Abstract
Endothelial barrier restoration reverses microvessel hyperpermeability and facilitates recovery from lung injury. Because inhibiting connexin 43 (Cx43)-dependent interendothelial communication blunts hyperpermeability in single microvessels, we determined whether endothelial Cx43 levels correlate with changes in microvessel permeability during recovery from lung injury. Toward this, bacterial endotoxin was instilled intratracheally into rat lungs, and at different durations postinstillation the lungs were isolated and blood perfused. Microvessel Cx43 expression was quantified by in situ immunofluorescence and microvessel permeability via a fluorescence method. To supplement the immunofluorescence data, protein levels were determined by immunoblots of lung tissue from endotoxin-instilled rats. Immunofluorescence and immunoblot together revealed that both Cx43 expression and microvessel permeability increased above baseline within a few hours after endotoxin instillation but declined progressively over the next few days. On day 5 postendotoxin, microvessel Cx43 declined to negligible levels, resulting in complete absence of intermicrovessel communication determined by photolytic uncaging of Ca2+. However, by day 14, both Cx43 expression and microvessel permeability returned to baseline levels. In contrast to Cx43, expression of microvessel vascular endothelial (VE)-cadherin, a critical determinant of vascular barrier integrity, exhibited an inverse trend by initially declining below baseline and then returning to baseline at a longer duration. Knockdown of vascular Cx43 by tail vein injection of Cx43 shRNA increased VE-cadherin expression, suggesting that reduction in Cx43 levels may modulate VE-cadherin levels in lung microvessels. Together, the data suggest that endotoxin challenge initiates interrelated changes in microvessel Cx43, VE-cadherin, and microvessel permeability, with changes in Cx43 temporally leading the other responses.
Keywords: connexin 43, Ca2+ communication, immunofluorescence, rat, uncaging, vascular endothelial cadherin, short-hairpin RNA
pulmonary vascular hyperpermeability and attendant alveolar edema are major characteristics of acute lung injury (ALI) (2, 22). Loss of endothelial barrier function and thus increase in vascular permeability result from disruption of interendothelial adherens junctions (AJ), of which vascular endothelial (VE)-cadherin is a major component (7, 10, 18, 23, 35). Restoration of lung endothelial barrier and resolution of alveolar edema are critical to reestablishing normal lung function (2, 17).
Several signaling pathways have been implicated in the disruption of AJ and associated structures during inflammation (3, 5, 12, 23, 30). However, it is not clear whether these pathways play a role in recovery from injury. Different sets of genes are upregulated during the proinflammatory and recovery phases, but a cohort of genes play a role in both phases. These sets of genes are regulated by the transcription factor NF-κB, whose contribution to inflammation is well established (21, 29). In endothelial cells, NF-κB regulates protein expression during both injury and recovery (20). Thus, levels of proteins that are active during both injury and recovery may be continually modulated. Hence, quantifying expression levels at regular intervals after initiation of injury is a necessary step in identifying players that contribute to all phases of inflammation.
Connexin 43 (Cx43), a predominant vascular connexin (38), plays a role in mediating lung microvessel permeability (26). Inhibiting Cx43-dependent interendothelial communication (IEC) blunted microvessel hyperpermeability due to airway instillation of hydrochloric acid (26–28). The blunting was evident even at sites of direct injury (26, 27). Recent evidence suggests that Cx43 is upregulated in tandem with increases in permeability of endothelial monolayers treated with inflammatory mediators (25). Thus, Cx43 appears to contribute to both increases and reductions in vascular permeability. Interestingly, Cx43 levels are modulated by NF-κB in different cell types (19, 37). Thus, Cx43 levels may be progressively modified during injury and recovery in tandem with changes in microvessel permeability that occur during this process.
To elucidate these unknowns, we instilled bacterial endotoxin (LPS) into rat lungs and quantified Cx43 expression at different intervals from initiation of injury to complete recovery. In addition, at the same intervals, we quantified microvessel VE-cadherin expression and single microvessel permeability using our fluorescence-based method (26). To define changes associated with modulation in Cx43 expression, we quantified VE-cadherin expression and microvessel permeability after knocking down vascular Cx43 in vivo using shRNA. Collectively, the data revealed that endothelial Cx43 expression changes continually during both the injury and recovery phases. These changes inversely correlated with similar continual modulations in microvessel VE-cadherin expression and lung microvessel barrier strength.
MATERIALS AND METHODS
Animals.
All studies were approved by the Institutional Animal Care and Use Committee of the University of Tennessee Health Science Center. Adult Sprague-Dawley male rats weighing 250–300 g, unless noted otherwise, were used in the study. They were given access ad libitum to food and water and placed on a 12-h light-dark cycle.
Reagents and plasmids.
LPS from E. coli (serotype 0111:B4), the nuclear stain, Hoechst-33342, and FITC-dextran 20 kDa (FDx20; 0.5 mg/ml) were from Sigma Aldrich (St. Louis, MO). Cx43 (13–8300) and VE-cadherin primary antibodies were from Invitrogen (Carlsbad, CA) and Santa Cruz Biotechnology (Santa Cruz, CA), respectively. Alexa Fluor 488-conjugated goat anti-mouse secondary Ab, fluo4 AM, and nitrophenyl-EGTA AM were from Invitrogen. Agents and fluorophores were infused into microvessels in Ca2+-rich HEPES-buffered vehicle (150 mM Na+, 5 mM K+, 1.0 mM Ca2+, 1 mM Mg2+, 10 mM glucose, and 20 mM HEPES) with 4% dextran and 1% fetal bovine serum at a final pH of 7.4. Cx43 shRNA plasmid was purchased from Santa Cruz Biotechnology and packaged into lentiviral vectors by the University of Tennessee Viral Vector Core (Memphis, TN). P-lenti-GFP lentiviral vector was from the University of Tennessee Viral Vector Core. ViraDuctin lentivirus transduction kit was purchased from Cell Biolabs (San Diego, CA).
LPS instillation in rats.
LPS (2 mg/kg) dissolved in sterile PBS (volume 1 μl/g body wt) was instilled intratracheally into rats under isoflurane anesthesia. Following LPS instillation, animals were allowed to recover for either 4 h, 24 h, 72 h, 5 days, or 14 days.
Lung preparation.
Rats were anesthetized for preparation of isolated blood-perfused lungs, as described previously (14, 16, 26). Briefly, anesthetized rats were exsanguinated by cardiac puncture. The chest cavity was opened, and cannulae were placed in the trachea, left atrium, and pulmonary artery. The lungs and heart were excised en bloc and continuously pump-perfused at 14 ml/min with autologous blood warmed to 37°C. The lungs were constantly inflated at an airway pressure of 5 cm H2O. The pulmonary artery and left atrial pressures were maintained at 10 and 3 cm H2O, respectively. The lungs were positioned on a microscope stage. The lung surface was kept moist with saline throughout the experiment.
Microvessel permeability.
Permeability of single microvessels in isolated blood-perfused rat lungs was determined as reported (26). Briefly, a PE10 (BD Biosciences, Sparks, MD) microcatheter was introduced through the left atrial cannula, and blood cell-free conditions were established by flushing with HEPES-buffered Ringer's solution into microvessels in a small portion of the lung. A video of the procedure along with photographs showing the final size of the blood-free region was reported recently (15). To quantify microvessel permeability, the fluorescent tracer FDx20 was infused into the cleared microvessels, and the FDx20 fluorescence was captured at regular intervals (1 image/min) using a wide-angle microscope (Olympus BX61-WI). After 1 h, the FDx20 infusion was stopped and HEPES-buffered saline infusion resumed to wash off the luminal FDx20. The captured images were analyzed using Metamorph image analysis software (Molecular Devices, Sunnyvale, CA) to determine the ratio of peak to residual FDx20 fluorescence (normalized fluorescence) in single microvessels. Agonist-induced changes in the normalized fluorescence ratio were interpreted to indicate modulation in single microvessel permeability by the agonist, as reported (26).
In situ immunofluorescence.
We determined expression of endothelial Cx43 and VE-cadherin in microvessels of intact blood-perfused lungs by in situ immunofluorescence, as described previously (14, 16). Briefly, a small region of the lung was made blood free by infusing Ringer's through a left atrial microcatheter, as described above. Microvessels in this region were fixed and permeabilized with paraformaldehyde and Triton X, respectively. Following a 30-min infusion of blocking buffer containing 5% bovine serum albumin, the appropriate primary antibody (30 min), Ringer's solution (30 min), fluorophore-tagged secondary antibody (30 min), and Ringer's solution (30 min) again were infused sequentially. Nuclear stain Hoechst-33342 was infused together with the secondary antobody. Subsequently, the fluorescence images of microvessels were captured using a confocal microscope (Zeiss LSM710). Fluorescence along the wall of single microvessels was determined by drawing a line along the vessel wall and calculating the average gray levels along that line using Metamorph. Only vessels in the middle two-thirds portion of an image were used in analysis to exclude heterogeneously fluorescent regions at the periphery of the image frame due to lung curvature and large image field (600 × 600 μm) used. In images with low secondary Ab fluorescence, the nuclear stain was used to define vessel location. Multiple vessels in a single image frame were analyzed, and the average fluorescence per image frame was quantified. The procedure was repeated for several images captured at different sites within the experimental lung region. The average fluorescence from all the vessels in all the image frames was taken as the immunofluorescence data for that lung and treatment protocol.
Ca2+ uncaging.
Ca2+ communication in lung microvessels was determined as reported (28). Briefly, we coloaded microvessels with the Ca2+ indicator fluo4 (10 μM) and the Ca2+ cage NP-EGTA (75 μM). Then, we exposed microvessels to a focal beam of UV light (355 nm) for 10–15 s using a flash photolysis unit (Rapp OptoElectronic) and quantified the resulting increase in fluo4 fluorescence at the uncaging site and in adjacent microvessels. The images were analyzed using Metamorph. Distances from the uncaging site to remote vessels were determined via a calibrated line drawn along vessels, as reported (28).
Transient knockdown of vascular Cx43 in vivo.
Sprague-Dawley rats (50–80 g) were anesthetized and maintained under anesthesia via isoflurane inhalation. Through the tail vein, the rats were injected with either 1) a mixture of Cx43 shRNA lentiviral vector (1 × 109 TU/ml; 20 μl) and ViraDuctin (2 μl in 100 μl PBS), 2) a mixture of p-lenti GFP lentiviral vector (1 × 109 TU/ml; 20 μl) and ViraDuctin (2 μl in 100 μl of PBS), or 3) ViraDuctin (2 μl in 100 μl PBS) alone as control. Postinjection, the rats were allowed to recover for 72 h. Subsequently, the lungs of the rats were isolated and prepared for immunofluorescence imaging.
Western blots.
To determine protein expression, peripheral regions of lungs were dissected out and homogenized in RIPA buffer with a protease inhibitor cocktail (Thermo Scientific). Protein concentrations were measured using the Bio-Rad protein assay kit and a SmartSpec 3000 Spectrophotometer (Bio-Rad, Hercules, CA). Samples containing ∼50 μg of total protein were separated using 4–20% ExpressPlus PAGE gels (GenScript, Piscataway, NJ) and transferred onto nitrocellulose membranes. The membranes were then blocked for 1 h in Tris-buffered saline containing 0.05% Tween-20 and nonfat powdered milk. The blots were probed with primary antibodies overnight at 4°C. Following a wash, the membranes were incubated in horseradish peroxidase-conjugated secondary antibodies for 45 min at room temperature. The blots were developed using chemiluminescent detection reagents (G-Biosciences, St. Louis, MO).
Statistics.
All data are reported as means ± SE. All multiple groups were compared with Kruskal-Wallis one-way ANOVA on ranks followed by pairwise multiple comparisons by Dunn's or Neumann-Kuels method. Two groups were compared by rank sum test or t-test.
RESULTS
Temporal changes in Cx43 expression in lung microvessels of LPS-treated animals.
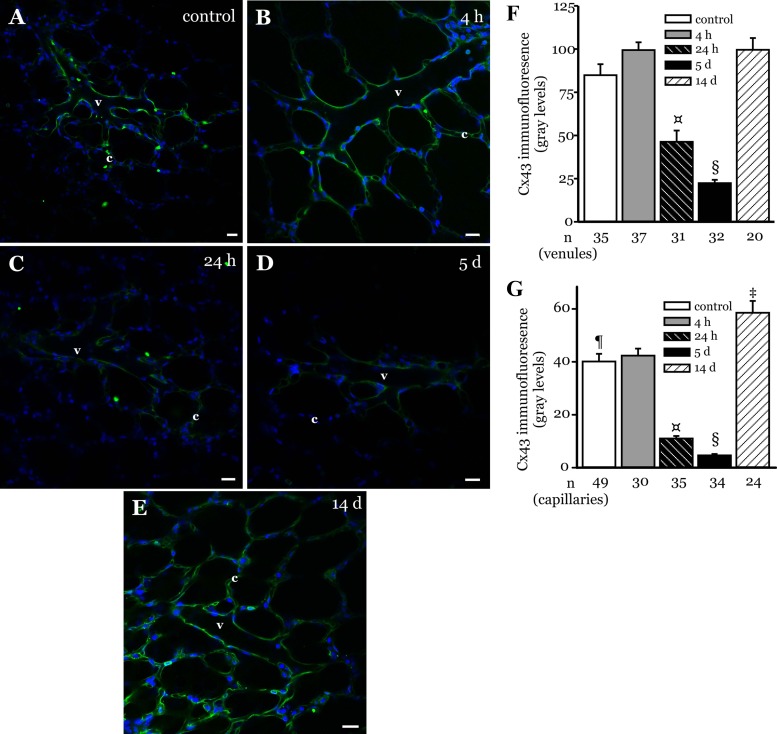
Microvessel Cx43 expression in LPS-treated lungs was determined using in situ immunofluorescence (14). Confocal immunofluorescence images revealed that Cx43 expression was higher at 4 h post-LPS instillation compared with control levels (Fig. 1, A and B). The expression began to decline at 24 h postinstillation (Fig. 1C). At 5 days postinstillation, Cx43 expression declined to just above detectable limits in microvessels (Fig. 1D). However, at 14 days postinstillation, the expression returned to baseline levels (Fig. 1E). These data suggest that microvessel Cx43 expression varied during recovery from LPS-injury. The expression pattern varied in both capillary and venular segments. The overall magnitude of expression was higher in venules compared with capillaries (Fig. 1, F and G). In addition, in capillaries the Cx43 expression declined faster initially and increased above baseline levels at 14 days postinstillation (Fig. 1, F and G).
Fig. 1.
Connexin 43 (Cx43) expression in LPS-instilled lungs. A–E: confocal images show immunofluorescence of Cx43 (green) in microvessels of lungs from rats instilled with LPS (2 mg/ml at 1 μl/g body wt) and allowed to recover for the duration shown. Control lungs did not receive any instillation. Nuclei appear in blue. Bar, 20 μm. v, Venule; c, capillary. (n = 3 lungs each). F and G: bar graphs show Cx43 expression quantified along the walls of venules (F) and capillaries (G) in the image field. n = no. of vessels analyzed. §P < 0.05 compared with all other treatment groups; ¤P < 0.05 compared with control, 4-h and 14-day treatment groups; ¶P < 0.05 compared with 14-day treatment group; ‡P < 0.05 compared with 4-h treatment group (3 lungs/treatment group).
IEC in lung microvessels of LPS-treated animals.
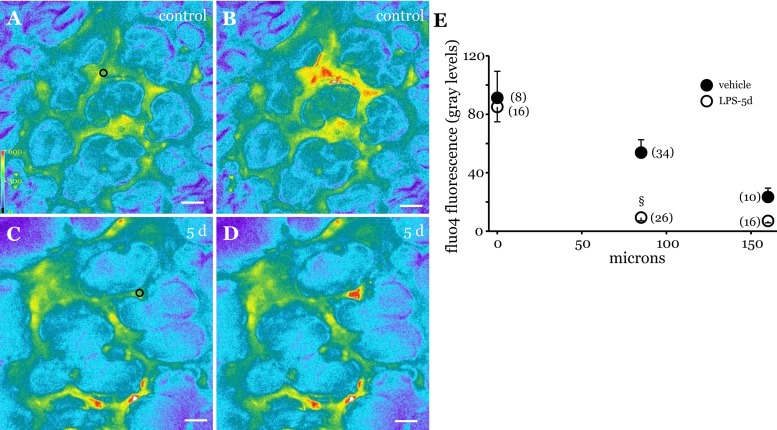
To determine whether the low levels of Cx43 evident at 5 days post-LPS treatment limited IEC, we increased Ca2+ at focal locations in microvessels by Ca2+ uncaging, as reported previously (28). In untreated lungs, Ca2+ uncaging increased Ca2+ both at the site of uncaging and in remote vessels (Fig. 2, A and B). The increase in Ca2+ decayed progressively with increasing distance from the uncaging site (Fig. 2E), as in our previous report (28). In contrast, at 5 days post-LPS, the Ca2+ increase was restricted to the site of uncaging (Fig. 2, C and D). Moreover, no increase in Ca2+ was evident in vessels adjacent to the uncaging site, indicating the absence of Ca2+ communication in microvessels after 5 days of recovery (Fig. 2E). These data suggest that 5 days after LPS treatment both endothelial Cx43 levels and Cx43-dependent IEC had declined to near-zero levels.
Fig. 2.
Ca2+ communication in microvessels of LPS-treated lungs. A–D: fluorescence images show fluo4 fluorescence in microvessels at baseline (A and C) and after uncaging (B and D) in control (A and B) and 5-day post-LPS lungs (C and D). In A and C, ○ represents site of uncaging. Bar, 30 μm. E: graph shows uncaging-induced changes in microvessel fluo4 fluorescence at various distances from the uncaging site in control (●) and 5-day post-LPS lungs (○). §P < 0.05 compared with the corresponding control response. No. of vessels analyzed for each data point indicated in parentheses (3 lungs/treatment group).
Changes in vascular permeability in lung microvessels in LPS-treated animals.
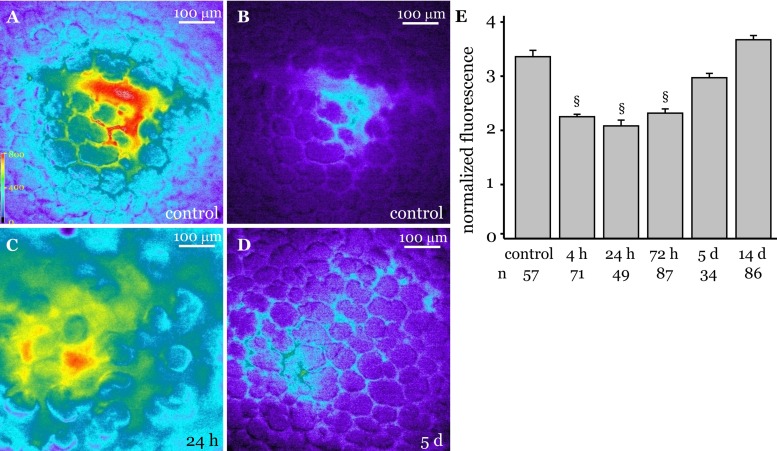
We reported that, in endothelial-specific Cx43 knockout mice, thrombin-induced increases in microvessel filtration coefficient were blunted (28). Hence, to determine whether the microvessel barrier was similarly modified in response to changes in Cx43 levels, we quantified single microvessel permeability in LPS-instilled lungs using our recently reported fluorescence-based method (Fig. 3, A–D) (26). Microvessel permeability increased at 4 h post-LPS instillation (Fig. 3E). The permeability remained elevated at 24 and 72 h postinstillation (Fig. 3E), as indicated by a high residual FDx20 fluorescence (Fig. 3C). However, at 5 days postinstillation, the permeability was low and close to baseline levels (Fig. 3, D and E). At 14 days postinstillation, microvessel permeability returned to baseline levels (Fig. 3E). Together the data suggest that microvessel permeability varies throughout recovery. Moreover, microvessel permeability returned to baseline levels at the same time endothelial Cx43 declined to the lowest level during recovery.
Fig. 3.
Microvessel permeability in LPS-instilled lungs. Permeability of single microvessels in isolated blood-perfused rat lungs was determined by infusing FITC-tagged dextran 20 kDa (FDx20) into the microvessels, followed by a 10-min Ringer's washoff, as detailed in materials and methods. Fluorescence images were captured during the FDx20 infusion and subsequent washoff. A: image shows maximal FDx20 fluorescence in microvessels during FDx20 infusion in Ringer's instilled (control) lungs. B–D: images show residual FDx20 fluorescence after a Ringer's wash to remove luminal FDx20 in control lungs (B) and LPS-instilled lungs that were allowed to recover for 24 h (C) and 5 days (D). E: bar graph shows ratio of maximal to residual FDx20 fluorescence (normalized fluorescence) in single lung microvessels of rats allowed to recover from LPS challenge for durations shown. §P < 0.05 compared with all other treatment groups. n, no. of vessels analyzed. (3 lungs/treatment group).
Temporal changes in VE-cadherin expression in lung microvessels of LPS-treated animals.
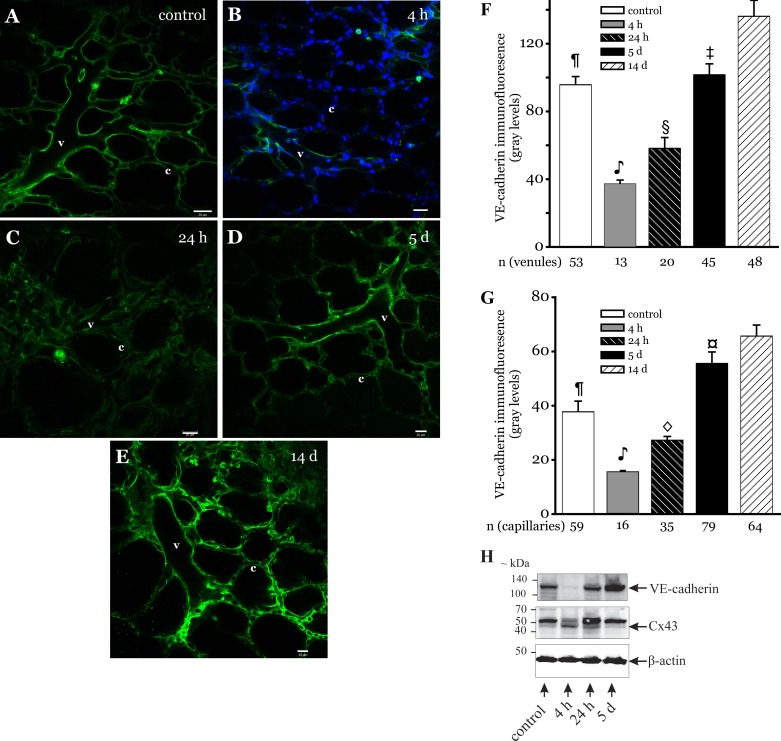
Since, VE-cadherin plays a major role in microvessel barrier integrity, we determined VE-cadherin expression in microvessels of LPS-instilled lungs. In situ immunofluorescence images show that VE-cadherin expression varied during the recovery (Fig. 4, A–E). At 4 h post-LPS, VE-cadherin expression was low and below the microscope detection limits in several vessels (Fig. 4B). In addition, the expression was lower compared with control and other longer recovery periods (Fig. 4, F and G). At 24 h postinstillation, VE-cadherin expression remained lower compared with control levels (Fig. 4, C, F, and G). However, at 5 days postinstillation, the expression level increased above control in both capillaries and venules (Fig. 4, D, F, and G). At 14 days postinstillation, the expression was higher but not significantly different compared with that at the 5-day recovery period (Fig. 4, E–G). To supplement the immunofluorescence results, rats were instilled with LPS and allowed to recover for 4 h, 24 h, and 5 days. The peripheral regions were collected from rat lungs and prepared for Western blots, as outlined in materials and methods. As shown in Fig. 4H, Cx43 (∼43 kDa) protein expression levels at 4 h post-LPS were significantly increased compared with all of the other treatment periods, whereas VE-cadherin (∼132 kDa) levels were decreased. In contrast, at 5 days post-LPS, Cx43 protein expression levels decreased whereas VE-cadherin levels increased compared with all other treatment periods. Apart from the ∼43-kDa Cx43 immunoreactive band, the antibody used in this study also detected a ∼52-kDa band. The identity of the ∼52-kDa band is uncertain. Cx43 immunoreactive band pattern changes detected by the antibody used in this study have been linked to stimulus-induced Cx43 phosphorylation in the lung fibroblast cell line (8). However, the bands representing the phosphorylated Cx43 species are between 34 and 48 kDa (8). Hence, the ∼52-kDa band detected here was likely a result of nonspecific binding.
Fig. 4.
Vascular endothelial (VE)-cadherin expression in LPS-instilled lungs. A–E: confocal images show immunofluorescence of VE-cadherin (green) in microvessels of lungs from rats that were instilled with LPS (2 mg/ml at 1 μl/g body wt) and allowed to recover for the durations shown. Bar, 20 μm (n = 3 lungs each). F and G: bar graphs show VE-cadherin expression quantified along the walls of venules (F) and capillaries (G) in the image field. Nuclei appear in blue. Nuclear staining was omitted in some images to improve clarity of VE-cadherin staining. n = No. of vessels analyzed. 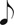 P < 0.05 compared with all groups except for the 24-h treatment group; §P < 0.01 compared with control, 5-day, and 14-day treatment groups; ¶P < 0.001 compared with 14-day treatment group; ‡P < 0.01 compared with 14-day treatment group; ◇P < 0.01 compared with 4-h, 5-day, and 14-day treatment groups; ¤P < 0.01 compared with control treatment group (3 lungs/treatment group). H: rats were instilled with LPS (2 mg/kg) and allowed to recover for 4 h, 24 h, and 5 days. Control rats were instilled with saline (volume 1 μl/g body wt). After the treatment duration, lungs were isolated and the peripheral regions of the lungs dissected and homogenized. The homogenized lung tissue was then prepared for immunoblot analysis of the indicated proteins, as outlined in materials and methods. Arrows point to β-actin, Cx43 (∼43 kDa), and VE-cadherin (∼132 kDa) bands. The Cx43 antibody used in this study also detected a nonspecific band at ∼52 kDa. Lung tissue from 1 rat/treatment period. Treatments and blots were repeated twice.
P < 0.05 compared with all groups except for the 24-h treatment group; §P < 0.01 compared with control, 5-day, and 14-day treatment groups; ¶P < 0.001 compared with 14-day treatment group; ‡P < 0.01 compared with 14-day treatment group; ◇P < 0.01 compared with 4-h, 5-day, and 14-day treatment groups; ¤P < 0.01 compared with control treatment group (3 lungs/treatment group). H: rats were instilled with LPS (2 mg/kg) and allowed to recover for 4 h, 24 h, and 5 days. Control rats were instilled with saline (volume 1 μl/g body wt). After the treatment duration, lungs were isolated and the peripheral regions of the lungs dissected and homogenized. The homogenized lung tissue was then prepared for immunoblot analysis of the indicated proteins, as outlined in materials and methods. Arrows point to β-actin, Cx43 (∼43 kDa), and VE-cadherin (∼132 kDa) bands. The Cx43 antibody used in this study also detected a nonspecific band at ∼52 kDa. Lung tissue from 1 rat/treatment period. Treatments and blots were repeated twice.
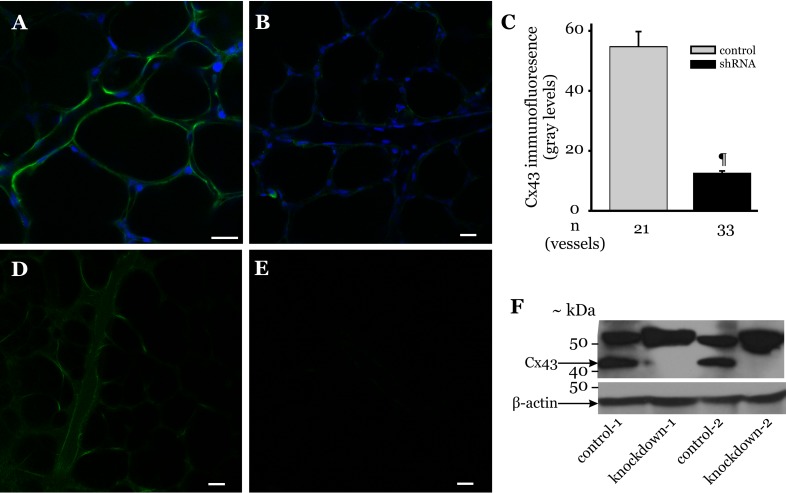
Cx43 knockdown in lung microvessels in vivo.
To determine whether changes in microvessel Cx43 levels correlated with the changes in VE-cadherin expression, we knocked down Cx43 in rat lung microvessels via tail vein injection of the Cx43 shRNA lentiviral vector. After 72 h, lungs were isolated from these animals and probed for microvessel Cx43 expression. In Cx43 shRNA-treated animals, lung microvessel Cx43 immunofluorescence was low (Fig. 5B). Quantifying the immunofluorescence revealed that shRNA treatment reduced microvessel Cx43 expression by >75% compared with that for control treatment (Fig. 5C). To further confirm that vascular injection of lentiviral vectors modifies protein expression in lung microvessels, we injected green fluorescent protein (GFP) lentiviral vector via tail vein of rats. After 72 h postinjection, GFP fluorescence was clearly detectable in microvessels of lungs isolated from these animals (Fig. 5D), suggesting that lentiviral vectors injected into the systemic circulation were transduced into lung microvessels. No fluorescence was evident in microvessels of rats injected with vehicle (Fig. 5E). To supplement the immunofluorescence data, lung tissue was collected from rats treated with Cx43 shRNA-treated lungs and prepared for Western blots, as outlined in materials and methods. The immunoblots also show that Cx43 was completely knocked down in Cx43 shRNA-treated lungs (Fig. 5F), thus supporting the immunofluorescence results. Cx43 knockdown did not alter the band appearing at ∼52 kDa, further confirming that this band detected by the antibody used in this study was nonspecific.
Fig. 5.
Knockdown of Cx43 in intact lung microvessels. A and B: rats were injected with either Cx43 shRNA or vehicle, as outlined in materials and methods. Microvessel Cx43 expression in lungs isolated from these animals was determined by in situ immunofluorescence. Immunofluorescence images show Cx43 expression (green) in vehicle- (A) and Cx43 shRNA-treated lung microvessels (B). Blue staining indicates nuclei. Bar, 20 μm. C: bar graph shows Cx43 expression quantified along margins of microvessels within an image field (>3 images/treatment group were analyzed). n = No. of vessels analyzed. ¶P < 0.001 compared with control treatment (3 lungs/treatment group). D and E: rats injected with P-lenti green fluorescent protein (GFP) lentiviral vector or vehicle, as outlined in materials and methods. Image shows GFP fluorescence in microvessels of lungs isolated from vector- (D) and vehicle-treated rats (E) repeated in 3 animals/treatment group. F: rats were injected with Cx43 shRNA lentiviral vector (1 × 109 TU/ml; 20 μl) and ViraDuctin (2 μl in 100 μl PBS) via the tail vein and allowed to recover for 72 h. Control rats were injected with ViraDuctin (2 μl in 100 μl PBS). After 72 h, the lungs were isolated and the peripheral regions of the lungs dissected and homogenized. The homogenized lung tissue was then prepared for immunoblot analysis of the indicated proteins, as outlined in materials and methods. The Cx43 antibody used in this study also detected a nonspecific band at ∼52 kDa; 2 rats/treatment protocol (indicated by the no. 1 or 2 in the figure). Each lane represents data from 1 rat.
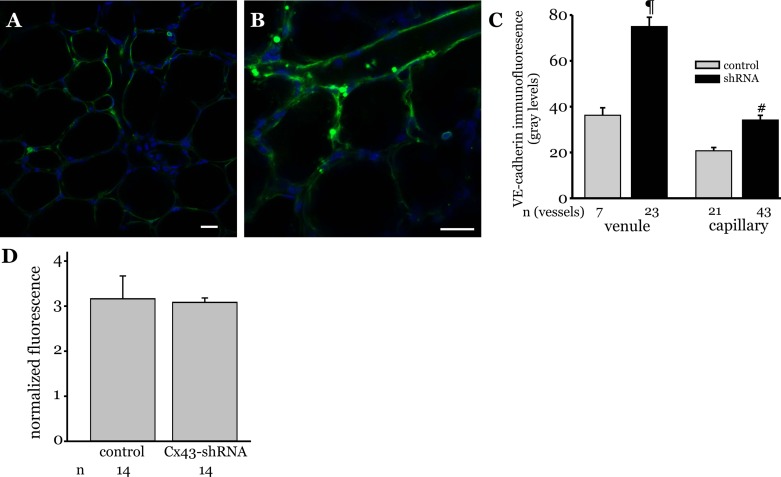
Lung microvessel VE-cadherin expression in Cx43 shRNA-treated rats.
To determine whether reduction in microvessel Cx43 levels modify VE-cadherin expression, we knocked down Cx43 in rat lung microvessels using Cx43 shRNA lentiviral vector and then probed for lung microvessel VE-cadherin expression after 72 h. Cx43 shRNA treatment increased VE-cadherin expression in lung microvessels compared with vehicle treatment (Fig. 6, A and B). Quantifying the immunofluorescence revealed that Cx43 shRNA treatment doubled VE-cadherin expression levels in both venules and capillaries (Fig. 6C), suggesting that reducing Cx43 expression increased VE-cadherin expression in lung microvessels. To determine whether Cx43 knockdown modified microvessel permeability, single lung microvessel permeability was determined using the FDx20 fluorescence method. The ratio of maximal to residual fluorescence was similar in microvessels of Cx43 shRNA-treated lungs compared with vehicle-treated controls, suggesting that Cx43 knockdown did not alter basal permeability in lung microvessels.
Fig. 6.
VE-cadherin expression in lung microvessels of Cx43 shRNA treated rats. A and B: rats were injected with either Cx43 shRNA or vehicle, as outlined in materials and methods. Microvessel VE-cadherin expression in lungs isolated from these animals was determined by in situ immunofluorescence. Immunofluorescence images show VE-cadherin expression (green) in vehicle- (A) and Cx43 shRNA-treated lung microvessels (B). Blue staining indicates nuclei. Bar, 20 μm. C: bar graph shows VE-cadherin expression quantified along margins of venules and capillaries within an image field (>3 images per Cx43 shRNA treatment group and 2 images per vehicle treatment group were analyzed). n = No. of vessels analyzed. ¶ and #P < 0.001 compared with control (3 lungs/treatment). D: rats were injected with Cx43 shRNA lentiviral vector (1 × 109 TU/ml; 20 μl) via the tail vein and allowed to recover for 72 h. After 72 h, isolated blood-perfused lung preparations were used to determine permeability in single microvessels by infusing FDx20 into the microvessels, followed by a 10-min Ringer's washoff, as detailed in materials and methods. Bar graph shows ratio of maximal to residual FDx20 fluorescence (normalized fluorescence) in single lung microvessels of control and lentiviral-treated rats (2 rats/treatment group). Permeability determination was repeated 3 times. n = No. of single microvessels analyzed.
DISCUSSION
This study provides evidence of a novel relationship among Cx43, VE-cadherin, and microvessel barrier integrity. Thus, during recovery from endotoxin-induced lung injury, 1) endothelial Cx43 declined progressively, and this decline occurred in tandem with reductions in microvessel barrier permeability; and 2) changes in microvessel barrier strength and endothelial VE-cadherin levels paralleled each other. Finally, in vivo knockdown of vascular Cx43 resulted in greater VE-cadherin expression in lung microvessels. Together, the data suggest for the first time that, during recovery from lung injury, reduction in endothelial Cx43 levels associates inversely with microvessel VE-cadherin expression and thus restoration of microvessel barrier integrity.
VE-cadherin clustering in AJ at cell-cell contacts is a critical determinant of endothelial barrier integrity (7, 10, 23, 35). Inflammatory agents, including LPS, perturb VE-cadherin clustering, disrupt AJs, and thus destabilize the barrier and promote permeability (5, 9, 17, 30, 33). In contrast, signaling that limit loss of VE-cadherin at AJ promotes endothelial barrier stability (3, 4, 12, 32). Conversely, improvements in endothelial barrier will require restoration of microvessel VE-cadherin expression. The present data show that changes in microvessel VE-cadherin expression directly correlated with changes in microvessel permeability. Thus, the data suggest that the magnitude of microvessel VE-cadherin expression is in itself a marker for microvessel barrier integrity. In addition to VE-cadherin clustering at cell junctions, the actin cytoskeleton, β-catenin, and p120-catenin determine endothelial barrier stability (9, 17, 30, 33). Thus, complete restoration of AJs and hence, microvessel barrier integrity may require reestablishing the balance among the various components involved. The role of the other components and timeline of their changes during recovery needs to be further elucidated.
Cx43 shRNA delivery via tail vein injection reduced Cx43 expression in lung microvessels. Use of shRNA lentiviral vectors for gene knockdown in endothelial cells in vitro is well reported (6, 21, 25). However, for in vivo gene knockdown, other groups have utilized either intranasal or intravenous delivery of shRNA (13, 24, 31, 39). However, the current study provides the first direct evidence of the effectiveness of shRNA delivery into the systemic circulation to knock down genes in lung microvessels. The knockdown efficiency of >75% is comparable with that reported for in vitro knockdown protocols (6, 20). The induction of de novo GFP expression in lung microvessels further supports the conclusion that tail vein delivery of shRNA lentiviral vectors is effective in modulating protein expression in lung microvessels.
Knockdown of Cx43 in lung microvessels increased VE-cadherin expression. Thus, the present findings suggest that loss of Cx43 augments VE-cadherin production in microvessels. Current understanding indicates that connexin expression level is defined by cadherins and other components of AJ. Thus, loss of cadherin or other AJ components induces a concomitant loss of connexin expression (19, 29, 36, 37). However, whether changes in connexin expression lead to changes in cadherin expression hitherto remains unknown. Thus, the present findings show for the first time that reduction in connexin levels correlates with a concomitant increase in cadherin expression.
Gene expression profiling and other studies suggest that Cx43 levels increase during inflammation (1, 11, 25). LPS increases nuclear translocation of NF-κB (14), which regulates Cx43 expression in different tissues (19, 37). LPS also increases endothelial permeability and downregulates VE-cadherin expression, likely via the action of Src kinases (5, 34). Thus, the initial responses following LPS challenge are likely initiated by the direct action of LPS on endothelial cells.
At 24 h post-LPS, Cx43 levels had reversed from their 4-h post-LPS levels and declined below baseline. Recently, it has been postulated that in endothelial cells subjected to endotoxemia, transition from injury to repair phase occurs at about 24 h (20). This time point also marked the switch by NF-κB from proinjury to barrier repair functions. Thus, the decline in endothelial Cx43 levels by 24 h post-LPS is a likely indication that the transition to repair phase had already commenced in these cells.
However, in contrast to Cx43, both VE-cadherin and permeability levels at 24 h post-LPS remained at the 4-h level. Thus, there is a difference in the kinetics of decay and restoration between Cx43 and VE-cadherin. Our Cx43 knockdown experiments suggest that loss of endothelial Cx43 increases VE-cadherin, even in the absence of an inflammatory stimulus. Our previous studies suggest that inhibition of IEC reduces permeability (26). Thus, for VE-cadherin levels to increase and microvessel permeability to decrease, it is likely that Cx43 levels will first have to fall below a certain threshold. The present experiments do not define this threshold, although it is low enough to block IEC. Since this threshold was likely not reached at 24 h, VE-cadherin levels remained at low values, leading to temporal differences in the decay and restoration between Cx43 and VE-cadherin, respectively. Reversal of endotoxemia-induced vascular leak in heart and lungs occurred 3 days after the switch from proinjury to prorepair phase in endothelial cells (20). These data are in agreement with the present findings that VE-cadherin levels and microvessel permeability returned to baseline 3 days after the beginning of the decline in Cx43 levels.
The above interpretation for the lack of synchrony in changes between Cx43 and VE-cadherin inherently assumes that loss of Cx43 leads to VE-cadherin increase and microvessel barrier improvement. This assumption is based on the data that Cx43 depletion increases VE-cadherin levels. This augmentation of VE-cadherin occurred despite the absence of an inflammatory stimulus. These data imply that the increase in VE-cadherin is initiated by an inflammation-independent transcription event, which requires further elucidation.
Although vascular Cx43 knockdown augmented VE-cadherin expression in lung microvessels, the baseline microvessel permeability was similar to that for Cx43-containing microvessels. Thus, augmenting VE-cadherin does not further reduce permeability below baseline levels. This interpretation is supported by data showing that the increase in VE-cadherin levels above control at 14 days post-LPS does not lower microvessel permeability below control levels. It is likely that the increase in VE-cadherin that follows loss of IEC is effective only in lowering hyperpermeability due to LPS or inflammatory stimuli. However, the transient knockdown model used in this study limits testing this possibility. Inducible gene knockdown animal models may be better suited to address this issue.
During recovery, changes in Cx43 and VE-cadherin expressions were greater in capillaries compared with that in venules. Thus, at 5 days post-LPS, Cx43 expression declined about 10-fold below control in capillaries compared with a threefold decline in venules. However, the absolute expression levels were lower in capillaries. In addition, permeability was only marginally different between capillaries and venules at the different time points. Thus, it is possible that the lower protein expression levels required larger changes to elicit similar reductions in permeability in capillaries.
In conclusion, our data reveal that endothelial Cx43 and VE-cadherin levels vary during recovery from endotoxin-induced lung injury. In addition, reductions in Cx43 levels paralleled the decline in microvessel permeability. Reductions in Cx43 during both recovery and knockdown of Cx43 using shRNA led to increased expression of VE-cadherin in lung microvessels. Together, the data strongly allude to the possibility that endothelial Cx43 and thus IEC play a major role in restoring endothelial barrier integrity during recovery from injury.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-75503, startup funds from Department of Physiology, University of Tennessee Health Science Center (UTHSC), and a grant incentive from the Office of Research, UTHSC, to K. Parthasarathi.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.K., R.E., J.M., A.A., and K.P. performed experiments; K.K., R.E., and K.P. analyzed data; K.K., A.A., and K.P. interpreted results of experiments; K.K. and K.P. prepared figures; K.K. drafted manuscript; K.K. and K.P. edited and revised manuscript; K.K., R.E., A.A., and K.P. approved final version of manuscript; K.P. conception and design of research.
ACKNOWLEDGMENTS
We sincerely thank Micheal Nguyen for constructing the animal surgery and instillation platforms and the instillation devices used in this study.
REFERENCES
- 1.Altemeier WA, Matute-Bello G, Gharib SA, Glenny RW, Martin TR, Liles WC. Modulation of lipopolysaccharide-induced gene transcription and promotion of lung injury by mechanical ventilation. J Immunol 175: 3369–3376, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharya J, Matthay MA. Regulation and repair of the alveolar-capillary barrier in acute lung injury. Annu Rev Physiol 75: 593–615, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Birukova AA, Tian X, Tian Y, Higginbotham K, Birukov KG. Rap-afadin axis in control of Rho signaling and endothelial barrier recovery. Mol Biol Cell 24: 2678–2688, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bogatcheva NV, Zemskova MA, Kovalenkov Y, Poirier C, Verin AD. Molecular mechanisms mediating protective effect of cAMP on lipopolysaccharide (LPS)-induced human lung microvascular endothelial cells (HLMVEC) hyperpermeability. J Cell Physiol 221: 750–759, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatterjee A, Snead C, Yetik-Anacak G, Antonova G, Zeng J, Catravas JD. Heat shock protein 90 inhibitors attenuate LPS-induced endothelial hyperpermeability. Am J Physiol Lung Cell Mol Physiol 294: L755–L763, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Chava KR, Tauseef M, Sharma T, Mehta D. Cyclic AMP response element-binding protein prevents endothelial permeability increase through transcriptional controlling p190RhoGAP expression. Blood 119: 308–319, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corada M, Zanetta L, Orsenigo F, Breviario F, Lampugnani MG, Bernasconi S, Liao F, Hicklin DJ, Bohlen P, Dejana E. A monoclonal antibody to vascular endothelial-cadherin inhibits tumor angiogenesis without side effects on endothelial permeability. Blood 100: 905–911, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Cruciani V, Mikalsen SO. Stimulated phosphorylation of intracellular connexin43. Exp Cell Res 251: 285–298, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Di Lorenzo A, Lin MI, Murata T, Landskroner-Eiger S, Schleicher M, Kothiya M, Iwakiri Y, Yu J, Huang PL, Sessa WC. eNOS-derived nitric oxide regulates endothelial barrier function through VE-cadherin and Rho GTPases. J Cell Sci 126: 5541–5552, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giannotta M, Trani M, Dejana E. VE-cadherin and endothelial adherens junctions: active guardians of vascular integrity. Dev Cell 26: 441–454, 2013. [DOI] [PubMed] [Google Scholar]
- 11.Grigoryev DN, Ma SF, Irizarry RA, Ye SQ, Quackenbush J, Garcia JG. Orthologous gene-expression profiling in multi-species models: search for candidate genes. Genome Biol 5: R34, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grinnell KL, Chichger H, Braza J, Duong H, Harrington EO. Protection against LPS-induced pulmonary edema through the attenuation of protein tyrosine phosphatase-1B oxidation. Am J Respir Cell Mol Biol 46: 623–632, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin LY, Li CF, Zhu GF, Wu CT, Wang J, Yan SF. Effect of siRNA against NF-κB on sepsis-induced acute lung injury in a mouse model. Mol Med Rep 10: 631–637, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kandasamy K, Bezavada L, Escue RB, Parthasarathi K. Lipopolysaccharide induces endoplasmic store Ca2+-dependent inflammatory responses in lung microvessels. PLoS One 8: e63465, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kandasamy K, Parthasarathi K. Quantifying single microvessel permeability in isolated blood-perfused rat lung preparation. J Vis Exp 30: e51552, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kandasamy K, Sahu G, Parthasarathi K. Real-time imaging reveals endothelium-mediated leukocyte retention in LPS-treated lung microvessels. Microvasc Res 83: 323–331, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knezevic N, Tauseef M, Thennes T, Mehta D. The G protein betagamma subunit mediates reannealing of adherens junctions to reverse endothelial permeability increase by thrombin. J Exp Med 206: 2761–2777, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Komarova Y, Malik AB. Regulation of endothelial permeability via paracellular and transcellular transport pathways. Annu Rev Physiol 72: 463–493, 2010. [DOI] [PubMed] [Google Scholar]
- 19.Liao CK, Jeng CJ, Wang HS, Wang SH, Wu JC. Lipopolysaccharide induces degradation of connexin43 in rat astrocytes via the ubiquitin-proteasome proteolytic pathway. PLoS One 8: e79350, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu G, Ye X, Miller EJ, Liu SF. NF-κB-to-AP-1 switch: a mechanism regulating transition from endothelial barrier injury to repair in endotoxemic mice. Sci Rep 4: 5543, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu SF, Malik AB. NF-κB activation as a pathological mechanism of septic shock and inflammation. Am J Physiol Lung Cell Mol Physiol 290: L622–L645, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest 122: 2731–2740, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev 86: 279–367, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Morizono K, Xie Y, Ringpis GE, Johnson M, Nassanian H, Lee B, Wu L, Chen IS. Lentiviral vector retargeting to P-glycoprotein on metastatic melanoma through intravenous injection. Nat Med 11: 346–352, 2005. [DOI] [PubMed] [Google Scholar]
- 25.O'Donnell JJ 3rd, Birukova AA, Beyer EC, Birukov KG. Gap junction protein connexin43 exacerbates lung vascular permeability. PLoS One 9: e100931, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parthasarathi K. Endothelial connexin43 mediates acid-induced increases in pulmonary microvascular permeability. Am J Physiol Lung Cell Mol Physiol 303: L33–L42, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parthasarathi K, Bhattacharya J. Localized acid instillation by a wedged-catheter method reveals a role for vascular gap junctions in spatial expansion of acid injury. Anat Rec (Hoboken) 94: 1585–1591, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parthasarathi K, Ichimura H, Monma E, Lindert J, Quadri S, Issekutz A, Bhattacharya J. Connexin 43 mediates spread of Ca2+-dependent proinflammatory responses in lung capillaries. J Clin Invest 116: 2193–2200, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rahman A, Fazal F. Blocking NF-κB: an inflammatory issue. Proc Am Thorac Soc 8: 497–503, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rajput C, Kini V, Smith M, Yazbeck P, Chavez A, Schmidt T, Zhang W, Knezevic N, Komarova Y, Mehta D. Neural Wiskott-Aldrich syndrome protein (N-WASP)-mediated p120-catenin interaction with Arp2-Actin complex stabilizes endothelial adherens junctions. J Biol Chem 288: 4241–4250, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sawant DA, Tharakan B, Hunter FA, Smythe WR, Childs EW. Role of β-catenin in regulating microvascular endothelial cell hyperpermeability. J Trauma 70: 481–487; discussion 487–488, 2011. [DOI] [PubMed] [Google Scholar]
- 32.Schulte D, Kuppers V, Dartsch N, Broermann A, Li H, Zarbock A, Kamenyeva O, Kiefer F, Khandoga A, Massberg S, Vestweber D. Stabilizing the VE-cadherin-catenin complex blocks leukocyte extravasation and vascular permeability. EMBO J 30: 4157–4170, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Setyawati MI, Tay CY, Chia SL, Goh SL, Fang W, Neo MJ, Chong HC, Tan SM, Loo SC, Ng KW, Xie JP, Ong CN, Tan NS, Leong DT. Titanium dioxide nanomaterials cause endothelial cell leakiness by disrupting the homophilic interaction of VE-cadherin. Nat Commun 4: 1673, 2013. [DOI] [PubMed] [Google Scholar]
- 34.Severgnini M, Takahashi S, Tu P, Perides G, Homer RJ, Jhung JW, Bhavsar D, Cochran BH, Simon AR. Inhibition of the Src and Jak kinases protects against lipopolysaccharide-induced acute lung injury. Am J Respir Crit Care Med 171: 858–867, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Vincent PA, Xiao K, Buckley KM, Kowalczyk AP. VE-cadherin: adhesion at arm's length. Am J Physiol Cell Physiol 286: C987–C997, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Wei CJ, Francis R, Xu X, Lo CW. Connexin43 associated with an N-cadherin-containing multiprotein complex is required for gap junction formation in NIH3T3 cells. J Biol Chem 280: 19925–19936, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Wu X, Huang W, Luo G, Alain LA. Hypoxia induces connexin 43 dysregulation by modulating matrix metalloproteinases via MAPK signaling. Mol Cell Biochem 384: 155–162, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yeh HI, Rothery S, Dupont E, Coppen SR, Severs NJ. Individual gap junction plaques contain multiple connexins in arterial endothelium. Circ Res 83: 1248–1263, 1998. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Jiang G, Sauler M, Lee PJ. Lung endothelial HO-1 targeting in vivo using lentiviral miRNA regulates apoptosis and autophagy during oxidant injury. FASEB J 27: 4041–4058, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]