Abstract
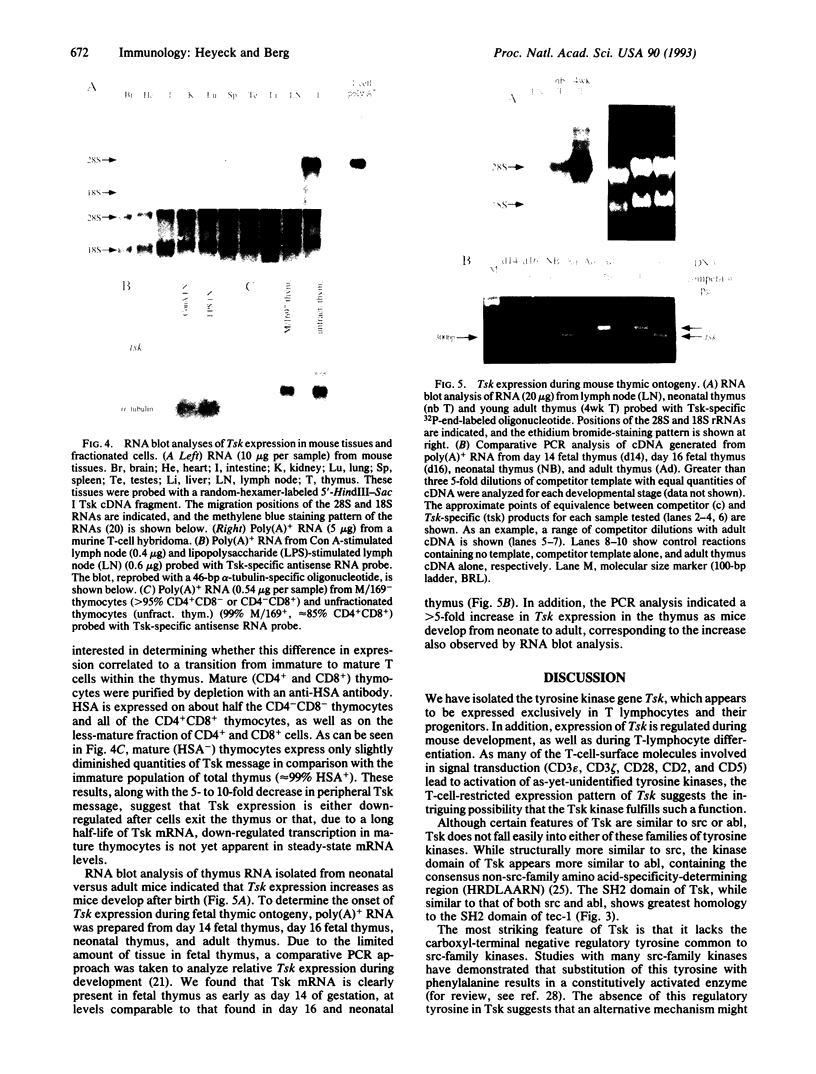
Protein-tyrosine kinases have been implicated in signal transduction in T lymphocytes after stimulation of many cell-surface molecules, including the T-cell antigen receptor, CD4, CD8, CD2, CD5, and CD28. Yet the identities of many of these tyrosine kinases remain unknown. We have isolated a murine tyrosine kinase gene, called Tsk for T-cell-specific kinase, that appears to be exclusively expressed in T lymphocytes. The Tsk cDNA clone encodes a polypeptide of 70 kDa, which is similar in sequence to both the src and abl families of tyrosine kinases. Sequence comparisons also indicate that Tsk contains one src-homology region 2 domain and one src-homology 3 domain but lacks the negative regulatory tyrosine (src Tyr-527) common to src-family kinases. In addition, Tsk expression is developmentally regulated. Steady-state Tsk mRNA levels are 5- to 10-fold higher in thymocytes than in peripheral T cells and increase in the thymus during mouse development from neonate to adult. Furthermore, Tsk is expressed in day 14 fetal thymus, suggesting a role for Tsk in early T-lymphocyte differentiation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberola-Ila J., Places L., Cantrell D. A., Vives J., Lozano F. Intracellular events involved in CD5-induced human T cell activation and proliferation. J Immunol. 1992 Mar 1;148(5):1287–1293. [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Appleby M. W., Gross J. A., Cooke M. P., Levin S. D., Qian X., Perlmutter R. M. Defective T cell receptor signaling in mice lacking the thymic isoform of p59fyn. Cell. 1992 Sep 4;70(5):751–763. doi: 10.1016/0092-8674(92)90309-z. [DOI] [PubMed] [Google Scholar]
- Becker-André M., Hahlbrock K. Absolute mRNA quantification using the polymerase chain reaction (PCR). A novel approach by a PCR aided transcript titration assay (PATTY). Nucleic Acids Res. 1989 Nov 25;17(22):9437–9446. doi: 10.1093/nar/17.22.9437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierer B. E., Sleckman B. P., Ratnofsky S. E., Burakoff S. J. The biologic roles of CD2, CD4, and CD8 in T-cell activation. Annu Rev Immunol. 1989;7:579–599. doi: 10.1146/annurev.iy.07.040189.003051. [DOI] [PubMed] [Google Scholar]
- Blackman M., Kappler J., Marrack P. The role of the T cell receptor in positive and negative selection of developing T cells. Science. 1990 Jun 15;248(4961):1335–1341. doi: 10.1126/science.1972592. [DOI] [PubMed] [Google Scholar]
- Chan A. C., Irving B. A., Fraser J. D., Weiss A. The zeta chain is associated with a tyrosine kinase and upon T-cell antigen receptor stimulation associates with ZAP-70, a 70-kDa tyrosine phosphoprotein. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9166–9170. doi: 10.1073/pnas.88.20.9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke M. P., Abraham K. M., Forbush K. A., Perlmutter R. M. Regulation of T cell receptor signaling by a src family protein-tyrosine kinase (p59fyn). Cell. 1991 Apr 19;65(2):281–291. doi: 10.1016/0092-8674(91)90162-r. [DOI] [PubMed] [Google Scholar]
- Crabtree G. R. Contingent genetic regulatory events in T lymphocyte activation. Science. 1989 Jan 20;243(4889):355–361. doi: 10.1126/science.2783497. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Irving B. A., Weiss A. The cytoplasmic domain of the T cell receptor zeta chain is sufficient to couple to receptor-associated signal transduction pathways. Cell. 1991 Mar 8;64(5):891–901. doi: 10.1016/0092-8674(91)90314-o. [DOI] [PubMed] [Google Scholar]
- Jackson P., Baltimore D. N-terminal mutations activate the leukemogenic potential of the myristoylated form of c-abl. EMBO J. 1989 Feb;8(2):449–456. doi: 10.1002/j.1460-2075.1989.tb03397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway C. A., Jr The T cell receptor as a multicomponent signalling machine: CD4/CD8 coreceptors and CD45 in T cell activation. Annu Rev Immunol. 1992;10:645–674. doi: 10.1146/annurev.iy.10.040192.003241. [DOI] [PubMed] [Google Scholar]
- June C. H., Fletcher M. C., Ledbetter J. A., Samelson L. E. Increases in tyrosine phosphorylation are detectable before phospholipase C activation after T cell receptor stimulation. J Immunol. 1990 Mar 1;144(5):1591–1599. [PubMed] [Google Scholar]
- June C. H., Ledbetter J. A., Gillespie M. M., Lindsten T., Thompson C. B. T-cell proliferation involving the CD28 pathway is associated with cyclosporine-resistant interleukin 2 gene expression. Mol Cell Biol. 1987 Dec;7(12):4472–4481. doi: 10.1128/mcb.7.12.4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J. M., Mardon G., Bishop J. M., Varmus H. E. The first seven amino acids encoded by the v-src oncogene act as a myristylation signal: lysine 7 is a critical determinant. Mol Cell Biol. 1988 Jun;8(6):2435–2441. doi: 10.1128/mcb.8.6.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D., Samelson L. E. T cell antigen receptor activation pathways: the tyrosine kinase connection. Cell. 1991 Mar 8;64(5):875–878. doi: 10.1016/0092-8674(91)90310-u. [DOI] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneur F., Klausner R. D. Activation of T cells by a tyrosine kinase activation domain in the cytoplasmic tail of CD3 epsilon. Science. 1992 Jan 3;255(5040):79–82. doi: 10.1126/science.1532456. [DOI] [PubMed] [Google Scholar]
- Lu Y., Granelli-Piperno A., Bjorndahl J. M., Phillips C. A., Trevillyan J. M. CD28-induced T cell activation. Evidence for a protein-tyrosine kinase signal transduction pathway. J Immunol. 1992 Jul 1;149(1):24–29. [PubMed] [Google Scholar]
- Mano H., Ishikawa F., Nishida J., Hirai H., Takaku F. A novel protein-tyrosine kinase, tec, is preferentially expressed in liver. Oncogene. 1990 Dec;5(12):1781–1786. [PubMed] [Google Scholar]
- Molina T. J., Kishihara K., Siderovski D. P., van Ewijk W., Narendran A., Timms E., Wakeham A., Paige C. J., Hartmann K. U., Veillette A. Profound block in thymocyte development in mice lacking p56lck. Nature. 1992 May 14;357(6374):161–164. doi: 10.1038/357161a0. [DOI] [PubMed] [Google Scholar]
- Perlmutter R. M., Marth J. D., Ziegler S. F., Garvin A. M., Pawar S., Cooke M. P., Abraham K. M. Specialized protein tyrosine kinase proto-oncogenes in hematopoietic cells. Biochim Biophys Acta. 1989 Feb;948(3):245–262. doi: 10.1016/0304-419x(89)90001-2. [DOI] [PubMed] [Google Scholar]
- Reynolds P. J., Lesley J., Trotter J., Schulte R., Hyman R., Sefton B. M. Changes in the relative abundance of type I and type II lck mRNA transcripts suggest differential promoter usage during T-cell development. Mol Cell Biol. 1990 Aug;10(8):4266–4270. doi: 10.1128/mcb.10.8.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodewald H. R., Moingeon P., Lucich J. L., Dosiou C., Lopez P., Reinherz E. L. A population of early fetal thymocytes expressing Fc gamma RII/III contains precursors of T lymphocytes and natural killer cells. Cell. 1992 Apr 3;69(1):139–150. doi: 10.1016/0092-8674(92)90125-v. [DOI] [PubMed] [Google Scholar]
- Romeo C., Seed B. Cellular immunity to HIV activated by CD4 fused to T cell or Fc receptor polypeptides. Cell. 1991 Mar 8;64(5):1037–1046. doi: 10.1016/0092-8674(91)90327-u. [DOI] [PubMed] [Google Scholar]
- Samelson L. E., Phillips A. F., Luong E. T., Klausner R. D. Association of the fyn protein-tyrosine kinase with the T-cell antigen receptor. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4358–4362. doi: 10.1073/pnas.87.11.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. A., Williams G. T., Kingston R., Jenkinson E. J., Owen J. J. Antibodies to CD3/T-cell receptor complex induce death by apoptosis in immature T cells in thymic cultures. Nature. 1989 Jan 12;337(6203):181–184. doi: 10.1038/337181a0. [DOI] [PubMed] [Google Scholar]
- Stein P. L., Lee H. M., Rich S., Soriano P. pp59fyn mutant mice display differential signaling in thymocytes and peripheral T cells. Cell. 1992 Sep 4;70(5):741–750. doi: 10.1016/0092-8674(92)90308-y. [DOI] [PubMed] [Google Scholar]
- Takei F., Secher D. S., Milstein C., Springer T. Use of a monoclonal antibody specifically non-reactive with T cells to delineate lymphocyte subpopulations. Immunology. 1981 Mar;42(3):371–378. [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe P., Freeman G. J., Nadler L. M., Fletcher M. C., Kamoun M., Turka L. A., Ledbetter J. A., Thompson C. B., June C. H. Antibody and B7/BB1-mediated ligation of the CD28 receptor induces tyrosine phosphorylation in human T cells. J Exp Med. 1992 Apr 1;175(4):951–960. doi: 10.1084/jem.175.4.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veillette A., Zúiga-Pflücker J. C., Bolen J. B., Kruisbeek A. M. Engagement of CD4 and CD8 expressed on immature thymocytes induces activation of intracellular tyrosine phosphorylation pathways. J Exp Med. 1989 Nov 1;170(5):1671–1680. doi: 10.1084/jem.170.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener A. M., Letourneur F., Hoeveler A., Brocker T., Luton F., Malissen B. The T cell receptor/CD3 complex is composed of at least two autonomous transduction modules. Cell. 1992 Jan 10;68(1):83–95. doi: 10.1016/0092-8674(92)90208-t. [DOI] [PubMed] [Google Scholar]
- Yue C. C. Novel putative protein kinase clones from a rat large granular lymphocyte tumor cell line. Mol Immunol. 1991 Apr-May;28(4-5):399–408. doi: 10.1016/0161-5890(91)90153-b. [DOI] [PubMed] [Google Scholar]