Abstract
Human jejunum smooth muscle cells (SMCs) and interstitial cells of Cajal (ICCs) express the SCN5A-encoded voltage-gated, mechanosensitive sodium channel NaV1.5. NaV1.5 contributes to small bowel excitability, and NaV1.5 inhibitor ranolazine produces constipation by an unknown mechanism. We aimed to determine the presence and molecular identity of Na+ current in the human colon smooth muscle and to examine the effects of ranolazine on Na+ current, mechanosensitivity, and smooth muscle contractility. Inward currents were recorded by whole cell voltage clamp from freshly dissociated human colon SMCs at rest and with shear stress. SCN5A mRNA and NaV1.5 protein were examined by RT-PCR and Western blots, respectively. Ascending human colon strip contractility was examined in a muscle bath preparation. SCN5A mRNA and NaV1.5 protein were identified in human colon circular muscle. Freshly dissociated human colon SMCs had Na+ currents (−1.36 ± 0.36 pA/pF), shear stress increased Na+ peaks by 17.8 ± 1.8% and accelerated the time to peak activation by 0.7 ± 0.3 ms. Ranolazine (50 μM) blocked peak Na+ current by 43.2 ± 9.3% and inhibited shear sensitivity by 25.2 ± 3.2%. In human ascending colon strips, ranolazine decreased resting tension (31%), reduced the frequency of spontaneous events (68%), and decreased the response to smooth muscle electrical field stimulation (61%). In conclusion, SCN5A-encoded NaV1.5 is found in human colonic circular smooth muscle. Ranolazine blocks both peak amplitude and mechanosensitivity of Na+ current in human colon SMCs and decreases contractility of human colon muscle strips. Our data provide a likely mechanistic explanation for constipation induced by ranolazine.
Keywords: colon, smooth muscle, sodium channel, SCN5A, ranolazine
Sodium Channel NaV1.5 Is Functionally Significant in Human GI Smooth Muscle
ion channels are directly involved in the regulation of electrical properties and excitability of electrically active tissues such as smooth muscle and nerves. GI smooth muscle excitability relies on multiple types of ion channels (3). Intracellular recordings from human small intestine smooth muscle strips have shown that voltage-gated sodium channels (NaV) are involved in the regulation of resting membrane potential and slow wave frequency and morphology (10, 22). We have previously shown that SCN5A-encoded NaV1.5, a voltage-gated sodium selective ion channel, is responsible for the sodium (Na+) current in human jejunum circular smooth muscle cells (SMC) and interstitial cells of Cajal (ICC) (10, 21, 22). Sodium current is also present in human colon SMC, but the molecular nature of this current is unclear (23, 24, 25).
Smooth Muscle Voltage-Gated Sodium Channels Are Mechanosensitive
Mechanical stimuli are important in the GI tract and known to affect the electrophysiology of smooth muscle (11). Voltage-gated sodium channels, including NaV1.5 in the GI tract, are mechanosensitive (10, 15, 20). Mechanical stimulation with shear stress increases peak Na+ currents in the human jejunum circular SMC and ICC (17, 21). In heterologous expression systems, mechanical stimuli significantly increase peak NaV1.5 currents, accelerate channel activation and inactivation, and increase single channel activity at resting potentials (5, 7, 17). Stretch increases slow wave frequency in the human jejunum (22). It is currently unknown whether Na+ current in the human colon is also mechanosensitive.
SCN5A Mutations in IBS Patients Lead to Abnormal NaV1.5 Function
Studies suggest that NaV1.5 is clinically relevant in GI functional disorders (12) such as irritable bowel syndrome (IBS) (4, 12, 17). A proportion of IBS patients have SCN5A mutations that lead to abnormal NaV1.5 function (4, 17). A majority of these SCN5A missense mutations cause a loss-of-function NaV1.5 phenotype in vitro, and the loss of NaV1.5 activity is associated with a constipation-predominant IBS in the majority of these IBS patients. Importantly, restoration of NaV1.5 function in a patient leads to improvement of constipation (4).
Ranolazine Is a NaV1.5 Inhibitor Associated with Constipation
Ranolazine is a piperazine derivative NaV1.5 inhibitor that blocks NaV1.5 voltage-dependent peak current and mechanosensitivity (7). Ranolazine is clinically available as an anti-anginal therapy with a particular advantage over other anti-anginal therapies in that it does not decrease heart rate and blood pressure (14). Ranolazine blocks NaV1.5 at an IC50 ∼135 μM (9) and has a low antagonist activity against L-type voltage-gated calcium channels (CaV1.x) [IC50 ∼300 μM (1)], consistent with its clinical lack of effect on blood pressure (8). Intriguingly, multiple large-scale clinical and postmarketing trials showed that constipation is one of the most commonly reported side effects of ranolazine, with a severalfold higher incidence for ranolazine than for placebo (14). It is unclear whether NaV1.5 currents are present in the human colon and whether blockade of these currents contributes to constipation related to ranolazine use.
The goals of this study were to examine the molecular identity of the Na+ current and examine the effect of ranolazine on Na+ current, mechanosensitivity, and contractility in human colonic circular smooth muscle cells and muscle strips.
METHODS
The Institutional Review Boards of the respective institutions approved the use of normal human colonic tissue resected as part of the surgical procedure for nonobstructing colon cancer.
Tissue Dissection
Colon specimens not involved by cancer (at least 10 cm away) were harvested directly into chilled F12 buffer solution (F-12: Gibco, Invitrogen, Grand Island, NY; 10 ml/l antibiotic antimicotic A5955: Sigma, St. Louis, MO; 14 mmol/l NaHCO3; pH 7.35) and transported to the laboratory within 20 min. Colonic tissue was transferred from F12, cut along the mesentery, and pinned mucosa side down onto a Sylgard (Dow Corning, Midland, MI)-coated dish filled with ice-cold Krebs solution (in mM, 137.4 Na+, 5.9 K+, 2.5 Ca2+, 1.2 Mg2+, 134 Cl−, 15.5 NaHCO3, 1.2 H2PO4−, 11.5 glucose, pH 7.4). The mucosa was cut away with a sharp scissors and discarded. The remaining tissue was repinned, serosa side up. The longitudinal muscle layer was peeled away from the circular layer with forceps. Circular muscle strips were shaved off of the connective tissue with a scalpel.
Reverse Transcription PCR
RT-PCR was carried out on RNA isolated from colonic smooth muscle strips using specific primers designed against the human SCN5A sequence as described before (21, 22).
Western Blots
Tissue preparation.
Flash-frozen samples of human heart and colon circular muscle were homogenized in 1 ml of homogenization buffer [0.025 M Tris-HCl, 0.15 M NaCl, 0.001 M EDTA, 1% NP-40, 5% glycerol, pH 7.4, with protease inhibitors (Sigma)] using a handheld homogenizer. The homogenates were centrifuged at high speed for 30 min at 4°C and the supernatant quantitated for protein concentration (ThermoScientific BCA Kit).
Immunoblotting.
Forty micrograms of human colon circular muscle lysates and 10 μg of human heart lysate (not boiled) were loaded for each sample and electrophoresed on a 4–15% Tris-glycine gel. Proteins were transferred to nitrocellulose (0.4 μm; BioRad) and blocked for 1 h in 5% NFDM at 4°C. Blots were incubated in anti-Nav1.5 Ig (Covance) or anti-GAPDH (Fitzgerald) overnight at 4°C. Blots were washed and incubated with the appropriate horseradish peroxidase (HRP)-conjugated secondary antibody (Jackson Laboratories) for 2 h at 4°C. Blots were washed and developed using the BioRad Clarity ECL kit and the BioRad Gel Documentation System.
Human Colon Smooth Muscle Cell Dissociation
Single smooth muscle cells from the human colonic circular layer were obtained by a method adapted from that previously described (10, 19). Circular smooth muscle strips were dissected out as described above in Tissue Dissection. Circular muscle layer strips were placed temporarily into a separate Krebs bath during dissection, then minced in 35 × 10 mm cell culture dishes filled with a Ca2+-free Hanks solution (Cellgro, Herndon, VA) supplemented with 1.5–2.0 mg/ml papain and 0.5 mg/ml dl-dithiothreitol (dTT; Sigma). The tissue was transferred to a test tube and stirred for 3 min at 800 rpm at 32°C on an isotemp magnetic stir plate (Fisher Scientific, Pittsburgh, PA). Next, cells were spun (1,000 rpm or 170 g for 90 s), and the supernatant was replaced with a collagenase solution (Hanks; 1.0 mg/ml collagenase type II; 2.0 mg/ml BSA; 5.0 mg/ml dTT). Colon cells in collagenase were returned to the hotplate and stirred at 600 rpm in the test tube at 32°C. After cells began to appear in the top fraction of the supernatant, the cells were spun as described above and rinsed twice with fresh enzyme-free Hanks. The cells were returned to the stir plate (450–500 rpm), and at 5-min intervals, a 7-ml cell suspension was transferred into tubes containing 2 ml modified minimal essential medium [10.4 g/l MEM (Sigma, St. Louis, MO) containing (in mM) 4.17 Na+, 0.5 Ca2+, 0.5 Mg2+, 2 Cl−, 4.17 HCO3−, 10 HEPES, pH 7.2, 276 mOsm].
HEK293 Cell Transfection
L-type Ca2+ channel subunits α1C and β2 were cotransfected with green fluorescent protein into HEK293 cells and voltage-clamp recordings were performed and recorded as previously described (13).
Whole Cell Electrophysiology
A Sutter Instruments P-97 puller (Sutter Instrument, Novato, CA) was used to pull electrodes from Kimble KG-12 glass. Electrodes were coated with heat-cured R6101 compound (Dow Corning, Midland, MI). Axon 200A amplifier, Digidata 1322A, and Clampex 10 (Molecular Devices, Union City, CA) software were used for voltage-clamp and data acquisition.
Electrophysiology protocols.
Whole cell inward currents were measured by standard patch-clamp technique as described previously (21). Cells were held at −100 mV and stepped to −80 through 35 mV in 5-mV steps. Start-to-start time was 1 s. Mechanical stimulation was induced by bath flow of solution through a 0.7-ml elliptical bath chamber, calibrated at 10 ml/min.
Human Ascending Colon Muscle Strips Contractility
Human colon strips were collected as above. Muscle strips (2 × 10 mm) were oriented in circular smooth muscle axis on the organ chamber and equilibrated over 60 min. Ranolazine (300 μM) and TTX (1 μM) were added to the bath solution. In specific experiments, electrical field stimulation applied using 150 V, 3 ms, 20 Hz, and 20 s duration. Data were acquired using LabChart software (ADInstruments, Colorado Springs, CO).
Drugs and Solutions
Electrophysiology.
The intracellular solution contained (in mM) 130 Cs+, 125 methanesulfonate, 20 Cl−, 5 Na+, 5 Mg2+, 5 HEPES, 2 EGTA, 2.5 ATP, 0.1 GTP. pH was 7.0, and osmolality was 300 mmol/kgH2O. Extracellular NaCl Ringer solution contained (in mM) 149.2 Na+, 159 Cl−, 4.74 K+, 2.54 Ca2+, 5 glucose, and 5 HEPES. For ion substitution experiments, Na+ was replaced with 149.2 mM N-methyl-d-glucamine (NMDG+), or Ca2+ was replaced with 2.54 mM Mn2+. Ranolazine was diluted to 50 μM in bath solution from a 10 mmol/l ethanol stock.
Muscle strips.
Bath solution was Krebs (in mM, 120.4 Na+, 5.9 K+, 2.5 Ca2+, 1.2 Mg2+, 15.5 NaHCO3, 1.2 NaH2PO4, 11.5 glucose, pH 7.4). Ranolazine was used at a final working concentration of 300 μM.
Data Analysis
Data were analyzed using Clampfit 10 software (Molecular Devices, Sunnyvale, CA), Microsoft Excel 2003 (Microsoft, Redmond, WA) and SigmaPlot 12 (SPSS, Chicago, IL).
Electrophysiology.
Inward currents displaying fast components were measured within the first 10 ms of the voltage step. For current-voltage graphs, the maximum inward current of a single control trace was normalized to 1 using the equation: , where INORM is normalized current, IV is measured current at a given voltage, and IMAX is maximum peak inward current of the control trace. Thus peaks of all other traces per cell were expressed as a fraction of 1. Statistical comparisons were performed on raw data using a paired two-tailed Student's t-test. Statistical significance was accepted when P < 0.05. Data are expressed as mean values ± SE.
Muscle strips.
Resting muscle tone was measured as baseline tension of two spans ≥10 min immediately before and ∼30 min after addition of ranolazine (300 μM). Contractile activity was measured as the contraction frequency and average contraction amplitude over two spans ≥30 min before and after addition of the drug. Response to electrical field stimulation (EFS) was measured as the difference between EFS peak and baseline tension prior to stimulus.
RESULTS
Voltage-Gated Sodium Current Is Present in Human Colon Smooth Muscle Cells
We carried out voltage-clamp experiments in whole cell configuration to characterize the inward currents from human colon circular layer smooth muscle cells (SMCs) (Fig. 1A). A robust inward current had a fast component in 35/44 (80%) cells, a slow component in 30/44 (68%) cells, and both components in 25/44 (57%) cells (Fig. 1A).
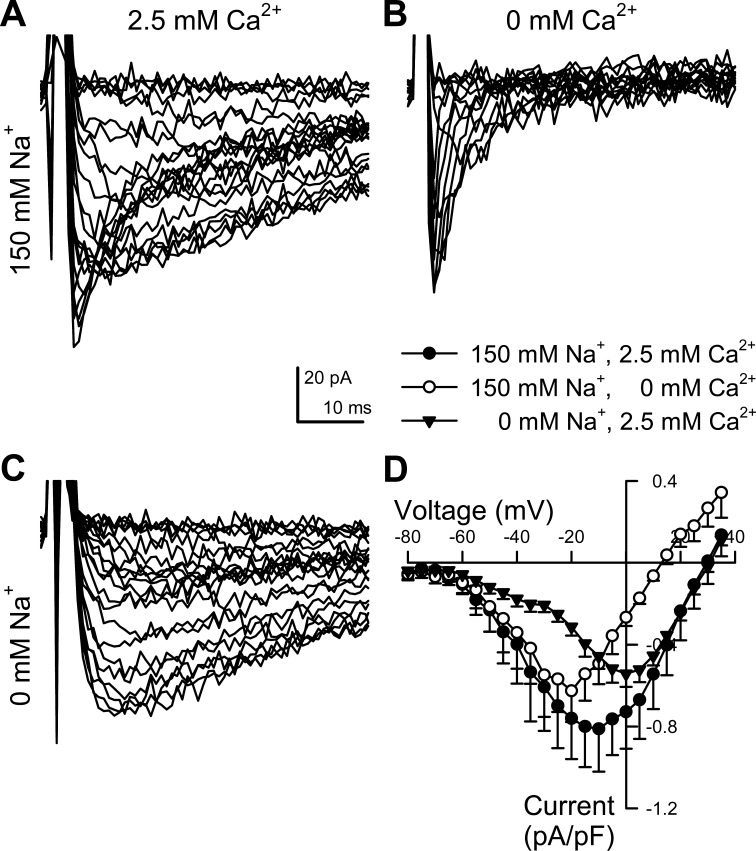
Fig. 1.
Fast Na+ and slow Ca2+ components contribute to total inward current in human colon myocytes. A–C: representative whole cell inward currents recorded from a human colonic circular smooth muscle cell evoked by stepping for 50 ms to −80 through +35 mV in 5-mV intervals from the −100 mV holding potential. Intracellular solution contained 5 mM Na+ and no added Ca2+, while the extracellular solution containing 150 mM Na+ and 2.5 mM Ca2+ was exchanged. A: total inward current. B: isolation of the fast component of inward current from the same cell as A by replacing extracellular Ca2+ with Mn2+. C: isolation of slow component of inward current from the same cell as A and B by replacing extracellular Na+ with NMDG+. D: average current-voltage relationships of peak inward currents (●, as shown in A), fast (○, B) and slow (▼, C) components of inward current, normalized to the conductance of each of cell. n = 6 cells.
We next aimed to determine which ion species were involved in carrying these inward currents. The slower component peaked around 0 mV and was eliminated by the replacement of Ca2+ by Mn2+ (Fig. 1, C and D), suggesting that this current was due to voltage-gated calcium channels (CaV). The faster component peaked at −20 mV and remained after exchanging Ca2+ with Mn2+ (Fig. 1, B and D), but it was eliminated by the replacement of Na+ by NMDG+ (Fig. 1C). These results suggest that the faster current was carried by voltage-gated sodium channels (NaV).
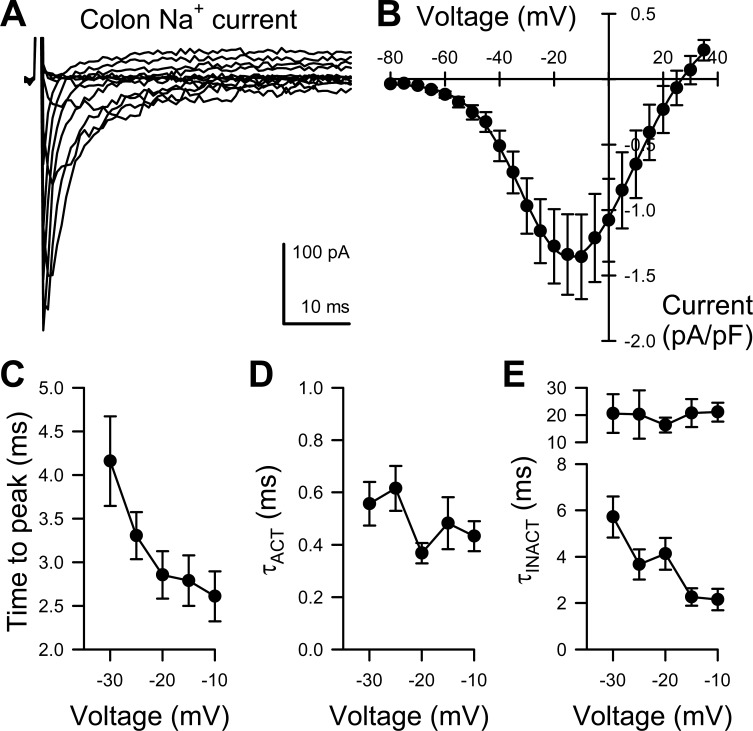
We then analyzed the voltage-dependence and kinetics of the NaV currents with the slower Ca2+ currents eliminated using Mn2+ (Fig. 2A). We fit these voltage-dependent currents using three exponentials (one time constant for activation, the other two time constants for inactivation) and the peaks of the voltage-dependent currents with a two-state Boltzmann function. The current-voltage data were fit by using two-state Boltzmann curves with half-point of the voltage dependence of activation at −37.4 ± 1.7 mV, slope 7.9 ± 0.8 (Fig. 2B). At peak, these currents activated with a time constant of 0.43 ± 0.06 ms, and inactivated with time constants of 2.2 ± 0.5 ms and 21.1 ± 3.5 ms (Fig. 2, C–E). The voltage dependence of activation and inactivation is shown in Fig. 2B.
Fig. 2.
Functional parameters of Na+ current from human colon myocytes. A: representative traces of human colon myocyte Na+ current evoked by steps from −100 mV to −80 through +35 mV in 5-mV intervals. B: average current-voltage relationships of peak inward Na+ currents from myocytes of the human colon normalized to the capacitance of each of cell. C–E: kinetic properties of peak inward Na+ currents from myocytes of the human colon comparing time-to-peak activation (C), and time constants of activation (D), fast and slow inactivation (E) (n = 13).
SCN5A-Encoded NaV1.5 Is Present in the Circular Smooth Muscle of Human Colon
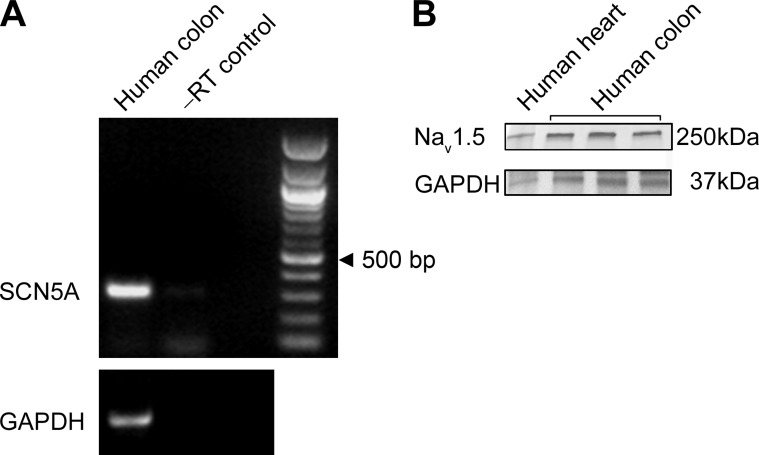
We next aimed to determine whether SCN5A, which encodes NaV1.5, is present in circular smooth muscle cells. Using RT-PCR on the smooth muscle layer with previously validated primers we found evidence for SCN5A mRNA in human colonic smooth muscle (Fig. 3A). We also performed Western blots on human heart and human colon circular smooth muscle and demonstrate the presence of the NaV1.5 protein in human colon circular smooth muscle (Fig. 3B).
Fig. 3.
SCN5A is expressed in human colonic smooth muscle. A: reverse transcription PCR (RT-PCR) using primers specific for SCN5A was carried out on RNA extracted from the human colonic smooth muscle layer. Identity of the band was confirmed by sequencing. From left to right, human colon smooth muscle, negative control lacking reverse transcriptase, blank lane, DNA ladder. GAPDH was used as a positive control for extraction. Black arrowhead points to 500 bp. B: protein immunoblots from human heart and human colon circular smooth muscle from three different samples demonstrate expression of Nav1.5.
Na+ Channel in Circular Smooth Muscle of Human Colon is Mechanosensitive
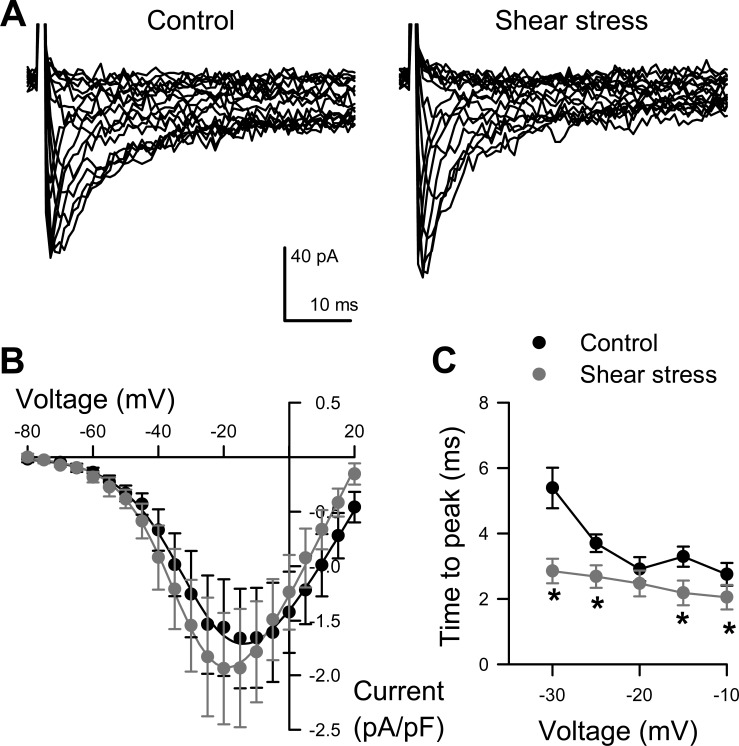
We next aimed to determine whether the human colon smooth muscle cell Na+ current is mechanosensitive using shear stress as a model of mechanostimulation. Voltage-clamped colonic smooth muscle cells were superfused with NaCl Ringer (bath) solution at a rate of 10 ml/min. In response to shear stress, peak Na+ current increased 18 ± 2%, from −1.73 ± 0.45 pA/pF at rest to −2.03 ± 0.54 pA/pF with shear stress (n = 6, P < 0.05, Fig. 4, A and B). Shear stress also accelerated the activation time to peak at voltages between −30 and −10 mV (−30 mV, from 5.4 ± 0.6 ms at rest to 2.9 ± 0.4 ms with shear stress; n = 5, P < 0.05, Fig. 4C).
Fig. 4.
Na+ current in human colonic myocytes is shear sensitive. A: representative traces of human colon myocyte Na+ current evoked by steps from −100 mV to −80 through +35 mV in 5-mV intervals, recorded with extracellular solution and perfused at 0 (Control, left) or 10 (Shear stress, right) ml/min. B: average peak current-voltage relationships before (black circles) or during (gray circles) perfusion. Solid lines are Boltzmann fits. C, Time to peak activation. n = 6 cells, *P < 0.05 by 2-tailed Student's t-test.
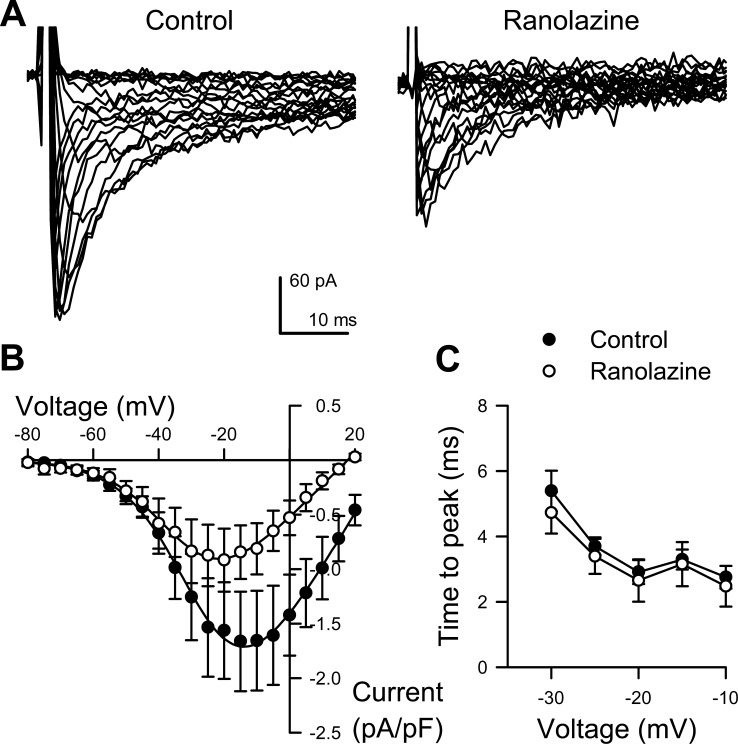
Ranolazine Decreases NaV Peak Current in Circular Smooth Muscle of the Human Colon
Ranolazine is a clinically employed NaV1.5-selective modulator which is known to inhibit NaV1.5 peak and late currents (1). We next examined the effect of ranolazine on inward currents from human colonic myocytes (Fig. 5). Addition of 50 μM ranolazine to the extracellular solution reversibly decreased peak Na+ currents by 43 ± 9%, from −1.73 ± 0.45 to −0.95 ± 0.28 pA/pF (n = 6, P < 0.05; Fig. 5, A and B). The kinetics of activation and inactivation remained unchanged (n = 6, P > 0.05; Fig. 5C). We tested the effects of ranolazine at this concentration on L-type Ca2+ channels in HEK cells, and ranolazine did not block L-type Ca2+ current (−3.6 ± 0.6 pA/pF before vs. −3.7 ± 0.5 pA/pF after ranolazine, +4.5 ± 9.3% change, n = 5 P > 0.05). Therefore, ranolazine decreases peak Na+ current in human colon smooth muscle cells.
Fig. 5.
Ranolazine blocks the human colon smooth muscle cell Na+ currents. A, Representative traces human colon myocyte Na+ current evoked by steps from −100 mV to −80 through +35 mV in 5 mV intervals, recorded with 0 (Control, left) or 50 μmol/l ranolazine (Ranolazine, right). B: average peak current-voltage relationships before without (●) or with (○) drug. Solid lines are Boltzmann fits. C: time to peak activation at multiple voltages (n = 4).
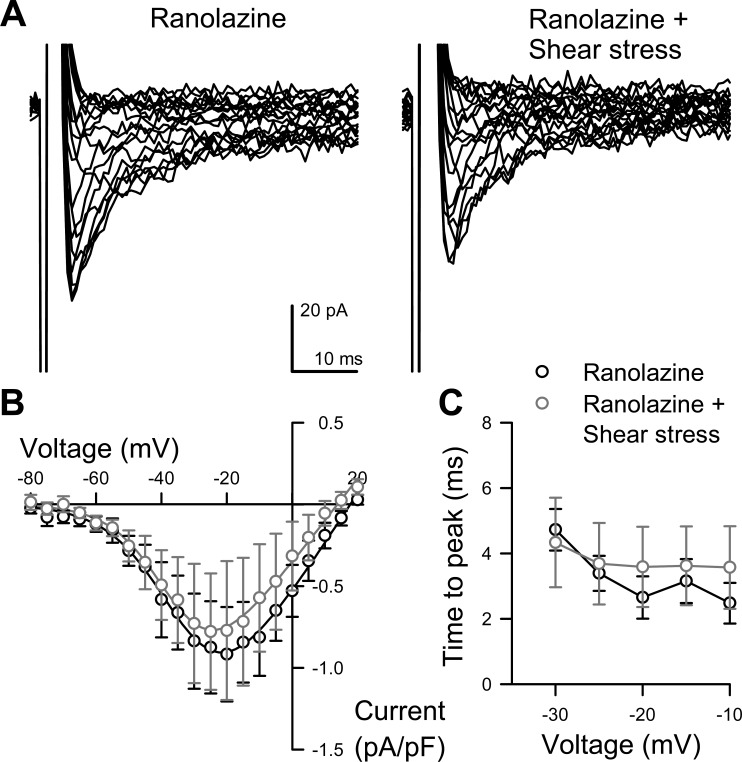
Ranolazine Blocks the Mechanosensitivity of NaV Current in Human Colon Smooth Muscle Cells
To study whether ranolazine also affected mechanosensitivity of colonic Na+ current, we shear-stressed smooth muscle cells while in voltage-clamp in the presence or absence of 50 μM ranolazine. Ranolazine inhibited the increase in peak Na+ current induced by shear (−0.94 ± 0.43 pA/pF at rest with ranolazine, to −0.86 ± 0.38 pA during shear with ranolazine; n = 4, P > 0.05; Fig. 6, A and B). Ranolazine also blocked the acceleration in activation caused by shear (3.6 ± 1.1 ms at rest with ranolazine, to 3.6 ± 1.2 ms during shear with ranolazine, n = 3, P > 0.05; Fig. 6C). Therefore, ranolazine not only decreased peak Na+ current (Fig. 5) but also separately inhibited the shear response (Fig. 6) in human colonic circular smooth muscle.
Fig. 6.
Ranolazine blocks shear sensitivity of Na+ current. A: representative traces of human colon myocyte Na+ current evoked by steps from −100 mV to −80 through +35 mV in 5-mV intervals, recorded with 50 μM ranolazine in the extracellular solution and perfused at 0 (Ranolazine, left) or 10 ml/min (Ranolazine + Shear stress, right). B: average peak current-voltage relationships with drug and before (black-outlined circles) or during (gray-outlined circles) perfusion. Solid lines are Boltzmann fits. C: time to peak activation at multiple voltages. n = 4.
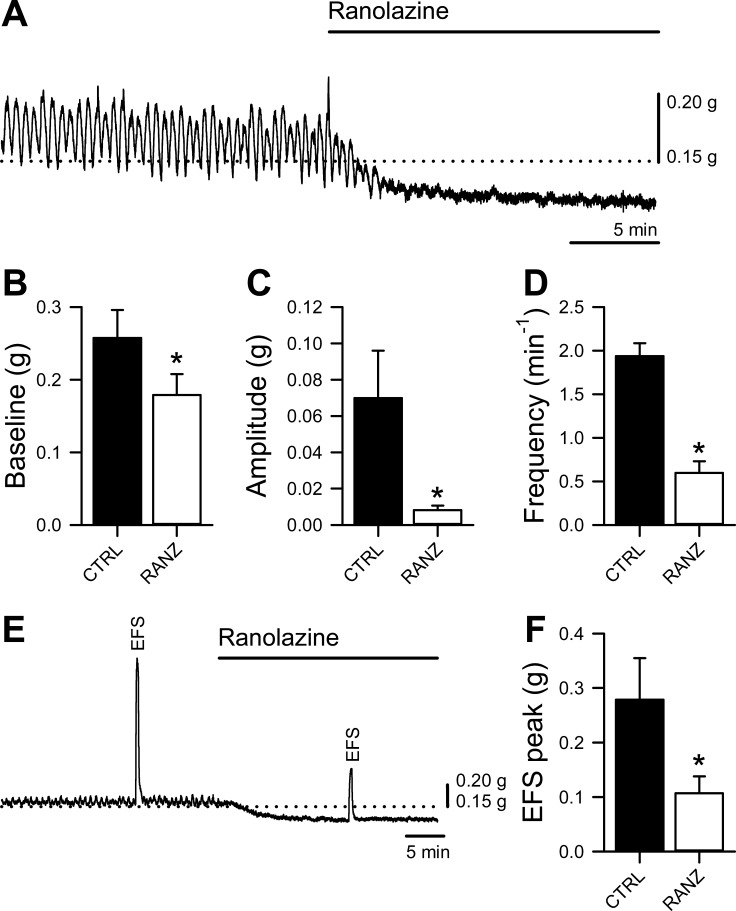
Ranolazine Decreases Contractility in Human Ascending Colon Muscle Strips
We next examined ascending human colonic contractility in response to ranolazine (Fig. 7). After a period of stabilization, ranolazine (300 μM) was added to the ascending human colon strips oriented along the circular smooth muscle axis. Ranolazine decreased resting tension from 0.26 ± 0.04 to 0.18 ± 0.03 g or by 29 ± 4% (n = 19, P = 0.00016) (Fig. 7, A and B), decreased the mean contraction amplitude from 0.070 ± 0.026 to 0.008 ± 0.002 g or by 73 ± 5% (n = 14, P = 0.034) (Fig. 7, A and C), and decreased the frequency of the spontaneous activity from 1.94 ± 0.15/min to 0.60 ± 0.13/min or by 68 ± 7% (n = 14, P = 0.00001) (Fig. 7, A and D). These data demonstrate that ranolazine decreases the resting tone and spontaneous activity in the human ascending colon.
Fig. 7.
Ranolazine decreases human colon smooth muscle contractility. A: representative muscle bath contractility traces from a human ascending colon muscle strip before and after the addition of 300 μM ranolazine, showing a decrease in resting tone and spontaneous contractions. B–D: mean baseline tension (B), contraction amplitude (C), and contraction frequency (D). E: representative muscle bath contractility traces from a human ascending colon muscle strip before and after the addition of 300 μM ranolazine, showing a decrease in peak response to EFS. F: mean peak response to electrical field stimulation (EFS) of 9–19 strips from 10 preparations. *P < 0.05 by 2-tailed Student's t-test.
Stimulated responses were also diminished by ranolazine in experiments using EFS with smooth muscle stimulation parameters. EFS-induced contractions were assayed, then 300 μM ranolazine was added to the bath and EFS was repeated. In these experiments EFS-induced contractions were decreased from 0.28 ± 0.08 to 0.11 ± 0.03 g or by 59 ± 9% (n = 9, P = 0.013) (Fig. 7, E and F). These data show that ranolazine inhibits excitability and contractility of the human ascending colon.
DISCUSSION
Voltage-sensitive sodium currents of unknown identity are present in rat and human colon smooth muscle cells (25). In the human jejunum, voltage-sensitive sodium currents are carried by SCN5A-encoded NaV1.5, which is expressed in smooth muscle cells (10) and interstitial cells of Cajal (22). In the human jejunum circular smooth muscle strips, inhibition of Na+ currents by lidocaine (22) or ion substitution (2, 22) leads to a decrease in smooth muscle excitability. In patients with irritable bowel syndrome (IBS) loss of NaV1.5 function due to mutations is associated with constipation (4). Ranolazine, a novel clinically approved NaV1.5 antagonist, is also associated with constipation (14). In this study we found that in human colonic circular smooth muscle SCN5A-encoded NaV1.5 contributes to a voltage-dependent and mechanosensitive Na+ current, and that ranolazine blocks both peak and mechanosensitivity of this Na+ current and also decreases contractility of human colon muscle strips.
SCN5A-Encoded NaV1.5 Contributes to the Fast Voltage-Dependent Sodium Current in the Human Colon Smooth Muscle Cells
Our data show that there was a robust voltage-sensitive inward current (−1.36 ± 0.36 pA/pF) in human colon smooth muscle cells. We used ion substitution to determine that the faster activating and inactivating current was carried by sodium. We present several lines of evidence that NaV1.5 contributes significantly to inward Na+ current in these smooth muscle cells. First, RT-PCR and Western blots of human colon circular smooth muscle identified SCN5A and NaV1.5, respectively (Fig. 3). Second, the voltage dependence and kinetic parameters of this Na+ current are consistent with the published parameters of NaV1.5 (Figs. 1 and 2) (10). Third, these NaV currents were sensitive to shear to the same extent as the NaV1.5 currents elsewhere in smooth muscle (Fig. 4) (22) and when NaV1.5 were heterologously expressed in HEK-293 cells (17). Fourth, NaV1.5-selective inhibitor ranolazine decreased Na+ peak currents and Na+ current mechanosensitivity to the same extent as in other studies (Fig. 5) (7). Therefore, our data show that SCN5A-encoded NaV1.5 contributes significantly to the inward Na+ current in the human circular smooth muscle cells.
Ranolazine Inhibits NaV Current in Human Colon Smooth Muscle Cells
Ranolazine is a clinically approved piperazine derivative NaV1.5 antagonist (1). Previous studies show that the IC50 for ranolazine block of NaV1.5 peak is ∼135 μM (9), and our results here in human colon myocytes are consistent, showing a 43.2 ± 9.3% inhibition by 50 μM ranolazine (Fig. 5) without affecting voltage sensitivity of activation or the kinetics of activation and fast inactivation.
Ranolazine Inhibits NaV Mechanosensitivity in Human Colon Smooth Muscle Cells
Mechanical sensitivity of ion channels is important since ion channels are directly involved in smooth muscle excitability. Acute mechanical stimulation of NaV1.5 is excitatory, with both shear stress and pressure significantly increasing peak currents and accelerating kinetics (5, 7). Further, NaV1.5 mechanosensitivity may be important in GI motility, which is abnormal in some IBS-linked mutations (17). We have previously shown that ranolazine (7) and local anesthetics (6) inhibit NaV1.5 mechanosensitivity. These data in human colon myocytes are consistent, showing that 50 μM ranolazine blocks shear-induced increase in peak NaV currents to a similar degree previously reported for NaV1.5 channels (Fig. 6).
Ranolazine Decreases Contractility of Human Colon Smooth Muscle Strips
Previous studies in vitro and in vivo suggest that ranolazine decreases vascular and bladder smooth muscle excitability and tension (16, 18). In this study we extend these findings to the GI tract. We demonstrate that ranolazine decreases contractility of the human colon muscle strips (Fig. 7). Ranolazine decreased resting tension by 29% (Fig. 7, A and B), and the amplitude and frequency of spontaneous contractile activity by 73% and 68%, respectively (Fig. 7, A, C, and D). It also decreased evoked activity in response to electrical field stimulation (EFS) by 59% (Fig. 7, E and F). These results complement the electrophysiology data and together suggest that ranolazine significantly decreases excitability and contractility of the human colon smooth muscle. Future studies will have to determine if other targets also contribute to the contractility effect of ranolazine.
Ranolazine Block of Human Colon Smooth Muscle Cell NaV Current May Contribute to the Constipation Phenotype Observed in Clinical Studies
Since block of NaV currents in smooth muscle decreases excitability (2, 22), and NaV1.5 loss-of-function is associated with constipation-predominant IBS, while restoration of NaV1.5 current improves constipation (4), inhibition of NaV1.5 peak currents and NaV1.5 mechanosensitivity by ranolazine may provide a mechanism for ranolazine-induced constipation.
In summary, we demonstrate the presence of the voltage-gated mechanosensitive SCN5A-encoded NaV1.5 in the circular smooth muscle layer of human colon. The NaV1.5 peak currents and mechanosensitivity in human colon smooth muscle cells were inhibited by ranolazine which also decreased contractility. These findings suggest that the mechanism of constipation due to ranolazine may be due to block of NaV1.5 and its mechanosensitivity by this drug. These findings also open the door for novel diagnostic and therapeutic options in GI disorders targeting NaV1.5.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-57266 and DK-57061.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
L.N., P.R.S., R.E.K., A.B., and G.F. conception and design of research; L.N., P.R.S., R.E.K., A.M., C.E.B., R.R.C., D.W.L., E.J.D., C.F.K., P.J.M., A.B., and G.F. analyzed data; L.N., P.R.S., R.E.K., A.B., and G.F. interpreted results of experiments; L.N., P.R.S., A.B., and G.F. drafted manuscript; L.N., P.R.S., A.B., and G.F. edited and revised manuscript; L.N., P.R.S., P.-L.R., R.E.K., A.M., C.E.B., R.R.C., D.W.L., E.J.D., C.F.K., P.J.M., A.B., and G.F. approved final version of manuscript; P.R.S., P.-L.R., R.E.K., A.M., C.E.B., R.R.C., D.W.L., E.J.D., C.F.K., and P.J.M. performed experiments; P.R.S. prepared figures.
ACKNOWLEDGMENTS
We thank Dr. D. Linden for valuable consultations, G. Stoltz for tissue dissection and cell dissociation, and K. Zodrow for secretarial assistance.
REFERENCES
- 1.Antzelevitch C, Belardinelli L, Zygmunt AC, Burashnikov A, Di Diego JM, Fish JM, Cordeiro JM, Thomas G. Electrophysiological effects of ranolazine, a novel antianginal agent with antiarrhythmic properties. Circulation 110: 904–910, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barajas-Lopez C, Den Hertog A, Huizinga JD. Ionic basis of pacemaker generation in dog colonic smooth muscle. J Physiol 416: 385–402, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beyder A, Farrugia G. Targeting ion channels for the treatment of gastrointestinal motility disorders. Therap Adv Gastroenterol 5: 5–21, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beyder A, Mazzone A, Strege PR, Tester DJ, Saito YA, Bernard CE, Enders FT, Ek WE, Schmidt PT, Dlugosz A, Lindberg G, Karling P, Ohlsson B, Gazouli M, Nardone G, Cuomo R, Usai-Satta P, Galeazzi F, Neri M, Portincasa P, Bellini M, Barbara G, Camilleri M, Locke GR 3rd, Talley NJ, D'Amato M, Ackerman MJ, Farrugia G. Loss-of-function of the voltage-gated sodium channel NaV1.5 (channelopathies) in patients with irritable bowel syndrome. Gastroenterology 146: 1659–1668, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beyder A, Rae JL, Bernard C, Strege PR, Sachs F, Farrugia G. Mechanosensitivity of Nav1.5, a voltage-sensitive sodium channel. J Physiol 588: 4969–4985, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beyder A, Strege PR, Bernard C, Farrugia G. Membrane permeable local anesthetics modulate NaV 1.5 mechanosensitivity. Channels (Austin) 6: 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beyder A, Strege PR, Reyes S, Bernard C, Terzic A, Makielski J, Ackerman M, Farrugia G. Ranolazine decreases mechanosensitivity of the voltage-gated sodium ion channel NaV1.5: a novel mechanism of drug action. Circulation 125: 2698–2706, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng CY, Kuang SJ, Rao F, Yang H, Fang XH, Shan ZX, Li XH, Zhou ZL, Lin QX, Yang M, Wu SL, Yu XY, Lin SG. Effect of ranolazine on rat intrarenal arteries in vitro. Eur J Pharmacol 683: 211–216, 2012. [DOI] [PubMed] [Google Scholar]
- 9.Fredj S, Sampson KJ, Liu H, Kass RS. Molecular basis of ranolazine block of LQT-3 mutant sodium channels: evidence for site of action. Br J Pharmacol 148: 16–24, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holm AN, Rich A, Miller SM, Strege P, Ou Y, Gibbons S, Sarr MG, Szurszewski JH, Rae JL, Farrugia G. Sodium current in human jejunal circular smooth muscle cells. Gastroenterology 122: 178–187, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Kraichely RE, Farrugia G. Mechanosensitive ion channels in interstitial cells of Cajal and smooth muscle of the gastrointestinal tract. Neurogastroenterol Motil 19: 245–252, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Locke GR 3rd, Ackerman MJ, Zinsmeister AR, Thapa P, Farrugia G. Gastrointestinal symptoms in families of patients with an SCN5A-encoded cardiac channelopathy: evidence of an intestinal channelopathy. Am J Gastroenterol 101: 1299–1304, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Lyford GL, Strege PR, Shepard A, Ou Y, Ermilov L, Miller SM, Gibbons SJ, Rae JL, Szurszewski JH, Farrugia G. α1C (CaV1.2) L-type calcium channel mediates mechanosensitive calcium regulation. Am J Physiol Cell Physiol 283: C1001–C1008, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Nash DT, Nash SD. Ranolazine for chronic stable angina. Lancet 372: 1335–1341, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Ou Y, Gibbons SJ, Miller SM, Strege PR, Rich A, Distad MA, Ackerman MJ, Rae JL, Szurszewski JH, Farrugia G. SCN5A is expressed in human jejunal circular smooth muscle cells. Neurogastroenterol Motil 14: 477–486, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Panfili M, Iafrate M, Marzot F, Secco S, De Rosa G, Groppa F, Padrini R. Ranolazine-induced severe bladder hypotonia. Ann Pharmacother 46: e24, 2012. [DOI] [PubMed] [Google Scholar]
- 17.Saito YA, Strege PR, Tester DJ, Locke GR 3rd, Talley NJ, Bernard CE, Rae JL, Makielski JC, Ackerman MJ, Farrugia G. Sodium channel mutation in irritable bowel syndrome: evidence for an ion channelopathy. Am J Physiol Gastrointest Liver Physiol 296: G211–G218, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sicouri S, Glass A, Belardinelli L, Antzelevitch C. Antiarrhythmic effects of ranolazine in canine pulmonary vein sleeve preparations. Heart Rhythm 5: 1019–1026, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strege PR, Bernard CE, Kraichely RE, Mazzone A, Sha L, Beyder A, Gibbons SJ, Linden DR, Kendrick ML, Sarr MG, Szurszewski JH, Farrugia G. Hydrogen sulfide is a partially redox-independent activator of the human jejunum Na+ channel, NaV1. 5. Am J Physiol Gastrointest Liver Physiol 300: G1105–G1114, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strege PR, Holm AN, Rich A, Miller SM, Ou Y, Sarr MG, Farrugia G. Cytoskeletal modulation of sodium current in human jejunal circular smooth muscle cells. Am J Physiol Cell Physiol 284: C60–C66, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Strege PR, Mazzone A, Kraichely RE, Sha L, Holm AN, Ou Y, Lim I, Gibbons SJ, Sarr MG, Farrugia G. Species dependent expression of intestinal smooth muscle mechanosensitive sodium channels. Neurogastroenterol Motil 19: 135–143, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Strege PR, Ou Y, Sha L, Rich A, Gibbons SJ, Szurszewski JH, Sarr MG, Farrugia G. Sodium current in human intestinal interstitial cells of Cajal. Am J Physiol Gastrointest Liver Physiol 285: G1111–G1121, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Xiong Z, Sperelakis N, Noffsinger A, Fenoglio-Preiser C. Ca2+ currents in human colonic smooth muscle cells. Am J Physiol Gastrointest Liver Physiol 269: G378–G385, 1995. [DOI] [PubMed] [Google Scholar]
- 24.Xiong Z, Sperelakis N, Noffsinger A, Fenoglio-Preiser C. Changes in calcium channel current densities in rat colonic smooth muscle cells during development and aging. Am J Physiol Cell Physiol 265: C617–C625, 1993. [DOI] [PubMed] [Google Scholar]
- 25.Xiong Z, Sperelakis N, Noffsinger A, Fenoglio-Preiser C. Fast Na+ current in circular smooth muscle cells of the large intestine. Pflügers Arch 423: 485–491, 1993. [DOI] [PubMed] [Google Scholar]