Abstract
Butyrate is a major metabolite in colonic lumen. It is produced from bacterial fermentation of dietary fiber. Butyrate has been shown to stimulate electroneutral sodium absorption through its regulation on sodium/hydrogen exchanger 3 (NHE3). Although NHE8, the newest addition of intestinal NHE family, is involved in sodium absorption in the intestinal tract, whether butyrate modulates NHE8 expression in the intestinal epithelial cells is not known. In the current study, we showed that butyrate treatment strongly induced NHE8 protein and NHE8 mRNA expression in human intestinal epithelial cells. Transfection with the human NHE8 promoter reporter constructs showed that butyrate treatment stimulated reporter gene expression at an amount comparable with its stimulation of NHE8 mRNA expression. Interestingly, a similar result was also observed in human NHE8 promoter transfected cells after trichostatin (TSA) treatment. Gel mobility shift assay identified an enhanced Sp3 protein binding on the human NHE8 basal promoter region upon butyrate stimulation. Furthermore, Sp3 acetylation modification is involved in butyrate-mediated NHE8 activation in Caco-2 cells. Our findings suggest that the mechanism of butyrate action on NHE8 expression involves enhanced Sp3 interaction at the basal promoter region of the human NHE8 gene promoter to activate NHE8 gene transcription. Thus butyrate is involved in intestinal regulation of NHE8 resulting enhanced sodium absorption.
Keywords: butyrate, NHE8, intestine, Sp3, acetylation
butyrate, a member of the short-chain fatty acid family, is produced in large amounts in the colonic lumen by bacterial fermentation of carbohydrates (7). It is important for mucosal homeostasis by modulating cell differentiation and apoptosis (12, 13). Butyrate is the preferred fuel for the colonic epithelial cells and also has significant effects on maintaining colonic health (22). Studies showed that lack of short-chain fatty acids could result in colonic inflammation, such as seen in diversion colitis (11). Decreased availability of luminal butyrate and/or impaired butyrate metabolism have also been found to contribute to ulcerative colitis and pseudomembranous colitis (10, 25).
In the intestine, butyrate stimulates sodium absorption by increasing the activity of the epithelial sodium channel (ENaC) and sodium/hydrogen exchanger 3 (NHE3). Unlike sodium channels, which transport sodium following concentration gradient, NHEs mediate the movement of extracellular Na+ into cells in exchange for intracellular H+. Multiple NHE isoforms are identified in the intestinal epithelial cells. NHE1 is located at the basolateral membrane in the intestinal epithelial cell and is involved in intracellular pH regulation and cell volume regulation. NHE2, NHE3, and NHE8 are expressed at the apical membrane in the intestinal epithelial cells and participate in neutral sodium absorption (29, 36). Studies have shown only NHE3, not NHE1 or NHE2, is regulated by butyrate. Because NHE8, the most recently identified NHE, also contributes to sodium absorption (28, 29) and is highly expressed in the distal intestine where the butyrate content is also high (19, 24, 34), we postulated that butyrate also regulates NHE8 expression in the intestine.
The present study confirmed that butyrate also has an effect on NHE8 expression in human intestinal epithelial cells. Butyrate stimulates intestinal NHE8 expression in Caco-2 cells through activating NHE8 gene transcription, and the increased NHE8 transcription activation is a result of enhanced Sp3 protein interaction on NHE8 gene basal promoter region. These findings suggest that butyrate may be an important player in regulating intestinal NHE8 gene transcription. Thus NHE8 is likely a novel target for the beneficial effect of short chain fatty acids on sodium absorption in the intestine.
MATERIALS AND METHODS
Cell culture.
Human intestinal epithelial cells (Caco-2) were purchased from American Type Culture Collection (ATCC, Manassas, VA) and cultured according to ATCC guidelines. Cells were cultured in MEM-NEAA medium (HyClone; GE Healthcare Life Sciences, Logan, UT) containing 20% fetal bovine serum (HyClone) and 1% penicillin-streptomycin (HyClone) at 37°C in a 95% air-5% CO2 atmosphere and passaged every 48 to 72 h. For butyrate treatment experiments, cells were incubated with 10 mM sodium butyrate (Sigma-Aldrich, St. Louis, MO) for 16 h before they were harvested. For the trichostatin A (TSA) (Cell Signaling Technology, Danvers, MA) study, cells were treated with 1 μM TSA for 16 h before they were harvested.
Protein purification, immnuoprecipitation, and Western blot analysis.
Total protein was prepared in radioimmunoprecipitation assay buffer as previously described (6) and used for Western blot detection for NHE8 protein and β-actin protein. Nuclear protein was extracted according to previously described method (31) and used for gel mobility shift assay (GMSA) and Western blot detection of Sp3 protein and TATA binding protein (TBP). For immunoprecipitation, cell lysates were immunoprecipitated with Sp3 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and used for Western blot detection of acetylated Sp3 protein. For Western blot detection, NHE8 antibody was used as a 1:3,000 dilution to detect NHE8 protein (29). β-Actin antiserum (Sigma) was used as a 1:5,000 dilution to determine β-actin protein abundance. TBP antiserum (Abcam, Cambridge, MA) was used as a 1:2,000 dilution to determine TBP abundance. Sp3 antiserum (Santa Cruz Biotechnology) was used as a 1:3,000 dilution to determine Sp3 protein abundance. Acetylated-lysine antibody (Cell Signaling Technology) was used as a 1:1,000 dilution to detect acetylated protein. Western detection was performed with the BM Chemiluminescence Western Blotting Kit (Roche Diagnostics, Indianapolis, IN). For NHE8 protein expression level quantitation, a ratio of NHE8 protein intensity over β-actin protein intensity was used. For Sp3 protein assay, a ratio of Sp3 protein intensity over TBP protein intensity was used.
RNA purification and PCR analysis to detect NHE8 expression.
RNA was purified from Caco-2 cells using TRIzol reagent (Invitrogen, Carlsbad, CA). Total RNA (500 ng) was used for reverse transcription reaction. TaqMan technology was used to determine the expression levels of NHE8 using human NHE8 and TBP primers from Applied Biosystems (Foster City, CA). Resulting data were analyzed using the comparative cycle threshold (Ct) method. The target gene cycle thresholds are adjusted relative to a calibrator (normalized Ct value obtained from control groups) and expressed as 2−ΔΔCt (Applied Biosystems User Bulletin no. 2: Rev B “Relative Quantitation of Gene Expression”). TBP data was used as an endogenous reference to normalize expression levels.
Promoter-reporter construct preparation, transient transfection, and functional promoter analysis.
The human NHE8 gene promoter constructs (pGL3b/−32 and pGL3b/−1905) were made by PCR method. The pGL3b/−32 construct was made as previously described (27). To make pGL3b/-1905 construct, the 5'-flanking region of NHE8 gene was PCR amplified from human genomic DNA by using primers with adapters containing Kpn I and Bgl II restriction enzyme sites (Forward primer TGGGTACCGCCTGAATCCCAGCCTT covering NHE8 gene promoter region −1905 bp to −1889 bp; Reverse primer ACTGAGATCTCCTGCGGAGCTTCCAGGAC covering NHE8 gene +77 bp to +96 bp; bold letters indicate introduced restriction enzyme sites and underlined regions indicate human NHE8 5'-flanking sequences). This PCR fragment was directionally cloned into the pGL3-basic vector. Both promoter constructs were confirmed by sequencing. For transfection experiments, Caco-2 cells were cultured in 24-well plates. When cell density reached 60% to 70%, Caco-2 cells were transfected with promoter construct using Effectene (Qiagen, Valencia, CA) according to the manufacturer's instructions. Promoter reporter assay was performed 40 h after transfection using a dual luciferase assay kit according to the manufacturer's instructions (Promega, Madison, WI). Luciferase activities were measured with a luminometer (Femtomaster FB 12; Berthold Detection System, Pforzheim, Germany). Renilla luciferase activity driven by pRL-CMV (Promega) was used as an internal control to calculate the relative luciferase activity. To test the effect of sodium butyrate or TSA on human NHE8 promoter activity, transfected cells were treated with 10 mM sodium butyrate or 1 μM TSA for 16 h before promoter reporter assay.
GMSA.
Synthetic double-stranded DNA oligonucleotides that span the targeted promoter region (−18 GCCGAGGCCCCGCCTCCCGCTCTCGCC +7) were end-labeled with γ[32P]-ATP, and 4 μg of nuclear extract were incubated with 1 ng of labeled probe in GMSA binding buffer containing 10 mM HEPES (pH 7.5), 1 mM EDTA, 50 mM NaCl, 1 mM dithiothreitol, and 50 μg/ml poly[d(I-C)]. After incubation at room temperature for 20 min, the mixture was electrophoresed on a 6% polyacrylamide gel in 0.25× Tris-boric acid-EDTA buffer. Gels were dried and exposed to X-ray film. For competition experiments, a 200-fold molar excess of unlabeled probe was added to the reaction mixture before the labeled probe was added. For supershift assays, 4 μg of Sp3 or Sp1 antibody (Santa Cruz Biotechnology) were added to the reaction mixtures.
Statistical analysis.
Student's t-test was used to compare values of the experimental data. P values <0.05 were considered significant.
RESULTS
Effect of butyrate on NHE8 protein expression in Caco-2 cells.
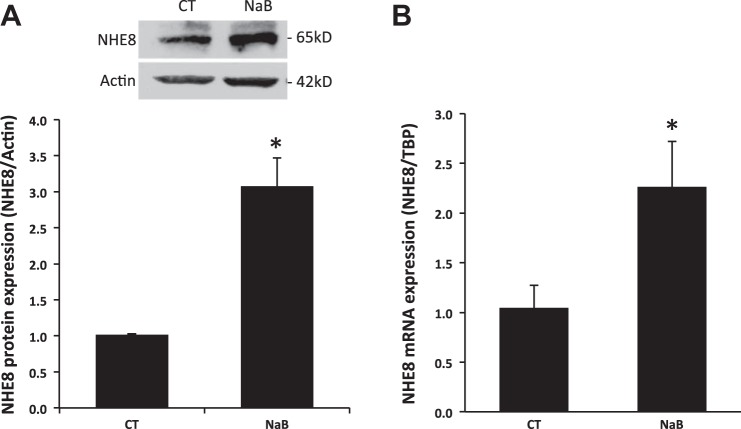
The expression of NHE8 protein in Caco-2 cells exposed to standard or butyrate-containing medium was assessed by Western blot. Sixteen hours after the exposure to butyrate, cells were harvested and total cell lysate was used for Western blot to determine NHE8 protein abundance. As shown in Fig. 1A, butyrate treatment significantly increased NHE8 protein expression by about threefold (1.02 ± 0.01 in control cells vs. 3.08 ± 0.39 in butyrate treated cells; n = 4, p = 0.002).
Fig. 1.
Effect of butyrate on the endogenous sodium/hydrogen exchanger 8 (NHE8) expression in human intestinal epithelial cells. Caco-2 cells were cultured in standard medium or butyrate-containing medium (10 mM, 16 h). Total protein was prepared and used for Western blot. RNA was isolated and used for real-time PCR. NHE8 immunoreactive protein abundance is indicated by the ratio of optical densities of the NHE8 band to that of the β-actin band. Comparative cycle threshold (Ct) method was used to calculate NHE8 mRNA expression. Results are means ± SE from 3 to 4 separate experiments. *P < 0.05 for control (CT) vs. butyrate treatment (NaB). A: effect of butyrate on NHE8 protein expression in Caco-2 cells. B: effect of butyrate on NHE8 mRNA expression in Caco-2 cells. TBP, TATA binding protein.
Effect of butyrate on NHE8 mRNA expression in Caco-2 cells.
Because NHE8 protein abundance was increased in butyrate-treated cells, we tested whether NHE8 mRNA expression is also increased in these cells. RNA was purified from Caco-2 cells exposed to standard or butyrate-containing medium and was used for Real-Time PCR to compare the abundance of NHE8 mRNA. As shown in Fig. 1B, NHE8 mRNA expression in Caco-2 cells was also significantly increased by butyrate treatment (1.05 ± 0.23 in control cells vs. 2.26 ± 0.45 in butyrate-treated cells; n = 3, p = 0.037).
Effect of butyrate on human NHE8 gene promoter activity.
To explore whether butyrate-mediated NHE8 expression is due to increased gene transcription, Caco-2 cells were transfected with human NHE8 gene promoter constructs followed by 16 h of treatment with 10 mM butyrate before analyzing promoter activity. As shown in Fig. 2, reporter gene expression driven by human NHE8 promoter was significantly induced in butyrate-treated Caco-2 cells. Approximately over twofold induction in the promoter activity was seen in human NHE8 gene promoter constructs pGL3b/-32 and pGL3b/-1905 (2.12 ± 0.31 fold induction in pGL3b/-32 promoter construct and 2.86 ± 0.44 fold induction in pGL3b/-1905 promoter construct; n = 3, p ≤ 0.01).
Fig. 2.
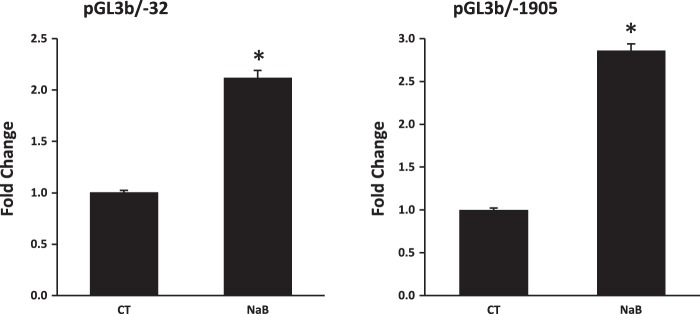
Effect of butyrate on human NHE8 gene promoter activity. Cells were cotransfected with pRL-CMV and pGL3 basic (pGL3b) or human NHE8 promoter constructs (pGL3b/-32, pGL3b/-1905). Butyrate was applied 16 h before measuring promoter activities. Promoter activity is calculated as a relative activity, a ratio of firefly luciferase activity driven by NHE8 promoter over Renilla luciferase activity driven by CMV promoter. The degree of stimulation on NHE8 promoter activity is shown as the ratio of relative activity in butyrate-treated cells over relative activity in control cells. Results are means ± SE from 3 separate experiments. *P < 0.05 for CT vs. NaB.
Identification of trans-factor and cis-element involved in butyrate response of the human NHE8 promoter.
GMSA was used to study the DNA/protein interaction involved in butyrate response. Promoter assay indicated that the shortest human NHE8 gene promoter construct pGL3b/-32 was responsive to butyrate treatment; thus we focused on determining the DNA/protein interaction at this promoter region. Previous studies showed that pGL3b/-32 contains a GC-box; this region recruits Sp3 protein to activate basal NHE8 gene transcription (27), so we tested whether butyrate-stimulated human NHE8 basal promoter activity was due to an increase in nuclear protein binding at this DNA region. As shown in Fig. 3, GMSA results suggested that butyrate enhanced the DNA-protein interaction at the basal promoter region of the human NHE8 gene, and this interaction could be blocked by GC-box consensus DNA oligos. Supershift experiments indicated that this DNA/protein complex could be further shifted by Sp3 antibody but not by Sp1 antibody in nuclear protein isolated from control and butyrate-treated cells. Western blot confirmed that Sp3 protein was enriched in nuclear extracts isolated from butyrate-treated Caco-2 cells (1.11 ± 0.02 in control cells vs. 1.83 ± 0.06 in butyrate-treated cells, n = 4, p = 0.016) (Fig. 4).
Fig. 3.
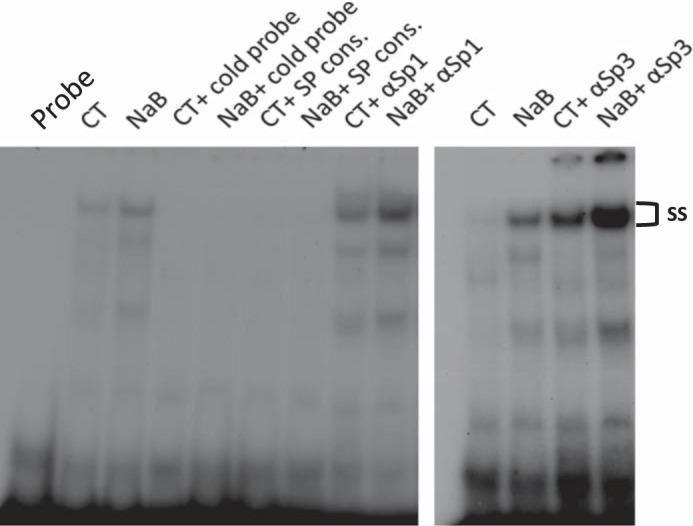
Identification of DNA/protein interaction involving butyrate regulation at the human NHE8 gene promoter by gel mobility shift assays (GMSAs). Oligo DNA was end-labeled with γ32P-ATP and used as a probe in GMSAs. Nuclear protein was isolated from CT or NaB. GMSAs were performed as indicated in the method section. GC-box consensus oligo (SP Cons.) was used to compete Sp protein family binding. Sp1 and Sp3 antibodies (αSp1 and αSp3) were used for supershift experiments in the presence of labeled probe. ss, Supershift complex.
Fig. 4.
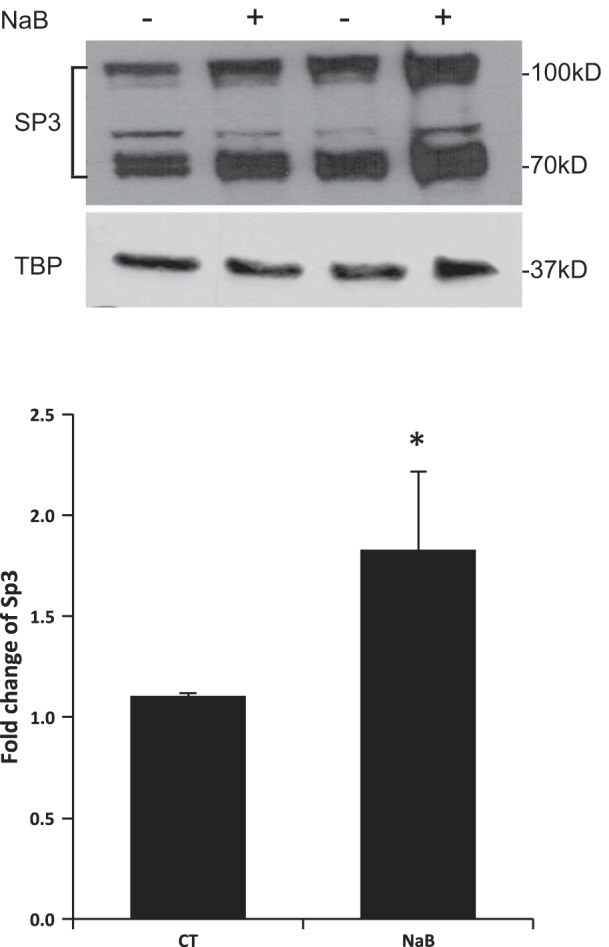
Effect of butyrate on Sp3 protein in human intestinal epithelial cells. Caco-2 cells were cultured in standard medium or butyrate-containing medium (10 mM, 16 h). Nuclear protein was isolated and used for Western blot detection. Sp3 protein abundance is calculated as a ratio of Sp3 intensity over TBP protein intensity. The degree of Sp3 protein expression is shown as the ratio of Sp3 protein abundance in butyrate-treated cells over Sp3 protein abundance in control cells. Results are means ± SE from 4 separate experiments. *P < 0.05 for CT vs. NaB. Top: a representative Western image; bottom: summary results of Sp3 protein abundance in the nuclei.
Sp3 protein acetylation is involved in butyrate-mediated NHE8 expression regulation.
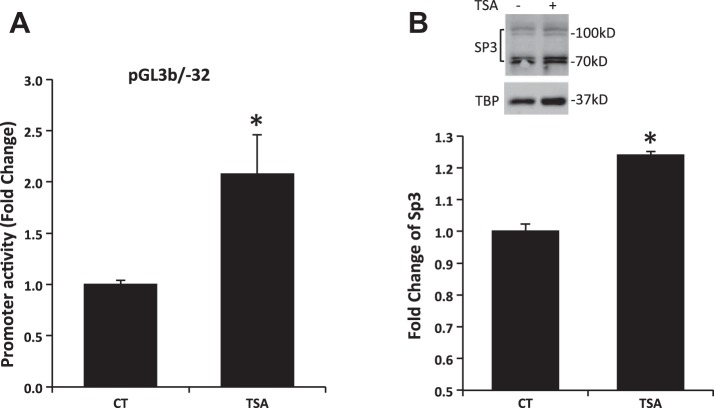
To assess whether acetylation is involved in butyrate-mediated NHE8 expression activation, Caco-2 cells were transfected with human NHE8 gene promoter construct pGL3b/-32 and then were treated with 1 μM TSA for 16 h before promoter reporter assay. As shown in Fig. 5A, the human NHE8 gene promoter activity was increased over twofold after TSA treatment (1.00 ± 0.04 in control groups vs. 2.08 ± 0.38 in TSA-treated groups; n = 4, P = 0.016). Western blot also detected an increased Sp3 protein nuclear localization in TSA-treated cells (1.00 ± 0.02 in control groups vs. 1.24 ± 0.01 in TSA-treated groups; n = 3, P = 0.001; Fig. 5B). Furthermore, acetylated-lysine antibody detected abundant Sp3 protein acetylation in Sp3 immunoprecipitated protein preps after cells exposed to butyrate or TSA (Fig. 6).
Fig. 5.
Effect of trichostatin (TSA) on human NHE8 gene promoter activity and Sp3 protein expression. A: effect of TSA on human NHE8 gene promoter is shown. Cells were cotransfected with pRL-CMV and pGL3 basic (pGL3b) or human NHE8 promoter construct pGL3b/-32. TSA (1 μM) was applied 16 h before measuring promoter activities. The degree of stimulation is shown as the ratio of relative luciferase activity in TSA-treated cells over relative luciferase activity in control cells. Results are means ± SE from 4 separate experiments. *P < 0.05 for CT vs. TSA treatment (TSA). B: effect of TSA on Sp3 protein expression. Caco-2 cells were cultured in standard medium or TSA-containing medium (1 μM, 16 h). Nuclear protein was isolated and used for Western blot detection. Sp3 protein abundance is calculated as a ratio of Sp3 intensity over TBP protein intensity. The degree of Sp3 protein expression is shown as the ratio of Sp3 protein abundance in TSA-treated cells over Sp3 protein abundance in control cells. Results are means ± SE from 3 separate experiments. *P < 0.05 for CT vs. TSA. B, top: representative Western image; B, bottom: summary results of Sp3 protein expression in nuclear extracts.
Fig. 6.
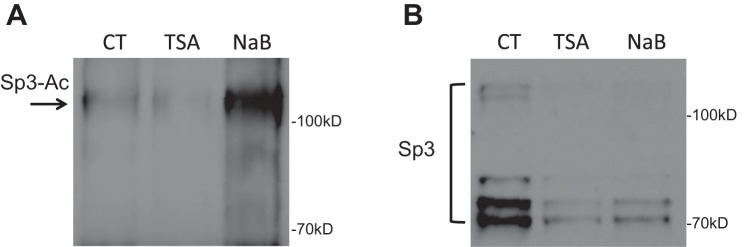
Sp3 protein acetylation detection. Caco-2 cells were treated with butyrate and TSA. Total protein was immunoprecipitated with Sp3 antibody. The immunoprecipitated protein was then separated on PAGE gel and transferred to nitrocellulose membrane. The acetylated Sp3 protein was detected using anti-acetyl lysine antibody (A), and the total Sp3 protein was detected using Sp3 antibody (B).
DISCUSSION
The intestinal epithelium plays an important role in barrier protection and in the transport of water, nutrients, and electrolytes. Apically expressed NHEs (NHE2, NHE3, and NHE8) are the major players for electroneutral sodium absorption in the intestinal tract. Importantly, NHE-mediated sodium absorption during intestinal maturation is regulated by growth factors (14, 30, 32) and steroids (8, 15, 33). Inflammatory cytokine and nutritional factors also play roles in regulating NHE function (1, 5, 16, 27, 35).
Butyrate, a pleiotropic byproduct of bacterial fermentation, also plays a role in regulating intestinal sodium absorption (2, 3, 18, 20, 21). It is known to regulate intestinal sodium absorption by stimulating ENaC (37) and NHE3 (16, 20) and not NHE2, but no study determined whether it would have any effect on NHE8, the latest characterized intestinal NHE. Previous studies showed that NHE3 activity was enhanced in rats fed with 5% pectin-supplemented diet and in butyrate-treated cultured intestinal epithelial cells (17, 20). Now we showed that butyrate treatment also significantly induced the expression of NHE8 in Caco-2 cells. NHE8 protein expression was increased by 200%, and NHE8 mRNA expression was increased by 120% after butyrate treatment. These observations suggest that butyrate could stimulate NHE8 expression at both mRNA and protein levels. Interestingly, the colonic NHE8 expression is increased after weanling (34), and the colonic butyrate production is also increased alongside the weaning process with the introduction of solid foods and the development of a more diverse, adult-type microbiota (19, 24). This correlation suggests that butyrate might contribute to the ontogenic NHE8 expression in the colon.
Because butyrate affects NHE8 expression at both protein and mRNA levels, we suspected that butyrate exerts transcriptional regulation on NHE8 expression. To dissect the mechanism of this stimulation, we first conducted promoter reporter assay in Caco-2 cells by transfecting cells with human NHE8 gene promoter constructs. As expected, butyrate treatment increased NHE8 gene promoter activity by more than twofold in cells transfected with pGL3b/-1905 and pGL3b/-32 promoter constructs. These observations indicated that butyrate indeed regulated NHE8 gene expression by activating NHE8 gene promoter. Because pGL3/-32 promoter construct contains the minimal promoter sequence required for basal NHE8 gene expression activation in the epithelial cells and butyrate treatment enhanced this promoter construct activity, the effect of butyrate on NHE8 gene expression is mediated by increasing basal NHE8 gene transcription in intestinal epithelial cells.
We have reported previously that Sp3 is an essential transcriptional factor to activate the basal NHE8 gene promoter (27). Because butyrate also stimulated NHE8 basal promoter activity, we suspected that Sp3 binding region might be involved. To address this question, we isolated nuclear protein from Caco-2 cells and performed GMSAs with DNA oligos spanning the basal promoter region of the human intestinal NHE8 gene. GMSA results showed that an increased DNA-protein interaction was detected at the human NHE8 proximal promoter region (−18 bp/+7 bp) after butyrate treatment. Supershift studies with an anti-Sp3 antibody indicated that the Sp3 protein bind to this DNA sequence in the human NHE8 proximal promoter, suggesting that Sp3 protein is the key transcription factor to activate human NHE8 expression under butyrate treatment condition.
The fact that GMSA suggests Sp3 is the key transcription factor for butyrate regulation of NHE8 supports our hypothesis that butyrate might contribute to the ontogenic NHE8 expression in the colon. We have previously identified that Sp3 protein is an essential transcription factor for activating human NHE8 gene expression (27). In relation to the interaction between NHE8 promoter DNA and Sp3 protein, this observation suggests that the interaction is also modulated by other factors such as butyrate.
Enhanced Sp3 protein binding on target gene promoter after butyrate treatment is not unique to NHE8. In fact, this form of transcriptional regulation was observed in modulating intestinal ENaC, the human insulin-like growth factor binding protein-3, and intestinal NHE3. However, the mechanisms that butyrate stimulated Sp3 binding on target gene promoter are not the same. Sp3 acetylation stimulates ENaC expression but represses human insulin-like growth factor binding protein-3 expression upon butyrate stimulation (26, 37). On the other hand, PKA activation is involved in increased NHE3 expression upon butyrate treatment (16). In butyrate-mediated NHE8 gene expression increased Sp3 binding on the human NHE8 basal promoter region is correlated with the increased Sp3 protein nuclear translocation. Because butyrate could modify Sp3 protein by acetylation mechanism or by PKA mediated mechanism, we tested whether Sp3 acetylation was involved by treating cells with TSA, a histone deacetylase (HDAC) inhibitor. TSA treatment increased the human NHE8 promoter activity with similar levels observed in butyrate treated cells. Using Sp3 antibody immunoprecipitation and acetylation antibody detection, we detected acetylated Sp3 protein in butyrate- and TSA-treated cells. Thus butyrate-induced NHE8 expression in Caco-2 cells is most likely mediated by enhanced interaction between NHE8 promoter DNA and acetylated Sp3 protein. It is also interesting to note that butyrate has stronger acetylation action on Sp3 protein than TSA in Caco-2 cells.
In summary, we showed that the intestinal NHE8 expression is enhanced by butyrate in human intestinal epithelial cells. We also identified that acetylated Sp3 is a key transcription factor involved in butyrate-mediated NHE8 gene expression upregulation. This study, for the first time, demonstrated a role of acetylation of Sp3 in regulating intestinal NHE8 expression at the basal gene transcription level. These results also identified a new target gene of short chain fatty acids on intestinal sodium absorption.
GRANTS
This investigation was funded by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-073638.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: H.X. and F.K.G. conception and design of research; H.X., A.M., J.L., and Y.Z. performed experiments; H.X., A.M., J.L., and Y.Z. analyzed data; H.X., A.M., and J.L. interpreted results of experiments; H.X. prepared figures; H.X. drafted manuscript; H.X. and F.K.G. edited and revised manuscript; H.X. and F.K.G. approved final version of manuscript.
REFERENCES
- 1.Amin MR, Orenuga T, Tyagi S, Dudeja PK, Ramaswamy K, Malakooti J. Tumor necrosis factor-α represses the expression of NHE2 through NF-κB activation in intestinal epithelial cell model, C2BBe1. Inflamm Bowel Dis 17: 720–731, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Binder HJ, Mehta P. Short-chain fatty acids stimulate active sodium and chloride absorption in vitro in the rat distal colon. Gastroenterology 96: 989–996, 1989. [DOI] [PubMed] [Google Scholar]
- 3.Choshniak I, Mualem R. SCFA and electrolyte absorption in the colon of three rodent species. Comp Biochem Physiol A Physiol 118: 381–384, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Chung YS, Song IS, Erickson RH, Sleisenger MH, Kim YS. Effect of growth and sodium butyrate on brush border membrane-associated hydrolases in human colorectal cancer cell lines. Cancer Res 45: 2976–2982, 1985. [PubMed] [Google Scholar]
- 5.Clayburgh DR, Musch MW, Leitges M, Fu YX, Turner JR. Coordinated epithelial NHE3 inhibition and barrier dysfunction are required for TNF-mediated diarrhea in vivo. J Clin Invest 116: 2682–2694, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins JF, Xu H, Kiela PR, Zeng J, Ghishan FK. Functional and molecular characterization of NHE3 expression during ontogeny in rat jejunal epithelium. Am J Physiol Cell Physiol 273: C1937–C1946, 1997. [DOI] [PubMed] [Google Scholar]
- 7.Cummings JH. Short chain fatty acids in the human colon. Gut 22: 763–779, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grahammer F, Henke G, Sandu C, Rexhepaj R, Hussain A, Friedrich B, Risler T, Metzger M, Just L, Skutella T, Wulff P, Kuhl D, Lang F. Intestinal function of gene-targeted mice lacking serum- and glucocorticoid-inducible kinase 1. Am J Physiol Gastrointest Liver Physiol 290: G1114–G1123, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Gum JR, Kam WK, Byrd JC, Hicks JW, Sleisenger MH, Kim YS. Effects of sodium butyrate on human colonic adenocarcinoma cells. Induction of placental-like alkaline phosphatase. J Biol Chem 262: 1092–1097, 1987. [PubMed] [Google Scholar]
- 10.Gustafsson A, Lund-Tonnesen S, Berstad A, Midtvedt T, Norin E. Faecal short-chain fatty acids in patients with antibiotic-associated diarrhoea, before and after faecal enema treatment. Scand J Gastroenterol 33: 721–727, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Harig JM, Soergel KH, Komorowski RA, Wood CM. Treatment of diversion colitis with short-chain-fatty acid irrigation. N Engl J Med 320: 23–28, 1989. [DOI] [PubMed] [Google Scholar]
- 12.Heerdt BG, Houston MA, Augenlicht LH. Potentiation by specific short-chain fatty acids of differentiation and apoptosis in human colonic carcinoma cell lines. Cancer Res 54: 3288–3293, 1994. [PubMed] [Google Scholar]
- 13.Heerdt BG, Houston MA, Augenlicht LH. Short-chain fatty acid-initiated cell cycle arrest and apoptosis of colonic epithelial cells is linked to mitochondrial function. Cell Growth Differ 8: 523–532, 1997. [PubMed] [Google Scholar]
- 14.Khurana S, Nath SK, Levine SA, Bowser JM, Tse CM, Cohen ME, Donowitz M. Brush border phosphatidylinositol 3-kinase mediates epidermal growth factor stimulation of intestinal NaCl absorption and Na+/H+ exchange. J Biol Chem 271: 9919–9927, 1996. [DOI] [PubMed] [Google Scholar]
- 15.Kiela PR, Guner YS, Xu H, Collins JF, Ghishan FK. Age- and tissue-specific induction of NHE3 by glucocorticoids in the rat small intestine. Am J Physiol Cell Physiol 278: C629–C637, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Kiela PR, Hines ER, Collins JF, Ghishan FK. Regulation of the rat NHE3 gene promoter by sodium butyrate. Am J Physiol Gastrointest Liver Physiol 281: G947–G956, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Krishnan S, Rajendran VM, Binder HJ. Apical NHE isoforms differentially regulate butyrate-stimulated Na absorption in rat distal colon. Am J Physiol Cell Physiol 285: C1246–C1254, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Krishnan S, Ramakrishna BS, Binder HJ. Stimulation of sodium chloride absorption from secreting rat colon by short-chain fatty acids. Dig Dis Sci 44: 1924–1930, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Midtvedt AC, Midtvedt T. Production of short chain fatty acids by the intestinal microflora during the first 2 years of human life. J Pediatr Gastroenterol Nutr 15: 395–403, 1992. [DOI] [PubMed] [Google Scholar]
- 20.Musch MW, Bookstein C, Xie Y, Sellin JH, Chang EB. SCFA increase intestinal Na absorption by induction of NHE3 in rat colon and human intestinal C2/bbe cells. Am J Physiol Gastrointest Liver Physiol 280: G687–G693, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Ramakrishna BS, Nance SH, Roberts-Thomson IC, Roediger WE. The effects of enterotoxins and short-chain fatty acids on water and electrolyte fluxes in ileal and colonic loops in vivo in the rat. Digestion 45: 93–101, 1990. [DOI] [PubMed] [Google Scholar]
- 22.Roediger WE. Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut 21: 793–798, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanderson IR. Short chain fatty acid regulation of signaling genes expressed by the intestinal epithelium. J Nutr 134: 2450S–2454S, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Tuohy K, Del Rio D. Diet-Microbe Interactions in the Gut: Effects on Human Health and Disease. Academic Press, 2014, p. 233. [Google Scholar]
- 25.Wachtershauser A, Stein J. Rationale for the luminal provision of butyrate in intestinal diseases. Eur J Nutr 39: 164–171, 2000. [DOI] [PubMed] [Google Scholar]
- 26.White NR, Mulligan P, King PJ, Sanderson IR. Sodium butyrate-mediated Sp3 acetylation represses human insulin-like growth factor binding protein-3 expression in intestinal epithelial cells. J Pediatr Gastroenterol Nutr 42: 134–141, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Xu H, Chen H, Dong J, Li J, Chen R, Uno JK, Ghishan FK. Tumor necrosis factor-α downregulates intestinal NHE8 expression by reducing basal promoter activity. Am J Physiol Cell Physiol 296: C489–C497, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu H, Chen H, Dong J, Lynch R, Ghishan FK. Gastrointestinal distribution and kinetic characterization of the sodium-hydrogen exchanger isoform 8 (NHE8). Cell Physiol Biochem 21: 109–116, 2008. [DOI] [PubMed] [Google Scholar]
- 29.Xu H, Chen R, Ghishan FK. Subcloning, localization, and expression of the rat intestinal sodium-hydrogen exchanger isoform 8. Am J Physiol Gastrointest Liver Physiol 289: G36–G41, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Xu H, Collins JF, Bai L, Kiela PR, Lynch RM, Ghishan FK. Epidermal growth factor regulation of rat NHE2 gene expression. Am J Physiol Cell Physiol 281: C504–C513, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Xu H, Inouye M, Hines ER, Collins JF, Ghishan FK. Transcriptional regulation of the human NaPi-IIb cotransporter by EGF in Caco-2 cells involves c-myb. Am J Physiol Cell Physiol 284: C1262–C1271, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Xu H, Zhang B, Li J, Chen H, Tooley J, Ghishan FK. Epidermal growth factor inhibits intestinal NHE8 expression via reducing its basal transcription. Am J Physiol Cell Physiol 299: C51–C57, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu H, Zhang B, Li J, Chen H, Wang C, Ghishan FK. Transcriptional inhibition of intestinal NHE8 expression by glucocorticoids involves Pax5. Am J Physiol Gastrointest Liver Physiol 299: G921–G927, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu H, Zhang B, Li J, Wang C, Chen H, Ghishan FK. Impaired mucin synthesis and bicarbonate secretion in the colon of NHE8 knockout mice. Am J Physiol Gastrointest Liver Physiol 303: G335–G343, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang W, Shen Z, Martens H. An energy-rich diet enhances expression of Na+/H+ exchanger isoform 1 and 3 messenger RNA in rumen epithelium of goat. J Anim Sci 90: 307–317, 2012. [DOI] [PubMed] [Google Scholar]
- 36.Zachos NC, Tse M, Donowitz M. Molecular physiology of intestinal Na+/H+ exchange. Annu Rev Physiol 67: 411–443, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Zeissig S, Fromm A, Mankertz J, Weiske J, Zeitz M, Fromm M, Schulzke JD. Butyrate induces intestinal sodium absorption via Sp3-mediated transcriptional up-regulation of epithelial sodium channels. Gastroenterology 132: 236–248, 2007. [DOI] [PubMed] [Google Scholar]