Abstract
Biliary atresia (BA), a neonatal obstructive cholangiopathy, remains the most common indication for pediatric liver transplantation in the United States. In the murine model of BA, Rhesus rotavirus (RRV) VP4 surface protein determines biliary duct tropism. In this study, we investigated how VP4 governs induction of murine BA. Newborn mice were injected with 16 strains of rotavirus and observed for clinical symptoms of BA and mortality. Cholangiograms were performed to confirm bile duct obstruction. Livers and bile ducts were harvested 7 days postinfection for virus titers and histology. Flow cytometry assessed mononuclear cell activation in harvested cell populations from the liver. Cytotoxic NK cell activity was determined by the ability of NK cells to kill noninfected cholangiocytes. Of the 16 strains investigated, the 6 with the highest homology to the RRV VP4 (>87%) were capable of infecting bile ducts in vivo. Although the strain Ro1845 replicated to a titer similar to RRV in vivo, it caused no symptoms or mortality. A Ro1845 reassortant containing the RRV VP4 induced all BA symptoms, with a mortality rate of 89%. Flow cytometry revealed that NK cell activation was significantly increased in the disease-inducing strains and these NK cells demonstrated a significantly higher percentage of cytotoxicity against noninfected cholangiocytes. Rotavirus strains with >87% homology to RRV's VP4 were capable of infecting murine bile ducts in vivo. Development of murine BA was mediated by RRV VP4-specific activation of mononuclear cells, independent of viral titers.
Keywords: cholangiocytes, natural killer cells, rotavirus, RRV, VP4
biliary atresia (BA) is a neonatal disease characterized by obstructive cholangiopathy of extrahepatic bile ducts, leading to chronic cholestasis and biliary cirrhosis. The incidence is estimated at 1 in 8,000–15,000 live births (27). Afflicted infants present with jaundice, acholic stool, and failure to thrive. Early diagnosis and surgical intervention with a Kasai portoenterostomy to establish biliary drainage are essential. Even after successful drainage, the progressive nature of this disease causes end-stage liver disease requiring liver transplantation for survival. BA remains the most common indication for liver transplantation in children (2). The importance of understanding the pathogenesis of BA is crucial such that alternative treatment options can be developed.
The etiology of BA remains unknown but likely is multifactorial and may include viral infection of the biliary tree (6, 7, 16, 20, 28) with subsequent immune-mediated duct injury (4, 15, 26). Several viruses have been isolated from the livers of BA patients including group C rotaviruses (20). A well-established murine model of BA is employed to study disease pathogenesis (21). Intraperitoneal injection of neonatal BALB/c mice with Rhesus rotavirus (RRV) leads to symptoms (jaundice, acholic stools, bilirubinuria) and bile duct obstruction that mimic human disease (1, 18). Previous studies demonstrated the unique tropism of RRV to the cholangiocyte (biliary epithelial cells) (12, 23) and that development of the murine model is virus strain dependent (1). In a previous study, it was shown that a simian rotavirus strain, SA11-FM (having a fast moving VP4 gene) induced symptoms, obstruction, and mortality whereas another similar simian strain, SA11-SM (having a different VP4 gene that was slow moving when run on a polyacrylamide gel), showed some clinical signs of hepatobiliary injury without significant mortality (1). The mouse strain EDIM (epizootic diarrhea of infant mice) and the human strain Wa did not induce the obstructive cholangiopathy (1), thus suggesting that strain-specific characteristics are critical in inducing the murine model of BA. Interestingly, the murine cholangiocyte strain-specific tropism was recapitulated by using primary human biliary epithelial cells, thus supporting the theory of a hepatotropic virus triggering human BA (5).
Rotavirus is a double-stranded RNA virus of the Reoviridae family and is comprised of 11 gene segments that code for six structural and six nonstructural proteins. To determine which RRV gene segment impacts disease pathogenesis, a series of single gene reassortants were generated, and when tested in vivo it was revealed that the RRV VP4 protein, a surface spike protein involved in cell attachment and infectivity, was a major determinant in the pathogenesis of murine BA (29).
The aim of this study was to determine how VP4 governs disease pathogenesis. To do so, 16 rotavirus strains derived from seven mammalian hosts (bovine, canine, caprine, human, murine, porcine, and simian) were tested. We found that viruses with higher homology to RRV VP4 induced murine BA and that RRV VP4 governed viral replication as well as NK cell cholangiocyte-specific cytotoxicity.
MATERIALS AND METHODS
Ethics statement.
All animal research was performed in accordance with regulations and protocols approved by the Institutional Animal Care and Use Committee (IACUC) at Cincinnati Children's Hospital Medical Center (protocol number IACUC2013-0039) which adheres to the NIH OLAW regulation (Animal Assurance number A3108) and the Animal Welfare Act (certification number 31-8-001).
Cells, viruses, and animals.
MA104 cells (BioWhittaker, Walkersville, MD) were grown in Dulbecco's modified Eagle's medium (DMEM) (Cellgro) supplemented with 10% fetal bovine serum (FBS) (GIBCO BRL, Gaithersburg, MD), 0.01% penicillin-streptomycin (GIBCO BRL), 0.01% l-glutamine (GIBCO BRL), and 0.005% amphotericin B (Cellgro). The mouse cholangiocyte cell line (mCL) generously provided by the laboratory of James Boyer (Yale Liver Care Center, Hartford, CT) was cultured as described previously (12). Sixteen culture-adapted rotavirus strains were grown in MA104 cells and used in this study: NCDV, WC-3, ST-3, VA-70, DS-1, WI-61, EMcN, EB, OSU, and RRV (Monica McNeal, Cincinnati Children's Hospital Medical Center); CU-1 (ATCC, Manassas, VA): TUCH (Dr. Karol Sestak, Tulane National Primate Research Center and Tulane University School of Medicine); PA260 (Dr. Vito Matella, University of Bari Aldo Moro); GRV (Dr. Osamu Nakagomi, Nagasaki University); KU (Dr. Koki Taniguchi, Fujita Health University); and Ro1845 (Yasutaka Hoshino, National Institute of Allergy and Infectious Diseases). Breeding pairs of BALB/c mice (Harlan Laboratories, Indianapolis, IN) were kept in microisolator cages in a virus-free environment with free access to sterilized chow and water. The mice were bred, and litters of more than four pups were used.
Generation, purification, and analysis of reassortant.
An Ro1845R(VP4) reassortant was generated by coinfection of MA104 cells, the standard kidney epithelial cell line used to maintain rotavirus strains in cell culture. MA104 cells were seeded in polystyrene tubes in 2 ml DMEM containing 10% FBS. Monolayers were coinfected with RRV and Ro1845 at varying multiplicities of infection. After 1 h of absorption, serum-free DMEM plus 4 μg/ml trypsin (1:250) (Invitrogen, Carlsbad, CA) was added. At 24 and 48 h, the cultures were evaluated for cytopathic effect (CPE), and they were frozen when CPE was complete.
The reassortant was plaque purified in confluent cell culture plates containing MA104 cells in DMEM. The cells were infected with 0.2 ml of serially diluted virus supernatants for 1 h, overlaid with 5 ml of medium containing 0.2% agarose (Lonza, Rockland, ME), and incubated for 3–4 days at 37°C. Plaques were picked and amplified in MA104 cells. Viral RNA was extracted, and the double-stranded RNA (dsRNA) segments were visualized by SDS-PAGE and silver staining as described previously (10). The migration patterns of the reassortant dsRNA genes were compared with those for the parental strains, and gene assignments were made based on RRV gene segment migration. Progeny with single-gene substitutions were identified and grown up. The parental origins of the single-gene reassortants were reconfirmed by gel electrophoresis and sequencing after amplification.
Phylogenetic analysis.
The VP4 gene sequences of the various strains of rotavirus were analyzed by state-of-the-art phylogenetic analysis methods, including PhyML. Gene sequences of gene segment 4 from each strain of rotavirus were obtained from GenBank: NCDV[AB119636], WC3[AY050271], CU-1[D13401], GRV[AB055967], ST-3[L33895], VA-70[AJ540229], WI-61[EF672619], Ro1845[EU708893], DS-1[HQ650119], PA260[HQ661115], KU[M21014], EMcN[AY267006], EB[U08419], OSU[X13190], RRV[AY033150], and TUCH[AY596189].
Murine model of BA.
Newborn pups were injected intraperitoneally with each of the various strains at a dose of 1.5 × 106 focus-forming units (FFU) per mouse within 24 h of birth, a dose at which 100% of pups develop symptoms of BA with RRV. Saline-injected pups served as controls. Weights, clinical signs of hepatobiliary injury (i.e., jaundice, acholic stools, and bilirubinuria), and survival were recorded for 21 days. The presence of bilirubin in the urine was detected quantitatively by use of commercially available urine dipsticks (Bayer, Elkhart, IN). Pups were monitored twice daily. Any animals which presented with >20% weight loss, anorexia, nonresponsiveness, or reaching a point of moribund were euthanized by carbon dioxide inhalation followed by cervical dislocation. There was no requirement for analgesia or anesthesia in this protocol.
Histology of extrahepatic bile duct and liver and immunohistochemical analysis for the presence of rotavirus in liver.
Extrahepatic bile ducts and livers were harvested from a subset of mice 7 days PI and preserved in formalin. Serial sections of livers and bile ducts underwent hematoxylin and eosin staining. Liver tissue harvested from mice 7 days PI was embedded in Histo Prep (Fisher Scientific, Pittsburgh, PA) over dry ice. Samples were stored at −80°C until they were sectioned for histological analysis. Frozen samples were cut in 7-μm sections and placed on microscope slides, fixed in cold acetone, and air dried. Sections were rehydrated in PBS and blocked with 5% donkey serum plus 3% bovine serum albumin in PBS. Slides were incubated overnight at 4°C with rabbit anti-rotavirus IgG (1:100) and rat anti-CK-19 (1:75) primary antibodies diluted in Dako antibody diluent (Dako, Carpinteria, CA), then washed and incubated with 488 anti-rat (1:250) and 594 anti-rabbit (1:500) secondary antibodies diluted in Dako antibody diluent (Dako) for 1.5 h at room temperature. Sections were washed in PBS and coverslipped with Vectashield mounting medium with DAPI (H-1200; Vector Laboratories, Burlingame, CA).
Focus-forming assay.
Subsets of BALB/c pups were injected with each of the different rotavirus strains; extrahepatic bile duct and liver samples were collected at 1 and 7 days postinoculation (PI). Tissues were weighed (wet weight), homogenized in Earle's balanced salt solution, and stored at −20°C until analyzed for the presence of infectious rotavirus by a focus-forming assay (FFA) as previously described (17).
Flow cytometry.
Livers were harvested from pups 7 days PI and pooled two per sample in RPMI media containing 2% FBS. Samples were then minced, moved through an 18-gauge needle three times, and passed through a 40-μm filter. Following centrifugation at 2,000 rpm, 33% Percoll (GE Healthcare, Uppsala, Sweden) was used to purify the mononuclear cells. Pellets were then treated with red blood cell lysis buffer, washed two times, and suspended in 1× PBS + 1% FBS. Each well of a 96-well v-bottom plate was seeded with 1 × 106 cells, treated with Fc block (BD Biosciences, San Jose, CA), and incubated at 4°C for 30 min. A panel of 1:100 dilution of anti-mouse CD49b (an NK cell marker) and anti-mouse CD69 (a marker of activation) (eBioscience, San Diego, CA) was added for 30 min of incubation at 4°C. Cells were washed twice and filtered through a 40-μm cell strainer into a tube containing 4% paraformaldehyde to fix cells. Samples were read on the Accuri C6 flow cytometer (BD, Franklin Lakes, NJ) and analyzed with FlowJo version 7.6.5 (FlowJo).
EliSpot for detection of IFN-γ.
NK cells were harvested from the livers of four infected pups at 7 days PI and collected individually in serum-free DMEM. Samples were then minced, passed through an 18-gauge needle three times, and filtered through a 40-μm filter. Following centrifugation at 2,000 rpm, 33% Percoll (GE Healthcare, Uppsala, Sweden) was used to purify the mononuclear cells. Pellets were treated with red blood cell lysis buffer, washed two times, and resuspended in 1× PBS + 0.5% BSA + 2 mM EDTA. Cells were incubated with NK cell-specific CD49b antibody-coated microbeads (Miltenyi, Auburn, CA) and isolated by column purification using LS columns (Miltenyi). Purity of isolation was determined to be greater than 95% by flow cytometry by use of an anti-CD49b antibody (data not shown). Cells were plated on a 96-well MultiScreen IP plate (EMD Millipore, Billerica, MA) coated with IFN-γ capture antibody from EliSpot Ready-SET-Go kit (88-7384-21, eBioscience). Plates were treated according to manufacturer's instructions. Briefly, plates were incubated overnight at 37°C, washed with PBS + Tween 20, and incubated overnight at 4°C with detection antibody. Plates were then washed four times with PBS + Tween 20, incubated with avidin-horseradish peroxidase, and developed with 3-amino-9-ethyl carbazole (Sigma, diluted in 0.1 M acetate solution). Finally, plates were read on a EliSpot reader (Cellular Technology, Shaker Heights, OH) and analyzed with Immunospot 4.0 software.
Nonradioactive cytotoxic NK cell assay.
NK cells were harvested as described above and plated in 96-well round bottom plates at increasing ratios along with mCl cells at a constant density of 1 × 104 cells/well. Plates were incubated at 37°C for 5 and 24 h followed by brief centrifugation at 250 g for 4 min and transfer of 50 μl supernatant to a new 96-well plate. Fifty microliters of substrate solution was added to each well from the CytoTox 96 NonRadioactive Cytotoxicity Assay Kit (Promega, Madison, WI). Plates were incubated at room temperature protected from light for 30 min followed by the addition of stop solution. Optical densities were read at 490 nm immediately on Synergy H1 Hybrid Reader (BioTek, Winooski, VT).
Statistical analysis.
Assessment of the development of symptoms and mortality rates following inoculation with each rotavirus strain was based on experimental groups of at least 15 pups each. The findings were presented as percentages of pups expressing at least two disease symptoms and percent survival. Analysis of noncontinuous variables was performed by χ2 and Fisher exact testing. Each subset of tissues utilized for histology and viral assays were derived from at least six pups. These continuous variables were analyzed for variance, with post hoc testing where appropriate, and expressed as means ± standard error of the mean. The flow data were analyzed with an unpaired t-test between each group. A P value of less than 0.05 was considered significant.
RESULTS
Phylogenetic analysis revealed VP4 homology between select rotavirus strains.
The relationship between different viral strains and how they evolved is often determined by analyzing homologous gene sequences. PhyML is a computer software application that can produce phylogenetic trees using the statistical principle of maximum likelihood (11). This program was utilized along with amino acid sequences published in GenBank to evaluate the phylogenetic relationship between different strains of rotavirus based on their VP4 sequence. The resulting phylogenetic tree along with a VP4 relationship to RRV is shown (Fig. 1A). Homology to RRV's VP4 is detailed in Table 1.
Fig. 1.

PhyML analysis of rotavirus VP4 and bile duct viral titers 1 and 7 days after infection. A: PhyML analysis was performed to determine the homology of Rhesus rotavirus (RRV) VP4 with other strains. B: a focus-forming assay (FFA) was performed on bile ducts harvested on postinoculation (PI) days 1 and 7 from newborn pups inoculated with 1.5 × 106 focus-forming units (FFU)/pup of each strain of rotavirus. Six strains were able to infect the bile ducts and replicate to similar titers at 1 day PI with higher viral yields at 7 days PI: RRV, GRV, PA260, Ro1845, Cu-1, and TUCH. *P < 0.05 vs. RRV on PI day 1, #P < 0.05 vs. RRV on PI day 7; n = 6–10 mice.
Table 1.
Signs of biliary obstruction, mortality rates and percent homology of different strains' VP4 protein to RRV's VP4
| Strain (host) | % of Mice with Symptoms* | Mortality Rate, %† | VP4 % Homology to RRV VP4‡ |
|---|---|---|---|
| RRV (simian) | 100 | 80 | 100 |
| GRV (capra) | 100 | 100 | 94.8 |
| PA260 (homo sapiens) | 10 | 0 | 91.4 |
| Ro1845 (homo sapiens) | 0 | 0 | 90.6 |
| CU-1 (canine) | 94 | 19 | 90.3 |
| TUCH (simian) | 0 | 0 | 87.1 |
| NCDV (bovine) | 0 | 0 | 85.6 |
| OSU (porcine) | 0 | 0 | 82.7 |
| EMcN (murine) | 0 | 0 | 78.5 |
| EB (murine) | 0 | 0 | 77.8 |
| WC-3 (bovine) | 0 | 0 | 75.4 |
| ST-3 (homo sapiens) | 0 | 0 | 74.7 |
| DS-1 (homo sapiens) | 0 | 0 | 72.6 |
| WI-61 (homo sapiens) | 0 | 0 | 72.2 |
| KU (homo sapiens) | 0 | 0 | 72.0 |
| VA-70 (homo sapiens) | 0 | 0 | 71.9 |
Groups of 15–20 pups per strain were injected within 24 h of birth with 1.5 × 106 focus-forming units of rotavirus and were monitored for symptoms of biliary atresia (BA) for 21 days.
Showing 2 or more signs of biliary obstruction.
Over 21 days.
VP4 amino acids sequence comparisons to Rhesus rotavirus (RRV) 's VP4 sequence.
Symptoms of BA and mortality following inoculation with rotavirus strains.
Rotavirus-inoculated neonatal mice were observed for symptoms of bile duct obstruction and mortality. Three strains were able to induce symptoms of hepatobiliary obstruction (Table 1). Inoculation with the capra rotavirus strain GRV manifested disease in 100% of pups. The canine strain CU-1 induced symptoms in 94% of mice with a 19% mortality rate. The human rotavirus strain PA260 caused only transient symptoms for 1 day in 10% of mice.
Several rotavirus strains are able to infect and replicate in bile ducts.
To determine the in vivo infectivity of different strains of rotavirus, bile ducts were harvested from pups 1 and 7 days PI and virus titer was assessed by FFA. These days were chosen because day 1 demonstrates the ability of the virus strains to reach the bile duct whereas day 7 postinfection has previously been shown to be the peak of rotavirus replication within the hepatobiliary system (1). As shown in Fig. 1B, six strains were able to infect the bile ducts and replicate to similar titers at 1 day PI with higher viral yields at 7 days PI. GRV and CU-1 strains replicated to titers similar to RRV (5.95 × 105 and 1.72 × 105 vs. 1.13 × 105 FFU/mg). Lower viral yields were seen with PA260 (1.79 × 104 FFU/mg), which demonstrated a low rate of symptoms, and with TUCH (1.76 × 104 FFU/mg), which does not induce the model as previously reported (29). Interestingly, the human strain Ro1845 replicated to high titers by PI day 7 (2.12 × 105 FFU/mg) but did not cause disease symptoms in the pups.
Immunohistochemistry confirms the presence of rotavirus in the liver parenchyma.
The presence of each rotavirus strain in the liver 7 days PI was investigated. Immunofluorescence of RRV, GRV, CU-1, PA260, and Ro1845 demonstrated colocalization with CK-19, a marker for biliary epithelium (Fig. 2).
Fig. 2.
Immunofluorescence detection of rotavirus in the liver parenchyma. Livers harvested 7 days PI, stained for CK-19 (green), rotavirus (red), and nucleus (blue) and overlaid to demonstrate colocalization (yellow-orange), indicated by arrows, confirmed the presence of rotavirus in the portal triad of pups inoculated with RRV, GRV, CU-1, PA260. and Ro1845. Magnification ×20.
Obstructive cholangiopathy is seen in symptomatic pups.
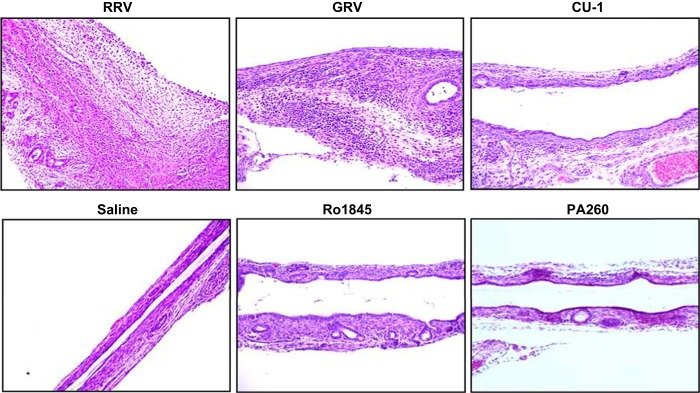
Histological evaluation of livers from pups inoculated with strains causing symptoms of BA demonstrated periportal inflammation with RRV and GRV infection and to a lesser extent in CU-1, but not in Ro1845 (Fig. 3). Complete bile duct obstruction was seen with RRV and GRV inoculation, but not in CU-1 or Ro1845 (Fig. 4). To confirm these findings, cholangiograms were performed that demonstrated complete obstructive cholangiopathy in RRV- and GRV-infected mice whereas CU-1- and Ro1845-infected mice have patent bile ducts (Fig. 5).
Fig. 3.
Histological appearance of liver parenchyma following rotavirus infection. Representative hematoxylin and eosin (H&E)-stained livers from mice 7 days PI with various rotavirus strains show periportal inflammation (black arrows) only in strains that are capable of inducing biliary atresia (BA) [RRV and capra rotavirus strain (GRV)]. Inflammation was seen to a lesser extent with CU-1 but was absent in Ro1845, PA260, and saline controls. There were 15–20 mice in each group. Magnification ×20.
Fig. 4.
Histological appearance of the extrahepatic bile ducts following infection with various rotavirus strains. Representative H&E stained bile duct sections 7 days PI with rotavirus strains shows bile duct obstruction only in those strains that cause symptoms and mortality (RRV and GRV) but a patent bile duct was seen in the other strains that do not induce the model, including CU-1, PA260, Ro1845 and saline controls. Magnification ×10; n = 15–20 mice.
Fig. 5.
Cholangiograms demonstrating biliary obstruction. Methylene blue dye was injected into the gall bladders of mice 12–14 days postinoculation. Bile duct obstruction was seen in strains that cause symptoms and mortality (RRV and GRV). Bile ducts remained patent after infection with CU-1 and Ro1845. There were 5–10 mice in each group.
Ro1845 reassortant containing the RRV VP4 induces BA symptoms in infected mice.
The Ro1845 strain infects the bile ducts in vivo and replicated to similar viral titers as RRV by 7 days PI but did not cause biliary symptoms or mortality. To confirm that RRV VP4 is required for disease development, a novel reassortant strain with an Ro1845 viral background containing a single RRV VP4 gene [Ro1845R(VP4)] was generated (Fig. 6A). When inoculated with Ro1845R(VP4) reassortant, neonatal mice developed symptoms in 92% of mice with a mortality rate of 89% (Fig. 6B). The mortality rate was slightly higher than RRV's but not significant different. Viral titers of bile ducts from Ro1845R(VP4) reassortant-infected mice 7 days PI (3.47 × 105 FFU·ml−1·mg−1) were not significantly different from RRV or Ro1845 (1.13 × 105 vs. 2.12 × 105 FFU·ml−1·mg−1) (P = 0.249) (Fig. 6C). Cholangiograms performed on mice inoculated with Ro145 and Ro1845R(VP4) reassortant showed bile duct obstruction only in the Ro1845R(VP4)-infected mice (Fig. 6D).
Fig. 6.
In vivo experiments with the Ro1845R(VP4) reassortant. A: dsRNAs extracted from the single-gene reassortant were separated by SDS-PAGE and silver stained to reveal the migration rates of the gene segments. Arrow indicates gene substitution. B: pups inoculated with the Ro1845R(VP4) reassortant induced symptoms of BA and mortality similar to RRV (n = 23 to 36 pups). *P < 0.05 vs. RRV, #P < 0.05 vs. Ro1845R(VP4). C: FFA revealed Ro1845R(VP4) was able to replicate in bile ducts to the same high titer 7 days PI as RRV and Ro1845 (P = 0.249; n = 6–10). D: representative cholangiograms demonstrated patency of the bile duct in pups infected with Ro1845 but obstruction of the bile duct in pups infected with Ro1845R(VP4) 12 days PI (n = 5–10).
RRV-VP4 induces activation of NK cells and IFN-γ production.
Increased numbers of activated NK cells have been found in the livers of RRV-infected mice at the time of bile duct obstruction (7 days PI), implicating them as effector cells in the pathogenesis of experimental BA (24, 25). Neonatal mice were inoculated with RRV, Ro1845, CU-1, and GRV. Saline-injected mice served as negative controls. The BA model-inducing strains, RRV and GRV, had a significantly higher amount of activated NK cells (73.4 ± 0.1 and 82.5 ± 1.2%, respectively) over Ro1845 and saline (64.4 ± 3.2 and 58.4 ± 0.3%, respectively) (P < 0.05). Interestingly, CU-1, which caused symptoms but no obstruction, had 63.6 ± 2.4% activated NK cells [significantly lower amount than RRV (P < 0.05)]. We next sought to determine whether this increase is mediated by RRV VP4 gene. A significantly increased number of activated NK cells were noted in the BA model-inducing strains RRV and Ro1845R(VP4) but not in Ro1845 or saline controls (P < 0.05) (Fig. 7, A and B). As determined by EliSpot, purified NK cells from livers harvested at PI day 7 with RRV and Ro1845R(VP4) produced increased amounts of IFN-γ compared with those harvested from Ro1845-inoculated mice (P < 0.05) (Fig. 7C).
Fig. 7.
NK cell activation and IFN-γ production is increased in the presence of the RRV VP4. A and B: flow cytometry of lymphocyte population harvested from the livers of 7 days PI pups illustrated a significant increase in the number of activated NK cells present in the model-inducing strains RRV and Ro1845R(VP4) over saline controls, but not in Ro1845. Red quadrants show the percentage of CD69+ cells in the CD49b+ population (2 livers per sample and 3 samples per strain). C: EliSpot data obtained from purified NK cells isolated from RRV and Ro1845R(VP4) shows increased production of IFN-γ. *P < 0.05; n = 4.
NK cells harvested from infected mice induce cholangiocyte death in vitro.
Purified NK cells harvested from pups 7 days PI were cocultured with cholangiocytes at increasing ratios for 5 and 24 h. NK cells from RRV- and Ro1845R(VP4)-infected mice were capable of killing uninfected cholangiocytes at a significantly higher percentage compared with Ro1845 at both time points (P < 0.05) (Fig. 8).
Fig. 8.

Cytotoxicity of NK cells obtained from RRV and Ro1845R(VP4) livers. NK cells harvested from the model-inducing strains RRV and Ro1845R(VP4) showed a significantly higher killing of uninfected cholangiocytes compared with those harvested from Ro1845 or saline controls. All strains where significantly different vs. saline. *P < 0.05; n = 4.
DISCUSSION
Previous studies demonstrated that induction of murine BA was rotavirus strain dependent (1). In this study, we demonstrated that the ability to cause the model is not restricted to RRV but may lie within the VP4 gene/protein. Virus strains with greater than 87% homology to RRV's VP4 demonstrated an ability to infect the bile ducts of pups. GRV with a 94.8% homology to RRV-VP4 was able to replicate to high titers in vivo, induce an obstructive cholangiopathy along with a mortality rate similar to that caused by RRV. Infection with the canine strain CU-1 (90.3% homologous with RRV-VP4), caused a high rate of symptoms but mortality was low similar to what was seen in previous studies with SA11-SM (1). Other viruses that have lower homology to RRV-VP4, such as PA260 and Ro1845, did not induce the model. Although obstructive cholangiopathy was not seen in CU-1, PA260, and Ro1845, these strains were able to replicate to appreciable titers in murine bile ducts. These data suggest that the presence of high virus titers within the biliary system is not enough by itself to induce the obstructive model.
Earlier work by our group compared the ability of RRV and TUCH, another simian rotavirus strain, to induce the model. Pups infected with TUCH did not develop any symptoms of BA or mortality. Generating a series of single reassortants we identified that the TUCH reassortant with a RRV-VP4 [TUCHR(VP4)] was able to induce the model whereas the parent strain TUCH did not. In contrast, an RRV reassortant with a TUCH-VP4 [RRVT(VP4)] did not develop any symptoms or mortality (29). In the previous studies TUCH replicated to a lower level both in vitro and in vivo compared with RRV, raising the possibility that reduced pathogenicity might be related to viral titer. In this study, we used another rotavirus strain, Ro1845, which was able to replicate to high titers both in vivo and in vitro (data not shown), similar to RRV, but did not induce the model. Although Ro1845 localized to the periportal region on immunohistochemistry, no inflammation or bile duct obstruction was observed. To further examine the role of the RRV VP4, a reassortant was created with a Ro1845 parent strain containing the RRV VP4. Viral titers from harvested bile ducts were comparable between RRV, Ro1845, and Ro1845R(VP4). However, in contrast to the parent strain, the Ro1845R(VP4) reassortant caused symptoms in 92% of inoculated mice with a mortality rate of 89%. With RRV and Ro1845's VP4 protein differing in only 9.4% homology, this suggests that there might be a specific amino acid sequence on RRV's VP4 that drives BA model induction.
A previous study performed by Feng et al. (9) demonstrated that both VP4 and NSP1 genes from RRV were required for replication within the mouse biliary tract when comparing RRV and the bovine strain UK. NSP1 has previously been shown to inhibit interferon regulatory factors to suppress type I interferon production (8, 22). To date, not all of the rotavirus strains examined in this study have had their NSP1 gene sequenced; thus we cannot rule out the potential involvement of NSP1 in the strains' ability to replicate within biliary cells. Although NSP1 might be important in viral replication in the biliary tract, it may not be involved in the pathogenesis of biliary obstruction. A previous study from our laboratory using single gene reassortants demonstrated no significant difference in the ability to induce or prevent the murine model of BA as a result of the NSP1 gene (29). Interestingly, the homology of the NSP1 found in RRV vs. PA260 is only 40.2%, but both the strains can replicate within the bile ducts of newborn mice.
Viral infections as well as chronic inflammatory and autoimmune-mediated bile duct injury were implicated in development of BA, with several studies demonstrating lymphocyte infiltrates surrounding the bile ducts in these patients (3, 4, 13, 19). Mack et al. (15) demonstrated that bile duct epithelial-specific T cell-mediated autoimmunity plays a role at least in part for bile duct injury in the murine model of BA. Furthermore, oligoclonal expansion of CD8+ T cells in the liver and extrahepatic bile ducts of patients undergoing surgery for BA indicate that T cells are activated in response to specific antigenic stimulation (14). Natural killer cells, present in the portal tract of patients with BA, have been shown to be activated, implicating the role of the innate immune system (25). We demonstrated NK cell activation following the inoculation with RRV and the reassortant containing the RRV VP4: Ro1845R(VP4). These NK cells also exhibited increased ability to kill uninfected cholangiocytes in coculture. This supports the hypothesis that a specific amino acid sequence on RRV's VP4 leads to increased activation of immune cells.
We previously reported the importance of VP4 in determining tropism for the cholangiocyte as well as the ability to induce murine BA (29). These results begin to define a pathogenic mechanism by which a specific sequence within RRV VP4 gene drives immune cell activation, leading to the development of experimental BA.
GRANTS
This project was funded by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) R01 DK-091566. It was supported in part by NIDDK P30 DK078392 (Research Flow Cytometry Core of the Digestive Disease Research Core Center in Cincinnati).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
A.W., S.K.M., B.D., A.C., I.L., and P.D. performed experiments; A.W., S.K.M., B.D., A.C., C.S.L., J.M., M.M., and G.T. analyzed data; A.W., S.K.M., B.D., A.C., C.S.L., J.M., and G.T. drafted manuscript; A.W., S.K.M., B.D., A.C., C.S.L., I.L., P.D., J.M., M.M., K.S., and G.T. edited and revised manuscript; A.W., S.K.M., B.D., A.C., C.S.L., I.L., P.D., J.M., M.M., K.S., and G.T. approved final version of manuscript; S.K.M., B.D., M.M., K.S., and G.T. conception and design of research; S.K.M., B.D., A.C., M.M., K.S., and G.T. interpreted results of experiments; S.K.M., B.D., C.S.L., and J.M. prepared figures.
ACKNOWLEDGMENTS
We thank Dr. Jelle Matthijnssens for assistance in obtaining several of the rotavirus strains tested.
REFERENCES
- 1.Allen SR, Jafri M, Donnelly B, McNeal M, Witte D, Bezerra J, Ward R, Tiao GM. Effect of rotavirus strain on the murine model of biliary atresia. J Virol 81: 1671–1679, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balistreri WF, Grand R, Hoofnagle JH, Suchy FJ, Ryckman FC, Perlmutter DH, Sokol RJ. Biliary atresia: current concepts and research directions. Summary of a symposium. Hepatology 23: 1682–1692, 1996. [DOI] [PubMed] [Google Scholar]
- 3.Bezerra JA. Potential etiologies of biliary atresia. Pediatr Transplant 9: 646–651, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Bezerra JA, Tiao G, Ryckman FC, Alonso M, Sabla GE, Shneider B, Sokol RJ, Aronow BJ. Genetic induction of proinflammatory immunity in children with biliary atresia. Lancet 360: 1653–1659, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Coots A, Donnelly B, Mohanty SK, McNeal M, Sestak K, Tiao G. Rotavirus infection of human cholangiocytes parallels the murine model of biliary atresia. J Surg Res 177: 275–281, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Tommaso AM, Andrade PD, Costa SC, Escanhoela CA, Hessel G. High frequency of human cytomegalovirus DNA in the liver of infants with extrahepatic neonatal cholestasis. BMC Infect Dis 5: 108, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drut R, Drut RM, Gomez MA, Cueto Rua E, Lojo MM. Presence of human papillomavirus in extrahepatic biliary atresia. J Pediatr Gastroenterol Nutr 27: 530–535, 1998. [DOI] [PubMed] [Google Scholar]
- 8.Feng N, Sen A, Nguyen H, Vo P, Hoshino Y, Deal EM, Greenberg HB. Variation in antagonism of the interferon response to rotavirus NSP1 results in differential infectivity in mouse embryonic fibroblasts. J Virol 83: 6987–6994, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng N, Sen A, Wolf M, Vo P, Hoshino Y, Greenberg HB. Roles of VP4 and NSP1 in determining the distinctive replication capacities of simian rotavirus RRV and bovine rotavirus UK in the mouse biliary tract. J Virol 85: 2686–2694, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenberg HB, Vo PT, Jones R. Cultivation and characterization of three strains of murine rotavirus. J Virol 57: 585–590, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guindon S, Delsuc F, Dufayard JF, Gascuel O. Estimating maximum likelihood phylogenies with PhyML. Methods Mol Biol 537: 113–137, 2009. [DOI] [PubMed] [Google Scholar]
- 12.Jafri M, Donnelly B, Allen S, Bondoc A, McNeal M, Rennert PD, Weinreb PH, Ward R, Tiao G. Cholangiocyte expression of α2β1-integrin confers susceptibility to rotavirus-induced experimental biliary atresia. Am J Physiol Gastrointest Liver Physiol 295: G16–G26, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mack CL. The pathogenesis of biliary atresia: evidence for a virus-induced autoimmune disease. Semin Liver Dis 27: 233–242, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mack CL, Falta MT, Sullivan AK, Karrer F, Sokol RJ, Freed BM, Fontenot AP. Oligoclonal expansions of CD4+ and CD8+ T-cells in the target organ of patients with biliary atresia. Gastroenterology 133: 278–287, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mack CL, Tucker RM, Lu BR, Sokol RJ, Fontenot AP, Ueno Y, Gill RG. Cellular and humoral autoimmunity directed at bile duct epithelia in murine biliary atresia. Hepatology 44: 1231–1239, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahjoub F, Shahsiah R, Ardalan FA, Iravanloo G, Sani MN, Zarei A, Monajemzadeh M, Farahmand F, Mamishi S. Detection of Epstein Barr virus by chromogenic in situ hybridization in cases of extra-hepatic biliary atresia. Diagn Pathol 3: 19, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McNeal MM, Broome RL, Ward RL. Active immunity against rotavirus infection in mice is correlated with viral replication and titers of serum rotavirus IgA following vaccination. Virology 204: 642–650, 1994. [DOI] [PubMed] [Google Scholar]
- 18.Mohanty SK, Donnelly B, Bondoc A, Jafri M, Walther A, Coots A, McNeal M, Witte D, Tiao GM. Rotavirus replication in the cholangiocyte mediates the temporal dependence of murine biliary atresia. PloS One 8: e69069, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohya T, Fujimoto T, Shimomura H, Miyano T. Degeneration of intrahepatic bile duct with lymphocyte infiltration into biliary epithelial cells in biliary atresia. J Pediatr Surg 30: 515–518, 1995. [DOI] [PubMed] [Google Scholar]
- 20.Riepenhoff-Talty M, Gouvea V, Evans MJ, Svensson L, Hoffenberg E, Sokol RJ, Uhnoo I, Greenberg SJ, Schakel K, Zhaori G, Fitzgerald J, Chong S, el-Yousef M, Nemeth A, Brown M, Piccoli D, Hyams J, Ruffin D, Rossi T. Detection of group C rotavirus in infants with extrahepatic biliary atresia. J Infect Dis 174: 8–15, 1996. [DOI] [PubMed] [Google Scholar]
- 21.Riepenhoff-Talty M, Schaekel K, Clark HF, Mueller W, Uhnoo I, Rossi T, Fisher J, Ogra PL. Group A rotaviruses produce extrahepatic biliary obstruction in orally inoculated newborn mice. Pediatr Res 33: 394–399, 1993. [DOI] [PubMed] [Google Scholar]
- 22.Sen A, Feng N, Ettayebi K, Hardy ME, Greenberg HB. IRF3 inhibition by rotavirus NSP1 is host cell and virus strain dependent but independent of NSP1 proteasomal degradation. J Virol 83: 10322–10335, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shivakumar P, Campbell KM, Sabla GE, Miethke A, Tiao G, McNeal MM, Ward RL, Bezerra JA. Obstruction of extrahepatic bile ducts by lymphocytes is regulated by IFN-gamma in experimental biliary atresia. J Clin Invest 114: 322–329, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shivakumar P, Sabla G, Mohanty S, McNeal M, Ward R, Stringer K, Caldwell C, Chougnet C, Bezerra JA. Effector role of neonatal hepatic CD8+ lymphocytes in epithelial injury and autoimmunity in experimental biliary atresia. Gastroenterology 133: 268–277, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shivakumar P, Sabla GE, Whitington P, Chougnet CA, Bezerra JA. Neonatal NK cells target the mouse duct epithelium via Nkg2d and drive tissue-specific injury in experimental biliary atresia. J Clin Invest 119: 2281–2290, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sokol RJ, Mack C. Etiopathogenesis of biliary atresia. Semin Liver Dis 21: 517–524, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Sokol RJ, Shepherd RW, Superina R, Bezerra JA, Robuck P, Hoofnagle JH. Screening and outcomes in biliary atresia: summary of a National Institutes of Health workshop. Hepatology 46: 566–581, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tyler KL, Sokol RJ, Oberhaus SM, Le M, Karrer FM, Narkewicz MR, Tyson RW, Murphy JR, Low R, Brown WR. Detection of reovirus RNA in hepatobiliary tissues from patients with extrahepatic biliary atresia and choledochal cysts. Hepatology 27: 1475–1482, 1998. [DOI] [PubMed] [Google Scholar]
- 29.Wang W, Donnelly B, Bondoc A, Mohanty SK, McNeal M, Ward R, Sestak K, Zheng S, Tiao G. The rhesus rotavirus gene encoding VP4 is a major determinant in the pathogenesis of biliary atresia in newborn mice. J Virol 85: 9069–9077, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]