion injury and cardiovascular toxicity from excessive levels of inflammatory cytokine activation and catecholamines, among other contributing factors. Similar mechanisms occur in myocardial dysfunction after cardiopulmonary bypass, in sepsis, and in stress-induced cardiomyopathy. Hemodynamic stabilization after resuscitation from cardiac arrest involves restoration of preload, vasopressors to support arterial pressure, and inotropic support if needed to reverse the effects of myocardial dysfunction and improve systemic perfusion. Further research is needed to define the role of postarrest myocardial dysfunction on cardiac arrest outcomes and identify therapeutic strategies.
1. Introduction
Cardiac arrest (CA) is a leading cause of death in the United States, affecting more than half a million Americans each year [1–4]. Survival rates after CA remain poor even after achieving return of spontaneous circulation (ROSC), and approximately 60% of patients admitted to the hospital after CA die from complications [1–4]. Deaths within the first 24 hours after ROSC typically result from refractory shock producing recurrent CA or multiorgan system failure (MOSF), while later deaths result from neurological injury [5–7]. Most deaths after in-hospital CA (IHCA) result from refractory shock, recurrent CA, and MOSF, while most deaths after out-of-hospital CA (OHCA) result from neurological injury [5–8]. Postcardiac arrest syndrome (PCAS) refers to the constellation of abnormalities that develops after resuscitation from CA, including neurological dysfunction, postarrest myocardial dysfunction (PAMD), systemic ischemic/reperfusion injury (IRI), and persistent precipitating pathology [9, 10]. PAMD results from acute cardiac injury from CA resuscitation superimposed on the acute or chronic cardiac condition that caused CA. Mechanisms of PAMD overlap with those producing cardiac dysfunction during myocardial infarction (MI), sepsis, and stress-induced cardiomyopathy and after cardiopulmonary bypass (CPB). Hemodynamic instability and shock after CA may result from PAMD and/or from systemic vasodilation from systemic inflammatory response syndrome (SIRS) [11–14]. In this review, we will discuss the epidemiology, pathophysiology, and management of PAMD and shock after ROSC.
2. Epidemiology of PAMD and Shock after ROSC
The true incidence of PAMD after CA in humans remains uncertain due to the small sample sizes, variable definitions, and inconsistent cardiac function assessment in published studies (Table 1) [8, 14–17]. Manifestations of PAMD include low cardiac index (CI), left ventricular systolic dysfunction (LVSD), left ventricular (LV) diastolic dysfunction, and/or right ventricular dysfunction. Echocardiography is the first-line diagnostic test for PAMD, and reduced left ventricular ejection fraction (LVEF) is the most commonly reported manifestation of PAMD. Human studies suggest that two-thirds of patients resuscitated from CA have LVSD within the first 24 hours after ROSC, with a mean LVEF of approximately 40% ± 5% (Table 1) [8, 14–21]. Shock and vasopressor dependence after ROSC are not surrogates for PAMD because they may result from vascular dysfunction without PAMD [14]. PAMD does not reliably predict vasopressor requirements and has not been consistently linked with adverse outcomes when corrected for severity of CA and presence of shock and vasopressor support. It remains uncertain whether PAMD directly impairs survival and recovery after CA or whether development of PAMD merely reflects a greater degree of ischemic injury sustained during severe CA. Rearrest early after ROSC appears to occur in at least 6% of transported post-ROSC patients [22]. As myocardial dysfunction predisposes to sudden death, it is likely that a portion of early post-ROSC rearrests and deaths result directly from underlying PAMD [8].
Table 1.
Incidence of left ventricular systolic dysfunction in adult survivors of cardiac arrest. LVEF = left ventricular ejection fraction, LVSD = left ventricular systolic dysfunction (LVEF < 50–60%), and NR = not reported.
| Study | Year | Number of patients | % LVSD | Mean LVEF |
|---|---|---|---|---|
| Laurent et al. [14] | 2002 | 148 | NR | 37.6% |
| Ruiz-Bailén et al. [15] | 2005 | 29 | 69% | 42% |
| Chang et al. [17] | 2007 | 58 | NR | 53.7% |
| Gonzalez et al. [8] | 2008 | 84 | NR | 32% |
| Gaieski et al. [18] | 2009 | 22 | NR | 36.9% |
| Dumas et al. [16] | 2012 | 308 | 72% | NR |
| Bro-Jeppesen et al. [20] | 2014 | 154 | NR | 37% |
| Bro-Jeppesen et al. [21] | 2015 | 523 | 75% | NR |
| Ameloot et al. [19] | 2015 | 82 | NR | 42% |
2.1. Low Cardiac Output after CA
In 2002, Laurent et al. reported hemodynamic data in 165 OHCA survivors who underwent systematic coronary angiography [14]. Hemodynamic instability requiring pulmonary artery catheter (PAC) placement and vasopressor support occurred in 55% of patients, predicted by a higher cumulative epinephrine dose and number of countershocks during cardiopulmonary resuscitation (CPR). Hypotension with a low CI (mean 2 L/min/m2) developed 6–8 hours after intensive care unit arrival despite aggressive fluid resuscitation (median 8 liters over 72 hours) for low cardiac filling pressures. Vasopressor requirements peaked at 24 hours, with a progressive increase in CI and a reduction in systemic vascular resistance (SVR) leading to persistent vasopressor requirements for up to 72 hours despite normalization of CI. Persistently low CI at 24 hours was associated with early death due to MOSF, but the surviving patients had restoration of normal hemodynamics by 72 hours. Mean LVEF at coronary angiography was lower in patients with hemodynamic instability (32% versus 43%), although only half of these patients had an acute coronary occlusion. Neurologic outcomes did not differ based on the presence or absence of hemodynamic instability.
Oksanen et al. reported on 47 patients who underwent PAC placement during therapeutic hypothermia (TH) after resuscitation from VF OHCA [23]. A low CI (<1.5 L/min/m2) developed in 66% during the first 12 hours after ROSC, with nadir CI values at 6 hours; the remaining patients without apparent PAMD had mean CI in the 1.5–2 L/min/m2 range. Low CI resulted from reduced stroke volume (SV) index and low heart rate (HR) that responded to low-dose dobutamine. There were no clinical, laboratory, or hemodynamic predictors of low CI, and low CI did not predict clinical adverse outcomes. Trzeciak et al. reported on a highly selected subset of 333 CA survivors undergoing invasive hemodynamic assessment with a PAC from a registry of 8736 total CA patients [24]. The initial CI was below 2.5 L/min/m2 in 49% of these patients and below 2.0 L/min/m2 in 28%; low CI was not a risk factor for adverse outcomes, although the requirement for inotropic support did increase mortality. A significant limitation of these studies is the selective monitoring of CI.
2.2. Abnormal Systolic Function
PAMD was first described in swine as decreased LVEF (from 55% to 20%) and increased LV end diastolic pressure within 30 min of ROSC that recovered to baseline within 48 hours [25, 26]. In 2005, Ruiz-Bailén et al. reported on serial echocardiography in 29 CA survivors without cardiac etiology or prior cardiac disease [15]. At 24 hours, an LVEF <55% was identified in 69% of patients, with a mean LVEF of 28% in these patients with PAMD and a mean LVEF of 42% overall. LVEF at 24 hours was higher in survivors than in nonsurvivors (38% versus 22%), but there were no significant predictors of reduced LVEF at 24 hours. Echocardiographic LVEF increased each week with normalization over the first month in survivors; nonsurvivors who underwent serial echocardiography did not have an improvement in LVEF. Apical segments displayed more severe wall motion abnormalities (WMA) with sparing of basal segments, a finding also seen in stress cardiomyopathy [27].
Preexisting LVSD cannot be reliably distinguished from reversible PAMD as the cause of reduced LVEF after ROSC in CA survivors without acute MI and may be more prognostically important. In 2008, Gonzalez et al. reported on 613 patients who had an echocardiogram within 3 months prior to IHCA [8]. LVEF decreased by one-quarter from its baseline value (from 43% prior to IHCA to 32% after IHCA) in the 84 patients who had an echocardiogram within 72 hours after IHCA, with a similar relative reduction in LVEF regardless of prearrest LVEF. Prearrest LVEF <45% was a predictor of lower survival after IHCA, and patients with LVSD prior to IHCA were more likely to die of refractory shock after ROSC.
In 2012, Dumas et al. reported on 422 OHCA survivors without obvious noncardiac arrest etiology who underwent early coronary angiography [16]. A reduced LVEF <40% was present in 34% of patients at the time of coronary angiography, including 17% of patients with recent coronary occlusion and 36% of patients without. Gaieski et al. performed echocardiography in 15 patients within 6 hours after OHCA, revealing a mean LVEF of 39% that improved to 43% at 72 hours in the 10 survivors who underwent repeat echocardiography [18]. Ameloot et al. reported a mean LVEF of 42% in 82 patients after ROSC, with a lower mean LVEF of 34% in the subgroup of patients with low ScvO2 ≤66% that correlated with a lower mean cardiac output (CO) of 3.2 L/min [19].
The most comprehensive study of PAMD comes from a subset of 171 patients enrolled in the Targeted Temperature Management (TTM) study comparing 36°C versus 33°C who underwent serial echocardiography and PAC placement [20]. Mean LVEF was 35–39% upon ICU admission and increased slightly to 39–42% (mean 4% increase) by 48 hours, with a greater increase in the 36°C group. The peak systolic myocardial tissue Doppler (s′) velocity and tricuspid annular plane systolic excursion (TAPSE) values were reduced on admission and increased by 48 hours. The CI was lower in the 33°C group despite similar vasopressor requirements, LVEF, TAPSE, and s′ values, primarily due to reduced HR with a lesser reduction in SV and similar mean arterial pressure (MAP) due to higher SVR. In the overall TTM study, LVEF on the first day was severely reduced (<30%) in 28% of patients and moderately reduced (30–50%) in 48%, with normal LVEF (>50%) in only 25% [21]. LVEF distribution did not differ between patients with higher and lower vasopressor requirements or between target temperature groups, emphasizing the dissociation between LVSD and systemic hemodynamics.
2.3. Abnormal Diastolic Function
Profound diastolic dysfunction was first demonstrated in animal models of PAMD prior to its description in humans [26, 28]. In 2007, Chang et al. performed echocardiography at 6 hours after ROSC in 58 OHCA survivors, reporting LVEF as a measure of LV systolic function and isovolumetric relaxation time (IVRT) as a measure of LV diastolic function [17]. Prior MI and higher epinephrine doses were associated with lower LVEF, and LVEF below 40% was associated with worse survival and lower rates of neurological recovery on univariate but not multivariate analysis. A prolonged IVRT ≥100 ms (reflecting diastolic dysfunction) was associated with noncardiac etiology of arrest and nonshockable arrest rhythm and remained an independent predictor of poor survival after adjustment for age, initial cardiac rhythm, epinephrine dose, and CPR duration. In the study by Bro-Jeppesen et al., early mitral annular diastolic tissue Doppler (e′) velocity was reduced immediately after ROSC and increased over the first 48 hours, suggesting transient myocardial diastolic dysfunction mirroring the systolic dysfunction reflected by reduced s′ velocities [20].
2.4. Hypotension and Shock after ROSC
Arterial hypotension with systolic blood pressure (SBP) <90–100 mmHg or mean arterial pressure (MAP) <60–65 mmHg is present in 47–73% of patients after ROSC, and vasopressor support is required in 52–72% of CA survivors [14, 17, 21, 24, 29–32]. Hypotension, shock, and the need for vasopressor support after ROSC consistently predict worse overall or neurologically intact survival after CA, with an inverse association between MAP and survival [19, 21, 24, 29–35]. Patients who require multiple and/or more potent vasopressors have worse outcomes, and the cardiovascular SOFA score carries the greatest prognostic value of all the SOFA subscores in patients with MOSF after CA [21, 34–36]. Shock after ROSC produces recurrent CA and MOSF and may impair brain perfusion and neurological recovery [37]. Survivors with favorable neurological outcomes have higher MAP and less hypotension than nonsurvivors and patients with poor neurological outcomes, even among patients requiring vasopressor support [19, 24, 31–33]. Hypotension may simply be an overall marker of CA severity, but disrupted cerebral blood flow autoregulation after ROSC may lead to cerebral hypoperfusion during hypotension [38]. Up to 35–80% of patients require inotropic support after ROSC, although rates are highly variable between studies [24, 39–41].
Laurent et al. first demonstrated that shock after CA and ROSC evolves from a low-output state with low CI from PAMD to a vasodilated state with low SVR, combined with a need for significant ongoing fluid resuscitation from abnormal vasodilation and capillary leak from SIRS, mimicking septic shock [14, 23]. Post-ROSC shock often develops after a brief “honeymoon period” lasting up to 6 hours, followed by a low-output state and then worsening vasodilation with increasing vasopressor requirements peaking at 24 hours and gradual resolution over the subsequent 24–48 hours [14, 23]. Higher initial lactate levels predict higher vasopressor doses, suggesting that a greater initial ischemic insult leads to cardiovascular failure [21, 42, 43].
3. Pathophysiology of PAMD and Shock after ROSC
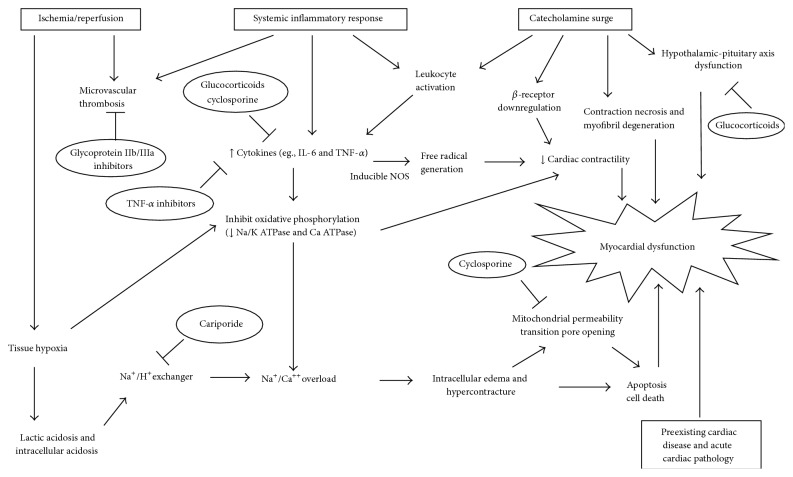
Multiple interacting processes contribute to the reversible deterioration of cardiac function after CA, leading to acute cardiac dysfunction superimposed on underlying structural heart disease (Figure 1). The triggering etiology of CA often produces cardiac dysfunction, but these acute and chronic cardiac conditions are conceptually distinct from true PAMD and are more appropriately considered as precipitating pathology. Three major pathways contribute to PAMD—cardiovascular IRI, catecholamine-induced myocardial injury, and cytokine-mediated cardiovascular dysfunction [44]. PAMD shares pathophysiological and clinical features with three better-characterized conditions, namely, post-CPB myocardial dysfunction, stress-induced cardiomyopathy, and septic cardiomyopathy, respectively [27, 45, 46]. Microvascular dysfunction, adrenal insufficiency, mitochondrial dysfunction, cardiac stunning from direct-current countershocks, and cardiovascular effects of iatrogenic interventions including TH further contribute to PAMD and shock after ROSC [44]. Current management of PAMD and shock is supportive and therapies targeting the underlying pathophysiology have not yet been investigated in clinical studies with patient-centered outcomes. Prevention of PAMD will require interventions targeting multiple pathways in order to produce clinical benefits, and PAMD remains a promising area of postresuscitation research.
Figure 1.
Pathophysiologic mechanisms involved in postarrest myocardial dysfunction. Boxes represent major contributing etiologies. Circles represent therapeutic interventions explored in experimental models of cardiac arrest.
3.1. Ischemia/Reperfusion Injury
IRI is one of the primary underlying mechanisms linking CA to MOSF, PAMD, and shock [9, 44]. IRI produces myocardial injury during MI and cardiac stunning after CPB via overlapping cellular mechanisms [45, 47]. Unlike focal myocardial ischemia due to MI, the entire myocardium is affected in CA and after CPB, leading to transient but global changes in cardiac systolic and diastolic function. Ischemia produces cellular energy depletion and lactic acidosis from anaerobic metabolism. Cellular energy depletion leads to failure of the membrane Na/K ATPase pump with intracellular sodium overload and cell edema that is worsened by sodium influx through the membrane Na/H exchanger (NHE) due to intracellular acidosis [47, 48]. Intracellular sodium accumulation induces calcium influx through the Na/Ca exchanger, leading to myocardial cellular calcium overload exacerbated by failure of the Ca ATPase due to energy depletion [47, 48]. Intracellular calcium overload produces harmful effects including downstream activation of calcineurin and initiation of cellular apoptosis by opening of the mitochondrial permeability transition pore (MPTP), along with impaired diastolic relaxation and predisposition to arrhythmias [49]. The cellular and hemodynamic effects of cardiovascular IRI overlap with the adverse effects of persistent lactic acidosis [21, 42, 43, 48]. With restoration of blood flow after transient ischemia, overproduction of toxic reactive oxygen species (ROS) leads to a second wave of cellular injury [47]. Profound myocardial cellular energy depletion leads to tetanic cardiac muscle contraction leading to progressive myocardial wall thickening and reduction in cavity volume, a potentially irreversible state called ischemic contracture [50].
Cyclosporine is a calcineurin inhibitor that ameliorates the adverse effects of cellular calcium overload by inhibiting MPTP opening and apoptosis, in addition to anti-inflammatory effects [51, 52]. Cyclosporine prevents IRI in preclinical animal models of PAMD and humans with MI and those undergoing CPB. Preclinical animal models have shown an improvement in PAMD after cyclosporine administration during CA [51–53]. A rat study by Huang et al. showed improved LV systolic function, cardiac output, and mortality when cyclosporine was administered during CA but not when cyclosporine was administered after ROSC [52]. A rabbit study by Cour et al. showed similar improvements in post-ROSC survival and PAMD when cyclosporine was administered at the establishment of reflow [51]. Both studies linked the beneficial effects of cyclosporine to inhibition of MPTP opening [51, 52]. Gill et al. improved cardiac and mitochondrial function in piglets subjected to asphyxial CA who received cyclosporine [53]. Piot et al. demonstrated significant reduction in infarct size in acute MI patients who received cyclosporine compared to placebo, leading to improvements in LV remodeling [54, 55]. Recent studies have shown reductions in myocardial injury with administration of cyclosporine in humans undergoing CPB [56, 57]. These preclinical studies in multiple animal models of CA along with human data in similar disease processes make cyclosporine a promising agent for prevention of PAMD.
The NHE is another potential therapeutic target for prevention of cellular injury during IRI. Multiple animal studies have shown improvements in PAMD and/or mortality with administration of NHE inhibitors (such as cariporide) during CA, including improved hemodynamics and reductions in LVSD and/or arrhythmias [58–64]. Mentzer et al. reported the effects of cariporide in the large EXPEDITION study of patients undergoing CPB, demonstrating a reduction in myocardial injury biomarkers but an increased rate of mortality and cerebrovascular events with cariporide [65]. This human study showing increased neurologic injury with cariporide has reduced enthusiasm for the use of this drug to prevent PAMD, given the importance of neurologic injury for prognosis after CA. Animal studies suggest a beneficial effect of the traditional Chinese medicine Shen-Fu on PAMD via inhibition of IRI and myocardial apoptosis [66, 67].
3.2. Inflammatory Cardiovascular Dysfunction
Systemic IRI after ROSC triggers release of inflammatory cytokines leading to SIRS that mimics sepsis, even in the absence of infection [11–13, 68]. The inflammatory response after ROSC is characterized by polymorphonuclear leukocyte activation, adhesion molecule expression, ROS production from inducible nitric oxide synthase (iNOS), and release of cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) [11, 13, 44, 69, 70]. Like sepsis and the vasoplegia that can occur after CPB, the SIRS that follows ROSC produces pathological vasodilation, depressed cardiac function, and MOSF from direct myocardial depression by cytokines and uncontrolled vasodilation resulting from iNOS activation [12, 46, 71]. Various cytokines have direct depressant effects on cardiac myocyte contractility, contributing to both systolic and diastolic dysfunction in septic cardiomyopathy [46, 72–74]. The intensity of the inflammatory response in both septic shock and postarrest syndrome may explain the high associated mortality in these conditions. Cytokine overproduction also occurs after CPB, and anti-inflammatory therapy with corticosteroids and other agents can reduce myocardial dysfunction after CPB in animal models [71, 75–78]. Corticosteroid treatment in humans undergoing CPB also reduces levels of inflammatory markers and appears to be associated with clinical benefits including reduced need for vasopressors and fewer arrhythmias [71, 78]. Bro-Jeppesen et al. found that IL-6 levels predicted vasopressor requirements and mortality in the TTM trial, confirming the importance of inflammatory mediators in shock after ROSC [13, 68]. Other studies have confirmed the importance of IL-6 levels for predicting MOSF and outcomes after ROSC, with a less consistent association between C-reactive protein levels and adverse outcomes [79, 80]. Therapies targeting cytokine removal have shown some promise for treatment of circulatory dysfunction after CA, suggesting that inflammation may be a modifiable risk factor for death and PAMD [81]. Mitochondrial dysfunction can result from excess cytokine activity as well as the cellular effects of IRI and oxidative stress from elevated ROS, contributing further to myocardial dysfunction via impaired energy metabolism [82, 83]. Mitochondrial dysfunction can impair cellular oxygen utilization, leading to lactic acidosis despite adequate tissue perfusion, a state characterized by high SvO2 levels and poor prognosis [19, 83].
TNF-α is a major mediator of cytokine-induced cardiovascular dysfunction that directly impairs cardiac contractility, beta-adrenergic responsiveness, and mitochondrial function [70, 73, 82, 84]. Biologic inhibitors of TNF-α, including infliximab and etanercept, have shown benefit in preclinical animal models of CA. Administration of infliximab during the periarrest period improved cardiovascular function in pigs, as demonstrated by improved MAP, SV, and short term survival [70, 85–87]. Etanercept failed to reproduce the benefits seen with infliximab in the same model [86]. Inhibition of cytokine production may contribute to the improvements in cardiovascular function seen with cyclosporine and corticosteroids after CA in animal models and limited human studies [51–53, 88, 89]. The no-reflow phenomenon, characterized by impaired or absent microvascular perfusion despite restoration of macrovascular flow, can occur in the brain and other organs after resuscitation from CA as it does in the myocardium after reperfusion therapy in acute MI [90, 91]. Endothelial damage from IRI and cytokine activation produces abnormal vascular permeability, coagulation cascade activation, tissue edema, and microvascular occlusion that further impair tissue perfusion [12, 44]. GpIIb/IIIa inhibitors such as abciximab and eptifibatide have improved myocardial microvascular perfusion in preclinical animal models of PAMD, without clear improvements in cardiac function or systemic microcirculatory perfusion [92, 93]. Similarly, improvements in microvascular function with GLP-1 infusion failed to reduce PAMD in pigs [94].
3.3. Catecholamine-Induced Cardiac Dysfunction
Catecholamine-mediated cardiotoxicity is another major mechanism contributing to PAMD. Excess levels of catecholamines (particularly epinephrine) can provoke cardiac dysfunction, including stress-induced (takotsubo) cardiomyopathy [27]. High doses of epinephrine (as are administered during CPR) can provoke stress-induced cardiomyopathy in humans [95]. Higher epinephrine doses during CPR predict PAMD in human studies and epinephrine given during resuscitation increases severity of PAMD in animal studies, an effect ameliorated by beta-blockade [14, 17, 96–98]. Catecholamine excess produces myocardial injury and cardiac dysfunction through multiple mechanisms including calcium overload, ROS overproduction, and beta-receptor downregulation and desensitization [27, 99]. Beta-receptor downregulation also occurs in animal models of PAMD in the absence of epinephrine treatment and has also been documented in myocardial dysfunction after CPB [100, 101]. Despite theoretical beta-receptor downregulation in PAMD, most patients respond well to low doses of beta-agonists such as dobutamine [23, 26, 102–104]. The apex of the left ventricle possesses a higher beta-adrenergic receptor concentration, explaining the predisposition to apical hypokinesis seen in stress cardiomyopathy and some studies of human PAMD [15, 27, 99].
Recent observational studies have called the use of epinephrine during CPR into question, showing higher rates of ROSC but lower rates of neurologically intact and overall survival [105]. No difference in mortality was seen with higher epinephrine doses in randomized trials compared to standard dose epinephrine during CPR [106, 107]. Studies using less beta-adrenergic vasopressors such as norepinephrine or vasopressin during CPR likewise have not shown consistent effects on mortality when compared to epinephrine, although certain subgroups appeared more likely to benefit in the case of vasopressin [107, 108]. Effects on myocardial function were not explicitly examined in the majority of these studies, although one study found a potentially harmful effect of epinephrine on post-ROSC hemodynamics with lower CI in patients who had received higher cumulative epinephrine doses during CPR [109]. Reducing epinephrine doses during CPR has the potential to reduce the severity of cardiovascular failure after ROSC.
3.4. Relative Vasopressin and Cortisol Deficiency
In two studies, Mentzelopoulos et al. randomized a total of 368 patients suffering IHCA to epinephrine alone or epinephrine with vasopressin and methylprednisolone during CPR, followed by ongoing hydrocortisone therapy or placebo after ROSC [88, 89]. The vasopressin and corticosteroids groups needed less epinephrine during CPR and had higher rates of ROSC and reduced need for vasopressors after ROSC, with improved functional and overall survival as seen in a pilot study [88, 89]. It remains uncertain whether the benefits seen in these studies were due to a harmful effect of epinephrine or a beneficial effect of vasopressin and/or corticosteroids. Prior studies have demonstrated endocrine dysfunction with relative deficiency of vasopressin and cortisol after ROSC, allowing for physiological repletion to have synergistic effects on shock reversal as seen in the studies by Mentzelopoulos [88, 89, 110–114]. Vasoplegia after ROSC may be associated with a relative vasopressin deficiency, as seen in vasodilatory shock from sepsis or after CPB [71, 110, 115]. Low-dose vasopressin has proven effective for shock reversal in all of these vasoplegic states by augmenting adrenergic vasoconstriction and opposing pathological vasodilation, although effects on mortality have been less consistent [71, 115, 116]. Recent animal studies have demonstrated that vasopressin may inhibit downstream receptor second messenger cascades to potentially ameliorate cellular toxicity from excessive beta-adrenergic stimulation [117]. Abnormalities of adrenal function leading to functional adrenal insufficiency appear common after CA, with greater abnormalities identified in nonsurvivors [111–114]. Similar abnormalities of adrenal function are well described in septic shock, and the same low-dose hydrocortisone regimens have proven effective for shock reversal in septic shock and post-ROSC shock [88, 89, 118].
3.5. Additional Factors Contributing to PAMD and Shock after ROSC
Several other iatrogenic factors can contribute to cardiovascular dysfunction after CA. The administration of direct-current countershocks during CPR is known to produce myocardial stunning [119]. Animal models have demonstrated that countershocks decrease cardiac contractility, decrease CI, and increase LV end diastolic pressure in a manner dependent on energy and waveform [120, 121]. Human studies show deterioration in hemodynamics and cardiac function after countershocks delivered by implantable defibrillators [122]. Increased number of countershocks is associated with PAMD in some studies, although more countershocks may be a marker of longer CPR duration (as is true for higher cumulative epinephrine dose) [14].
Several medications commonly administered after CA may affect cardiovascular function, including antiarrhythmics and sedatives. Antiarrhythmics such as amiodarone and beta-blockers have negative inotropic effects which may impair systemic hemodynamics in the setting of PAMD. Propofol often produces hypotension from systemic vasodilation and direct myocardial depression and may impair the response to vasopressors and inotropes, particularly in patients with cardiovascular dysfunction [123–126]. Post-ROSC patients receiving propofol and remifentanil had higher rates of hypotension and greater need for vasopressors than patients sedated with midazolam and fentanyl, despite similar outcomes [127]. Adverse hemodynamic effects, particularly vasodilatory hypotension, can be seen with other sedatives and intravenous antiepileptic drugs such as phenytoin and valproic acid. Despite the necessity of vasopressors to maintain systemic hemodynamics in many patients after ROSC, excessive use of these drugs may impair microvascular function and tissue perfusion, in addition to provoking recurrent arrhythmias and potentially increasing the risk of adverse outcomes [36, 128, 129].
TH and TTM have become central to reducing neurological injury and improving outcomes after OHCA [130–133]. Mild TH alters systemic hemodynamics and myocardial performance and has improved PAMD in animal models [134]. The effects of TH on isolated myocardium include increased inotropy and impaired diastolic relaxation, but reduced HR and increased SVR dominate the clinical hemodynamic effects of TH [134]. Bernard et al. demonstrated that patients randomized to TH had significantly lower CI, higher SVR, and lower HR during the first 12 hours after ROSC without a significant difference in MAP or SV [130]. In this study, patients receiving TH had improved clinical outcomes, suggesting that hemodynamic changes resulting from TH are not harmful per se. Observational studies have shown similar vasopressor requirements in patients receiving TH versus normothermia, with persistence of vasopressor dependence after rewarming in patients receiving TH [36, 41, 135]. On the contrary, patients in the TTM trial randomized to 33°C had increased vasopressor requirements compared to the 36°C group despite similar MAP [21]. In addition, patients with shock in the 33°C group of the TTM trial had higher lactate levels and a trend to worse outcomes when adjusted for baseline characteristics [37]. This supports the use of TTM to 36°C in patients after ROSC independent of the presence of shock or vasopressor dependence and suggests caution when using mild TH to 33°C in patients with shock after ROSC. Interestingly, small studies of overt cardiogenic shock (including patients after CA) have shown improvements in hemodynamics after induction of mild TH, without apparent adverse effects [136–138].
4. Therapeutic Approach to PAMD and Shock after ROSC
There are no randomized, controlled clinical trials examining different treatment approaches or interventions for PAMD and shock after CA. Early goal-directed therapy (EGDT) has been advocated for hemodynamic optimization of shock after CA based on similarities to septic shock, although recent sepsis studies have failed to show that EGDT improves outcomes [9, 10, 18, 39, 40, 139]. Observational studies show reduced mortality after instituting EGDT protocols in post-CA patients as part of a multifactorial quality improvement strategy including TH and routine coronary angiography [18, 39, 40]. It is difficult to draw conclusions regarding the effects of the EGDT protocol itself on outcomes in the context of these complex interventions.
4.1. Optimizing Preload
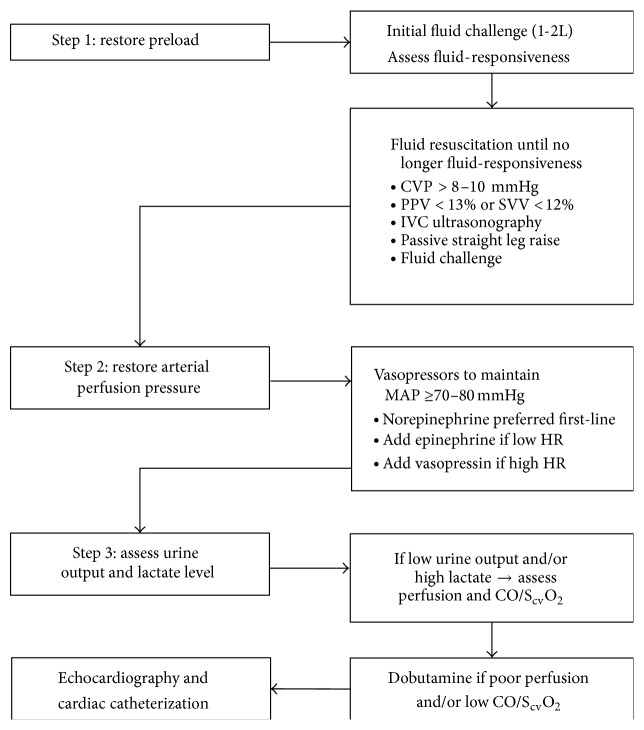
Restoration of adequate preload is the first step in resuscitation of patients with hypotension, shock, or low CO after ROSC (Figure 2) [10]. Large volumes of fluid may be required to maintain adequate CO due to systemic capillary leak from systemic IRI and cytokine release [14]. Initial resuscitation with 1-2 L of isotonic crystalloid is recommended in hypotensive patients after ROSC [10]. Early aggressive fluid resuscitation targeting hemodynamic goals may reduce overall fluid requirements. A central venous pressure of 8–12 mmHg is recommended by guidelines and has been used as a fluid resuscitation endpoint in most studies of EGDT after CA [9, 10, 18, 20, 39]. Our institutional protocol involves use of dynamic measures such as pulse pressure and stroke volume variation to assess fluid-responsiveness due to the limitations of central venous pressure as a measure of preload, particularly in the setting of cardiac dysfunction. Diastolic dysfunction after ROSC predisposes patients to both inadequate CO during relative hypovolemia and pulmonary edema from aggressive fluid administration [140].
Figure 2.
Suggested early goal-directed hemodynamic optimization strategy for patients with hypotension or hypoperfusion after return of spontaneous circulation following cardiac arrest. CVP, central venous pressure; PPV, pulse pressure variation; SVV, stroke volume variation; IVC, inferior vena cava; MAP, mean arterial pressure; HR, heart rate; ScvO2, central venous oxygen saturation; CO, cardiac output.
4.2. Restoring Arterial Pressure
Vasopressor support can counteract the pathologic vasodilation resulting from vascular IRI and inflammatory cytokine release after ROSC, although no randomized studies have explicitly studied specific vasopressors after CA [141]. The need for vasopressors to restore MAP and support tissue perfusion (Figure 2) often lasts for approximately 48–72 hours, even after CO normalizes [14]. Arterial pressure monitoring is prudent for hemodynamically unstable patients with PAMD or shock requiring vasopressor support. Use of norepinephrine as a first-line vasopressor is supported by studies showing favorable outcomes with lower risk of arrhythmias in heterogeneous shock patients receiving norepinephrine [129, 142]. Dopamine is a suboptimal vasopressor based on its lower efficacy and increased risk of tachyarrhythmias and mortality in cardiogenic shock patients in the SOAP-II study [129]. Our institutional protocol is to add epinephrine as the second-line vasopressor for patients with refractory shock, low CO, and/or bradycardia. The optimal HR for patients after CA remains unknown, and many patients tolerate bradycardia remarkably well if they can maintain CO by increasing SV, especially in the presence of diastolic dysfunction or during hypothermia. Vasopressin can be added to counteract refractory vasoplegia in patients with preserved CO and/or tachycardia and may be useful in patients with recurrent tachyarrhythmias due to its lack of proarrhythmic effects [116]. Low-dose hydrocortisone can be added for patients not responding promptly to standard vasopressor therapy and has proven efficacy for reversal of refractory vasodilatory shock [88, 89, 118]. In addition to relative adrenal insufficiency, ionized hypocalcemia and lactic acidosis with severe acidemia are frequent contributors to refractory shock after ROSC [21, 43, 111–114, 143].
The optimal MAP for patients after ROSC remains uncertain, with no consistency between published protocols for hemodynamic support after ROSC. Current American Heart Association guidelines recommend maintaining systolic BP goal ≥90 mmHg and MAP ≥65 mmHg [9, 10]. A MAP ≥70 mmHg is associated with better outcomes after CA, while a MAP <65 mmHg has been associated with poor outcomes and impaired cerebral oximetry [19, 21, 32]. One study reported maximal survival in patients with a MAP range of 76–86 mmHg and maximal cerebral oximetry values with a higher MAP range of 87–101 mmHg [19]. Several authors have recommended a MAP goal ≥80 mmHg to prevent cerebral hypoperfusion in the presence of impaired cerebral blood flow autoregulation after CA [18, 32, 38]. However, elevating the MAP from 70 mmHg to 90 mmHg using norepinephrine failed to improve cerebral oximetry after CA in a small study [144]. Our institutional protocol is to maintain MAP ≥80 mmHg after ROSC, except in patients with severe shock requiring high doses of vasopressor agents when a lower goal of ≥65–70 mmHg is used to avoid excessive vasopressor doses. Vasodilator and/or beta-blocker therapy to maintain MAP ≤100 mmHg is reasonable to reduce myocardial afterload and oxygen demand in patients who remain hypertensive after adequate sedation [9]. One study showed worse outcomes and poorer cerebral oximetry in patients with MAP >100 mmHg after ROSC [19].
4.3. Supporting Tissue Perfusion
Inotropic support may be required to treat persistently low CO after fluid resuscitation (Figure 2), potentially warranting PAC insertion [141]. Indications for inotropic support for shock after CA remain uncertain, although EGDT protocols often recommend inotropic support to augment low CO and/or low ScvO2 [18, 39]. Inotropic agents can aggravate tachyarrhythmias or myocardial ischemia, and no CO value is optimal for all patients [134]. Inotropic agents should be reserved for patients with impaired end-organ perfusion in addition to inadequate CO and/or systemic oxygen delivery, that is, low urine output and/or persistent lactic acidosis in the presence of a low CO or SvO2. Artificially augmenting CO with inotropic support based on low ScvO2 is unlikely to be beneficial in the absence of impaired end-organ perfusion [134]. Reasonable therapeutic goals for inotropic support include a urine output ≥0.5–1 mL/kg/h (up to 1.5 mL/kg/h during TH) and ScvO2 ≥70% with a declining or normal lactate [9, 10]. One study found higher survival in patients with a SvO2 of 67–72%, with optimal cerebral oximetry at SvO2 values of 70–75%; elevated SvO2 values >75% may suggest failure of end-organ oxygen utilization due to mitochondrial dysfunction or microvascular shunting, with an adverse prognosis [19]. Dobutamine doses of 2–5 mcg/kg/min are usually effective for augmenting CO, with no added efficacy and more adverse effects at doses >10 mcg/kg/min [10, 23, 26, 102–104]. The vasodilatory properties of dobutamine may be useful for improving splanchnic perfusion in patients requiring vasopressors [145]. Low-dose dopamine or epinephrine can augment CO and HR in hypotensive patients while avoiding the vasodilatory effects of dobutamine that can exacerbate hypotension when SVR is low [10]. Milrinone carries a higher risk of vasodilatory hypotension but retains efficacy despite beta-receptor downregulation and is less likely to provoke tachyarrhythmias or increase myocardial oxygen demand in selected patients [146].
Patients who have suffered CA due to massive acute MI may develop refractory cardiogenic shock, with a very high mortality rate despite medical therapy [147]. In selected patients, mechanical circulatory support can restore hemodynamic stability and end-organ perfusion [148]. The intra-aortic balloon pump (IABP) appears to provide relatively minimal augmentation of MAP and CO [148]. The large IABP-SHOCK-II trial failed to show a mortality benefit from the use of IABP in revascularized patients with cardiogenic shock after MI [147]. These findings likely apply to patients with PAMD and shock after CA due to MI, because 45% of enrolled patients had been resuscitated from CA. Animal studies suggest greater efficacy of dobutamine than IABP for augmenting hemodynamics after ROSC [104]. The Impella percutaneous left ventricular assist device may be an alternative to IABP after ROSC that provides more robust hemodynamic support [149]. Venoarterial extracorporeal membrane oxygenator (ECMO) pumps have been used as rescue therapy for refractory CA or severe cardiogenic shock after ROSC, and preliminary data suggest that appropriately selected patients can be stabilized on ECMO and survive despite shock refractory to maximal medical therapy [150].
5. Conclusion
PAMD is a multifactorial syndrome developing from the interaction between prearrest cardiac pathology and intra-arrest cardiac insults. PAMD has been reported in up to two-thirds of patients resuscitated from CA, even in the absence of prior cardiac disease. Systolic dysfunction of variable severity is commonly identified, with diastolic dysfunction less frequently reported. PAMD may lead to impaired CO requiring vasoactive support, but shock after ROSC is typically dominated by pathologic vasodilation which persists after normalization of CO. The adverse prognostic value of shock and vasopressor dependency after ROSC is clear, although the contribution of PAMD to adverse outcomes remains uncertain. The pathophysiology of PAMD overlaps with myocardial dysfunction developing as a result of IRI seen after CPB, cytokine excess seen in sepsis, and catecholamine toxicity as in stress-induced cardiomyopathy. Echocardiography is the primary tool for diagnosing PAMD, with invasive hemodynamic monitoring typically warranted for patients with PAMD or shock after ROSC. Treatment of PAMD is similar to other forms of shock, including optimization of preload, restoration of perfusion pressure, and augmentation of contractility to ensure tissue perfusion. Future research is needed to explore the independent relationship between PAMD and outcomes after CA, in addition to the optimal approach to management.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Mozaffarian D., Benjamin E. J., Go A. S., et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–e322. doi: 10.1161/cir.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.McNally B., Robb R., Mehta M., et al. Out-of-hospital cardiac arrest surveillance—cardiac arrest registry to enhance survival (CARES), United States, October 1, 2005–December 31, 2010. Morbidity and Mortality Weekly Report: Surveillance Summaries. 2011;60:1–19. [PubMed] [Google Scholar]
- 3.Merchant R. M., Yang L., Becker L. B., et al. Incidence of treated cardiac arrest in hospitalized patients in the United States. Critical Care Medicine. 2011;39(11):2401–2406. doi: 10.1097/ccm.0b013e3182257459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Girotra S., Nallamothu B. K., Spertus J. A., Li Y., Krumholz H. M., Chan P. S. Trends in survival after in-hospital cardiac arrest. The New England Journal of Medicine. 2012;367(20):1912–1920. doi: 10.1056/nejmoa1109148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laver S., Farrow C., Turner D., Nolan J. Mode of death after admission to an intensive care unit following cardiac arrest. Intensive Care Medicine. 2004;30(11):2126–2128. doi: 10.1007/s00134-004-2425-z. [DOI] [PubMed] [Google Scholar]
- 6.Dragancea I., Rundgren M., Englund E., Friberg H., Cronberg T. The influence of induced hypothermia and delayed prognostication on the mode of death after cardiac arrest. Resuscitation. 2013;84(3):337–342. doi: 10.1016/j.resuscitation.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 7.Lemiale V., Dumas F., Mongardon N., et al. Intensive care unit mortality after cardiac arrest: the relative contribution of shock and brain injury in a large cohort. Intensive Care Medicine. 2013;39(11):1972–1980. doi: 10.1007/s00134-013-3043-4. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez M. M., Berg R. A., Nadkarni V. M., et al. Left ventricular systolic function and outcome after in-hospital cardiac arrest. Circulation. 2008;117(14):1864–1872. doi: 10.1161/CIRCULATIONAHA.107.740167. [DOI] [PubMed] [Google Scholar]
- 9.Neumar R. W., Nolan J. P., Adrie C., et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation. 2008;118(23):2452–2483. doi: 10.1161/CIRCULATIONAHA.108.190652. [DOI] [PubMed] [Google Scholar]
- 10.Peberdy M. A., Callaway C. W., Neumar R. W., et al. Part 9: post-cardiac arrest care: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(3):S768–S786. doi: 10.1161/circulationaha.110.971002. [DOI] [PubMed] [Google Scholar]
- 11.Adrie C., Adib-Conquy M., Laurent I., et al. Successful cardiopulmonary resuscitation after cardiac arrest as a ‘sepsis-like’ syndrome. Circulation. 2002;106(5):562–568. doi: 10.1161/01.cir.0000023891.80661.ad. [DOI] [PubMed] [Google Scholar]
- 12.Adrie C., Laurent I., Monchi M., Cariou A., Dhainaou J.-F., Spaulding C. Postresuscitation disease after cardiac arrest: a sepsis-like syndrome? Current Opinion in Critical Care. 2004;10(3):208–212. doi: 10.1097/01.ccx.0000126090.06275.fe. [DOI] [PubMed] [Google Scholar]
- 13.Bro-Jeppesen J., Kjaergaard J., Wanscher M., et al. The inflammatory response after out-of-hospital cardiac arrest is not modified by targeted temperature management at 33°C or 36°C. Resuscitation. 2014;85(11):1480–1487. doi: 10.1016/j.resuscitation.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Laurent I., Monchi M., Chiche J.-D., et al. Reversible myocardial dysfunction in survivors of out-of-hospital cardiac arrest. Journal of the American College of Cardiology. 2002;40(12):2110–2116. doi: 10.1016/s0735-1097(02)02594-9. [DOI] [PubMed] [Google Scholar]
- 15.Ruiz-Bailén M., Aguayo de Hoyos E., Ruiz-Navarro S., et al. Reversible myocardial dysfunction after cardiopulmonary resuscitation. Resuscitation. 2005;66(2):175–181. doi: 10.1016/j.resuscitation.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Dumas F., Manzo-Silberman S., Fichet J., et al. Can early cardiac troponin I measurement help to predict recent coronary occlusion in out-of-hospital cardiac arrest survivors? Critical Care Medicine. 2012;40(6):1777–1784. doi: 10.1097/ccm.0b013e3182474d5e. [DOI] [PubMed] [Google Scholar]
- 17.Chang W.-T., Ma M. H.-M., Chien K.-L., et al. Postresuscitation myocardial dysfunction: correlated factors and prognostic implications. Intensive Care Medicine. 2007;33(1):88–95. doi: 10.1007/s00134-006-0442-9. [DOI] [PubMed] [Google Scholar]
- 18.Gaieski D. F., Band R. A., Abella B. S., et al. Early goal-directed hemodynamic optimization combined with therapeutic hypothermia in comatose survivors of out-of-hospital cardiac arrest. Resuscitation. 2009;80(4):418–424. doi: 10.1016/j.resuscitation.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 19.Ameloot K., Meex I., Genbrugge C., et al. Hemodynamic targets during therapeutic hypothermia after cardiac arrest: a prospective observational study. Resuscitation. 2015;91:56–62. doi: 10.1016/j.resuscitation.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 20.Bro-Jeppesen J., Hassager C., Wanscher M., et al. Targeted temperature management at 33 degrees C versus 36 degrees C and impact on systemic vascular resistance and myocardial function after out-of-hospital cardiac arrest: a sub-study of the Target Temperature Management Trial. Circulation Cardiovascular Interventions. 2014;7:663–672. doi: 10.1161/CIRCINTERVENTIONS.114.001556. [DOI] [PubMed] [Google Scholar]
- 21.Bro-Jeppesen J., Annborn M., Hassager C., et al. Hemodynamics and vasopressor support during targeted temperature management at 33 degrees C versus 36 degrees C after out-of-hospital cardiac arrest: a post hoc study of the target temperature management trial. Critical Care. 2015;43:318–327. doi: 10.1097/CCM.0000000000000691. [DOI] [PubMed] [Google Scholar]
- 22.Hartke A., Mumma B. E., Rittenberger J. C., Callaway C. W., Guyette F. X. Incidence of re-arrest and critical events during prolonged transport of post-cardiac arrest patients. Resuscitation. 2010;81(8):938–942. doi: 10.1016/j.resuscitation.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oksanen T., Skrifvars M., Wilkman E., Tierala I., Pettilä V., Varpula T. Postresuscitation hemodynamics during therapeutic hypothermia after out-of-hospital cardiac arrest with ventricular fibrillation: a retrospective study. Resuscitation. 2014;85(8):1018–1024. doi: 10.1016/j.resuscitation.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 24.Trzeciak S., Jones A. E., Kilgannon J. H., et al. Significance of arterial hypotension after resuscitation from cardiac arrest. Critical Care Medicine. 2009;37(11):2895–2903. doi: 10.1097/ccm.0b013e3181b01d8c. [DOI] [PubMed] [Google Scholar]
- 25.Kern K. B., Hilwig R. W., Rhee K. H., Berg R. A. Myocardial dysfunction after resuscitation from cardiac arrest: an example of global myocardial stunning. Journal of the American College of Cardiology. 1996;28(1):232–240. doi: 10.1016/0735-1097(96)00130-1. [DOI] [PubMed] [Google Scholar]
- 26.Kern K. B., Hilwig R. W., Berg R. A., et al. Postresuscitation left ventricular systolic and diastolic dysfunction: treatment with dobutamine. Circulation. 1997;95(12):2610–2613. doi: 10.1161/01.cir.95.12.2610. [DOI] [PubMed] [Google Scholar]
- 27.Boland T. A., Lee V. H., Bleck T. P. Stress-induced cardiomyopathy. Critical Care Medicine. 2015;45(3):686–693. doi: 10.1097/ccm.0000000000000851. [DOI] [PubMed] [Google Scholar]
- 28.Xu T., Tang W., Ristagno G., Wang H., Sun S., Weil M. H. Postresuscitation myocardial diastolic dysfunction following prolonged ventricular fibrillation and cardiopulmonary resuscitation. Critical Care Medicine. 2008;36(1):188–192. doi: 10.1097/01.ccm.0000295595.72955.7c. [DOI] [PubMed] [Google Scholar]
- 29.Kaji A. H., Hanif A. M., Thomas J. L., Niemann J. T. Out-of-hospital cardiac arrest: early in-hospital hypotension versus out-of-hospital factors in predicting in-hospital mortality among those surviving to hospital admission. Resuscitation. 2011;82(10):1314–1317. doi: 10.1016/j.resuscitation.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 30.Kilgannon J. H., Roberts B. W., Reihl L. R., et al. Early arterial hypotension is common in the post-cardiac arrest syndrome and associated with increased in-hospital mortality. Resuscitation. 2008;79(3):410–416. doi: 10.1016/j.resuscitation.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beylin M. E., Perman S. M., Abella B. S., et al. Higher mean arterial pressure with or without vasoactive agents is associated with increased survival and better neurological outcomes in comatose survivors of cardiac arrest. Intensive Care Medicine. 2013;39(11):1981–1988. doi: 10.1007/s00134-013-3075-9. [DOI] [PubMed] [Google Scholar]
- 32.Kilgannon J. H., Roberts B. W., Jones A. E., et al. Arterial blood pressure and neurologic outcome after resuscitation from cardiac arrest. Critical Care Medicine. 2014;42(9):2083–2091. doi: 10.1097/ccm.0000000000000406. [DOI] [PubMed] [Google Scholar]
- 33.Young M. N., Hollenbeck R. D., Pollock J. S., et al. Higher achieved mean arterial pressure during therapeutic hypothermia is not associated with neurologically intact survival following cardiac arrest. Resuscitation. 2015;88:158–164. doi: 10.1016/j.resuscitation.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roberts B. W., Kilgannon J. H., Chansky M. E., et al. Multiple organ dysfunction after return of spontaneous circulation in postcardiac arrest syndrome. Critical Care Medicine. 2013;41(6):1492–1501. doi: 10.1097/ccm.0b013e31828a39e9. [DOI] [PubMed] [Google Scholar]
- 35.Torgersen C., Meichtry J., Schmittinger C. A., et al. Haemodynamic variables and functional outcome in hypothermic patients following out-of-hospital cardiac arrest. Resuscitation. 2013;84(6):798–804. doi: 10.1016/j.resuscitation.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 36.Bro-Jeppesen J., Kjaergaard J., Søholm H., et al. Hemodynamics and vasopressor support in therapeutic hypothermia after cardiac arrest: prognostic implications. Resuscitation. 2014;85(5):664–670. doi: 10.1016/j.resuscitation.2013.12.031. [DOI] [PubMed] [Google Scholar]
- 37.Annborn M., Bro-Jeppesen J., Nielsen N., et al. The association of targeted temperature management at 33 and 36°C with outcome in patients with moderate shock on admission after out-of-hospital cardiac arrest: a post hoc analysis of the Target Temperature Management trial. Intensive Care Medicine. 2014;40(9):1210–1219. doi: 10.1007/s00134-014-3375-8. [DOI] [PubMed] [Google Scholar]
- 38.Sundgreen C., Larsen F. S., Herzog T. M., Knudsen G. M., Boesgaard S., Aldershvile J. Autoregulation of cerebral blood flow in patients resuscitated from cardiac arrest. Stroke. 2001;32(1):128–132. doi: 10.1161/01.str.32.1.128. [DOI] [PubMed] [Google Scholar]
- 39.Walters E. L., Morawski K., Dorotta I., et al. Implementation of a post-cardiac arrest care bundle including therapeutic hypothermia and hemodynamic optimization in comatose patients with return of spontaneous circulation after out-of-hospital cardiac arrest: a feasibility study. Shock. 2011;35(4):360–366. doi: 10.1097/shk.0b013e318204c106. [DOI] [PubMed] [Google Scholar]
- 40.Sunde K., Pytte M., Jacobsen D., et al. Implementation of a standardised treatment protocol for post resuscitation care after out-of-hospital cardiac arrest. Resuscitation. 2007;73(1):29–39. doi: 10.1016/j.resuscitation.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 41.Huynh N., Kloke J., Gu C., et al. The effect of hypothermia ‘dose’ on vasopressor requirements and outcome after cardiac arrest. Resuscitation. 2013;84(2):189–193. doi: 10.1016/j.resuscitation.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cocchi M. N., Miller J., Hunziker S., et al. The association of lactate and vasopressor need for mortality prediction in survivors of cardiac arrest. Minerva Anestesiologica. 2011;77(11):1063–1071. [PubMed] [Google Scholar]
- 43.Donnino M. W., Andersen L. W., Giberson T., et al. Initial lactate and lactate change in post-cardiac arrest: a multicenter validation study. Critical Care Medicine. 2014;42(8):1804–1811. doi: 10.1097/ccm.0000000000000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chalkias A., Xanthos T. Pathophysiology and pathogenesis of post-resuscitation myocardial stunning. Heart Failure Reviews. 2012;17(1):117–128. doi: 10.1007/s10741-011-9255-1. [DOI] [PubMed] [Google Scholar]
- 45.Hausenloy D. J., Boston-Griffiths E., Yellon D. M. Cardioprotection during cardiac surgery. Cardiovascular Research. 2012;94(2):253–265. doi: 10.1093/cvr/cvs131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Antonucci E., Fiaccadori E., Donadello K., Taccone F. S., Franchi F., Scolletta S. Myocardial depression in sepsis: from pathogenesis to clinical manifestations and treatment. Journal of Critical Care. 2014;29(4):500–511. doi: 10.1016/j.jcrc.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 47.Ibáñez B., Heusch G., Ovize M., Van de Werf F. Evolving therapies for myocardial ischemia/reperfusion injury. Journal of the American College of Cardiology. 2015;65(14):1454–1471. doi: 10.1016/j.jacc.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 48.Kimmoun A., Novy E., Auchet T., Ducrocq N., Levy B. Hemodynamic consequences of severe lactic acidosis in shock states: from bench to bedside. Critical Care. 2015;19, article 175 doi: 10.1186/s13054-015-0896-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heusch G., Boengler K., Schulz R. Inhibition of mitochondrial permeability transition pore opening: the holy grail of cardioprotection. Basic Research in Cardiology. 2010;105(2):151–154. doi: 10.1007/s00395-009-0080-9. [DOI] [PubMed] [Google Scholar]
- 50.Klouche K., Weil M. H., Sun S., et al. Evolution of the stone heart after prolonged cardiac arrest. Chest. 2002;122(3):1006–1011. doi: 10.1378/chest.122.3.1006. [DOI] [PubMed] [Google Scholar]
- 51.Cour M., Loufouat J., Paillard M., et al. Inhibition of mitochondrial permeability transition to prevent the post-cardiac arrest syndrome: a pre-clinical study. European Heart Journal. 2011;32(2):226–235. doi: 10.1093/eurheartj/ehq112. [DOI] [PubMed] [Google Scholar]
- 52.Huang C.-H., Tsai M.-S., Hsu C.-Y., et al. Post-cardiac arrest myocardial dysfunction is improved with cyclosporine treatment at onset of resuscitation but not in the reperfusion phase. Resuscitation. 2011;82(supplement 2):S41–S47. doi: 10.1016/s0300-9572(11)70150-2. [DOI] [PubMed] [Google Scholar]
- 53.Gill R. S., Lee T.-F., Manouchehri N., et al. Postresuscitation cyclosporine treatment attenuates myocardial and cardiac mitochondrial injury in newborn piglets with asphyxia-reoxygenation. Critical Care Medicine. 2013;41(4):1069–1074. doi: 10.1097/ccm.0b013e3182746704. [DOI] [PubMed] [Google Scholar]
- 54.Piot C., Croisille P., Staat P., et al. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. The New England Journal of Medicine. 2008;359(5):473–481. doi: 10.1056/nejmoa071142. [DOI] [PubMed] [Google Scholar]
- 55.Mewton N., Croisille P., Gahide G., et al. Effect of Cyclosporine on Left Ventricular Remodeling After Reperfused Myocardial Infarction. Journal of the American College of Cardiology. 2010;55(12):1200–1205. doi: 10.1016/j.jacc.2009.10.052. [DOI] [PubMed] [Google Scholar]
- 56.Chiari P., Angoulvant D., Mewton N., et al. Cyclosporine protects the heart during aortic valve surgery. Anesthesiology. 2014;121(2):232–238. doi: 10.1097/aln.0000000000000331. [DOI] [PubMed] [Google Scholar]
- 57.Hausenloy D. J., Kunst G., Boston-Griffiths E., et al. The effect of cyclosporin-A on peri-operative myocardial injury in adult patients undergoing coronary artery bypass graft surgery: a randomised controlled clinical trial. Heart. 2014;100(7):544–549. doi: 10.1136/heartjnl-2013-304845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liakopoulos O. J., Hristov N., Buckberg G. D., Triana J., Trummer G., Allen B. S. Resuscitation after prolonged cardiac arrest: effects of cardiopulmonary bypass and sodium-hydrogen exchange inhibition on myocardial and neurological recovery. European Journal of Cardio-Thoracic Surgery. 2011;40(4):978–984. doi: 10.1016/j.ejcts.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Radhakrishnan J., Kolarova J. D., Ayoub I. M., Gazmuri R. J. AVE4454B—a novel sodium-hydrogen exchanger isoform-1 inhibitor—compared less effective than cariporide for resuscitation from cardiac arrest. Translational Research. 2011;157(2):71–80. doi: 10.1016/j.trsl.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ayoub I. M., Kolarova J., Gazmuri R. J. Cariporide given during resuscitation promotes return of electrically stable and mechanically competent cardiac activity. Resuscitation. 2010;81(1):106–110. doi: 10.1016/j.resuscitation.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ayoub I. M., Kolarova J., Kantola R. L., Sanders R., Gazmuri R. J. Cariporide minimizes adverse myocardial effects of epinephrine during resuscitation from ventricular fibrillation. Critical Care Medicine. 2005;33(11):2599–2605. doi: 10.1097/01.ccm.0000186773.88576.83. [DOI] [PubMed] [Google Scholar]
- 62.Gazmuri R. J., Ayoub I. M., Kolarova J. Myocardial protection during resuscitation from cardiac arrest. Current Opinion in Critical Care. 2003;9(3):199–204. doi: 10.1097/00075198-200306000-00005. [DOI] [PubMed] [Google Scholar]
- 63.Lin X., Kraut J. A., Wu D. Coadministration of a Na+-H+ exchange inhibitor and sodium bicarbonate for the treatment of asphyxia-induced cardiac arrest in piglets. Pediatric Research. 2014;76(2):118–126. doi: 10.1038/pr.2014.65. [DOI] [PubMed] [Google Scholar]
- 64.Lin X., Lee D., Wu D. Protective effects of NHE1 inhibition with sabiporide in an experimental model of asphyxia-induced cardiac arrest in piglets. Resuscitation. 2013;84(4):520–525. doi: 10.1016/j.resuscitation.2012.08.334. [DOI] [PubMed] [Google Scholar]
- 65.Mentzer R. M., Jr., Bartels C., Bolli R., et al. Sodium-hydrogen exchange inhibition by cariporide to reduce the risk of ischemic cardiac events in patients undergoing coronary artery bypass grafting: results of the EXPEDITION study. The Annals of Thoracic Surgery. 2008;85(4):1261–1270. doi: 10.1016/j.athoracsur.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 66.Gu W., Li C., Yin W., Guo Z., Hou X., Zhang D. Shen-fu injection reduces postresuscitation myocardial dysfunction in a porcine model of cardiac arrest by modulating apoptosis. Shock. 2012;38(3):301–306. doi: 10.1097/shk.0b013e31825f6632. [DOI] [PubMed] [Google Scholar]
- 67.Ji X.-F., Yang L., Zhang M.-Y., Li C.-S., Wang S., Cong L.-H. Shen-Fu injection attenuates postresuscitation myocardial dysfunction in a porcine model of cardiac arrest. Shock. 2011;35(5):530–536. doi: 10.1097/shk.0b013e31820e2058. [DOI] [PubMed] [Google Scholar]
- 68.Bro-Jeppesen J., Kjaergaard J., Wanscher M., et al. Systemic inflammatory response and potential prognostic implications after out-of-hospital cardiac arrest: a substudy of the target temperature management trial. Critical Care Medicine. 2015;43(6):1223–1232. doi: 10.1097/CCM.0000000000000937. [DOI] [PubMed] [Google Scholar]
- 69.Geppert A., Zorn G., Karth G. D., et al. Soluble selectins and the systemic inflammatory response syndrome after successful cardiopulmonary resuscitation. Critical Care Medicine. 2000;28(7):2360–2365. doi: 10.1097/00003246-200007000-00030. [DOI] [PubMed] [Google Scholar]
- 70.Niemann J. T., Rosborough J. P., Youngquist S., et al. Cardiac function and the proinflammatory cytokine response after recovery from cardiac arrest in swine. Journal of Interferon & Cytokine Research. 2009;29(11):749–757. doi: 10.1089/jir.2009.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Omar S., Zedan A., Nugent K. Cardiac vasoplegia syndrome: pathophysiology, risk factors and treatment. The American Journal of the Medical Sciences. 2015;349(1):80–88. doi: 10.1097/maj.0000000000000341. [DOI] [PubMed] [Google Scholar]
- 72.Pathan N., Hemingway C. A., Alizadeh A. A., et al. Role of interleukin 6 in myocardial dysfunction of meningococcal septic shock. The Lancet. 2004;363(9404):203–209. doi: 10.1016/s0140-6736(03)15326-3. [DOI] [PubMed] [Google Scholar]
- 73.Kumar A., Thota V., Dee L., Olson J., Uretz E., Parrillo J. E. Tumor necrosis factor alpha and interleukin 1beta are responsible for in vitro myocardial cell depression induced by human septic shock serum. The Journal of Experimental Medicine. 1996;183(3):949–958. doi: 10.1084/jem.183.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Landesberg G., Gilon D., Meroz Y., et al. Diastolic dysfunction and mortality in severe sepsis and septic shock. European Heart Journal. 2012;33(7):895–903. doi: 10.1093/eurheartj/ehr351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zahler S., Massoudy P., Hartl H., Hähnel C., Meisner H., Becker B. F. Acute cardiac inflammatory responses to postischemic reperfusion during cardiopulmonary bypass. Cardiovascular Research. 1999;41(3):722–730. doi: 10.1016/S0008-6363(98)00229-6. [DOI] [PubMed] [Google Scholar]
- 76.Duffy J. Y., McLean K. M., Lyons J. M., Czaikowski A. J., Wagner C. J., Pearl J. M. Modulation of nuclear factor-kappaB improves cardiac dysfunction associated with cardiopulmonary bypass and deep hypothermic circulatory arrest. Critical Care Medicine. 2009;37(2):577–583. doi: 10.1097/ccm.0b013e318194ab65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liakopoulos O. J., Teucher N., Mühlfeld C., et al. Prevention of TNFalpha-associated myocardial dysfunction resulting from cardiopulmonary bypass and cardioplegic arrest by glucocorticoid treatment. European Journal of Cardio-Thoracic Surgery. 2006;30(2):263–270. doi: 10.1016/j.ejcts.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 78.Murphy G. S., Whitlock R. P., Gutsche J. T., Augoustides J. G. T. Steroids for adult cardiac surgery with cardiopulmonary bypass: update on dose and key randomized trials. Journal of Cardiothoracic and Vascular Anesthesia. 2013;27(5):1053–1059. doi: 10.1053/j.jvca.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 79.Vaahersalo J., Skrifvars M. B., Pulkki K., et al. Admission interleukin-6 is associated with post resuscitation organ dysfunction and predicts long-term neurological outcome after out-of-hospital ventricular fibrillation. Resuscitation. 2014;85(11):1573–1579. doi: 10.1016/j.resuscitation.2014.08.036. [DOI] [PubMed] [Google Scholar]
- 80.Dell'anna A. M., Bini Vinotti J., Beumier M., et al. C-reactive protein levels after cardiac arrest in patients treated with therapeutic hypothermia. Resuscitation. 2014;85(7):932–938. doi: 10.1016/j.resuscitation.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 81.Laurent I., Adrie C., Vinsonneau C., et al. High-volume hemofiltration after out-of-hospital cardiac arrest: a randomized study. Journal of the American College of Cardiology. 2005;46(3):432–437. doi: 10.1016/j.jacc.2005.04.039. [DOI] [PubMed] [Google Scholar]
- 82.Moe G. W., Marin-Garcia J., Konig A., Goldenthal M., Lu X., Feng Q. In vivo TNF-α inhibition ameliorates cardiac mitochondrial dysfunction, oxidative stress, and apoptosis in experimental heart failure. American Journal of Physiology—Heart and Circulatory Physiology. 2004;287(4):H1813–H1820. doi: 10.1152/ajpheart.00036.2004. [DOI] [PubMed] [Google Scholar]
- 83.Han F., Da T., Riobo N. A., Becker L. B. Early mitochondrial dysfunction in electron transfer activity and reactive oxygen species generation after cardiac arrest. Critical Care Medicine. 2008;36(11):S447–S453. doi: 10.1097/ccm.0b013e31818a8a51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gulick T., Chung M. K., Pieper S. J., Lange L. G., Schreiner G. F. Interleukin 1 and tumor necrosis factor inhibit cardiac myocyte β-adrenergic responsiveness. Proceedings of the National Academy of Sciences of the United States of America. 1989;86(17):6753–6757. doi: 10.1073/pnas.86.17.6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Niemann J. T., Youngquist S., Rosborough J. P., Shah A. P., Phan Q. T., Filler S. G. Infliximab attenuates early myocardial dysfunction after resuscitation in a swine cardiac arrest model. Critical Care Medicine. 2010;38(4):1162–1167. doi: 10.1097/ccm.0b013e3181d44324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Youngquist S. T., Niemann J. T., Shah A. P., Thomas J. L., Rosborough J. P. A comparison of etanercept vs. infliximab for the treatment of post-arrest myocardial dysfunction in a swine model of ventricular fibrillation. Resuscitation. 2013;84(7):999–1003. doi: 10.1016/j.resuscitation.2012.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Niemann J. T., Youngquist S. T., Shah A. P., Thomas J. L., Rosborough J. P. TNF-α blockade improves early post-resuscitation survival and hemodynamics in a swine model of ischemic ventricular fibrillation. Resuscitation. 2013;84(1):103–107. doi: 10.1016/j.resuscitation.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mentzelopoulos S. D., Malachias S., Chamos C., et al. Vasopressin, steroids, and epinephrine and neurologically favorable survival after in-hospital cardiac arrest: a randomized clinical trial. The Journal of the American Medical Association. 2013;310(3):270–279. doi: 10.1001/jama.2013.7832. [DOI] [PubMed] [Google Scholar]
- 89.Mentzelopoulos S. D., Zakynthinos S. G., Tzoufi M., et al. Vasopressin, epinephrine, and corticosteroids for in-hospital cardiac arrest. Archives of Internal Medicine. 2009;169(1):15–24. doi: 10.1001/archinternmed.2008.509. [DOI] [PubMed] [Google Scholar]
- 90.Fischer M., Hossmann K.-A. No-reflow after cardiac arrest. Intensive Care Medicine. 1995;21(2):132–141. doi: 10.1007/bf01726536. [DOI] [PubMed] [Google Scholar]
- 91.Durante A., Camici P. G. Novel insights into an ‘old’ phenomenon: the no reflow. International Journal of Cardiology. 2015;187:273–280. doi: 10.1016/j.ijcard.2015.03.359. [DOI] [PubMed] [Google Scholar]
- 92.Kern K. B., Sasaoka T., Higashi H., Hilwig R. W., Berg R. A., Zuercher M. Post-resuscitation myocardial microcirculatory dysfunction is ameliorated with eptifibatide. Resuscitation. 2011;82(1):85–89. doi: 10.1016/j.resuscitation.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 93.Padosch S. A., Teschendorf P., Fuchs A., et al. Effects of abciximab on postresuscitation microcirculatory dysfunction after experimental cardiac arrest in rats. Resuscitation. 2010;81(2):255–259. doi: 10.1016/j.resuscitation.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 94.Dokken B. B., Hilwig W. R., Teachey M. K., et al. Glucagon-like peptide-1 (GLP-1) attenuates post-resuscitation myocardial microcirculatory dysfunction. Resuscitation. 2010;81(6):755–760. doi: 10.1016/j.resuscitation.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 95.Abraham J., Mudd J. O., Kapur N., Klein K., Champion H. C., Wittstein I. S. Stress cardiomyopathy after intravenous administration of catecholamines and beta-receptor agonists. Journal of the American College of Cardiology. 2009;53(15):1320–1325. doi: 10.1016/j.jacc.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 96.Tang W., Weil M. H., Sun S., Noc M., Yang L., Gazmuri R. J. Epinephrine increases the severity of postresuscitation myocardial dysfunction. Circulation. 1995;92(10):3089–3093. doi: 10.1161/01.CIR.92.10.3089. [DOI] [PubMed] [Google Scholar]
- 97.Pellis T., Weil M. H., Tang W., et al. Evidence favoring the use of an α 2-selective vasopressor agent for cardiopulmonary resuscitation. Circulation. 2003;108(21):2716–2721. doi: 10.1161/01.cir.0000096489.40209.dd. [DOI] [PubMed] [Google Scholar]
- 98.Zhang Q., Li C. Combination of epinephrine with esmolol attenuates post-resuscitation myocardial dysfunction in a porcine model of cardiac arrest. PLoS ONE. 2013;8(12) doi: 10.1371/journal.pone.0082677.e82677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Paur H., Wright P. T., Sikkel M. B., et al. High levels of circulating epinephrine trigger apical cardiodepression in a beta2-adrenergic receptor/Gi-dependent manner: a new model of takotsubo cardiomyopathy. Circulation. 2012;126(6):697–706. doi: 10.1161/circulationaha.112.111591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ji X.-F., Shuo W., Yang L., Li C.-S. Impaired beta-adrenergic receptor signalling in post-resuscitation myocardial dysfunction. Resuscitation. 2012;83(5):640–644. doi: 10.1016/j.resuscitation.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 101.Gerhardt M. A., Booth J. V., Chesnut L. C., et al. Acute myocardial β-adrenergic receptor dysfunction after cardiopulmonary bypass in patients with cardiac valve disease. Duke Heart Center Perioperative Desensitization Group. Circulation. 1998;98(19):II275–II281. [PubMed] [Google Scholar]
- 102.Vasquez A., Kern K. B., Hilwig R. W., Heidenreich J., Berg R. A., Ewy G. A. Optimal dosing of dobutamine for treating post-resuscitation left ventricular dysfunction. Resuscitation. 2004;61(2):199–207. doi: 10.1016/j.resuscitation.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 103.Meyer R. J., Kern K. B., Berg R. A., Hilwig R. W., Ewy G. A. Post-resuscitation right ventricular dysfunction: delineation and treatment with dobutamine. Resuscitation. 2002;55(2):187–191. doi: 10.1016/s0300-9572(02)00204-6. [DOI] [PubMed] [Google Scholar]
- 104.Tennyson H., Kern K. B., Hilwig R. W., Berg R. A., Ewy G. A. Treatment of post resuscitation myocardial dysfunction: aortic counterpulsation versus dobutamine. Resuscitation. 2002;54(1):69–75. doi: 10.1016/s0300-9572(02)00055-2. [DOI] [PubMed] [Google Scholar]
- 105.Atiksawedparit P., Rattanasiri S., McEvoy M., Graham C. A., Sittichanbuncha Y., Thakkinstian A. Effects of prehospital adrenaline administration on out-of-hospital cardiac arrest outcomes: a systematic review and meta-analysis. Critical Care. 2014;18, article 463 doi: 10.1186/s13054-014-0463-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vandycke C., Martens P. High dose versus standard dose epinephrine in cardiac arrest—a meta-analysis. Resuscitation. 2000;45(3):161–166. doi: 10.1016/s0300-9572(00)00188-x. [DOI] [PubMed] [Google Scholar]
- 107.Callaham M., Madsen C. D., Barton C. W., Saunders C. E., Pointer J. A randomized clinical trial of high-dose epinephrine and norepinephrine vs standard-dose epinephrine in prehospital cardiac arrest. Journal of the American Medical Association. 1992;268(19):2667–2672. doi: 10.1001/jama.268.19.2667. [DOI] [PubMed] [Google Scholar]
- 108.Layek A., Maitra S., Pal S., Bhattacharjee S., Baidya D. K. Efficacy of vasopressin during cardio-pulmonary resuscitation in adult patients: a meta-analysis. Resuscitation. 2014;85(7):855–863. doi: 10.1016/j.resuscitation.2014.03.303. [DOI] [PubMed] [Google Scholar]
- 109.Rivers E. P., Wortsman J., Rady M. Y., Blake H. C., McGeorge F. T., Buderer N. M. The effect of the total cumulative epinephrine dose administered during human CPR on hemodynamic, oxygen transport, and utilization variables in the postresuscitation period. Chest. 1994;106(5):1499–1507. doi: 10.1378/chest.106.5.1499. [DOI] [PubMed] [Google Scholar]
- 110.Lindner K. H., Haak T., Keller A., Bothner U., Lurie K. G. Release of endogenous vasopressors during and after cardiopulmonary resuscitation. Heart. 1996;75(2):145–150. doi: 10.1136/hrt.75.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ito T., Saitoh D., Takasu A., Kiyozumi T., Sakamoto T., Okada Y. Serum cortisol as a predictive marker of the outcome in patients resuscitated after cardiopulmonary arrest. Resuscitation. 2004;62(1):55–60. doi: 10.1016/j.resuscitation.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 112.Pene F., Hyvernat H., Mallet V., et al. Prognostic value of relative adrenal insufficiency after out-of-hospital cardiac arrest. Intensive Care Medicine. 2005;31(5):627–633. doi: 10.1007/s00134-005-2603-7. [DOI] [PubMed] [Google Scholar]
- 113.Kim J. J., Lim Y. S., Shin J. H., et al. Relative adrenal insufficiency after cardiac arrest: impact on postresuscitation disease outcome. The American Journal of Emergency Medicine. 2006;24(6):684–688. doi: 10.1016/j.ajem.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 114.Miller J. B., Donnino M. W., Rogan M., Goyal N. Relative adrenal insufficiency in post-cardiac arrest shock is under-recognized. Resuscitation. 2008;76(2):221–225. doi: 10.1016/j.resuscitation.2007.07.034. [DOI] [PubMed] [Google Scholar]
- 115.Russell J. A. Bench-to-bedside review: vasopressin in the management of septic shock. Critical Care. 2011;15(4, article 126) doi: 10.1186/cc8224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mayr V., Luckner G., Jochberger S., et al. Arginine vasopressin in advanced cardiovascular failure during the post-resuscitation phase after cardiac arrest. Resuscitation. 2007;72(1):35–44. doi: 10.1016/j.resuscitation.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 117.Tilley D. G., Zhu W., Myers V. D., et al. Beta-adrenergic receptor-mediated cardiac contractility is inhibited via vasopressin type 1A-receptor-dependent signaling. Circulation. 2014;130:1800–1811. doi: 10.1161/circulationaha.114.010434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Marik P. E., Pastores S. M., Annane D., et al. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care Medicine. Critical Care Medicine. 2008;36(6):1937–1949. doi: 10.1097/ccm.0b013e31817603ba. [DOI] [PubMed] [Google Scholar]
- 119.Gazmuri R. J. Effects of repetitive electrical shocks on postresuscitation myocardial function. Critical Care Medicine. 2000;28(11):N228–N232. doi: 10.1097/00003246-200011001-00016. [DOI] [PubMed] [Google Scholar]
- 120.Xie J., Weil M. H., Sun S., et al. High-energy defibrillation increases the severity of postresuscitation myocardial dysfunction. Circulation. 1997;96(2):683–688. doi: 10.1161/01.cir.96.2.683. [DOI] [PubMed] [Google Scholar]
- 121.Tang W., Weil M. H., Sun S. Low-energy biphasic waveform defibrillation reduces the severity of postresuscitation myocardial dysfunction. Critical Care Medicine. 2000;28(11):N222–N224. doi: 10.1097/00003246-200011001-00014. [DOI] [PubMed] [Google Scholar]
- 122.Toh N., Nishii N., Nakamura K., et al. Cardiac dysfunction and prolonged hemodynamic deterioration after implantable cardioverter-defibrillator shock in patients with systolic heart failure. Circulation: Arrhythmia and Electrophysiology. 2012;5(5):898–905. doi: 10.1161/circep.111.970285. [DOI] [PubMed] [Google Scholar]
- 123.Bilotta F., Fiorani L., La Rosa I., Spinelli F., Rosa G. Cardiovascular effects of intravenous propofol administered at two infusion rates: a transthoracic echocardiographic study. Anaesthesia. 2001;56(3):266–271. doi: 10.1046/j.1365-2044.2001.01717-5.x. [DOI] [PubMed] [Google Scholar]
- 124.Niwa H., Tanimoto A., Sugimura M., Morimoto Y., Hanamoto H. Cardiovascular effects of epinephrine under sedation with nitrous oxide, propofol, or midazolam. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontology. 2006;102:e1–e9. doi: 10.1016/j.tripleo.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 125.Sprung J., Ogletree-Hughes M. L., McConnell B. K., Zakhary D. R., Smolsky S. M., Moravec C. S. The effects of propofol on the contractility of failing and nonfailing human heart muscles. Anesthesia and Analgesia. 2001;93(3):550–559. doi: 10.1097/00000539-200109000-00006. [DOI] [PubMed] [Google Scholar]
- 126.Kanaya N., Gable B., Wickley P. J., Murray P. A., Damron D. S. Experimental conditions are important determinants of cardiac inotropic effects of propofol. Anesthesiology. 2005;103(5):1026–1034. doi: 10.1097/00000542-200511000-00017. [DOI] [PubMed] [Google Scholar]
- 127.Bjelland T. W., Dale O., Kaisen K., et al. Propofol and remifentanil versus midazolam and fentanyl for sedation during therapeutic hypothermia after cardiac arrest: a randomised trial. Intensive Care Medicine. 2012;38(6):959–967. doi: 10.1007/s00134-012-2540-1. [DOI] [PubMed] [Google Scholar]
- 128.De Backer D., Creteur J., Silva E., Vincent J.-L. Effects of dopamine, norepinephrine, and epinephrine on the splanchnic circulation in septic shock: which is best? Critical Care Medicine. 2003;31(6):1659–1667. doi: 10.1097/01.ccm.0000063045.77339.b6. [DOI] [PubMed] [Google Scholar]
- 129.De Backer D., Biston P., Devriendt J., et al. Comparison of dopamine and norepinephrine in the treatment of shock. The New England Journal of Medicine. 2010;362(9):779–789. doi: 10.1056/nejmoa0907118. [DOI] [PubMed] [Google Scholar]
- 130.Bernard S. A., Gray T. W., Buist M. D., et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. The New England Journal of Medicine. 2002;346(8):557–563. doi: 10.1056/nejmoa003289. [DOI] [PubMed] [Google Scholar]
- 131.The Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. The New England Journal of Medicine. 2002;346(8):549–556. doi: 10.1056/nejmoa012689. [DOI] [PubMed] [Google Scholar]
- 132.Rittenberger J. C., Callaway C. W. Temperature management and modern post-cardiac arrest care. The New England Journal of Medicine. 2013;369(23):2262–2263. doi: 10.1056/nejme1312700. [DOI] [PubMed] [Google Scholar]
- 133.Nielsen N., Wetterslev J., Cronberg T., et al. Targeted temperature management at 33°C versus 36°C after cardiac arrest. The New England Journal of Medicine. 2013;369(23):2197–2206. doi: 10.1056/nejmoa1310519. [DOI] [PubMed] [Google Scholar]
- 134.Giraud R., Siegenthaler N., Bendjelid K. Cardiac index during therapeutic hypothermia: which target value is optimal? Critical Care. 2013;17, article 214 doi: 10.1186/cc12523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Roberts B. W., Kilgannon J. H., Chansky M. E., et al. Therapeutic hypothermia and vasopressor dependency after cardiac arrest. Resuscitation. 2013;84(3):331–336. doi: 10.1016/j.resuscitation.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 136.Stegman B., Aggarwal B., Senapati A., Shao M., Menon V. Serial hemodynamic measurements in post-cardiac arrest cardiogenic shock treated with therapeutic hypothermia. European Heart Journal: Acute Cardiovascular Care. 2015;4(3):263–269. doi: 10.1177/2048872614547688. [DOI] [PubMed] [Google Scholar]
- 137.Schmidt-Schweda S., Ohler A., Post H., Pieske B. Moderate hypothermia for severe cardiogenic shock (COOL Shock Study I & II) Resuscitation. 2013;84(3):319–325. doi: 10.1016/j.resuscitation.2012.09.034. [DOI] [PubMed] [Google Scholar]
- 138.Zobel C., Adler C., Kranz A., et al. Mild therapeutic hypothermia in cardiogenic shock syndrome. Critical Care Medicine. 2012;40(6):1715–1723. doi: 10.1097/CCM.0b013e318246b820. [DOI] [PubMed] [Google Scholar]
- 139.Angus D. C., Barnato A. E., Bell D., et al. A systematic review and meta-analysis of early goal-directed therapy for septic shock: the ARISE, ProCESS and ProMISe Investigators. Intensive Care Medicine. 2015 doi: 10.1007/s00134-015-3822-1. [DOI] [PubMed] [Google Scholar]
- 140.Kim F., Nichol G., Maynard C., et al. Effect of prehospital induction of mild hypothermia on survival and neurological status among adults with cardiac arrest: a randomized clinical trial. Journal of the American Medical Association. 2014;311(1):45–52. doi: 10.1001/jama.2013.282173. [DOI] [PubMed] [Google Scholar]
- 141.Kakavas S., Chalkias A., Xanthos T. Vasoactive support in the optimization of post-cardiac arrest hemodynamic status: from pharmacology to clinical practice. European Journal of Pharmacology. 2011;667(1–3):32–40. doi: 10.1016/j.ejphar.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 142.de Backer D., Aldecoa C., Njimi H., Vincent J.-L. Dopamine versus norepinephrine in the treatment of septic shock: a meta-analysis. Critical Care Medicine. 2012;40(3):725–730. doi: 10.1097/ccm.0b013e31823778ee. [DOI] [PubMed] [Google Scholar]
- 143.Gando S., Igarashi M., Kameue T., Nanzaki S. Ionized hypocalcemia during out-of-hospital cardiac arrest and cardiopulmonary resuscitation is not due to binding by lactate. Intensive Care Medicine. 1997;23(12):1245–1250. doi: 10.1007/s001340050493. [DOI] [PubMed] [Google Scholar]
- 144.Bouzat P., Suys T., Sala N., Oddo M. Effect of moderate hyperventilation and induced hypertension on cerebral tissue oxygenation after cardiac arrest and therapeutic hypothermia. Resuscitation. 2013;84(11):1540–1545. doi: 10.1016/j.resuscitation.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 145.Duranteau J., Sitbon P., Teboul J. L., et al. Effects of epinephrine, norepinephrine, or the combination of norepinephrine and dobutamine on gastric mucosa in septic shock. Critical Care Medicine. 1999;27(5):893–900. doi: 10.1097/00003246-199905000-00021. [DOI] [PubMed] [Google Scholar]
- 146.Niemann J. T., Garner D., Khaleeli E., Lewis R. J. Milrinone facilitates resuscitation from cardiac arrest and attenuates postresuscitation myocardial dysfunction. Circulation. 2003;108(24):3031–3035. doi: 10.1161/01.cir.0000101925.37174.85. [DOI] [PubMed] [Google Scholar]
- 147.Thiele H., Zeymer U., Neumann F.-J., et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. The New England Journal of Medicine. 2012;367(14):1287–1296. doi: 10.1056/nejmoa1208410. [DOI] [PubMed] [Google Scholar]
- 148.Rihal C. S., Naidu S. S., Givertz M. M., et al. 2015 SCAI/ACC/HFSA/STS Clinical Expert Consensus Statement on the Use of Percutaneous Mechanical Circulatory Support Devices in Cardiovascular Care: Endorsed by the American Heart Assocation, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; Affirmation of Value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d'intervention. Journal of the American College of Cardiology. 2015;65(19):e7–e26. doi: 10.1016/j.jacc.2015.03.036. [DOI] [PubMed] [Google Scholar]
- 149.Manzo-Silberman S., Fichet J., Mathonnet A., et al. Percutaneous left ventricular assistance in post cardiac arrest shock: comparison of intra aortic blood pump and IMPELLA Recover LP2.5. Resuscitation. 2013;84(5):609–615. doi: 10.1016/j.resuscitation.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 150.Xie A., Phan K., Yi-Chin Tsai M., Yan T. D., Forrest P. Venoarterial extracorporeal membrane oxygenation for cardiogenic shock and cardiac arrest: a meta-analysis. Journal of Cardiothoracic and Vascular Anesthesia. 2015;29(3):637–645. doi: 10.1053/j.jvca.2014.09.005. [DOI] [PubMed] [Google Scholar]