Abstract
1,25-Dihydroxyvitamin D 3 is immunosuppressive both in vivo and in vitro. Topical vitamin D analogs such as calcipotriol alter keratinocyte function, but their effects on cutaneous immune responses are less well understood. We demonstrate that exposure of the skin to calcipotriol before transcutaneous immunization with OVA protein and CpG adjuvant prevents Ag-specific CD8+ T cell priming coincident with Langerhans cell depletion in the skin. Immunization through calcipotriol-treated skin induces CD4+CD25+ regulatory T cells (Treg) that prevent subsequent Ag-specific CD8+ T cell proliferation and IFN-γ production. Treg induced by calcipotriol are able to inhibit the induction and the elicitation of protein contact hypersensitivity. Topical calcipotriol treatment also induces RANKL (receptor activator of NF-κB ligand) expression by keratinocytes, a TNF family member involved in modulation of skin dendritic cells. UV light B induces Ag-specific tolerance when it is applied before transcutaneous immunization. We suggest that UV light B-induced tolerance is induced via a vitamin D receptor-dependent mechanism as vitamin D receptor (VDR) knockout mice fail to increase FoxP3+ Treg in their peripheral draining lymph node following irradiation. Additionally, keratinocytes of VDR−/− mice fail to induce RANKL upon UV irradiation or calcipotriol treatment. The in vivo expansion of Ag-specific Treg with the topical application of the vitamin D analog calcipotriol followed by transcutaneous immunization is a simple method to augment functional Ag-specific CD4+CD25+Foxp3+ Treg populations and mimics Ag-specific UV-induced tolerance.
The CD4+CD25+ regulatory T cells (Treg)3 play a significant role in maintaining peripheral tolerance (1). Strategies to expand these cells or to induce the differentiation of such cells in vivo are being pursued to treat allergic and autoimmune disease. The activated metabolite of vitamin D (1,25-dihydroxyvitamin D3, VD3) exerts actions through its nuclear receptor, the VD3 receptor (VDR) (2, 3). Expression of VDR on immune cells such as dendritic cells and T cells suggests that the function of VD3 extends well beyond bone metabolism and calcium homeostasis. A number of studies have shown that VD3 is immunosuppressive in both humans and animals. VD3 has an inhibitory effect on dendritic cell (DC) maturation and differentiation in vitro, which results in a marked decrease in T cell stimulatory capacity (4). Furthermore, dietary VD3 analog supplementation expands Treg and prevents auto-immune diabetes in the NOD mouse (5). Use of such analogs for immunomodulation in vivo has been hampered by concern over toxicities, including hypercalcemia; however, topical analogs of VD3 such as calcipotriol (MC903) have been used clinically for >10 years in the treatment of psoriasis without systemic toxicity (6). The effect of this topical treatment on immune suppression and Treg has not been explored.
The skin is an excellent organ for immunization. Exposure of protein Ag through the skin induces dominant Th2 T cell and Ab responses and is termed transcutaneous immunization (TCI) (7). Concurrent topical adjuvant application can promote Th1 skewing and safely increase the amplitude of the response (8). The skin likewise is an ideal site for tolerance induction, as UV light (UV) exposure of skin suppresses T cell-mediated immune responses, and TCI through UV-exposed skin results in the generation of Ag-specific Treg (9). Keratinocytes up-regulate RANKL (receptor activator of NF-κB ligand) expression upon UV exposure, promoting the expansion of FoxP3+ Treg in mouse skin-draining lymph nodes (LN) (10) and possibly enabling the generation of Ag-specific Treg. Given the oncogenicity of UV light, we investigated whether topical application of the VD3 analog calcipotriol before protein TCI would affect the outcome of immunization, and if Ag-specific Treg could be generated. Herein, we demonstrate that TCI through skin treated with the VD3 analog calcipotriol abolishes Ag-specific CD8+ T cell priming and further induces CD4+CD25+ Treg, thereby promoting Ag-specific tolerance. Both UV and calcipotriol treatment induce RANKL expression in the skin. VDR-deficient mice fail to up-regulate keratinocyte RANKL expression in response to UV or calcipotriol and do not demonstrate Treg accumulation in the skin-draining LN. This work suggests that UV-mediated RANKL expression may be induced through cutaneous VDR stimulation, and it proposes a new and practical method of inducing Ag-specific Treg in vivo.
Materials and Methods
Mice
C57BL/6 mice were purchased from Charles Rivers Laboratories. OT-I, OT-II, and FoxP3gfp mice (11) were purchased from The Jackson Laboratory. VDR knockout (KO) mice (12) (on a mixed C57BL/6 and DB-1 background) were kindly provided Dr. S. Kato (University of Tokyo). VDR genotypes were determined on genomic DNA from tail clips as previously reported (13). VDR KO mice were raised from weaning on 2% calcium and 20% lactose rescue diets (14). All animal experiments were conducted according to institutional animal care protocol requirements.
Vitamin D treatment
Calcipotriol (Donovex; Leo Pharma) ointment (50 μg/g) or plasticized base (5% polyethylene glycol and 95% mineral oil) was applied 30 mg/ mouse on the shaved dorsal skin of mice before TCI.
UV radiation
UV radiation was provided by four FS40 TL2 lamps (National Biological). FS40 lamps emit 3% in the UVC range, 45% in the UVB range, and 52% in the UVA range. The emission peak is at 310 nm (in the UVB range). The irradiance of the source at the center averaged 10 J/m2/s, as measured by an IL400A radiometer, using an SEL 240 UVB detector (International Light). Groups of mice were anesthetized and subsequently irradiated on shaved dorsal skin over 4 consecutive days (daily 1200 J/m2).
Peptides and proteins
OVA protein (OVA-V; Sigma-Aldrich) and BSA (Roche Diagnostics) were used as topical immunogens.
Adoptive transfer of T cells
OT-I T cells were isolated from the pooled LN and spleen of naive OT-I mice. CD8+ T cells purified by positive selection using CD8 microbeads (Miltenyi Biotec) to >90% purity were labeled with CFSE (Molecular Probes) and 5 × 106 cells were injected into the tail vein of C57BL/6 mice on the second day of calcipotriol treatment. Mice were immunized 24 h after the last calcipotriol treatment. Mice were euthanized and skin-draining LN were analyzed 3 days following immunization (day 7).
For adoptive transfer of CD4+ T cells, peripheral LN and spleens were pooled and CD4+ T cells were negatively selected to >95% purity using an enrichment kit (StemCell Technologies); 5 × 106 CD4+ cells were adoptively transferred. CD4+CD25+ T cells were positively purified from peripheral LN and spleen using microbeads (Miltenyi Biotec) to a purity of >90%. CD4+CD25− cells were negatively selected; 2 × 106 cells (or as indicated for titration) were transferred. OT-I cells were also cotransferred as indicated. Mice were immunized 24 h after the transfer and skin-draining LN were harvested 3 days later.
Transcutaneous immunization
Immunization was performed as described (9) 1 day after the calcipotriol or vehicle treatment. Following the removal of calcipotriol or plasticized base and tape stripping to remove the stratum corneum, animals were immunized with OVA (500 μg) and 500 μg of CpG (oligodeoxynucleotide 1826, 5′-TCCATGACGTTCCTGACGTT-3′, prepared by the Oligonucleotide Synthesis Facility of the University of British Columbia). All immunogens were applied in 50 μl of PBS followed by tape occlusion for 24–48 h. Application of plasticized base (control) before TCI did not affect immune responses to TCI.
Flow cytometry
Cells were analyzed using a FACSCalibur flow cytometer (BD Biosciences) and CellQuest software following staining. Abs to CD8 (clone 53-6.7), CD4 (clone RM 4-5), IFN-γ (clone MG1.2), CD45 RA B220 (clone RA3-6B2), CD25 (clone PC16), and CD152 (clone UC10-4F10-11) were from BD Pharmingen. The Ab for FoxP3 (clone FJK-16s) was from eBioscience. Peptide-specific transgenic CD8+ T cells were identified using a PE-conjugated Kb-OVA tetramer (made in-house). Intracellular cytokine staining for IFN-γ was performed as described (9). Foxp3 staining was performed without in vitro stimulation.
Epidermal sheet preparation
Epidermal sheets were prepared, stained with anti-mouse I-Ab (clone AF6-120.1; BD Pharmingen) as described (9), and images were captured with a Zeiss Axioplan epifluorescent microscope equipped with a COHO-CCD camera.
Protein contact hypersensitivity (CHS)
OVA protein CHS responses were induced and measured as described (9). In experiments involving the transfer of cells, donor mice were treated with calcipotriol or vehicle (no treatment) and then immunized over the treated skin once with OVA protein (500 μg) and CpG (500 μg) beginning 24 h after calcipotriol treatment. Four days later, CD4+CD25+ cells were isolated from LN and spleens. Recipient mice were immunized with OVA and CpG on the back. CD4+CD25+ donor-derived cells (2 × 106) were transferred into recipient mice 4 days after recipient mouse immunization. One day later, mice were challenged with either OVA or BSA protein and ear swelling was compared with the contralateral untreated ear. To assess the Ag specificity of the response, BSA protein was also used for immunization.
Immunohistochemistry
Mice were treated daily with calcipotriol for 3 days or were UV irradiated (9). Staining of RANKL was performed on treated formalin-fixed skin using Ab to RANKL (clone 12A668; Imgenex), secondary biotin-labeled goat anti-mouse Ab, and 3,3′-diaminobenzidine tetrahydrochloride as a fluorochrome.
Statistical analysis
Groups were compared using two-tailed Student’s t tests and results were displayed using Prism 3 (GraphPad Software).
Results
Topical calcipotriol prevents the transcutaneous priming of CTL to protein Ag
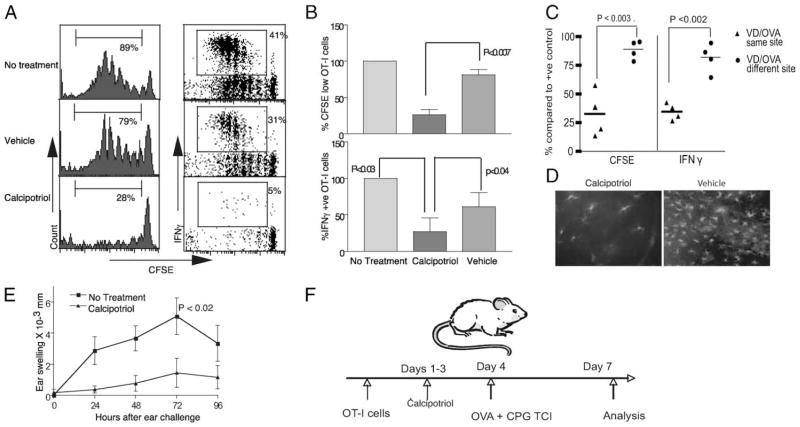
Calcipotriol is a VD3 analog with low-calcemic activity used topically for the treatment of psoriasis (6). Application of calcipotriol in the setting of psoriasis alters keratinocyte function and may have a direct suppressive effect on immune cells. To determine the effect of calcipotriol on skin immune responses, we studied the effect of this drug on protein TCI. Application of protein Ag to tape-stripped skin in mice with the coadministration of the TLR9 agonist CpG induces Ag-specific CTL (15). We used the adoptive transfer of naive Ag-specific T cells to follow CTL responses to topical Ag following calcipotriol administration. OT-I CD8+ T cells, which respond to the immunodominant Kb-restricted OVA-derived epitope, were adoptively transferred into naive hosts. Recipients were treated with three daily applications of calcipotriol ointment or control vehicle. OVA protein with CpG adjuvant was next applied to the treated skin. Labeling of the naive OT-I CD8+ T cells with CFSE allowed the detection of proliferation and intracellular cytokine analysis and detection of CTL precursors. The level of proliferation and IFN-γ production in the skin-draining LN was determined 3 days following immunization (Fig. 1F). TCI through calcipotriol, but not vehicle-treated skin, inhibited the priming of OVA-specific CTL (Fig. 1, A and B). The preventive effect of calcipotriol on OT-I proliferation was only observed when OVA Ag was applied at the same location as the calcipotriol treatment (the back) but not at a different site (the abdomen; Fig. 1C). Thus, the inhibitory effect of calcipotriol on CD8+ T cell immune activation is a local effect. Importantly, CTL priming by TCI through calcipotriol treatment is impaired even in the presence of an artificially high frequency of CTL precursors (OT-I cells). The inhibition of T cell priming was not global, as CD4+ T cell expansion to TCI was not affected (data not shown).
FIGURE 1.
Effect of topical calcipotriol upon the priming of CD8+ T cells to cutaneous Ag. CFSE+OT-I CD8+ T cells were transferred into naive B6 mice that were then treated three times daily on the back with 30 mg of calcipotriol ointment 50 μg/g or vehicle (plasticized base). Animals were next topically immunized (TCI) with OVA protein (500 μg) and CpG (500 μg). OT-I cells from the draining LN were assessed for proliferation and IFN-γ production 3 days later as indicated in F. A, Representative histograms and dot plots gated for CD8+ cells. Percentages indicate the proportion of CFSE+ cells that have divided at least once and express IFN-γ, respectively. B, Proliferation and IFN-γ production normalized to OVA-immunized but previously untreated mice. C, Suppressive effect of calcipotriol on OT-I proliferation and IFN-γ production was observed only when subsequent immunization was performed at the same site (back (same site) vs back and abdomen (different site); n = 4). D, Calcipotriol application resulted in depletion of MHC-II+ cells in skin epidermal sheets (n = 2–4). E, Calcipotriol treatment before immunization inhibited induction of OVA protein CHS as measured by ear swelling in mice following OVA application to the ears (n = 6 mice/group; sum of two experiments); all error bars represent SEM.
In vitro studies have demonstrated that DC function and phenotype may be modified directly by VD3 (4). Human (6) and murine Langerhans cells (LC) express vitamin D receptors that may modulate their function (16). Treatment of epidermal cells with VD3 in vitro inhibits the production of keratinocyte-derived GM-CSF, an activation factor for LCs, which results in suppression of the ability of LC to stimulate allogeneic T cell proliferation (17). Additionally, a direct immune-suppressive effect of VD3 treatment on purified LCs derived from mouse skin has been shown (18). To investigate the effect of calcipotriol on murine LC in vivo, the frequency of these cells within epidermal sheets was assessed following drug application. The number of MHC-II+ cells (LC) was visibly diminished in epidermal sheets 24 h after repeated treatment with calcipotriol when compared with vehicle (Fig. 1D). Thus, topical calcipotriol administration inhibits the generation of subsequent CD8+ T cell responses to TCI and results in diminished numbers of intraepidermal LC.
Application of protein with adjuvant to tape-stripped skin induces CTL that mediate protein CHS (9). To determine the physiologic significance of inhibition of CTL priming by calcipotriol, the elicitation of a protein CHS response following priming was studied (Fig. 1E). Mice were treated with calcipotriol or vehicle and then immunized with protein Ag. Five days after immunization, mice were challenged with OVA on the ears and the resultant ear swelling was measured. Mice treated with calcipotriol before OVA sensitization had significantly reduced ear swelling compared with control OVA only-sensitized mice without prior calcipotriol treatment. These results demonstrate that topical calcipotriol impairs the CTL priming response to cutaneous Ag (induction of protein CHS), and they support a recent human study in which topical application of calcipotriol diminished the induction of CHS to dinitrofluorobenzene (19). This also indicates that physiologically significant alteration in skin immune function is an important mode of action of this drug.
CD4+CD25+ T cells induced by immunization through calcipotriol-treated skin transfer tolerance in an Ag-specific manner
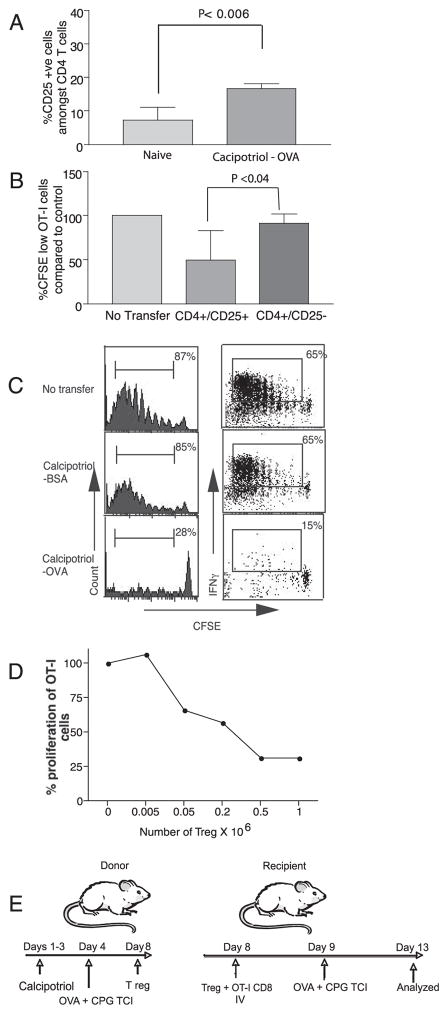
Polyclonal Treg prevent autoimmunity and control host immune responses (20). Topical application of soluble VD3 induced the activation of CD4+CD25+ Treg in the draining LN of BALB/c mice. These activated Treg were able to suppress the proliferation of transgenic OVA-specific DO11.1 CD4 T cells in vitro (21). We have previously demonstrated that TCI through UV light-treated skin can be used to expand Ag-specific Treg (9). UV irradiation induces biosynthesis of the activated form of vitamin D in the skin. We next sought to determine whether topical calcipotriol treatment before TCI could likewise expand Ag-specific Treg. Preliminary experiments demonstrated that the adoptive transfer of purified CD4+ T cells from animals treated with calcipotriol and immunized to OVA by TCI into naive hosts would inhibit subsequent CTL priming to OVA Ag (unpublished data). Application of calcipotriol and subsequent OVA TCI resulted in an increase in the fraction of CD4+CD25+ T cells in the draining LN (Fig. 2A). To identify the cellular subset of CD4+ T cells responsible for the transfer of tolerance, 2 × 106 purified CD4+CD25+ T cells or CD4+CD25− T cells from mice immunized to OVA by TCI following calcipotriol application were transferred into naive mice. Recipient mice were next immunized with OVA, and CTL priming was assessed by OT-I transfer (Fig. 2B, as depicted in Fig. 2E). CD4+CD25+ T cells, but not CD4+CD25− T cells from previously calcipotriol-treated and OVA-immunized animals, inhibited the priming of OT-I cells. The suppression of OVA-specific OT-I cells mediated by adoptive transfer of CD4+CD25+ cells was Ag-specific, as no suppression was induced when these cells were transferred from animals immunized to BSA by TCI after calcipotriol treatment as compared with cells from animals immunized to OVA (Fig. 2C). In these experiments as few as 5 × 104 Treg were sufficient to transfer the tolerance and inhibit OT-I proliferative responses (Fig. 2D). Thus, Ag-specific Treg induced by TCI through calcipotriol-treated skin are highly efficient in suppressing the priming of potentially damaging Ag-specific CTL.
FIGURE 2.
Topical calcipotriol followed by TCI induces Ag-specific CD4+CD25+ Treg. A, The fraction of CD25+ T cells among CD4+ T cells in the skin-draining LN was determined after three daily applications of calcipotriol, followed by OVA immunization (n = 6/group). B, Effect of CD4+ T cell subsets transferred from calcipotriol-treated mice upon CD8+ T cell priming responses in previously naive mice. CD4+CD25+ T cells or CD4+CD25− T cells from calcipotriol-treated and OVA-immunized donors were purified from peripheral LN and spleen 4 days after immunization and were transferred into recipients along with OT-1 CD8+ T cells (as depicted in E). Recipients were next immunized with OVA/CpG. CD4+ CD25+ T cells from calcipotriol and OVA-immunized mice suppressed proliferation of OT-I cells (n = 6/group; ordinate shows percentage control response). C, Ag specificity of CD4+CD25+ cells. No inhibition of CD8+ T cell proliferation or IFN-γ production was noted when 2 × 106 purified CD4+CD25+ T cells from mice treated with calcipotriol and immunized with BSA were transferred (n = 4). D, Calcipotriol-induced Treg efficiently prevented OT-I cell proliferation in vivo. As few as 5 × 104 cells were sufficient to suppress the proliferation of 5 × 106 adoptively transferred OT-I cells.
Immunization through calcipotriol-treated skin induces Treg that prevent the elicitation of CHS responses
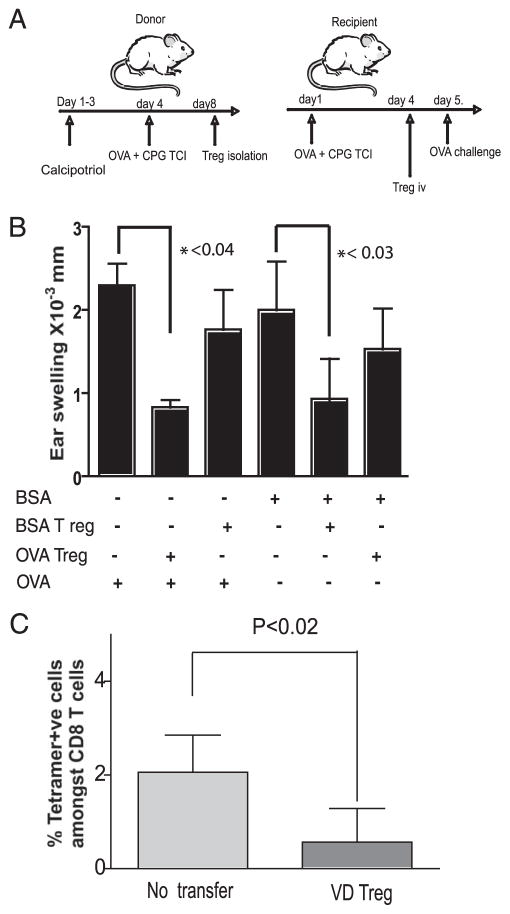
The Treg induced by TCI through calcipotriol-treated skin were capable of preventing the priming of CTL following topical TLR9 agonist application (Fig. 2). Treg induced through the skin following UV light have been reported to home poorly to the skin and to be unable to prevent the elicitation of conventional CHS unless they are directly transferred into the skin (22). However, UV-induced VD3 programs DC to enhance the epidermotropism of T cells (23) by promoting the induction of CCR10 expression on the T cells. We thus asked whether the Treg induced by TCI following topical calcipotriol and transferred i.v. could inhibit the elicitation of a protein CHS response in the skin. Mice were immunized to OVA or BSA by standard TCI. Four days later, these mice received CD4+CD25+ T cells by i.v. adoptive transfer from calcipotriol-treated and OVA-immunized or BSA-immunized mice. One day following this adoptive transfer, immunized mice were challenged with OVA or BSA protein on the ears, and protein CHS responses, as demonstrated by ear swelling, were determined (as depicted in Fig. 3A). The transfer of Treg from TCI mice following calcipotriol treatment prevented the elicitation of the protein CHS response in an Ag-specific fashion, as only the ear swelling elicitation response with the relevant protein (either BSA or OVA) was significantly inhibited (Fig. 3B). Treg expanded in vivo in this way are thus able to prevent the elicitation of an established immune response in an Ag-specific manner, suggesting that this method of tolerance induction may be of value in the inhibition of established immunity (such as autoimmunity).
FIGURE 3.
Calcipotriol-induced Treg inhibit Ag-specific CTL priming and CHS elicitation responses in vivo. A and B, Calcipotriol-induced Treg suppressed CHS response in an Ag-specific manner. CD4+CD25+ cells were isolated from the LN and spleen of donor mice (either treated with calcipotriol or untreated) 4 days after OVA or BSA immunization and were transferred into (primed) recipients immunized 4 days previously with either OVA or BSA (as depicted in A). The following day, protein CHS responses were elicited using the same protein that was used for immunization of recipients. Data are representative of two experiments (n = 5 mice/group). C, Calcipotriol-induced Treg suppressed CTL expansion. Donor mice were immunized with OVA after 3 days of topical calcipotriol administration. Four days later, 2 × 106 CD4+CD25+ cells were isolated form peripheral LN and spleen and transferred into naive recipients. Recipients were then immunized with topical OVA 1 day after transfer and reimmunized (boosted) on day 7. Kb OVA-specific CTL in the skin-draining LN were enumerated by tetramer staining on day 13. The fraction of OVA-specific CTL among CD8+ T cells was significantly less in groups receiving Treg compared with controls.
To confirm that calcipotriol-induced Treg are able to inhibit the priming of endogenously generated Ag-specific CTL, Treg induced by calcipotriol were assessed for their ability to suppress the priming and expansion of OVA-specific CTL in OVA TCI mice. The adoptive transfer of 2 × 106 CD4+CD25+ OVA and calcipotriol-induced Treg prevented the expansion of Kb OVA tetramer-positive CD8+ T cells in C57BL/6 mice subsequently immunized with OVA and boosted on day 7 (Fig. 3C). Such OVA-specific CTL (roughly 2% of the CD8+ T cells) were readily detected in the skin-draining LN by tetramer analysis 13 days after immunization in controls that did not receive adoptively transferred Treg.
Immunization through calcipotriol-treated skin expands Ag-specific FoxP3+ Treg
FoxP3 is a master regulator for Treg, required for both the development and function of CD4+CD25+ Treg (24). We next determined the fraction of FoxP3+CD25+ cells among CD4+ T cells within the draining LN following topical calcipotriol treatment. Application of calcipotriol and protein Ag (with CpG adjuvant) doubled the fraction of CD25+FoxP3+ cells among CD4+ T cells within the draining LN postimmunization (Fig. 4, A and B). This increase was noted with either OVA or BSA Ags. To determine whether Ag-specific stimulation enhanced the expansion of Ag-specific Treg, we used OT-II mice that are transgenic for the CD4+ T cells responding to the immunodominant I-Ab-restricted epitope of OVA. Whereas naive OT-II mice had a lower fraction of CD4+CD25+FoxP3+ T cells than did wild-type mice, this fraction increased 3-fold following TCI with calcipotriol and OVA compared with an undetectable expansion noted when BSA was used as Ag. B6 mice did not demonstrate a difference in the TCI-induced expansion of the FoxP3+ Treg population when either OVA or BSA was used as Ag. This demonstrates that TCI through calcipotriol-treated skin directly promotes the expansion of Ag-specific FoxP3+ Treg. Expansion of FoxP3+ Treg was not mediated by calcipotriol alone or immunization of the skin with OVA alone in B6 mice and only combined treatment resulted in a significant expansion of this population (Fig. 4C). In these studies, CpG adjuvant was used topically a priori to enhance CTL generation. The CpG used in these experiments enhanced the expansion of Treg to topical OVA in OT-II mice, possibly due to enhanced Ag transfer, but CpG treatment was not a requirement for the Treg expansion (Fig. 4C). To explore potential mechanisms mediated by calcipotriol-stimulated Treg to induce suppression, we examined the phenotype of draining LN resident and splenic Treg following 4 days of topical calcipotriol treatment and OVA-TCI. To easily identify Treg, we used recently described FoxP3-GFP reporter mice (11). Although naive FoxP3 expressing Treg constitutively expressed CTLA-4, calcipotriol and OVA TCI resulted in a significant up-regulation of this molecule on these cells (Figs. 4, D and E).
FIGURE 4.
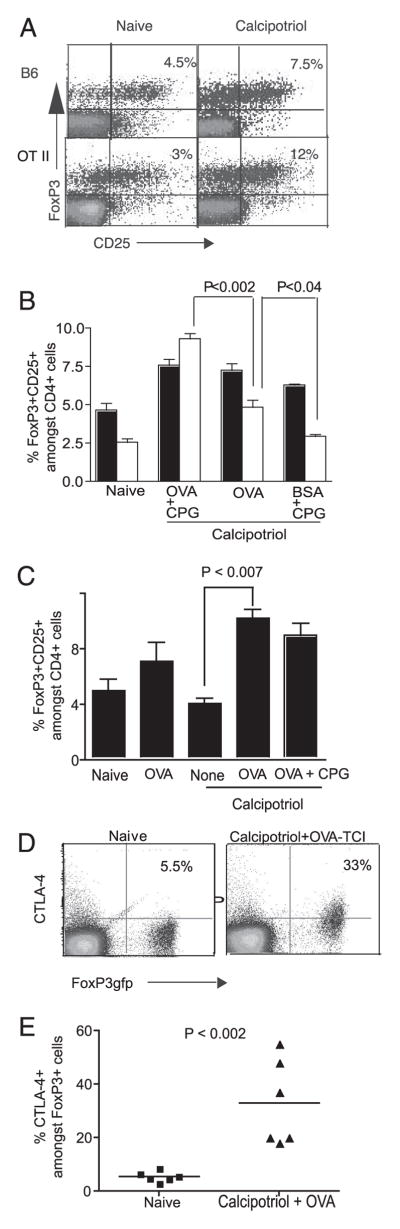
Effect of topical calcipotriol and Ag immunization upon CD4+CD25+FoxP3+ T cells. A, Calcipotriol treatment before TCI expands FoxP3+ Treg. Wild-type (B6) or OT-II TCR transgenic mice were either treated with topical calcipotriol for 3 days or untreated and then immunized with OVA and CpG on the following day. Draining LN were then examined for CD4, CD25, and FoxP3 expression on day 4 (n = 3; representative of two experiments). B, OVA Ag-specific Treg are inducible by TCI following calcipotriol treatment. The proportion of CD25+FoxP3+ cells among CD4+ T cells was increased following topical calcipotriol and OVA treatment. This increase was Ag specific in OT-II mice (open bars) and occurred in an Ag-nonspecific fashion in B6 mice (filled bars) (n = 3; representative of two experiments). C, OVA immunization following calcipotriol treatment resulted in a significant increase in the number of Treg compared with calcipotriol treatment alone. The proportion of CD25+FoxP3+ cells among CD4+ T cells in the skin-draining LN was determined 4 days after the topical treatment of B6 mice as indicated. A significant expansion of the proportion of Treg occurred following combined application of calcipotriol and OVA Ag with or without application of topical CpG (n = 4; representative of two experiments). D and E, OVA immunization following calcipotriol treatment up-regulates CTLA-4 in Treg. FoxP3gfp mice were either treated with calcipotriol followed by OVA immunization or left untreated. Four days after immunization, mononuclear cells were isolated from the peripheral LN and spleen, were stained for CTLA-4, and FoxP3-expressing cells were identified by green fluorescence (D). The scatter plot in E includes data from two experiments.
UV-mediated FoxP3+ Treg activation is VDR dependent in vivo
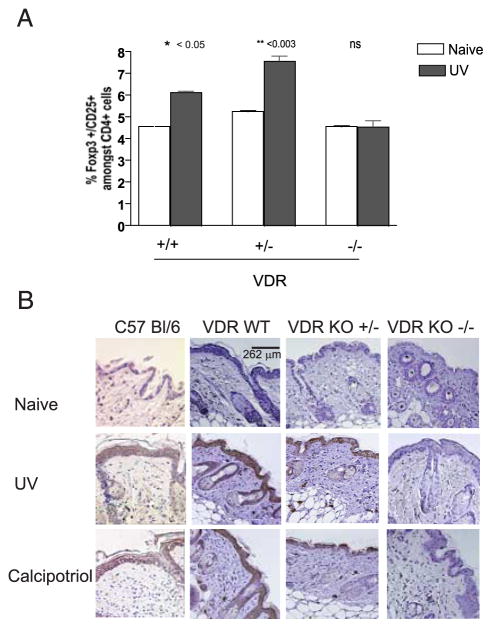
UV irradiation of the skin enhances the relative fraction and number of FoxP3+ Treg in skin-draining LN (9). These cells mediate transferable suppression of CTL. We have shown that topical calcipotriol, a VD3 analog, likewise prevents the priming of CTL in response to locally administered Ag and may be used to induce Ag-specific Treg following TCI. To determine whether VD3 signaling is required for the UV-mediated activation of Treg, we studied the effect of UV irradiation on the frequency of Treg in vitamin D receptor-deficient animals (VDR KO) (12). VDR KO mice, in contrast to wild-type and heterozygote (VDR−/+) animals, failed to increase FoxP3+ Treg in their skin-draining LN when analyzed 3 days following UV irradiation. These data suggest that Treg induction and the suppressive effect of UV irradiation occur through the VDR (Fig. 5A).
FIGURE 5.
Induction of Treg and RANKL is VDR dependent. A, VDR is required for the induction of CD4+CD25+FoxP3+ cells mediated by UVB. Mice were treated with UVB (1000 J/m2) for 4 successive days or were untreated. Three days later, the proportion of CD4+CD25+FoxP3+ cells among CD4+ T cells in the peripheral LN was determined (n = 3; representative of two experiments). B, VDR expression is required for UV induction of RANKL expression. Mice were treated with UVB or calcipotriol. Three days later, skin samples were harvested and RANKL expression was then determined by immunohistochemistry. Calcipotriol induced keratinocyte RANKL expression equivalent to the induction by UV light through VD receptor. Images are representative of three animals per group.
Keratinocytes expression of RANKL following calcipotriol treatment or UV treatment is VDR dependent
UV-induced expression of RANKL, a TNF-family molecule, by keratinocytes appears critical in the modulation of skin DC function to permit the expansion of Treg within the draining LN (10). Topical calcipotriol induces the expression of immunomodulatory proteins by keratinocytes (25). Likewise, VD3 may promote RANKL expression. We thus investigated the expression of RANKL in the skin following topical calcipotriol application. Three daily applications of calcipotriol to murine skin resulted in high levels of RANKL protein expression as determined by immunohistochemistry (Fig. 5B, bottom panel). This was comparable to the levels obtained following UV light exposure (Fig. 5 B, middle panel). Thus, both UV and calcipotriol induce keratinocyte RANKL. This suggests that, in addition to possible direct effects upon the skin resident DC, topical calcipotriol may modulate Treg by altering DC activation indirectly through the modulation of keratinocyte RANKL expression. To determine whether VDR expression affects RANKL induction, VDR KO mice, heterozygotes, and wild-type mice were treated with calcipotriol or were UV irradiated. Three days later, skin samples were taken from treated sites and compared with control untreated mice for the expression of RANKL. Treatment of VDR KO mice with either UV or calcipotriol failed to induce RANKL expression (Fig. 5B, rightmost column). This demonstrates that RANKL induction in keratinocytes by either UV or calcipotriol is dependent on VDR signaling.
Discussion
The suppression of cytotoxic T cell responses by Treg is essential to homeostasis and the control of autoimmune disease. Despite experimental success with different methods of Treg induction, a safe, simple, and Ag-specific mode of inhibiting immune responses has not yet been achieved. Treg make up only 5–10% and 1–2% of the CD4+ T cell pool in mice and humans, respectively, and thus therapeutic applications have been limited by prohibitively low numbers of Ag-specific Treg. Protocols for the robust expansion of Ag-specific Treg are therefore urgently required. The development of an efficient method for the expansion of Ag-specific Treg from a polyclonal pool has been challenging. An in vitro expansion protocol involving the alternation of Ag-specific stimulation and polyclonal stimulation with the usage of Abs to CD28 and CD3 (26) in the presence of high-dose IL-2 has been described but is costly and inefficient. Optimizing the expansion of Treg in vivo may be a more viable approach to developing Treg therapies.
UVB has long been known to have immunosuppressive properties (27) and potently inhibits the induction of CTL responses in mice (9). The use of UV irradiation for Ag-specific tolerance induction in humans has practical limitations and raises concerns over the oncogenicity of UV administration. One potential strategy for safely harnessing the tolerogenic effects of UV light may be through the use of vitamin D analogs. UV light plays an essential role in the biosynthesis of vitamin D, as UV irradiation of the skin triggers conversion of vitamin D to its activated form VD3. This molecule can then exert its function through the VDR, which is widely expressed in the body. Indeed, its presence on a variety of immune cells initiated interest in this molecule as an immune system regulator, independent of its recognized role in regulating bone metabolism (28, 29). The immunosuppressive potential of VD3 has been clearly demonstrated both in vitro and in vivo (4, 5). Calcipotriol, a synthetic analog of VD3 lacking systemic calcitropic effects, has been used extensively by clinicians in the management of skin conditions such as psoriasis (6). In this study we explored the suppressive effects of topical calcipotriol treatment on immune responses to cutaneously applied Ag.
TCI involves using skin DC to present Ag to T cells in the draining LN, thereby activating CD8+ CTL and CD4+ T cells. In our experiments with the adoptive transfer of transgenic CD8+ OT-I T cells, OT-I cell priming in response to TCI was inhibited after repeated daily topical calcipotriol treatment (Fig. 1, A and B). These cells were unable to proliferate or produce significant amounts of IFN-γ. These suppressive effects of calcipotriol treatment upon TCI could be due to direct or indirect effects on APC, direct suppression of CTL, inhibition of CD4+ helper T cells, and/or induction of suppressor T cells. However, calcipotriol was not generally immunosuppressive, as CD4+ T cell proliferative responses were not inhibited (data not shown). Calcipotriol administration prevented the priming of CTL even when the Ag-specific precursor frequency was artificially high following OT-1 adoptive transfer. Calcipotriol also suppressed the expansion of CTL when naive mice were immunized and boosted with OVA TCI (Fig. 3A), demonstrating that suppression by calcipotriol can inhibit the generation of CTL derived from physiologic numbers of Ag-specific CTL precursors. The suppressive effect of calcipotriol is a local effect, as application of Ag to an area untreated with calcipotriol did not result in suppression of OT-1 proliferation or cytotoxicity (Fig. 1C). The suppressive effects of calcipotriol were evaluated in an in vivo model of CHS (Fig. 1E). We found that calcipotriol treatment before OVA sensitization prevented an ear-swelling response, illustrating the potential utility of this drug to prevent the induction of CHS.
Topical calcipotriol administration significantly reduced the number of MHC-II+ cells in epidermal sheets, corresponding with the previously described effects of VD3 on LC (30). Further study is required to explore whether the observed disappearance of LC is due to apoptosis, migration, or to the down-regulation of MCH-II expression. Treatment of purified skin-derived LC with VD3 inhibits expression of MHC-II, CD40, CD54, CD80, CD86, migratory capacity, and cytokine production (18). VD3 has multiple effects on myeloid DC, including the induction of the potentially immunoregulatory ILT3 (31) and CCL22 up-regulation (32), a cytokine attracting Treg. In vitro stimulation of VDR on DC can also promote the induction of CD4+CD25+ T cells (5, 33). Thus, the alteration of LC by calcipotriol may explain some of its direct immunosuppressive effects.
TCI (with protein Ag and CpG adjuvant) through topical calcipotriol-reated skin increased the frequency of CD4+CD25+ Treg in draining LN. This led us to explore whether topical administration of calcipotriol promoted Ag-specific Treg induction. The discovery of FoxP3 as the key transcription factor controlling Treg development resulted in significant advances in Treg immunobiology (34). FoxP3 was highly expressed in the CD4+ CD25+ cell population in all of our mice, regardless of treatment (Fig. 3B). However, FoxP3-xpressing CD4+CD25+ Treg isolated from BSA-tolerized donor mice were not capable of preventing the OT-I response to OVA-TCI in recipients (Fig. 2C), suggesting that TCI and calcipotriol-induced Treg act in an Ag-specific manner. The Treg induced by calcipotriol and TCI are highly potent, and as few as 5 × 104 T cells transferred was enough to suppress the OT-I response (Fig. 2D). In our CHS model, the transfer of 2 × 106 Treg induced by OVA-TCI or BSA-TCI was able to reduce ~80% of the ear CHS response to their respective Ags; however, a small amount of suppression (20–25%) was also seen when an irrelevant Ag was used (Fig. 3B). This demonstrates the potency of the Ag-specific Treg generated and suggests that Treg with a broader (Ag nonspecific) suppressive function may also be generated. The Ag-independent suppressive activity of these cells (Fig. 2C) was likely not detected in the OT-1 adoptive transfer experiments due to the high frequency of Ag-specific CTL precursors requiring suppression. Ag specificity of our Treg was further confirmed by the increase in FoxP3 expression seen within transgenic OT-II CD4+ T cells when treating OT-II mice with OVA protein, an effect that was not seen when BSA was used (Fig 4B). In contrast, Gorman et al. (21) have shown that application of topical VD3 without Ag immunization increased the suppressive ability of Treg in vitro without increasing their frequency in vivo. Treg induced by this group had general, Ag-independent suppressive activity both in vitro and in vivo. Our use of topical Ag and TLR9 agonist following topical calcipotriol administration may explain our ability to detect Ag specificity and an increase in frequency of Treg (Fig. 4C). Treg induce their suppressive function via multiple mechanisms: 1) cell-cell contact and interaction between CTLA-4 on the surface and its ligands CD80 and CD86 on DC or effector T cells, or 2) secretion of suppressive cytokines (IL-10 or TGF-β) (reviewed in Ref. 35). CTLA-4 has recently been shown to be essential for the Treg-mediated inhibition of autoimmunity (36). Treg induced by calcipotriol treatment followed by OVA-TCI demonstrated a significant increase in surface CTLA-4 molecule expression (Fig. 4, D and E). The control of CD8 T cell responses by CD25+ Treg has been well described (37). Future studies will be required to determine whether the induced Treg interact with APC or directly with potential effector T cells in vivo.
Remarkably, the induction of FoxP3+ Treg by UV irradiation was VDR dependent, as UV-irradiated VDR−/− mice failed to show any increase in this subset (Fig. 5A). Although synthesis of activated VD3 is dependent on UV irradiation, and the immunosuppressive effects of UV and VD3 have been recently linked (21), UV-induced Treg induction has not been previously shown to be VDR dependent. To explore the common pathway that UV and VD3 share in induction of Treg, we looked at the expression of RANKL, a member of the TNF-β family that is induced by both UVB (10) and VD3 (38). Expression of RANKL on keratinocytes along with interaction with its receptor on APC have been shown to be critical for induction of Treg following UV light exposure (10). We document up-regulation in C57BL/6 mice after UV or calcipotriol treatment. RANKL up-regulation was abrogated in VDR−/− mice, suggesting that both UV- and calcipotriol-induced RANKL expression occurs through the VDR (Fig. 5B). Failure of FoxP3 up-regulation in VDR−/− mice could therefore be due to absence of RANKL expression (Fig. 5B). UV light induces immunosuppression by multiple pathways, including keratinocyte-mediated cytokine production and the local induction of cis-urocanic acid (reviewed in Ref. 27). While we demonstrate a possible role for VDR in the generation of UV-induced Treg, the role of VDR in these other immunomodulatory skin responses remains to be determined.
Herein, we demonstrate that calcipotriol treatment prevents the subsequent priming of CD8+ T cells to topical Ags. Topical calcipotriol increases the proportion of Treg in the skin-draining LN. Furthermore, TCI through calcipotriol-treated skin induces Ag-specific tolerance mediated by CD4+CD25+FoxP3+ cells. These cells have the ability to prevent the further priming of potentially damaging CD8+ T cells in an Ag-specific manner and are able to inhibit an established immune response in the form of protein CHS. A physiologically relevant expansion of Ag-specific Treg is promoted by a topical drug (calcipotriol) that has been in clinical use for other indications for more than a decade.
There are multiple parallels between this phenomenon and the observed Treg induction following UV light exposure as detailed in our work (9) and as exemplified by the induction of keratinocyte RANKL expression (10). Recently, there has been a renewed interest in the importance of vitamin D, and possibly sun-induced vitamin D production, on the control of autoimmune disease. This work demonstrates that topical vitamin D expands Treg and can be harnessed to do so in an Ag-specific manner. Taken together, our results show a profound inhibitory effect of a topical vitamin D analog on CD8+ T cell priming and activation. These effects may not only lead to a potential therapeutic use for Treg against allergic and autoimmune-based diseases, but may promote the development of a strategy for enhanced CTL response to cutaneous tumors or bacterial Ag vaccination via the selective blockade of VDR.
Footnotes
This work was supported by the Canadian Institutes of Health Research (MOP-81083), Juvenile Diabetes Research Foundation International (Award 4-2004-223), and the Canadian Dermatology Foundation. J.P.D. is a Senior Scholar of the Michael Smith Foundation for Health Research.
Abbreviations used in this paper: Treg, regulatory T cell; CHS, contact hypersensitivity; DC, dendritic cell; KO, knockout; LC, Langerhans cell; LN, lymph node; VD3, 1,25-dihydroxyvitamin D3; RANKL, receptor activator of NF-κB ligand; TCI, transcutaneous immunization; VDR, vitamin D receptor.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 2.Carlberg C, Bendik I, Wyss A, Meier E, Sturzenbecker LJ, Grippo JF, Hunziker W. Two nuclear signalling pathways for vitamin D. Nature. 1993;361:657–660. doi: 10.1038/361657a0. [DOI] [PubMed] [Google Scholar]
- 3.Green S. Nuclear hormone receptors: promiscuous liaisons. Nature. 1993;361:590–591. doi: 10.1038/361590a0. [DOI] [PubMed] [Google Scholar]
- 4.Penna G, Adorini L. 1α,25-Dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164:2405–2411. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- 5.Gregori S, Giarratana N, Smiroldo S, Uskokovic M, Adorini L. A 1α,25-dihydroxyvitamin D3 analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes. 2002;51:1367–1374. doi: 10.2337/diabetes.51.5.1367. [DOI] [PubMed] [Google Scholar]
- 6.Kragballe K. Calcipotriol: a new drug for topical psoriasis treatment. Pharmacol Toxicol. 1995;77:241–246. doi: 10.1111/j.1600-0773.1995.tb01020.x. [DOI] [PubMed] [Google Scholar]
- 7.Glenn GM, Kenney RT, Hammond SA, Ellingsworth LR. Transcutaneous immunization and immunostimulant strategies. Immunol Allergy Clin North Am. 2003;23:787–813. doi: 10.1016/s0889-8561(03)00094-8. [DOI] [PubMed] [Google Scholar]
- 8.Kahlon R, Hu Y, Orteu CH, Kifayet A, Trudeau JD, Tan R, Dutz JP. Optimization of epicutaneous immunization for the induction of CTL. Vaccine. 2003;21:2890–2899. doi: 10.1016/s0264-410x(03)00141-5. [DOI] [PubMed] [Google Scholar]
- 9.Ghoreishi M, Dutz JP. Tolerance induction by transcutaneous immunization through ultraviolet-irradiated skin is transferable through CD4+ CD25+ T regulatory cells and is dependent on host-derived IL-10. J Immunol. 2006;176:2635–2644. doi: 10.4049/jimmunol.176.4.2635. [DOI] [PubMed] [Google Scholar]
- 10.Loser K, Mehling A, Loeser S, Apelt J, Kuhn A, Grabbe S, Schwarz T, Penninger JM, Beissert S. Epidermal RANKL controls regulatory T-cell numbers via activation of dendritic cells. Nat Med. 2006;12:1372–1379. doi: 10.1038/nm1518. [DOI] [PubMed] [Google Scholar]
- 11.Haribhai D, Lin W, Relland LM, Truong N, Williams CB, Chatila TA. Regulatory T cells dynamically control the primary immune response to foreign antigen. J Immunol. 2007;178:2961–2972. doi: 10.4049/jimmunol.178.5.2961. [DOI] [PubMed] [Google Scholar]
- 12.Yoshizawa T, Handa Y, Uematsu Y, Takeda S, Sekine K, Yoshihara Y, Kawakami T, Arioka K, Sato H, Uchiyama Y, et al. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat Genet. 1997;16:391–396. doi: 10.1038/ng0897-391. [DOI] [PubMed] [Google Scholar]
- 13.Kallay E, Pietschmann P, Toyokuni S, Bajna E, Hahn P, Mazzucco K, Bieglmayer C, Kato S, Cross HS. Characterization of a vitamin D receptor knockout mouse as a model of colorectal hyperproliferation and DNA damage. Carcinogenesis. 2001;22:1429–1435. doi: 10.1093/carcin/22.9.1429. [DOI] [PubMed] [Google Scholar]
- 14.Song Y, Kato S, Fleet JC. Vitamin D receptor (VDR) knockout mice reveal VDR-independent regulation of intestinal calcium absorption and ECaC2 and calbindin D9k mRNA. J Nutr. 2003;133:374–380. doi: 10.1093/jn/133.2.374. [DOI] [PubMed] [Google Scholar]
- 15.Klimuk SK, Najar HM, Semple SC, Aslanian S, Dutz JP. Epicutaneous application of CpG oligodeoxynucleotides with peptide or protein antigen promotes the generation of CTL. J Invest Dermatol. 2004;122:1042–1049. doi: 10.1111/j.0022-202X.2004.22411.x. [DOI] [PubMed] [Google Scholar]
- 16.Meindl S, Rot A, Hoetzenecker W, Kato S, Cross HS, Elbe-Burger A. Vitamin D receptor ablation alters skin architecture and homeostasis of dendritic epidermal T cells. Br J Dermatol. 2005;152:231–241. doi: 10.1111/j.1365-2133.2005.06392.x. [DOI] [PubMed] [Google Scholar]
- 17.Kowitz A, Greiner M, Thieroff-Ekerdt R. Inhibitory effect of 1α,25- dihydroxyvitamin D3 on allogeneic lymphocyte stimulation and Langerhans cell maturation. Arch Dermatol Res. 1998;290:540–546. doi: 10.1007/s004030050349. [DOI] [PubMed] [Google Scholar]
- 18.Fujita H, Asahina A, Komine M, Tamaki K. The direct action of 1α,25(OH)2-vitamin D3 on purified mouse Langerhans cells. Cell Immunol. 2007;245:70–79. doi: 10.1016/j.cellimm.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Hanneman KK, Scull HM, Cooper KD, Baron ED. Effect of topical vitamin D analogue on in vivo contact sensitization. Arch Dermatol. 2006;142:1332–1334. doi: 10.1001/archderm.142.10.1332. [DOI] [PubMed] [Google Scholar]
- 20.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 21.Gorman S, Kuritzky LA, Judge MA, Dixon KM, McGlade JP, Mason RS, Finlay-Jones JJ, Hart PH. Topically applied 1,25- dihydroxyvitamin D3 enhances the suppressive activity of CD4+CD25+ cells in the draining lymph nodes. J Immunol. 2007;179:6273–6283. doi: 10.4049/jimmunol.179.9.6273. [DOI] [PubMed] [Google Scholar]
- 22.Schwarz A, Maeda A, Schwarz T. Alteration of the migratory behavior of UV-induced regulatory T cells by tissue-specific dendritic cells. J Immunol. 2007;178:877–886. doi: 10.4049/jimmunol.178.2.877. [DOI] [PubMed] [Google Scholar]
- 23.Sigmundsdottir H, Pan J, Debes GF, Alt C, Habtezion A, Soler D, Butcher EC. DCs metabolize sunlight-induced vitamin D3 to “program” T cell attraction to the epidermal chemokine CCL27. Nat Immunol. 2007;8:285–293. doi: 10.1038/ni1433. [DOI] [PubMed] [Google Scholar]
- 24.Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445:766–770. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 25.Li M, Hener P, Zhang Z, Kato S, Metzger D, Chambon P. Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc Natl Acad Sci USA. 2006;103:11736–11741. doi: 10.1073/pnas.0604575103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peters JH, Hilbrands LB, Koenen HJ, Joosten I. Ex vivo generation of human alloantigen-specific regulatory T cells from CD4posCD25high T cells for immunotherapy. PLoS ONE. 2008;3:e2233. doi: 10.1371/journal.pone.0002233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schade N, Esser C, Krutmann J. Ultraviolet B radiation-induced immunosuppression: molecular mechanisms and cellular alterations. Photochem Photobiol Sci. 2005;4:699–708. doi: 10.1039/b418378a. [DOI] [PubMed] [Google Scholar]
- 28.Bhalla AK, Amento EP, Clemens TL, Holick MF, Krane SM. Specific high-affinity receptors for 1,25-dihydroxyvitamin D3 in human peripheral blood mononuclear cells: presence in monocytes and induction in T lymphocytes following activation. J Clin Endocrinol Metab. 1983;57:1308–1310. doi: 10.1210/jcem-57-6-1308. [DOI] [PubMed] [Google Scholar]
- 29.Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983;221:1181–1183. doi: 10.1126/science.6310748. [DOI] [PubMed] [Google Scholar]
- 30.Dam TN, Moller B, Hindkjaer J, Kragballe K. The vitamin D3 analog calcipotriol suppresses the number and antigen-presenting function of Langerhans cells in normal human skin. J Investig Dermatol Symp Proc. 1996;1:72–77. [PubMed] [Google Scholar]
- 31.Penna G, Roncari A, Amuchastegui S, Daniel KC, Berti E, Colonna M, Adorini L. Expression of the inhibitory receptor ILT3 on dendritic cells is dispensable for induction of CD4+Foxp3+ regulatory T cells by 1,25-dihydroxyvitamin D3. Blood. 2005;106:3490–3497. doi: 10.1182/blood-2005-05-2044. [DOI] [PubMed] [Google Scholar]
- 32.Penna G, Amuchastegui S, Giarratana N, Daniel KC, Vulcano M, Sozzani S, Adorini L. 1,25-Dihydroxyvitamin D3 selectively modulates tolerogenic properties in myeloid but not plasmacytoid dendritic cells. J Immunol. 2007;178:145–153. doi: 10.4049/jimmunol.178.1.145. [DOI] [PubMed] [Google Scholar]
- 33.Adorini L, Penna G, Giarratana N, Uskokovic M. Tolerogenic denritic cells induced by vitamin D receptor ligands enhance regulatory T cells inhibiting allograft rejection and autoimmune diseases. J Cell Biochem. 2003;88:227–233. doi: 10.1002/jcb.10340. [DOI] [PubMed] [Google Scholar]
- 34.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 35.von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat Immunol. 2005;6:338–344. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]
- 36.Friedline RH, Brown DS, Nguyen H, Kornfeld H, Lee J, Zhang Y, Appleby M, Der SD, Kang J, Chambers CA. CD4+ regulatory T cells require CTLA-4 for the maintenance of systemic tolerance. J Exp Med. 2009;206:421–434. doi: 10.1084/jem.20081811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piccirillo CA, Shevach EM. Cutting edge: control of CD8+ T cell activation by CD4+CD25+ immunoregulatory cells. J Immunol. 2001;167:1137–1140. doi: 10.4049/jimmunol.167.3.1137. [DOI] [PubMed] [Google Scholar]
- 38.Theill LE, Boyle WJ, Penninger JM. RANK-L and RANK: T cells, bone loss, and mammalian evolution. Annu Rev Immunol. 2002;20:795–823. doi: 10.1146/annurev.immunol.20.100301.064753. [DOI] [PubMed] [Google Scholar]