MRI-based markers can distinguish patients with schizophrenia from healthy controls. Koutsouleris et al. now report a diagnostic signature that distinguishes major depression/bipolar disorder from schizophrenia in 80%/74% of cases. Classification accuracy generalizes to early phases of psychosis, and is moderated by disease stage, age of onset and accelerated brain ageing.
Keywords: multivariate pattern classification, imaging, schizophrenia, mood disorders, at-risk mental states for psychosis
MRI-based markers can distinguish patients with schizophrenia from healthy controls. Koutsouleris et al. now report a diagnostic signature that distinguishes major depression/bipolar disorder from schizophrenia in 80%/74% of cases. Classification accuracy generalizes to early phases of psychosis, and is moderated by disease stage, age of onset and accelerated brain ageing.
Abstract
Magnetic resonance imaging-based markers of schizophrenia have been repeatedly shown to separate patients from healthy controls at the single-subject level, but it remains unclear whether these markers reliably distinguish schizophrenia from mood disorders across the life span and generalize to new patients as well as to early stages of these illnesses. The current study used structural MRI-based multivariate pattern classification to (i) identify and cross-validate a differential diagnostic signature separating patients with first-episode and recurrent stages of schizophrenia (n = 158) from patients with major depression (n = 104); and (ii) quantify the impact of major clinical variables, including disease stage, age of disease onset and accelerated brain ageing on the signature’s classification performance. This diagnostic magnetic resonance imaging signature was then evaluated in an independent patient cohort from two different centres to test its generalizability to individuals with bipolar disorder (n = 35), first-episode psychosis (n = 23) and clinically defined at-risk mental states for psychosis (n = 89). Neuroanatomical diagnosis was correct in 80% and 72% of patients with major depression and schizophrenia, respectively, and involved a pattern of prefronto-temporo-limbic volume reductions and premotor, somatosensory and subcortical increments in schizophrenia versus major depression. Diagnostic performance was not influenced by the presence of depressive symptoms in schizophrenia or psychotic symptoms in major depression, but earlier disease onset and accelerated brain ageing promoted misclassification in major depression due to an increased neuroanatomical schizophrenia likeness of these patients. Furthermore, disease stage significantly moderated neuroanatomical diagnosis as recurrently-ill patients had higher misclassification rates (major depression: 23%; schizophrenia: 29%) than first-episode patients (major depression: 15%; schizophrenia: 12%). Finally, the trained biomarker assigned 74% of the bipolar patients to the major depression group, while 83% of the first-episode psychosis patients and 77% and 61% of the individuals with an ultra-high risk and low-risk state, respectively, were labelled with schizophrenia. Our findings suggest that neuroanatomical information may provide generalizable diagnostic tools distinguishing schizophrenia from mood disorders early in the course of psychosis. Disease course-related variables such as age of disease onset and disease stage as well alterations of structural brain maturation may strongly impact on the neuroanatomical separability of major depression and schizophrenia.
Introduction
Psychiatric diagnoses arise from complex clinical processes and hence are prone to errors (Freedman et al., 2013), depending on the patient’s symptoms, the interviewer’s experience and the classification systems’ normative validity. Biological data so far only served the exclusion of somatic pathologies, leaving the question whether individualized differential diagnosis could benefit from the analysis of complex neurodiagnostic ‘patterns’ unanswered (Fu and Costafreda, 2013; Perkins et al., 2014). Furthermore, pattern analysis could unveil overlaps between and heterogeneity within diagnoses, thus promoting the revision of psychiatric nosology, and ultimately the convergence of neuroscientific and clinical observation (Krystal and State, 2014).
Phenomenological heterogeneity particularly characterizes schizophrenic psychoses and mood disorders (Murray et al., 2005; Linscott and Os, 2010): affective symptoms are a core feature of prodromal (Schultze-Lutter et al., 2007; Addington et al., 2014) and established schizophrenia (Baynes et al., 2000; Marengo et al., 2000; Chemerinski et al., 2008; Romm et al., 2010; Cotton et al., 2012; Sönmez et al., 2013) while psychotic symptoms frequently coalesce with mania and depression (Ohayon and Schatzberg, 2002; Goodwin and Jamison, 2007). At the brain level, heterogeneity appears as subgrouping and cross-nosological effects, including neuroanatomical correlates of different symptom dimensions (Koutsouleris et al., 2008; Nenadic et al., 2012; Zhang et al., 2014), overlapping and segregating structural abnormalities (Bora et al., 2008, 2010; Yu et al., 2010; Du et al., 2012; Hulshoff Pol et al., 2012) and gradual transitions of brain activation patterns between diagnostic entities (Brandt et al., 2014). Clinically, this heterogeneity may contribute to diagnostic uncertainty along the diversity of possible disease trajectories (Baca-Garcia et al., 2007; Pope et al., 2013; Salvatore et al., 2013). Scientifically, it challenged the detection of diagnostically specific neurobiological markers and hence questioned the validity of the current disease taxonomy (Linscott and Os, 2010; Keshavan and Brady, 2011), suggesting that unipolar depression, bipolar disorder and schizophrenia may represent ‘stages’ or ‘domains’ along a phenotypic and neurobiological disease continuum (Häfner et al., 2005; Green et al., 2009; Lin et al., 2013).
To simultaneously address this debate and close the ‘translational gap’ between neurobiological findings and their clinical application, researchers increasingly used multivariate pattern analysis to quantify the sensitivity, specificity and generalizability of diagnostic brain signatures (Bray et al., 2009; Fu and Costafreda, 2013) rather than describing them in terms of their constituents’ group-level significance (Davatzikos, 2004). Using multivariate pattern analysis, the field recently demonstrated a high separability of different neuropsychiatric conditions versus healthy controls, thus foreshadowing a potential translation of neuroimaging findings into diagnostic tools (Orrù et al., 2012; Kambeitz et al., 2015). However, doubts remain whether multivariate pattern analysis-based biomarkers are really useful in discriminating neuropsychiatric illness from mental well-being, or whether they are rather needed as objective tools for a more reliable ‘differential diagnosis’ (Savitz et al., 2013). Initial findings suggest that neuroimaging may aid in individually separating schizophrenia from bipolar disorder (Schnack et al., 2014) and major depression (Ota et al., 2013) or bipolar from unipolar depression (Mourão-Miranda et al., 2012; Grotegerd et al., 2013; Serpa et al., 2014). However, as these studies focused on pairwise comparisons it remains unclear how the reported neurodiagnostic signatures would perform in patients with ‘intermediate’ phenotypes and early disease states as well as in populations broadly covering the different age windows of these phenotypes.
An established approach to measure how clinical intersections, disease stages and age windows impact on neurodiagnostic performance is to investigate these variables along a single disease dimension, which is first spanned by ‘extreme’ or clearly distinct clinical phenotypes and then applied to the ‘intermediate’ or moderating conditions. This approach has been used in the dementia field where morphometric patterns distinguishing patients with Alzheimer’s disease from healthy controls were used to quantify disease progression and severity in patients with mild cognitive impairment (Davatzikos et al., 2009). In the psychosis field, Fan et al. (2008b) used this framework to trace the neuroanatomical schizophrenia signature in unaffected first-degree relatives of patients with schizophrenia, indicating that the latter display intermediate neuroanatomical phenotypes between patients and controls. Herein, we took a similar approach to explore the hypothesis that the neuroanatomical signatures of major depression, bipolar disorder, the at-risk mental states for psychosis (ARMS) and schizophrenia lie along a single direction spanned by major depression and schizophrenia as the two end points of this continuum. Therefore, we first measured the single-subject separability of stable schizophrenia versus major depression in a representative database of 262 patients using MRI-based multivariate pattern analysis and then quantified differential diagnostic scores of independent persons with high-risk or first-episode states of psychosis (n = 112) as well as patients with bipolar disorder (n = 35). Second, we evaluated whether neurodiagnostic classification was moderated by important variables such as age of disease onset, disease stage and ‘accelerated ageing’ effects (Koutsouleris et al., 2013) as well as cross-sectional psychopathological profiles overlapping between major depression and schizophrenia. We expected classification performance to be moderated by gradients of neuroanatomical schizophrenia likeness increasing (i) from at-risk states to established schizophrenia; (ii) from major depression, over bipolar disorder to schizophrenia; and (iii) from later to earlier disease onsets across the life span.
Materials and methods
Participants
Patients with schizophrenia and major depression were examined at the Department of Psychiatry and Psychotherapy, Ludwig-Maximilian-University Munich (LMU) using the Structured Clinical Interview for DSM-IV – Axis I & II Disorders (SCID-I/-II), the review of records and psychotropic medications and a semi-standardized assessment of the psychiatric and somatic history. Patients’ symptoms were evaluated using standard psychometric scales (Table 1). Patients received a consensus diagnosis by two experienced psychiatrists at study inclusion and were excluded in case of an unstable SCID diagnosis over a 4-year follow-up period. Further exclusion criteria were: (i) a history of (a) schizoaffective and/or bipolar disorder, (b) traumatic brain injury with loss of consciousness, mental retardation, anorexia nervosa, delirium, dementia, amnestic disorders, personality disorders, substance dependence, as defined by DSM-IV, (c) previous electroconvulsive treatments, and (d) somatic conditions affecting the CNS; as well as (ii) insufficient knowledge of German, IQ <70, and age <18 or >65. Eleven patients with major depression fulfilled criteria for psychotic depression (DSM-IV: 296.24/.34). Psychotic psychopathology in the major depression group was further quantified by computing a composite Z-score from the Hamilton Depression Rating Scale items ‘feelings of guilt’, ‘hypochondriasis’, ‘depersonalization and derealization’ and ‘paranoid symptoms’. This score was significantly elevated in patients with psychotic major depression [psychotic major depression: mean (SD) = 1.2 (1.0); non-psychotic major depression: −0.2 (0.9); T = 4.5, P < 0.001]. In the schizophrenia group the severity of depressive symptoms was measured by summing the Positive and Negative Symptom Scale (PANSS items) ‘somatic concern’, ‘anxiety’, ‘guilt feelings’ and ‘depression’ and Z transforming this PANSS depression subscale score (Kontaxakis et al., 2000; El Yazaji et al., 2002).
Table 1.
Sociodemographic and clinical characteristics of study groups
| Sociodemographic and clinical variables | Training and cross-validation database |
Independent validation database |
||||||
|---|---|---|---|---|---|---|---|---|
| MD | SZ | T | P | BIP | FEP | ARMS-E | ARMS-L | |
| n | 104 | 158 | 35 | 23 | 21 | 68 | ||
| n Basel [%] / Munich [%] | 0 / 100 | 0 / 100 | 0 / 100 | 100 / 0 | 0 / 100 | 54 / 46 | ||
| Mean age at baseline [yrs] (SD) | 42.3 (12.0) | 30.8 (10.0) | 8.1‡ | <.001 | 39 (9.6) | 26.8 (6.5) | 25.6 (5.6) | 24.6 (5.9) |
| Sex (male) [%] | 50 | 74 | 15.8† | <.001 | 51 | 74 | 48 | 68 |
| Handedness (right) [%] | 95 | 91 | 1.5† | ns | 85 | 78 | 81 | 90 |
| BMI [kg/m2] (SD) | 24.7 (4.6) | 24.3 (4.4) | 0.7‡ | ns | 25.6 (3.6) | – | 21.1 (2.4) | 23.0 (3.3)m |
| Schooling [yrs] (SD) | 10.6 (2.0) | 10.6 (2.1) | 0.2‡ | ns | 11.6 (1.6) | 10.0 (1.6) | 11.5 (2.9) | 11.1 (1.5) |
| Nicotine [cig./day] | 9.7 (13.4) | 13.2 (13.7) | −1.96‡ | ns | 11.4 (13.1) | – | 7.0 (9.9) | 7.0 (9.8)m |
| Alcohol [g/day] | 11.2 (21.1) | 11.2 (25.5) | −0.01‡ | ns | 5.6 (20.6) | – | 2.9 (5.6) | 7.7 (15.3)m |
| Mean age of disease onset [yrs] (SD; median) | 36.5 (12.0) | 25.5 (8.0) | 8.1‡ | <.001 | 26.1 (9.1) | – | – | – |
| Mean illness duration [yrs] (SD) | 6.0 (7.8) | 4.5 (7.0) | 1.4‡ | ns | 13.9 (9.2) | – | – | – |
| Current treatment with typical antipsychotics [%] | 10.0 | 30.7 | 17.7† | <.001 | 0.0 | 0.0 | – | – |
| Current treatment with atypical antipsychotics [%] | 9.0 | 67.3 | 86.7† | <.001 | 40.0 | 39.1 | – | – |
| Current chlorpromazine equivalents [mg/d] | 43.1 (162.0) | 346.3 (373.4) | −8.6‡ | <.001 | 189.2 (322.9) | 244.0 (163) | – | – |
| Current treatment with antidepressants [%] | 73.1 | 7.9 | 156.7† | <.001 | 16.7 | 21.7 | 23.8 | 12.9 |
| Current treatment with mood stabilizers [%] | 13.0 | 3.3 | 8.1† | <.01 | 66.7 | 0.0 | 0.0 | 0.0 |
| Current treatment with lithium [%] | 7.0 | 0.0 | 10.† | <.01 | 16.7 | 0.0 | 0.0 | 0.0 |
| Mean BPRS (SD) | – | 52.7 (13.6) | – | 41.9 (10.6)b | ||||
| Mean PANSS total (SD) | – | 52.6 (29.2) | – | – | – | – | 56.8 (14.0) | 62.0 (22.2) |
| Mean PANSS positive (SD) | – | 11.9 (8.0) | – | – | – | – | 9.86 (2.6) | 13.7 (4.5) |
| Mean PANSS negative (SD) | – | 15.2 (9.7) | – | – | – | – | 14.9 (6.7) | 15.7 (8.5) |
| Mean PANSS general psychopathology (SD) | – | 25.6 (16.1) | – | – | – | – | 32.0 (7.9) | 32.6 (11.1) |
| Mean SANS (SD) | – | 45.0 (26.8) | – | – | – | 10.0 (5.3) | – | 9.5 (5.4)b |
| Mean HDRS (SD) | 21.3 (9.5) | – | – | – | 9.7 (9.8) | – | – | – |
| Mean YMRS (SD) | 11.0 (12.0) | – | – | – | ||||
| BrainAGE [yrs] (SD) | 4.0 (6.2) | 6.0 (6.0) | −2.55‡ | <.05 | 3.8 (6.5) | 5.1 (8.5) | −1.5 (7.7) | 2.7 (6.8) |
Descriptive analyses between major depression and schizophrenia patient groups were performed by means of χ2-tests for categorical data (†) and t-tests for continuous data (‡) t-tests. BMI = body mass index; BPRS = Brief Psychiatric Rating Scale; cig. = cigarettes; HDRS = Hamilton Depression Rating Scale; SD = standard deviation; YMRS = Young Mania Rating Scale. m data only available for the Munich subjects. b data only available for the Basel subjects.
Patients with an illness duration of <1 year, no previous inpatient treatment and <12 months (life-time) psychopharmacological treatment (antipsychotics in schizophrenia, antidepressants in major depression) were assigned to first episode subgroups, or to recurrently-ill (recurrent episode) samples, if they did not fulfil these criteria. These first episode criteria were chosen to mitigate potential secondary disease effects (e.g. continuous medication and frequent hospitalization) on brain structure in the respective major depression and schizophrenia subgroups. Illness duration was the time between MRI scanning and disease onset defined retrospectively by the onset of symptoms paralleled by a general decline in social and role functioning (Lieberman et al., 2001). Following these definitions, the mean (SD) illness duration in the major depression/schizophrenia (MDFE/SZFE) samples was 0.34 (0.24)/0.37 (0.68) years, while the respective values for the MDRE/SZRE were 9.19 (8.22)/7.25 (7.14) years. Diagnosis had no significant main (F = 1.53, P = 0.217) or interaction effects (F = 1.63, P = 0.203) on illness duration in the first episode and recurrent episode samples.
The schizophrenia versus major depression classifier was independently validated in 23 patients with first episode psychosis (FEP) (Yung et al., 1998) recruited at the Department of Psychiatry, University of Basel and 89 ARMS individuals pooled across the Ludwig-Maximilian-University (LMU; n = 52) and Basel (n = 37) early recognition services, which were detailed in previous work (Koutsouleris et al., 2009) (Supplementary material and Table 1). ARMS individuals were stratified into (i) an early ARMS (n = 21) defined either by predictive basic symptoms or a Global Functioning-Trait criterion; and (ii) late ARMS (ARMS, n = 68) defined by attenuated or brief limited intermittent psychotic symptoms, which closely corresponded to internationally established high-risk criteria (Yung et al., 1998; Klosterkötter et al., 2001). Psychosis developed in 4.8% and 47.1% of early ARMS and late ARMS individuals, respectively, over a follow-up period of 4.5 years (n = 33, 87.9% diagnosed as schizophrenia). At MRI, 61% and 95% of FEP and ARMS individuals, respectively, were antipsychotic-naïve (FEP: six with antipsychotic treatment for <1 month, and three for 1–3 months; ARMS: four treated with low-dose atypical antipsychotics for <3 weeks). The diagnosis of patients with FEP was evaluated 5 years after baseline and all examined subjects met DSM-IV criteria for schizophrenic psychosis.
Furthermore, classifier validation involved 35 patients from LMU with an established SCID diagnosis of bipolar disorder (Table 1), who did not meet exclusion criteria i(b)–(d) and ii. Thirty and five of these patients fulfilled criteria for bipolar I and II disorder, respectively, with bipolar I patients showing depressive (n = 11), manic (n = 12), mixed episodes (n = 3) and euthymic states (n = 4). Psychotic episodes were present in six patients with bipolar I (four and two patients with manic and depressive states, respectively).
Finally, 437 healthy volunteers previously described in Koutsouleris et al. (2013) and scanned at the same Munich scanner as the patient cohorts were used to correct the patients’ MRI data for age and sex effects as detailed below. The study was approved by each centre’s local ethics committee. Written informed consent was obtained from each participant before inclusion.
MRI data acquisition and preprocessing
Study participants were scanned using two SIEMENS MAGNETOM VISION 1.5 T scanners located at the University Hospital Basel and the Department of Radiology, Ludwig-Maximilian-University. In Basel, a T1-weighted 3D volumetric spoiled gradient recalled echo sequence generated 176 contiguous slices using the following protocol: echo time 4 ms; repetition time, 9.7 ms; flip angle, 12; field of view, 25.6 × 25.6 cm, matrix, 200 × 256; voxel dimensions, 1.28 × 1.0 × 1.0 mm. In Munich, a T1-weighted 3D-MPRAGE sequence was used: echo time, 4.9 ms; repetition time, 11.6 ms; field of view, 230 mm; matrix, 512 × 512; 126 contiguous axial slices; voxel dimensions, 0.45 × 0.45 × 1.5 mm. No calibration of MRI scanners was performed before or during the recruitment period.
MRI preprocessing first involved the segmentation of T1-weighted images into grey and white matter as well as CSF using the VBM8 toolbox (Koutsouleris et al., 2013; Supplementary material) (Gaser, 2009). Then, the high-dimensional DRAMMS (Ou et al., 2011, 2014) algorithm registered each grey matter map to the single-subject MNI template. Resulting deformations and warped tissue maps were used to compute grey matter maps for a Regional Analysis of brain Volumes in Normalized Space (GM-RAVENS) (Davatzikos et al., 2001).
Correction for age and sex effects
To remove age- and sex-related differences between patient groups while retaining disease-associated neuroanatomical variation, the following strategy (Dukart et al., 2011) was used. First, we calculated voxel-level β-coefficients for age and sex in our healthy control subjects’ GM-RAVENS maps using partial correlation analysis. These coefficients described maps of (i) grey matter volume change from 18- to 65-year-old healthy control subjects; and (ii) grey matter volume differences between male and female healthy control subjects. Then we residualized the patient data using these coefficients to correct for age- and sex effects not attributable to disease-related factors. This strategy was validated as shown in the Supplementary material.
Differential diagnostic pattern classification
We implemented a fully automated machine learning pipeline that extracted neuroanatomical features from the GM-RAVENS maps and generated decision rules from these features to individually distinguish patients with major depression from those with schizophrenia. To strictly separate the training process from the evaluation of the classifier’s generalizability, the pipeline was embedded into a repeated, double cross-validation (CV) framework (Filzmoser et al., 2009) (Supplementary material), as detailed previously (Koutsouleris et al., 2012; Borgwardt et al., 2013). More specifically, the following analysis steps were wrapped into a 10 × 10-fold cross-validation cycle at the outer (CV2) and the inner (CV1) levels of repeated double CV: the training subjects’ GM-RAVENS maps were initially corrected for age and sex effects (see above) and then scaled voxel-wise to [0, 1]. To reduce the maps’ dimensionality and discard noisy information, principal component analysis (PCA) (Hansen et al., 1999) projected correlated voxel sets to 170 uncorrelated eigenvariates, thus retaining 80% of the variance in each CV1 training partition. Correction, scaling, and PCA parameters were applied to the CV1 test data. Then, in each training partition, PCA features entered a recursive feature elimination algorithm (Guyon et al., 2002) that used a linear support vector machine (Fan et al., 2008a) to remove those eigenvariates that impaired separability on the respective CV1 test data (support vector machine penalty parameter: C = 1).
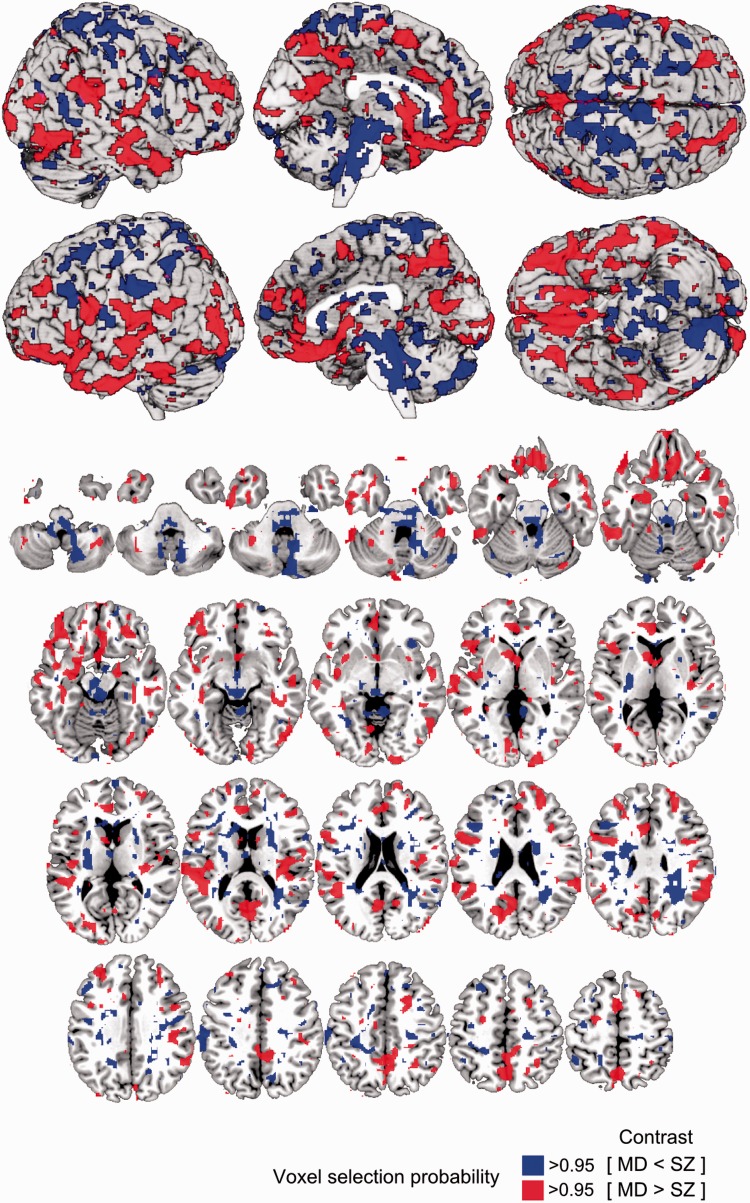
This process was repeated for all CV1 partitions, thus creating 100 diagnostic models for each CV2 partition. To obtain CV2 test predictions, the respective GM-RAVENS data were first processed using the correction, scaling and PCA parameters of each CV1 training partition, and then classified using the learned decision rules. Classification produced decision scores measuring the neuroanatomical schizophrenia versus major depression likeness of a given subject. Finally, a CV2 test case’s group membership was predicted by an ensemble classifier that averaged the decision scores of those 1000 CV1 base learners in the repeated double CV, in which the subject had not been involved in the training process (Supplementary material). The bipolar disorder, FEP, early ARMS and late ARMS samples were processed identically to the CV2 test subjects. Finally, the classifier’s decision function was visualized in Fig. 3 and the underlying patterns of volumetric differences were quantified in Supplementary Fig. 7.
Figure 3.
Voxel probability map (VPM) of reliable contributions to the major depression versus schizophrenia decision boundary. Voxels with a probability of >50% were overlaid on the single subject MNI template using the MRIcron software package (http://www.mccauslandcenter.sc.edu/mricro/mricron/). Methodological descriptions on how the voxel probability maps were computed can be found in the Supplementary material. MD = major depression; SZ = schizophrenia.
Testing differential diagnostic gradients in the ARMS and patient cohorts
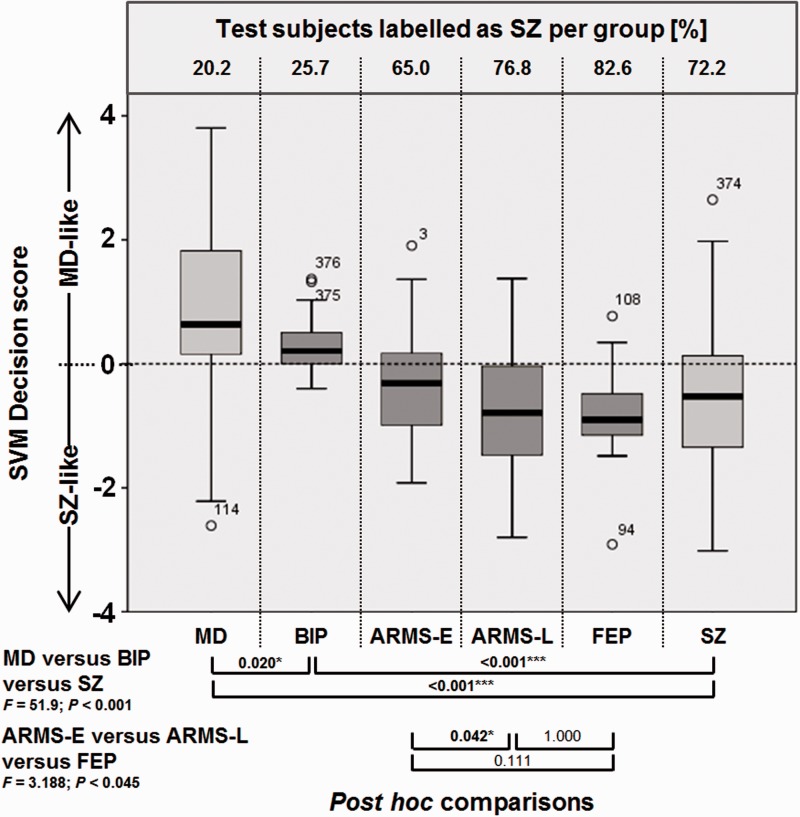
The decision scores generated by the differential diagnostic classifier entered ANOVAs that tested the hypotheses of neuroanatomical schizophrenia likeness increasing (i) from the major depression, through the bipolar disorder, to the schizophrenia group; and (ii) from the early ARMS, through the late ARMS to the FEP sample. In case of significant omnibus test statistics (P < 0.05), post hoc tests were carried out to evaluate pairwise differences at P < 0.05, corrected for multiple comparisons using Tukey’s HSD test (Fig. 1). Furthermore, a supplementary analysis was carried out in the ARMS sample to explore whether neurodiagnostic scores predicted a subsequent transition to psychosis (Supplementary material) or functional outcome as measured by the Global Assessment of Functioning Score at follow-up (Supplementary Fig. 5).
Figure 1.
Box plot comparison and ANOVAs of support vector machine (SVM) decision values. Box plot includes the major depression (MD) and schizophrenia (SZ) training database (light grey) and independent validation data consisting of bipolar disorder (BIP), ARMS (early, E and late, L) and FEP samples (dark grey). Box plots describe decision value distributions in terms of 5%, 25%, 50%, 75% and 95% confidence intervals. Frequency of schizophrenia-positive diagnosis is measured as percentage of subjects per group labelled as schizophrenia by the classifier (top of the box plot chart). P-values of post hoc comparisons in both ANOVAs are provided below and were corrected for multiple comparisons using Tukey’s HSD method (SPSS version 20, IBM Inc.).
Testing clinical and brain structural moderators of neurodiagnostic classification
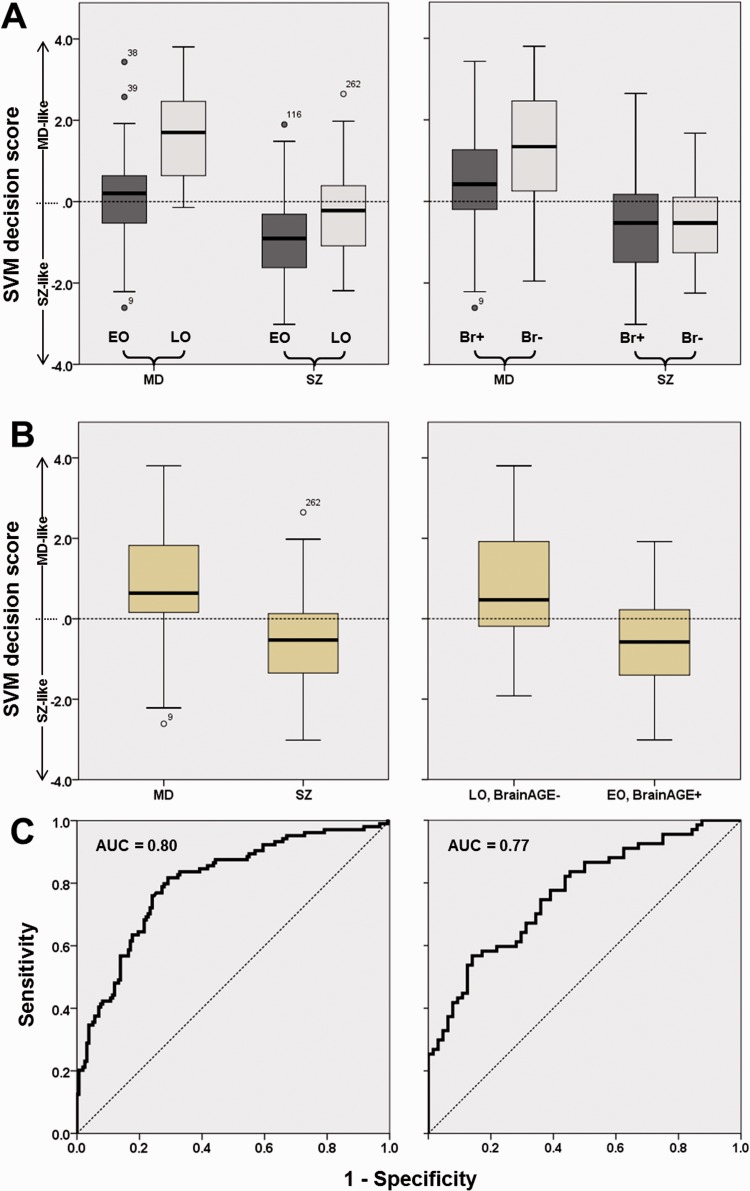
Potential moderating effects of disease stage on classification performance were evaluated at P < 0.05 by stratifying major depression and schizophrenia patients into first episode versus recurrent episode subgroups and performing a χ2 test on the misclassification error in these samples. Then, the impact of age of onset and BrainAGE (Brain Age Gap Estimation) (Koutsouleris et al., 2013) on decision scores was investigated by median-splitting the schizophrenia and major depression groups according to the latter two variables. Main and interactions effects between decision scores and the factors ‘Diagnosis’, ‘Early versus Late onset’, ‘Low versus High BrainAGE’ were assessed at P < 0.05 using the General Linear Model (Table 3 and Fig. 2A). Further analyses evaluated if classification of early-onset/high-BrainAGE patients versus late-onset/low-BrainAGE patients equalled diagnostic categorization (Fig. 2B and C). Based on these analyses, we assessed the separability within and between onset-defined diagnostic subgroups by performing pairwise support vector machine analyses as described above (Table 2 and Supplementary Fig. 6).
Table 2.
Diagnostic performance
| Dataset | TP | TN | FP | FN | Sens [%] | Spec [%] | BAC [%] | FPR [%] | PPV [%] | NPV [%] | DOR |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cross-validation | 83 | 114 | 44 | 21 | 79.8 | 72.2 | 76.0 | 27.8 | 65.4 | 84.4 | 10.2 |
| MD [+1] versus SZ [−1] | |||||||||||
| Age-of-onset stratified multi-group classifier | |||||||||||
| MD-E versus SZ-E | 38 | 51 | 24 | 14 | 73.1 | 68.0 | 70.5 | 30.2 | 61.3 | 78.5 | 5.8 |
| MD-E versus MD-L | 42 | 45 | 7 | 10 | 80.8 | 86.5 | 83.7 | 13.5 | 85.7 | 81.8 | 27.0 |
| MD-E versus SZ-L | 31 | 42 | 34 | 21 | 59.6 | 55.3 | 57.4 | 44.7 | 47.7 | 66.7 | 1.82 |
| SZ-E versus MD-L | 67 | 52 | 0 | 8 | 89.3 | 100.0 | 94.7 | 0.0 | 100.0 | 86.7 | – |
| SZ-E versus SZ-L | 47 | 47 | 29 | 28 | 62.7 | 61.8 | 62.3 | 38.2 | 61.8 | 62.7 | 2.72 |
| MD-L versus SZ-L | 46 | 64 | 12 | 6 | 88.5 | 84.2 | 86.3 | 15.8 | 79.3 | 91.4 | 40.9 |
| MD versus SZ (collapsed) | 79 | 104 | 47 | 25 | 76.0 | 68.9 | 72.4 | 31.1 | 62.7 | 80.6 | 6.99 |
The performance of the MRI diagnostic system was evaluated by means of sensitivity (Sens), specificity (Spec), balanced accuracy (BAC), false positive rate (FPR), positive/negative predictive value (PPV / NPV) and Diagnostic Odds Ratio (DOR). These measures were calculated from the confusion matrix containing the number of true positives (TP), false negatives (FN), true negatives (TN) and false positives (FP). MD-E = early-onset major depression; MD-L = late-onset major depression; SZ-E = early-onset schizophrenia; SZ-L = late-onset schizophreia.
Figure 2.
Box plot and receiver operator characteristics analyses comparing the separability of diagnosis-based versus age of onset and BrainAGE-based patient groups. (A) Effects of age of onset (left; early, EO and late, LO) and BrainAGE (right) on diagnostic separability in major depression (MD) versus schizophrenia (SZ) patients. (B) Diagnostic separability of diagnostic groups (major depression versus schizophrenia, left) versus separability in cross-nosological patient groups (right) defined by early versus late disease onset and high versus low BrainAGE (Br+ versus Br−). (C) Receiver operator characteristics analyses of MRI-based decision scores in the classification of diagnosis (left) and EO/Br+ versus LO/Br− groups. SVM = support vector machine; AUC = area under the curve.
We explored potential moderating effects of psychometric psychosis on neurodiagnostic classification in major depression by comparing the decision scores of patients with high (n = 17) versus low (n = 16) scores on the standardized composite scale of Hamilton-Depression-Rating Scale items 2, 15, 19 and 20. These major depression subgroups were identified by thresholding the composite scale at Z > 1 and Z < −1. The same procedure was used to measure the effect of psychometric depression on neurodiagnostic classification in schizophrenia: identical Z thresholds were applied to the standardized PANSS depression subscale and neuroanatomical decision scores were compared between the resulting schizophrenia subgroups with high (n = 30) and low (n = 20) depression scores. Finally, correlations between the decision scores and additional clinical variables of the major depression and schizophrenia samples were analysed in Supplementary Table 1. This supplementary analysis also explored whether neurodiagnostic scores were associated with verbal IQ in a subgroup of patients with schizophrenia stratified for early versus late disease onset.
Results
Sociodemographic and clinical variables
Schizophrenia and major depression patient groups did not differ regarding handedness, BMI, schooling years, nicotine or alcohol consumption (Table 1). Patient groups differed in the prescribed antipsychotic, antidepressant and mood-stabilizing medications. However, all of these variables had no effect on neurodiagnostic decision scores (Supplementary Table 1). Group-level differences were observed for age at scan, sex and age of disease onset, but not illness duration. Finally, schizophrenia patients had a higher mean (SD) BrainAGE score of +5.99 (6.00) compared to patients with major depression [+4.04 (6.19)].
Neuroanatomical classification and influence of moderating variables
The MRI classifier diagnosed unseen major depression and schizophrenia patients with a balanced accuracy of 76% (sensitivity/specificity = 79.8%/72.2%, diagnostic odds ratio = 10.2; Table 2). Recurrently-ill patients were more likely misclassified compared to first-episode patients (error rates MDFE/SZFE: 15.0%/11.5%; MDRE/SZRE: 23.4%/28.8%; χ2 = 6.6; P = 0.010). The neuroanatomical decision function (Fig. 3) involved grey matter reductions in schizophrenia versus major depression covering the perisylvian structures (inferior frontal, insular, supramarginal, angular, superior temporal and temporopolar cortices) with extensions to the orbitofrontal, inferior temporal and medial temporal cortices. Further reductions covered the ventromedial prefrontal, anterior cingulate, medial parietal, occipital and dorsolateral prefrontal cortices. Grey matter reductions in major depression versus schizophrenia were localized in a spatially distinct pattern including the brainstem regions, cerebellum, periventricular areas and the somatosensory cortices, extending to the premotor, parietal and supplementary motor areas.
The medians of age of onset/BrainAGE used to stratify patients were 36.3/+3.57 in the major depression group and 23.8/+5.62 in the schizophrenia sample. The General Linear Model evaluating effects of diagnosis, age of onset and BrainAGE factors on diagnostic scores detected significant main effects as well as a significant interaction between the ‘diagnosis’ and ‘early versus late’ factors (Table 3). Box plot analyses showed that early disease onset and high BrainAGE increased schizophrenia likeness in both disease groups, with this effect being more pronounced in major depression compared to schizophrenia (Fig. 2A). Using the diagnostic decision scores, late-onset, low-BrainAGE patients were separable from early-onset, high-BrainAGE patients to a similar degree [area under the curve (AUC) = 0.77] as major depression from schizophrenia patients (AUC = 0.80, Fig. 2B and C). Finally, the onset-stratified subgroup classification showed (i) a better separability of early versus late-onset major depression patients (balanced accuracy = 83.7%) than early versus late-onset schizophrenia patients (62.3%, Table 2); and (ii) a particularly low separability of early-onset major depression versus late-onset schizophrenia patients (57.4%). When diagnostic subgroup probabilities were collapsed into major depression versus schizophrenia diagnoses, the balanced accuracy was lower (72.2%) than in the original whole-group analysis.
Table 3.
Moderators of MRI-based differential diagnosis
| Data set | E | SE | 95%-CI Low / Up | F | P |
|---|---|---|---|---|---|
| Main effects | |||||
| MD versus SZ [mean difference] | 1.49 | 0.13 | 1.24 / 1.75 | 134.0 | <.001 |
| MD [marginal mean] | 0.89 | 0.10 | 0.70 / 1.09 | ||
| SZ [marginal mean] | −0.60 | 0.08 | −0.76 / −0.44 | ||
| Early versus late-onset | −1.03 | 0.13 | −1.29 / −0.78 | 62.6 | <.001 |
| Early-onset | −0.37 | 0.09 | −0.55 / −0.19 | ||
| Late-onset | 0.66 | 0.09 | 0.48 / 0.85 | ||
| Low versus High BrainAGE | 0.41 | 0.13 | 0.15 / 0.67 | 9.8 | .002 |
| Low BrainAGE | 0.35 | 0.09 | 0.17 / 0.53 | ||
| High BrainAGE | −0.06 | 0.09 | −0.24 / 0.13 | ||
| Two-way interaction effects | |||||
| MD versus SZ × Early versus Late-Onset | 8.3 | 0.004 | |||
| MD × Early-Onset | 0.19 | 0.14 | −0.09 / 0.47 | ||
| MD × Late-Onset | 1.59 | 0.14 | 1.32 / 1.88 | ||
| SZ × Early-Onset | −0.93 | 0.12 | −1.16 / −0.70 | ||
| SZ × Late-Onset | −0.27 | 0.12 | −0.50 / −0.04 | ||
| MD versus SZ × Low versus High BrainAGE | 3.7 | 0.056 | |||
| MD × Low BrainAGE | 1.22 | 0.14 | 0.95 / 1.50 | ||
| MD × High BrainAGE | 0.56 | 0.12 | 0.29 / 0.84 | ||
| SZ × Low BrainAGE | −0.52 | 0.12 | −0.74 / −0.29 | ||
| SZ × High BrainAGE | −0.68 | 0.12 | −0.91 / −0.44 |
Main and two-way interaction effects of diagnosis, early versus late disease onset and low versus high BrainAGE on diagnostic scores were analysed using univariate linear modelling in SPSS (version 20, IBM Inc.). E = estimate (mean difference or marginal mean); SE = standard error; 95%-CI Low/Up = 95% confidence interval with lower and upper bounds; F = F-statistic; MD = major depression; SZ = schizophrenia.
The comparison of the neurodiagnostic scores in schizophrenia patients with high versus low psychometric depression scores [mean (SD): −0.59 (1.30) versus −0.57 (0.98)] did not yield significant differences (T = −0.04; P = 0.966). Similarly, major depression patients with high psychometric psychosis scores did not significantly differ from patients with low scores [mean (SD): 1.64 (1.41) versus 0.77 (1.17); T = 1.88; P = 0.071]. Additionally, major depression patients with versus without a DSM-IV diagnosis of psychotic depression did not differ in their neurodiagnostic scores [1.25 (1.63) versus 0.84 (1.24); T = 0.98; P = 0.331] or in their misclassification rates (20.0% versus 20.2%; χ2 = 0.00; P = 1.000). Finally, increasing neurodiagnostic schizophrenia likeness in patients with early-onset schizophrenia was significantly associated with lower verbal IQ (r = 0.46, P = 0.013), whereas this correlation was not observed in late-onset schizophrenia (r = 0.03, P = 0.906; see Supplementary material).
Presence of a schizophrenia-like neuroanatomical signature in ARMS, FEP and bipolar disorder subjects
Decision scores obtained from the independent validation data showed that schizophrenia likeness was most pronounced in the Basel FEP patients (86.9% labelled as schizophrenia, Fig. 1) followed by the cross-centre late ARMS group (77.9%) and the Munich early ARMS sample (61.0%). Schizophrenia likeness was lower in the Munich bipolar disorder group (25.7%) resulting in 74% of these patients being classified as having major depression. Significant group differences were detected in all pairwise post hoc contrasts of the major depression versus bipolar disorder versus schizophrenia comparison (Fig. 1). In the early ARMS versus late ARMS versus FEP analysis, we observed a significant increase of schizophrenia likeness in the late ARMS compared to the early ARMS group (P = 0.042) with the former being on par with the FEP sample (P = 1.000). Finally, we did not find significant differences between subsequent converters versus non-converters to psychosis. However, increasing schizophrenia likeness at baseline predicted the ARMS individuals’ GAF scores at follow-up with R2 = 0.204 (P = 0.018, Supplementary Fig. 5).
Discussion
This is to our knowledge the first structural MRI study to report a cross-validated, single-subject separability of 76% in a representative cohort of patients with a stable diagnosis of either schizophrenia or major depression. This finding is in keeping with the balanced accuracy of 78% reported by Ota et al. (2013) who examined 25 age-matched female patients with schizophrenia or major depression using fractional anisotropy and grey matter volumes in predefined regions of interest. We observed that neuroanatomical markers successfully generalized to patients with first-episode psychosis who were examined at an independent centre using a different MRI protocol and were prospectively diagnosed with schizophrenia. Validation also showed that diagnostic sensitivity extended to the ARMS and grew with symptomatic proximity to overt psychosis. Strikingly, the neurodiagnostic classifier assigned 74% of patients with bipolar disorder to the major depression group, suggesting that schizophrenia may be differentiated from mood disorders at the single-subject level. In addition, we did not find evidence that neurodiagnostic classification was significantly influenced by the presence of psychotic symptoms in major depression or depressive symptoms in schizophrenia patients, nor by life-style factors or different medications at the time of MRI scanning (Supplementary Table 1). However, we identified a neuroanatomical signature shared by schizophrenia and major depression patients with an average disease onset at 26.5 years (Supplementary Table 2) and accelerated brain ageing effects (Koutsouleris et al., 2013), which led to a ‘non-separability’ of these subgroups.
Our findings partly agree with recent studies that used structural imaging data (Ota et al., 2013) or near-infrared spectroscopy (Takizawa et al., 2014) to differentiate between functional psychoses at the single-subject level. However, comparability to these studies is limited because we did not mitigate naturally occurring demographic differences between schizophrenia and major depression by studying matched patient samples. Instead, we adjusted our data using a representative database of healthy controls that fully covered the age range of our patient population (Dukart et al., 2011). Therefore, potential confounds like divergent illness durations and equalized sex distributions were avoided a priori. This approach facilitated the evaluation of the neurodiagnostic pattern, its presence in partly overlapping clinical phenotypes and its clinical moderators across the adult life span. First, we identified a pattern of perisylvian, prefrontal and temporo-limbic grey matter volume reductions used by the classifier to separate schizophrenia from major depression at the single-subject level. Similar patterns were repeatedly described to distinguish patients with schizophrenia from healthy controls (Honea et al., 2005; Bora et al., 2011) and were interpreted in line with a disconnection syndrome (Friston, 1999) underlying the cognitive, perceptual and thought disturbances of psychosis (Modinos et al., 2012; Sans-Sansa et al., 2013). Our finding of a grey matter volume ‘reduction’ signature (Supplementary Fig. 7) in schizophrenia compared to major depression patients, who were on average 11.5 years older, adds to the concept of schizophrenia being a neurodevelopmentally-mediated, cognitive illness (Kahn and Keefe, 2013) marked by more unfavourable disease outcomes compared to unipolar depression (Harrow et al., 2000). In contrast, somatosensory, periventricular and subcortical abnormalities distinguished major depression from schizophrenia in line with previously reported structural abnormalities in major white matter tracts of depressed patients (Disabato et al., 2014; Guo et al., 2014). Alterations of these regions may subserve core features of depression, such as psychomotor and mood disturbances (Serafini et al., 2011).
Second, the high rate of major depression classifications in our bipolar disorder sample suggests that bipolar disorder and unipolar depression share a common structural denominator different from schizophrenia, in line with Kraepelin’s original dichotomic concept of functional psychoses (Kraepelin, 1899). This finding agrees with initial reports of a good single-subject separability of schizophrenia and bipolar disorder based on structural (Schnack et al., 2014) or functional MRI (Costafreda et al., 2011). It may also point to an increased sensitivity of multivariate pattern analysis techniques in detecting points of rarity in high-dimensional neuroimaging data compared to univariate methods, which frequently reported considerable overlaps between bipolar disorder and schizophrenia (Arnone et al., 2009). On the other hand, we found a significant difference in the neurodiagnostic scores of the major depression and bipolar disorder groups (Fig. 1), which adds to the growing evidence for structural and functional brain signatures separating these two largely overlapping conditions (Almeida and Phillips, 2013; Redlich et al., 2014). However, it remains unclear whether the configuration of schizophrenia, bipolar disorder and major depression found in our neuroanatomical analysis is conserved in functional neuroimaging domains. Hence, future studies are needed that simultaneously acquire structural and functional imaging data from larger samples of bipolar and schizoaffective patients and directly quantify the neurodiagnostic separability of these ‘intermediate’ phenotypes in the multi-modal imaging space (Lawrie et al., 2011). This will provide us with a comprehensive picture of continuities and discontinuities in the functional psychoses spectrum (Laursen et al., 2009).
Third, we found that an ‘earlier disease occurrence’ correlated with lower differential diagnostic accuracy, rendering major depression patients with earlier disease onsets inseparable from schizophrenia patients—despite distinct cross-sectional phenotypes. This observation was corroborated by the high separability of age of onset-defined major depression samples (83.7%, Table 2) compared to the respective schizophrenia subgroups (62.3%), which suggests that neuroanatomical surrogates of depressive syndromes strongly covary with the disease onset axis. This hypothesis has recently received support from studies showing a pronounced thinning in prefrontal, cingulate, precuneal and inferior temporal cortices of early versus late-onset major depression patients and healthy controls (Truong et al., 2013) (Supplementary Fig. 6). Hence, our results may point to more disrupted neurodevelopmental processes in early depression, potentially manifesting as an accelerated brain ageing effect (Koutsouleris et al., 2013). These processes may also be linked to a more severe clinical phenotype of depression, entailing greater illness severity, higher relapse rates, more cognitive disturbances, as well as overall poorer disease outcomes and higher familial co-aggregation with schizophrenia (Maier et al., 1993; Zisook et al., 2004; Dekker et al., 2007; Korten et al., 2012). Hence, the overlaps between schizophrenia and early-onset depression may lead to a diagnostic dilemma, particularly in the early phases of these illnesses when overt psychotic symptoms have not yet evolved or patients are not explored during psychotic phases. Our results indicate that this challenge cannot be resolved by our neuroanatomical classifier, which would frequently diagnose these early-onset depressed patients with schizophrenia. Thus, it remains to be elucidated whether different imaging modalities and combinations thereof may help increase the diagnostic specificity in the neurobiological classification of early-onset depression.
Fourth, we observed an increasing schizophrenia likeness from the early ARMS to the FEP individuals, suggesting that the neurodiagnostic fingerprint of schizophrenia is already detectable in persons with basic symptoms, and further intensifies as attenuated, brief limited intermittent and frank psychotic symptoms emerge. This finding agrees with previous studies reporting longitudinal grey matter volume changes in the ARMS, indicating a progressive course of neuroanatomical alterations as the at-risk state evolves into overt psychosis (Koutsouleris et al., 2010; Cannon et al., 2015). However, due to the cross-sectional design of our study, it remains unclear (i) whether the increase of schizophrenia likeness along these early states of psychosis also occurs at the level of neuroanatomical disease trajectories; and (ii) which protective factors contribute to a non-conversion to psychosis despite the presence of the schizophrenia-specific pattern in a given patient. Beyond the arbitrary clinical endpoint of disease transition [see Supplementary material and Yung et al. (2010)], our supplementary results indicate that an increased schizophrenia likeness in the ARMS may be associated with poorer functional outcomes at follow-up, suggesting that the identified MRI pattern may not only have differential diagnostic validity but also potential prognostic relevance.
Finally, one caveat has to be considered when interpreting our findings: different medication and treatment histories in our schizophrenia versus major depression groups may have influenced the separability of our patients as long-standing antipsychotic treatment has been previously shown to interact with disease-related brain changes (Ho et al., 2011). Although life-time medication data were not available for the current data set, the high diagnostic sensitivity in our minimally-treated ARMS and FEP groups argues against major treatment effects on our results. Furthermore, our finding of a significantly higher classification performance in first-episode compared to recurrently-ill patients is at odds with the expectation that relapsing illness stages and—in consequence—accumulating disease-specific treatment effects would increase the neuroanatomical gaps between schizophrenia and unipolar depression. The higher diagnostic error in the recurrently-ill patient sample could be interpreted as a ‘dilution effect’, which may arise from increasing neuroanatomical heterogeneity as patients evolve along divergent disease trajectories. Hence, this heterogeneity may result from (i) structural brain variation linked to differential disease courses (Mourao-Miranda et al., 2012) and treatment outcomes (Palaniyappan et al., 2013); (ii) distinct neuroanatomical correlates of positive, negative, disorganized and depressive subsyndromes of schizophrenia, as revealed by factor analytic studies (Koutsouleris et al., 2008; Nenadic et al., 2010, 2012; Zhang et al., 2014); and (iii) temporal shifts of these profiles over time, with negative and depressive symptoms becoming increasingly prominent in the course of the disease (Salvatore et al., 2013). Nevertheless, our results did not support a moderating or ‘diluting’ effect of depressive/psychotic syndromes in schizophrenia/major depression on neurodiagnostic classification performance. Thus, the strong impact of longitudinal disease variables such as age of disease onset and disease stage may suggest that the identified neuroanatomical biomarker is linked to the temporal and neurodevelopmental characteristics of these clinical phenotypes rather than to their cross-sectional psychopathological features (Gogtay et al., 2011).
In summary, our findings partly confirm and partly question the Kraepelinian dichotomy of functional psychoses into schizophrenia and affective disorders. This is not surprising if one considers the plethora of studies in support of either a phenomenological and neurobiological continuum or a division between these two nosological groups (Kotov et al., 2013). Our results suggest that the diagnostic boundaries drawn by a neuroanatomical disease signature become increasingly porous as patients develop depressive disorders at younger ages, potentially mediated by a cross-nosological disruption of neurodevelopmental processes. This gradient of diagnostic uncertainty does not only challenge clinical and biomarker-based diagnosis, it highlights also the utility of pattern recognition methods to probe the neurological basis of psychiatric illnesses and potentially refine nosological disease constructs.
Funding
This work was supported by The German Association for Psychiatry, Psychotherapy and Psychosomatics with a travel grant (NeuroImaging Prize) to N.K. C.D was supported by NIH grant R01-AG14971 for participation in the analyses and writing of the manuscript.
Supplementary material
Supplementary material is available at Brain online.
Glossary
Abbreviations
- ARMS =
at-risk mental state;
- BrainAGE =
Brain Age Gap Estimation;
- CV =
cross-validation;
- FEP =
first episode psychosis;
- PANSS =
Positive and Negative Symptom Scale;
- RAVENS =
regional analysis of brain volumes in normalized space
References
- Addington J, Shah H, Liu L, Addington D. Reliability and validity of the Calgary Depression Scale for Schizophrenia (CDSS) in youth at clinical high risk for psychosis. [Internet] Schizophr Res. 2014;153:64–7. doi: 10.1016/j.schres.2013.12.014. Available from: http://dx.doi.org/10.1016/j.schres.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida JR, Cardoso de, Phillips ML. Distinguishing between unipolar depression and bipolar depression: current and future clinical and neuroimaging perspectives. [Internet] Biol Psychiatry. 2013;73:111–18. doi: 10.1016/j.biopsych.2012.06.010. Available from: http://dx.doi.org/10.1016/j.biopsych.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone D, Cavanagh J, Gerber D, Lawrie SM, Ebmeier KP, McIntosh AM. Magnetic resonance imaging studies in bipolar disorder and schizophrenia: meta-analysis. [Internet] Br J Psychiatry. 2009;195:194–201. doi: 10.1192/bjp.bp.108.059717. Available from: http://dx.doi.org/10.1192/bjp.bp.108.059717. [DOI] [PubMed] [Google Scholar]
- Baca-Garcia E, Perez-Rodriguez MM, Basurte-Villamor I, Fernandez del Moral AL, Jimenez-Arriero MA, Gonzalez de Rivera JL, et al. Diagnostic stability of psychiatric disorders in clinical practice. [Internet] Br J Psychiatry. 2007;190:210–16. doi: 10.1192/bjp.bp.106.024026. Available from: http://dx.doi.org/10.1192/bjp.bp.106.024026. [DOI] [PubMed] [Google Scholar]
- Baynes D, Mulholland C, Cooper SJ, Montgomery RC, MacFlynn G, Lynch G, et al. Depressive symptoms in stable chronic schizophrenia: prevalence and relationship to psychopathology and treatment. Schizophr Res. 2000;45:47–56. doi: 10.1016/s0920-9964(99)00205-4. [DOI] [PubMed] [Google Scholar]
- Bora E, Fornito A, Radua J, Walterfang M, Seal M, Wood SJ, et al. Neuroanatomical abnormalities in schizophrenia: a multimodal voxelwise meta-analysis and meta-regression analysis. [Internet] Schizophr Res. 2011;127:46–57. doi: 10.1016/j.schres.2010.12.020. Available from: http://dx.doi.org/10.1016/j.schres.2010.12.020. [DOI] [PubMed] [Google Scholar]
- Bora E, Fornito A, Yücel M, Pantelis C. Voxelwise meta-analysis of gray matter abnormalities in bipolar disorder. [Internet] Biol Psychiatry. 2010;67:1097–105. doi: 10.1016/j.biopsych.2010.01.020. Available from: http://dx.doi.org/10.1016/j.biopsych.2010.01.020. [DOI] [PubMed] [Google Scholar]
- Bora E, Yucel M, Fornito A, Berk M, Pantelis C. Major psychoses with mixed psychotic and mood symptoms: are mixed psychoses associated with different neurobiological markers? [Internet] Acta Psychiatr Scand. 2008;118:172–87. doi: 10.1111/j.1600-0447.2008.01230.x. Available from: http://dx.doi.org/10.1111/j.1600-0447.2008.01230.x. [DOI] [PubMed] [Google Scholar]
- Borgwardt S, Koutsouleris N, Aston J, Studerus E, Smieskova R, Rössler A, et al. Distinguishing prodromal from first-episode psychosis using neuroanatomical single-subject pattern recognition. Schizophr Bull. 2013;39:1105–14. doi: 10.1093/schbul/sbs095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt CL, Eichele T, Melle I, Sundet K, Server A, Agartz I, et al. Working memory networks and activation patterns in schizophrenia and bipolar disorder: comparison with healthy controls. [Internet] Br J Psychiatry. 2014;204:290–8. doi: 10.1192/bjp.bp.113.129254. Available from: http://dx.doi.org/10.1192/bjp.bp.113.129254. [DOI] [PubMed] [Google Scholar]
- Bray S, Chang C, Hoeft F. Applications of multivariate pattern classification analyses in developmental neuroimaging of healthy and clinical populations. [Internet] Front Hum Neurosci. 2009;3:32. doi: 10.3389/neuro.09.032.2009. Available from: http://dx.doi.org/10.3389/neuro.09.032.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Chung Y, He G, Sun D, Jacobson A, van Erp TGM, et al. Progressive reduction in cortical thickness as psychosis develops: a multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biol Psychiatry. 2015;77:147–57. doi: 10.1016/j.biopsych.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemerinski E, Bowie C, Anderson H, Harvey PD. Depression in schizophrenia: methodological artifact or distinct feature of the illness? [Internet] J Neuropsychiatry Clin Neurosci. 2008;20:431–40. doi: 10.1176/appi.neuropsych.20.4.431. Available from: http://dx.doi.org/10.1176/appi.neuropsych.20.4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costafreda SG, Fu CHY, Picchioni M, Toulopoulou T, McDonald C, Kravariti E, et al. Pattern of neural responses to verbal fluency shows diagnostic specificity for schizophrenia and bipolar disorder. [Internet] BMC Psychiatry. 2011;11:18. doi: 10.1186/1471-244X-11-18. Available from: http://dx.doi.org/10.1186/1471-244X-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton SM, Lambert M, Schimmelmann BG, Mackinnon A, Gleeson JFM, Berk M, et al. Depressive symptoms in first episode schizophrenia spectrum disorder. [Internet] Schizophr Res. 2012;134:20–6. doi: 10.1016/j.schres.2011.08.018. Available from: http://dx.doi.org/10.1016/j.schres.2011.08.018. [DOI] [PubMed] [Google Scholar]
- Davatzikos C. Why voxel-based morphometric analysis should be used with great caution when characterizing group differences. [Internet] Neuroimage. 2004;23:17–20. doi: 10.1016/j.neuroimage.2004.05.010. Available from: http://dx.doi.org/10.1016/j.neuroimage.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Davatzikos C, Genc A, Xu D, Resnick SM. Voxel-based morphometry using the RAVENS maps: methods and validation using simulated longitudinal atrophy. [Internet] Neuroimage. 2001;14:1361–9. doi: 10.1006/nimg.2001.0937. Available from: http://dx.doi.org/10.1006/nimg.2001.0937. [DOI] [PubMed] [Google Scholar]
- Davatzikos C, Xu F, An Y, Fan Y, Resnick SM. Longitudinal progression of Alzheimer’s-like patterns of atrophy in normal older adults: the SPARE-AD index [Internet] Brain. 2009;132:2026–35. doi: 10.1093/brain/awp091. Available from: http://brain.oxfordjournals.org/cgi/content/abstract/132/8/2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker MC, Ferdinand RF, Lang NDJ van, Bongers IL, Ende J, van der, Verhulst FC. Developmental trajectories of depressive symptoms from early childhood to late adolescence: gender differences and adult outcome. [Internet] J Child Psychol Psychiatry. 2007;48:657–66. doi: 10.1111/j.1469-7610.2007.01742.x. Available from: http://dx.doi.org/10.1111/j.1469-7610.2007.01742.x. [DOI] [PubMed] [Google Scholar]
- Disabato BM, Morris C, Hranilovich J, D’Angelo GM, Zhou G, Wu N, et al. Comparison of brain structural variables, neuropsychological factors, and treatment outcome in early-onset versus late-onset late-life depression. [Internet] Am J Geriatr Psychiatry. 2014;22:1039–46. doi: 10.1016/j.jagp.2013.02.005. Available from: http://dx.doi.org/10.1016/j.jagp.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du MY, Wu QZ, Yue Q, Li J, Liao Y, Kuang WH, et al. Voxelwise meta-analysis of gray matter reduction in major depressive disorder. [Internet] Prog Neuropsychopharmacol Biol Psychiatry. 2012;36:11–16. doi: 10.1016/j.pnpbp.2011.09.014. Available from: http://dx.doi.org/10.1016/j.pnpbp.2011.09.014. [DOI] [PubMed] [Google Scholar]
- Dukart J, Schroeter ML, Mueller K Alzheimer’s Disease Neuroimaging Initiative. Age correction in dementia–matching to a healthy brain. PLoS One. 2011:6: e22193. doi: 10.1371/journal.pone.0022193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Yazaji M, Battas O, Agoub M, Moussaoui D, Gutknecht C, Dalery J, et al. Validity of the depressive dimension extracted from principal component analysis of the PANSS in drug-free patients with schizophrenia. Schizophr Res. 2002:56: 121–7. doi: 10.1016/s0920-9964(01)00247-x. [DOI] [PubMed] [Google Scholar]
- Fan R, Chang K, Hsieh C, Wang X, Lin C. LIBLINEAR: a library for large linear classification. J Mach Learn Res. 2008a;9:1871–4. [Google Scholar]
- Fan Y, Gur RE, Gur RC, Wu X, Shen D, Calkins ME, et al. Unaffected family members and schizophrenia patients share brain structure patterns: a high-dimensional pattern classification study. [Internet] Biol Psychiatry. 2008b;63:118–24. doi: 10.1016/j.biopsych.2007.03.015. Available from: http://dx.doi.org/10.1016/j.biopsych.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filzmoser P, Liebmann B, Varmuza K. Repeated double cross validation. J. Chemometrics. 2009;23:160–71. [Google Scholar]
- Freedman R, Lewis DA, Michels R, Pine DS, Schultz SK, Tamminga CA, et al. The initial field trials of DSM-5: new blooms and old thorns. [Internet] Am J Psychiatry. 2013;170:1–5. doi: 10.1176/appi.ajp.2012.12091189. Available from: http://dx.doi.org/10.1176/appi.ajp.2012.12091189. [DOI] [PubMed] [Google Scholar]
- Friston KJ. Schizophrenia and the disconnection hypothesis. Acta Psychiatr Scand Suppl. 1999;395:68–79. doi: 10.1111/j.1600-0447.1999.tb05985.x. [DOI] [PubMed] [Google Scholar]
- Fu CHY, Costafreda SG. Neuroimaging-based biomarkers in psychiatry: clinical opportunities of a paradigm shift. Can J Psychiatry. 2013;58:499–508. doi: 10.1177/070674371305800904. [DOI] [PubMed] [Google Scholar]
- Gaser C. Voxel-Based Morphometry Toolbox, version 8 (VBM8): [Internet] 2009 Available from: http://dbm.neuro.uni-jena.de. [Google Scholar]
- Goodwin F, Jamison K. Manic-Depressive Illness: Bipolar Disorders and Recurrent Depression. New York: Oxford University Press; 2007. [Google Scholar]
- Green EK, Grozeva D, Jones I, Jones L, Kirov G, Caesar S, et al. The bipolar disorder risk allele at CACNA1C also confers risk of recurrent major depression and of schizophrenia. [Internet] Mol. Psychiatry. 2009 doi: 10.1038/mp.2009.49. Available from: http://dx.doi.org/10.1038/mp.2009.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotegerd D, Stuhrmann A, Kugel H, Schmidt S, Redlich R, Zwanzger P, et al. Amygdala excitability to subliminally presented emotional faces distinguishes unipolar and bipolar depression: An fMRI and pattern classification study. [Internet] Hum Brain Mapp. 2013 doi: 10.1002/hbm.22380. Available from: http://dx.doi.org/10.1002/hbm.22380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Vyas NS, Testa R, Wood SJ, Pantelis C. Age of onset of schizophrenia: perspectives from structural neuroimaging studies. [Internet] Schizophr Bull. 2011;37:504–13. doi: 10.1093/schbul/sbr030. Available from: http://dx.doi.org/10.1093/schbul/sbr030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Liu F, Xun G, Hu M, Guo X, Xiao C, et al. Disrupted white matter integrity in first-episode, drug-naive, late-onset depression. [Internet] J Affect Disord. 2014;163:70–5. doi: 10.1016/j.jad.2014.03.044. Available from: http://dx.doi.org/10.1016/j.jad.2014.03.044. [DOI] [PubMed] [Google Scholar]
- Guyon I, Weston J, Barnhill S, Vapnik V. Gene Selection for Cancer Classification using Support Vector Machines [Internet] Mach. Learn. 2002;46:389–22. Available from: http://dx.doi.org/10.1023/A:1012487302797. [Google Scholar]
- Häfner H, Maurer K, Trendler G, an der Heiden W, Schmidt M, Könnecke R. Schizophrenia and depression: challenging the paradigm of two separate diseases–a controlled study of schizophrenia, depression and healthy controls. [Internet] Schizophr. Res. 2005;77:11–24. doi: 10.1016/j.schres.2005.01.004. Available from: http://dx.doi.org/10.1016/j.schres.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Hansen LK, Larsen J, Nielsen FA, Strother SC, Rostrup E, Savoy R, et al. Generalizable patterns in neuroimaging: how many principal components? [Internet] Neuroimage. 1999;9:534–44. doi: 10.1006/nimg.1998.0425. Available from: http://dx.doi.org/10.1006/nimg.1998.0425. [DOI] [PubMed] [Google Scholar]
- Harrow M, Grossman LS, Herbener ES, Davies EW. Ten-year outcome: patients with schizoaffective disorders, schizophrenia, affective disorders and mood-incongruent psychotic symptoms. Br J Psychiatry. 2000;177:421–6. doi: 10.1192/bjp.177.5.421. [DOI] [PubMed] [Google Scholar]
- Hulshoff Pol HE, Baal GCM van, Schnack HG, Brans RGH, van der Schot AC, Brouwer RM, et al. Overlapping and segregating structural brain abnormalities in twins with schizophrenia or bipolar disorder. [Internet] Arch Gen Psychiatry. 2012;69:349–59. doi: 10.1001/archgenpsychiatry.2011.1615. Available from: http://dx.doi.org/10.1001/archgenpsychiatry.2011.1615. [DOI] [PubMed] [Google Scholar]
- Ho B-C, Andreasen NC, Ziebell S, Pierson R, Magnotta V. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. [Internet] Arch Gen Psychiatry. 2011;68:128–37. doi: 10.1001/archgenpsychiatry.2010.199. Available from: http://dx.doi.org/10.1001/archgenpsychiatry.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. [Internet] Am. J. Psychiatry. 2005;162:2233–45. doi: 10.1176/appi.ajp.162.12.2233. Available from: http://dx.doi.org/10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- Kahn RS, Keefe RSE. Schizophrenia is a cognitive illness: time for a change in focus. [Internet] JAMA Psychiatry. 2013;70:1107–12. doi: 10.1001/jamapsychiatry.2013.155. Available from: http://dx.doi.org/10.1001/jamapsychiatry.2013.155. [DOI] [PubMed] [Google Scholar]
- Kambeitz J, Kambeitz-Ilankovic L, Leucht S, Wood S, Davatzikos C, Malchow B, et al. Detecting Neuroimaging Biomarkers for Schizophrenia: A Meta-Analysis of Multivariate Pattern Recognition Studies. [Internet] Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.22. Available from: http://dx.doi.org/10.1038/npp.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klosterkötter J, Hellmich M, Steinmeyer EM, Schultze-Lutter F. Diagnosing schizophrenia in the initial prodromal phase. Arch. Gen. Psychiatry. 2001;58:158–64. doi: 10.1001/archpsyc.58.2.158. [DOI] [PubMed] [Google Scholar]
- Kontaxakis VP, Havaki-Kontaxaki BJ, Stamouli SS, Margariti MM, Collias CT, Christodoulou GN. Comparison of four scales measuring depression in schizophrenic inpatients. Eur Psychiatry. 2000:15: 274–7. doi: 10.1016/s0924-9338(00)00232-7. [DOI] [PubMed] [Google Scholar]
- Koutsouleris N, Gaser C, Jäger MM, Bottlender R, Frodl T, Holzinger S, et al. Structural correlates of psychopathological symptom dimensions in schizophrenia: A voxel-based morphometric study. [Internet] Neuroimage. 2008;39:1600–12. doi: 10.1016/j.neuroimage.2007.10.029. Available from: http://dx.doi.org/10.1016/j.neuroimage.2007.10.029. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Brady R. Biomarkers in schizophrenia: we need to rebuild the Titanic. World Psychiatry. 2011:10: 35–6. doi: 10.1002/j.2051-5545.2011.tb00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korten NCM, Comijs HC, Lamers F, Penninx BWJH. Early and late onset depression in young and middle aged adults: Differential symptomatology, characteristics and risk factors? [Internet] Journal of Affective Disorders. 2012;138:259–67. doi: 10.1016/j.jad.2012.01.042. Available from: http://www.sciencedirect.com/science/article/pii/S0165032712000894. [DOI] [PubMed] [Google Scholar]
- Kotov R, Leong SH, Mojtabai R, Erlanger AC, Fochtmann LJ, Constantino E, et al. Boundaries of schizoaffective disorder: revisiting Kraepelin. JAMA Psychiatry. 2013;70:1276–86. doi: 10.1001/jamapsychiatry.2013.2350. [DOI] [PubMed] [Google Scholar]
- Koutsouleris N, Gaser C, Bottlender R, Davatzikos C, Decker P, Jäger M, et al. Use of neuroanatomical pattern regression to predict the structural brain dynamics of vulnerability and transition to psychosis. [Internet] Schizophr Res. 2010;123:175–87. doi: 10.1016/j.schres.2010.08.032. Available from: http://dx.doi.org/10.1016/j.schres.2010.08.032. [DOI] [PubMed] [Google Scholar]
- Koutsouleris N, Meisenzahl E, Davatzikos C, Bottlender R, Frodl T, Scheuerecker J, et al. Use of neuroanatomical pattern classification to identify subjects in at-risk mental states of psychosis and predict disease transition. [Internet] Arch Gen Psychiatry. 2009;66:700–2. doi: 10.1001/archgenpsychiatry.2009.62. Available from: http://dx.doi.org/10.1001/archgenpsychiatry.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsouleris N, Borgwardt S, Studerus E, Smieskova R, Meisenzahl E, Riecher A. MRI-based prediction of psychosis across populations: findings from the Munich and Basel early recognition services. 2012 in prep. [Google Scholar]
- Koutsouleris N, Davatzikos C, Borgwardt S, Gaser C, Bottlender R, Frodl T, et al. Accelerated Brain Aging in Schizophrenia and Beyond: A Neuroanatomical Marker of Psychiatric Disorders. [Internet] Schizophr Bull. 2013 doi: 10.1093/schbul/sbt142. Available from: http://dx.doi.org/10.1093/schbul/sbt142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraepelin E. Psychiatrie. 6th edn. Germany: Barth: Leipzig; 1899. [Google Scholar]
- Krystal JH, State MW. Psychiatric Disorders: Diagnosis to Therapy. [Internet] Cell. 2014;157:201–14. doi: 10.1016/j.cell.2014.02.042. Available from: http://dx.doi.org/10.1016/j.cell.2014.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman J, Chakos M, Wu H, Alvir J, Hoffman E, Robinson D, et al. Longitudinal study of brain morphology in first episode schizophrenia. Biol. Psychiatry. 2001:49: 487–99. doi: 10.1016/s0006-3223(01)01067-8. [DOI] [PubMed] [Google Scholar]
- Laursen TM, Agerbo E, Pedersen CB. Bipolar disorder, schizoaffective disorder, and schizophrenia overlap: a new comorbidity index. [Internet] J Clin Psychiatry. 2009;70:1432–8. doi: 10.4088/JCP.08m04807. Available from: http://dx.doi.org/10.4088/JCP.08m04807. [DOI] [PubMed] [Google Scholar]
- Lawrie SM, Olabi B, Hall J, McIntosh AM. Do we have any solid evidence of clinical utility about the pathophysiology of schizophrenia? World Psychiatry. 2011;10:19–31. doi: 10.1002/j.2051-5545.2011.tb00004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A, Reniers RLEP, Wood SJ. Clinical staging in severe mental disorder: evidence from neurocognition and neuroimaging. [Internet] Br J Psychiatry Suppl. 2013;54:s11–17. doi: 10.1192/bjp.bp.112.119156. Available from: http://dx.doi.org/10.1192/bjp.bp.112.119156. [DOI] [PubMed] [Google Scholar]
- Linscott RJ, van Os J. Systematic reviews of categorical versus continuum models in psychosis: evidence for discontinuous subpopulations underlying a psychometric continuum. Implications for DSM-V, DSM-VI, and DSM-VII. [Internet] Annu Rev Clin Psychol. 2010;6:391–419. doi: 10.1146/annurev.clinpsy.032408.153506. Available from: http://dx.doi.org/10.1146/annurev.clinpsy.032408.153506. [DOI] [PubMed] [Google Scholar]
- Maier W, Lichtermann D, Minges J, Hallmayer J, Heun R, Benkert O, et al. Continuity and discontinuity of affective disorders and schizophrenia. Results of a controlled family study. Arch Gen Psychiatry. 1993;50:871–83. doi: 10.1001/archpsyc.1993.01820230041004. [DOI] [PubMed] [Google Scholar]
- Marengo J, Harrow M, Herbener ES, Sands J. A prospective longitudinal 10-year study of schizophrenia’s three major factors and depression. Psychiatry Res. 2000;97:61–77. doi: 10.1016/s0165-1781(00)00218-3. [DOI] [PubMed] [Google Scholar]
- Modinos G, Costafreda SG, van Tol M-J, McGuire PK, Aleman A, Allen P. Neuroanatomy of auditory verbal hallucinations in schizophrenia: A quantitative meta-analysis of voxel-based morphometry studies. [Internet] Cortex. 2012;49:146–55. doi: 10.1016/j.cortex.2012.01.009. Available from: http://dx.doi.org/10.1016/j.cortex.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Mourão-Miranda J, Almeida JRC, Hassel S, Oliveira L de, Versace A, Marquand AF, et al. Pattern recognition analyses of brain activation elicited by happy and neutral faces in unipolar and bipolar depression. [Internet] Bipolar Disord. 2012;14:451–60. doi: 10.1111/j.1399-5618.2012.01019.x. Available from: http://dx.doi.org/10.1111/j.1399-5618.2012.01019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourao-Miranda J, Reinders AATS, Rocha-Rego V, Lappin J, Rondina J, Morgan C, et al. Individualized prediction of illness course at the first psychotic episode: a support vector machine MRI study. [Internet] Psychol Med. 2012;42:1037–47. doi: 10.1017/S0033291711002005. Available from: http://dx.doi.org/10.1017/S0033291711002005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray V, McKee I, Miller P, Young D, Muir W, Pelosi A, et al. Dimensions and classes of psychosis in a population cohort: a four-class, four-dimension model of schizophrenia and affective psychoses. Psychol Med. 2005:35: 499–510. doi: 10.1017/s0033291704003745. [DOI] [PubMed] [Google Scholar]
- Nenadic I, Gaser C, Sauer H. Heterogeneity of brain structural variation and the structural imaging endophenotypes in schizophrenia. [Internet] Neuropsychobiology. 2012;66:44–9. doi: 10.1159/000338547. Available from: http://dx.doi.org/10.1159/000338547. [DOI] [PubMed] [Google Scholar]
- Nenadic I, Sauer H, Gaser C. Distinct pattern of brain structural deficits in subsyndromes of schizophrenia delineated by psychopathology. [Internet] Neuroimage. 2010;49:1153–60. doi: 10.1016/j.neuroimage.2009.10.014. Available from: http://dx.doi.org/10.1016/j.neuroimage.2009.10.014. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Schatzberg AF. Prevalence of depressive episodes with psychotic features in the general population. Am J Psychiatry. 2002:159: 1855–61. doi: 10.1176/appi.ajp.159.11.1855. [DOI] [PubMed] [Google Scholar]
- Orrù G, Pettersson-Yeo W, Marquand AF, Sartori G, Mechelli A. Using Support Vector Machine to identify imaging biomarkers of neurological and psychiatric disease: a critical review. [Internet] Neurosci Biobehav Rev. 2012;36:1140–52. doi: 10.1016/j.neubiorev.2012.01.004. Available from: http://dx.doi.org/10.1016/j.neubiorev.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Ota M, Ishikawa M, Sato N, Hori H, Sasayama D, Hattori K, et al. Discrimination between schizophrenia and major depressive disorder by magnetic resonance imaging of the female brain. [Internet] J Psychiatr Res. 2013;47:1383–8. doi: 10.1016/j.jpsychires.2013.06.010. Available from: http://dx.doi.org/10.1016/j.jpsychires.2013.06.010. [DOI] [PubMed] [Google Scholar]
- Ou Y, Sotiras A, Paragios N, Davatzikos C. DRAMMS: Deformable registration via attribute matching and mutual-saliency weighting. [Internet] Med Image Anal. 2011;15:622–39. doi: 10.1016/j.media.2010.07.002. Available from: http://dx.doi.org/10.1016/j.media.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou Y, Akbari H, Bilello M, Da X, Davatzikos C. Comparative evaluation of registration algorithms in different brain databases with varying difficulty: results and insights. [Internet] IEEE Trans Med Imaging. 2014;33:2039–65. doi: 10.1109/TMI.2014.2330355. Available from: http://dx.doi.org/10.1109/TMI.2014.2330355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaniyappan L, Marques TR, Taylor H, Handley R, Mondelli V, Bonaccorso S, et al. Cortical folding defects as markers of poor treatment response in first-episode psychosis. [Internet] JAMA Psychiatry. 2013;70:1031–40. doi: 10.1001/jamapsychiatry.2013.203. Available from: http://dx.doi.org/10.1001/jamapsychiatry.2013.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins DO, Jeffries CD, Addington J, Bearden CE, Cadenhead KS, Cannon TD, et al. Towards a psychosis risk blood diagnostic for persons experiencing high-risk symptoms: preliminary results from the NAPLS project. [Internet] Schizophr Bull. 2015 doi: 10.1093/schbul/sbu099. 41: 419–28. Available from: http://dx.doi.org/10.1093/schbul/sbu099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope MA, Joober R, Malla AK. Diagnostic stability of first-episode psychotic disorders and persistence of comorbid psychiatric disorders over 1 year. Can J Psychiatry. 2013;58:588–94. doi: 10.1177/070674371305801008. [DOI] [PubMed] [Google Scholar]
- Redlich R, Almeida JJR, Grotegerd D, Opel N, Kugel H, Heindel W, et al. Brain morphometric biomarkers distinguishing unipolar and bipolar depression. A voxel-based morphometry-pattern classification approach. [Internet] JAMA Psychiatry. 2014;71:1222–30. doi: 10.1001/jamapsychiatry.2014.1100. Available from: http://dx.doi.org/10.1001/jamapsychiatry.2014.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romm KL, Rossberg JI, Berg AO, Barrett EA, Faerden A, Agartz I, et al. Depression and depressive symptoms in first episode psychosis. [Internet] J Nerv Ment Dis. 2010;198:67–71. doi: 10.1097/NMD.0b013e3181c81fc0. Available from: http://dx.doi.org/10.1097/NMD.0b013e3181c81fc0. [DOI] [PubMed] [Google Scholar]
- Salvatore P, Baldessarini RJ, Khalsa H-MK, Amore M, Di Vittorio C, Ferraro G, et al. Predicting diagnostic change among patients diagnosed with first-episode DSM-IV-TR major depressive disorder with psychotic features. [Internet] J Clin Psychiatry. 2013;74:723–31. doi: 10.4088/JCP.12m08328. quiz 731. Available from: http://dx.doi.org/10.4088/JCP.12m08328. [DOI] [PubMed] [Google Scholar]
- Sans-Sansa B, McKenna PJ, Canales-Rodríguez EJ, Ortiz-Gil J, López-Araquistain L, Sarró S, et al. Association of formal thought disorder in schizophrenia with structural brain abnormalities in language-related cortical regions. [Internet] Schizophr Res. 2013;146:308–13. doi: 10.1016/j.schres.2013.02.032. Available from: http://dx.doi.org/10.1016/j.schres.2013.02.032. [DOI] [PubMed] [Google Scholar]
- Schnack HG, Nieuwenhuis M, Haren NEM van, Abramovic L, Scheewe TW, Brouwer RM, et al. Can structural MRI aid in clinical classification? A machine learning study in two independent samples of patients with schizophrenia, bipolar disorder and healthy subjects. [Internet] Neuroimage. 2014;84:299–306. doi: 10.1016/j.neuroimage.2013.08.053. Available from: http://dx.doi.org/10.1016/j.neuroimage.2013.08.053. [DOI] [PubMed] [Google Scholar]
- Serpa M, Ou Y, Schaufelberger M, Doshi J, Ferreira L, Machado-Vieira R, et al. Neuroanatomical classification in a population-based sample of psychotic major depression and bipolar I disorder with 1 year of diagnostic stability. Biomed Res Int. 2014;214:706157. doi: 10.1155/2014/706157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz JB, Rauch SL, Drevets WC. Clinical application of brain imaging for the diagnosis of mood disorders: the current state of play. [Internet] Mol Psychiatry. 2013;18:528–39. doi: 10.1038/mp.2013.25. Available from: http://dx.doi.org/10.1038/mp.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultze-Lutter F, Ruhrmann S, Picker H, Reventlow HG von, Brockhaus-Dumke A, Klosterkötter J. Basic symptoms in early psychotic and depressive disorders. [Internet] Br J Psychiatry Suppl. 2007;51:s31–7. doi: 10.1192/bjp.191.51.s31. Available from: http://dx.doi.org/10.1192/bjp.191.51.s31. [DOI] [PubMed] [Google Scholar]
- Serafini G, Pompili M, Innamorati M, Fusar-Poli P, Akiskal HS, Rihmer Z, et al. Affective temperamental profiles are associated with white matter hyperintensity and suicidal risk in patients with mood disorders. [Internet] J Affect Disord. 2011;129:47–55. doi: 10.1016/j.jad.2010.07.020. Available from: http://dx.doi.org/10.1016/j.jad.2010.07.020. [DOI] [PubMed] [Google Scholar]
- Sönmez N, Romm KL, Andreasssen OA, Melle I, Røssberg JI. Depressive symptoms in first episode psychosis: a one-year follow-up study. [Internet] BMC Psychiatry. 2013;13:106. doi: 10.1186/1471-244X-13-106. Available from: http://dx.doi.org/10.1186/1471-244X-13-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa R, Fukuda M, Kawasaki S, Kasai K, Mimura M, Pu S, et al. Neuroimaging-aided differential diagnosis of the depressive state. [Internet] Neuroimage. 2014;85 (Pt 1):498–507. doi: 10.1016/j.neuroimage.2013.05.126. Available from: http://dx.doi.org/10.1016/j.neuroimage.2013.05.126. [DOI] [PubMed] [Google Scholar]
- Truong W, Minuzzi L, Soares CN, Frey BN, Evans AC, MacQueen GM, et al. Changes in cortical thickness across the lifespan in major depressive disorder. [Internet] Psychiatry Res. 2013;214:204–11. doi: 10.1016/j.pscychresns.2013.09.003. Available from: http://dx.doi.org/10.1016/j.pscychresns.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Yu K, Cheung C, Leung M, Li Q, Chua S, McAlonan G. Are bipolar disorder and schizophrenia neuroanatomically distinct? An anatomical likelihood meta-analysis. [Internet] Front Hum Neurosci. 2010;4:189. doi: 10.3389/fnhum.2010.00189. Available from: http://dx.doi.org/10.3389/fnhum.2010.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung AR, Phillips LJ, McGorry PD, McFarlane CA, Francey S, Harrigan S, et al. Prediction of psychosis. A step towards indicated prevention of schizophrenia. Br. J. Psychiatry Suppl. 1998;172:14–20. [PubMed] [Google Scholar]
- Yung AR, Nelson B, Thompson A, Wood SJ. The psychosis threshold in Ultra High Risk (prodromal) research: is it valid? [Internet] Schizophr Res. 2010;120:1–6. doi: 10.1016/j.schres.2010.03.014. Available from: http://dx.doi.org/10.1016/j.schres.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Zhang T, Koutsouleris N, Meisenzahl E, Davatzikos C. Heterogeneity of structural brain changes in subtypes of schizophrenia revealed using magnetic resonance imaging pattern analysis. Schizophr Bull. 2015 doi: 10.1093/schbul/sbu136. 41: 74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zisook S, Rush AJ, Albala A, Alpert J, Balasubramani GK, Fava M, et al. Factors that differentiate early vs. later onset of majordepression disorder. [Internet] Psychiatry Res. 2004;129:127–40. doi: 10.1016/j.psychres.2004.07.004. Available from: http://dx.doi.org/10.1016/j.psychres.2004.07.004. [DOI] [PubMed] [Google Scholar]