Abstract
Biopolymer homeostasis underlies the health of organisms, and protective osmolytes have emerged as one strategy used by Nature to preserve biopolymer homeostasis. However, a great deal remains unknown about the mechanism of action of osmolytes. Trehalose, as a prominent example, stabilizes proteins against denaturation by extreme temperature and denaturants, preserves membrane integrity upon freezing or in dry conditions, inhibits polyQ-mediated protein aggregation, and suppresses the aggregation of denatured proteins. The underlying thermodynamic mechanisms of such diverse effects of trehalose remain unclear or controversial. In this study, we applied the surface-additive method developed in the Record laboratory to attack this issue. We characterized the key features of trehalose-biopolymer preferential interactions and found that trehalose has strong unfavorable interactions with aliphatic carbon and significant favorable interactions with amide/anionic oxygen. This dissection has allowed us to elucidate the diverse effects of trehalose and to identify the crucial functional group(s) responsible for its effects. With (semi)quantitative thermodynamic analysis, we discovered that 1) the unfavorable interaction of trehalose with hydrophobic surfaces is the dominant factor in its effect on protein stability, 2) the favorable interaction of trehalose with polar amides enables it to inhibit polyQ-mediated protein aggregation and the aggregation of denatured protein in general, and 3) the favorable interaction of trehalose with phosphate oxygens, together with its unfavorable interaction with aliphatic carbons, enables trehalose to preserve membrane integrity in aqueous solution. These results provide a basis for a full understanding of the role of trehalose in biopolymer homeostasis and the reason behind its evolutionary selection as an osmolyte, as well as for a better application of trehalose as a chemical chaperone.
Introduction
Biopolymer homeostasis is the basis of the health of organisms. In particular, perturbations of proteostasis have been implicated in many chronic diseases in humans (1,2). Protective osmolytes have been selected by Nature as one strategy to prevent many organisms from perturbation of biopolymer homeostasis (3,4), and thus, people have a growing interest in osmolytes (5,6) as a potential strategy to retard protein misfolding/aggregation and so to alleviate the pathology of conformational diseases. In addition, osmolytes have wide application in the production of pharmaceutical proteins and as agents to promote protein crystallization (7). However, a lot is still unknown about the mechanism of action of osmolytes and, hence, about the reason behind their evolutionary selection. Osmolytes, and chemical chaperones in general, work as perturbing solutes instead of as site-binding ligands, and they exert their effects by modulating local solution conditions, leading to changes in the free energy of the biopolymer components. Known mechanisms of osmolyte effects as developed in the Timasheff (8,9) and Bolen (10) laboratories do not fully explain the diverse effects of the osmolyte trehalose, a disaccharide of glucose. Trehalose has been selected in evolution by many organisms to cope with osmotic stress, to protect proteins from denaturation by extreme temperature, to preserve membrane integrity in freezing and dry conditions, and to counteract the effects of denaturants (4). Moreover, trehalose has been reported to inhibit polyQ-mediated protein aggregation in vitro and in vivo and to alleviate polyQ-mediated pathology in a mouse model of Huntington disease (11,12). Trehalose can also suppress the aggregation of denatured proteins. In so doing, it interferes with protein refolding by maintaining the protein in a partially folded state, but the native state can be recovered after removing trehalose (13). Numerous studies have been performed to look into the mechanism of trehalose’s effect on protein stability (14–16), membrane integrity (17,18), and protein aggregation (13). However, we still lack a sound thermodynamic explanation, and the physical properties of trehalose that underlie its diverse effects remain unknown. In this study, using the surface-additive method developed in the Record laboratory and especially advanced in recent years (19–21), we identified key features of trehalose-biopolymer preferential interactions, on the basis of which we were able to interpret thermodynamically various trehalose effects. Briefly, unfavorable interaction of trehalose with hydrophobic surfaces is the dominant factor in its stabilizing effect on globular proteins, and favorable interactions of trehalose with polar amide groups determine its inhibition of polyQ-mediated protein aggregation. Together these two factors make trehalose (at low concentration) a general inhibitor of aggregation of denatured protein and thereby of protein refolding, whereas its favorable interactions with phosphate oxygens and unfavorable interactions with aliphatic carbons make trehalose a perfect cosolvent for membrane integrity.
In analyzing perturbing small solute or cosolvent effects on a particular biopolymer process, two thermodynamic strategies have been widely used: preferential interaction analysis (9,22) and the group transfer free-energy study (23,24). The preferential interaction coefficient is the fundamental thermodynamic quantity (transfer free energy is quantitatively related) used to analyze the effect on any biopolymer equilibrium due to changes in the concentration of a perturbing solute (22,25). In contrast to direct interactions possibly visualized by spectroscopic methods (which provide some valuable microscopic detail), preferential interaction coefficients reflect the relative strength of solute-water and solute-biopolymer interactions; as interpreted in the solute partitioning model (21,26,27), the preferential interaction coefficient reflects the relative distribution of the solute between the biopolymer vicinity and the bulk solvent. Therefore, preferential exclusion does not necessarily exclude the possibility of direct interaction of solute with biopolymer, and vice versa. The molecular mechanism of the solute effect could be clarified by statistical mechanical analysis of information on molecular interactions at the microscopic level to derive the preferential interaction coefficient.
Unlike earlier studies of solute effects using preferential interaction analysis, where the water-accessible protein surface was basically treated as homogeneous (9), the surface-additive method (19–21) takes into account explicitly the heterogeneity of a biopolymer surface and thus allows one to understand varying effects of a particular solute on different biopolymer processes (28). The surface-additive method combines the idea of surface analysis of protein stability and the solute effect (29,30) and the idea of surface heterogeneity implied by numerous previous studies exhibiting either differential preferential interactions of a small solute with different types of biopolymer surface (31–34) or differential energetic contributions to the solute effect from different types of biopolymer functional groups (10,35–37).
In common with the group transfer free-energy study (GTFE) (23,24) for the additivity assumption and the heterogeneity treatment of biopolymer surfaces, the surface-additive method decomposes preferential interaction on the basis of biopolymer functional groups (or water-accessible surface types), whereas the GTFE decomposes transfer free energy on the basis of amino acid side chains plus peptide backbone units. Therefore, the surface-additive method provides a direct view of the regularity of preferential interaction of small solutes with biopolymer surface types and facilitates comparison among biopolymer functional groups of their energetic contribution to the solute effect on biopolymer processes. The independence of functional groups in terms of their preferential interactions with solute allows a more concise way of analyzing the solute effect.
Materials and Methods
Chemicals
All chemicals were from Sigma-Aldrich (St. Louis, MO) and all chemicals have purity >99% or 99.5%, except butyramide (>98%).
Analysis
The small-solute effect on a particular biopolymer conformational transition process, describing how small-solute concentration (m3) affects the standard free-energy change of the process, (= −RTlnKobs), or shifts the equilibrium of the process, Kobs (the observed equilibrium concentration quotient), is determined by the differential influence of the solute on the chemical potential of the product and the reactant, , as in the equation (20)
| (1) |
where R is the gas constant, T stands for thermodynamic temperature, and the derivatives are taken at constant T and pressure (P). The m-value has been widely used to quantify small-solute effects on protein unfolding processes. The quantity expresses the dependence of the chemical potential of a biopolymer (at constant T, P, and m2) with respect to the small-solute concentration (in this manuscript, subscripts 2 and 3 represent the biopolymer or model compound and trehalose, respectively), and it is the determining factor of the conventional preferential interaction coefficient, (the derivative of m3 with respect to m2 at constant T, P, and the solute chemical potential μ3) (9,22,38). In the solute partitioning model (21,26,27), preferential interaction of a small solute with a biopolymer gains its molecular interpretation: a thermodynamically favorable interaction ( < 0) corresponds to preferential accumulation ( > 0) of the solute in the local domain of the biopolymer surface; a thermodynamically unfavorable interaction ( > 0) corresponds to preferential exclusion ( < 0) from the local domain; and no preferential interaction (, = 0) indicates that the interaction of the solute with water and with biopolymer is thermodynamically indifferent and thus that the distribution of the solute is random between the vicinity of the biopolymer surface and the bulk water. Equation 1 reveals that a process is promoted if the solute is less excluded from or more accumulated at the product than the reactant.
In the surface-additive method, to interpret or predict solute-biopolymer preferential interactions or the small-solute effect on a particular process, one sums up preferential interactions of the solute with all kinds of water-accessible biopolymer surface (changes) involved, assuming surface additivity (20):
| (2) |
where ASA stands for water-accessible surface area, and the subscript i denotes a functional group of the biopolymer, classified as aliphatic carbon, aromatic carbon, hydroxyl oxygen, amide oxygen, amide nitrogen, anionic carboxylate oxygen, cationic nitrogen, or anionic phosphate oxygen. The preferential interaction potential () expresses the contribution to μ23 by 1 Å2 of the solvent-accessible functional group and is obtained by regression analysis of preferential interactions of solute with model compounds () as a function of ASA composition of model compounds (Eq. 2). Model compounds were selected to represent certain groups of biopolymer surfaces and to be suitable for osmometry study. The term νionβion is added to account for the contribution to preferential interactions of counterions if possible, with stoichiometric number νion of inorganic ions and the unit contribution of βion.
Osmometry
Osmometry (39,40) was utilized to determine the preferential interaction, , of small solutes with model compounds using the equation
| (3) |
In this method, excess osmolality, ΔOsm (= Osm(m2,m3) – Osm(m2) – Osm(m3)), is determined by measuring the osmolality of corresponding solutions (Osm(m2,m3) for a solute-model compound-water three-component solution and Osm(m2) and Osm(m3) for the corresponding two-component solution), and fitting the two-component data to a quadratic function for the purpose of calculating ΔOsm. The value of is obtained from the plot of ΔOsm versus as the slope using linear fitting.
Sample preparation and osmometer reading
A freezing-point depression osmometer (OM806, Loser Messtechnik, Berlin, Germany) was used to measure solution osmolality. Compared with a vapor pressure osmometer (27,33), the freezing-point depression osmometer allows a larger working volume (100 μL) and is thus a better choice (for a potentially higher preparation accuracy) for model compounds, which, unlike precious protein, are not subject to availability issues. No difference was observed for two-component urea data obtained from these two kinds of osmometers. The osmometer was calibrated at zero, 300, and 900 mOsm using NaCl standard solutions provided by Loser Messtechnik.
Stock solutions of trehalose and model compound (10–30 mL) were prepared gravimetrically, and ∼1 mL of sample solution was prepared by gravimetrically mixing trehalose stock and/or model-compound stock with water. The sample solution was prepared at ambient temperature (20°C). Multiple readings on the osmometer were carried out for each sample solution. To determine for the interaction of trehalose with a model compound, a series of sample solutions was made with m2 ∼0.15−0.5 m and m3 ∼0.25−0.45 m.
A self-writing program in Excel using the algebra for multiple linear regression analysis (41) was utilized in this study for two-component osmolality data fitting, and for determining and by fitting ΔOsm with m2m3 and fitting with surface composition , respectively. The standard deviation for multiple reading of osmolality was used in fitting, and error propagation analysis (41) was performed wherever needed. Preferential interactions are often reported as normalized terms (by RT) in this study.
Results
Preferential interactions of trehalose with model compounds
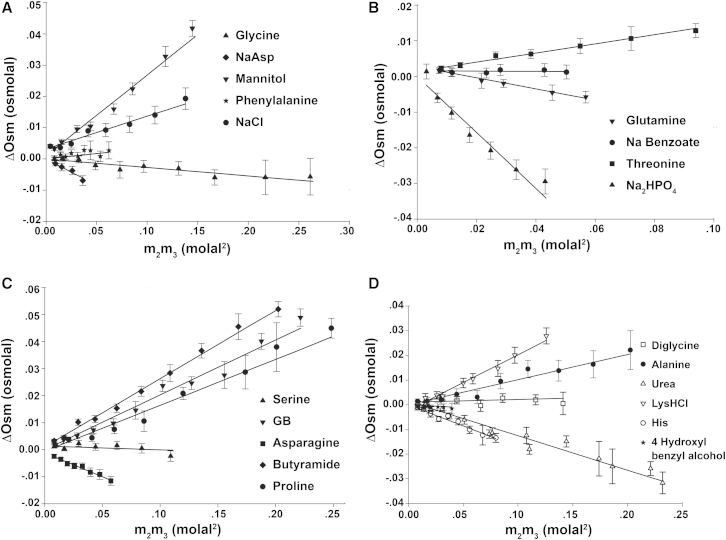
To determine preferential interactions of trehalose with biopolymer functional groups, model compounds were selected to represent certain protein functional groups and phosphate oxygen and to have sufficient solubility in water (Table 1). Osmometry was performed over a range of trehalose (m3) and model compound (m2) concentrations to determine preferential interactions () of trehalose with individual model compounds, as shown in Fig. 1. In Fig. 1, excess osmolality, ΔOsm, is plotted versus concentration product m2m3, and as expected from Eq. 3, the experimentally determined ΔOsm varies linearly with m2m3 with a slope value equivalent to . The linearity of the osmometry plots in Fig. 1 is a demonstration that the osmometry method is reliable in trehalose-model compound systems over the investigated concentration range (39,40), and it also suggests no concentration dependence on m2 and m3 of preferential interactions in the concentration range studied.
Table 1.
Values of for preferential interactions of trehalose with model compounds
| Model Compound | Experimentala (m−1) | Predictedb (m−1) |
|---|---|---|
| Glycine | −0.027 ± 0.008 | −0.017 ± 0.005 |
| Proline | 0.17 ± 0.01 | 0.162 ± 0.005 |
| Alanine | 0.102 ± 0.013 | 0.061 ± 0.006 |
| Glycine betaine | 0.203 ± 0.009 | 0.213 ± 0.008 |
| Mannitol | 0.258 ± 0.013 | 0.264 ± 0.013 |
| Serine | −0.014 ± 0.015 | −0.008 ± 0.005 |
| Threonine | 0.128 ± 0.019 | 0.053 ± 0.006 |
| Diglycine | 0.012 ± 0.014 | 0.013 ± 0.012 |
| Phenylalanine | 0.035 ± 0.027 | 0.058 ± 0.022 |
| Na benzoate | −0.004 ± 0.038 | −0.046 ± 0.026 |
| Urea | −0.139 ± 0.009 | −0.139 ± 0.009 |
| Glutamine | −0.156 ± 0.031 | −0.159 ± 0.012 |
| Asparagine | −0.177 ± 0.026 | −0.180 ± 0.013 |
| Butyramide | 0.252 ± 0.010 | 0.252 ± 0.009 |
| Na aspartate | −0.168 ± 0.029 | −0.107 ± 0.021 |
| LysHCl | 0.225 ± 0.025 | 0.253 ± 0.020 |
| NaCl | 0.105 ± 0.016 | 0.093 ± 0.015 |
| Na2HPO4 | −0.785 ± 0.071 | −0.709 ± 0.050 |
| Hisc | −0.166 ± 0.012 | |
| 4-hydroxybenzyl alcoholc | −0.023 ± 0.043 |
The error comes from the linear fitting of ΔOsm versus m2m3, using the error of ΔOsm propagated from the standard deviation of multiple readings of osmolality of corresponding solutions.
The error is the result of error propagation of the uncertainty on the preferential interaction potential.
Data from these model compounds have not been used in global fitting.
Figure 1.
Determination of preferential interactions () of trehalose with model compounds by osmometry. The excess osmolality, ΔOsm, is plotted versus the concentration product, m2m3. The error bar is propagated from the standard deviation of the multiple readings of osmolality of corresponding solutions for calculating ΔOsm. Lines are linear fits to Eq. 3, with the value of given by the slope. The master plot is divided into four subplots (A–D) for clarity.
Qualitative feature of trehalose preferential interactions with biopolymer functional groups
In Fig. 1, the plots of ΔOsm versus m2m3 span the slope values from negative to near zero to positive values, indicating that trehalose has different preferential interactions with different biopolymer functional groups or their combinations, which provides direct evidence of heterogeneity of trehalose-biopolymer surface preferential interactions. Investigation of the osmometry plots (Fig. 1) and preferential interaction values, (Table 1), reveals some features of trehalose preferential interactions with biopolymer functional groups. 1) Trehalose has a favorable interaction with urea (negative ), which implies that trehalose is preferentially accumulated at polar amide surfaces, since urea is dominated by the amide group (Table S1 in the Supporting Material). 2) Unlike urea, butyramide has a positive , and its major difference from urea is that it has considerably more aliphatic C ASA, which implies that trehalose has an unfavorable interaction with or is preferentially excluded from aliphatic C surface. 3) A negative was often observed for compounds either having an amide group or one more carboxylate oxygen or having phosphate oxygen.
Preferential interactions of trehalose with biopolymer functional groups
Using Eq. 2 and the ASA composition of model compounds (Table S1), we performed regression analysis for data (Table 1) and obtained preferential interaction potentials for each functional group (Table 2). The fitting quality is justified by the agreement between experimental and predicted (Fig. 2 and Table 1) using fitted preferential interaction potentials and ASA compositions of the model compounds. Consistent with our qualitative analyses above, trehalose is preferentially excluded from aliphatic C and preferentially accumulated at amide/carboxylate/phosphate O and amide N, has minimal preferential interaction with hydroxyl O, and is preferentially excluded from cationic N. The interaction of trehalose with aromatic rings exhibits complication. In the above regression analysis, global fitting without or with either of the four aromatic model compounds produces no difference for nonaromatic functional groups, whereas trehalose shows an unfavorable interaction with benzene C in Phe and Na benzoate and a favorable interaction with aromatic C in the imidazole of His (only C in the ring is counted as an aromatic atom in this fitting) or in the phenol of 4-hydroxyl benzyl alcohol (4BA). In addition, the difference between Phe and Na benzoate is smaller than that between His and 4BA. We thus chose Phe and Na benzoate in global fitting to determine the interaction potential of trehalose with aromatic C (appropriate for Phe) and with nonaromatic groups and used fitted parameters for nonaromatic groups to calculate the interaction potential of trehalose with aromatic atoms of the imidazole (His) or phenol ring (4BA) (Table 2). In this calculation, the ASA for the whole imidazole ring (N and C) is counted as aromatic ASA for His, and the amide feature of N is also considered simultaneously (Table S1). In so doing, the difference between imidazole and phenol is largely reduced. Though our modified ASA calculation works for His in this case, our data indicate that aromatic C behaves differently in different aromatic groups, which suggests that atom-wise additivity may not always work well for complicated functional groups such as heterocyclic compounds. In that case, we propose group-wise additivity (for example, the imidazole group) in combination with atom-wise additivity.
Table 2.
Preferential interaction potential for trehalose
| (m−1 Å−2) | |
|---|---|
| Aliphatic C | 22.4 ± 0.8 |
| Aromatic C | 5.9 ± 1.5a, −8.9 ± 1.6b |
| Hydroxyl O | −0.8 ± 0.7 |
| Amide O | −19.6 ± 5.4 |
| Amide N | −4.7 ± 2.2 |
| Carboxylate O | −28.2 ± 2.1 |
| Cationic N | 12.9 ± 2.1 |
| Phosphate O | −59.2 ± 5.1 |
| (m−1) | |
| Na+ | 0.09 ± 0.03 |
| Cl− | 0.00 ± 0.03 |
Except for the second value of Aromatic C, the error is given by multiple linear regression analysis of as a linear combination of the ASA composition of model compounds, using the uncertainty of as reported in Table 1.
For interaction of trehalose with benzene C of Phe.
For interaction of trehalose with aromatic ring atoms in imidazole (N and C) or phenol; calculated as the average value for His and 4BA.
Figure 2.
Comparison of predicted and experimental , or m-value/RT. The absolute values of model compounds (Table 1) were used in log-scale plot. Experimental μ23 of trehalose with native RNase A (8) and BSA (27) and trehalose m−value for unfolding RNase A (8) or adenylate kinase (AK) (10) were as reported, with corresponding predictions in Table S2. The line represents the equality of predicted and experimental values. (Inset) Model compound data in linear scale.
Discussion
Predicting m-values or preferential interactions of native proteins
Using the preferential interaction potential of trehalose (Table 2) and applying the surface-additive method (Eq. 2), we predicted the trehalose m-values for unfolding of RNase A and adenylate kinase, as well as preferential interactions of trehalose with native bovine serum albumin (BSA) and RNase A. The predicted data are plotted versus reported experimental data in Fig. 2 and compiled in Table S2. A good agreement is obtained between prediction and experimental measurements.
Comparison of trehalose with other osmolytes
Fig. 3 A shows that trehalose behaves similarly to urea (42) (with larger magnitude), but differently from glycine betaine (GB) (20) and proline (43), in interactions with amide/carboxylate/phosphate O and with cationic N. On the other hand, trehalose exhibits a much stronger unfavorable interaction with aliphatic C than either GB or proline, both in contrast to the favorable interaction of urea with aliphatic C. To compare with sucrose and sorbitol data (44), we predicted (using Eq. 2 with ASA composition (43)) preferential interactions () of amino acids and backbone unit, and calculated the corresponding transfer free energy at 1 M trehalose from (45) and the transfer free energy for side chains. As shown in Table S3 and Fig. 3 B, compared to sucrose and sorbitol, 1) trehalose has a significantly stronger unfavorable interaction with nonpolar side chains; 2) trehalose shows a much stronger favorable interaction with the Gln or Asn side chain, although the three osmolytes have similar interactions with other polar or negatively charged side chains; and 3) trehalose behaves differently for Lys and Arg. In short, trehalose is probably unique in its highly unfavorable interaction with aliphatic C and significant favorable interaction with amide groups.
Figure 3.
Comparison of trehalose with other osmolytes and with GTFE data. (A) Preferential interaction potential data. The error bar is from regression analysis or as reported. Interaction of trehalose with benzene C is shown as black and with aromatic ring atoms of imidazole or phenol as white in the aromatic C position. (B) Predicted transfer free-energy data of trehalose is compared with that of sorbitol and sucrose. (C) Predicted or experimental transfer free-energy data of trehalose is compared with that from GTFE.
Comparison of the osmometry study with the GTFE
In Fig. 3 C and Table S3, the predicted (as in Fig. 3 B) and experimental (from Table 1) transfer free energy of side chains and backbone unit at 1 M trehalose is compared with those from the GTFE (46,47). We observed that 1) both studies show an unfavorable interaction of trehalose with typical nonpolar side chains; 2) like osmometry, GTFE shows a favorable interaction of trehalose with amide in the case of Gln (though the two studies differ with regard to Asn); 3) for the remaining polar and negatively charged side chains, both methods show similar interactions; 4) osmometry presents a less unfavorable interaction of trehalose with backbone; 5) osmometry and GTFE present contrary interactions for Lys and Arg; and 6) a much larger difference is observed for transfer free energies of amino acids (Table S3). Overall, agreement is observed in some cases, whereas osmometry exhibits more internal consistency. The difference between the two methods has been investigated (43,45) and the discrepancy between the data (Fig. 3 C) is worth further exploration.
Trehalose effects on globular protein stability
Using the same analysis as Guinn et al. (42), we predicted the m-value for the trehalose effect on unfolding of an average globular protein involving 1000 Å2 of ASA change and estimated that 1 molal trehalose at 37°C can stabilize an average globular protein by 0.69 kcal/mol relative to its unfolded state. Compared to urea, GB, and proline (20,42,43) (Fig. 4 A and Table S4), trehalose generates a three- to fourfold stronger effect, consistent with the general observation that trehalose is a strong protein stabilizer (4).
Figure 4.
Osmolyte effect on protein stability. (A) m-values for unfolding an average globular protein of 1000 Å2 of ΔASA for trehalose, urea (42), GB (20), and proline (43). (B) Contribution to the m-value from functional groups. The error is propagated from the uncertainty of the preferential interaction potential.
As revealed by Fig. 4 B and Table S4, the stabilization mechanism of trehalose lies in its exclusion from the surface exposed upon unfolding, as observed by the Timasheff group (8). The dominant stabilization effect comes from preferential exclusion of trehalose from the hydrophobic surface exposed upon unfolding, whereas exposure of polar amide surface, largely attributed to peptide backbone, contributes an opposing effect with much smaller magnitude. On the contrary, the stabilizing effect of GB and proline has a significant contribution from their overall unfavorable interaction with the amide surface. Thus, the surface-additive method allows us to dissect the subtle differences between the working mechanisms of different solutes. Trehalose is probably unique (Fig. 3): its favorable interaction with the backbone amide is overtaken by its unfavorable interaction with the hydrophobic surface. Gekko’s data (48) may imply that inositol also has a favorable interaction with the amide surface. Our data do not support the idea (10) that the stabilizing effect of protecting osmolytes arises predominantly from unfavorable interaction of these solutes with the peptide backbone (comprising both polar amide and aliphatic C surfaces) exposed upon unfolding.
The protein unfolded state is a function of solution conditions (49–51) and is expected to be more compact in trehalose than the chemically denatured state due to the large unfavorable interaction of trehalose with aliphatic C (the dominant ASA of the unfolded state (52)), as is consistent with the observation that trehalose lowered the heat capacity change of unfolding a series of globular proteins (14). The predicted trehalose effect using an extended model for the unfolded state would tend to be overestimated, whereas the qualitative conclusion remains.
Trehalose preserves the integrity of biomembranes
Trehalose can protect biomembranes in freezing and in the dry state (4,53), and this ability of trehalose is attributed to 1) its interaction (through H-bonding) with the headgroups of the phospholipid bilayer (18,54), and 2) its vitrification property, namely, that trehalose can form amorphous glasses and thereby reduce the structural fluctuation of biopolymers (53). We propose here a thermodynamic analysis for the effect of trehalose on membrane integrity at solution conditions.
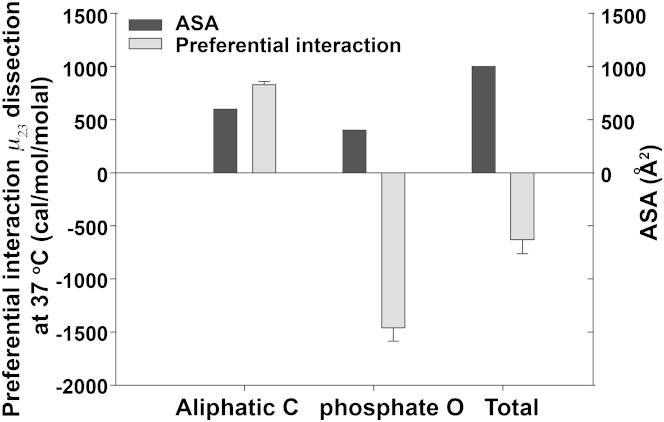
Using Eq. 2 and assuming two-fifths of the bilayer headgroup is contributed by the phosphate O and three-fifths by the aliphatic C (estimated using the quasimolecular volumes of the choline and phosphate groups of a bilayer structure of phosphatidylcholine (PC) (55) with a sphere assumption, and treating the cationic nitrogen as water-inaccessible), we conclude that at 37°C, 1 molal trehalose can lower the free energy of a lipid bilayer (of a size corresponding to 1000 Å2 of water-accessible headgroups) by 632 cal/mol, and that trehalose is preferentially accumulated at the lipid bilayer (Table S4 and Fig. 5), as reported (17). The stabilization primarily arises from favorable interactions between trehalose and the phosphate O, with aliphatic C contributing opposing but smaller effects. Moreover, any disruption of the membrane bilayer is thermodynamically unfavorable due to the resulting unfavorable interaction of trehalose with exposed aliphatic C of hydrocarbon tails.
Figure 5.
Preferential interactions of trehalose with a lipid bilayer of 1000 Å2 ASA of the headgroup and its functional groups. The error is propagated from the uncertainty of the preferential interaction potential.
Due to fewer aliphatic carbons in the headgroup of phosphatidylethanolamine (PE) or phosphatidylglycerol (PG) than in PC, a higher affinity of trehalose with PE or PG membrane is expected and has been suggested (56). To our knowledge, trehalose is the only osmolyte that shows highly unfavorable interaction with aliphatic C and a favorable interaction with phosphate O (cf. Fig. 3), making it a perfect cosolvent for preserving membrane integrity.
Trehalose delays aggregation of polyQ-containing protein
Trehalose is reported to inhibit polyQ-mediated protein aggregation and to alleviate polyQ-mediated pathology (11,12). Here, we offer a semiquantitative thermodynamic interpretation by showing how trehalose may differentially affect the extended state (the ground state of polyQ sequences (57)) and the β-hairpin conformation in polyQ sequences. For the precursor to aggregation, a β-sheet is chosen because it is crucial for aggregation and pathology of polyQ-containing proteins (58–62).
We constructed an extended conformation of polyQ36 using Discovery Studio (Accelrys, San Diego, CA) (Supporting Material), calculated its ASA (63), and quantified its preferential interaction with trehalose (as well as GB, proline, and urea for comparison) using Eq. 2. As shown in Table S5 and Fig. 6, only trehalose and urea make the extended state more stable, and the resulting effect of trehalose is determined by its favorable interaction with polar amide, which overtakes its unfavorable interaction with aliphatic C. At 37°C, 1 molal trehalose can reduce the free energy of polyQ36 by 1.2 kcal/mol or by 33 cal/mol/glutamine. This effect is expected to be larger regarding the actual compactness of the polyQ sequences in water (57), which results in a reduced ASA ratio of aliphatic C compared to amide.
Figure 6.
Osmolyte effect on the free energy of the extended state and β-hairpin of glutamine sequences. β-hairpins 1 (4FEB (64)), 2 (1ubq (69)), 3 (1fxi (70)), and 4 (1le0 (71)) are used for modeling and analysis (Table S5). The error is propagated from the uncertainty of the preferential interaction potential.
With no β-sheet structure reported for polyQ sequences, we took the β-hairpin for mutant polyQ (64) and mutated known β-hairpins by replacing amino acids in the sheet regions with glutamine (using Discovery Studio, Supporting Material), and calculated the Gln ASA in the sheet regions and corresponding preferential interactions with solutes. As shown in Fig. 6 and Table S5, 1 molal trehalose makes the sheet regions of β-hairpins more stable by 1–16 cal/mol/glutamine at 37°C. This effect is significantly smaller than the stabilizing effect of trehalose on the extended state. Urea has an effect similar to that of trehalose, whereas GB and proline make the extended state more unstable than β sheet.
In short, trehalose disfavors the transition of a polyQ sequence to β-sheet, largely because of the favorable interaction of trehalose with polar amide and the relative increase of amide ASA in ployQ. Thus, trehalose could inhibit nucleation and subsequent aggregation of polyQ-containing proteins. Trehalose may also retard aggregation by blocking the formation of intermolecular H-bonding due to its favorable interaction with amide.
Trehalose delays aggregation of denatured globular proteins
Singer and Lindquist (13) reported that trehalose suppressed the aggregation of denatured proteins and their refolding, and proposed that trehalose could exert its effect by binding to the denatured state. The concentration of trehalose (13) is probably not sufficient to overcome the denaturing effect of the remaining GdnHCl, but local hydrophobic collapse was facilitated due to unfavorable interaction of trehalose with aliphatic C, resulting in a partially folded state. Meanwhile, intermolecular H-bonding, and thus aggregation, is inhibited due to preferential accumulation of trehalose at backbone amide groups. Local hydrophobic collapse and trehalose-amide interactions are expected to kinetically interfere with protein refolding. We propose that osmolytes with no favorable interactions with the backbone amide would be less efficient than trehalose in suppressing aggregation of denatured proteins.
Conclusions
Using the surface-additive method (19,20), we characterized the preferential interaction potentials of trehalose with the significant functional groups of protein and biomembranes. Compared with other osmolytes, trehalose seems to be unique in that it has favorable interactions with amide groups and anionic O and a highly unfavorable interaction with aliphatic C. With this feature, we were able to investigate the mechanism by which trehalose stabilizes globular proteins, discover the mechanism by which trehalose preserves biomembrane integrity in aqueous solution, and provide an explanation for why trehalose inhibits polyQ-mediated aggregation and the aggregation of the denatured protein in general. Our study demonstrates the power of the surface-additive method to facilitate understanding of intriguing mechanisms of small-solute effects on various self-assembly processes of biopolymers.
Author Contributions
J.H. designed the research, J.H. and Z.L. performed the research, and J.H. and L.M.G. analyzed the data and wrote the article.
Acknowledgments
We thank M. Thomas Record, Jr., and his group members, Emily Zytkiewicz, Rituparna Sengupta, and Ben Knowles, for valuable conversations and an updated Gln pdb file. We thank the editor and reviewers for valuable suggestions.
This study was supported by the Innovation Program of Shanghai Municipal Education Commission (13YZ009) and the Innovation Foundation of Shanghai University (sdcx2012037) to J.H., and by National Institutes of Health grants GM027616 and OD000945) to L.M.G.
Editor: Rohit Pappu.
Footnotes
Zhicheng Liu’s present address is Mondelez Shanghai Food Corporate Management Co., Ltd., Shanghai 200031, China.
Supporting Materials and Methods and five tables are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(15)00549-4.
Supporting Citations
References (65–71) appear in the Supporting Material.
Supporting Material
References
- 1.Chiti F., Dobson C.M. Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 2.Balch W.E., Morimoto R.I., Kelly J.W. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- 3.Tapia H., Koshland D.E. Trehalose is a versatile and long-lived chaperone for desiccation tolerance. Curr. Biol. 2014;24:2758–2766. doi: 10.1016/j.cub.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Yancey P.H. Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J. Exp. Biol. 2005;208:2819–2830. doi: 10.1242/jeb.01730. [DOI] [PubMed] [Google Scholar]
- 5.Cortez L., Sim V. The therapeutic potential of chemical chaperones in protein folding diseases. Prion. 2014;8:197–202. doi: 10.4161/pri.28938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ignatova Z., Gierasch L.M. Inhibition of protein aggregation in vitro and in vivo by a natural osmoprotectant. Proc. Natl. Acad. Sci. USA. 2006;103:13357–13361. doi: 10.1073/pnas.0603772103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajan R.S., Tsumoto K., Arakawa T. Chemical and pharmacological chaperones: application for recombinant protein production and protein folding diseases. Curr. Med. Chem. 2011;18:1–15. doi: 10.2174/092986711793979698. [DOI] [PubMed] [Google Scholar]
- 8.Xie G., Timasheff S.N. The thermodynamic mechanism of protein stabilization by trehalose. Biophys. Chem. 1997;64:25–43. doi: 10.1016/s0301-4622(96)02222-3. [DOI] [PubMed] [Google Scholar]
- 9.Timasheff S.N. Control of protein stability and reactions by weakly interacting cosolvents: the simplicity of the complicated. Adv. Protein Chem. 1998;51:355–432. doi: 10.1016/s0065-3233(08)60656-7. [DOI] [PubMed] [Google Scholar]
- 10.Auton M., Rösgen J., Bolen D.W. Osmolyte effects on protein stability and solubility: a balancing act between backbone and side-chains. Biophys. Chem. 2011;159:90–99. doi: 10.1016/j.bpc.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanaka M., Machida Y., Nukina N. Trehalose alleviates polyglutamine-mediated pathology in a mouse model of Huntington disease. Nat. Med. 2004;10:148–154. doi: 10.1038/nm985. [DOI] [PubMed] [Google Scholar]
- 12.Yang C.R., Yu R.K. Intracerebral transplantation of neural stem cells combined with trehalose ingestion alleviates pathology in a mouse model of Huntington’s disease. J. Neurosci. Res. 2009;87:26–33. doi: 10.1002/jnr.21817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singer M.A., Lindquist S. Multiple effects of trehalose on protein folding in vitro and in vivo. Mol. Cell. 1998;1:639–648. doi: 10.1016/s1097-2765(00)80064-7. [DOI] [PubMed] [Google Scholar]
- 14.Kaushik J.K., Bhat R. Why is trehalose an exceptional protein stabilizer? An analysis of the thermal stability of proteins in the presence of the compatible osmolyte trehalose. J. Biol. Chem. 2003;278:26458–26465. doi: 10.1074/jbc.M300815200. [DOI] [PubMed] [Google Scholar]
- 15.Seo J.A., Hédoux A., Descamps M. Thermal denaturation of β-lactoglobulin and stabilization mechanism by trehalose analyzed from Raman spectroscopy investigations. J. Phys. Chem. B. 2010;114:6675–6684. doi: 10.1021/jp1006022. [DOI] [PubMed] [Google Scholar]
- 16.Chen L., Cabrita G.J., Melo E.P. Stabilization of the ribosomal protein S6 by trehalose is counterbalanced by the formation of a putative off-pathway species. J. Mol. Biol. 2005;351:402–416. doi: 10.1016/j.jmb.2005.05.056. [DOI] [PubMed] [Google Scholar]
- 17.Andersen H.D., Wang C., Westh P. Reconciliation of opposing views on membrane-sugar interactions. Proc. Natl. Acad. Sci. USA. 2011;108:1874–1878. doi: 10.1073/pnas.1012516108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crowe J.H., Crowe L.M., Chapman D. Preservation of membranes in anhydrobiotic organisms: the role of trehalose. Science. 1984;223:701–703. doi: 10.1126/science.223.4637.701. [DOI] [PubMed] [Google Scholar]
- 19.Pegram L.M., Record M.T., Jr. Thermodynamic origin of hofmeister ion effects. J. Phys. Chem. B. 2008;112:9428–9436. doi: 10.1021/jp800816a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Capp M.W., Pegram L.M., Record M.T., Jr. Interactions of the osmolyte glycine betaine with molecular surfaces in water: thermodynamics, structural interpretation, and prediction of m-values. Biochemistry. 2009;48:10372–10379. doi: 10.1021/bi901273r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Record M.T., Jr., Guinn E., Capp M. Introductory lecture: interpreting and predicting Hofmeister salt ion and solute effects on biopolymer and model processes using the solute partitioning model. Faraday Discuss. 2013;160:9–44. doi: 10.1039/c2fd20128c. discussion 103–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Record M.T., Jr., Zhang W., Anderson C.F. Analysis of effects of salts and uncharged solutes on protein and nucleic acid equilibria and processes: a practical guide to recognizing and interpreting polyelectrolyte effects, Hofmeister effects, and osmotic effects of salts. Adv. Protein Chem. 1998;51:281–353. doi: 10.1016/s0065-3233(08)60655-5. [DOI] [PubMed] [Google Scholar]
- 23.Nozaki Y., Tanford C. The solubility of amino acids and related compounds in aqueous urea solutions. J. Biol. Chem. 1963;238:4074–4081. [PubMed] [Google Scholar]
- 24.Auton M., Bolen D.W. Application of the transfer model to understand how naturally occurring osmolytes affect protein stability. Methods Enzymol. 2007;428:397–418. doi: 10.1016/S0076-6879(07)28023-1. [DOI] [PubMed] [Google Scholar]
- 25.Wyman J., Jr. Linked functions and reciprocal effects in hemoglobin: a second look. Adv. Protein Chem. 1964;19:223–286. doi: 10.1016/s0065-3233(08)60190-4. [DOI] [PubMed] [Google Scholar]
- 26.Record M.T., Jr., Anderson C.F. Interpretation of preferential interaction coefficients of nonelectrolytes and of electrolyte ions in terms of a two-domain model. Biophys. J. 1995;68:786–794. doi: 10.1016/S0006-3495(95)80254-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Courtenay E.S., Capp M.W., Record M.T., Jr. Vapor pressure osmometry studies of osmolyte-protein interactions: implications for the action of osmoprotectants in vivo and for the interpretation of “osmotic stress” experiments in vitro. Biochemistry. 2000;39:4455–4471. doi: 10.1021/bi992887l. [DOI] [PubMed] [Google Scholar]
- 28.Pegram L.M., Wendorff T., Record M.T., Jr. Why Hofmeister effects of many salts favor protein folding but not DNA helix formation. Proc. Natl. Acad. Sci. USA. 2010;107:7716–7721. doi: 10.1073/pnas.0913376107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee B., Richards F.M. The interpretation of protein structures: estimation of static accessibility. J. Mol. Biol. 1971;55:379–400. doi: 10.1016/0022-2836(71)90324-x. [DOI] [PubMed] [Google Scholar]
- 30.Myers J.K., Pace C.N., Scholtz J.M. Denaturant m values and heat capacity changes: relation to changes in accessible surface areas of protein unfolding. Protein Sci. 1995;4:2138–2148. doi: 10.1002/pro.5560041020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Felitsky D.J., Cannon J.G., Record M.T., Jr. The exclusion of glycine betaine from anionic biopolymer surface: why glycine betaine is an effective osmoprotectant but also a compatible solute. Biochemistry. 2004;43:14732–14743. doi: 10.1021/bi049115w. [DOI] [PubMed] [Google Scholar]
- 32.Courtenay E.S., Capp M.W., Record M.T., Jr. Thermodynamic analysis of interactions between denaturants and protein surface exposed on unfolding: interpretation of urea and guanidinium chloride m-values and their correlation with changes in accessible surface area (ASA) using preferential interaction coefficients and the local-bulk domain model. Proteins. 2000;41(Suppl. 4):72–85. doi: 10.1002/1097-0134(2000)41:4+<72::aid-prot70>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 33.Hong J., Capp M.W., Record M.T., Jr. Preferential interactions of glycine betaine and of urea with DNA: implications for DNA hydration and for effects of these solutes on DNA stability. Biochemistry. 2004;43:14744–14758. doi: 10.1021/bi049096q. [DOI] [PubMed] [Google Scholar]
- 34.Hong J., Capp M.W., Record M.T., Jr. Use of urea and glycine betaine to quantify coupled folding and probe the burial of DNA phosphates in lac repressor-lac operator binding. Biochemistry. 2005;44:16896–16911. doi: 10.1021/bi0515218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rees W.A., Yager T.D., von Hippel P.H. Betaine can eliminate the base pair composition dependence of DNA melting. Biochemistry. 1993;32:137–144. doi: 10.1021/bi00052a019. [DOI] [PubMed] [Google Scholar]
- 36.Scholtz J.M., Barrick D., Baldwin R.L. Urea unfolding of peptide helices as a model for interpreting protein unfolding. Proc. Natl. Acad. Sci. USA. 1995;92:185–189. doi: 10.1073/pnas.92.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zou Q., Habermann-Rottinghaus S.M., Murphy K.P. Urea effects on protein stability: hydrogen bonding and the hydrophobic effect. Proteins. 1998;31:107–115. [PubMed] [Google Scholar]
- 38.Casassa E.F., Eisenberg H. Thermodynamic analysis of multicomponent solutions. Adv. Protein Chem. 1964;19:287–395. doi: 10.1016/s0065-3233(08)60191-6. [DOI] [PubMed] [Google Scholar]
- 39.Robinson R.A., Stokes R.H. Activity coefficients in aqueous solutions of sucrose, mannitol, and their mixtures at 25° C. J. Phys. Chem. 1961;65:1954–1958. [Google Scholar]
- 40.Anderson C.F., Record M.T., Jr. Gibbs-Duhem-based relationships among derivatives expressing the concentration dependences of selected chemical potentials for a multicomponent system. Biophys. Chem. 2004;112:165–175. doi: 10.1016/j.bpc.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 41.Bevington P.R., Robinson D.K. McGraw-Hill; New York: 1992. Data Reduction and Error Analysis for the Physical Sciences. [Google Scholar]
- 42.Guinn E.J., Pegram L.M., Record M.T., Jr. Quantifying why urea is a protein denaturant, whereas glycine betaine is a protein stabilizer. Proc. Natl. Acad. Sci. USA. 2011;108:16932–16937. doi: 10.1073/pnas.1109372108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diehl R.C., Guinn E.J., Record M.T., Jr. Quantifying additive interactions of the osmolyte proline with individual functional groups of proteins: comparisons with urea and glycine betaine, interpretation of m-values. Biochemistry. 2013;52:5997–6010. doi: 10.1021/bi400683y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Auton M., Bolen D.W. Predicting the energetics of osmolyte-induced protein folding/unfolding. Proc. Natl. Acad. Sci. USA. 2005;102:15065–15068. doi: 10.1073/pnas.0507053102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cannon J.G., Anderson C.F., Record M.T., Jr. Urea-amide preferential interactions in water: quantitative comparison of model compound data with biopolymer results using water accessible surface areas. J. Phys. Chem. B. 2007;111:9675–9685. doi: 10.1021/jp072037c. [DOI] [PubMed] [Google Scholar]
- 46.Auton M., Bolen D.W., Rösgen J. Structural thermodynamics of protein preferential solvation: osmolyte solvation of proteins, aminoacids, and peptides. Proteins. 2008;73:802–813. doi: 10.1002/prot.22103. [DOI] [PubMed] [Google Scholar]
- 47.Auton M., Bolen D.W. Additive transfer free energies of the peptide backbone unit that are independent of the model compound and the choice of concentration scale. Biochemistry. 2004;43:1329–1342. doi: 10.1021/bi035908r. [DOI] [PubMed] [Google Scholar]
- 48.Gekko K. Mechanism of polyol-induced protein stabilization: solubility of amino acids and diglycine in aqueous polyol solutions. J. Biochem. 1981;90:1633–1641. doi: 10.1093/oxfordjournals.jbchem.a133638. [DOI] [PubMed] [Google Scholar]
- 49.Hong J., Gierasch L.M. Macromolecular crowding remodels the energy landscape of a protein by favoring a more compact unfolded state. J. Am. Chem. Soc. 2010;132:10445–10452. doi: 10.1021/ja103166y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tucker M.J., Oyola R., Gai F. Conformational distribution of a 14-residue peptide in solution: a fluorescence resonance energy transfer study. J. Phys. Chem. B. 2005;109:4788–4795. doi: 10.1021/jp044347q. [DOI] [PubMed] [Google Scholar]
- 51.Choy W.Y., Mulder F.A., Kay L.E. Distribution of molecular size within an unfolded state ensemble using small-angle x-ray scattering and pulse field gradient NMR techniques. J. Mol. Biol. 2002;316:101–112. doi: 10.1006/jmbi.2001.5328. [DOI] [PubMed] [Google Scholar]
- 52.Courtenay E.S., Capp M.W., Record M.T., Jr. Thermodynamics of interactions of urea and guanidinium salts with protein surface: relationship between solute effects on protein processes and changes in water-accessible surface area. Protein Sci. 2001;10:2485–2497. doi: 10.1110/ps.ps.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Crowe L.M. Lessons from nature: the role of sugars in anhydrobiosis. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002;131:505–513. doi: 10.1016/s1095-6433(01)00503-7. [DOI] [PubMed] [Google Scholar]
- 54.Doxastakis M., Sum A.K., de Pablo J.J. Modulating membrane properties: the effect of trehalose and cholesterol on a phospholipid bilayer. J. Phys. Chem. B. 2005;109:24173–24181. doi: 10.1021/jp054843u. [DOI] [PubMed] [Google Scholar]
- 55.Wiener M.C., White S.H. Structure of a fluid dioleoylphosphatidylcholine bilayer determined by joint refinement of x-ray and neutron diffraction data. III. Complete structure. Biophys. J. 1992;61:434–447. doi: 10.1016/S0006-3495(92)81849-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abusharkh S.E., Erkut C., Fahmy K. The role of phospholipid headgroup composition and trehalose in the desiccation tolerance of Caenorhabditis elegans. Langmuir. 2014;30:12897–12906. doi: 10.1021/la502654j. [DOI] [PubMed] [Google Scholar]
- 57.Wetzel R. Physical chemistry of polyglutamine: intriguing tales of a monotonous sequence. J. Mol. Biol. 2012;421:466–490. doi: 10.1016/j.jmb.2012.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nagai Y., Inui T., Toda T. A toxic monomeric conformer of the polyglutamine protein. Nat. Struct. Mol. Biol. 2007;14:332–340. doi: 10.1038/nsmb1215. [DOI] [PubMed] [Google Scholar]
- 59.Kar K., Hoop C.L., Wetzel R. β-hairpin-mediated nucleation of polyglutamine amyloid formation. J. Mol. Biol. 2013;425:1183–1197. doi: 10.1016/j.jmb.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buchanan L.E., Carr J.K., Zanni M.T. Structural motif of polyglutamine amyloid fibrils discerned with mixed-isotope infrared spectroscopy. Proc. Natl. Acad. Sci. USA. 2014;111:5796–5801. doi: 10.1073/pnas.1401587111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schneider R., Schumacher M.C., Baldus M. Structural characterization of polyglutamine fibrils by solid-state NMR spectroscopy. J. Mol. Biol. 2011;412:121–136. doi: 10.1016/j.jmb.2011.06.045. [DOI] [PubMed] [Google Scholar]
- 62.Zhang Q.C., Yeh T.L., Poirier M.A. A compact beta model of huntingtin toxicity. J. Biol. Chem. 2011;286:8188–8196. doi: 10.1074/jbc.M110.192013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsodikov O.V., Record M.T., Jr., Sergeev Y.V. Novel computer program for fast exact calculation of accessible and molecular surface areas and average surface curvature. J. Comput. Chem. 2002;23:600–609. doi: 10.1002/jcc.10061. [DOI] [PubMed] [Google Scholar]
- 64.Kim M. Beta conformation of polyglutamine track revealed by a crystal structure of Huntingtin N-terminal region with insertion of three histidine residues. Prion. 2013;7:221–228. doi: 10.4161/pri.23807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Estrada J., Bernadó P., Sancho J. ProtSA: a web application for calculating sequence specific protein solvent accessibilities in the unfolded ensemble. BMC Bioinformatics. 2009;10:104. doi: 10.1186/1471-2105-10-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chatani E., Hayashi R., Ueki T. Conformational strictness required for maximum activity and stability of bovine pancreatic ribonuclease A as revealed by crystallographic study of three Phe120 mutants at 1.4 Å resolution. Protein Sci. 2002;11:72–81. doi: 10.1110/ps.31102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Müller C.W., Schulz G.E. Structure of the complex between adenylate kinase from Escherichia coli and the inhibitor Ap5A refined at 1.9 Å resolution. A model for a catalytic transition state. J. Mol. Biol. 1992;224:159–177. doi: 10.1016/0022-2836(92)90582-5. [DOI] [PubMed] [Google Scholar]
- 68.Bujacz A. Structures of bovine, equine and leporine serum albumin. Acta Crystallogr. D Biol. Crystallogr. 2012;68:1278–1289. doi: 10.1107/S0907444912027047. [DOI] [PubMed] [Google Scholar]
- 69.Vijay-Kumar S., Bugg C.E., Cook W.J. Structure of ubiquitin refined at 1.8 Å resolution. J. Mol. Biol. 1987;194:531–544. doi: 10.1016/0022-2836(87)90679-6. [DOI] [PubMed] [Google Scholar]
- 70.Tsukihara T., Fukuyama K., Matsubara H. Structure of the [2Fe-2S] ferredoxin I from the blue-green alga Aphanothece sacrum at 2.2 Å resolution. J. Mol. Biol. 1990;216:399–410. doi: 10.1016/S0022-2836(05)80330-4. [DOI] [PubMed] [Google Scholar]
- 71.Cochran A.G., Skelton N.J., Starovasnik M.A. Tryptophan zippers: stable, monomeric β-hairpins. Proc. Natl. Acad. Sci. USA. 2001;98:5578–5583. doi: 10.1073/pnas.091100898. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.