Abstract
Tissue transglutaminase 2 (TG2) is an enzyme with multiple functions, including catalysis of serotonin conjugation to proteins (serotonylation). Previous research indicates that TG2 expression is upregulated in human asthma and in the lung endothelium of ovalbumin (OVA)-challenged mice. It is not known whether endothelial cell TG2 is required for allergic inflammation. Therefore, to determine whether endothelial cell TG2 regulates allergic inflammation, mice with an endothelial cell-specific deletion of TG2 were generated, and these mice were sensitized and challenged in the airways with OVA. Deletion of TG2 in endothelial cells blocked OVA-induced serotonylation in lung endothelial cells, but not lung epithelial cells. Interestingly, deletion of endothelial TG2 reduced allergen-induced increases in respiratory system resistance, number of eosinophils in the bronchoalveolar lavage, and number of eosinophils in the lung tissue. Endothelial cell deletion of TG2 did not alter expression of adhesion molecules, cytokines, or chemokines that regulate leukocyte recruitment, consistent with other studies, demonstrating that deletion of endothelial cell signals does not alter lung cytokines and chemokines during allergic inflammation. Taken together, the data indicate that endothelial cell TG2 is required for allergic inflammation by regulating the recruitment of eosinophils into OVA-challenged lungs. In summary, TG2 functions as a critical signal for allergic lung responses. These data identify potential novel targets for intervention in allergy/asthma.
Keywords: airway responsiveness, allergy, endothelium, lung, transglutaminase 2
the incidence of asthma and allergic disease in several countries, including the United States, has dramatically increased in the last 40 years (31, 64, 66). Furthermore, the World Health Organization has reported that the prevalence of asthma from 1950 to the present has increased in many countries, including those with high, intermediate, or low rates of asthma (13). Therefore, it is important to identify novel targets for regulation of allergic airway disease by delineating mechanisms of allergic inflammation.
In airways of asthmatic patients, there is increased expression of transglutaminase 2 (TG2) (34). TG2 is expressed by endothelial cells, epithelial cells, monocytes, and other cell types (6, 36). TG2 is a multifunctional enzyme that can catalyze the serotonin transamidation of glutamines (serotonylation) (34). Serotonylation regulates cell signaling and actin polymerization (8, 25, 33, 67). It is reported that, in mice, daily intraperitoneal administration of a peptide inhibitor (KVLDGQDP) of TG2 blocks ovalbumin (OVA)-induced lung inflammation (40, 41). However, in these studies, the in vivo cell/tissue targets of the peptide are not known. We reported that, in mice, allergen challenge increases endothelial cell serotonylation and TG2 expression, but not epithelial cell serotonylation (1). There are two forms of transglutaminase, TG1 and TG2 (7, 11). Lung endothelial cells express TG2, but not TG1 (7). The substrate serotonin is present in endothelial cells, because endothelial cells have tryptophan-metabolizing enzymes for synthesis of serotonin and express some of the serotonin receptors (26, 37, 47–49). Therefore, in vivo localized serotonin is available for TG2-catalyzed serotonylation in endothelial cells. We previously reported that in vitro inhibition of endothelial cell TG2 with cystamine blocks TNFα-induced serotonylation in endothelial cells and blocks leukocyte transendothelial migration across endothelial cell monolayers (1). Thus, TG2 is increased in humans and mice during allergic responses, and TG2 regulates leukocyte transendothelial migration in vitro. However, it is not known whether endothelial cell TG2 is required for allergic inflammation in vivo.
To determine whether endothelial cell TG2 is required for allergic lung inflammation, we generated mice with an endothelial cell-specific deletion of TG2. In this report, we demonstrate that endothelial cell TG2 is necessary for recruitment of leukocytes and allergen-induced airway hyperresponsiveness during allergic inflammation.
METHODS
Mice.
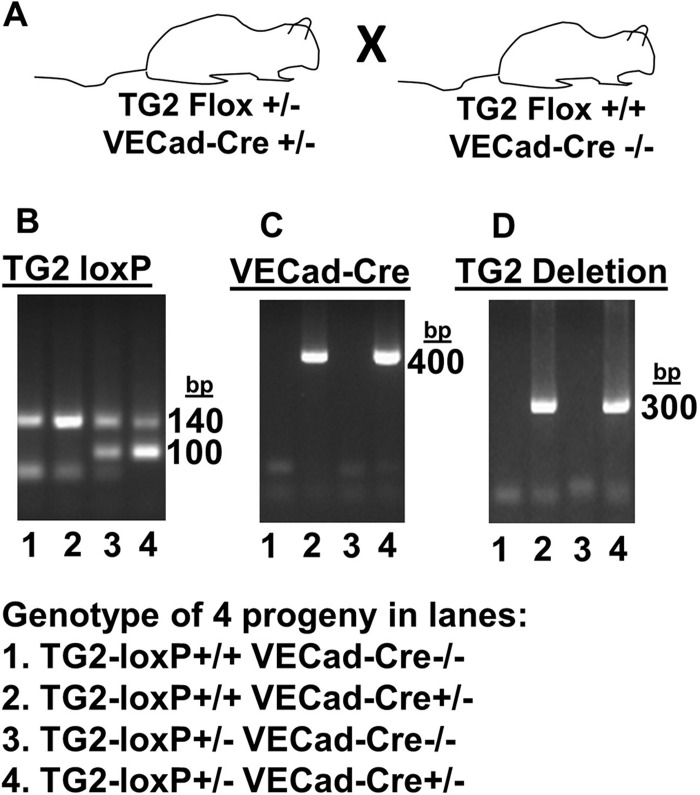
Animal studies were approved by the Institutional Review Committee for Animals at Northwestern University. Deletion of TG2 in the endothelium was accomplished by breeding two strains of mice. C57BL/6 mice with the TG2 locus flanked with loxP sites (TG2loxP/+) (56) were generously provided by Dr. Siiri E. Iismaa and Dr. Robert M. Graham (Victor Chang Cardiac Research Institute, Sydney, Australia) via Gail V. W. Johnson (University of Rochester, Rochester, NY) (19). These mice were bred with mice expressing Cre under the endothelial cell-expressing promoter vascular endothelial (VE)-cadherin-Cre-recombinase (Jackson Laboratories) to generate mice with a constitutive deletion of TG2 specifically in endothelial cells. Mice were genotyped according to methods described elsewhere (19, 56). Endothelial cell-specific TG2−/− mice and littermate controls were generated by breeding male and female mice expressing TG2flox+/− VE-cadherin-Cre+/− (VECad-Cre) with mice expressing TG2flox+/+ VECad-Cre−/− (Fig. 1A). The littermates used for the studies were designated wild-type [WT (TG2flox+/+ VECad-Cre−/−)] mice, mice with heterozygous endothelial expression of TG2 [eTG2+/− (TG2flox+/− VECad-Cre+/−)], and endothelial cell TG2-deficient [eTG2−/− (TG2flox+/+ VECad-Cre+/−)] mice.
Fig. 1.
Genotypes detected by PCR. A: breeding scheme for mice. B: PCR shows wild-type (WT) transglutaminase 2 (TG2) at 100 bp and TG2-loxP at 140 bp. C: PCR for vascular endothelial cadherin Cre (VECad-Cre). D: PCR for deletion of TG2. In B–D, each lane is from DNA of a representative mouse that was analyzed for TG2-loxP, VECad-Cre, and TG2 deletion. TG2-loxP+/+ VECad-Cre+/− are designated as eTG2−/− mice. TG2-loxP+/+ VECad-Cre−/− are designated as WT TG2 mice. TG2-loxP+/− VECad-Cre+/− are designated as eTG2+/− mice.
Immunomagnetic isolation of endothelial cells.
Lungs were minced and placed in RPMI medium containing 0.2 mg/ml DNase I (catalog no. 10104159001, Roche) and 1 mg/ml collagenase (catalog no. 11088874103, Roche) for 1 h at 37°C. Cells were pressed through a 40-μm mesh, and red blood cells were lysed using BD Pharm Lyse solution. Cells were washed, suspended in 250 μl of PBS-0.5% BSA, and incubated with 25 μl of a FcR blocking reagent (catalog no. 130-092-575, Miltenyi Biotec) for 30 min on ice and washed. Cells were suspended in 50 μl of PBS-0.5% BSA and labeled for 30 min with 8 μl of Alexa Fluor 647-conjugated rat anti-mouse PECAM-1 (CD31; catalog no. 102415, Biolegend) and 8 μl of Alexa Fluor 488-conjugated rat anti-mouse endoglin (CD105; catalog no. 120405, Biolegend). Cells were washed and resuspended in 1 ml of degassed PBS-0.5% BSA and 20 μl of goat anti-rat IgG magnetic microbeads (catalog no. 130-048501, Miltenyi Biotec) for 15 min. The endothelial cells were isolated using a VarioMACS separator (catalog no. 130-090-282, Miltenyi Biotec) with magnetic columns (catalog no. 130-042-201, Miltenyi Biotec). The positively selected fraction was assessed for endothelial cell purity using flow cytometry to quantify endoglin and PECAM-1 double-positive cells. Endothelial cell fractions were >85% pure.
Western blot for TG2.
Cell lysates were resolved using 4–15% SDS-PAGE (Bio-Rad). After resolution, gel contents were transferred to nitrocellulose membranes using the semidry method according to the manufacturer's instructions (Bio-Rad). Membranes were blocked in Tris-buffered saline plus 0.1% Tween 20 (TBS-T) and 5% BSA for 1 h at room temperature. Membranes were washed with TBS-T three times for 5 min and then incubated in TBS-T-5% BSA with 10 μl of rabbit anti-mouse anti-TG2 (catalog no. D11A6, Cell Signaling Technology) overnight at 4°C with gentle agitation on an orbital shaker. Membranes were washed with TBS-T three times for 5 min and then incubated in TBS-T-5% BSA with 10 μl of goat anti-rabbit IgG horseradish peroxidase-linked secondary antibody (catalog no. 70746, Cell Signaling Technology) for 1 h at room temperature on an orbital shaker. After the membranes were washed with TBS-T three times for 5 min, the enhanced chemiluminescence kit (catalog no. RPN2132, Amersham) was used for autoradiographic detection of membrane proteins. Then the membranes were stripped of bound antibody by incubation in 20 ml of Restore Western blot stripping buffer (Pierce, Rockford, IL) for 15 min on an orbital shaker. Blots were washed three times for 5 min with TBS-T. Equal protein loading was assessed by labeling blots with rabbit anti-mouse anti-β-actin antibody (catalog no. 3700, Cell Signaling Technology). β-Actin labeling and detection were accomplished as described above, except a TBS-T solution containing 5% nonfat dry milk, instead of 5% BSA, was used. Densitometry was performed using ImageJ software (National Institutes of Health). Data are presented as the ratio of the fold increase in relative intensity of the TG2 band to its corresponding β-actin band.
OVA administration in vivo.
C57BL/6 mice were used, because the TG2 floxed mice are on the C57BL/6 background and C57BL/6 mice are commonly used to examine regulation of the serotonin pathway in vivo (14, 32, 35, 51, 57, 62, 63). Mice (8–10 per group) received intraperitoneal injections (200 μl) of aluminum hydroxide-adsorbed (alum) chicken egg OVA fraction V (10 μg; catalog no. 5503, Sigma)-alum or saline-alum on days 1 and 8 (3). OVA grade V was used for sensitization, because it contains low endotoxin levels, which are required for adequate OVA sensitization (28); in contrast, high levels of endotoxin suppress the OVA response (28). On days 15, 18, and 20, mice received intranasal OVA fraction V (150 μg) in saline or saline alone. At 24 h after the last treatment, the mice were weighed and tissues were collected.
Peripheral blood eosinophils were counted in a 1:10 dilution in Discombe's stain (1:1:8 acetone-2% aqueous eosin-distilled water) (23, 27). The eosin-positive eosinophils were counted using a hemocytometer (catalog no. 0267110, Fisher Scientific) (3). Bronchoalveolar lavage (BAL) cells were counted and cytospun for differential counts (3). Cytospins of BAL cells were differentially stained using the Diff-Quik staining kit (Dade Behring), and neutrophils, eosinophils, lymphocytes, and monocytes were identified and counted according to morphological criteria. The large left lung lobe was frozen in OCT, and tissue sections were prepared. Lung tissue sections were stained for eosinophils by fixation of frozen tissue sections in 100% ice-cold methanol for 15 min at room temperature. After methanol fixation, slides were incubated in a 1% eosin solution for 5 s, rinsed in 1× PBS, counterstained with 0.05% Methyl Green for 2 s, and then rinsed in deionized water. After the slides were allowed to air-dry overnight, they were mounted using Cytoseal coverslipping agent. Numbers of perivascular eosinophils were counted per high-power field.
Lung tissue immunofluorescence.
Methanol-fixed lung tissue sections were rehydrated in 1× PBS for 1 h. After rehydration, Super PAP Pen (catalog no. 71310, Electron Microscopy Sciences) was used to create a hydrophobic barrier around each tissue section on each slide. Sections were blocked for 30 min in 300 μl of PBS-BSA-azide containing 3 μl of goat and rat sera. Sections were then incubated for 80 min in 300 μl of PBS-0.3%BSA-0.15%azide containing 3 μl rabbit anti-mouse anti-TG2 (catalog no. D11A6, Cell Signaling Technology), rabbit anti-mouse anti-serotonin (catalog no. 8250-0004, AbD Serotec), or polyclonal rabbit (catalog no. ab27478, Abcam) antibody as an isotype control. After primary incubation, sections were washed three times with PBS-BSA-azide and then incubated for 30 min in 300 μl of PBS-BSA-azide containing polyclonal goat anti-rabbit antibody conjugated with Alexa Fluor 594. Sections were washed with PBS-BSA-azide prior to coverslip application with ProLong Gold antifade reagent with DAPI (catalog no. P36935, Life Technologies).
Quantitative PCR of whole lung cytokines, chemokines, and others.
Total RNA was extracted from 30 mg of lung tissue according to the manufacturer's instructions using the Qiagen RNeasy Mini Kit (catalog no. 74106). RNA quantity and quality were assessed using a NanoDrop spectrophotometer (Thermo Scientific). The qScript cDNA synthesis kit (Quanta Biosciences) was used to synthesize cDNA from 500 ng of lung RNA. Expression of IL-2, IL-4, IL-5, IL-17, TNFα, CCL11, CCL24, MUC5AC, and VCAM was quantified by real-time PCR on a 7500 Real Time PCR system (Applied Biosystems) using PerfeCTa IDT qPCR master mix (Quanta) and Prime Time qPCR probes (Integrated DNA Technologies). Thermocycling conditions were as follows: 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 s alternating with 60°C for 1 min. To compare expression levels, expression levels of genes of interest were normalized to levels of GAPDH.
OVA-induced airway hyperresponsiveness.
The mice (8–10 per group) were sensitized by intraperitoneal injection (200 μl) of OVA grade V (10 μg)-alum or saline-alum on days 1 and 8 (3). On days 15 and 18, the mice received intranasal endotoxin-free OVA fraction V (150 μg) in saline or saline alone. On day 20, the mice were anesthetized, tracheostomized, and mechanically ventilated. Mechanical ventilation and measurements of lung mechanics were performed using a flexiVent mouse ventilator (Scireq, Montreal, PQ, Canada) according to the manufacturer's instructions (65). A standard ventilation history for each mouse was obtained with two total lung capacity maneuvers before the mice were treated retro-orbitally with 500 μg of OVA fraction V in 50 μl of sterile saline to induce a response to antigen (24). Respiratory system resistance (RRS) was calculated using the flexiVent software by fitting the equation of motion of the linear single-compartment model of lung mechanics to the single-frequency forced-oscillation maneuver data. The data are presented as percentage of baseline RRS.
Statistical analyses.
Data are presented as means ± SE. The significance of difference between two groups was determined by Student's t-test. For multiple experimental groups with one treatment per group, data were analyzed with a one-way analysis of variance followed by Tukey's multiple-comparisons test (Prism, GraphPad Software, La Jolla, CA).
RESULTS
Endothelial cell-specific deletion of TG2 during allergic inflammation.
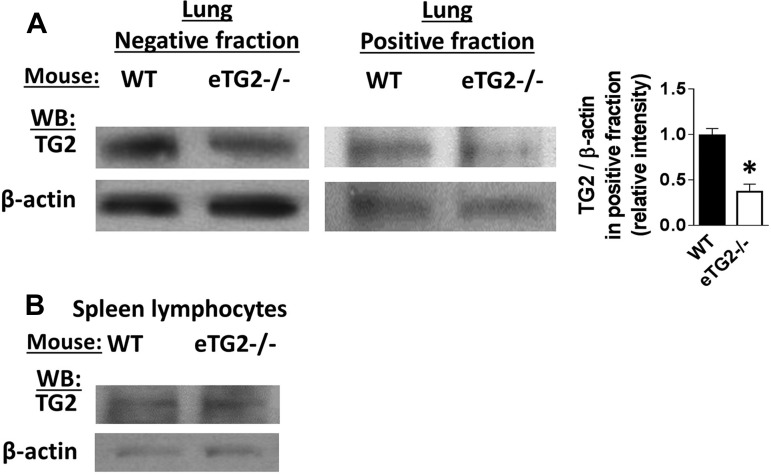
TG2 expression is upregulated in human asthma (34), and we previously reported that TG2 expression is increased in the lung endothelium of OVA-challenged mice (1). To determine whether TG2 in endothelial cells regulates inflammation during allergen challenge, we created an endothelial cell-specific constitutive knockout of TG2 under a VE-cadherin promoter for induction of the deletion of the floxed TG2 locus. Tail clips at weaning were used to select mice on the basis of gene expression of VE-cadherin-Cre, homozygous floxed TG2, and deletion of TG2 (Fig. 1). To determine the specificity of the TG2 deletion in endothelial cells, lung endothelial cells and spleen lymphocytes were isolated from the mice. The purity of endothelial cells was >80% as measured by flow cytometry for endothelial cell expression of CD31 and CD105 (data not shown). There was a significant reduction in the ratio of TG2 to β-actin expression in endothelial cells from eTG2−/− mice compared with WT control mice (Fig. 2A, positive fraction). There is a small amount of TG2 in the lung positive fraction from the eTG2−/− mice, which is likely because purity of isolated endothelial cells is >80%. As a control, lymphocytes (>95% purity) were isolated from spleens of the same animals and represented the hematopoietic compartment. Western blots for the relative ratio of TG2 expression to the control, β-actin, showed no differences in spleen lymphocyte TG2 protein levels between eTG2−/− and control mice (Fig. 2B).
Fig. 2.
TG2 expression in lung endothelial and spleen cells. A: lung endothelial cells were immunomagnetically selected using anti-PECAM-1 and anti-endoglin antibodies. Fractions were analyzed by flow cytometry, and the positive fraction was >80% endothelial cells (data not shown). The negative fraction is the flowthrough from the immunomagnetic column. Left: representative Western blots from a WT and eTG2−/− mouse for TG2 and β-actin for the endothelial cell-positive fraction and the column flowthrough (negative fraction). The light band in the positive fraction from the eTG2−/− mice is due to the 80% purity of the endothelial cells. Right: relative intensity of TG2-to-β-actin ratio in the positive fraction from the immunomagnetic cells, which contained >80% endothelial cells. Values are means ± SE. *P < 0.05. B: spleen cells are >90% lymphocytes and were examined for TG2 expression and β-actin by Western blotting.
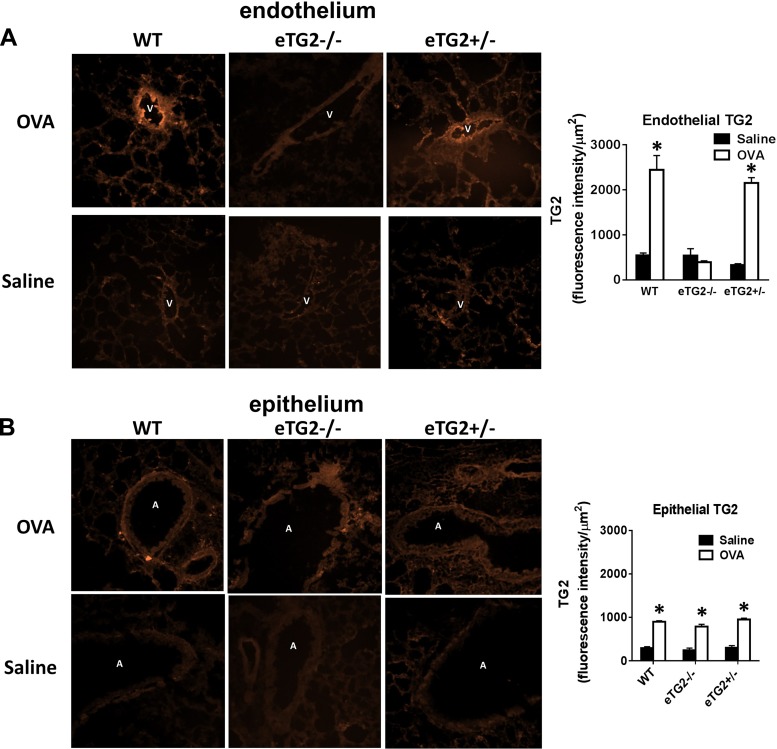
To determine local TG2 protein in endothelial and epithelial cells in the lungs of OVA-challenged and nonchallenged mice, lung tissue sections were immunolabeled with anti-TG2, which detects latent and active TG2. The relative fluorescence per area of venule endothelial cells, the site of leukocyte transendothelial migration, or epithelial cells was determined. OVA challenge increased endothelial cell expression of TG2 in the WT, but not eTG2−/−, mice (Fig. 3A). There was no difference in the expression of epithelial cell TG2 between the OVA-challenged groups (Fig. 3B).
Fig. 3.
TG2 expression in lung tissue sections. Frozen lung tissue sections were immunolabeled with anti-TG2. A and B: TG2 in lung endothelium and epithelium of WT, eTG2−/−, and eTG2+/− mice. Left: representative micrographs of a high-power field. Right: relative intensity of anti-TG2 immunolabeling per area of endothelium or airway epithelium. For each mouse, the average relative intensity was obtained from 3–5 lung venule endothelium or airway epithelium (n = 6–8 mice per group). Values are means ± SE. V, vessel lumen; A, airway; OVA, ovalbumin. *P < 0.05 vs. saline control.
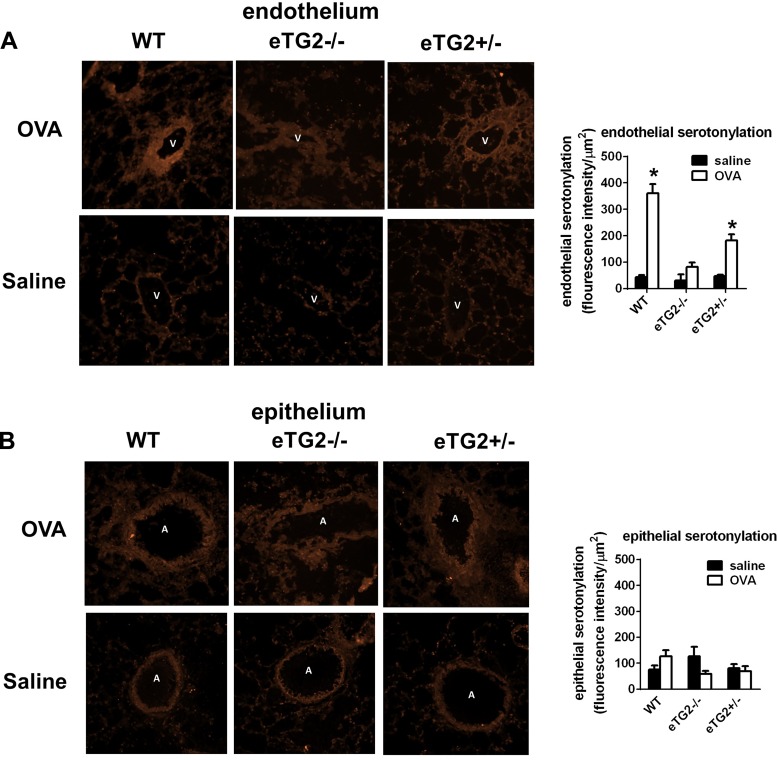
Since TG2 catalyzes localized serotonin transamidation of glutamines (serotonylation) and this serotonylation regulates cell functions (25, 41, 45, 59, 67), we determined whether endothelial cell serotonylation is reduced during allergic inflammation in eTG2−/− mice. Active, but not latent, TG2 catalyzes serotonylation (6, 36). To determine TG2 activity in the lung tissue sections, serotonylation in the tissue sections was examined by immunolabeling. OVA challenge increased endothelial cell serotonylation in the WT, but not eTG2−/−, mice (Fig. 4A). Serotonylation in the epithelium was not altered by OVA challenge or in the epithelium of eTG2−/− mice (Fig. 4B), consistent with no differences in TG2 expression in the epithelium among OVA-challenged groups (Fig. 3B).
Fig. 4.
Serotonylation in lung tissue sections. Frozen lung tissue sections were fixed, washed, rehydrated, and immunolabeled with anti-serotonin. A and B: serotonylation in lung endothelium and epithelium of WT, eTG2−/−, and eTG2+/− mice. Left: representative micrographs of a high-power field. Right: relative intensity of anti-serotonin immunolabeling per area of the venule endothelium or epithelium. For each mouse, the average relative intensity was obtained from 3–5 lung venule endothelium or airway epithelium (n = 6–8 mice per group). Values are means ± SE. V, vessel lumen; A, airway. *P < 0.05 vs. saline control.
Endothelial cell deletion of TG2 decreases number of eosinophils during allergic lung inflammation.
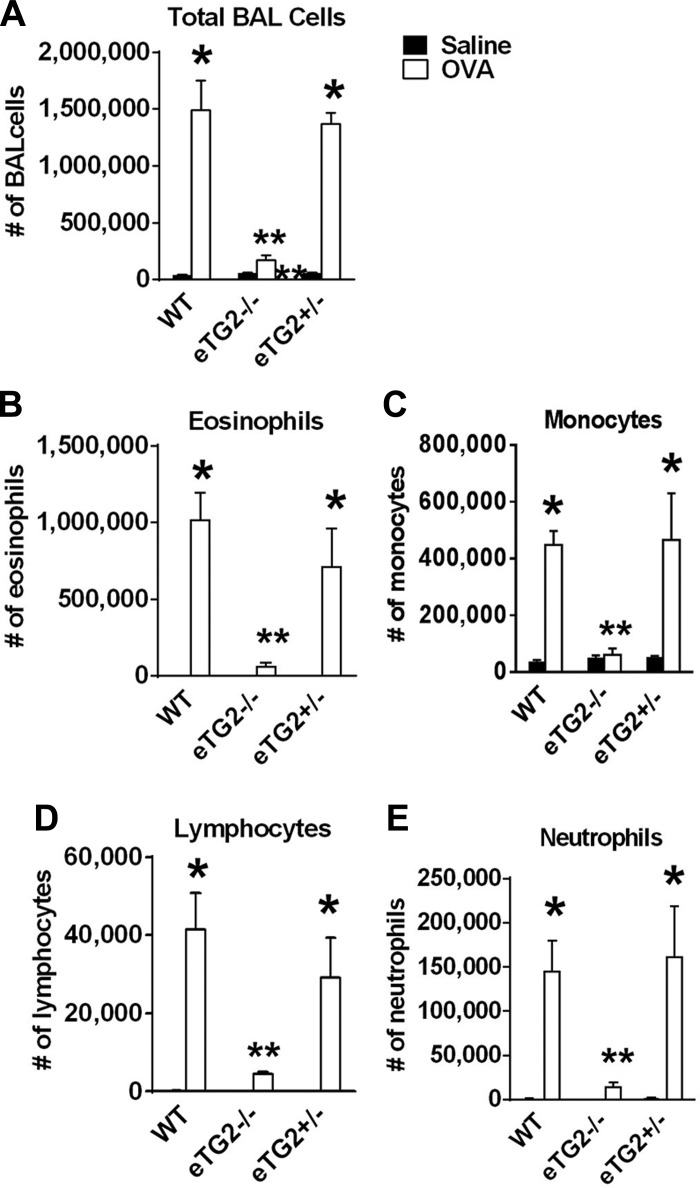
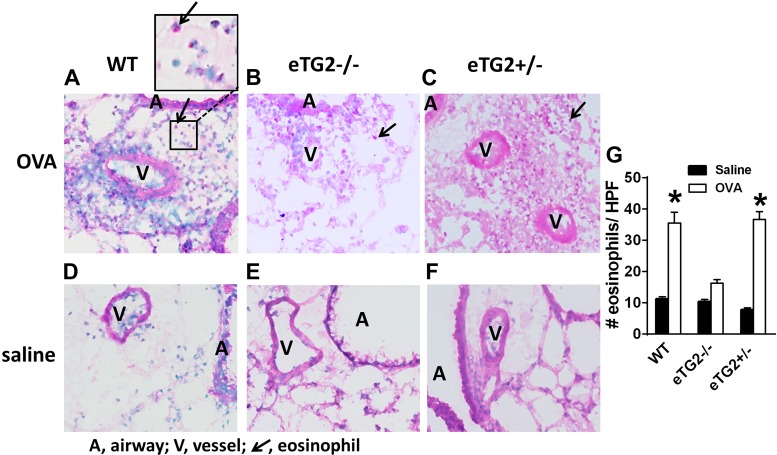
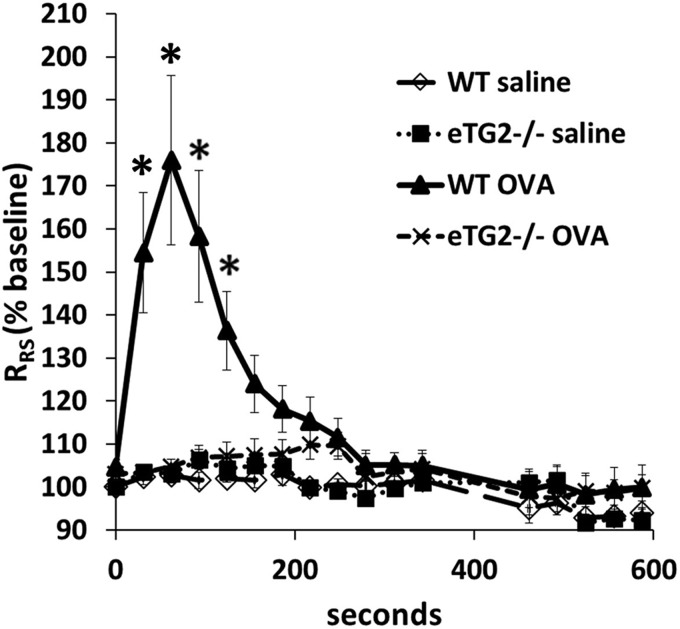
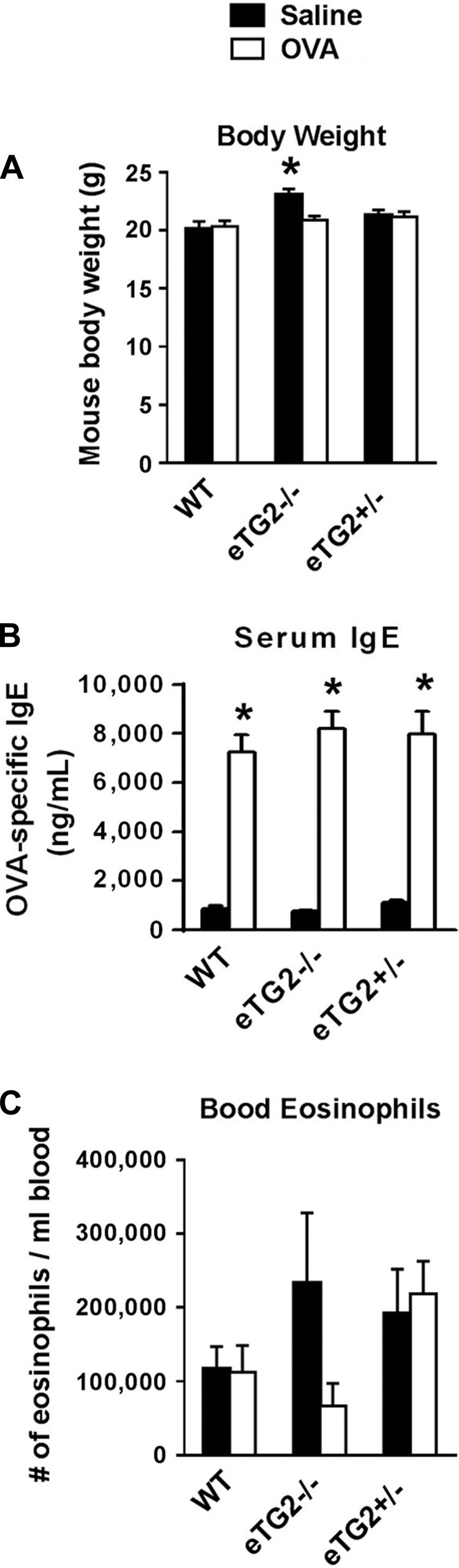
OVA-challenged eTG2−/− mice had a significant decrease in total BAL leukocytes (Fig. 5) compared with OVA-challenged control mice. There was a significant decrease in eosinophils and monocytes/macrophages, as well as a decrease in the low numbers of lymphocytes and neutrophils in BAL, in the eTG2−/− mice (Fig. 5). In addition, in lung tissue sections, there was a decrease in eosinophils in the OVA-challenged eTG2−/− mice compared with the OVA-challenged WT mice (Fig. 6). The number of eosinophils in the OVA-challenged eTG2+/− mice was not different from that in the OVA-challenged WT mice (Figs. 5 and 6). Allergen-stimulated airway hyperresponsiveness was assessed as previously described (1, 24). Furthermore, OVA-induced airway hyperresponsiveness was decreased in the OVA-treated eTG2−/− mice (Fig. 7). There were no differences in blood eosinophils (Fig. 8C) and production of OVA-specific IgE antibodies (Fig. 8B) among the OVA-challenged groups. Body weight was similar among the groups of mice (Fig. 8A), indicating that endothelial TG2 deficiency and allergic inflammation did not alter body weight. Thus, endothelial cell deletion of TG2 blocks OVA-induced lung inflammation and airway hyperresponsiveness.
Fig. 5.
Deletion of endothelial cell TG2 reduced numbers of bronchoalveolar (BAL) cells. BAL cells were collected, cytospun, stained with hematoxylin and eosin, and counted according to standard morphological criteria. Saline bars that are not visible are too small to be seen. Values are means ± SE. *P > 0.05 vs. WT saline control. **P < 0.05 vs. OVA-challenged WT and OVA-challenged eTG2+/− groups.
Fig. 6.
Deletion of endothelial TG2 decreases number of lung tissue eosinophils. A–F: hematoxylin-eosin-stained lung tissue sections. Shown are representative micrographs of high-power fields. G: number of eosinophils per high-power field (HPF). Values are means ± SE. *P < 0.05 vs. WT saline.
Fig. 7.
Deletion of endothelial cell TG2 blocked antigen-induced airway hyperresponsiveness. Allergen-induced airway hyperresponsiveness was measured using a flexiVent ventilator. Antigen-induced airway hyperresponsiveness was induced by retro-orbital administration of 500 μg of OVA. Control mice were treated with saline. Respiratory system resistance (RRS) is presented as percentage of baseline. Peak 176% for the WT OVA group is 1.0 ± 0.01 RRS at time 0 to 1.76 ± 0.20 at peak change in RRS. *P < 0.05 vs. WT saline control.
Fig. 8.
Body weight, OVA-specific IgE, and blood eosinophils were not altered in the eTG2−/− mice. Samples were from mice in Fig. 5. A: body weight of mice at the time of tissue collection. B: OVA-specific IgE in the serum from the mice determined by ELISA. C: numbers of Discombe-stained blood eosinophils. Values are means ± SE. *P < 0.05 vs. corresponding saline group.
Endothelial cell deletion of TG2 does not alter production of cytokines or chemokines that mediate allergic inflammation.
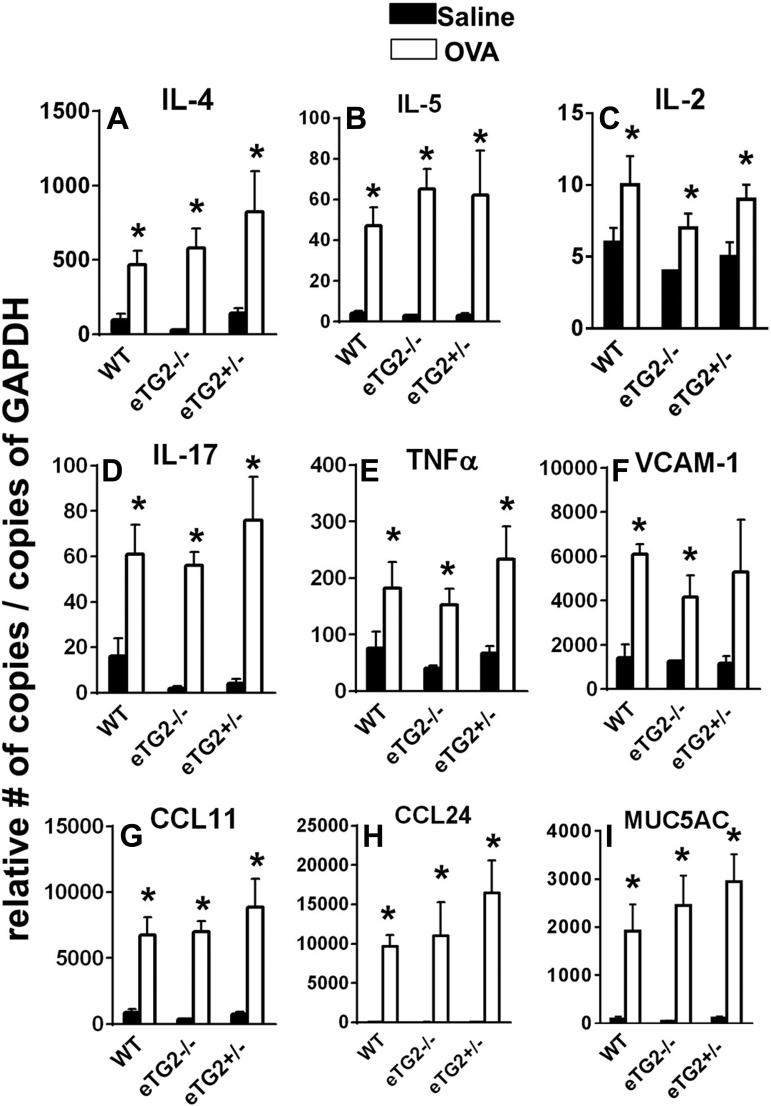
We determined whether cytokines and chemokines that regulate leukocyte infiltration in allergic inflammation (40, 42, 53) were altered in OVA-challenged eTG2−/− mice. OVA challenge to eTG2−/−, TG2+/−, and WT control mice increased RNA expression of several mediators of allergic inflammation, including cytokines (IL-4, IL-5, IL-17, and TNFα), chemokines (CCL11 and CCL24), and mucin (MUC5AC), compared with the corresponding saline treatment groups (Fig. 9). There were also small increases in low levels of IL-2 compared with the corresponding saline treatment group (Fig. 9). However, there were no differences in these mediators among the OVA-challenged groups (Fig. 9). Consistent with no change in the cytokines, expression of the adhesion molecule VCAM-1, which is induced by cytokines, was not altered by the TG2 deletion in the OVA-challenged groups (Fig. 9). In summary, without altering lung cytokines and chemokines, deletion of TG2 in the endothelium decreased leukocyte recruitment into the lung and airway hyperresponsiveness during OVA challenge.
Fig. 9.
Endothelial cell deletion of TG2 did not alter lung cytokines, chemokines, MUC5AC, or VCAM-1. Samples were collected from mice in Fig. 5, and lung mediators were examined by quantitative RT-PCR. Values are means ± SE. *P < 0.05 vs. corresponding saline control.
DISCUSSION
Endothelial cells have an active function in leukocyte recruitment to inflammatory sites (22). We demonstrate in this report that TG2 in endothelial cells is required for leukocyte recruitment and endothelial cell serotonylation. In these studies, eTG2−/− mice, which had a deletion of TG2 specifically in endothelial cells, displayed reduced numbers of inflammatory cells in the lung, reduced OVA-stimulated lung endothelial serotonylation, and reduced OVA-stimulated airway hyperresponsiveness. The inhibition of inflammation in eTG2−/− mice occurred without affecting blood eosinophil numbers or expression of mediators for eosinophil recruitment, including the chemokines CCL11 and CCL24, or the endothelial cell adhesion molecule for eosinophil recruitment, VCAM-1. Thus, eosinophils and mediators for eosinophil recruitment were available, but recruitment of the eosinophils into the tissue was reduced. This is consistent with our reports of a reduction in the number of eosinophils in the lung and a reduction in airway hyperresponsiveness without an alteration of lung adhesion molecules, cytokines, or chemokines in other mouse models with deletions in endothelial cell signaling molecules during allergic inflammation (3, 9, 10, 22, 39, 60). This is the first report that endothelial cell-specific deletion of TG2 in vivo blocks allergic inflammation.
In asthmatic patients, TG2 is elevated in human airway cells, and it has been reported that bronchial epithelial tissue expresses TG2 (34). We have demonstrated in mice that allergen challenge increases TG2 expression and serotonylation in endothelial cells, whereas epithelial cell serotonylation is not significantly altered during allergic airway responses (1). In the current studies of eTG2−/− mice, the airway epithelium expressed TG2 and the TG2 expression was slightly increased in the OVA-challenged WT and OVA-challenged eTG2−/− mice. However, epithelial cell serotonylation was not induced in OVA-challenged eTG2−/−, eTG2+/−, or WT mice compared with saline-treated mice. The finding that TG2 is mostly in a latent transglutaminase form until activated (6, 36) suggests that OVA challenge stimulates an increase in epithelial TG2 protein expression, but the increased TG2 is most likely in a latent form, because there is no increase in TG2-mediated epithelial cell serotonylation. In contrast, during allergic inflammation, endothelial cell TG2 expression is increased and serotonylation is increased, suggesting that the endothelial cell TG2 is in an active form.
In other reports, in mice, global inhibition of TG2 blocks inflammation. Briefly, in vivo administration of the chemical inhibitor of TG2 cystamine blocks OVA-induced allergic inflammation in the lung (58). In an IgE-induced passive cutaneous anaphylaxis model in mice, sensitization with antigen-specific IgE followed by administration of cystamine and then antigen challenge blocked antigen-stimulated passive anaphylaxis (29, 40, 41), suggesting that TG2 is at least involved in the response to antigen challenge. Mice with a full gene knockout of TG2 have reduced neutrophil recruitment during endotoxemia (12) or reduced OVA-induced lung inflammation (58). Also, mice receiving daily intraperitoneal administration of a peptide inhibitor (KVLDGQDP) of TG2 exhibit reduced OVA-induced lung inflammation, reduced passive cutaneous anaphylaxis, or reduced atopic dermatitis (29, 40, 41). In studies with the TG2 peptide inhibitor, antigen-stimulated lung expression of VCAM-1, IL-4, IL-5, and IL-13 is also reduced (40, 41). This differs from our finding of no change in these cytokines in mice with endothelial cell-specific deletion of TG2. This difference is likely because TG2 is ubiquitous and expressed by leukocytes, epithelium, endothelium, and other cell types (6, 36) and because, in the studies with peptides, inhibitors, or full TG2 knockout mice, the in vivo cell/tissue targets that were necessary for the inhibition of cytokines are not known.
It has been reported that, in vitro, antibody blockade of transglutaminase on the endothelial cell surface blocks CD8+ T cell transendothelial migration but anti-transglutaminase treatment of CD8+ cells does not have an effect (54). We have reported that pretreatment of the endothelial cells in vitro with the TG2 inhibitor cystamine blocks eosinophil or lymphocyte transendothelial migration without altering eosinophil and lymphocyte binding to the endothelial cells and without altering VCAM-1 expression by the endothelial cells or endothelial cell expression of the chemokine monocyte chemoattractant protein-1 (1), suggesting that TG2 regulates endothelial cell signaling. During leukocyte transendothelial migration, leukocyte binding to vascular adhesion molecules activates signals in the endothelial cells that are required for leukocyte transendothelial migration. In the OVA model of allergic inflammation, eosinophils migrate on VCAM-1 (16), lymphocytes and neutrophils migrate on ICAM-1 and partially migrate on VCAM-1 (16, 38, 44), and monocytes can migrate on VCAM-1 and ICAM-1 (50). Neutrophils also can migrate on PECAM-1 (18, 61). Thus the inhibition of OVA-stimulated recruitment of eosinophils in eTG2−/− mice without altering expression of VCAM-1, CCL11, and CCL24 is consistent with our report that, in vitro, pharmacological inhibition of endothelial TG2 in cultured endothelial cells blocks leukocyte migration across endothelial cells (1). These studies suggest that endothelial cell TG2 has a role in vivo in endothelial cell function during leukocyte transendothelial migration on vascular adhesion molecules.
Endothelial cells express intracellular and cell surface TG2 that may be in a latent or active state (6, 11, 30). In the cytoplasm of cells, the transglutaminase activity of TG2 is primarily in a latent form until activated (6, 36). Cytoplasmic transglutaminase activity of TG2 is activated by high concentrations of calcium (6, 36). TG2 is also activated by reactive oxygen species in fibroblasts (43) or by PKC in rat embryonic cardiomyoblast-derived H9c2 cells (5). We previously reported that TG2-mediated serotonylation in endothelial cells is increased in vitro and in vivo during VCAM-1-mediated leukocyte transendothelial migration (1). Interestingly, we have also demonstrated that VCAM-1 signaling activates calcium fluxes (21), generation of reactive oxygen species (20, 22, 52), and oxidative activation of PKCα (2). This suggests that VCAM-1 signaling through calcium, reactive oxygen species, or PKCα may activate latently synthesized intracellular TG2 in endothelial cells during VCAM-1-mediated leukocyte recruitment to inflammatory sites. This is consistent with serotonylation regulation of cell signaling and our reports that signaling in the endothelium in vivo is required for VCAM-1-dependent eosinophil recruitment in allergic lung inflammation (3, 9, 10, 22, 39, 60). Although we previously reported that serotonylation is required for in vitro transendothelial migration (1) and report here that OVA-challenged eTG2−/− mice have reduced allergic inflammation and endothelial serotonylation, it is possible that nonserotonylation functions of TG2 participate in allergic inflammation and allergen-induced airway hyperresponsiveness. Mechanisms for activation of endothelial cell TG2 and mechanisms for TG2 function during VCAM-1-dependent leukocyte transendothelial migration in vitro and in vivo are under investigation.
Active TG2 is a multifunctional enzyme that has transglutaminase activity, noncatalytic matrix cross-linking activity, G protein activity, and intracellular calcium signaling functions (6, 36). On the basal surface of adherent cells, TG2 regulates focal adhesions through interactions with integrins (4). TG2 also interacts with integrins on the surface of leukocytes for the regulation of leukocyte function during transendothelial migration (6, 36). One function of TG2 in the cytoplasm is TG2 interaction with stress fibers to regulate cell signals (17). In addition to TG2 G protein activity (46, 55), TG2-catalyzed serotonylation activates small-molecular-weight G proteins (25, 41, 45, 59, 67). Intracellular TG2-mediated serotonylation regulates the function of signaling proteins (25, 41, 45, 59, 67), and it has been reported that endothelial cell signals are required for eosinophil recruitment in allergic lung inflammation (3, 9, 10, 22, 39, 60).
In conclusion, we have demonstrated that endothelial cell TG2 has a critical role in allergic inflammation and airway hyperresponsiveness in vivo. Since TG2 has a regulatory function in the endothelium during leukocyte recruitment in vivo and in vitro, these studies are likely of general relevance to regulation of leukocyte recruitment in inflammation. These novel data identify endothelial cell TG2 as a potential target for intervention in the limitation of allergic inflammation (15, 16).
GRANTS
These studies were supported by National Institutes of Health Grant R01 HL-111624.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the authors.
AUTHOR CONTRIBUTIONS
F.S., H.A.-V., J.C., L.M.-N., and J.M.C.-M. performed the experiments; F.S., H.A.-V., J.C., L.M.-N., and J.M.C.-M. analyzed the data; F.S., H.A.-V., J.C., G.M.M., and J.M.C.-M. interpreted the results of the experiments; F.S., J.C., and J.M.C.-M. prepared the figures; F.S. and J.M.C.-M. drafted the manuscript; F.S., J.C., G.M.M., and J.M.C.-M. edited and revised the manuscript; F.S., H.A.-V., J.C., L.M.-N., G.M.M., and J.M.C.-M. approved the final version of the manuscript; J.M.C.-M. developed the concept and designed the research.
REFERENCES
- 1.Abdala-Valencia H, Berdnikovs S, McCary CA, Urick D, Mahadevia R, Marchese ME, Swartz K, Wright L, Mutlu GM, Cook-Mills JM. Inhibition of allergic inflammation by supplementation with 5-hydroxytryptophan. Am J Physiol Lung Cell Mol Physiol 303: L642–L660, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdala-Valencia H, Cook-Mills JM. VCAM-1 signals activate endothelial cell protein kinase Cα via oxidation. J Immunol 177: 6379–6387, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdala-Valencia H, Earwood J, Bansal S, Jansen M, Babcock G, Garvy B, Wills-Karp M, Cook-Mills JM. Nonhematopoietic NADPH oxidase regulation of lung eosinophilia and airway hyperresponsiveness in experimentally induced asthma. Am J Physiol Lung Cell Mol Physiol 292: L1111–L1125, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akimov SS, Krylov D, Fleischman LF, Belkin AM. Tissue transglutaminase is an integrin-binding adhesion coreceptor for fibronectin. J Cell Biol 148: 825–838, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Almami I, Dickenson JM, Hargreaves AJ, Bonner PL. Modulation of transglutaminase 2 activity in H9c2 cells by PKC and PKA signalling: a role for transglutaminase 2 in cytoprotection. Br J Pharmacol 171: 3946–3960, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bakker EN, Pistea A, VanBavel E. Transglutaminases in vascular biology: relevance for vascular remodeling and atherosclerosis. J Vasc Res 45: 271–278, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Baumgartner W, Golenhofen N, Weth A, Hiiragi T, Saint R, Griffin M, Drenckhahn D. Role of transglutaminase 1 in stabilisation of intercellular junctions of the vascular endothelium. Histochem Cell Biol 122: 17–25, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Baumgartner W, Weth A. Transglutaminase 1 stabilizes β-actin in endothelial cells correlating with a stabilization of intercellular junctions. J Vasc Res 44: 234–240, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Berdnikovs S, Abdala-Valencia H, Cook-Mills JM. Endothelial cell PTP1B regulates leukocyte recruitment during allergic inflammation. Am J Physiol Lung Cell Mol Physiol 304: L240–L249, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berdnikovs S, Abdala-Valencia H, McCary C, Somand M, Cole R, Garcia A, Bryce P, Cook-Mills JM. Isoforms of vitamin E have opposing immunoregulatory functions during inflammation by regulating leukocyte recruitment. J Immunol 182: 4395–4405, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bergamini CM, Griffin M, Pansini FS. Transglutaminase and vascular biology: physiopathologic implications and perspectives for therapeutic interventions. Curr Med Chem 12: 2357–2372, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Bijli KM, Kanter BG, Minhajuddin M, Leonard A, Xu L, Fazal F, Rahman A. Regulation of endothelial cell inflammation and lung polymorphonuclear lymphocyte infiltration by transglutaminase 2. Shock 42: 562–569, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bousquet J, Bousquet PJ, Godard P, Daures JP. The public health implications of asthma. Bull World Health Organ 83: 548–554, 2005. [PMC free article] [PubMed] [Google Scholar]
- 14.Carneiro AM, Airey DC, Thompson B, Zhu CB, Lu L, Chesler EJ, Erikson KM, Blakely RD. Functional coding variation in recombinant inbred mouse lines reveals multiple serotonin transporter-associated phenotypes. Proc Natl Acad Sci USA 106: 2047–2052, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaplin DD. Cell cooperation in development of eosinophil-predominant inflammation in airways. Immunol Res 26: 55–62, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Chin JE, Hatfield CA, Winterrowd GE, Brashler JR, Vonderfecht SL, Fidler SF, Griffin RL, Kolbasa KP, Krzesicki RF, Sly LM, Staite ND, Richards IM. Airway recruitment of leukocytes in mice is dependent on α4-integrins and vascular cell adhesion molecule-1. Am J Physiol Lung Cell Mol Physiol 272: L219–L229, 1997. [DOI] [PubMed] [Google Scholar]
- 17.Chowdhury ZA, Barsigian C, Chalupowicz GD, Bach TL, Garcia-Manero G, Martinez J. Colocalization of tissue transglutaminase and stress fibers in human vascular smooth muscle cells and human umbilical vein endothelial cells. Exp Cell Res 231: 38–49, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Christofidou-Solomidou M, Nakada MT, Williams J, Muller WA, DeLisser HM. Neutrophil platelet endothelial cell adhesion molecule-1 participates in neutrophil recruitment at inflammatory sites and is down-regulated after leukocyte extravasation. J Immunol 158: 4872–4878, 1997. [PubMed] [Google Scholar]
- 19.Colak G, Johnson GV. Complete transglutaminase 2 ablation results in reduced stroke volumes and astrocytes that exhibit increased survival in response to ischemia. Neurobiol Dis 45: 1042–1050, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cook-Mills JM. VCAM-1 signals during lymphocyte migration: role of reactive oxygen species. Mol Immunol 39: 499–508, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cook-Mills JM, Johnson JD, Deem TL, Ochi A, Wang L, Zheng Y. Calcium mobilization and Rac1 activation are required for VCAM-1 (vascular cell adhesion molecule-1) stimulation of NADPH oxidase activity. Biochem J 378: 539–547, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cook-Mills JM, Marchese ME, Abdala-Valencia H. Vascular cell adhesion molecule-1 expression and signaling during disease: regulation by reactive oxygen species and antioxidants. Antioxid Redox Signal 15: 1607–1638, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Copeland D, Grove DI. Effects of Toxoplasma gondii (Gleadle strain) on the host-parasite relationship in trichinosis. Int J Parasitol 9: 205–211, 1979. [DOI] [PubMed] [Google Scholar]
- 24.Cyphert JM, Kovarova M, Allen IC, Hartney JM, Murphy DL, Wess J, Koller BH. Cooperation between mast cells and neurons is essential for antigen-mediated bronchoconstriction. J Immunol 182: 7430–7439, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dai Y, Dudek NL, Patel TB, Muma NA. Transglutaminase-catalyzed transamidation: a novel mechanism for Rac1 activation by 5-hydroxytryptamine2A receptor stimulation. J Pharmacol Exp Ther 326: 153–162, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dempsie Y, MacLean MR. Pulmonary hypertension: therapeutic targets within the serotonin system. Br J Pharmacol 155: 455–462, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Discombe G. Criteria of eosinophilia. Lancet 1: 195, 1946. [DOI] [PubMed] [Google Scholar]
- 28.Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, Toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med 196: 1645–1651, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eom S, Kim Y, Kim M, Park D, Lee H, Lee YS, Choe J, Kim YM, Jeoung D. Transglutaminase II/microRNA-218/-181a loop regulates positive feedback relationship between allergic inflammation and tumor metastasis. J Biol Chem 289: 29483–29505, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faye C, Inforzato A, Bignon M, Hartmann DJ, Muller L, Ballut L, Olsen BR, Day AJ, Ricard-Blum S. Transglutaminase-2: a new endostatin partner in the extracellular matrix of endothelial cells. Biochem J 427: 467–475, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friebele E. The attack of asthma. Environ Health Perspect 104: 22–25, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gardier AM. Mutant mouse models and antidepressant drug research: focus on serotonin and brain-derived neurotrophic factor. Behav Pharmacol 20: 18–32, 2009. [DOI] [PubMed] [Google Scholar]
- 33.Guilluy C, Eddahibi S, Agard C, Guignabert C, Izikki M, Tu L, Savale L, Humbert M, Fadel E, Adnot S, Loirand G, Pacaud P. RhoA and Rho kinase activation in human pulmonary hypertension: role of 5-HT signaling. Am J Respir Crit Care Med 179: 1151–1158, 2009. [DOI] [PubMed] [Google Scholar]
- 34.Hallstrand TS, Wurfel MM, Lai Y, Ni Z, Gelb MH, Altemeier WA, Beyer RP, Aitken ML, Henderson WR. Transglutaminase 2, a novel regulator of eicosanoid production in asthma revealed by genome-wide expression profiling of distinct asthma phenotypes. PLos One 5: e8583, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Izikki M, Hanoun N, Marcos E, Savale L, Barlier-Mur AM, Saurini F, Eddahibi S, Hamon M, Adnot S. Tryptophan hydroxylase 1 knockout and tryptophan hydroxylase 2 polymorphism: effects on hypoxic pulmonary hypertension in mice. Am J Physiol Lung Cell Mol Physiol 293: L1045–L1052, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Kanchan K, Fuxreiter M, Fesus L. Physiological, pathological, and structural implications of non-enzymatic protein-protein interactions of the multifunctional human transglutaminase 2. Cell Mol Life Sci 6: 6, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kawahara K, Hashiguchi T, Kikuchi K, Tancharoen S, Miura N, Ito T, Oyama Y, Nawa Y, Biswas KK, Meng X, Morimoto Y, Shrestha B, Sameshima H, Maruyama I. Induction of high mobility group box 1 release from serotonin-stimulated human umbilical vein endothelial cells. Int J Mol Med 22: 639–644, 2008. [PubMed] [Google Scholar]
- 38.Keramidaris E, Merson TD, Steeber DA, Tedder TF, Tang ML. L-selectin and intercellular adhesion molecule 1 mediate lymphocyte migration to the inflamed airway/lung during an allergic inflammatory response in an animal model of asthma. J Allergy Clin Immunol 107: 734–738, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Keshavan P, Deem TL, Schwemberger SJ, Babcock GF, Cook-Mills JM, Zucker SD. Unconjugated bilirubin inhibits VCAM-1-mediated transendothelial leukocyte migration. J Immunol 174: 3709–3718, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Kim DY, Park BS, Hong GU, Lee BJ, Park JW, Kim SY, Ro JY. Anti-inflammatory effects of the R2 peptide, an inhibitor of transglutaminase 2, in a mouse model of allergic asthma, induced by ovalbumin. Br J Pharmacol 162: 210–225, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim Y, Eom S, Kim K, Lee YS, Choe J, Hahn JH, Lee H, Kim YM, Ha KS, Ro JY, Jeoung D. Transglutaminase II interacts with rac1, regulates production of reactive oxygen species, expression of snai1, secretion of Th2 cytokines and mediates in vitro and in vivo allergic inflammation. Mol Immunol 47: 1010–1022, 2010. [DOI] [PubMed] [Google Scholar]
- 42.Lampinen M, Carlson M, Hakansson LD, Venge P. Cytokine-regulated accumulation of eosinophils in inflammatory disease. Allergy 59: 793–805, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Lee ZW, Kwon SM, Kim SW, Yi SJ, Kim YM, Ha KS. Activation of in situ tissue transglutaminase by intracellular reactive oxygen species. Biochem Biophys Res Commun 305: 633–640, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Lehmann JC, Jablonski-Westrich D, Haubold U, Gutierrez-Ramos JC, Springer T, Hamann A. Overlapping and selective roles of endothelial intercellular adhesion molecule-1 (ICAM-1) and ICAM-2 in lymphocyte trafficking. J Immunol 171: 2588–2593, 2003. [DOI] [PubMed] [Google Scholar]
- 45.Liu Y, Wei L, Laskin DL, Fanburg BL. Role of protein transamidation in serotonin-induced proliferation and migration of pulmonary artery smooth muscle cells. Am J Respir Cell Mol Biol 44: 548–555, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lorand L, Graham RM. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol 4: 140–156, 2003. [DOI] [PubMed] [Google Scholar]
- 47.MaassenVanDenBrink A, Centurion D, Villalon CM. Crosstalk of vascular 5-HT1 receptors with other receptors: clinical implications. Neuropharmacology 55: 986–993, 2008. [DOI] [PubMed] [Google Scholar]
- 48.MacLean MR. Pulmonary hypertension and the serotonin hypothesis: where are we now? Int J Clin Pract Suppl 27–31, 2007. [DOI] [PubMed] [Google Scholar]
- 49.MacLean MR, Dempsie Y. Serotonin and pulmonary hypertension—from bench to bedside? Curr Opin Pharmacol 9: 281–286, 2009. [DOI] [PubMed] [Google Scholar]
- 50.Maslin CL, Kedzierska K, Webster NL, Muller WA, Crowe SM. Transendothelial migration of monocytes: the underlying molecular mechanisms and consequences of HIV-1 infection. Curr HIV Res 3: 303–317, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Mason SS, Baker KB, Davis KW, Pogorelov VM, Malbari MM, Ritter R, Wray SP, Gerhardt B, Lanthorn TH, Savelieva KV. Differential sensitivity to SSRI and tricyclic antidepressants in juvenile and adult mice of three strains. Eur J Pharmacol 602: 306–315, 2009. [DOI] [PubMed] [Google Scholar]
- 52.Matheny HE, Deem TL, Cook-Mills JM. Lymphocyte migration through monolayers of endothelial cell lines involves VCAM-1 signaling via endothelial cell NADPH oxidase. J Immunol 164: 6550–6559, 2000. [DOI] [PubMed] [Google Scholar]
- 53.Menzies-Gow A, Ying S, Phipps S, Kay AB. Interactions between eotaxin, histamine and mast cells in early microvascular events associated with eosinophil recruitment to the site of allergic skin reactions in humans. Clin Exp Allergy 34: 1276–1282, 2004. [DOI] [PubMed] [Google Scholar]
- 54.Mohan K, Pinto D, Issekutz TB. Identification of tissue transglutaminase as a novel molecule involved in human CD8+ T cell transendothelial migration. J Immunol 171: 3179–3186, 2003. [DOI] [PubMed] [Google Scholar]
- 55.Nakaoka H, Perez DM, Baek KJ, Das T, Husain A, Misono K, Im MJ, Graham RM. Gh: a GTP-binding protein with transglutaminase activity and receptor signaling function. Science 264: 1593–1596, 1994. [DOI] [PubMed] [Google Scholar]
- 56.Nanda N, Iismaa SE, Owens WA, Husain A, Mackay F, Graham RM. Targeted inactivation of Gh/tissue transglutaminase II. J Biol Chem 276: 20673–20678, 2001. [DOI] [PubMed] [Google Scholar]
- 57.Neal KB, Parry LJ, Bornstein JC. Strain-specific genetics, anatomy and function of enteric neural serotonergic pathways in inbred mice. J Physiol 587: 567–586, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oh K, Seo MW, Lee GY, Byoun OJ, Kang HR, Cho SH, Lee DS. Airway epithelial cells initiate the allergen response through transglutaminase 2 by inducing IL-33 expression and a subsequent Th2 response. Respir Res 14: 35, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park D, Choi SS, Ha KS. Transglutaminase 2: a multi-functional protein in multiple subcellular compartments. Amino Acids 39: 619–631, 2010. [DOI] [PubMed] [Google Scholar]
- 60.Pero RS, Borchers MT, Spicher K, Ochkur SI, Sikora L, Rao SP, Abdala-Valencia H, O'Neill KR, Shen H, McGarry MP, Lee NA, Cook-Mills JM, Sriramarao P, Simon MI, Birnbaumer L, Lee JJ. Gαi2-mediated signaling events in the endothelium are involved in controlling leukocyte extravasation. Proc Natl Acad Sci USA 104: 4371–4376, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schaefer A, Te Riet J, Ritz K, Hoogenboezem M, Anthony EC, Mul FP, de Vries CJ, Daemen MJ, Figdor CG, van Buul JD, Hordijk PL. Actin-binding proteins differentially regulate endothelial cell stiffness, ICAM-1 function and neutrophil transmigration. J Cell Sci 127: 4470–4482, 2014. [DOI] [PubMed] [Google Scholar]
- 62.Solberg LC, Valdar W, Gauguier D, Nunez G, Taylor A, Burnett S, Arboledas-Hita C, Hernandez-Pliego P, Davidson S, Burns P, Bhattacharya S, Hough T, Higgs D, Klenerman P, Cookson WO, Zhang Y, Deacon RM, Rawlins JN, Mott R, Flint J. A protocol for high-throughput phenotyping, suitable for quantitative trait analysis in mice. Mamm Genome 17: 129–146, 2006. [DOI] [PubMed] [Google Scholar]
- 63.Tenner K, Qadri F, Bert B, Voigt JP, Bader M. The mTPH2 C1473G single nucleotide polymorphism is not responsible for behavioural differences between mouse strains. Neurosci Lett 431: 21–25, 2008. [DOI] [PubMed] [Google Scholar]
- 64.van Schayck CP, Smit HA. The prevalence of asthma in children: a reversing trend. Eur Respir J 26: 647–650, 2005. [DOI] [PubMed] [Google Scholar]
- 65.Vanoirbeek JA, Rinaldi M, De Vooght V, Haenen S, Bobic S, Gayan-Ramirez G, Hoet PH, Verbeken E, Decramer M, Nemery B, Janssens W. Noninvasive and invasive pulmonary function in mouse models of obstructive and restrictive respiratory diseases. Am J Respir Cell Mol Biol 42: 96–104, 2010. [DOI] [PubMed] [Google Scholar]
- 66.Vollmer WM, Osborne ML, Buist AS. 20-Year trends in the prevalence of asthma and chronic airflow obstruction in an HMO. Am J Respir Crit Care Med 157: 1079–1084, 1998. [DOI] [PubMed] [Google Scholar]
- 67.Watts SW, Priestley JR, Thompson JM. Serotonylation of vascular proteins important to contraction. PLos One 4: e5682, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]