Abstract
Purpose
To determine the effect of proparacaine-induced topical anesthesia on episcleral venous pressure (EVP).
Methods
In anesthetized rabbits (n = 11), EVP was measured with a servonull micropressure system, with glass pipettes with 2- to 3-μm tips used to cannulate episcleral veins. Additional measurements included arterial, intraocular, and orbital venous pressures obtained by direct cannulation, to assess the ocular pressure gradients, and carotid blood flow and heart rate, to verify preparation stability. The protocol entailed 5 to 10 minutes of stable baseline recording followed by topical application of proparacaine (0.5%, 10 μL) with continued measurements for another 5 to 15 minutes.
Results
Baseline EVP without topical anesthesia was 12.3 ± 1.1 mm Hg. EVP decreased significantly to 8.7 ± 0.9 mm Hg within minutes after application of proparacaine. A small decrease also occurred in intraocular pressure. All other measured variables were unchanged.
Conclusions
These results suggest that the episcleral circulation is under tonic neural control and that either an upstream resistance site is under tonic vasodilatory control or a downstream site is under vasoconstrictor control.
Steady state intraocular pressure (IOP) is determined by the rate of aqueous humor production and the ease of aqueous outflow through the trabecular and uveoscleral pathways. Aqueous leaving the eye via the trabecular pathway depends on the pressure gradient from the anterior chamber (the IOP) to the recipient episcleral veins (EVP).1 The important role of EVP in IOP homeostasis is evident in the modified Goldmann equation: IOP = [(Fac − Fu) ÷ C] + EVP, where Fac is the aqueous flow through the anterior chamber, Fu is the aqueous flow through the uveoscleral pathway, and C is the trabecular conductance or facility.2 The EVP in a normal eye is generally assumed to be approximately 9 mm Hg.3 If so, the Goldmann equation indicates that EVP accounts for more than 50% of a normal IOP of 15 mm Hg.
The nominal EVP of 9 mm Hg is based on numerous studies using both direct and indirect measurement techniques; however, EVP is difficult to measure and the physiology and pharmacology of the episcleral circulation are poorly understood.4–7 The episcleral circulation is complex and has numerous arteriovenous anastomoses (AVAs).8–13 To add further complexity, the blood supply to this network comes from vessels originating inside and outside the eye.8 As a result, blood flow changes in episcleral supply vessels can affect the rate of aqueous entering the episcleral venous system. Moreover, there is strong evidence that the episcleral circulation is richly innervated and that a variety of vasoactive neurotransmitters are present (e.g., nitric oxide, acetylcholine, norepinephrine, vasoactive intestinal peptide, neuropeptide Y, and substance P), but their influence on EVP remains to be determined.10,14
As a first step in trying to understand the neural control of EVP, we sought to block it with the commonly used topical ocular anesthetic proparacaine. 15,16 To measure EVP, we chose a servonull micropressure system that permits cannulation of individual vessels and provides continuous measurements. The technique has been used to measure EVP in monkeys and rabbits.17,18
Methods
The animal procedures were approved by the local Institutional Animal Care and Use Committee and conducted in accordance with the Guide for the Care and Use of Laboratory Animals and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. At the end of the experiments, all animals were euthanatized with an overdose of pentobarbital without regaining consciousness.
Animal Preparation
New Zealand albino rabbits (2–3 kg) of both sexes were housed in the vivarium with food and water available ad libitum before the experiments. Animals were anesthetized with pentobarbital sodium (30 mg/kg IV, supplemented as needed) and paralyzed with gallamine triethiodide (1 mg/kg) to eliminate eye movement. To estimate the ocular mean arterial pressure (MAP), we inserted a catheter into the right ear artery and connected it to a pressure transducer positioned at the same height above the heart as the eye. The animals were intubated through tracheostomy and respired with room air. Expired PCO2 was monitored (SurgiVet V9004; Sims BCI, Inc., Waukesha, WI) and maintained between 39 and 44 mm Hg. During the tracheotomy procedure, the right common carotid was isolated for measurement of carotid blood flow (BFcar) with a transit-time ultrasound flow probe (2PSB; Transonic Systems, Ithaca, NY) and flowmeter (TS420; Transonic Systems). The BFcar was used as an index of cerebral perfusion and to trigger a digital cardiotachometer (Chart, ver. 5.5.6; ADInstruments Colorado Springs, CO) to measure heart rate (HR) as an index of cardiac performance. A heating pad was used to maintain normal body temperature (38–39°C). All intravenous injections were given via cannulas placed in the marginal ear veins. After the initial surgical preparation, the animal was mounted in a stereotaxic head holder, and the orbital venous sinus was cannulated with a 23-gauge needle inserted through the posterior supraorbital foramen to measure orbital venous pressure (OVP) with a second pressure transducer.19 The right eye was then cannulated with a 23-gauge needle inserted into the vitreous cavity through the pars plana to measure the intraocular pressure (IOP) with a third pressure transducer. All parameters were monitored in real time and digitally recorded (Chart, ver 5.5.6; ADInstruments; Macintosh G4 computer; Apple, Inc., Cupertino, CA).
EVP Measurements
EVP was measured with a micropipette-based servonull pressure system (model 900A; World Precision Instruments, Sarasota, FL). The micropipettes were made from borosilicate glass capillary tubes (1.2 mm) pulled on a pipette puller (model P-87; Sutter Instrument Co., Novata, CA), with the tips triple beveled in three planes to achieve an inner diameter of 2 to 4 μm on a micropipette beveler (BV-10; Sutter Instrument Co.) with a grinding plate (104-F; Sutter Instrument Co.). The pipettes were filled with a 2-M sodium-chloride solution and connected to the servonull pressure system. The ground wire was placed within the dermal opening of the tracheostomy, to complete the circuit loop of the pressure system. The eye was fixed to a stationary ring with 5-0 suture ties through the conjunctiva at 2, 6, and 10 o’clock, to minimize respiratory and cardiac synchronous movement. The ring was fixed to a magnetic holder, and the eye was held throughout the experiment in its natural position. After the eye was stabilized, a small portion of conjunctiva was cut near the superior limbus to expose the episcleral veins, and the conjunctival flaps were used to form a small (3 × 3 mm) calibration well that was filled with 0.9% sodium-chloride solution. Guided by a surgical microscope (Surgioscope DFV; model MC-M3101XY; WPI), the pipette tip was first advanced into the well where it was calibrated in saline solution at zero pressure, and then it was further advanced into an episcleral vein. The episcleral veins were identified by the streaming of aqueous in the flow of blood and cannulated near their exit point from the sclera.
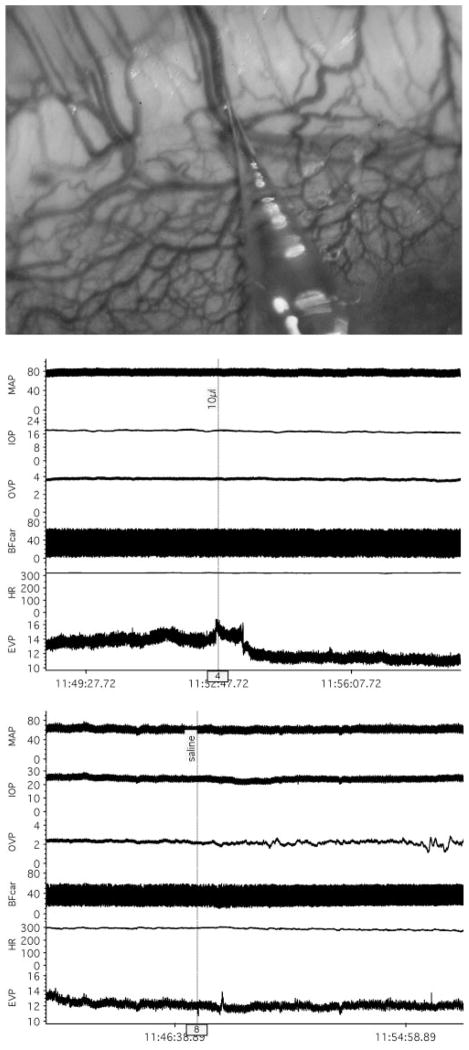
After an episcleral vein was cannulated, 10 to 15 minutes of baseline data were obtained before drug application. Once sufficient baseline data were obtained, 10 μL of proparacaine hydrochloride (0.5%; Bausch & Lomb, Tampa, FL) was placed locally on the episcleral vascular plexus at the site of the cannulated vessel, and the measurements continued for a minimum of another 10 to 15 minutes. Figure 1 shows an experimental tracing that illustrates the protocol.
Figure 1.
Top: photograph of rabbit episcleral circulation with micropipette vein cannulation. Middle: representative tracings of MAP (mm Hg), IOP (mm Hg), OVP (mm Hg), BFcar (mL/min), HR (bpm), and EVP (mm Hg) showing response to topical proparacaine (10 μL). Bottom: tracings from a different animal showing lack of response to topical saline.
Data Analysis
Measured variables were averaged over the 5- to 10-minute baseline period before proparacaine and for 5 to 10 minutes once the EVP response stabilized after proparacaine. The averaged values were compiled in a spreadsheet and analyzed by a two-tailed, paired t-test. A Bonferroni corrected P < 0.0083 was considered significant. Data are presented as the mean ± SE.
Results
The photograph in Figure 1 (top) shows a representative region of the rabbit episcleral circulation and a vein cannulated with a micropipette for EVP measurement with the servonull system. The cornea is located in the lower right corner of the photograph, and the cannulated vein arises from deeper in the sclera and travels posteriorly. The dense circumferential limbal vascular plexus is also evident. The middle set of experimental tracings shows a typical robust decrease in EVP in response to topical proparacaine, which did not affect the other measured variables. The bottom set of tracings shows no response to a topical application of saline, which was done to verify that the proparacaine response was not simply due to the application of an exogenous fluid. Periodic saline irrigation was given in all experiments to maintain corneal hydration and consistently caused no response (data not shown).
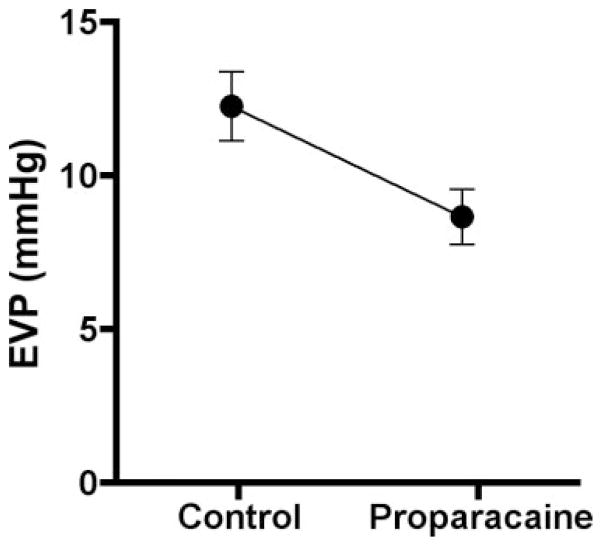
Figure 2 and Table 1 present the mean values for the measured variables before and after proparacaine. There were no significant changes in MAP (−1% ± 1%), OVP (−21% ± 9%), BFcar (−12% ± 2%), or HR (−1% ± 1%). However, the EVP was significantly affected, decreasing from 12.26 ± 1.13 mm Hg before proparacaine to 8.67 ± 0.90 mm Hg afterward (−27% ± 6%, P = 0.0049). A significant decrease also occurred in IOP (−9% ± 2%).
Figure 2.
Effect of topical proparacaine on EVP (n = 11; P = 0.0049).
Table 1.
Proparacaine’s Effects
| Parameter | Control | Proparacaine | P |
|---|---|---|---|
| MAP (mmHg, n = 11) | 75.48 ± 1.56 | 74.73 ± 1.36 | 0.286 |
| IOP (mmHg, n = 11) | 17.73 ± 1.06 | 16.22 ± 1.08 | 0.004 |
| OVP (mmHg, n = 8) | 1.89 ± 0.39 | 1.62 ± 0.38 | 0.011 |
| BFcar (mL/min, n = 11) | 27.69 ± 2.07 | 24.44 ± 1.73 | 0.106 |
| HR (bpm, n = 11) | 291.52 ± 7.13 | 288.17 ± 6.95 | 0.148 |
The OVP cannula clotted in three animals, and those data are not included. The Bonferroni-corrected P threshold is 0.0083.
Discussion
In his original observations of aqueous veins, Ascher5 noted increased erythrocyte flux in response to topical anesthetics. Subsequent anatomic studies showing innervation of episcleral arterioles, AVAs, and venules are consistent with Ascher’s observation,10,14 and it is not surprising that the EVP changed in response to a local anesthetic in the present study. However, to the best of our knowledge, the observation that local anesthesia decreases EVP is new information.
We chose proparacaine because it is a commonly used topical ocular anesthetic and it is less irritating than other available local anesthetics such as lidocaine, tetracaine, or procaine (Novocain).15 Local anesthetics exert their desired anesthetic effect by blocking the conduction of neural action potentials by closing voltage-gated sodium channels; however, they are not selective for afferent nerves and block conduction in efferent nerves as well.15,16,20 We assume that the efferent block is primarily responsible for the EVP response to proparacaine. However, local anesthetics can also affect endothelial cells21–23 as well as vascular smooth muscle calcium homeostasis,24–26 and these non-neural affects may also have contributed. Another caveat that should be noted is that the animals were under general anesthesia with pentobarbital, which may have blunted the EVP response.
The decrease in EVP after proparacaine suggests that the episcleral circulation is under tonic neural control in this animal model, but it does not indicate the site or nature of the control. Although the episcleral circulation is too complex (Fig 1, top) for a simplistic hemodynamic analysis, some general comments can be made. The pressure in any vessel is set by the vessel’s flow rate and the upstream and downstream resistances. If the flow rate is constant, a decrease in pressure can occur if upstream resistance increases or if downstream resistance decreases. A more complex scenario would be for both resistances to decrease, but downstream to a greater extent. If the upstream resistance sites from the episcleral veins (i.e., the arterioles and AVAs) receive tonic neural dilator input (e.g., nitrergic nerves), then local anesthesia would decrease EVP. Conversely, if the downstream resistance sites (i.e., the larger conduit veins traveling to the orbital venous sinus) are under tonic neural constrictor tone (e.g., sympathetic nerves), EVP would also decline with local anesthesia. If both upstream and downstream resistance sites have neural constrictor tone, EVP would decrease if the downstream site resistance declined more with local anesthesia.
Determining which resistance sites and which vasodilator or constrictor nerves are involved in the EVP decrease with local anesthesia requires further study. Current drug treatments for glaucoma focus on aqueous production, trabecular facility, and uveoscleral outflow to lower IOP. EVP is not now deliberately targeted for its antihypertensive effect, but the present results and the Goldmann equation suggest that lowering EVP may be a feasible approach to lowering IOP.
It should be noted that the decrease in IOP in response to proparacaine was significant, but less than the decrease in EVP, because we deliberately tried to anesthetize only the small region at the point of episcleral vein cannulation. Indeed, we had hoped that only EVP would change, so that any concomitant changes in aqueous dynamics would be avoided. It may be that anesthesia of the entire anterior eye surface lowers IOP, but it would be necessary to evaluate all facets of aqueous dynamics to interpret the response.
A practical aspect of the current results is that EVP measurements in humans and many animal studies were performed with a venomanometer, typically with the eye anesthetized.6,27 If humans and other species respond to topical anesthesia as does the anesthetized rabbit, the EVP values in the literature may be lower than in the unanesthetized eye. In addition, EVP measurements before and after giving an antagonist (e.g., timolol) are unlikely to detect the antagonist’s response if the nerves are anesthetized, and similarly, the responses to agonists (e.g., brimonidine) are likely to be blunted. Last, aqueous dynamics calculations (e.g., uveoscleral outflow) may be problematic if based on anesthetized EVP measurements (or an assumed EVP measurement) with other parameters measured with the eye unanesthetized.
Acknowledgments
Supported by National Eye Institute Grant EY09702, the van Heuven Endowment, the San Antonio Lions and Lions International, and a Lew Wasserman award and an unrestricted grant from Research to Prevent Blindness, Inc.
The authors thank Alma Maldonado for excellent technical assistance.
Footnotes
Disclosure: D.O. Zamora, None; J.W. Kiel, None
References
- 1.Goldmann H. Abflussdruck, minutenvolumen und widerstand der kammerwasser-stromung des menschen. Doc Ophthalmol. 1951;5–6:278–356. doi: 10.1007/BF00143664. [DOI] [PubMed] [Google Scholar]
- 2.Gabelt BT, Kiland JA, Tian B, Kaufman PL. Aqueous humor: secretion and dynamics. In: Tasman W, Jaeger EA, editors. Duane’s Clinical Ophthalmology [DVD] Philadelphia: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 3.Gabelt BT, Kaufman PL. Aqueous Humor hydrodynamics. In: Kaufman P, Alm A, editors. Adler’s Physiology of the Eye: Clinical Application. 10. St Louis: Mosby; 2003. pp. 237–289. [Google Scholar]
- 4.Ascher KW. The aqueous veins: 1. Physiologic importance of visible elimination of intraocular fluid. Am J Ophthalmol. 1942;25:1174–1209. doi: 10.1016/j.ajo.2018.05.025. [DOI] [PubMed] [Google Scholar]
- 5.Ascher KW. Aqueous veins. II. Local, pharmacologic effects on aqueous veins. III Glaucoma and aqueous veins. Am J Ophthalmol. 1942;25:1301–1315. [Google Scholar]
- 6.Zeimer RC. Episcleral venous pressure. In: Ritch R, Shields M, Krupin T, editors. The Glaucomas. St Louis: CV Mosby; 1989. pp. 249–255. [Google Scholar]
- 7.Brubaker RF. Determination of episcleral venous pressure in the eye Arch. Ophthalmol. 1967;77:110–114. doi: 10.1001/archopht.1967.00980020112024. [DOI] [PubMed] [Google Scholar]
- 8.Rohen JW, Funk RHW. Vasculature of the anterior eye segment. Prog Retin Eye Res. 1994;13:653–685. [Google Scholar]
- 9.Rohen JW, Funk RHW. Functional morphology of the episcleral vasculature in rabbits and dogs: presence of arteriovenous anastomoses. J Glaucoma. 1994;3:51–57. [PubMed] [Google Scholar]
- 10.Selbach JM, Schonfelder U, Funk RH. Arteriovenous anastomoses of the episcleral vasculature in the rabbit and rat eye. J Glaucoma. 1998;7:50–57. [PubMed] [Google Scholar]
- 11.Funk RHW, Gehr J, Rohen JW. Short-term hemodynamic changes in episcleral arteriovenous anastomoses correlate with venous pressure and IOP changes in the albino rabbit. Cur Eye Res. 1996;15:87–93. doi: 10.3109/02713689609017615. [DOI] [PubMed] [Google Scholar]
- 12.Funk RH, Rohen JW. Scanning electron microscopic study of episcleral arteriovenous anastomoses in the owl and cynomolgus monkey. Curr Eye Res. 1996;15:321–327. doi: 10.3109/02713689609007627. [DOI] [PubMed] [Google Scholar]
- 13.Funk RHW, Rohen JW. In vivo observations of the episcleral vasculature in the albino rabbit. J Glaucoma. 1994;3:44–50. [PubMed] [Google Scholar]
- 14.Selbach JM, Rohen JW, Steuhl KP, Lütjen-Drecoll E. Angioarchitecture and innervation of the primate anterior episclera. Curr Eye Res. 2005;30:337–344. doi: 10.1080/02713680590934076. [DOI] [PubMed] [Google Scholar]
- 15.Catterall W, Makie K. Local anesthetics. In: Hardman J, Limbird L, editors. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 10. New York: McGraw-Hill; 2001. pp. 367–384. [Google Scholar]
- 16.Mindel JS. Pharmacology of local anesthetics. In: Tasman W, Jaeger EA, editors. Duane’s Ophthalmology. Philadelphia: Lippincott Williams and Wilkins; 2006. [DVD] [Google Scholar]
- 17.Maepea O, Bill A. The pressures in the episcleral veins, Schlemm’s Canal and the trabecular meshwork in monkeys: effects of changes in intraocular pressure. Exp Eye Res. 1989;49:645–663. doi: 10.1016/s0014-4835(89)80060-0. [DOI] [PubMed] [Google Scholar]
- 18.Reitsamer HA, Posey M, Kiel JW. Effects of a topical alpha2 adrenergic agonist on ciliary blood flow and aqueous production in rabbits. Exp Eye Res. 2006;82:405–415. doi: 10.1016/j.exer.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 19.Reitsamer HA, Kiel JW. A rabbit model to study orbital venous pressure, intraocular pressure, and ocular hemodynamics simultaneously. Invest Ophthalmol Vis Sci. 2002;43:3728–3734. [PubMed] [Google Scholar]
- 20.Acquadro MA, Kim JC, Kini MM. Anesthetics. In: Albert D, Jakobiec F, editors. Principles and Practice of Ophthalmology: Basic Sciences. Philadelphia: WB Saunders; 1994. pp. 929–940. [Google Scholar]
- 21.Lan W, Harmon DC, Wang JH, Shorten GD, Redmond PH. Activated endothelial interleukin-1beta, -6, and -8 concentrations and intercellular adhesion molecule-1 expression are attenuated by lidocaine. Anesth Analg. 2005;100:409–412. doi: 10.1213/01.ANE.0000142113.39092.87. [DOI] [PubMed] [Google Scholar]
- 22.Lan W, Harmon D, Wang JH, Shorten G, Redmond P. The effect of lidocaine on neutrophil CD11b/CD18 and endothelial ICAM-1 expression and IL-1beta concentrations induced by hypoxia-reoxygenation. Eur J Anaesthesiol. 2004;21:967–972. doi: 10.1017/s0265021504000353. [DOI] [PubMed] [Google Scholar]
- 23.de Klaver MJ, Weingart GS, Obrig TG, Rich GF. Local anesthetic-induced protection against lipopolysaccharide-induced injury in endothelial cells: the role of mitochondrial adenosine triphosphate-sensitive potassium channels. Anesth Analg. 2006;102:1108–1113. doi: 10.1213/01.ane.0000200310.39031.1f. [DOI] [PubMed] [Google Scholar]
- 24.Tokinaga Y, Ogawa K, Yu J, et al. Mechanism of the ropivacaine-induced increase in intracellular Ca2+ concentration in rat aortic smooth muscle. Acta Anaesthesiol Scand. 2007;51:1155–1160. doi: 10.1111/j.1399-6576.2007.01390.x. [DOI] [PubMed] [Google Scholar]
- 25.Huang Y, Lau CW, Chan FL, Yao XQ. Contribution of nitric oxide and K+ channel activation to vasorelaxation of isolated rat aorta induced by procaine. Eur J Pharmacol. 1999;367:231–237. doi: 10.1016/s0014-2999(98)00950-9. [DOI] [PubMed] [Google Scholar]
- 26.Curtis TM, Tumelty J, Stewart MT, et al. Modification of smooth muscle Ca2+-sparks by tetracaine: evidence for sequential RyR activation. Cell Calcium. 2008;43:142–154. doi: 10.1016/j.ceca.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 27.Zeimer RC, Gieser DK, Wilensky JT, et al. A practical venomanometer; measurement of episcleral venous pressure and assessment of the normal range. Arch Ophthalmol. 1983;101:1447–1449. doi: 10.1001/archopht.1983.01040020449024. [DOI] [PubMed] [Google Scholar]