Abstract
The time required for the effective clearance of pleural adhesions/organization after intrapleural fibrinolytic therapy (IPFT) is unknown. Chest ultrasonography and computed tomography (CT) were used to assess the efficacy of IPFT in a rabbit model of tetracycline-induced pleural injury, treated with single-chain (sc) urokinase plasminogen activators (scuPAs) or tissue PAs (sctPA). IPFT with sctPA (0.145 mg/kg; n = 10) and scuPA (0.5 mg/kg; n = 12) was monitored by serial ultrasonography alone (n = 12) or alongside CT scanning (n = 10). IPFT efficacy was assessed with gross lung injury scores (GLIS) and ultrasonography scores (USS). Pleural fluids withdrawn at 0–240 min and 24 h after IPFT were assayed for PA and fibrinolytic activities, α-macroglobulin/fibrinolysin complexes, and active PA inhibitor 1 (PAI-1). scuPA and sctPA generated comparable steady-state fibrinolytic activities by 20 min. PA activity in the scuPA group decreased slower than the sctPA group (kobs = 0.016 and 0.042 min−1). Significant amounts of bioactive uPA/α-macroglobulin (but not tPA; P < 0.05) complexes accumulated at 0–40 min after IPFT. Despite the differences in intrapleural processing, IPFT with either fibrinolysin was effective (GLIS ≤ 10) in animals imaged with ultrasonography only. USS correlated well with postmortem GLIS (r2 = 0.85) and confirmed relatively slow intrapleural fibrinolysis after IPFT, which coincided with effective clearance of adhesions/organization at 4–8 h. CT scanning was associated with less effective (GLIS > 10) IPFT and higher levels of active PAI-1 at 24 h following therapy. We concluded that intrapleural fibrinolysis in tetracycline-induced pleural injury in rabbits is relatively slow (4–8 h). In CT-scanned animals, elevated PAI-1 activity (possibly radiation induced) reduced the efficacy of IPFT, buttressing the major impact of active PAI-1 on IPFT outcomes.
Keywords: fibrinolytic therapy, fibrinolysis, plasminogen activator inhibitor 1, pleural injury, urokinase, tissue plasminogen activator
intrapleural fibrinolytic therapy (IPFT) activates the endogenous fibrinolytic system, resolving intrapleural adhesions and complex fibrinous deposits that sequester pockets of inflammation loculations, thus improving drainage and clinical outcome, in part by decreasing surgical interventions (10). There are multiple reports of successful (88–100% efficacy) IPFT in adult (1, 7, 8, 16, 17, 29, 42, 47, 48, 66, 67, 72) and in pediatric (2, 3, 6, 11, 20, 40, 41, 58, 59, 61, 63, 67) patients with empyema although outcomes in adult patients are inconsistent in these trials. Thus IPFT represents a less invasive and costly alternative to video-assisted thoracic surgery or other surgical interventions, which demonstrate comparable efficacy (16, 44, 45, 58, 60, 71) in pediatric practice. IPFT also represents a preferred choice in high-risk (1, 22) and otherwise inoperable patients (10, 43, 49, 56, 57, 69) or those with empyema/loculation who refuse surgery. The reasons for the inconsistent results of IPFT in adult patients remain unclear but likely reflect the empiric approaches that are currently used. Current dosing of IPFT differs by up to two orders of magnitude, variable dwell times (1–4 h) of chest tube clamping after IPFT, and dosing schedules (1–8 treatments, every 4–24 h) (1, 4–7, 15, 16, 28, 46, 50, 53, 58, 61, 65, 66). The empiricism in large part is attributable to the lack of formal toxicological and dose-escalation studies of presently used IPFT agents in humans and our relatively limited understanding of the molecular mechanisms governing the efficacy of IPFT.
The tetracycline (TCN)-induced model of pleural injury in rabbits is characterized by extensive pleural adhesions and exudative pleural fluids (PFs) that are reliably induced within 24 h of injury with relatively little variability in individual rabbits (23, 24, 27). Although TCN-induced injury recapitulates the features of pleural injury in humans, there are certain limitations, which preclude a direct correlation with infectious injury in empyema. The rabbit model offers fundamental advantages for the evaluation of IPFT with human tissue plasminogen (PLG) activators (tPAs) and urokinase PAs (uPAs). Unlike mouse fibrin, the structure of rabbit fibrin is similar to that in humans (52). Mouse PA inhibitor 1 (PAI-1) notably differs from human PAI-1 (12), and human uPA possesses poor affinity to the murine uPA receptor (30). The TCN-induced model of pleural injury has therefore been successfully used to evaluate the intrapleural processing of single-chain uPA (scuPA) (35) and to improve the efficacy of IPFT by targeting active PAI-1 (14).
Previously, we showed that the fibrinolytic activity in the first 1.5 h after IPFT does not determine outcome and hypothesized that the time of successful fibrinolysis in TCN-induced pleural injury in rabbits is longer than 2 h (14, 35). We also found that the intrapleural level of active PAI-1 dramatically affects the half-life of fibrinolysins and outcome of IPFT and that in vivo PAI-1 neutralization improves IPFT outcomes and decreases the required effective dose of a fibrinolysin (14). In the present study, we used chest ultrasonography (US) and computed tomography (CT) imaging modalities to define the time course for effective intrapleural fibrinolysis after IPFT by using known minimal effective doses (MED) of sc tissue PA (sctPA) and scuPA in our well-characterized TCN-induced pleural injury in rabbits (14, 24, 31, 35). We found that the time needed for effective fibrinolysis for sctPA and scuPA were similar and protracted over several hours. Moreover, serial CT scanning in the TCN-induced model of pleural injury results in a local increase in the level of active PAI-1, which decreases the efficacy of IPFT.
MATERIALS AND METHODS
Proteins and reagents.
The scuPA used in this study was a generous gift from Dr. Jack Henkin, Abbott Laboratories (Chicago, IL). The activity standard for human two-chain (tc) uPA (100,000 IU/mg) was from Sekisui Diagnostics (Stanford, CT). Human recombinant sctPA (Activase) was from Genentech (San Francisco, CA). Wild-type recombinant human PAI-1, human fibrinogen, and FITC fibrinogen (3 mol fluorescein/mol of fibrinogen) were from Molecular Innovations (Novi, MI). Fluorogenic uPA and tPA substrates (Pefafluor uPA and tPA, respectively) were purchased from Centerchem (Norwalk, CT). Fluorogenic plasmin substrate, PLG, and plasmin were from Haematologic Technologies (HTI, Essex Junction, VT). Protein concentrations were determined using a BCA protein assay kit (Pierce, Rockford, IL). All in vitro and ex vivo experiments were carried out at pH 7.4 in either 50 mM phosphate or Hepes/NaOH buffers, with or without BSA (1 mg/ml).
Rabbit model of TCN-induced pleural injury.
All experiments involving animals were approved by the Institutional Animal Care and Utilization Committee of The University of Texas Health Science Center at Tyler and conformed to all applicable NIH guidelines. Female New Zealand white rabbits, weighing 3.0–3.6 kg, were used (n = 27). Pleural injury was induced by intrapleural administration of a single dose of TCN, as previously reported (14, 24). Single intrapleural doses of either scuPA (Abbott Laboratories) or tPA (Activase, Genentech), known to clear pleural organization (MEDs 0.5 mg/kg and 0.145 mg/kg, respectively) in the model (in n = 12 and 10 rabbits, respectively), or a vehicle control (n = 3) were administered 48 h after induction of pleural injury by TCN (26). Two animals were excluded from the sctPA/CT group; one had a large pneumothorax induced at the time of TCN administration precluding efficient collection, whereas TCN administration was unsuccessful in a different animal assigned to the US/CT group. The control vehicle animals received TCN-induced pleural injury but were not imaged. Treatments were administered using a catheter (18 gauge, 1.25 inches in length), which was cleared using 0.5 ml PBS. Anesthesia and postoperative pain medication were administered, as previously reported (31). During each preoperative and postoperative period, rabbits were carefully monitored for signs of overt pain or distress to ensure animal stability and comfort. Euthesol (0.25 ml/kg), administered intravenously, followed by exsanguination via the renal arteries, was used for euthanasia performed 24 h following the intrapleural interventions.
US was performed for all rabbits at 0, 2, 8, and 24 h (n = 22), as previously described (14). Additional US imaging was performed at 4 h (n = 16; scuPA/US n = 3, scuPA/US/CT n = 6, sctPA/US n = 3, sctPA/US/CT n = 4) and at 12 h (n = 12; scuPA/US n = 4, scuPA/US/CT n = 3, sctPA/US n = 3, sctPA/US/CT n = 2). Aliquots of PF were collected at 0 and 24 h from all animals (n = 22; scuPA/US n = 6; scuPA/US/CT n = 6; sctPA/US n = 6, sctPA/US/CT n = 4), at 10, 20, and 40 min (n = 16; scuPA/US n = 5, scuPA/US/CT n = 3, sctPA/US n = 6, sctPA/US/CT n = 2), and at 4 h (n = 5; scuPA/US/CT n = 3, sctPA/US/CT n = 2). Citrated cell-free fluids were immediately aliquoted and stored at −80°C (35). Four animals treated with intrapleural sctPA and six animals treated with scuPA were imaged with four sequential CT scans. CT was performed at baseline (before TCN injury), at an injured baseline (48 h after TCN and immediately before IPFT), and at 24 h after IPFT. Additional CT imaging was done after pleural effusions were evacuated using a 20-ml plastic syringe at 24 h. At 24 h after administration of the intrapleural interventions, the rabbits were euthanized, and gross outcomes of IPFT were visualized and scored using the gross lung injury score (GLIS) as previously described (14, 24, 31, 35).
US outcome assessments.
The progression of pleural injury was imaged by serial chest diagnostic and cine US in gently restrained, awake animals at 0, 2, 4, 8, 12, and 24 h after intrapleural delivery of either fibrinolysin. B-mode US was performed using the Logic e system (GE Healthcare, Milwaukee, WI), equipped with R5.2.x software and a multi-frequency transducer model 12L-RS (3.0–10.0 MHz) at a working frequency of 10 MHz (14). The right pleural space was scanned using the same mid-chest transverse and longitudinal planes in each prone animal. All sonographic imaging was performed by the same operator. A US score (USS) was developed based on the scoring of still images derived from the cine motion studies with atelectasis/consolidated lung associated with diffuse intrapleural density/fluid collections equivalent to a score of 50; lung attached to the heart or chest wall by fibrin strands and/or collections of solid intrapleural material scored 25; detectable but reduced visualization of retained collections scored 10; residual single strands or small webs scored 5; complete clearance, with no pleural adhesions, scored 0.
CT imaging.
CT imaging was done before induction of pleural injury, at 48 h after TCN but before IPFT administration, and at 72 h after removal of all PF amenable to aspiration after TCN but before death. CT images were obtained using a GE Discovery 750HD 64-slice CT scanner (General Electric Healthcare, Little Chalfont, UK), which reduces breathing artifact, with a technique of 80 kV, 320 mA, 2-mm slice thickness, and a lung window reconstruction algorithm. Images were imported into the treatment-planning software Eclipse (Varian Medical Systems, Palo Alto, CA) in which the lungs were outlined and lung volumes were recorded. Morphometric determinations of pleural thickness and the depth of underlying pneumonitis were assessed as previously described (70).
Metrics of pleural injury.
GLIS were determined at necropsy, 24 h after IPFT for each animal, as described (31, 35). The autopsy of each animal was carried out by the same surgeon, who did not see the results of the US until this study was being prepared for submission. Two to three different scientists performed GLIS scoring evaluation simultaneously with the surgeon averaging all of the data. Pleural injury at 24-h autopsy was also photographed as described previously (31). IPFT was considered successful with a GLIS < 10. Multiple visceral-parietal interconnected fibrin webs and sheets or “too numerous to count” strands correlated with a GLIS = 50. In morphometric analyses, rabbit lung tissue slides stained with hematoxylin and eosin were examined using a Nikon Eclipse Ti Microscope. Morphometric analyses of pleural thickness were obtained in 30 fields per animal studied at ×20 magnification. The thickness of the pleura was measured from the basement membrane to the external border of the visceral pleura on the ipsilateral side of the pleural injury.
uPA and tPA amidolytic activity assay.
The amidolytic activities of uPA and tPA in PFs were measured and analyzed as described elsewhere (37). Briefly, 50 μl of 0.2 mM fluorogenic substrate (Pefafluor uPA or tPA, respectively; Centerchem) in DPBS with 1 mg/ml BSA was added to samples of PF (0.05–2.00 ml) in 50 μl of the same buffer in white, 96-well, flat-bottom plates from Costar (Corning, NY). An increase in the fluorescence emission with time was monitored using a Varian Cary Eclipse fluorescence spectrophotometer (Varian, Lincolnshire, IL).
Formation of PA/α-macroglobulin complexes.
The observed first-order rate constants (kobs) for the intrapleural formation of α-macroglobulin (αM)/PA were determined from measurements of the amidolytic activities of uPA and tPA in PF in the presence of 20–50-fold excess of exogenous human PAI-1, as described elsewhere (35). The amidolytic activity in samples of PFs was measured before and after supplementation with recombinant human PAI-1, with values corresponding to the activities of αM/PA and total PA, respectively. A single exponential equation was fit to the time-dependences of the concentration of active free PA ([free PA] = [total PA] − [αM/PA]) or αM/PA using SigmaPlot 12.0.
PAI-1 activity assay.
Active PAI-1 in the PFs was determined by titrating the active inhibitor with solutions of uPA of known concentration, after which residual uPA amidolytic or PA activity was measured as previously described (36, 37).
PLG activation assay.
PA activity was measured as we previously reported (36). Briefly, PF (0.1–2.0 μl) was added to the mixture of Glu-PLG (100–250 nM) and fluorogenic plasmin substrate (SN-5) (0.2–0.5 mM) in 0.05 M phosphate buffer, pH 7.4, with 1 mg/ml BSA.
Measurements of fibrinolytic activity in PFs.
Fibrinolytic activity in PFs was measured using a FITC-fibrin assay as described previously (14, 36). Briefly, 100 μl of an equimolar mixture of unlabeled and FITC (3:1) fibrinogen (Molecular Innovations) (0.4 mg/ml) in 0.05 M Hepes/NaOH (pH 7.4, 20 mM NaCl, 5 mM CaCl2, room temperature) was transferred to black, 96-well flat-bottom plates from Costar. Human thrombin (10 μl, 10 nM) was added to each well and mixed. Plates were then dried overnight at room temperature in the dark. Formed FITC-fibrin films were washed three times with 0.3 ml of cold Hepes/NaOH buffer and stored at −20°C. Endogenous fibrinolytic activity in PFs was proportional to the rate of the increase in fluorescence emission of fluorescein at 512 nm (excitation at 490 nm) because of dequenching of fluorescein residues during degradation of FITC-fibrin by PFs.
Data analysis and statistics.
Levels of statistical significance were determined using Kruskal-Wallis one-way ANOVA on ranks and pairwise multiple-comparison procedures (Holm-Sidak method and Tukey's test). Data analysis was performed using SigmaPlot, v. 11, as previously described (14, 25).
RESULTS
Outcomes of IPFT in TCN-induced pleural injury in rabbits are adversely affected by imaging with multiple chest CT scans.
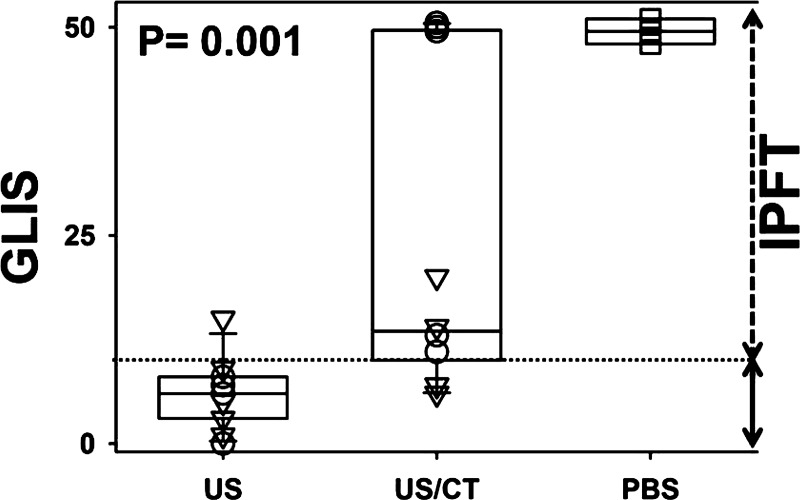
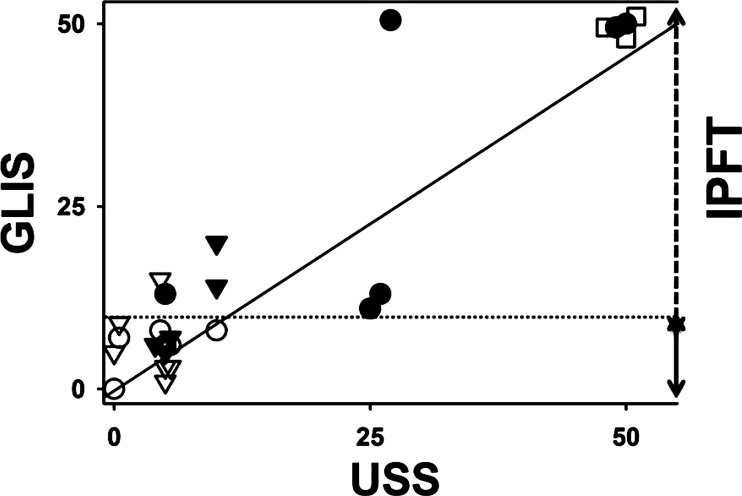
Animals with TCN-induced pleural injury were imaged with either chest US or US/CT, as described in materials and methods. Surprisingly, GLIS levels indicative of effective IPFT (GLIS ≤ 10) were found in rabbits imaged with US alone, whereas scores indicative of ineffective IPFT (10 < GLIS ≤ 50) were found in rabbits imaged with both US and serial chest CT scanning. The distributions of GLIS for the three groups of animals are shown in Fig. 1 [controls without treatment with PA (n = 3); animals treated with MED of fibrinolysins and imaged with US only (n = 12) and with US and CT (n = 10)]. Animals imaged with US/CT and treated with sctPA demonstrated a trend toward higher GLIS, and there was a statistically significant increase in GLIS (P < 0.05) indicative of unsuccessful IPFT (GLIS > 10) in the scuPA group (Fig. 1). As expected, the GLIS in the TCN-injured, imaging vehicle control group animals (n = 3) was maximal (indicating adhesions that were too numerous to count in each animal, GLIS = 50) (Fig. 1). Therefore, addition of serial chest CT imaging to chest US is associated with a decrease in the efficacy of IPFT in TCN-induced pleural injury and adversely impacts outcomes in animals treated with otherwise effective intrapleural doses of PAs. In all animals, injury resolution after IPFT was assessed using chest US (14), when sequentially applied over 24 h, which detected adhesions, lung consolidation/atelectasis, and pleural effusions (Fig. 2). The pleural thickness was below the limit of detection for US and CT in TCN-induced pleural injury in rabbits. By morphometric analyses (data not shown), no significant differences in the extent of pleural thickening were observed between the tPA- or scuPA-treated groups. The contralateral uninjured lungs did not typically demonstrate pleural thickening or pneumonitis although small amounts of PF were generally present in the contralateral left hemithorax attributable to the partial communication between the hemithoraces that is usually found in rabbits. We also tested the in vivo USS at 24 h and postmortem visual-based assessments (GLIS) for correlation (Fig. 3). The USS at 24 h correlated well (r2 = 0.85; Fig. 3) with GLIS, indicating that the USS comparably reflects the severity and extent of pleural injury in the same animals at 24 h after IPFT and immediately before euthanasia.
Fig. 1.
The effect of chest ultrasonography (US) alone and US combined with chest computed tomography (CT) (US/CT) on outcomes of single-chain (sc) urokinase plasminogen (PLG) activator (scuPA) and sc tissue PA (sctPA) intrapleural fibrinolytic therapy (IPFT) in rabbits with tetracycline (TCN)-induced pleural injury. Outcomes of IPFT with a minimal effective dose (MED) of scuPA or sctPA, assessed at 24 h by gross lung injury scores (GLIS) are shown. GLIS values were calculated as previously described (14, 31, 35) for animals treated with intrapleural MEDs of scuPA (n = 12, ○), sctPA (n = 10, ▽), or PBS vehicle (n = 3, □). Data are presented as box plots for animals subjected to US alone or US/CT. The range of GLIS values representing effective (GLIS ≤ 10) and ineffective (10 < GLIS ≤ 50) IPFT are shown as solid and dashed arrows on the right, respectively. Ineffective IPFT corresponds to values above the dotted line, GLIS = 10 (14, 31, 35). The boxes illustrate the interquartile ranges with whiskers showing 5% and 95% values, and the P value represents results for Kruskal-Wallis ANOVA rank tests. Pairwise multiple-comparison procedures (Dunn's method) showed a statistical difference (P < 0.05) for US vs. US/CT animals. There was no statistical difference between US/CT and PBS groups.
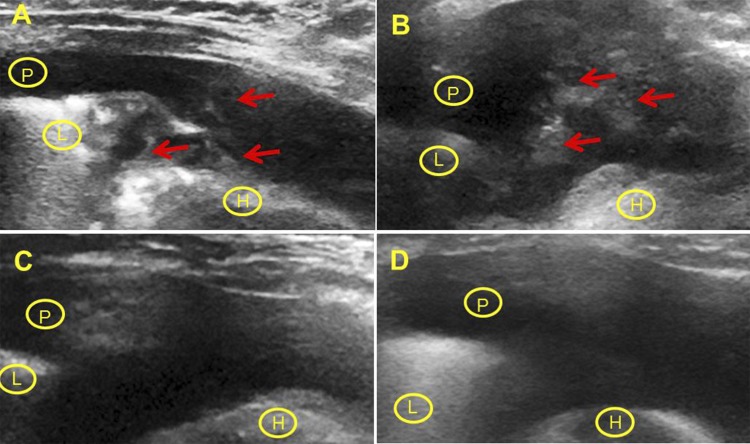
Fig. 2.
US imaging of the resolution of pleural injury after IPFT. Resolution of pleural injury was assessed using serial US imaging after IPFT. Static images representative of those derived from the cine imaging of the injured right hemithorax are shown. In each case, images that best recapitulated baseline imaging planes were used at each interval. Representative still images of injury before IPFT (score 50) (A), 4 h after treatment (score 25) (B), 8 h (score 10) and 24 h after treatment (score 0) with 0.5 mg/kg of scuPA (C and D, respectively) are shown. The underlying pleural effusion (P) provides an acoustic window for imaging the underlying lung (L) and heart (H). Arrows (red) indicate pleural adhesions/collections that are generally fibrous and organizing (27).
Fig. 3.
A strong linear correlation between GLIS and US scores (USS). USS were determined at 24 h after IPFT using the definitions described in materials and methods and Fig. 2 and plotted against GLIS (Fig. 1). The values of GLIS and USS for animals treated with scuPA, sctPA, and vehicle controls are shown as ● and ○, ▼ and ▽, and □, respectively. Open and closed symbols correspond to imaging with US alone and combined with CT, respectively. A solid line represents the best fit of a linear equation, USS = 0.92GLIS − 0.25, to the data (r2 = 0.85). The dotted line (GLIS = 10) and the right axis represent the ranges of GLIS for successful (GLIS < 10) and unsuccessful (10 < GLIS ≤ 50) IPFT.
USS at 0–24 h after IPFT reports slow fibrinolysis with MEDs of sctPA and scuPA and confirms adverse outcomes in animals imaged with serial CT.
USS was next used to evaluate and assess the severity of the pleural injury before and at 2, 4, 8, 12, and 24 h after IPFT. USS were determined for each intervention with US imaging alone and in combination with CT and plotted against time (Fig. 4, A and B, respectively). Reduction of USS was detectable at 2–4 h after intrapleural delivery of either fibrinolysin and approached the level of effective IPFT (USS ≤ 10) by 4–8 h in 11/12 animals imaged with US alone (Fig. 4A). However, the USS at 2–24 h also demonstrated a trend toward worsening injury in animals imaged with both US and CT, with most animals in this group showing ineffective responses (GLIS > 10) by 12 h (Fig. 4B). In contrast to the group scanned with US alone, there were no animals with either USS = 0 or GLIS = 0 in the CT group at 24 h (Fig. 4). Moreover, five US/CT animals had USS >10 (and comparable GLIS scores; Figs. 1 and 3) at 24 h, likewise indicative of unsuccessful IPFT. Together, these findings indicate that initial detection of pleural organization resolution by US was relatively protracted and only detectable hours after either tPA- or scuPA-based IPFT. Moreover, the changes in USS observed with MED scuPA and tPA (Fig. 4A) noninvasively define the time of effective fibrinolysis (TEF; the minimal time required to achieve successful clearance of pleural adhesions; USS ≤ 10). The TEFs ranged from 4–8 h (Fig. 4A), irrespective of the fibrinolysin used. Notably, multiple CT scans affected the outcomes of IPFT with MEDs of both fibrinolysins (Fig. 4B), strongly suggesting that intrapleural fibrinolysis was impaired.
Fig. 4.
Time course of the clearance of pleural adhesions after IPFT by USS. Changes in the USS with time following IPFT with MEDs of either scuPA (n = 6, ○) and sctPA (n = 6, ▽) with US imaging only (A) and in combination with serial CT scanning (scuPA, n = 6, ○, and sctPA, n = 4, ▽) (B). 48 h after induction of pleural injury with TCN, animals were treated with 0.5 mg/kg scuPA (n = 12) or 0.145 mg/kg tPA (n = 10), and the level of injury was assessed using US, as described in materials and methods, and as previously reported (14). The dotted line (USS = 10) and the right axis represent the ranges of USS for successful (USS < 10) and unsuccessful (10 < USS ≤ 50) IPFT. The data are presented as box plots using the same format as described in the legend of Fig. 1.
As anticipated, CT assessment demonstrated clear evidence of pleural injury, with readily apparent collections of PF associated with complex densities that were difficult to distinguish from parenchymal injury or atelectasis of the underlying lungs (Fig. 5). CT images clearly demonstrate changes in the affected lung and pleural space after induction of the injury and injured lung expansion after successful IPFT, which becomes evident upon PF drainage (Fig. 5, A–C). Moreover, CT image-based reconstruction of lung volumes (Fig. 5B) visualizes and supports these conclusions. However, a box plot of the reconstructed lung volumes (Fig. 5C) demonstrates insignificant changes in lung volume after treatment, whereas lung volumes after injury were reduced vs. baseline levels (P < 0.001). The inability of CT to discern changes in lung volume after IPFT related to the presence of large (>30 ml) pleural effusions, as confirmed by detection of anticipated increments in CT-derived lung volume after complete drainage of the injured right hemithorax. Although GLIS and USS more readily detect the outcomes of IPFT than CT imaging, they do not explain the underlying causes for the disparate responses to IPFT in US- vs. US/CT-imaged animals. To address this issue, intrapleural PA processing was evaluated in each group and compared to understand the basis for the relatively poorer outcomes of IPFT with combined US/CT imaging.
Fig. 5.
Assessment of the development and resolution of pleural injury using chest CT. A: total lung volumes of the injured right hemithoraces were determined by CT-derived reconstructions as described in materials and methods, with total lung volumes for scuPA-treated animals (n = 6) and sctPA-treated animals (n = 4) at baseline (I), 48 h after TCN-induced injury (II, injured), 24 h after treatment (III, without drainage of accessible pleural fluid, PF), or at 24 h after removal of accessible PF (IV, drained). Representative images are shown (of a total of 10 animals imaged with US/CT). B: representative thoracic CT images (2-mm slice thickness) are from a tPA-treated rabbit including cursor outlines (in blue) of the aerated lung and CT-derived lung reconstruction of the injured hemithorax (shown here on the left) and contralateral hemithorax (right) of the rabbits before initiation of injury (I), 48 h after intrapleural TCN injection (II), 24 h after treatment (72 h after TCN) before evacuation (III), and after removal of PF (IV). Pleuropulmonary densities representing an aggregate of PF, parenchymal lung inflammation, and atelectasis are apparent on the CT images in the injured hemithorax. C: lung total volume.
Serial chest CT scanning does not affect changes in intrapleural PA or fibrinolytic activities during the first 4 h after IPFT.
Changes in intrapleural PA (Fig. 6) and fibrinolytic (Fig. 7, A and B) activities following administration of IPFT were evaluated by analyzing aliquots of PF, as previously described (14, 35). PF PA activity progressively decreased over 40 min with approximate first-order rate constants (kobs) 0.016 and 0.042 min−1 in the scuPA- and tPA-treated animals in both the US and US/CT group (Fig. 6). Levels of PA activity in tPA-treated animals were significantly (P < 0.05) less at 10–40 min than those in the scuPA IPFT group (Fig. 6), reflecting both the lower specific PA activity of tPA and the relatively larger scuPA dose that was delivered. There was no significant difference in the change of PA activity between animals scanned with US alone or with US/CT. By 4 h after scuPA or tPA IPFT, the PA activity was significantly decreased vs. those observed at 10 min, P < 0.01. There were no significant differences in the levels of PA activity at 240 min between the scuPA- and tPA-treated animals. However, despite 90–95% of the initially administrated intrapleural PA activity being lost, it was detectable in both groups of animals at 4 h, providing complete activation of the PLG and providing maximal fibrinolytic activity. These results directly support our hypothesis (14, 35) that intrapleural fibrinolysis in TCN-induced pleural injury is relatively protracted after administration of IPFT.
Fig. 6.
Intrapleural inactivation of fibrinolysins during IPFT with a MED of scuPA (○ and ●) and sctPA (▽ and ▼). Changes in PA activity in rabbit PFs during IPFT of TCN-induced pleural injury in rabbits with scuPA (n = 8) and tPA (n = 8) are shown. PA activity in PF samples from animals subjected to US alone (○, n = 11) is shown. Animals imaged with US/CT (n = 5) are shown in closed symbols. PA activity was measured in aliquots, as described in materials and methods (14, 35).
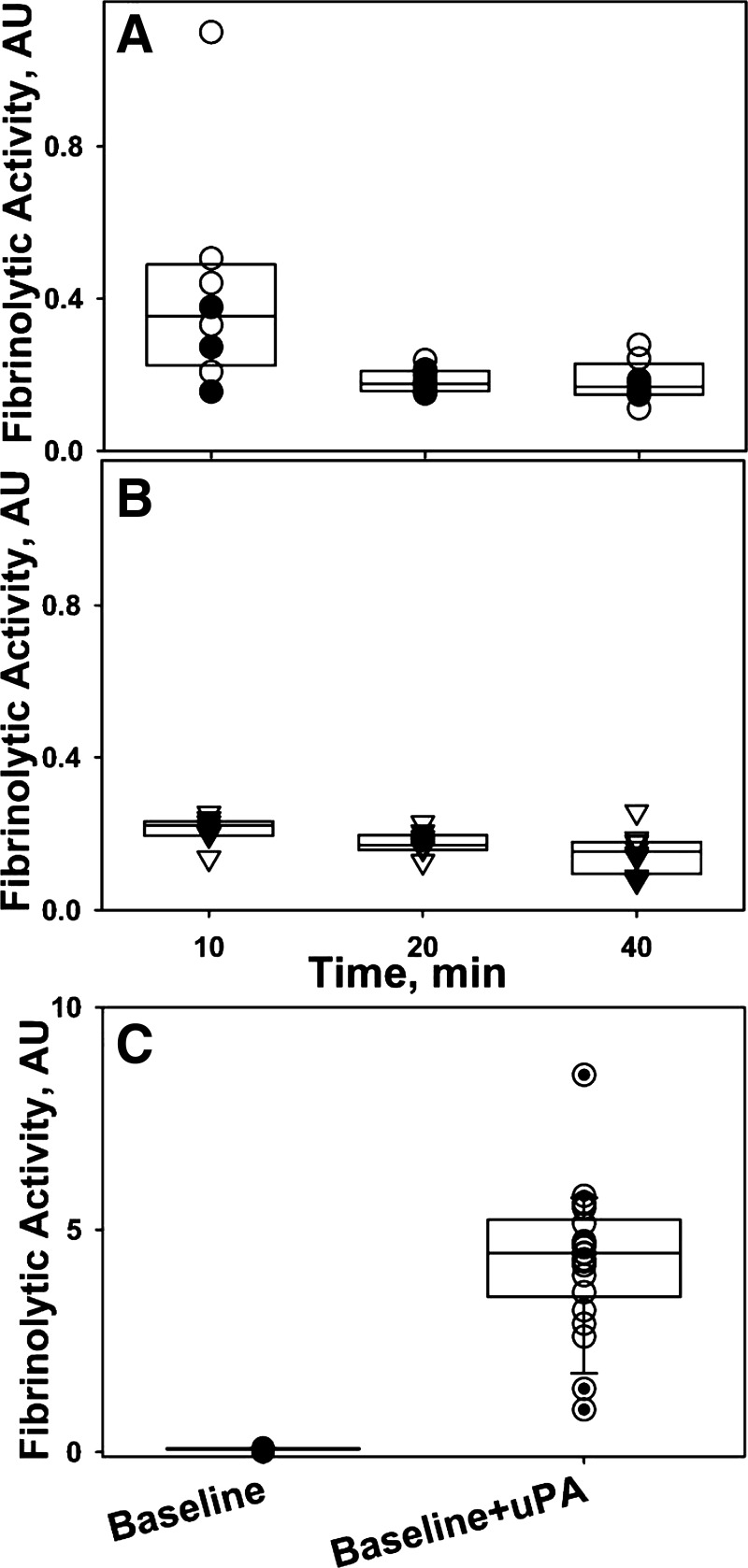
Fig. 7.
Changes in the fibrinolytic activity in PFs of animals treated with scuPA (A, n = 8) and sctPA (B, n = 8) at 0–40 min. Intrapleural fibrinolytic activity approaches a steady-state level, which is the same for both fibrinolysins but significantly lower than that expected from baseline PF analysis (C). Fibrinolytic activity in PFs withdrawn at 10, 20, and 40 min after IPFT with scuPA (A) or sctPA (B) and imaged by either US/CT (closed symbols) or US alone (open symbols) was determined using a FITC-fibrin film assay, as described previously (36). C: although fibrinolytic activity is suppressed at the baseline, ex vivo neutralization of the endogenous PA inhibitor 1 (PAI-1) and activation of accumulated PLG with uPA (or tPA; not shown) results in a higher burst of fibrinolytic activity by almost 2 orders of magnitude.
The changes of fibrinolytic activity in PFs at 10–40 min after intrapleural delivery of scuPA or tPA are shown in Fig. 7. Unlike PA activity, which was higher for the scuPA-treated group at 10–80 min (Fig. 6), intrapleural fibrinolytic activity was similar in the PFs of sctPA- and scuPA-treated animals (Fig. 7) from 20 min onward, indicating that PF fibrinolysis after IPFT is tightly regulated by the endogenous levels of PLG and its inhibitors. Notably, intrapleural fibrinolytic activity in scuPA-treated animals was significantly increased (P < 0.05) compared with the sctPA animals at 10 min (Fig. 7, A and B). Within the US or US/CT groups, there was no significant difference in the steady-state level of PF fibrinolytic activity up to 240 min after IPFT (not shown). The rate of inactivation of tPA and tcuPA by PAI-1 is diffusion limited (kass > 106 M−1·s−1) (21, 68); as a result, endogenous PAI-1 neutralization and PLG activation occur instantly. In contrast, the activation of endogenous PLG by scuPA is delayed (35) because of an equilibrium that favors the inactive conformation of the proenzyme (37). Thus slow (kon = 0.072 min−1) (37) formation of the active species of scuPA limits intrapleural PAI-1 neutralization and promotes a delay in subsequent activation of the endogenous PLG. Indeed, fibrinolytic activity in the PFs of scuPA-treated animals was higher at 10 min compared with sctPA-treated animals (Fig. 7, A and B; P < 0.05) before reaching steady-state levels at 20 min. Notably, baseline PFs, where both PA and fibrinolytic activities are suppressed (Fig. 7C), contain significant amounts of accumulated PLG. Indeed, the activation of endogenous PLG in baseline PFs by supplementation with exogenous PA, which mimics IPFT, resulted in an increase of nearly two orders of magnitude in the fibrinolytic activity from the limit of detection level (Fig. 7C). However, the fibrinolytic activity generated by bolus administration of IPFT (Fig. 7C) was rapidly decreased in vivo by the first 10–20 min after IPFT (Fig. 7, A and B) and approached the steady-state level, which is almost an order of magnitude less than was expected based on the ex vivo experiments (Fig. 7C). The observed difference in fibrinolytic activity induced by scuPA and tPA at 10 min likely reflects the delayed activation of PLG (and further inactivation of plasmin in PF) by scuPA in the presence of high levels of endogenous PAI-1 (35, 37). In contrast to the treated groups, both fibrinolytic and PA activities were suppressed at 0–24 h in the control (untreated) group (not shown) similar to that observed at the baseline for treated animals (Fig. 7C). Maximal injury scores (GLIS/USS = 50) observed at 24 h for the control animals (Figs. 1 and 3) clearly indicated that endogenous fibrinolytic activity cannot support spontaneous resolution of the TCN-induced pleural injury in rabbits.
Unlike uPA, intrapleural tPA generates few molecular-cage-type complexes with α-macroglobulin.
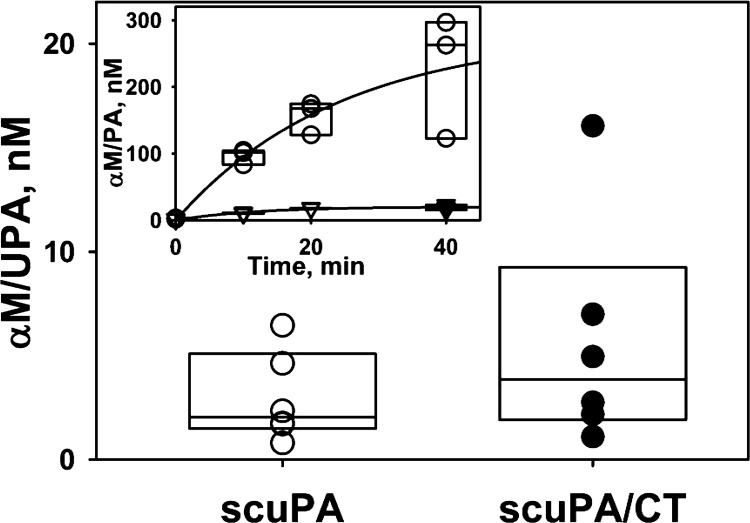
Intrapleural processing of scuPA yielded increasing levels of αM/uPA molecular-cage type complexes (Fig. 8, inset), which have previously been shown to be bioactive in PFs (14, 35, 37). αM/uPA is resistant to PAI-1 and retains amidolytic activity toward low-molecular-weight (LMW) fluorogenic substrates (37, 38). Thus, at any time point, total uPA amidolytic activity is a sum of free and αM-complexed enzyme. The activity measured in the presence of an excess of exogenous PAI-1 reflects the level of αM/uPA. The time-dependent changes of intrapleural αM/uPA reflect an exponential increase to a maximum level (Fig. 9, inset), [αM/uPA]t = [αM/uPA]max × [1-exp(−kobs × time)]; r2 = 0.95, where [αM/uPA]t, [αM/uPA]max, and kobs are molar concentrations of intrapleural αM/uPA at time t and at saturation and a pseudo first-order rate constant for the αM/uPA formation, respectively. The values of [αM/uPA]max and kobs were 160 ± 30 nM and 0.022 ± 0.008 min−1, respectively (Fig. 8, inset). In sharp contrast to scuPA treatment (Fig. 8, inset), there was little accumulation of PAI-1-resistant bioactive complexes in PFs of the sctPA-treated animals collected at 0–40 min. αM/uPA complexes, which have an intrapleural lifetime that exceeds that of uPA by almost an order of magnitude (35), were readily detectable in PFs of animals treated with scuPA 24 h after IPFT (Fig. 9). There was no free PA in these PFs, and the molecular-cage-type complexes with αM were detected only in PFs of animals treated with scuPA (Fig. 8). The intrapleural concentration of αM/uPA was almost three orders of magnitude (P < 0.05) less than the concentration of uPA at 10 min after injection. In contrast to uPA, the level of tPA activity resistant to PAI-1 at 24 h (not shown) was at the limit of detection. Increments of αM/uPA in scuPA IPFT-treated animals were independently confirmed in PFs of scuPA- vs. sctPA-treated animals by enzymographic analyses (not shown). There was no statistical difference in levels of αM/uPA complexes between scuPA groups scanned with US alone and with US/CT.
Fig. 8.
Intrapleural concentration of α-macroglobulin (αM)/uPA complexes at 24 h after scuPA IPFT with (●) or without (○) CT imaging (n = 6 for each group). Inset: accumulation of high levels of αM/uPA (○) and putative αM/tPA (▽) molecular-cage-type complexes detected in aliquots of pleural fluid. The concentration of the αM/uPA complexes was estimated from the residual amidolytic activity after incubation (10 min, 4°C) of a sample of PF with 100–150 nM of exogenous active human recombinant PAI-1, as previously described (37).
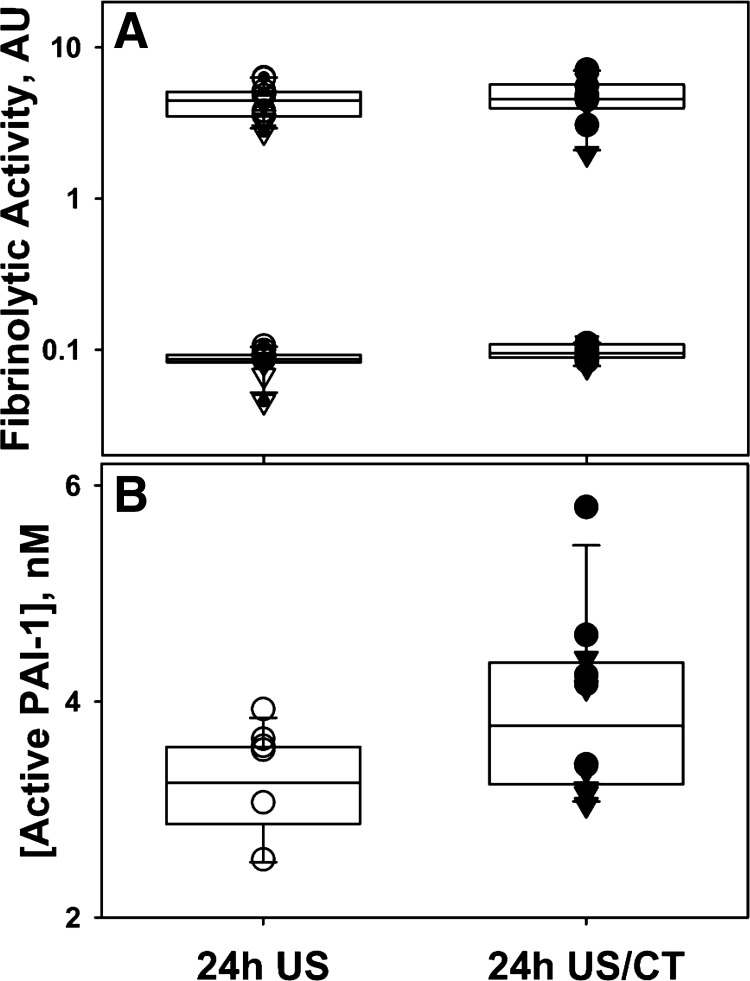
Fig. 9.
Suppression of fibrinolytic activity and accumulation of PLG (A) and active PAI-1 (B) in PF at 24 h after IPFT. Aliquots of PF of animals treated with scuPA (○ and ●) or sctPA (▽ and ▼) with (closed symbols) or without (open symbols) CT imaging were analyzed for fibrinolytic activity (A) or level of endogenous active PAI-1 (B). The fibrinolytic activity was measured with (A, top) or without (A, bottom) supplementation with exogenous uPA (5 nM) using a FITC-fibrin film assay (36). Formation of plasmin attributable to the activation of endogenous accumulated PLG results in an increase by almost 2 orders of magnitude in the fibrinolytic activity at 24 h. B: increased levels of active PAI-1 suppress fibrinolytic activity at 24 h after IPFT. Active PAI-1 was readily detectable in PFs obtained at 24 h following TCN-induced pleural injury in rabbits. The concentration of active PAI-1 in PF withdrawn 24 h after IPFT was determined as previously described (31). The concentration of active PAI-1 in animals imaged with CT was higher (P < 0.05).
CT imaging results in an increased level of active PAI-1 at 24 h after IPFT.
Both PA (not shown) and fibrinolytic (Fig. 9A; ● and ▼) activities were suppressed at 24 h after administration of scuPA or sctPA, as we previously reported (14), indicating that additional dwell time was unlikely to increase the efficacy of IPFT (further decrease GLIS) for any treatment. Complete inactivation of intrapleural PA results in rapid termination of fibrinolysis attributable to a lack of freshly synthesized plasmin and the accumulation of PLG. Indeed, supplementation of PFs collected at 24 h after IPFT with tcuPA (Fig. 9A; ○ and ▽) resulted in a significant increase in the fibrinolytic activity, as observed with baseline PF samples (Fig. 7C) (14). Elevated active PAI-1 plays a critical role in the inactivation of low-level PA activity generated endogenously and attributable to the slow degradation of the molecular-cage-type complexes with αM (14, 35). Thus, the higher the level of active PAI-1, the sooner the PA activity is completely inactivated and fibrinolysis terminated. Notably, concentrations of active PAI-1 in PF at 24 h were statistically increased in the US/CT group (Fig. 9B). Therefore, CT scanning resulted in increased intrapleural expression of active PAI-1 and its accumulation to a higher level at 24 h, which likely contributed to the adverse outcomes of IPFT in the scuPA- or tPA-treated US/CT groups.
DISCUSSION
The results of the present study clearly demonstrate that the rate of fibrinolysis during IPFT with known effective doses of fibrinolysin is relatively slow (minimal TEF; TEF = 4–8 h; Fig. 4A). The potential burst of fibrinolytic activity immediately after IPFT attributable to activation of the endogenous PLG (Fig. 7C) is followed by a rapid (kobs = 0.23 min−1 for scuPA and less for sctPA) fall to approximately equivalent steady-state levels for both fibrinolysins (Fig. 7, A and B). This steady-state level of intrapleural fibrinolytic activity appears to reflect a dynamic equilibrium between inhibition of fibrinolysis and activation of replenished PLG. Both steady-state fibrinolytic (not shown) and PA (Fig. 6) activities were durable through 4 h and thus detected in the samples from animals imaged with US/CT (Fig. 4B). A significant increase in the fibrinolytic activity in baseline PFs after supplementation with PA (Fig. 7C) most likely reflects a potential burst of fibrinolysis in the short period of time after fibrinolysin injection (10–20 min for scuPA and <10 min for sctPA; Fig. 7, A and B). However, as extrapolated from Fig. 7, the approximate areas under the curve (AUCs) for initial fibrinolytic activity (0 to 10–20 min) were three to five times smaller than those for the steady-state part (10–20 to 240–480 min; Fig. 4A). In contrast, the AUCs for animals from the control group (not treated with PA) were minimal because the fibrinolytic activity was at the level of detection. Interestingly, the steady-state levels of accumulated PLG at the baseline (Fig. 7C) and at 24 h (Fig. 9, A and B) were on the same order of magnitude, indicating that 1) there are mechanisms that prevent an uncontrolled increase in the level of endogenous PLG, and 2) it is unlikely that CT dramatically affects intrapleural PLG expression. Moreover, we have previously demonstrated that the level of total PLG/plasmin antigen in PFs during IPFT also does not vary significantly (14, 35). The results shown in Figs. 4, 6, and 7 suggest that there was a similarity between the changes in fibrinolytic activity at 0–240 min for animals imaged with US only (effective IPFT; GLIS ≤ 10) and with US/CT (ineffective IPFT; 10 < GLIS ≤ 50). Thus the intrapleural expression of PLG is not likely to depend on the type or dose of fibrinolysin. Although changes in the expression of other genes could potentially affect degradation of adhesions after CT imaging, endogenous active PAI-1 may function as an all-or-nothing switch for intrapleural PLG activation and fibrinolysis. As soon as endogenous active PAI-1 neutralizes the intrapleural PA activity, the dynamic equilibrium between the activation of newly synthesized PLG and inactivation of plasmin, which determines the steady-state fibrinolytic activity (Fig. 7, A and B), moves toward the latter, and fibrinolysis stops. Previously, we demonstrated that changes in the level of active PAI-1 in PF affect the half-life of intrapleural PA activity and the outcomes of IPFT (14, 35) and that neutralization of PAI-1 results in an increase in the efficacy of IPFT (14). Thus using PAI-1 inhibitors such as monoclonal antibodies, which redirect the reaction from the inhibitory to the substrate branch of the PAI-1 mechanism (32–34, 39), would likely improve outcomes of IPFT with CT imaging in a manner similar to that observed without CT (14). On the other hand, even if an initial short (0–10 min) burst in the intrapleural fibrinolytic activity occurs, it may contribute to, but does not determine, the effective IPFT attributable to the long TEF (4–8 h) observed in this model. The observed low rate of intrapleural fibrinolysis raises the possibility that relatively shorter IPFT dwell times could slow intrapleural fibrinolysis through removal of both plasmin and fibrinolysin, which could adversely impact outcomes.
US was applied in a diagnostic imaging mode and was sensitive enough to consistently detect changes in discrete pleural adhesions/collections amenable to imaging over time (Fig. 4, A and B). Use of the TCN model in these studies is justified by similarity between rabbit and human proteins of the fibrinolytic system and structure of fibrin (52) and also the relatively consistent time course of outcomes we observed over several years in individual rabbits (14, 24, 27, 31, 35). The USS was predicated by observing changes in adhesion density, size, and distribution. GLIS (31, 35), determined at the time of euthanasia in all animals, corroborated the USS results. Monitoring intrapleural fibrinolysis with US directly demonstrated slow clearance of fibrin deposition (Fig. 4) and a decrease in the efficacy of IPFT in animals that were subjected to CT scanning (Fig. 4B).
Active PAI-1 is one of the key profibrogenic molecules as well as a biomarker and a target for IPFT in TCN-induced pleural injury in rabbits (14, 31, 35). PAI-1 activity at 24 h after IPFT was significantly higher for animals imaged with US/CT (Fig. 9B; P < 0.05). Thus, although a number of other factors could have contributed to the response, increased intrapleural PAI-1 in animals with US/CT imaging predictably (14) coincided with worse IPFT outcomes. Notably, the pleural space and lung that was not subjected to TCN-induced injury did not develop fibrosis (not shown), indicating that CT exacerbates the effects of TCN rather than independently inducing pleural injury. The absorbed dose of radiation for each rabbit was 1.14 cGy per CT scan, which represents an effective dose of 1.37 mSV per scan to the lungs. Thus each rabbit received a total CT lung dose of 5–7 mSv because each underwent four CT scans. Because the last two CT scans were performed within an hour before euthanasia, we speculate that the two earlier CT scans (15–20 min before TCN injection and IPFT) could have major effects on IPFT and injury outcomes. These levels of radiation exposure typically do not induce pleuropulmonary injury, consistent with the lack of adverse effects on the contralateral lung. However, accumulating experimental and clinical evidence demonstrates that even the low doses of ionizing radiation used in CT produce free radicals and peroxides, which increase oxidative stress, and decrease the oxygen radical capacity, and an increase in γ-H2AX in blood indicating dsDNA breaks as soon as 1 h after CT (18, 19). Moreover, chemically compromised tissues such as the lungs of smokers, may similarly be more sensitive to radiation damage by chest CT (9). Thus we hypothesized that low-level CT radiation could affect production of reactive oxygen species at the injury site, induce local increase in PAI-1 expression, and exacerbate pleural injury. Nevertheless, despite the fact that TCN-induced pleural injury in rabbits recapitulates features of the human disease, it is premature to extrapolate from these results to clinical ramifications of CT imaging for patients with empyema. To the best of our knowledge, the effects of CT scanning, a technique widely used to image patients with empyema, on infectious pleural injury have not been investigated. Additional studies of the effect of CT imaging on pleural injury could prove to be of clinical utility.
Whereas the MEDs of scuPA and sctPA cleared adhesions in a comparable manner in animals not imaged with CT (Fig. 1, 4A), the intrapleural processing of these agents differed considerably. sctPA is sensitive to inhibition by active PAI-1 (64) but is relatively more fibrin specific (54) and partially protected from inhibition by PAI-1 when bound to fibrin (64). On the other hand, scuPA generates up to 250 nM of intrapleural bioactive αM/uPA that contributes to low-level, durable PA activity in PF over 24 h (14, 37). Molecular-cage-type (51) complexes between αM and endogenous or exogenous uPA have previously been found in both airway fluids and PFs from humans and rabbits (35, 37, 38). Whereas proteinases complexed with αMs interact with LMW molecules (substrates, inhibitors, ligands), they are sterically protected from high-molecular-weight (HMW) ligands (13, 55). Their activities toward HMW substrates and inhibitors, if they exist, are greatly suppressed (35, 62). Thus the loss of PA activity in scuPA-treated animals (Fig. 6) is accompanied by simultaneous accumulation of bioactive αM/uPA complexes (Fig. 8, inset), which have a considerably longer intrapleural half-life (35) and are found at nanomolar levels in the PFs at 24 h after IPFT (Fig. 8). Slow degradation of αM/uPA contributes to durable, low-level intrapleural PA activity (35). However, under conditions of elevated endogenous PAI-1, uPA, which formed because of slow intrapleural degradation of αM/uPA, becomes inactivated. Indeed, in the CT-imaged group, in which PAI-1 levels were elevated (Fig. 9B), the efficacy of IPFT was notably decreased (Figs. 1 and 4B). The U-shape of dependence of USS on the time observed for two animals treated with scuPA (Fig. 4B) could reflect the blockade of αM/uPA-derived PA by increased active PAI-1. However, intrapleural PAI-1 neutralization results in effective IPFT with a decrease in doses of fibrinolysins by almost an order of magnitude (14). These results support the critical role of active PAI-1 as a biomarker and a molecular target for IPFT in TCN-induced pleural injury. The lack of molecular-cage-type complexes with tPA could reflect the effect of tPA binding to intrapleural fibrin (64) on the reaction with αM. On the other hand, tPA bound to fibrin easily activates PLG, which incorporated into the fibrin structure, affecting the rate and efficacy of intrapleural fibrinolysis.
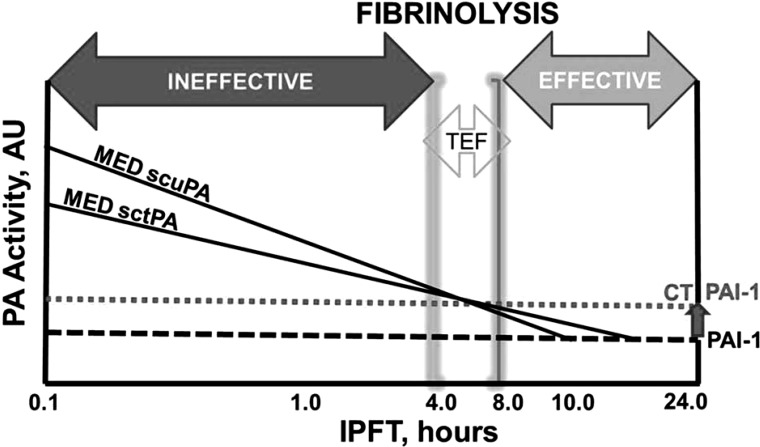
The proposed scheme of intrapleural fibrinolysis in rabbits with TCN-induced pleural injury during IPFT is shown in Fig. 10. Both PA and fibrinolytic activity are positive, whereas endogenous intrapleural PAI-1 (Fig. 10; gray dotted and black dashed lines represent active PAI-1 levels in animals imaged with and without CT, respectively) is neutralized. Activation of endogenous PLG results in durable fibrinolytic activity (Fig. 7, A and B) until the total PA activity exceeds the level of endogenous active PAI-1 (Fig. 10; to the left from intercepts of the MED sctPA or scuPA with dashed line). During the TEF (4–8 h) or later, fibrinolysis results in effective IPFT (Figs. 1 and 4A). However, under conditions of increased PAI-1 activity attributable to CT scanning (Fig. 9B), complete inactivation of PA activity occurs earlier (Fig. 10; intercepts of the MED sctPA or scuPA with the gray dotted line). As soon as PAI-1 inactivates residual PA, fibrinolysis stops; the balance between intrapleural fibrinolytic and profibrotic activities shifts toward the latter, and the efficacy of IPFT decreases (Figs. 1 and 4B).
Fig. 10.
Intrapleural fibrinolysis during IPFT with a MED of sctPA or scuPA in TCN-induced pleural injury in rabbits. A double logarithmic plot of relative activity (PA and PAI-1, left and right, respectively) vs. time of IPFT illustrates 3 putative phases of intrapleural fibrinolysis: ineffective 0–4 h; effective 8–24 h; and the intermediate phase, the minimal time of effective fibrinolysis (TEF, 4–8 h). The dotted and dashed lines represent the relative levels of endogenous active PAI-1 expression with and without CT scans, respectively. The solid lines represent intrapleural inactivation of the MED of scuPA or sctPA. Intrapleural PA activity is suppressed and fibrinolysis stops as soon as endogenous PAI-1 inactivates fibrinolysin (intercepts of solid and dotted or dashed lines). As long as the time of fibrinolysis (time for positive intrapleural PA activity) equals or exceeds the TEF (4–8 h), IPFT is successful. Shortening the intrapleural half-life of PA activity, attributable to increased levels of PAI-1 (intercepts of solid and dotted lines), decreased IPFT efficacy.
Although a MED of scuPA and tPA cleared most of the organizing adhesions over 24 h (Figs. 1 and 4A), at 48 h and for up to 72 h (23) after TCN-induced injury, this study demonstrates, for the first time, that discernable clearance of pleural adhesions is relatively slow, based on serial US imaging (Fig. 4). This noninvasive approach obviated the need to perform additional dedicated experiments in control groups, allowing us to conserve animals. The results obtained support the proposed mechanisms of intrapleural processing of scuPA (35) and the critical contribution of endogenous active PAI-1 to the outcome of IPFT in this model (14, 31, 35). Our results further buttress the concept that targeting active PAI-1 is a promising avenue to develop novel therapeutic interventions for the treatment of organizing or loculated pleural injury. Although the TCN-induced pleural injury model possesses a number of features of organizing human disease (24), the effects of PAI-1 targeting in empyema are most clinically germane and have yet to be tested.
GRANTS
This work was supported by NIH grant P50 HL107186-01 CADET I (S. Idell and A. Komissarov) and the Texas Lung Injury Institute, UTHSCT.
DISCLOSURES
Dr. Idell is the unpaid Chief Scientific Officer of Lung Therapeutics, serves on its board of directors, and has an equity position in the company, which was created to develop and commercialize single-chain urokinase and other agents for use in lung and pleural disease. His work on single-chain urokinase and pleural injury has been supported by grants from the National Institutes of Health and philanthropy.
AUTHOR CONTRIBUTIONS
A.A.K., G.F., and S.I. conception and design of research; A.A.K., G.F., A.O.A., A.B., W.M.B., C.S., and K.K. performed experiments; A.A.K. analyzed data; A.A.K. interpreted results of experiments; A.A.K. and G.F. prepared figures; A.A.K. drafted manuscript; A.A.K. and S.I. edited and revised manuscript; A.A.K., G.F., A.O.A., A.B., W.M.B., C.S., K.K., and S.I. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Ms. Sophia Karandashova, MS for the editorial help, Mr. Jarod Pamatmat, and Mr. Douglas Shryock for technical assistance.
REFERENCES
- 1.Bar I, Stav D, Fink G, Peer A, Lazarovitch T, Papiashvilli M. Thoracic empyema in high-risk patients: conservative management or surgery? Asian Cardiovasc Thorac Ann 18: 337–343, 2010. [DOI] [PubMed] [Google Scholar]
- 2.Barbato A, Panizzolo C, Monciotti C, Marcucci F, Stefanutti G, Gamba PG. Use of urokinase in childhood pleural empyema. Pediatr Pulmonol 35: 50–55, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Barnes NP, Hull J, Thomson AH. Medical management of parapneumonic pleural disease. Pediatr Pulmonol 39: 127–134, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Barthwal MS. Intrapleural fibrinolytic therapy in complicated parapneumonic effusion and empyema: present status. Indian J Chest Dis Allied Sci 50: 277–282, 2008. [PubMed] [Google Scholar]
- 5.Ben-Or S, Feins RH, Veeramachaneni NK, Haithcock BE. Effectiveness and risks associated with intrapleural alteplase by means of tube thoracostomy. Ann Thorac Surg 91: 860–863, 2011. [DOI] [PubMed] [Google Scholar]
- 6.Bianchini MA, Ceccarelli PL, Repetto P, Durante V, Biondini D, Bergamini B, Cacciari A. Once-daily intrapleural urokinase treatment of complicated parapneumonic effusion in pediatric patients. Turk J Pediatr 52: 274–277, 2010. [PubMed] [Google Scholar]
- 7.Bouros D, Antoniou KM, Chalkiadakis G, Drositis J, Petrakis I, Siafakas N. The role of video-assisted thoracoscopic surgery in the treatment of parapneumonic empyema after the failure of fibrinolytics. Surg Endosc 16: 151–154, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Bouros D, Schiza S, Tzanakis N, Drositis J, Siafakas N. Intrapleural urokinase in the treatment of complicated parapneumonic pleural effusions and empyema. Eur Respir J 9: 1656–1659, 1996. [DOI] [PubMed] [Google Scholar]
- 9.Brenner DJ. Radiation risks potentially associated with low-dose CT screening of adult smokers for lung cancer. Radiology 231: 440–445, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Cameron R, Davies HR. Intra-pleural fibrinolytic therapy versus conservative management in the treatment of adult parapneumonic effusions and empyema. Cochrane Database Syst Rev 16: CD002312, 2008. [DOI] [PubMed] [Google Scholar]
- 11.de Benedictis FM, De Giorgi G, Niccoli A, Troiani S, Rizzo F, Lemmi A. Treatment of complicated pleural effusion with intracavitary urokinase in children. Pediatr Pulmonol 29: 438–442, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Dewilde M, Van De Craen B, Compernolle G, Madsen JB, Strelkov S, Gils A, Declerck PJ. Subtle structural differences between human and mouse PAI-1 reveal the basis for biochemical differences. J Struct Biol 171: 95–101, 2010. [DOI] [PubMed] [Google Scholar]
- 13.Feinman RD, Wang D, Windwer SR, Wu K. The role of enzyme lysyl amino groups in the reaction with alpha 2-macroglobulin. Ann NY Acad Sci 421: 178–187, 1983. [DOI] [PubMed] [Google Scholar]
- 14.Florova G, Azghani A, Karandashova S, Schaefer C, Koenig K, Stewart-Evans K, Declerck PJ, Idell S, Komissarov AA. Targeting of plasminogen activator inhibitor 1 improves fibrinolytic therapy for tetracycline-induced pleural injury in rabbits. Am J Respir Cell Mol Biol 52: 429–437, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Froudarakis ME, Kouliatsis G, Steiropoulos P, Anevlavis S, Pataka A, Popidou M, Mikroulis D, Pneumatikos I, Bouros D. Recombinant tissue plasminogen activator in the treatment of pleural infections in adults. Respir Med 102: 1694–1700, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Gates RL, Caniano DA, Hayes JR, Arca MJ. Does VATS provide optimal treatment of empyema in children? A systematic review. J Pediatr Surg 39: 381–386, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Gebbink MF, Bouma B, Maas C, Bouma BN. Physiological responses to protein aggregates: fibrinolysis, coagulation and inflammation (new roles for old factors). FEBS Lett 583: 2691–2699, 2009. [DOI] [PubMed] [Google Scholar]
- 18.Halm BM, Franke AA, Lai JF, Li X, Custer LJ, Pagano I, Cooney RV, Turner HC, Brenner DJ. Pilot study for the establishment of biomarkers for radiation damage after computed tomography in children. Hawaii J Med Public Health 74: 112–119, 2015. [PMC free article] [PubMed] [Google Scholar]
- 19.Halm BM, Lai JF, Morrison CM, Pagano I, Custer LJ, Cooney RV, Franke AA. In vivo changes in plasma coenzyme Q10, carotenoid, tocopherol, and retinol levels in children after computer tomography. Arch Biochem Biophys 547: 37–43, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Handman HP, Reuman PD. The use of urokinase for loculated thoracic empyema in children: a case report and review of the literature. Pediatr Infect Dis J 12: 958–959, 1993. [PubMed] [Google Scholar]
- 21.Hekman CM, Loskutoff DJ. Kinetic analysis of the interactions between plasminogen activator inhibitor 1 and both urokinase and tissue plasminogen activator. Arch Biochem Biophys 262: 199–210, 1988. [DOI] [PubMed] [Google Scholar]
- 22.Huang HC, Chen HC, Fang HY, Lin YC, Wu CY, Cheng CY. Lung abscess predicts the surgical outcome in patients with pleural empyema. J Cardiothorac Surg 5: 88, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Idell S, Allen T, Chen S, Koenig K, Mazar A, Azghani A. Intrapleural activation, processing, efficacy, and duration of protection of single-chain urokinase in evolving tetracycline-induced pleural injury in rabbits. Am J Physiol Lung Cell Mol Physiol 292: L25–L32, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Idell S, Azghani A, Chen S, Koenig K, Mazar A, Kodandapani L, Bdeir K, Cines D, Kulikovskaya I, Allen T. Intrapleural low-molecular-weight urokinase or tissue plasminogen activator versus single-chain urokinase in tetracycline-induced pleural loculation in rabbits. Exp Lung Res 33: 419–440, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Idell S, Girard W, Koenig KB, McLarty J, Fair DS. Abnormalities of pathways of fibrin turnover in the human pleural space. Am Rev Respir Dis 144: 187–194, 1991. [DOI] [PubMed] [Google Scholar]
- 26.Idell S, Jun NM, Liao H, Gazar AE, Drake W, Lane KB, Koenig K, Komissarov A, Tucker T, Light RW. Single-chain urokinase in empyema induced by Pasturella multocida. Exp Lung Res 35: 665–681, 2009. [DOI] [PubMed] [Google Scholar]
- 27.Idell S, Mazar A, Cines D, Kuo A, Parry G, Gawlak S, Juarez J, Koenig K, Azghani A, Hadden W, McLarty J, Miller E. Single-chain urokinase alone or complexed to its receptor in tetracycline-induced pleuritis in rabbits. Am J Respir Crit Care Med 166: 920–926, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Islam S, Calkins CM, Goldin AB, Chen C, Downard CD, Huang EY, Cassidy L, Saito J, Blakely ML, Rangel SJ, Arca MJ, Abdullah F, St Peter SD. The diagnosis and management of empyema in children: a comprehensive review from the APSA Outcomes and Clinical Trials Committee. J Pediatr Surg 47: 2101–2110, 2012. [DOI] [PubMed] [Google Scholar]
- 29.Janda S, Swiston J. Intrapleural fibrinolytic therapy for treatment of adult parapneumonic effusions and empyemas: a systematic review and meta-analysis. Chest 142: 401–411, 2012. [DOI] [PubMed] [Google Scholar]
- 30.Jo M, Takimoto S, Montel V, Gonias SL. The urokinase receptor promotes cancer metastasis independently of urokinase-type plasminogen activator in mice. Am J Pathol 175: 190–200, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karandashova S, Florova G, Azghani AO, Komissarov AA, Koenig K, Tucker TA, Allen TC, Stewart K, Tvinnereim A, Idell S. Intrapleural adenoviral delivery of human plasminogen activator inhibitor-1 exacerbates tetracycline-induced pleural injury in rabbits. Am J Respir Cell Mol Biol 48: 44–52, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Komissarov AA, Andreasen PA, Bodker JS, Declerck PJ, Anagli JY, Shore JD. Additivity in effects of vitronectin and monoclonal antibodies against alpha-helix F of plasminogen activator inhibitor-1 on its reactions with target proteinases. J Biol Chem 280: 1482–1489, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Komissarov AA, Andreasen PA, Declerck PJ, Kamikubo Y, Zhou A, Gruber A. Redirection of the reaction between activated protein C and a serpin to the substrate pathway. Thromb Res 122: 397–404, 2008. [DOI] [PubMed] [Google Scholar]
- 34.Komissarov AA, Declerck PJ, Shore JD. Mechanisms of conversion of plasminogen activator inhibitor 1 from a suicide inhibitor to a substrate by monoclonal antibodies. J Biol Chem 277: 43858–43865, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Komissarov AA, Florova G, Azghani A, Karandashova S, Kurdowska AK, Idell S. Active α-macroglobulin is a reservoir for urokinase after fibrinolytic therapy in rabbits with tetracycline-induced pleural injury and in human pleural fluids. Am J Physiol Lung Cell Mol Physiol 305: L682–L692, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Komissarov AA, Florova G, Idell S. Effects of extracellular DNA on plasminogen activation and fibrinolysis. J Biol Chem 286: 41949–41962, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Komissarov AA, Mazar AP, Koenig K, Kurdowska AK, Idell S. Regulation of intrapleural fibrinolysis by urokinase-α-macroglobulin complexes in tetracycline-induced pleural injury in rabbits. Am J Physiol Lung Cell Mol Physiol 297: L568–L577, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Komissarov AA, Stankowska D, Krupa A, Fudala R, Florova G, Florence J, Fol M, Allen TC, Idell S, Matthay MA, Kurdowska AK. Novel aspects of urokinase function in the injured lung: role of α2-macroglobulin. Am J Physiol Lung Cell Mol Physiol 303: L1037–L1045, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Komissarov AA, Zhou A, Declerck PJ. Modulation of serpin reaction through stabilization of transient intermediate by ligands bound to alpha-helix F. J Biol Chem 282: 26306–26315, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Kornecki A, Sivan Y. Treatment of loculated pleural effusion with intrapleural urokinase in children. J Pediatr Surg 32: 1473–1475, 1997. [DOI] [PubMed] [Google Scholar]
- 41.Krishnan S, Amin N, Dozor AJ, Stringel G. Urokinase in the management of complicated parapneumonic effusions in children. Chest 112: 1579–1583, 1997. [DOI] [PubMed] [Google Scholar]
- 42.Lee KS, Im JG, Kim YH, Hwang SH, Bae WK, Lee BH. Treatment of thoracic multiloculated empyemas with intracavitary urokinase: a prospective study. Radiology 179: 771–775, 1991. [DOI] [PubMed] [Google Scholar]
- 43.Light RW. Parapneumonic effusions and empyema. Proc Am Thorac Soc 3: 75–80, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Marhuenda C, Barcelo C, Fuentes I, Guillen G, Cano I, Lopez M, Hernandez F, Perez-Yarza EG, Matute JA, Garcia-Casillas MA, Alvarez V, Moreno-Galdo A. Urokinase versus VATS for treatment of empyema: a randomized multicenter clinical trial. Pediatrics 134: e1301–e1307, 2014. [DOI] [PubMed] [Google Scholar]
- 45.Marhuenda C, Barcelo C, Molino JA, Guillen G, Moreno A, Martinez X. [Treatment of loculated parapneumonic empyema. Video assisted thoracoscopy or fibrinolytics?] An Pediatr (Barc) 75: 307–313, 2011. [DOI] [PubMed] [Google Scholar]
- 46.Maskell NA, Davies CW, Nunn AJ, Hedley EL, Gleeson FV, Miller R, Gabe R, Rees GL, Peto TE, Woodhead MA, Lane DJ, Darbyshire JH, Davies RJ; First Multicenter Intrapleural Sepsis Trial (MIST1) Group. Controlled trial of intrapleural streptokinase for pleural infection. N Engl J Med 352: 865–874, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Moulton JS, Benkert RE, Weisiger KH, Chambers JA. Treatment of complicated pleural fluid collections with image-guided drainage and intracavitary urokinase. Chest 108: 1252–1259, 1995. [DOI] [PubMed] [Google Scholar]
- 48.Moulton JS, Moore PT, Mencini RA. Treatment of loculated pleural effusions with transcatheter intracavitary urokinase. AJR Am J Roentgenol 153: 941–945, 1989. [DOI] [PubMed] [Google Scholar]
- 49.Ozol D, Oktem S, Erdinc E. Complicated parapneumonic effusion and empyema thoracis: microbiologic and therapeutic aspects. Respir Med 100: 286–291, 2006. [DOI] [PubMed] [Google Scholar]
- 50.Piccolo F, Pitman N, Bhatnagar R, Popowicz N, Smith NA, Brockway B, Nickels R, Burke AJ, Wong CA, McCartney R, Choo-Kang B, Blyth KG, Maskell NA, Lee YC. Intrapleural tissue plasminogen activator and deoxyribonuclease for pleural infection. An effective and safe alternative to surgery. Ann Am Thorac Soc 11: 1419–1425, 2014. [DOI] [PubMed] [Google Scholar]
- 51.Pizzo SV, Rajagopalan S, Roche PA, Fuchs HE, Feldman SR, Gonias SL. Specificity of alpha 2-macroglobulin covalent cross-linking for the active domain of proteinases. Biol Chem Hoppe Seyler 367: 1177–1182, 1986. [DOI] [PubMed] [Google Scholar]
- 52.Pretorius E, Humphries P, Ekpo OE, Smit E, van der Merwe CF. Comparative ultrastructural analyses of mouse, rabbit, and human platelets and fibrin networks. Microsc Res Tech 70: 823–827, 2007. [DOI] [PubMed] [Google Scholar]
- 53.Rahman NM, Maskell NA, West A, Teoh R, Arnold A, Mackinlay C, Peckham D, Davies CW, Ali N, Kinnear W, Bentley A, Kahan BC, Wrightson JM, Davies HE, Hooper CE, Lee YC, Hedley EL, Crosthwaite N, Choo L, Helm EJ, Gleeson FV, Nunn AJ, Davies RJ. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N Engl J Med 365: 518–526, 2011. [DOI] [PubMed] [Google Scholar]
- 54.Sakharov DV, Barrertt-Bergshoeff M, Hekkenberg RT, Rijken DC. Fibrin-specificity of a plasminogen activator affects the efficiency of fibrinolysis and responsiveness to ultrasound: comparison of nine plasminogen activators in vitro. Thromb Haemost 81: 605–612, 1999. [PubMed] [Google Scholar]
- 55.Salvesen GS, Sayers CA, Barrett AJ. Further characterization of the covalent linking reaction of alpha 2-macroglobulin. Biochem J 195: 453–461, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schweigert M, Solymosi N, Dubecz A, Beron M, Thumfart L, Oefner-Velano D, Stein HJ. Surgical management of pleural empyema in the very elderly. Ann R Coll Surg Engl 94: 331–335, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schweigert M, Solymosi N, Dubecz A, Fernandez MJ, Stadlhuber RJ, Ofner D, Stein HJ. Surgery for parapneumonic pleural empyema—What influence does the rising prevalence of multimorbidity and advanced age has on the current outcome? Surgeon S1479-666X(14)00069-9, 2014. [DOI] [PubMed] [Google Scholar]
- 58.Sonnappa S, Cohen G, Owens CM, van Doorn C, Cairns J, Stanojevic S, Elliott MJ, Jaffe A. Comparison of urokinase and video-assisted thoracoscopic surgery for treatment of childhood empyema. Am J Respir Crit Care Med 174: 221–227, 2006. [DOI] [PubMed] [Google Scholar]
- 59.Sonnappa S, Jaffe A. Treatment approaches for empyema in children. Paediatr Respir Rev 8: 164–170, 2007. [DOI] [PubMed] [Google Scholar]
- 60.St Peter SD, Tsao K, Spilde TL, Keckler SJ, Harrison C, Jackson MA, Sharp SW, Andrews WS, Rivard DC, Morello FP, Holcomb GW 3rd, Ostlie DJ. Thoracoscopic decortication vs tube thoracostomy with fibrinolysis for empyema in children: a prospective, randomized trial. J Pediatr Surg 44: 106–111, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stefanutti G, Ghirardo V, Barbato A, Gamba P. Evaluation of a pediatric protocol of intrapleural urokinase for pleural empyema: a prospective study. Surgery 148: 589–594, 2010. [DOI] [PubMed] [Google Scholar]
- 62.Straight DL, Hassett MA, McKee PA. Structural and functional characterization of the inhibition of urokinase by alpha 2-macroglobulin. Biochemistry 24: 3902–3907, 1985. [DOI] [PubMed] [Google Scholar]
- 63.Stringel G, Hartman AR. Intrapleural instillation of urokinase in the treatment of loculated pleural effusions in children. J Pediatr Surg 29: 1539–1540, 1994. [DOI] [PubMed] [Google Scholar]
- 64.Thelwell C, Longstaff C. The regulation by fibrinogen and fibrin of tissue plasminogen activator kinetics and inhibition by plasminogen activator inhibitor 1. J Thromb Haemost 5: 804–811, 2007. [DOI] [PubMed] [Google Scholar]
- 65.Thommi G, Shehan CJ, McLeay MT. Fibrinolytics in parapneumonic effusions/empyemas. Chest 146: e103–e104, 2014. [DOI] [PubMed] [Google Scholar]
- 66.Thommi G, Shehan JC, Robison KL, Christensen M, Backemeyer LA, McLeay MT. A double blind randomized cross over trial comparing rate of decortication and efficacy of intrapleural instillation of alteplase vs placebo in patients with empyemas and complicated parapneumonic effusions. Respir Med 106: 716–723, 2012. [DOI] [PubMed] [Google Scholar]
- 67.Thomson AH, Hull J, Kumar MR, Wallis C, Balfour Lynn IM. Randomised trial of intrapleural urokinase in the treatment of childhood empyema. Thorax 57: 343–347, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thorsen S, Philips M, Selmer J, Lecander I, Astedt B. Kinetics of inhibition of tissue-type and urokinase-type plasminogen activator by plasminogen-activator inhibitor type 1 and type 2. Eur J Biochem 175: 33–39, 1988. [DOI] [PubMed] [Google Scholar]
- 69.Tokuda Y, Matsushima D, Stein GH, Miyagi S. Intrapleural fibrinolytic agents for empyema and complicated parapneumonic effusions: a meta-analysis. Chest 129: 783–790, 2006. [DOI] [PubMed] [Google Scholar]
- 70.Tucker TA, Jeffers A, Alvarez A, Owens S, Koenig K, Quaid B, Komissarov AA, Florova G, Kothari H, Pendurthi U, Mohan Rao LV, Idell S. Plasminogen activator inhibitor-1 deficiency augments visceral mesothelial organization, intrapleural coagulation, and lung restriction in mice with carbon black/bleomycin-induced pleural injury. Am J Respir Cell Mol Biol 50: 316–327, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van Loo A, van Loo E, Selvadurai H, Cooper P, Van Asperen P, Fitzgerald DA. Intrapleural urokinase versus surgical management of childhood empyema. J Paediatr Child Health 50: 823–826, 2014. [DOI] [PubMed] [Google Scholar]
- 72.Zuckerman DA, Reed MF, Howington JA, Moulton JS. Efficacy of intrapleural tissue-type plasminogen activator in the treatment of loculated parapneumonic effusions. J Vasc Interv Radiol 20: 1066–1069, 2009. [DOI] [PubMed] [Google Scholar]