Abstract
Quantitative information about adhesion strength is a fundamental part of our understanding of cell-extracellular matrix (ECM) interactions. Adhesion assays should measure integrin-ECM bond strength, but reports now suggest that cell components remain behind after exposure to acute force for radial shear assays in the presence of divalent cations that increase integrin-ECM affinity. Here, we show that focal adhesion proteins FAK, paxillin, and vinculin but not the cytoskeletal protein actin remain behind after shear-induced detachment of HT1080 fibrosarcoma cells. Cytoskeletal stabilization increased attachment strength by eightfold, whereas cross-linking integrins to the substrate only caused a 1.5-fold increase. Reducing temperature—only during shear application—also increased attachment strength eightfold, with detachment again occurring between focal adhesion proteins and actin. Detachment at the focal adhesion-cytoskeleton interface was also observed in mouse and human fibroblasts and was ligand-independent, highlighting the ubiquity of this mode of detachment in the presence of divalent cations. These data show that the cytoskeleton and its dynamic coupling to focal adhesions are critically important for cell adhesion in niche with divalent cations.
Introduction
Integrin-mediated adhesion to extracellular matrix (ECM) occurs via complex molecular clusters called focal adhesions (FAs) that enable cells to transduce forces and signals to and from the cell’s surroundings. Proteins within FAs are intrinsically dynamic, with average integrin bond lifetimes on the order of seconds (1); thus, cell adhesion can only be achieved by the continuous binding, disengaging, and rebinding of many integrins to and from ECM, i.e., avidity. Single-molecule studies indicated that integrin binding affinity for ECM is highly influenced by niche conditions, i.e., cation type and concentration (2). Given the broad scope of cation-mediated cell processes (3), such reductionist experiments might be preferable; however, integrin affinity and avidity are internally regulated within FAs (4), and thus their response to cations has been demonstrated to differ in situ. For example, α5β1 integrin dominated adhesion of fibrosarcoma cells supported highest attachment strength in the presence of both Mg2+ and Ca2+ (5) unlike in single-molecule experiments (2); conversely, Ca2+ decreased attachment strength of fibroblasts (5). Although integrin affinity may be regulated differently in situ versus at the single-molecule level, the cytoskeleton has been identified as an important contributor to adhesion strength. In the presence of cations, cells detach by a peeling mechanism when subjected to shear, i.e., cells detach piecewise beginning with the side of the cells exposed to shear and subsequently undergo cytoskeletal remodeling (5,6). Because cytoskeletal remodeling is cell type, cation type, and ligand specific, these data suggest that shear force assays could reveal differences in cytoskeletal dynamics.
A variety of methods have been developed to quantify cell adhesion in situ after cell attachment. These range from bead binding assays, e.g., biomembrane force probes and optical tweezers, to whole cell-ECM interactions, e.g., micropipette aspiration and centrifugal or shear force assays. Most of these methods apply force to dissociate bonds shortly after attachment (seconds to hours) and over extremely short periods of time (milliseconds to minutes) (7,8). Besides the differences in duration of applied force, the environmental conditions during measurement also differ. Centrifugation assays are commonly performed at 4°C, whereas other adhesion assays specify room or physiological temperature. For those assays performed at nonphysiological temperature, significant changes in protein function, e.g., folding, metabolism, etc., could alter adhesion at the cell level. For example, ATP- regulated actin polymerization and depolymerization rates are significantly lowered at subphysiological temperatures (9). Recent work has also suggested that these temperature-sensitive cytoskeletal changes could play a crucial role in cell adhesion strength when integrins are in a high affinity state due to the cations present (5). The importance of cytoskeletal dynamics is also bolstered by recent observations, which challenge the current paradigm that force-induced cell detachment of integrin-mediated cell adhesion is limited by integrin bond strength, i.e., that attachment strength is a direct measure of integrin-ECM bond strength, e.g. (10–12). Although some quantitative assessments of cell adhesion are in agreement with this paradigm (13,14) others have detected cell components, i.e., integrins and other FA proteins, that remain on the substrate after cell detachment (5,15,16). Although these recent data would suggest that detaching cells break their connection to the ECM higher up than at the integrin-ECM interface, i.e., somewhere between the FA-cytoskeleton, the frequency of occurrence of this detachment mechanism and its functional impact on detachment strength has yet to be determined.
Here, we analyze the molecular mechanisms that control cellular detachment under an externally applied force. These data reveal that detaching cells leave a footprint containing FA proteins behind. Drug- and temperature-induced stabilization of the cytoskeleton significantly increases attachment strength. As this increased strength is several-fold higher than attachment strength of cells with integrins cross-linked to the substrate, this demonstrates that the cytoskeleton can disconnect from the FAs during cellular detachment. Our data further suggest that this disconnection mechanism represents a cellular function, which can differ between cell type and state, resulting in cell type specific differences in apparent attachment strength.
Materials and Methods
Cell culture and reagents
Mouse NIH 3T3 fibroblast cells, human WI38 fibroblast cells, and human HT1080 fibrosarcoma cells were obtained from American Type Culture Collection (ATCC, Manassas, VA) and cultured in their respective media, noting typical formulations from Life Technologies (Carlsbad, CA) (a comprehensive table of the media conditions can be found in (5)). All cells were cultured at 37°C in a humidified incubator containing 5% carbon dioxide. Integrins were cross-linked by DTSSP (3,3′-dithiobis(sulfosuccinimidylpropionate)) at 1 mM (Thermo Scientific, Carlsbad, CA). The F-Actin stabilizer phalloidin oleate (PO) was added at 100 μM (Sigma, St. Louis, MO). The inhibitors cytocholasin D and latrunculin A (Sigma) were added at 0.5 μM and 1 μM, respectively. All chemicals were added in serum free conditions (0.5 mM MgCl2 and 1 mM CaCl2 and 4.5 mg/ml dextrose) for times indicated. Unless otherwise noted, cell culture products purchased were from Life Technologies.
Cell adhesion strength
25-mm glass coverslips (Fisher Scientific, St. Louis, MO) were sonicated with ethanol and pure water before being used for incubation of 10 μg/ml human fibronectin (isolated from serum (17)) or 20 μg/ml type I collagen (rat tail, BD Biosciences, Franklin Lakes, NJ) for 60 min at room temperature. Under regular conditions cells were allowed to attach for 24 h at 37°C and 5% CO2. The coverslips were then mounted on a custom-built spinning disc device and dipped into the temperature-controlled spinning buffer (37°C). As spinning buffer, phosphate buffered saline (PBS) (without magnesium and calcium or with 0.5 mM MgCl2 and 1 mM CaCl2 (Cellgro, Manassas, VA). All spinning buffers contained 4.5 mg/ml Dextrose. Once immersed into the spinning buffer, coverslips were spun for 5 min at defined angular velocities and fixed with 3.7% formaldehyde immediately after spinning.
Quantification of adhesion strength
Shear stress τ by radial fluid motion over the surface of the coverslip was calculated according to (11) such that:
| (1) |
where r is the radial position from the center of the disk, ρ is the buffer density, μ is the buffer viscosity, and ω is the rotational speed. The viscosity and density of PBS are very similar to water (18,19) and because the viscosity is highly temperature-dependent, values were obtained as a function of temperature (20). To obtain quantitative information of adhesion strength, whole 25 mm coverslips were imaged at 10× magnification on a Nikon Ti-S microscope (Tokyo, Japan; ∼1000 individual images stitched together with Metamorph 7.6 software and custom macros (Molecular Devices, Sunnyvale, CA)) and analyzed using a custom written MATLAB program (The MathWorks, Natick, MA). In brief, the user defines the outer circle of the coverslip from a stitched overview image and the software then finds the position of each nucleus relative to the center of the coverslip. Cell densities as a function of radial position and subsequently shear, are stored and combined with other measurements, e.g., those obtained at different revolutions per minute. A sigmoidal fit is used to quantify values of adhesion strength and determine the statistical error of the fit. Additionally, to determine cell alignment, cell morphology was analyzed similarly as a function of shear for each cell when stained for actin cytoskeleton.
Immunofluorescence staining and focal adhesion analysis
Fixed cells were incubated for 10 min with 0.25% Triton X-100 followed by 1% albumin overnight at 4°C for blocking. Primary paxillin antibody (1:2000, ab32084, Abcam (Cambridge, MA)) was applied for 2 h at room temperature, and then a secondary AlexaFluor 488-conjugated antibody (1:2000, Invitrogen) was applied for 1 h or rhodamine phalloidin (1:2000 Invitrogen) and Hoechst 33342 (3.2 μM, Invitrogen (Carlsbad, CA)) for 30 min at room temperature. The cells were subsequently mounted with Fluoromount-G (Southern Biotech, Birmingham, AL). All buffers used contained 1 mM MgCl2. The samples were imaged by using a CARV II confocal (BD Biosciences) Nikon Eclipse Ti-S microscope equipped with a motorized, programmable stage using a Cool-Snap HQ camera (Photometrics, Tucson, AZ) and controlled by Metamorph 7.6 (Molecular Devices). A custom written MATLAB (The MathWorks) program was used to quantify cell area and focal adhesion number and size.
Green fluorescent protein (GFP) imaging
3T3 fibroblasts were transfected with GFP-Paxillin using Lipofectamine 2000 (Life Technologies). The full-length cDNA of Paxillin fused with enhanced GFP was subcloned into pCDNA3 vector (Invitrogen).
Western blotting and focal adhesion isolation
Focal adhesions were isolated using an established protocol (21). In brief, cells were exposed to ice-cold 2.5 mM triethanolamine inducing a hypotonic shock for 3 min. By rigorous pipetting with cold water containing protease inhibitor tablets (complete mini EDTA-free, Roche (Basel, Switzerland)), the remaining cell bodies were removed by hydrodynamic force. For samples subjected to shear, the center of the coverslips was covered with Parafilm that had been circularly cut using a crafting punch device (The Punch Bunch, Temple, TX) and removed before shear application to ensure that all cells were subjected to a minimum shear of 600 dynes/cm2. FAs were collected with mRIPA buffer (50 mM HEPES pH 7.5, 150 mM NaCl, 1.5 mM MgCl2, 1% Triton X-100, 1% Na-DOC, and 0.1% sodium dodecyl sulfate (SDS)) with 1 mM EGTA, 1 mM Na3VO4, 10 mM Na4P2O7, and 1 mM phenylmethanesulfonylfluoride for Western blots. Samples were run in 10% SDS-polyacrylamide gel electrophoresis (PAGE) gels at 150 V until proteins were separated and then transferred to polyvinylidene fluoride membranes (Bio-Rad, Hercules, CA) to be run at 100 V for 1 h 15 min in the transfer apparatus (Bio-Rad). The membranes were washed in Buffer A (25 mM Tris-HCl, 150 mM NaCl, and 0.1% Tween-20) 15% milk overnight at 4°C and then incubated for 2 h with the following antibodies: Vinculin (ab129002) at 1/10,000, focal adhesion kinase (FAK) (ab40794) at 1/500, Paxillin (ab32084) at 1/5000 (all from Abcam), GAPDH (MAB374) at 1/2500 (Millipore, Darmstadt, Germany), Fibronectin (R457) at 1/2000 (22), and Actin (JLA20) at 1/5000 (Millipore). After three 10-min washes with Buffer A, secondary goat antirabbit HRP (Bio-Rad) and anti-mouse HRP (Abcam) were used for incubation for 30 min. Immunoblots were visualized using ECL reagent (Thermo Fisher).
Statistical analysis
Nonparametric Kruskal-Wallis analysis of variance tests were used for all statistical analysis. All data in shear plots are expressed as mean ± standard deviation. Data in box plots are expressed as mean and the 10th and 90th percentile. All experiments were performed at least in triplicate and analyses represent hundreds of cells per condition.
Results
Focal adhesions remain on substrate after cell detachment
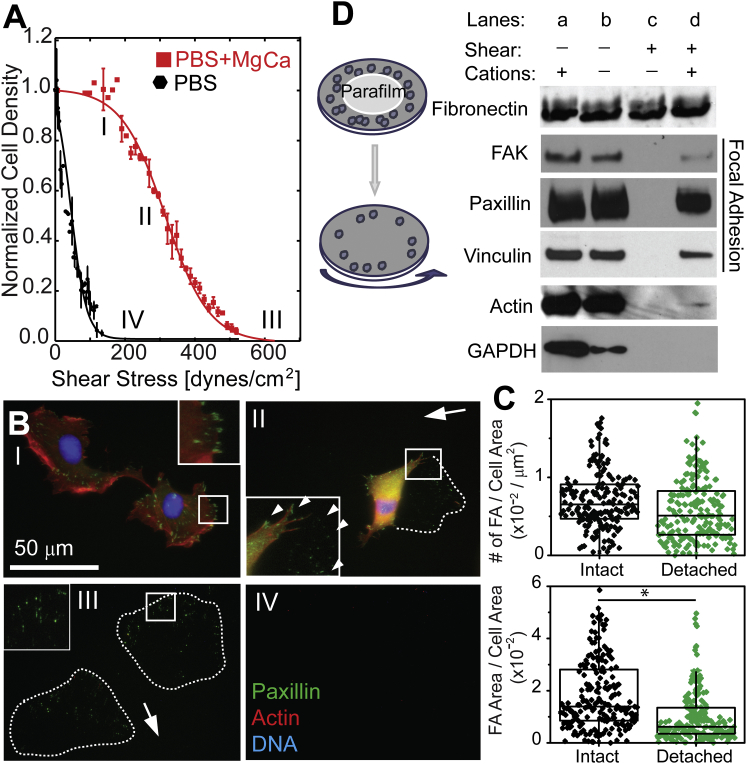
Adhesion strength, defined as the shear required to detach 50% of cells after 5 min of radially-dependent shear exposure, i.e., τ50, was assessed for human fibrosarcoma cells (HT1080) and found to vary 10-fold for cells with and without Mg+2 and Ca+2 supplemented shear buffer (Fig. 1 A; further annotated as MgCa for 0.5 mM Mg+2 and 1 mM Ca+2 and is the standard shear buffer condition). However, as cells detach from the substrate (Fig. 1 A, positions II and III), paxillin puncta were observed at the cells’ leading edge relative to the shear direction (Fig. 1 B, position II) or puncta resembling cellular footprints when completely detached (Fig. 1 B, position III). These paxillin puncta did not differ in number but were smaller in area compared to cells not exposed to shear (Fig. 1 C). Puncta were not observed for cells detaching in media lacking these cations (Fig. 1 B, position IV). Puncta were however also observed in the presence of Manganese (0.5mM Mn2+; Fig. S1 in the Supporting Material). Western blots in Fig. 1 D showed that cells ruptured by hypotonic shock (21) had reduced cytoplasmic components relative to cell lysate (lane b), cells subjected to only high shear lacking cations detached without leaving adhesion proteins (lane c), but cells detached in the presence of MgCa left additional FA components, e.g., FAK and vinculin, but minimal actin (lane d). These data suggest that FA proteins were firmly bound to the substrate and that rupture occurred at the interface between FAs and actin.
Figure 1.
Focal adhesion puncta remain postshear detachment. (A) Cell density is plotted as a function of shear for HT1080 cells attached to fibronectin in the presence (squares) or absence of cations (circles). T50 were 313 ± 16 and 24 ± 4 dynes/cm2 for cells sheared with or without cations, respectively. (B) Representative fluorescence images showing DNA (blue), actin (red), and paxillin (green) corresponding to the indicated shear regions and conditions in (A). (C) Focal adhesion density after application of high shear (>600 dynes/cm2) in PBS+MgCa, (top) based on number of discrete adhesions or (bottom) on the area of those adhesions versus cell area, was determined for intact cells or detached cells that left behind paxillin-containing puncta. For detached cells, the area was determined by the maximum extent of the puncta. For each condition, at least 200 intact cells from triplicate experiments were scanned and analyzed. ∗p < 0.05. (D) Western blots of proteins remaining on the fibronectin substrates for four different conditions: whole cell lysate (lane a), cells that were hypotonic shocked to remove soluble, cytosolic components (bane b), cells subjected to shear without or with cations (lanes c and d, respectively). To see this figure in color, go online.
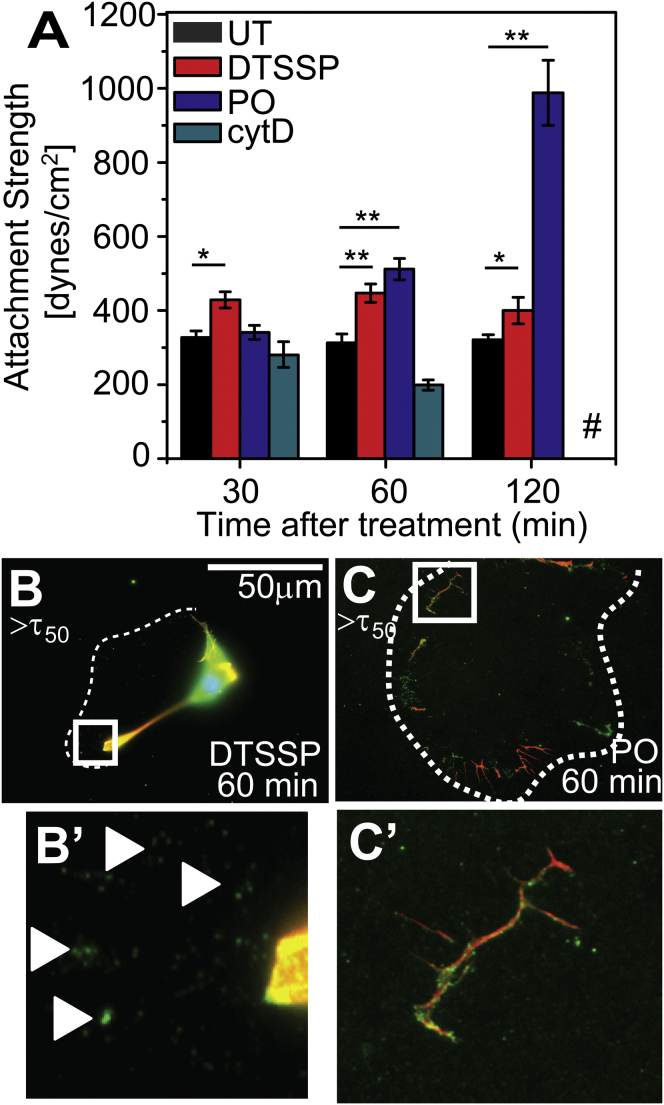
To better discern whether adhesions or the actin cytoskeleton limit adhesion strength, we treated cells with DTSSP (a cleavable homobifunctional protein cross-linking reagent) to cross-link integrins to the fibronectin-coated substrate (14) or cell-permeable PO to stabilize actin filaments, respectively, prior application of shear in PBS+MgCa conditions; note that DTSSP treatment cross-links integrins independent of cation concentrations implying that integrins, independent of their bound state, are sufficiently close to the substrate to be cross-linked to matrix proteins (Fig. S2 A). For PO treatment, cells remain viable even after short-term exposure (Fig. S2). Cross-linking integrins increased attachment strength by ∼30%, but stabilizing the actin cytoskeleton with PO increased attachment strength by ∼250% after 120 min of treatment. Depolymerizing actin had the inverse effect; cell adhesion strength was undetectable after sufficient incubation (Fig. 2 A). Because PO treatment blocks rhodamine phalloidin labeling, cells were fixed after 1 h after partial PO stabilization. Although we found that DTSSP-treated cells detached like untreated cells leaving paxillin puncta behind (Fig. 2, B and B′ arrowheads), PO-treated cells left significant portions of actin behind that colocalized with paxillin (Fig. 2, C and C′).
Figure 2.
Drug-induced stabilization of the cytoskeleton dramatically increases attachment strength. (A) Plot of the effects of drug treatment on attachment strength of HT1080 cells to fibronectin in PBS+MgCa. DTSSP was used to cross-link integrins to fibronectin. To stabilize the cytoskeleton phalloidin oleate was used (PO, third bar) and cytochalasin D (cytD, fourth bar) was used to destabilize the cytoskeleton. (#) indicates attachment strength below measurement threshold.∗p < 0.01, ∗∗p < 0.001. (B and C) Representative fluorescence images showing DNA (blue), actin (red), and paxillin (green) indicate that DTSSP-treated cells detach similar to untreated cells by leaving paxillin but not actin behind (B) and that PO-treated cells leave paxillin as well as actin behind after detachment (C). To see this figure in color, go online.
These data show that the actin cytoskeleton plays a crucial role in regulating attachment strength through drug treatments. However timing, dosing, and other side effects complicated this method, making an alternative method of stabilization desirable.
Temperature dependence of attachment strength
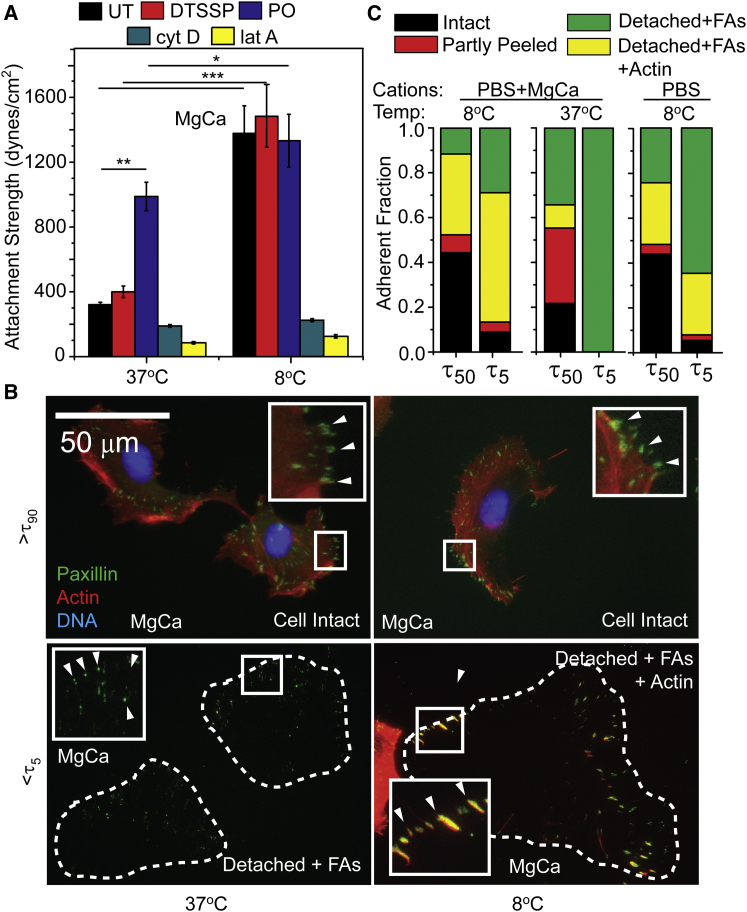
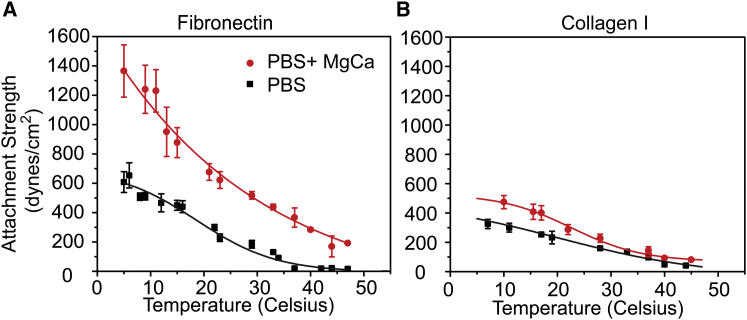
As a less intrusive means of stabilizing the cytoskeleton, media temperature was decreased only during shear application, which has been shown to limit actin depolymerization (9) but does not significantly alter focal adhesion assembly (Fig. S3). In PBS+MgCa conditions and at 8°C, HT1080 cell attachment strength increased for all conditions compared to 37°C except when the cytoskeleton was disassembled with cytocholasin D (cytoD) or latrunculin A (lat A) (Fig. 3 A). Although FAs and actin were stable at the lowest shear, >τ90, regardless of temperature, nearly complete cell detachment occurred with most paxillin puncta containing actin at 8°C with the highest shear, <τ5; at physiological temperature, paxillin puncta did not contain actin (Fig. 3 B). Quantification of these data in the presence of cations at 8°C shows that actin being left in adhesions is the predominant detachment mode but that either a change in temperature or MgCa cations can shift detachment from actin rupture to FAs (Fig. 3 C). Measurement temperature varies for cell attachment strength assays, e.g., centrifugation assays at 4°C (23) versus shear assays performed at room temperature (11), and thus we examined temperature dependence over the range used in these assays. There was a significant increase in attachment strength with temperature, which could be modulated by cation concentration and the matrix protein used as the adhesive substrate (Fig. 4). Interestingly, without cations, attachment strength at lower temperatures is higher than at physiological 37°C in the presence of cations, suggesting that lowered temperature prevents integrins from switching to a lower affinity confirmation. Overall, independent of cation concentration or ligand type, attachment strength increased with lowering temperature and thus these data further show the importance of the cytoskeleton to attachment strength and warrant a careful temperature control to ensure reliability and repeatability.
Figure 3.
Temperature-induced stabilization of the cytoskeleton dramatically increases attachment strength. (A) The attachment strength of HT1080 cells to fibronectin in PBS+MgCa conditions is shown after treatment with DTSSP (60 min), PO (120 min), cytD (60 min), or lat A (30 min). Temperature during shear application was either 37°C or 8°C. (B) Representative fluorescence images showing DNA (blue), actin (red), and paxillin (green) of cells subjected to shear at 37°C (left) or 8°C (right) at indicated shear in PBS+MgCa conditions, relative to their condition attachment strength, where <τ90 indicates that at least 90% of the cells remained attached, whereas >τ5 indicates >95% have detached. (C) Quantification of detachment mechanisms using at least 100 cells (or footprints) per condition. Cells were characterized as Intact (e.g., B, top), partly peeled defined as nucleus present and paxillin puncta were observed at cells’ leading edge relative to the shear direction (e.g., B, middle left), Detached+FAs, only paxillin puncta resembling cellular footprints visible (e.g., B, bottom left), Detached+FAs+Actin, paxillin puncta resembling cellular footprints and actin visible. To see this figure in color, go online.
Figure 4.
Attachment strength monotonically increases with decreasing temperature independent of substrate. Attachment strength of HT1080 cells in dependence of temperature with cations (circles) or without cations (squares) on fibronectin (A) or Collagen type 1 (B). To see this figure in color, go online.
FA fracture is preserved in other cell types
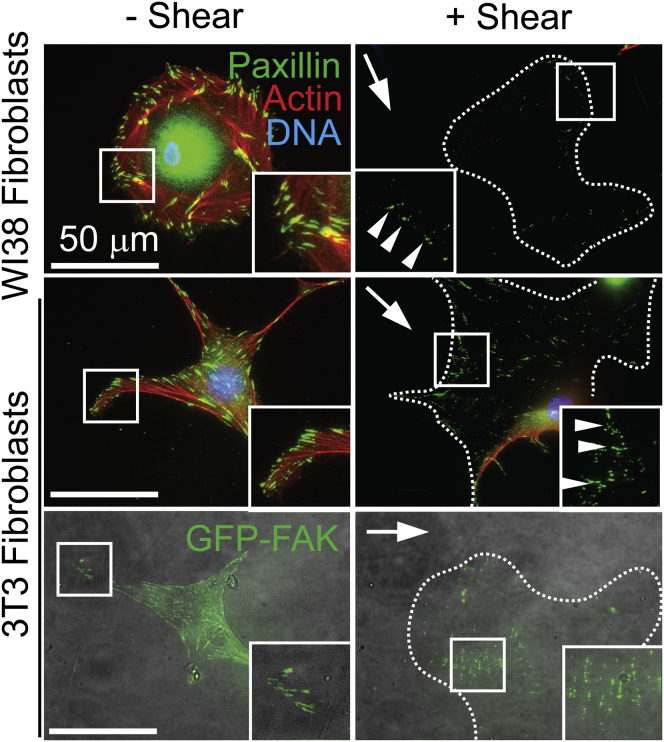
To determine how ubiquitous detachment modes are, human WI38 and mouse 3T3 fibroblasts were subject to high shear, <τ5, in the presence of MgCa at 37°C. As with fibrosarcoma cells (Fig. 1 B), fibroblasts that detached from the substrate left paxillin puncta bound to the substrate (Fig. 5, top and middle). To exclude complications from staining and examine other FA proteins, GFP-FAK transfected fibroblasts were also examined, and we found that they left GFP-FAK bound to substrates (Fig. 5, bottom). Similarly, attachment strength increased with reduced temperature during application of shear for both fibroblast lines (Fig. S4), in most cases more dramatically than with HT1080 cells and always dependent on cation, substrate, and temperature conditions (Fig. 6). Given that these cell types reside in a different niche in vivo, our data suggest that cell type specific differences correlate with such differences.
Figure 5.
Detachment by FA disconnection is ubiquitous in mammalian cells in media containing cations at 37°C. (Left) Fluorescence images of Wi38 and 3T3 fibroblasts on top and middle, respectively, were stained for DNA (blue), actin (red), and paxillin (green). On the bottom, 3T3 fibroblasts are shown in brightfield (gray) with the FAK (green) image overlaid. Images are shown without shear (left) and after shear-induced cell detachment (right). Arrows indicate shear direction. Arrowheads denote focal adhesion left on the coverslip after detachment. To see this figure in color, go online.
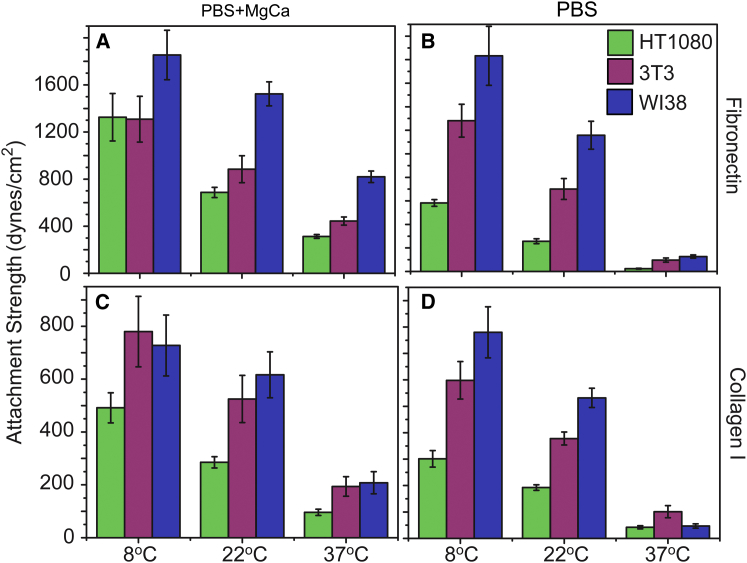
Figure 6.
Dependence of attachment strength on temperature is attachment strength ubiquitous in mammalian cells. Attachment strength in temperature dependence with cations (A and C) or without (B and D) for HT1080 fibrosarcoma cells, 3T3 mouse fibroblasts, and Wi38 human fibroblasts to fibronectin (A and B) and type 1 collagen (C and D). To see this figure in color, go online.
Discussion
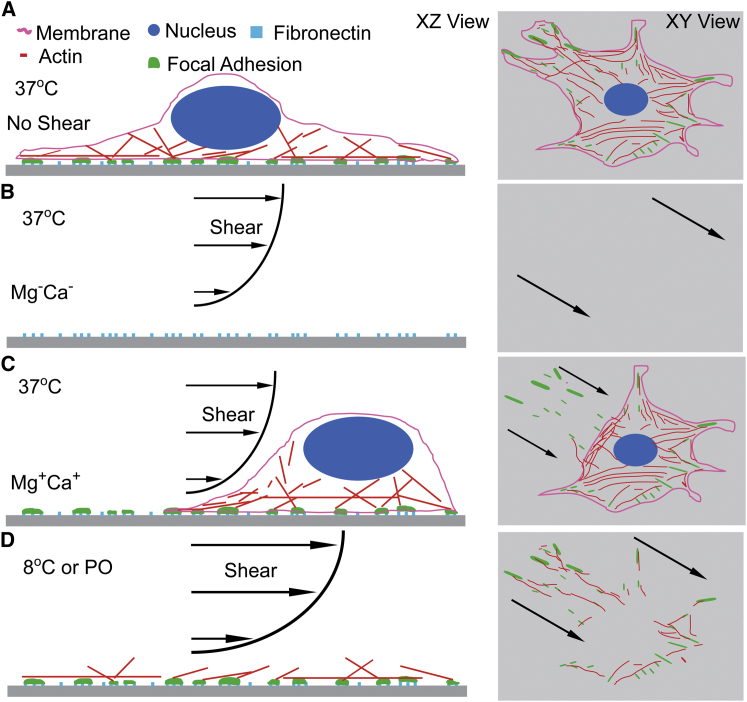
Quantification of adhesion is commonly used to understand cell mechanisms, and thus it is crucial to understand which variables adhesion assays measure. When cations are present during the application of shear at concentrations consistent with that observed in tissue (24,25), cells do not detach completely (Fig. 7). Instead, we observed that they leave behind a significant portion of their FAs, including Paxillin, Vinculin, and FAK, but not actin, which is consistent with other observations made without further quantification (5,15,16). These data are similar to the trailing edge of cell migration in two-dimensional, where cells also leave pieces of their FAs behind (26). We only observed complete cellular detachment without any FA proteins left on the substrate during application of shear in the absence of any cations, suggesting that integrin affinity was markedly reduced under this condition to allow cells to detach together with their integrins and FAs. This is in sharp contrast to the commonly held assumption that maximal integrin-ECM binding strength would limit cell adhesion strength and thus cell adhesion strength assays would measure integrin-ECM binding strength (10–12).
Figure 7.
Illustration of detachment mechanisms. Detachment modes are illustrated here and correspond to the (A) no shear condition, (B) shear without cations at 37°C, (C) shear with cations at 37°C, and (D) shear at 8°C or with actin stabilized by phalloidin oleate. To see this figure in color, go online.
Cell attachment strength may represent detachment by FA rupture
Previous reports using a rotating disc chamber, e.g. (11,14,27,28). or a centrifugation device (29) linked cell adhesion strength to the sum of individual integrin-ECM bond strengths. However much higher bond stabilities, and therefore cell adhesion strength, are suggested by theoretical models of multiple receptor-ligand pairs cooperating under external force (30–32). These models appear consistent with our observations that detachment by adhesion rupture is ubiquitous under defined cation conditions though it is difficult to directly apply these models to cell attachment as thousands of integrins cooperatively bind. To demonstrate their agreement in principle, we first cross-linked integrins to their ligands, which resulted in a modest increase in attachment strength and disconnecting at the FA-cytoskeleton interface. Conversely altering the cytoskeleton had much more drastic effects than cross-linking integrins; PO-stabilized actin increased attachment strength drastically more than cross-linking integrins, whereas preventing actin polymerization with cytochalasin D completely abolished attachment strength. However, the long incubation time required for PO treatment likely alters cell function and thus may prevent further analysis. Polymerization rates were also perturbed with temperature, with low temperatures limiting the cells’ ability to dynamically respond to force and creating qualitatively similar affects in cell detachment. Combined temperature and integrin cross-linking or actin stabilization did not increase attachment strength at low temperature suggesting that environmental conditions were sufficient to prevent actin depolymerization. At these conditions, integrin-ECM bond strength was still not limiting attachment strength, as cells left FA proteins as well as actin filaments behind. Together, these data suggest that the actin cytoskeleton disconnects in proximity to the FA during this mode of detachment, i.e., at the motor-clutch interface (33).
Adhesion strength differences arise from measurement conditions between experiments and cell type differences
As many quantitative adhesion strength assays operate over short periods of time (minutes), measurement conditions, such as cation concentrations or temperature, are often less controlled and may give rise to the variability observed between studies. For example, HT1080 attachment strength on fibronectin coverslips has ranged between 300 (5) and 700 dynes/cm2 (34) despite the same presence of cations. The data here provide a possible explanation; assay temperature was room (34) and physiological (5). When plotting these data on the adhesion strength versus temperature curve in Fig. 4 A, one can see how well attachment strength agrees with these prior reports. Adhesion experiments have commonly been conducted at not further specified room temperature (11,14,34), and at room temperature for 3T3 and WI38 fibroblasts, cation concentration and cation composition do not significantly affect adhesion strength unlike at physiological temperature (Figs. 6 and S4).
Although attachment strength generally increases with lower temperature for all tested cells, we noted some striking differences. HT1080 fibrosarcoma cells appear to be more sensitive to cation removal at physiological temperatures (5). Although 3T3 and WI38 fibroblasts are almost insensitive to cation removal at low temperature, attachment strength of HT1080 is reduced by >50% on both ligands, fibronectin and type I collagen (Fig. 6). As cellular functions should be halted at low temperatures, this may suggest unique integrin expression patterns between HT1080 cells and 3T3 or WI38 cells, which modifies the integrin affinities of one cell type to be more so than another.
Conclusions
Our data appears to support the notion that under certain high-affinity integrin conditions, attachment strength assays do not necessarily measure how strongly integrins bind the cells to their ECM; rather other weaker interfaces within the cell may rupture. Fig. 7 illustrates the different cell detachment mechanisms and the specific conditions where they occur. As classically described in the absence of cations and at 37°C, cells detach at their integrins during shear application, but we found that in the presence of cations, attachment strength increased and detachment occurred at the FA-actin interface. Further stabilization of the actin cytoskeleton, chemically or with temperature, resulted in rupture occurring within actin filaments. Given the ubiquity of these observations, these data suggest that adhesion assays must account for these environmental factors that may shift what the assay measures from assessing integrin binding strength to other internal structures.
Author Contributions
A.F. performed all experiments and analyzed data. A.F. and A.J.E. designed experiments and wrote the article.
Acknowledgments
The authors thank Drs. Caroline Damsky and David Strom for antibodies obtained via the Developmental Studies Hybridoma Bank under the auspices of the National Institute of Child Health and Human Development (NICHD) and maintained by The University of Iowa (Iowa City, IA). The spinning disc device was designed and manufactured by Jeremy Riley, Ryan Tam, Joe Shu, and the UCSD Campus Research Machine Shop. GFP-FAK was kindly provided by Dr. Shu Chien.
This work was supported by grants from the National Institutes of Health (DP2OD006460 and U54CA143803) and Department of Defense (W81XWH-13-1-0133).
Editor: Douglas Robinson.
Footnotes
Four figures are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(15)00585-8.
Supporting Material
References
- 1.Kong F., García A.J., Zhu C. Demonstration of catch bonds between an integrin and its ligand. J. Cell Biol. 2009;185:1275–1284. doi: 10.1083/jcb.200810002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell I.D., Humphries M.J. Integrin structure, activation, and interactions. Cold Spring Harb. Perspect. Biol. 2011;3:1–14. doi: 10.1101/cshperspect.a004994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lodish H. Macmillan; 2008. Molecular Cell Biology. [Google Scholar]
- 4.Hughes P.E., Pfaff M. Integrin affinity modulation. Trends Cell Biol. 1998;8:359–364. doi: 10.1016/s0962-8924(98)01339-7. [DOI] [PubMed] [Google Scholar]
- 5.Fuhrmann A., Li J., Engler A.J.A. Cation type specific cell remodeling regulates attachment strength. PLoS ONE. 2014;9:e102424. doi: 10.1371/journal.pone.0102424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griffin M.A., Engler A.J., Discher D.E. Patterning, prestress, and peeling dynamics of myocytes. Biophys. J. 2004;86:1209–1222. doi: 10.1016/S0006-3495(04)74195-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedrichs J., Legate K.R., Benoit M. A practical guide to quantify cell adhesion using single-cell force spectroscopy. Methods. 2013;60:169–178. doi: 10.1016/j.ymeth.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Garcia A.J., Gallant N.D. Stick and grip: measurement systems and quantitative analyses of integrin-mediated cell adhesion strength. Cell Biochem. Biophys. 2003;39:61–73. doi: 10.1385/CBB:39:1:61. [DOI] [PubMed] [Google Scholar]
- 9.Wendel H., Dancker P. Kinetics of actin depolymerization: influence of ions, temperature, age of F-actin, cytochalasin B and phalloidin. Biochim. Biophys. Acta. 1986;873:387–396. doi: 10.1016/0167-4838(86)90088-9. [DOI] [PubMed] [Google Scholar]
- 10.García A.J., Ducheyne P., Boettiger D. Quantification of cell adhesion using a spinning disc device and application to surface-reactive materials. Biomaterials. 1997;18:1091–1098. doi: 10.1016/s0142-9612(97)00042-2. [DOI] [PubMed] [Google Scholar]
- 11.Boettiger D. Quantitative measurements of integrin-mediated adhesion to extracellular matrix. Methods Enzymol. 2007;426:1–25. doi: 10.1016/S0076-6879(07)26001-X. [DOI] [PubMed] [Google Scholar]
- 12.Friedland J.C., Lee M.H., Boettiger D. Mechanically activated integrin switch controls alpha5beta1 function. Science. 2009;323:642–644. doi: 10.1126/science.1168441. [DOI] [PubMed] [Google Scholar]
- 13.Dumbauld D.W., Michael K.E., García A.J. Focal adhesion kinase-dependent regulation of adhesive forces involves vinculin recruitment to focal adhesions. Biol. Cell. 2010;102:203–213. doi: 10.1042/BC20090104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallant N.D., Michael K.E., García A.J. Cell adhesion strengthening: contributions of adhesive area, integrin binding, and focal adhesion assembly. Mol. Biol. Cell. 2005;16:4329–4340. doi: 10.1091/mbc.E05-02-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee A.M., Berny-Lang M.A., Newton P.K. A low-dimensional deformation model for cancer cells in flow. Phys. Fluids. 2012;24:81903-1–81903-14. doi: 10.1063/1.4748811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selhuber-Unkel C., Erdmann T., Spatz J.P. Cell adhesion strength is controlled by intermolecular spacing of adhesion receptors. Biophys. J. 2010;98:543–551. doi: 10.1016/j.bpj.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mao Y., Schwarzbauer J.E. Fibronectin fibrillogenesis, a cell-mediated matrix assembly process. Matrix Biol. 2005;24:389–399. doi: 10.1016/j.matbio.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Fluxion. Technical Note. Understanding effects of viscosity in the BioFlux system. 1–2.
- 19.Prot, C.M., V. Station, and T.V. Station. Application Note High Throughput Viscosity Measurement of Biologics Formulations.: 40–41.
- 20.Kestin J., Sokolov M., Wakeham W.A. Viscosity of liquid water in the range −8°C to 150°C. J. Phys. Chem. Ref. Data. 1978;7:941–948. [Google Scholar]
- 21.Seog J., Kuo J., Waterman C.M. Integrin and cell adhesion molecules. Microscopy. 2012;757:297–323. [Google Scholar]
- 22.Aguirre K.M., McCormick R.J., Schwarzbauer J.E. Fibronectin self-association is mediated by complementary sites within the amino-terminal one-third of the molecule. J. Biol. Chem. 1994;269:27863–27868. [PubMed] [Google Scholar]
- 23.McClay D.R., Hertzler P.L. Quantitative measurement of cell adhesion using centrifugal force. Curr. Protoc. Cell Biol. 2001;Chapter 9 doi: 10.1002/0471143030.cb0902s00. Unit 9.2. [DOI] [PubMed] [Google Scholar]
- 24.Seltzer M.H., Rosato F.E., Fletcher M.J. Serum and tissue calcium in human breast carcinoma. Cancer Res. 1970;30:615–616. [PubMed] [Google Scholar]
- 25.Seltzer M.H., Rosato F.E., Fletcher M.J. Serum and tissue magnesium levels in human breast carcinoma. J. Surg. Res. 1970;10:159–162. doi: 10.1016/0022-4804(70)90026-0. [DOI] [PubMed] [Google Scholar]
- 26.Palecek S.P., Schmidt C.E., Horwitz A.F. Integrin dynamics on the tail region of migrating fibroblasts. J. Cell Sci. 1996;109:941–952. doi: 10.1242/jcs.109.5.941. [DOI] [PubMed] [Google Scholar]
- 27.Boettiger D., Lynch L., Huber F. Distinct ligand-binding modes for integrin alpha(v)beta(3)-mediated adhesion to fibronectin versus vitronectin. J. Biol. Chem. 2001;276:31684–31690. doi: 10.1074/jbc.M103997200. [DOI] [PubMed] [Google Scholar]
- 28.Shi Q., Boettiger D. A novel mode for integrin-mediated signaling: tethering is required for phosphorylation of FAK Y397. Mol. Biol. Cell. 2003;14:4306–4315. doi: 10.1091/mbc.E03-01-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keselowsky B.G., Collard D.M., García A.J. Surface chemistry modulates fibronectin conformation and directs integrin binding and specificity to control cell adhesion. J. Biomed. Mater. Res. A. 2003;66:247–259. doi: 10.1002/jbm.a.10537. [DOI] [PubMed] [Google Scholar]
- 30.Williams P.M. Analytical descriptions of dynamic force spectroscopy: behaviour of multiple connections. Anal. Chim. Acta. 2003;479:107–115. [Google Scholar]
- 31.Sulchek T., Friddle R.W., Noy A. Strength of multiple parallel biological bonds. Biophys. J. 2006;90:4686–4691. doi: 10.1529/biophysj.105.080291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lo Schiavo V., Robert P., Bongrand P. Quantitative modeling assesses the contribution of bond strengthening, rebinding and force sharing to the avidity of biomolecule interactions. PLoS ONE. 2012;7:e44070. doi: 10.1371/journal.pone.0044070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan C., Odde D. Traction dynamics of filopodia on compliant substrates. Science. 2008;322:1687–1691. doi: 10.1126/science.1163595. [DOI] [PubMed] [Google Scholar]
- 34.Engler A.J., Chan M., Schwarzbauer J.E. A novel mode of cell detachment from fibrillar fibronectin matrix under shear. J. Cell Sci. 2009;122:1647–1653. doi: 10.1242/jcs.040824. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.