Abstract
A hallmark of liver fibrosis is the activation of hepatic stellate cells (HSC), which results in their production of fibrotic molecules, a process that is largely regulated by connective tissue growth factor (CCN2). CCN2 is increasingly expressed during HSC activation because of diminished expression of microRNA-214 (miR-214), a product of dynamin 3 opposite strand (DNM3os) that directly suppresses CCN2 mRNA. We show that an E-box in the miR-214 promoter binds the basic helix-loop-helix transcription factor, Twist1, which drives miR-214 expression and results in CCN2 suppression. Twist1 expression was suppressed in HSC of fibrotic livers or in cultured HSC undergoing activation in vitro or after treatment with ethanol. Furthermore, Twist1 decreasingly interacted with DNM3os as HSC underwent activation in vitro. Nanovesicular exosomes secreted by quiescent but not activated HSC contained high levels of Twist1, thus reflecting the suppression of cellular Twist1 during HSC activation. Exosomal Twist1 was intercellularly shuttled between HSC and stimulated expression of miR-214 in the recipient cells, causing expression of CCN2 and its downstream effectors to be suppressed. Additionally, the miR-214 E-box in HSC was also regulated by hepatocyte-derived exosomes, showing that functional transfer of exosomal Twist1 occurs between different cell types. Finally, the levels of Twist1, miR-214, or CCN2 in circulating exosomes from fibrotic mice reflected fibrosis-induced changes in the liver itself, highlighting the potential utility of these and other constituents in serum exosomes as novel circulating biomarkers for liver fibrosis. These findings reveal a unique function for cellular or exosomal Twist1 in CCN2-dependent fibrogenesis.
Keywords: connective tissue growth factor, CCN2, exosome, fibrosis, E-box
liver injury is characterized by a phenotypic and functional transformation of normally quiescent hepatic stellate cells (HSC) into α-smooth muscle actin (α-SMA)-expressing myofibroblastic cells, which promote wound closure and produce a collagen matrix that supports hepatocyte repopulation (9, 11). Whereas this activated HSC phenotype is relatively short lived in acute injury, it persists during chronic injury and results in unrelenting deposition of large amounts of collagen that is a hallmark of hepatic fibrosis, a serious pathology that compromises normal hepatic structure and function and is a harbinger of cirrhosis, hepatocarcinoma, and end-stage liver disease (10). Transforming growth factor-β (TGF-β) plays a central role in stimulating pathways of fibrogenesis in activated HSC, but it is a challenging therapeutic target because it also regulates critical immune responses and has important tumor-suppressive actions. On the other hand, the fibrogenic properties of TGF-β are mediated via connective tissue growth factor (CCN2, also known as CTGF), a complex matricellular molecule that is produced downstream of TGF-β and directly regulates many of the differentiated functions of activated HSC, including mitogenesis, chemotaxis, adhesion, matrigenesis, and fibrogenesis (15). CCN2 is produced at high levels in activated HSC, whereas its expression in quiescent HSC is substantially suppressed. We recently identified microRNA-214 (miR-214) as a direct negative regulator of CCN2 in primary mouse HSC or the human LX-2 HSC line (4). Via its direct binding of the CCN2 3′ untranslated region (UTR) in quiescent HSC, miR-214 inhibits CCN2 expression in HSC, whereas, in activated HSC, miR-214 expression is suppressed, thereby allowing CCN2 to be expressed. CCN2 and miR-214 are thus dynamically and reciprocally expressed as a function of HSC activation (4).
MiR-214 is located within the intron of the dynamin 3 (DNM3) gene and is encoded with miR-199a, producing a 7.9-kb noncoding DNM3 opposite strand transcript, termed DNM3os (27, 41). Because factors that drive DNM3os transcription will enhance miR-214-dependent suppression of CCN2 expression and thus have potential therapeutic utility, we sought to identify the element(s) in the miR-214 promoter and their associated transcription factor(s) that account for the high levels of miR-214 expression that occur in quiescent HSC. Here we show that an E-box in the miR-214 promoter is a binding site for the basic helix-loop-helix (bHLH) transcription factor, Twist1, which drives miR-214 promoter activity and miR-214 expression, resulting in CCN2 suppression. Functional assays show that Twist1 decreasingly interacts with DNM3os as HSC undergo culture-induced activation, consistent with the finding that HSC demonstrate an activation-dependent suppression of Twist1 expression. Moreover, nanovesicular exosomes secreted by quiescent HSC or hepatocytes contain Twist1, which is intercellularly shuttled to recipient HSC in which the E-box is targeted, resulting in regulation of the miR-214-CCN2 axis. Finally, serum exosomes contain Twist1, miR-214, or CCN2 at levels that reflect their fibrosis-induced changes in the liver, suggesting that the molecular payload in circulating exosomes offers new possibilities in the search for noninvasive biomarkers of liver fibrosis.
MATERIALS AND METHODS
Animal procedures.
Animal protocols were approved by the Institutional Animal Care and Use Committee of Nationwide Children's Hospital (Columbus, OH). Normal male Swiss Webster mice (6–8 wk) (n = 10) were injected intraperitoneally three times each week for 5 wk with either 30 μl of vegetable oil or a mixture of 4 μl carbon tetrachloride (CCl4; Sigma-Aldrich, St. Louis, MO) in 26 μl of vegetable oil. Upon death, blood was collected, and individual liver lobes were tied and harvested either immediately and snap-frozen in liquid nitrogen for subsequent RNA or protein extraction or after perfusion using PBS followed by 4% paraformaldehyde (Sigma-Aldrich) for histological analysis of fixed tissue. In an alternative model of liver injury, male FVB mice (6–8 wk) (n = 10) received intraperitoneal thioacetic acid (TAA; 100 mg/kg; Sigma-Aldrich) in saline three times per week for 5 wk. Control mice received intraperitoneal saline alone. Mice were killed 72 h after the last injection, livers were harvested, and RNA was isolated and processed for qRT-PCR. Some livers were fixed for histological analysis.
Cell culture.
Primary HSC were isolated, essentially as we have described (5), by buoyant-density centrifugation from normal male Swiss Webster mice (6–8 wk), and spent medium from the cultured cells was replaced with fresh DMEM/F12/10% FBS medium on day 1 and every other day as needed. HSC were split 1:3 every 5 days and used at passages 0–6 (P0–P6). Our previous studies showed that, when isolated from normal (nonfibrotic) animals, cells studied within 24 h of brief culture do not exhibit characteristics typical of activated cells (e.g., α-SMA, CCN2) and thereafter gradually transition to a highly activated phenotype over the ensuing 7–10 days of culture (5). In some experiments, triplicate wells of cells were incubated for up to 48 h in the presence of 0–50 mM ethanol. Cells were then evaluated for Twist1, CCN2, or miR-214 expression by qRT-PCR. Human LX-2 HSC were cultured as described (5). The AML12 mouse hepatocyte line was obtained from American Type Culture Collection (Manassas, VA).
Western blotting.
Western blots of whole-liver lysates (20 μg), freshly isolated or cultured HSC (20 μg), or exosomal proteins (5 μg; see below) were performed using anti-Twist1 (1:300; Abcam, Cambridge, MA), anti-CCN2 IgY [5 μg/ml; in house (4)], or anti-CD9 (1:400; LSBio, Seattle, WA), using our published protocols (5). Blots were stripped and incubated with anti-β-actin (1:2,000; Abcam) to verify equal loading among samples within individual experiments (data not shown).
Immunohistochemistry.
Fixed livers were incubated with anti-Twist1 (1:300, Abcam), anti-desmin (1:300, Abcam), anti-collagen α1(I) (1:250, Abcam), anti-α-SMA (1:1,000; Dako Cytomation, Glostrup, Denmark), or anti-CCN2 IgY (5 μg/ml) (4), followed by Alexa Fluor 488 goat-anti rabbit IgG and Alexa Fluor 568 goat-anti mouse IgG, or Alexa Fluor 647 goat-anti mouse IgG, or Alexa Fluor 568 goat-anti-chicken IgG (all at 1:1,000; Life Technologies, Grand Island, NY) for 1 h at room temperature. The slides were mounted with Vectashield Mounting Medium containing 4′,6-diamidino-2-phenylindole nuclear stain (Vector Laboratories, Burlingame, CA), and examined by confocal microscopy.
Cellular RNA extraction and qRT-PCR.
Total RNA from frozen liver tissues or mouse HSC or human LX-2 cells was extracted using a microRNeasy Plus kit (Qiagen, Valencia, CA) and was reverse transcribed using a miScript II RT kit (Qiagen) according to the manufacturers' protocols. Resulting transcripts were analyzed by qRT-PCR as described (4, 5) with primers for Twist1, CCN2, α-SMA, collagen α1(I), or miR-214 (Table 1). Each reaction was run in triplicate, and all samples were normalized to GAPDH. Negative controls were a nonreverse transcriptase reaction or a nonsample reaction.
Table 1.
Primers used for RT-PCR
| Primers |
||||
|---|---|---|---|---|
| Gene | GenBank Accession Number | Sense | Anti-Sense | Product Size, bp |
| Twist1 (mouse) | NM_011658 | 5′ CGACGACAGCCTGAGCAACA 3′ | 5′ TGCAGCTCCTCGTACGACTG 3′ | 293 |
| CCN2 (mouse) | NM_010217 | 5′ CACTCTGCCAGTGGAGTTCA 3′ | 5′ AAGATGTCATTGTCCCCAGG 3′ | 111 |
| miR-214 (mouse) | NR_029796 | 5′ ACAGCAGGCACAGACAGGCA 3′ | Universal anti-sense | 20 |
| Collagen α1(I) (mouse) | NM_007742 | 5′ GCCCGAACCCCAAGGAAAAGAAGC 3′ | 5′ CTGGGAGGCCTCGGTGGACATTAG 3′ | 148 |
| α−SMA (mouse) | NM_007392 | 5′GGCTCTGGGCTCTGTAAGG3′ | 52CTCTTGCTCTGGGCTTCATC3′ | 148 |
| GAPDH (mouse) | NM_002046 | 5′ TGCACCACCAACTGCTTAGC 3′ | 5′ GGCATGGACTGTGGTCATGAG 3′ | 87 |
CCN2, connective tissue growth factor; SMA, smooth muscle actin.
Twist1 overexpression or suppression in primary mouse HSC or human LX-2 cells.
Mouse Twist1 plasmids, Twist1 siRNA, or negative controls were obtained from Addgene (Cambridge, MA) or Santa Cruz Biotechnology (Santa Cruz, CA). To avoid off-target effects, the Twist1 siRNA preparation was comprised of three target-specific 20–25-nt siRNAs designed to knock down Twist1 gene expression. Primary mouse HSC or human LX-2 cells (105-106 cells) were transfected with 3.2 μg plasmid or 100 nM siRNA by electroporation using a Nucleofector Kit (Lonza, Koln, Germany) and incubated for 12 h in medium containing 10% FBS, which was then replaced with either fresh 10% FBS-enriched or serum-free medium. Transfection efficiency in cells was ∼40% in mHSC or 90% in LX-2 cells, as monitored by cotransfection with 0.8 μg of a green fluorescent protein (GFP)-expressing plasmid, pEGFP (Invitrogen, Carlsbad, CA). In some experiments, LX-2 cells were cotransfected for 12 h with 100 nM miR-214 antagomir (Qiagen). Western blot analysis of Twist1-transfected LX-2 cells was performed using anti-Twist1 (1:300, Abcam) or anti-CCN2 IgY (5 μg/ml) (4), using our published protocols (5).
Transfection of mouse primary HSC with pGL 4.11[Luc2P]-DNM3os promoter plasmids.
The DNM3os promoter (647 bp; Genbank SEQ ID: CM000994.2: 162217623:162225550:1) was amplified by PCR from primary mouse HSC genomic DNA using forward primer 5′- TAAAGCTTAAAGGGGGGAGCCCCAACTTATCTG- 3′ and reverse primer 5′-TACTCGAGTTCCTGCACCAGGGGCTTGT- 3′. The PCR fragment was digested with Hind III and Xho I, subcloned into pGL 4.11[Luc2P] Vector (Promega, Madison, WI), downstream of the Firefly luciferase reporter, and verified by DNA sequencing. A mutant DNM3os promoter containing a six-base point mutation (CATCTG → GCGGCC) in the E-box site (nt. 162217485-162217490) was amplified from the wild-type mouse DNM3os promoter using forward primer 5′- CATTACACGAAAAGCGGCCGTACCATTTTATGC- 3′ and reverse primer 5′- GCATAAAATGGTACGGCCGCTTTTCGTGTAATG- 3′ and verified by DNA sequencing. The mutant DNM3os promoter fragment was released with Hind III and Xho I.
Primary D6 mouse HSC were transfected with pGL 4.11[Luc2P]-DNM3os wild type or mutant plasmids, or vector alone. To control for transfection efficiency, cells were co- transfected with pRL-CMV vector (Promega) containing Renilla luciferase reporter gene. After 24 h, luciferase activity was measured in triplicate using an E1910 Dual Luciferase Reporter Assay System (Promega). Renilla luciferase activity was used for normalization, and Firefly luciferase activity in pGL 4.11[Luc2P]-DNM3os promoter-transfected cells was compared with that in mock-transfected cells.
EMSA.
5 × 107 primary mouse HSC (D1) were lysed in EMSA lysis buffer according to the manufacturer's recommendations (Thermo Scientific, Rockford, IL). After centrifugation (10,000 revolution/min, 1 min) of the cell lysate, the pellet (nuclei) was collected, resuspended in extraction buffer, and centrifuged (14,000 revolution/min, 5 min, 4°C), and the supernatant was used for EMSA.
Wild-type or mutant DNM3os promoters were labeled at their 3′ end using biotin (0.5 μM; Thermo Scientific) and terminal deoxynucleotidyltransferase (0.2 U/μl; Thermo Scientific), and purified using chloroform:isoamyl alcohol (1:1). Nuclear protein (1 μg) was incubated for 25 min at room temperature with labeled oligonucleotides (20 fmol/reaction assay) in binding buffer [50% glycerol, 1% NP-40, 100 mM MgCl2, 200 mM EDTA, 1× binding buffer, 1 M KCl, and 1 μg/μl Poly(dI:dC)]. For some groups, 1) 0.5 μg/μl anti-Twist1 antibody (Abcam) was added for super-shift evaluation, 2) 4 pmol unlabeled oligonucleotide was used to competitively inhibit formation of shifted complexes, or 3) nuclear protein was omitted to verify its requirement for complex formation. Samples were mixed with 5× loading buffer (Thermo Scientific) and electrophoresed on a 5% DNA retardation gel in 0.5× TBE buffer. Complexes were transferred and cross linked to a nylon membrane before incubation with streptavidin-horseradish peroxidase conjugate (1:300; Thermo Scientific) at 37°C for 30 min and analysis by chemiluminescence (Thermo Scientific).
Chromatin immunoprecipitation assay.
Chromatin immunoprecipitation (ChIP) assays were performed with an EpiTect ChIP kit (Qiagen). Briefly, 2 × 106 HSC were cross linked with 1% formaldehyde for 10 min at room temperature, after which the reaction was terminated with 0.125 M glycine. The cells were then isolated and sonicated on ice to generate DNA shear fragments of ∼200-1,000 bp. The lysates were pelleted, precleared, precipitated with 10 μg/ml Twist1 antibody (Millipore, Temecula, CA) or control IgG, and allowed to rotate overnight at 4°C with magnetic protein A beads (Qiagen). The immune complexes were collected and eluted using ChIP-grade proteinase K. The cross links were destroyed by heating the samples at 45°C for 30 min, and the DNA recovered underwent ChIP-PCR using DNM3os primers (see above) according to the manufacturers' instructions.
Analysis of exosomal Twist1.
Exosomes were isolated as described from HSC-conditioned medium on days 3 or 20. Cryogenic transmission electron microscopy of purified exosomes was performed as described (4). Exosomal Twist1 protein or mRNA were determined by, respectively, Western blot using anti-Twist1 (Abcam) or qRT-PCR, the latter of which was normalized to let-7a, which we determined to be an optimal exosomal housekeeping miR for these studies (data not shown). Exosome uptake in P6 HSC was shown by confocal microscopy of the cells after incubation for 12 h with exosomes isolated from day 3 HSC and subsequently stained with PKH26.
Exosomes were purified from primary HSC after transfection of day 6 cells for 48 h with or without 100 nM Twist1 siRNA. Suppressed exosomal Twist1 levels in the exosomes from siRNA-treated cells was confirmed by RT-PCR using exosomal let-7a as a reference control. Exosomal proteins were evaluated by Western blot as described above. Control or Twist1-deficient exosomes were added (3 μg/ml) for 48 h to P4 primary HSC, after which Twist1 levels in the cells were evaluated by RT-PCR or Western blot (see above). Cells were further analyzed by RT-PCR for miR-214, CCN2, α-SMA, or collagen α1(I).
Exosomal regulation of DNM3os was shown by assessment of dual luciferase activity in day 6 HSC transfected with pGL 4.11[Luc2P]-DNM3os wild-type or mutant plasmids, or vector alone, when cocultured for 24 h with day 1 HSC or AML12 hepatocytes that had been treated for 24 h with or without 100 μM GW4869, an inhibitor of nSMase2 that is required for biosynthesis of ceramide on which exosome production depends (3, 4).
Circulating exosomes were harvested using PureExo Exosome Isolation Kits (101Bio, Palo Alto, CA) from serum of mice treated for up to 5 wk with CCl4 as described above. Total RNA from exosomes in 200 μl of serum was prepared using miRNeasy mini kits (Qiagen) as described above. Each reaction was run in triplicate, and all samples were normalized to let-7a.
Statistical analysis.
All experiments were performed at least three times with triplicate measurements. For controls, error bars were derived by setting the mean value as 1 and defining variance of replicates from 1. Treatment groups were then expressed as fold of means ± SE. The data from qRT-PCR or luciferase activity assays were analyzed by Student's t-test using Sigma plot 12.0 software (SPSS, Chicago, IL), and P values <0.05 were considered statistically significant.
RESULTS
Suppression of Twist1 expression during fibrosing liver injury or during HSC activation in vivo or in vitro.
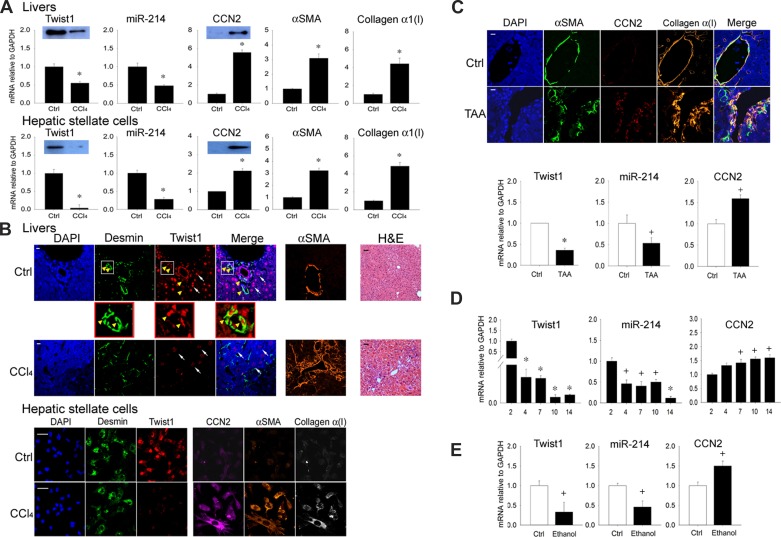
Analysis of total hepatic RNA showed that hepatic Twist1 expression was high in livers recovered from control oil-treated mice but was significantly decreased in livers from CCl4-treated mice (Fig. 1A). This response was associated with suppressed expression of hepatic miR-214 and stimulated expression of CCN2, α-SMA, or collagen α1(I) (Fig. 1A). Isolated activated HSC from this 5-wk injury model showed an overall similar expression pattern in that Twist1 or miR-214 were inhibited and CCN2, α-SMA, or collagen α1(I) were enhanced (Fig. 1A). Consistent with these findings, Western blot analysis showed that Twist1 protein levels were suppressed in fibrotic livers or in activated HSC recovered from fibrotic livers and that CCN2 protein levels increased under the same conditions (Fig. 1A). Immunostaining for Twist1 in liver sections showed that it was present in desmin-positive nonparenchymal cells (presumptive quiescent HSC) in control animals, but, after CCl4 injury, Twist1 staining was absent from activated HSC, which stained positively for α-SMA as well as desmin (Fig. 1B, top, arrows). Some parenchymal cells also strongly stained positively for Twist1, but this was only weakly reduced after CCl4 treatment (Fig. 1B, top). Nonetheless, because background hepatocyte staining might potentially confound our interpretation of Twist1 staining in HSC, we alternatively isolated HSC from the livers of control or fibrotic animals to verify their Twist1 status in vivo. As assessed by immunostaining, quiescent HSC isolated from control animals were positive for desmin or Twist1 but not for CCN2, α-SMA, or collagen α1(I). In contrast, activated HSC isolated from animals treated with CCl4 for 5 wk were positive for desmin, CCN2, α-SMA, or collagen α1(I) but not for Twist1 (Fig. 1B, bottom). Thus, because HSC activation in vivo was associated with the loss of Twist1 mRNA expression or protein production (Fig. 1, A and B), this phenomenon was the focus of the studies described herein.
Fig. 1.
Twist1 is expressed at high levels in normal liver or hepatic stellate cells (HSC) and is suppressed during fibrosis. A: expression of Twist1, connective tissue growth factor (CCN2), α-smooth muscle actin (α-SMA), or collagen α1(I) mRNA or microRNA-214 (miR-214) assessed by qRT-PCR and normalized to GAPDH mRNA after administration of oil or CCl4 for 5 wk determined for RNA from either liver tissue (top) or HSC isolated from the livers and maintained in culture for 24 h (bottom) (n = 5 independent experiments performed in triplicate, *P < 0.001 vs. oil control). Insets: detection of Twist1 or CCN2 by Western blotting of 20 μg total protein (β-actin was detected equally in each set of paired samples; data not shown). B, top: hematoxylin and eosin (H&E) staining or immunohistochemical detection of desmin, Twist1, or α-SMA in the livers of mice treated with oil or CCl4 for 5 wk. Immunostained specimens were also stained with 4′,6-diamidino-2-phenylindole (DAPI) nuclear stain (blue). In control livers, Twist1 staining was associated with the desmin-positive quiescent HSC population, with some staining also in hepatocytes. Fibrosis in response to CCl4 was accompanied by HSC activation, as shown by increased α-SMA staining in HSC and a reduction of Twist1 staining in both HSC and hepatocytes. The magnified parts of the figure illustrate Twist1 staining in desmin-positive HSC from normal liver. Bottom: immunocytochemical staining for desmin, α-SMA, Twist1, CCN2, or collagen α1 in HSC from control or CCl4-treated mice obtained as described in A (bars: 20 μm for immunostained sections; 50 μm for H&E-stained sections). C: immunohistochemical detection of α-SMA, CCN2, or collagen α1 (top: bar = 20 μm) or expression of Twist1, miR-214, or CCN2 (bottom) in the livers of mice treated with water (control) or thioacetic acid (TAA) for 5 wk to induce HSC activation and fibrosis (n = 5 independent experiments performed in triplicate, *P < 0.001, +P < 0.05 vs. no treatment). D: Twist1, miR-214, or CCN2 expression analyzed by qRT-PCR and normalized to GAPDH mRNA in primary HSC isolated from livers of normal mice and cultured for up to 14 days (n = 3 independent experiments performed in triplicate, *P < 0.001 vs. day 2, +P < 0.05 vs. day 2). E: qRT-PCR of Twist1, CCN2 mRNA, or miR-214, normalized to GAPDH mRNA, after day 3 primary mouse HSC were incubated in 1% serum for 24 h before 48-h treatment with 0 or 50 mM ethanol (n = 3 independent experiments performed in triplicate, +P < 0.05 vs. control).
In a TAA liver fibrosis model exhibiting enhanced staining in HSC for CCN2, α-SMA, or collagen α1(I), decreased expression of hepatic Twist1 mRNA or miR-214 and increased expression of hepatic CCN2 mRNA were also documented (Fig. 1C). Analysis of HSC isolated from normal livers and maintained in vitro showed that there was a large decrease in Twist1 expression between days 2 and 4 of culture and then a more gradual decline in its expression up to day 14 of culture as the cells became progressively activated and expressed decreasing levels of miR-214 and increasing levels of CCN2 (Fig. 1D). Treatment of day 3 primary HSC with ethanol resulted in decreased Twist1 or miR-214 expression and increased CCN2 expression (Fig. 1E). Thus the decreased Twist1 expression in HSC during culture-induced activation or in response to fibrosing stimulus was comparable to that observed in HSC undergoing activation in vivo during CCl4 injury.
Twist1 regulation of miR-214 expression and downstream CCN2 production.
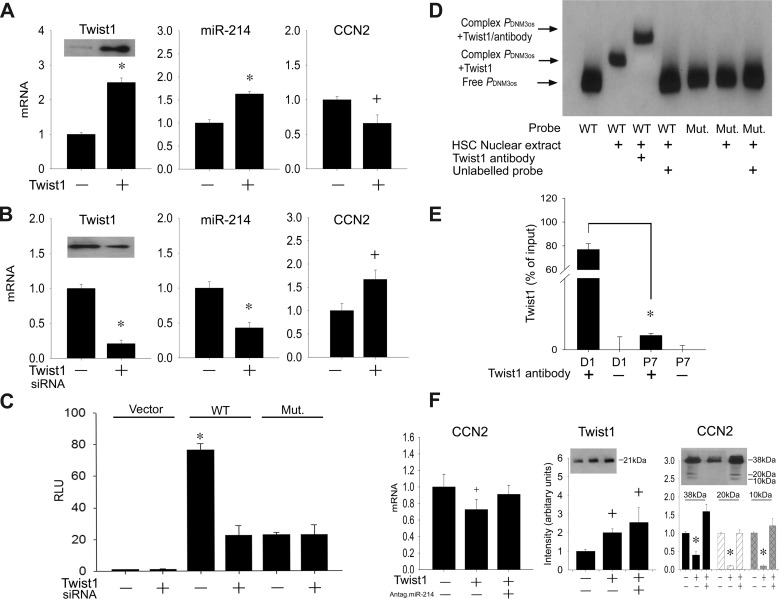
Transfection of Twist1 in highly activated HSC resulted in increased Twist1 mRNA or protein levels, causing miR-214 to be increased or CCN2 expression to be decreased (Fig. 2A). On the other hand, Twist siRNA treatment of day 6 HSC resulted in decreased Twist1 mRNA or protein levels, causing miR-214 expression to be decreased or CCN2 expression to be increased (Fig. 2B). Because miR-214 exerts inhibitory actions on the CCN2 3′UTR (4), these results suggested that Twist1 transcriptionally activates miR-214, which then acts to suppress CCN2 mRNA levels. This was supported by the finding that the activity in day 6 HSC of a wild-type DNM3os luciferase reporter was blocked by Twist1 siRNA, whereas the luciferase reporter activity of a mutant form of DNM3os lacking the E-box binding site for Twist1 was suppressed and insensitive to addition of Twist1 siRNA (Fig. 2C). To confirm a direct binding interaction in HSC between Twist1 and the E-box, an EMSA was performed that showed that a wild-type DNM3os probe formed a shifted complex in the presence of nuclear extracts that was supershifted by preincubation of the nuclear extract with a Twist1 antibody (Fig. 2D). On the contrary, no complexes were formed when DNM3os containing the E-box mutation was used as the probe (Fig. 2D). To verify that the binding between Twist1 and DNM3os occurred in living HSC and decreased as their level of activation was increased, a ChIP assay was performed that showed that Twist1-DNM3os binding was ∼100-fold greater in day 1 quiescent HSC than in highly activated P7 HSC (Fig. 2E). Collectively, these data show that, in quiescent mouse HSC, Twist1 transcriptionally activates the E-box in the miR-214 promoter and that the resulting high levels of miR-214 are inhibitory for CCN2 production. To confirm that the Twist1-miR-214-CCN2 axis is evolutionarily conserved in human HSC, LX-2 cells were analyzed for CCN2 expression downstream of Twist1. As shown in Fig. 2F, transfection of the cells with Twist1 resulted in reduced CCN2 mRNA expression or protein production, but this was reversed by cotransfection of the cells with a miR-214 antagomir showing that CCN2 inhibition by Twist1 is indirect and mediated through miR-214.
Fig. 2.
Twist1 regulates CCN2 production via transcriptional control of miR-214 E-box promoter element. Expression of Twist1, CCN2 mRNA, or miR-214 was assessed by qRT-PCR and normalized to GAPDH mRNA in passage 6 (P6) mouse HSC transfected with Twist1 (A) or day 6 primary mouse HSC transfected with 100 nM Twist1 siRNA (B) (n = 3 independent experiments performed in triplicate, *P < 0.001 vs. scramble, +P < 0.05 vs. scramble). Insets: detection of Twist1 by Western blotting of 20 μg total protein (staining for β-actin confirmed equal protein loading; data not shown). C: day 6 primary mouse HSC were transfected with parental pGL 4.11[Luc2P]-vector (Vector), pGL 4.11[Luc2P] containing wild-type mouse dynamin 3 opposite strand (DNM3os) promoter (WT), or a substitution mutation in the DNM3os promoter targeting the E-box (Mut.). After 36 h, firefly luciferase activity in cell lysates was measured and normalized to that of Renilla luciferase (n = 3 independent experiments performed in triplicate, *P < 0.001 vs. vector group). D: nuclear extracts from D1 mHSC underwent EMSA by incubation with oligonucleotide probes corresponding to the wild-type or mutated Twist1 binding site in the DNM3os promoter. Shifted complexes are indicative of binding interactions with the probe; the involvement of Twist1 is indicated by a supershifted complex using Twist1 antibody. Data are representative of 3 independent experiments. E: chromatin immunoprecipitation-PCR of DNM3os DNA in immune complexes generated using Twist1 antibody (+) to pull down endogenous Twist1-DNM3os complexes from day 1 or P7 HSC. Control reactions were performed with normal IgG (-), *P < 0.001. F: qRT-PCR (left) of CCN2 mRNA normalized to GAPDH mRNA, after LX-2 cells were transfected with Twist1 alone or together with miR-214 antagomir (n = 3 independent experiments performed in triplicate, +P < 0.05 vs. nontransfection). Western blot images and quantification thereof (middle and right) show that Twist1 transfection of LX-2 cells caused higher Twist1 but lower CCN2 protein levels but that CCN2 levels were restored to normal levels when Twist1-transfected cells were also transfected with a miR-214 antagomir. The CCN2 Western blot shows the principal CCN2 38-kDa protein and its 10–20-kDa proteolytic cleavage products.
Twist1 is shuttled between HSC in exosomes and targets the miR-214-CCN2 axis in recipient cells.
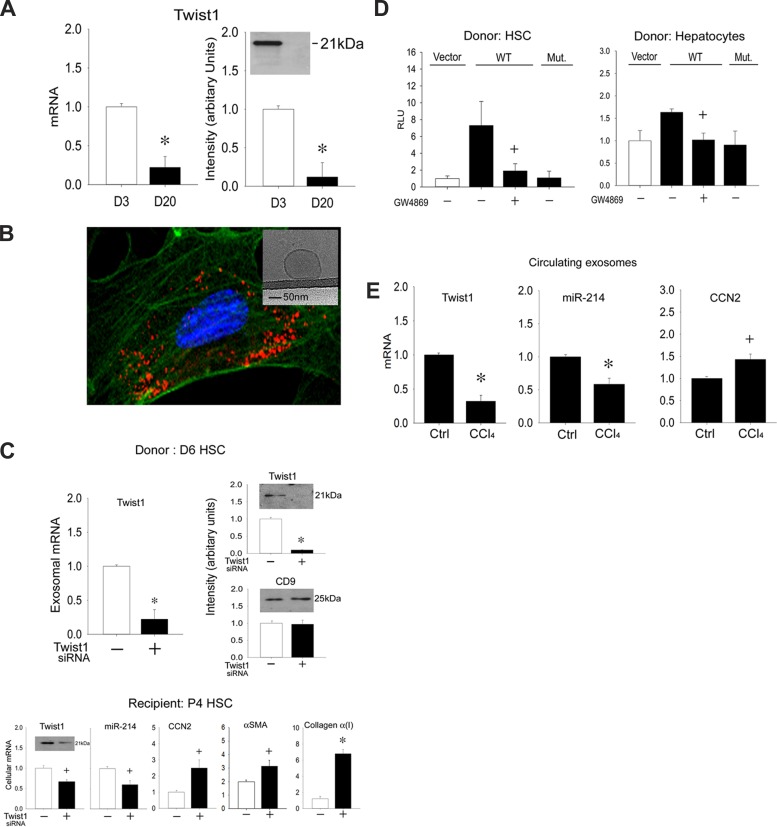
We have previously shown that HSC secrete nanovesicular exosomes (3, 4). In studying the identity of the molecular constituents of these exosomes, we found that they contained Twist1 mRNA or protein, the concentrations of which were reduced in exosomes from day 20 HSC compared with those from day 3 HSC (Fig. 3A), consistent with the activation-associated decrease documented earlier for cellular Twist1 (Fig. 1, D and E). Because purified HSC-derived exosomes stained with the fluorescent marker PKH26 for visualization were taken up by P4 HSC after incubation for 12 h (Fig. 3B), we investigated whether this process resulted in the delivery of endogenous exosomal Twist1 and its regulation of DNM3os in the recipient cells. First, because primary HSC at very early stages of culture are difficult to transfect, we used exosome donor cells on day 6 of culture because they are amenable to transfection and, importantly, still express readily detectable levels of Twist1 (see Fig. 1D). Compared with nontransfected controls, transfection of day 6 cells with Twist1 siRNA resulted in the production of exosomes that contained significantly diminished Twist1 mRNA or protein levels, whereas exosomal CD9 protein levels remained unchanged (Fig. 3C). Compared with the effects of control exosomes from day 6 HSC, which contained relatively high levels of endogenous Twist1, treatment of P4 recipient HSC (expressing negligible levels of endogenous Twist1) with Twist1-depleted exosomes from Twist1 siRNA-transfected HSC resulted in reduced Twist1 mRNA or protein in the recipient cells, and this was accompanied by decreased miR-214 expression and enhanced expression of CCN2, α-SMA, or collagen α1(I) (Fig. 3C). These data showed that, through its epigenetic regulation of miR-214, delivery of exosomal Twist1 serves to dampen expression of CCN2 and its downstream effectors, α-SMA or collagen α1(I).
Fig. 3.
Identification and intercellular transfer of exosomal Twist1. A: Twist1 mRNA assessed by qRT-PCR and normalized to let-7a (left) or protein assessed by Western blot (right, 5 μg total protein) in exosomes isolated from day 3 or day 20 mouse HSC (n = 3 independent experiments performed in triplicate, *P < 0.001 vs. day 3). B: P6 HSC were incubated for 12 h with exosomes purified from P6 HSC and subsequently stained with PKH-26. Cells were visualized for exosome fluorescence (red) and α-SMA immunofluorescence (green) by confocal microscopy. Inset: appearance of HSC-derived exosomes by cryogenic transmission electron microscopy. C: reduced Twist1 mRNA expression (top, left) or protein levels (top, right) in exosomes produced over 24 h after Twist1 siRNA transfection of day 6 donor HSC and the effect of these exosomes on Twist1, miR-214, CCN2, α-SMA, or collagen α(I) expression assessed by RT-PCR or Twist1 protein levels assessed by Western blot (20 μg total protein, for which β-actin signals were detected equally between samples; data not shown) after being added to recipient P4 HSC for 12 h (bottom). *P < 0.001 and +P < 0.05 vs. control exosomes from nontransfected cells. D: day 6 primary mouse HSC were transfected with parental pGL 4.11[Luc2P]-vector or pGL 4.11[Luc2P] containing WT or mutant mouse DNM3os promoter (see Fig. 2C) and cocultured for 24 h with D1 HSC (left) or AML12 mouse hepatocytes (right) that had been pretreated with or without GW4869 for 24 h. Firefly luciferase activity in cell lysates was measured and normalized to that of Renilla luciferase (n = 3 independent experiments performed in triplicate, +P < 0.05 vs. Vector). E: PureExo exosome isolation kit was used to isolate circulating exosomes from the serum of mice after 5-wk administration of oil or CCl4 Twist1, miR-214, or CCN2 expression in circulating exosomes analyzed by qRT-PCR and normalized to let-7a (n = 5 independent experiments performed in triplicate, *P < 0.001, +P < 0.05 vs. oil group).
When day 6 HSC were transfected with DNM3os promoter reporters, luciferase activity of the wild-type promoter was stimulated in the presence of cocultured day 1 HSC. This response was blocked when exosome production by the D1 cells was blocked using GW4869 [a chemical inhibitor that inhibits N-sphingomyelinase 2 and downstream exosome production (2–4, 19)], whereas the involvement of Twist1 in the response was shown by the lack of activity of the mutant DNM3os (Fig. 3D). Because some parenchymal cells were also observed to stain positively for Twist1 in liver sections (see Fig. 1B), we also investigated the ability of hepatocytes to deliver exosomal Twist1 to HSC in the same coculture system. These experiments, which were performed with AML12 cells, showed that hepatocytes were able to regulate wild-type but not mutant DNM3os in recipient cells and that this was GW4869 dependent (Fig. 3D). Collectively, these data showed that the E-box in DNM3os in HSC is regulated by Twist1 delivered intercellularly within exosomes that are secreted by other HSC or by hepatocytes.
Finally, in view of the emerging interest in using circulating exosomes for disease diagnosis or assessment (32), we analyzed Twist1, miR-214, or CCN2 expression in exosomes recovered from the circulation of mice during CCl4-induced fibrosis. As shown in Fig. 3E, experimental fibrosis was associated with a progressive decrease in circulating exosomal Twist1 or miR-214 and an increase in circulating exosomal CCN2, which paralleled their respective changes in the fibrosing livers themselves (Fig. 1B) (4).
DISCUSSION
Twist1 is a 21-kDa bHLH transcription factor that was discovered based on its induction of cell differentiation and control of dorsoventral patterning in Drosophila; Twist-null embryos are mesoderm deficient, have a twisted appearance, and subsequently die (35, 38). Although the mammalian counterparts, Twist1 and Twist2, also play a central role in cell fate determination, their actions are generally inhibitory during differentiation of muscle, bone, and other cells in the mesenchymal lineage (13, 20, 21, 25, 42). During embryogenesis, Twist1 is expressed at high levels and in a spatio-temporal manner that reflects its role in regulating genes that govern mesoderm specification or differentiation as well as mesenchymal tissue development (12, 30). In adults, Twist1 expression is low but present in stem cells of muscle, adipose tissue, bone marrow, and other mesenchymal tissues of mesoderm origin (17, 40). Twist1 gene mutations in humans result in Saethre-Chotzen syndrome (8, 14), and a similar condition occurs in Twist1-deficient mice (1). Twist1 expression is also strongly associated with cancers of the breast, prostate, esophagus, stomach, liver, pancreas, or bladder, and, although its mechanisms of action are variable, it regulates cancer cell senescence, apoptosis, resistance to chemotherapy, differentiation, invasiveness, and metastasis, the latter attributable to its ability to drive epithelial-mesenchymal transition (EMT) (33).
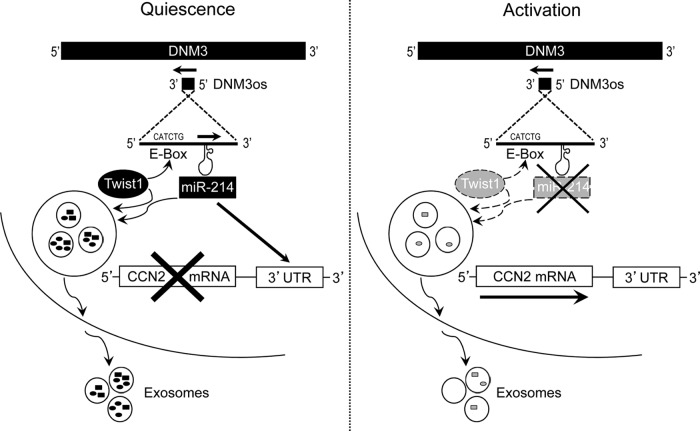
In these studies, we identified Twist1 as a product of quiescent HSC in adult mice, which indirectly inhibits CCN2 production through its transcriptional activation of miR-214, the latter of which suppresses CCN2 via direct binding to the CCN2 3′-UTR. During HSC activation, higher levels of CCN2 result, at least in part, from decreased expression of Twist1, resulting in reduced miR-214 transcription. The regulation by Twist1 of the miR-214-CCN2 axis in HSC thus reveals a hitherto unrecognized role for Twist1 as a suppressor of CCN2 expression and its downstream fibrogenic signals (Fig. 4). We further showed that this action of Twist1 is critically dependent on its activation of the E-box in the miR-214 promoter (Fig. 4), a mechanism that is also responsible for Twist1 regulation of miR-214/miR-199a during development (23). Our identification of miR-214 as a Twist1-regulated gene in HSC is consistent with previous studies showing that Twist regulates DNM3os or miR-199a/214 expression during development (23, 26, 27, 41) or in epithelial ovarian cancer cells differentiating from stem-like cells along a miR-199a/214-dependent axis (44). In the liver, miR-214 suppresses stem-like traits, invasion, or recurrence in human hepatocellular carcinoma (22, 28, 29), whereas miR-199a/214 is downregulated in rodent models of alcoholic steatohepatitis (7) or fibrosis (4). Forced overexpression of miR-214 in activated mouse HSC decreases expression of inflammation- or fibrosis-associated genes, including IL-1α or IL-10, integrin-α3 or integrin-β8, platelet-derived growth factor-α, matrix metalloproteinase-2, matrix metalloproteinase-8, or matrix metalloproteinase-13, tissue inhibitor of metalloproteinase 1, and CCN2 (4). Conversely, expression of miR-214-5p, which comprises the complementary sequence arising from the 5′ arm of the miR-214 hairpin, is enhanced in fibrotic liver and activated HSC and is associated with expression of fibrosis-related genes (16).
Fig. 4.
The Twist1-miR-214-CCN2 axis in HSC. In quiescent HSC, Twist1 is expressed at high levels and drives miR-214 expression through its binding of the E-box in DNM3os. One of the targets of miR-214 is the 3′-untranslated region (UTR) of CCN2, resulting in the inhibition of CCN2 in quiescent cells. Twist1 (these results) or miR-214 (4) are exported from quiescent HSC (or hepatocytes) (see Fig. 3D) in exosomes, allowing them to exert epigenetic effects on their targets (miR-214 or CCN2, respectively) after being shuttled to neighboring HSC, causing fibrogenic signaling to be suppressed. HSC activation is characterized by suppressed Twist1 production (the mediators of which have yet to be determined) and reduced activation of the E-box (dashed arrow). In turn, expression of miR-214 and its binding to the CCN2 3′-UTR are reduced, allowing CCN2 expression and its downstream fibrogenic cascades to be manifested. Twist1 or miR-214 is incorporated into exosomes at lower levels (dashed arrows) with the result that exosomes from activated HSC are less effective at suppressing the miR-214-CCN2 axis in neighboring HSC.
We are not aware of previous reports of Twist1 in adults in mesenchymal cell types, such as HSC that play specialized roles in wound healing or fibrosis. There is, however, evidence that, through its effects on EMT, Twist1 stimulates an epithelial contribution to fibrotic processes in the kidney, lung, and oral cavity (6, 31, 36). Although we observed some Twist1-positive hepatocytes in normal livers and the ability of hepatocyte-derived exosomal Twist1 to regulate miR-214 in HSC, the substantial suppression of hepatic Twist1 expression during experimental fibrosis coupled with emerging evidence that EMT does not contribute to hepatic fibrosis (43) highlights the need for further studies in this area. Even so, a role for epithelial Twist1 in more severe hepatic pathology is supported by studies of human hepatocellular carcinoma in which Twist1 stimulates metastasis and angiogenesis by reducing expression of E-cadherin, increasing expression of N-cadherin and vascular endothelial growth factor, and increasing cell motility and invasiveness (22, 28, 29). EMT and metastasis of cholangiocarcinoma are also dependent on a miR-214-Twist1 axis, but in this case Twist1 appears to be a target of miR-214 (24).
Another novel aspect of our studies was the identification of Twist1 as a component of HSC- or hepatocyte-derived exosomes that allowed for its intercellular shuttling to neighboring HSC. Exosomes are membranous nanovesicles that arise by inward budding of multivesicular bodies and are released extracellularly when these multivesicular bodies fuse internally with the plasma membrane; thereafter, exosomes traverse the intercellular spaces and may be taken up by neighboring cells (18, 37, 39). Exosomes contain a complex mixture of miRs, mRNAs, and proteins, and therefore their intercellular shuttling represents a communication pathway through which genetic, epigenetic, or proteomic information may be delivered from donor cells that impact gene expression in recipient cells (34). In this study, we showed that Twist1 is exosomally delivered to recipient-activated HSC in which it then targets the miR-214 promoter. Because miR-214 and CCN2 themselves are also individually and functionally delivered to HSC within exosomes (3, 4), the manifestation of fibrogenic pathways in any given HSC likely reflects the net action of its own cellular constituents in combination with those that are exosomally received from neighboring HSC and other cell types. Finally, because the fibrosis-related expression of hepatic Twist1, CCN2, or miR-214 was mirrored in circulating exosomes, evaluation of hepatic fibrosis may be possible based on the relative expression of a slate of signature molecular components in circulating exosomes; this approach, which is noninvasive or minimally invasive and can be undertaken repeatedly in individual patients, is rapidly emerging as a powerful diagnostic tool in other pathologies and diseases (32, 39).
In summary, through its transcriptional activation of the DNM3os E-box, cellular or exosomal Twist1 drives miR-214 expression and suppresses CCN2 production and downstream fibrogenic signaling. Twist1 is thus identified as a novel regulator of HSC function.
GRANTS
This work was supported by NIH/NIAAA grant 1RO1 AA021276 awarded to D. Brigstock.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: L.C. and D.R.B. conception and design of research; L.C., R.C., S.K., and A.C. performed experiments; L.C., R.C., S.K., A.C., and D.R.B. analyzed data; L.C., A.C., and D.R.B. interpreted results of experiments; L.C. prepared figures; L.C. and D.R.B. drafted manuscript; L.C. and D.R.B. edited and revised manuscript; L.C., R.C., S.K., A.C., and D.R.B. approved final version of manuscript.
REFERENCES
- 1.Bourgeois P, Bolcato-Bellemin AL, Danse JM, Bloch-Zupan A, Yoshiba K, Stoetzel C, Perrin-Schmitt F. The variable expressivity and incomplete penetrance of the twist-null heterozygous mouse phenotype resemble those of human Saethre-Chotzen syndrome. Hum Mol Genet 7: 945–957, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Chairoungdua A, Smith DL, Pochard P, Hull M, Caplan MJ. Exosome release of beta-catenin: a novel mechanism that antagonizes Wnt signaling. J Cell Biol 190: 1079–1091, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charrier A, Chen R, Chen L, Kemper S, Hattori T, Takigawa M, Brigstock DR. Exosomes mediate intercellular transfer of pro-fibrogenic connective tissue growth factor (CCN2) between hepatic stellate cells, the principal fibrotic cells in the liver. Surgery 156: 548–555, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen L, Charrier A, Zhou Y, Chen R, Yu B, Agarwal K, Tsukamoto H, Lee LJ, Paulaitis ME, Brigstock DR. Epigenetic regulation of connective tissue growth factor by MicroRNA-214 delivery in exosomes from mouse or human hepatic stellate cells. Hepatology 59: 1118–1129, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen L, Charrier AL, Leask A, French SW, Brigstock DR. Ethanol-stimulated differentiated functions of human or mouse hepatic stellate cells are mediated by connective tissue growth factor. J Hepatol 55: 399–406, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Das RK, Anura A, Pal M, Bag S, Majumdar S, Barui A, Chakraborty C, Ray AK, Sengupta S, Paul RR, Chatterjee J. Epithelio-mesenchymal transitional attributes in oral sub-mucous fibrosis. Exp Mol Pathol 95: 259–269, 2013. [DOI] [PubMed] [Google Scholar]
- 7.Dolganiuc A, Petrasek J, Kodys K, Catalano D, Mandrekar P, Velayudham A, Szabo G. MicroRNA expression profile in Lieber-DeCarli diet-induced alcoholic and methionine choline deficient diet-induced nonalcoholic steatohepatitis models in mice. Alcohol Clin Exp Res 33: 1704–1710, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.el Ghouzzi V, Le Merrer M, Perrin-Schmitt F, Lajeunie E, Benit P, Renier D, Bourgeois P, Bolcato-Bellemin AL, Munnich A, Bonaventure J. Mutations of the TWIST gene in the Saethre-Chotzen syndrome. Nat Genet 15: 42–46, 1997. [DOI] [PubMed] [Google Scholar]
- 9.Eng FJ, Friedman SL. Fibrogenesis I. New insights into hepatic stellate cell activation: the simple becomes complex. Am J Physiol Gastrointest Liver Physiol 279: G7–G11, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology 134: 1655–1669, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem 275: 2247–2250, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Gitelman I. Twist protein in mouse embryogenesis. Dev Biol 189: 205–214, 1997. [DOI] [PubMed] [Google Scholar]
- 13.Hebrok M, Wertz K, Fuchtbauer EM. M-twist is an inhibitor of muscle differentiation. Dev Biol 165: 537–544, 1994. [DOI] [PubMed] [Google Scholar]
- 14.Howard TD, Paznekas WA, Green ED, Chiang LC, Ma N, Ortiz de Luna RI, Garcia Delgado C, Gonzalez-Ramos M, Kline AD, Jabs EW. Mutations in TWIST, a basic helix-loop-helix transcription factor, in Saethre-Chotzen syndrome. Nat Genet 15: 36–41, 1997. [DOI] [PubMed] [Google Scholar]
- 15.Huang G, Brigstock DR. Regulation of hepatic stellate cells by connective tissue growth factor. Front Biosci 17: 2495–2507, 2012. [DOI] [PubMed] [Google Scholar]
- 16.Iizuka M, Ogawa T, Enomoto M, Motoyama H, Yoshizato K, Ikeda K, Kawada N. Induction of microRNA-214-5p in human and rodent liver fibrosis. Fibrogenesis Tissue Repair 5: 12, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isenmann S, Arthur A, Zannettino AC, Turner JL, Shi S, Glackin CA, Gronthos S. TWIST family of basic helix-loop-helix transcription factors mediate human mesenchymal stem cell growth and commitment. Stem Cells 27: 2457–2468, 2009. [DOI] [PubMed] [Google Scholar]
- 18.Johnstone RM. Exosomes biological significance: A concise review. Blood Cells Mol Dis 36: 315–321, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem 285: 17442–17452, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee MS, Lowe G, Flanagan S, Kuchler K, Glackin CA. Human Dermo-1 has attributes similar to twist in early bone development. Bone 27: 591–602, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Lee MS, Lowe GN, Strong DD, Wergedal JE, Glackin CA. TWIST, a basic helix-loop-helix transcription factor, can regulate the human osteogenic lineage. J Cell Biochem 75: 566–577, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Lee TK, Poon RT, Yuen AP, Ling MT, Kwok WK, Wang XH, Wong YC, Guan XY, Man K, Chau KL, Fan ST. Twist overexpression correlates with hepatocellular carcinoma metastasis through induction of epithelial-mesenchymal transition. Clin Cancer Res 12: 5369–5376, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Lee YB, Bantounas I, Lee DY, Phylactou L, Caldwell MA, Uney JB. Twist-1 regulates the miR-199a/214 cluster during development. Nucleic Acids Res 37: 123–128, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li B, Han Q, Zhu Y, Yu Y, Wang J, Jiang X. Down-regulation of miR-214 contributes to intrahepatic cholangiocarcinoma metastasis by targeting Twist. FEBS J 279: 2393–2398, 2012. [DOI] [PubMed] [Google Scholar]
- 25.Li L, Cserjesi P, Olson EN. Dermo-1: a novel twist-related bHLH protein expressed in the developing dermis. Dev Biol 172: 280–292, 1995. [DOI] [PubMed] [Google Scholar]
- 26.Loebel DA, O'Rourke MP, Steiner KA, Banyer J, Tam PP. Isolation of differentially expressed genes from wild-type and Twist mutant mouse limb buds. Genesis 33: 103–113, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Loebel DA, Tsoi B, Wong N, Tam PP. A conserved noncoding intronic transcript at the mouse Dnm3 locus. Genomics 85: 782–789, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Matsuo N, Shiraha H, Fujikawa T, Takaoka N, Ueda N, Tanaka S, Nishina S, Nakanishi Y, Uemura M, Takaki A, Nakamura S, Kobayashi Y, Nouso K, Yagi T, Yamamoto K. Twist expression promotes migration and invasion in hepatocellular carcinoma. BMC Cancer 9: 240, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niu RF, Zhang L, Xi GM, Wei XY, Yang Y, Shi YR, Hao XS. Up-regulation of Twist induces angiogenesis and correlates with metastasis in hepatocellular carcinoma. J Exp Clin Cancer Res 26: 385–394, 2007. [PubMed] [Google Scholar]
- 30.O'Rourke MP, Tam PP. Twist functions in mouse development. Int J Dev Biol 46: 401–413, 2002. [PubMed] [Google Scholar]
- 31.Pozharskaya V, Torres-Gonzalez E, Rojas M, Gal A, Amin M, Dollard S, Roman J, Stecenko AA, Mora AL. Twist: a regulator of epithelial-mesenchymal transition in lung fibrosis. PLoS One 4: e7559, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Properzi F, Logozzi M, Fais S. Exosomes: the future of biomarkers in medicine. Biomark Med 7: 769–778, 2013. [DOI] [PubMed] [Google Scholar]
- 33.Qin Q, Xu Y, He T, Qin C, Xu J. Normal and disease-related biological functions of Twist1 and underlying molecular mechanisms. Cell Res 22: 90–106, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramachandran S, Palanisamy V. Horizontal transfer of RNAs: exosomes as mediators of intercellular communication. Wiley Interdiscip Rev RNA 3: 286–293, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simpson P. Maternal-zygotic gene interactions during formation of the dorsoventral pattern in drosophila embryos. Genetics 105: 615–632, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun S, Du R, Xia L, Sun W, Zhai Y, Yu Y, Zhao A, Huang C, Ning X, Wang H. Twist is a new prognostic marker for renal survival in patients with chronic kidney disease. Am J Nephrol 35: 141–151, 2012. [DOI] [PubMed] [Google Scholar]
- 37.Thery C. Exosomes: secreted vesicles and intercellular communications. F1000 Biol Rep 3: 15, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thisse B, el Messal M, Perrin-Schmitt F. The twist gene: isolation of a Drosophila zygotic gene necessary for the establishment of dorsoventral pattern. Nucleic Acids Res 15: 3439–3453, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta 1820: 940–948, 2012. [DOI] [PubMed] [Google Scholar]
- 40.Wang SM, Coljee VW, Pignolo RJ, Rotenberg MO, Cristofalo VJ, Sierra F. Cloning of the human twist gene: its expression is retained in adult mesodermally-derived tissues. Gene 187: 83–92, 1997. [PubMed] [Google Scholar]
- 41.Watanabe T, Sato T, Amano T, Kawamura Y, Kawamura N, Kawaguchi H, Yamashita N, Kurihara H, Nakaoka T. Dnm3os, a non-coding RNA, is required for normal growth and skeletal development in mice. Dev Dyn 237: 3738–3748, 2008. [DOI] [PubMed] [Google Scholar]
- 42.Wolf C, Thisse C, Stoetzel C, Thisse B, Gerlinger P, Perrin-Schmitt F. The M-twist gene of Mus is expressed in subsets of mesodermal cells and is closely related to the Xenopus X-twi and the Drosophila twist genes. Dev Biol 143: 363–373, 1991. [DOI] [PubMed] [Google Scholar]
- 43.Xie G, Diehl AM. Evidence for and against epithelial-to-mesenchymal transition in the liver. Am J Physiol Gastrointest Liver Physiol 305: G881–G890, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yin G, Chen R, Alvero AB, Fu HH, Holmberg J, Glackin C, Rutherford T, Mor G. TWISTing stemness, inflammation and proliferation of epithelial ovarian cancer cells through MIR199A2/214. Oncogene 29: 3545–3553, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]