Abstract
P1066 is an open-label study of raltegravir in HIV+ youth, ages 4 weeks-18 years. Here we summarize P1066 pharmacokinetic (PK) data and a population PK model for the pediatric chewable tablet and oral granules. Raltegravir PK parameters were calculated using non-compartmental analysis. A two-compartment model was developed using data from P1066 and an adult study of the pediatric formulations. Inter-individual variability was described by an exponential error model, and residual variability was captured by an additive/proportional error model. Twelve-hour concentrations (C12hr) were calculated from the model-derived elimination rate constant and 8-hour observed concentration. Simulated steady-state concentrations were analyzed by non-compartmental analysis. Target area-under-the-curve (AUC0-12hr) and C12hr were achieved in each cohort. For the pediatric formulations, geometric mean AUC0-12hr values were 18.0–22.6 μM*hr across cohorts, and C12hr values were 71–130 nM, with lower coefficients of variation vs the film-coated tablet. A two-compartment model with first-order absorption adequately described raltegravir plasma PK in pediatric and adult patients. Weight was a covariate on clearance and central volume, and incorporated using allometric scaling. Raltegravir chewable tablets and oral granules exhibited PK parameters consistent with those from prior adult studies and older children in P1066, as well as lower variability than the film-coated tablet.
Keywords: Raltegravir, Pharmacokinetics, HIV/AIDS, Pediatrics, IMPAACT study
INTRODUCTION
Raltegravir is an integrase strand transfer inhibitor indicated for the treatment of HIV-1 infection in adults, children and infants above 4 weeks of age 1,2,3. In order to provide age-appropriate formulations of raltegravir across this range of ages, scored tablets are available for adults and adolescents, while chewable tablets are available for older children, and a granules for suspension (GFS) formulation has been developed for infants and toddlers in whom solid formulations are not appropriate due to their immature swallowing ability and the potential hazard of choking. The pharmacokinetics (PK), safety, and 48-week efficacy of raltegravir in children 2 through 18 years of age administered either the adult film-coated tablet or the pediatric chewable tablet in IMPAACT Protocol 1066 (P1066) have been recently reported 4.
In adults, raltegravir PK parameters exhibit considerable intra- and inter-subject variability 2,7–9, which has complicated the development of a population PK model to characterize the PK of the adult tablet formulation in patients, and contributes to difficulties in assessing the relevance of PK data obtained at single or minimal time points (e.g. sparse sampling). A population PK model based on data from six male healthy volunteers has recently been published 10, and a population PK model based on data in both HIV-positive patients and healthy subjects has been presented 11. However, an adequate model, has not been previously developed using a large dataset representing a mix of full-profile and sparse-sampling data collected from both healthy subjects and HIV-infected patients under a variety of dosing conditions during the raltegravir development program.
A greater emphasis has been placed on the importance of maintaining adequate raltegravir trough concentration (C12hr) based on results from the raltegravir 800 mg QD vs 400 mg BID study (QDMRK) 5,6. Specifically, results of this study and the associated PK/PD analyses indicated that failure to achieve undetectable HIV RNA levels appeared predominantly at high baseline HIV RNA levels in both treatment arms, and was associated with lower values of Ctrough in the 800 mg QD arm, with patients achieving Ctrough values below 45 nM at the greatest risk of treatment failure. Thus, interpretation of the PK results for alternative formulations or populations, such as those studied in P1066, should be done to ensure that the majority of subjects at recommended doses remain above this threshold value of 45 nM.
This report describes the PK data collected from HIV-infected children 4 weeks to <2 years of age who received the GFS formulation of raltegravir in P1066. Also included is a description of the development of a population PK model to describe the PK of the pediatric chewable tablet and GFS formulations, based on both data from P1066 and from Protocol 068, a formulation biocomparison study conducted in adult healthy volunteers where the adult tablet, chewable tablet and GFS formulation were also administered 12.
METHODS
IMPAACT Protocol 1066 (P1066, NCT00485264) is a Phase I/II, multicenter, open-label, noncomparative study to evaluate the safety, tolerability, pharmacokinetics (PK), and efficacy of raltegravir in HIV-infected children from 4 weeks through 18 years of age. P1066 was conducted at IMPAACT Network sites in the U.S., South Africa, Botswana, and Brazil after approvals were obtained from local institutional review boards and in-country ethics committees responsible for oversight of the study (Appendix). Written informed consent was obtained from the parents or legal guardians of the study subjects. Assent from the subject was also obtained if he or she was able to understand the nature, significance, and risks of the study.
Subjects enrolled in P1066 were stratified by age into six cohorts, to receive one of three formulations of raltegravir (Table 1); Cohorts I–III have been previously described 4. Cohort IV (6 months to <2 years of age) and Cohort V (4 weeks to <6 months of age) received raltegravir granules for suspension (GFS) formulation. In assessing medication compliance at each visit, the primary care giver completed an adherence questionnaire and site personnel collected the patients' opened study drug bottles (Cohorts I–III) or sachet boxes (Cohorts IV–V) and counted the returned and unused tablets or prepackaged GFS sachets to determine the total number of tablets taken or sachets used, since the last visit.
Table 1.
Raltegravir formulations studied in Protocol 1066 and recommended doses for pediatric patients
| Age | Cohort | Formulation and Recommended Dose |
|---|---|---|
| 12 to 18 years | I | 400 mg film-coated tablet twice daily |
| 6 to <12 years (≥25 kg) | IIA | |
|
| ||
| 6 to <12 years | IIB | ~6 mg/kg chewable tablet |
| 2 to <6 years | III | (up to a maximum of 300 mg) twice daily |
|
| ||
| 6 months to <2 years | IV | ~6 mg/kg granules for suspension |
| 4 weeks to <6 months | V | twice daily |
The PK criteria used for dose selection in Cohorts IV and V were a geometric mean exposure (AUC0-12hr) between 14 and 45 μM*hr and an approximate geometric mean C12hr ≥75 nM, which was chosen based on the variability in C12hr from previous raltegravir studies to result in the majority (>90%) of patients above the 45 nM threshold associated with a lower risk of treatment failure 6. This was done as a practical measure, as intensive PK was collected in between 8 and 12 subjects in each cohort, and thus criteria based on individual (rather than mean) values could be biased by a single outlier.
Pharmacokinetic Samples and Datasets
Data included in this analysis comes from a formulation comparison study in adult healthy volunteers (P068)12 and the pediatric PK, safety and efficacy study in HIV-infected children (P1066)4, where in both studies the adult tablet, chewable tablet and GFS formulations were used. Analyses of data from P1066 Cohorts I and IIA (children administered the adult tablets) have been previously described 4, and were not used for modeling analyses described in this report.
In P068, healthy adult subjects received a single 400 mg dose of raltegravir as the adult tablet, chewable tablets, or GFS formulation. Blood samples for PK analyses were collected within 3 hours before study drug administration and at 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, 16, 24, 32, 48, and 72 hours after dosing.
In P1066, PK evaluations were scheduled such that witnessed dosing of raltegravir was as close as possible to 12 hours after the previous dose. Whole blood samples for steady state plasma concentration evaluations were collected for intensive PK at 5–12 days after dosing began and for sparse PK analysis at weeks 4, 8, 12 and 24. The intensive PK samples were collected fasted at predose, 0.5, 1, 2, 3, 4, 6, 8, and 12 hr post-dose for Cohorts IIB and III; Cohort IV (predose, 0.5, 1, 2, 4 and 12 hr) and Cohort V (predose, 0.5, 1, between 3–5 and between 8–10 hr) did not require fasting but recommended feeding to be held for 30 minutes following dosing. The sparse PK samples were collected regardless of food at following time points: weeks 4 and 12, one sample at 10–14 hr post-dose, week 8, two samples (2 hr apart) at 0.5–6 hr post-dose, and week 24, two samples (2 hr apart) at 6–12 hr post-dose for Cohorts IIB-V.
In a separate substudy of P1066 designed to generate additional C12hr data in children ages 2 to <6 years receiving the chewable tablet, children already participating in P1066 in this age range (but who may have initially enrolled into either cohort III, IV or V) were enrolled to provide two additional C12hr samples, one following unobserved dosing and a second following witnessed dosing. The unobserved C12hr plasma sample was collected between 11 and 13 hours after the prior (unobserved) raltegravir dose, and just before an observed dose.
All PK samples in P1066 were shipped to the University of Alabama at Birmingham for real-time quantitation. The analytical method for the determination of raltegravir in human plasma involves isolation, via 96-well liquid-liquid extraction, of the analyte and internal standard from plasma, followed by HPLC-MS/MS analysis. The lower limit of quantitation for the plasma assay used in P1066 was 10 ng/mL (22.5 nM) and the linear calibration range was 10 to 10,000 ng/mL from a 200 μL plasma sample. For P068 the lower limit of quantitation was 2 ng/mL (4.5 nM) and the linear calibration range was 2 to 1000 ng/mL. More details regarding the bioanalysis can be found in the previously published methods 13,14.
Plasma concentrations below the limit of quantitation were treated as 50% of the assay lower limit of quantitation and included in the analysis dataset. Missing observations were excluded from the analysis. In P1066, observations greater than 20-fold higher than adjacent time points and not comparable to corresponding time points of other steady state samples were considered outliers and removed from the initial dataset during model development.
Non-model-based Pharmacokinetic Analyses
All intensive PK data was analyzed using non-compartmental analysis methods using either WinNonlin version 5.3 (Pharsight Corporation, Mountain View, CA) for all PK analyses through January 1st, 2013 or Phoenix WinNonlin version 6.3 (Certara USA, Inc., St. Louis, MO) after that date, uniformly weighting the data. The last intensive PK sample from subjects in Cohort V was collected at approximately 8 hours post dose as per protocol. Only 3 of the 11 Cohort V subjects had full 12-hour profiles; data for the initial 8 subjects that did not have an observed sample collected at 12 hours were modeled in order to more accurately estimate the C12hr. One subject in Cohort IV was also missing a PK sample at 12 hours post-dose; a modeled C12hr value was also used for this subject. Specifically, the model-estimated elimination rate constant for each individual was used with the last observed concentration at 8 hours to estimate the concentration at the 12-hour time point. Subsequently, sampling times were changed by protocol amendment to include a 12-hour point in an additional set of patients enrolled in Cohort V, hereafter referred to as the supplemental patient cohort V (N=3, had a full 12-hour profile collected).
Population Pharmacokinetic Model Analysis
The software package NONMEM, version VII (Globomax, 7250 Parkway Drive, Suite 430, Hanover, MD 21076 USA) was used in the analysis. Model fitting was performed in a UNIX environment with Intel FORTRAN Compiler, version 11.1 (Intel Corporation, 2200 Mission College Blvd., Santa Clara, CA 95054). Xpose (xpose.sourceforge.net), PsN (psn.sourceforge.net) and R (R-project, www.r-project.org) were used for the exploratory analysis and post-processing of NONMEM output, for example to assess goodness-of-fit.
Model development was performed in three steps consisting of structural model selection, covariate screening, and model refinement. Standard two-compartment structural models were explored with and without lag time, utilizing zero-order or first-order absorption, and log transforming the concentration data. For the pivotal models, reductions in OFV and residual error, and goodness-of-fit plots were used to diagnose the appropriateness of the structural model and covariate relationships.
Continuous covariates including weight, age and body surface area were included in the model using power equations after centering on the median. The following general equation was used to constrain the typical value of the parameter to be positive throughout the convergence process and to be consistent with the assumed log-normal distribution:
| (eq.1) |
where P* is a typical value of a PK parameter P, and θx and θy are fixed-effect parameters to be estimated. Additionally, more complicated equations for the maturation of clearance as a function of weight were examined, given the pediatric population. Categorical covariates including race, sex and food intake were incorporated into the model as categorical covariates as follows:
| (eq.2) |
, where Q is an index variable that has a value of 1 in the presence of the covariate, otherwise it has a value of 0.
Additive, proportional, and additive + proportional error models were explored for residual variability. Exponential error models were utilized for between-subject variability throughout the random effects model selection process. The appropriateness of the random effects models was assessed by examination of diagnostic plots.
The estimation method in NONMEM was first-order conditional estimation with interaction (FOCEI) with proportional and additive + proportional models for residual variability (log-additive models for residual variability of log transformed DV). Stability of a NONMEM model was assessed on the basis of acceptable basic goodness-of-fit plots, estimates of θ's not close to a boundary, condition number (ratio of largest to smallest eigenvalue) less than 1000, correlation less than 0.95 between any two parameters, and a stability check: the model finds the global minimum when the initial values are altered.
RESULTS
Twelve subjects (9 male, 3 female) were enrolled in P068; the mean age was 40 years (range 26 to 52 years), and the mean BMI was 26.4 kg/m2 (range 20.8 to 31.4 kg/m2). In P1066, 26 patients were treated in Cohorts IV and V (17 male, 9 female); 84.6% were Black/African American, the median age was 28 weeks, and mean weight was 8.5 kg (Cohort IV) and 5.5 kg (Cohort V). A total of 408 pharmacokinetic samples from 12 subjects from P068 and 740 PK samples from 65 subjects from P1066 (368 samples from sparsely collected PK and 372 samples from intensive PK) were included in the analysis. Out of the total of 1148 samples, 46 samples (4%) were below the limit of quantitation (BLOQ) and imputed to a value of ½ the assay limit of quantitation. 25 samples from P068 were BLOQ, primarily at the 72 hour time point. 21 samples from P1066 were BLOQ, 1 sample (0.2%) from the intensive PK collection (in Cohort III) and 20 samples (5%) from the sparse PK collection (3 out of 74 sparsely collected samples in Cohort IV and 1 out of 51 sparsely collected samples in Cohort V). A sensitivity analysis was conducted to either treat these samples as missing or impute their value as ½ the assay limit of quantitation, and model parameters were found to be similar in both instances.
Non-model-based Pharmacokinetic Analyses
The mean raltegravir PK parameters associated with the final recommended doses for Cohorts IV and V from P1066 are listed in Table 2. Variability in raltegravir PK parameter values with the recommended dosing regimen for the GFS (approximately 30–70% CV) is lower than observed in either pediatric patients or adults taking the film-coated tablet (90–220%) 4, and is similar to variability in children taking the chewable tablet (30–90%) 4. Note that C12hr values used for the noncompartmental analysis of the intensive PK profiles for subjects that did not have an observed sample drawn were calculated from the model-derived elimination rate constant and the observed concentration at the 8-hour time point.
Table 2.
Summary of raltegravir PK parameters following administration of final recommended doses in IMPAACT Protocol 1066
| Age | Cohort | Formulation | Final Recommen ded Dose | N* | Mean (%CV) Weight (kg) | Mean (%CV) Dose (mg/kg) | Observed Geometric Mean (%CV) AUC0-12hr (μM-hr) | Simulated Geometric Mean (%CV) AUC0-12hr (μM-hr) | Observed Geometric Mean (%CV) C12h (nM) | Simulated Geometric Mean (%CV) C12hr (nM) |
|---|---|---|---|---|---|---|---|---|---|---|
| 6 months to <2 years | IV | Granules for suspension | 6 mg/kg twice daily | 8 | 8.5 (34) | 5.9 (7) | 19.8 (34) | 18.5 (34) | 108.2 (52) | 149.1 (88) |
|
| ||||||||||
| 4 weeks to <6 months | V | Granules for suspension | 6 mg/kg twice daily | 11 | 5.5(22) | 5.7 (12) | 22.3 (40) | 21.6 (47) | 116.6 (68) | 140.0 (117) |
Number of patients with intensive PK results at the final recommended dose
Eleven subjects were enrolled into a separate substudy designed to assess observed trough concentrations in children 2 to 6 years of age administered the chewable tablet (regardless of age or cohort at time of enrollment). One subject had the unobserved trough collected at 6.3 hours post dose and the second observed sample was not collected. This participant was excluded. A total of 1 out of 20 (5%) trough concentrations fell below the 45 nM threshold for all samples collected, a rate similar to that seen with the currently marketed 400 mg twice-daily adult tablet formulation6. This occurred following the observed-dose trough collection, and was at a value of 36.0 nM. The median trough concentrations were 303.5 nM (134.8 ng/mL) and the geometric mean was 268.4 nM (119.2 ng/mL) at a mean time of 11.7 hours post dose. Variability in trough concentrations (%CV) was high at approximately 90%, but consistent with prior raltegravir data. The median trough concentrations for the unobserved and observed time points were similar at 302 and 309 nM, respectively. The variability, however, was considerably lower for trough concentrations collected following the observed dose (50% CV), as would be expected.
Pharmacokinetic parameter values at the administered dose of the GFS of approximately 6 mg/kg twice daily met the pre-specified PK targets used for dose selection in Cohorts IV and V (geometric mean AUC0-12hr between 14 and 45 μM*hr and an approximate geometric mean C12hr ≥75 nM), indicating that the recommended doses in Table 1 provide adequate raltegravir exposure in children 4 weeks to 2 years of age administered the GFS. Specifically, geometric mean AUC0-12hr values were 19.8 and 22.3 μM*hr for Cohorts IV and V, respectively, and geometric mean C12hr values were 108.2 and 116.6 nM for Cohorts IV and V, well above the 75 nM PK target and similar to those observed in older children administered the chewable tablet (71.2–129.6 nM) in Cohorts IIB and III.
Population Pharmacokinetic Model Analysis
A two-compartment model with first-order absorption adequately described raltegravir plasma pharmacokinetics in pediatric and adult patients dosed with the GFS and chewable tablet formulations. A bioavailability term (F1) with inter-individual variability was incorporated in the model to allow for scaling of the difference in relative bioavailability of the chewable tablet and GFS formulations (47% higher relative bioavailability for the GFS as compared to the chewable tablet), as observed in P068 12, and to incorporate inter-individual variation in relative bioavailability. The sparsely collected PK exhibit higher variability than that of intensive PK for both chewable tablet and GFS formulations. This difference incorporated into the model through different inter-individual variability terms for the absorption rate constant and different proportional residual error terms for intensive and sparse PK data, as the intensive PK samples were collected fasted and the sparse PK samples were collected regardless of food. The impact of food on the PK of raltegravir when administered as the chewable tablet in P068 has been demonstrated to slow the rate, but not impact the extent of absorption 12. Of the covariates evaluated, weight was a significant covariate on clearance and central volume, and was incorporated using allometric scaling (exponent of 0.75 on clearance terms, 1.0 on central volume). Attempts to fit, rather than fix to allometric exponents, the effect of weight did not result in improved model fits. Although age, weight, and body surface area (BSA) were all significantly correlated with clearance and volume during stepwise covariate model (SCM) building, the reduction in objective function value was less with age than that obtained using weight or BSA. BSA was a significant covariate on clearance and central volume relative to the base model, but the correlation between BSA and weight in this dataset was very high; thus only weight was further considered. Model development was completed using an initial dataset that excluded a number of outlier samples. In the final run, these outlier samples were included in the final model fit, and the model parameters were found to be similar to those obtained prior to inclusion of the outliers, indicating there were no influential outliers in the dataset. Thus, no outlier samples were excluded from the final dataset.
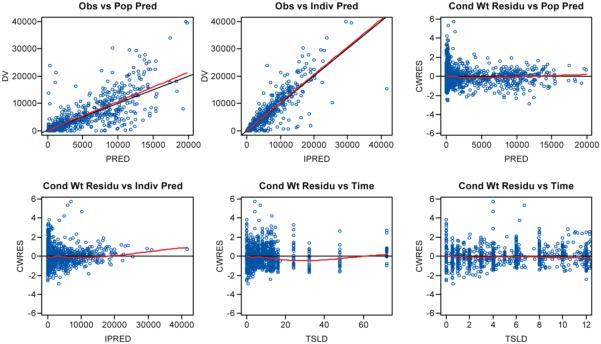
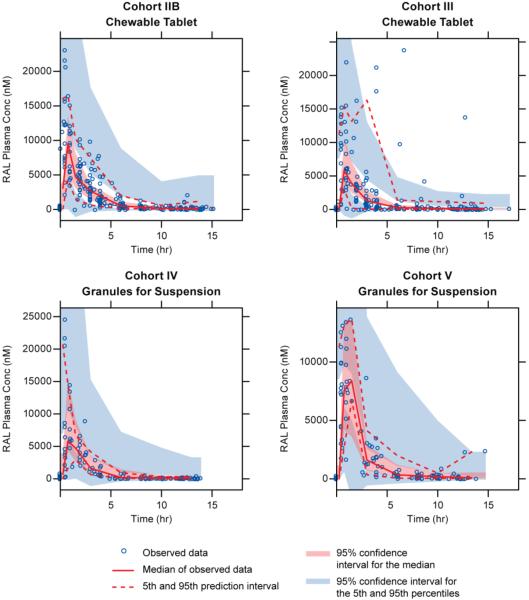
The final model (parameters shown in Table 3) was utilized to generate simulated profiles for all individuals in Cohorts IV and V, including predicted C12hr values for individuals who did not have an observed 12-hour sample collected during intensive PK sampling. As indicated in both the goodness-of-fit plots (Figure 1) and the visual predictive check (Figure 2), the model adequately describes the PK profiles of raltegravir in the studied population.
Table 3.
Final model parameter estimates and bootstrap confidence intervals of model parameters
| Parameters | Population estimates (%RSE) | Bootstrap median [90% CIa] of population estimates | IIVb (%RSE) | Bootstrap median [90% CI] of IIV |
|---|---|---|---|---|
| V2 (L) | 3.51 (22.4) | 3.54 [2.31, 4.67] | 107.7 (29.1) | 106.3 [79.7, 138.9] |
| V3 (L) | 27 (11.8) | 27.1 [22.5, 31.3] | --- | --- |
| CL (L/hr) | 9.72 (5.5) | 9.69 [8.96, 10.7] | 33.9 (26.4) | 33.3 [26.3, 40.7] |
| Q (L/hr) | 0.865 (12.6) | 0.861 [0.68, 1.07] | 55.9 (35.6) | 54.8 [35.4, 69.2] |
| KA (intensive PK) (1/hr) | 0.723 (5.4) | 0.726 [0.663, 0.801] | 31.8 (34.2) | 30.8 [17.2, 40.1] |
| KA (sparse PK) (1/hr) | 0.723 (5.4) | 0.726 [0.663, 0.801] | 94.5 (36.3) | 94.3 [66, 122] |
| F1 (bioavailability) | 1 (---) | 1 [1, 1] | 46.0 (26.9) | 44.9 [34.9, 54.4] |
| WT on CL (power) | 0.75 (fixed) | 0.75 [0.75, 0.75] | --- | --- |
| WT on Q (power) | 0.75 (fixed) | 0.75 [0.75, 0.75] | --- | --- |
| WT on V2 (power) | 1 (fixed) | 1 [1, 1] | --- | --- |
| Proportional error (intensive PK) | 0.15 (15.1) | 0.15 [0.12, 018] | --- | --- |
| Proportional error (sparse PK) | 0.47 (13.5) | 0.47 [0.38, 0.59] | --- | --- |
| Additive error | 4.9 (18.4) | 4.77 [3.61, 6.54] | --- | --- |
Median value and 90% confidence interval were calculated using 500 re-sampled and 74% converged bootstrapping runs. The lower and upper limits for 90% CI were calculated as 5th and 95th percentiles, respectively.
IIV: Interindividual variability, calculated as (variance)½*100(%).
Figure 1.
Goodness of fit plots for final model. From top left: observed (DV) vs population predictions (PRED), DV vs individual predictions (IPRED), conditionally weighted residuals (CWRES) vs PRED, CWRES vs IPRED, CWRES vs time since last dose (TSLD), and CWRES vs TSLD for TSLD below 12 hr.
Figure 2.
Visual predictive check from the final model stratified by P1066 cohort. Open circles represent observed concentrations; solid and dashed red lines represents the median and 5th/95th interval of the observed data, respectively; red and blue shaded areas represent the 95% confidence interval for the median and 5th/95th interval derived from 1000 simulations. TSLD = time since last dose.
DISCUSSION
P1066 was designed to determine the dose of 3 different age-appropriate formulations of raltegravir suitable for pediatric patients from 4 weeks through 18 years of age, and to assess its safety and efficacy over 240 weeks of therapy. The chewable tablet and GFS formulations exhibited more consistent and well-behaved concentration-time profiles relative to the current adult formulation. At the final selected doses, the PK for all cohorts met the geometric mean exposure (AUC0-12hr) and Ctrough targets, both those originally specified by protocol, and those updated based on emergent data from additional PK/PD analysis of the QDMRK study 6. Original protocol PK targets for Ctrough were to maintain a geometric mean C12hr above 33 nM, the in vitro IC95 value for raltegravir. However, results from the QDMRK study demonstrated that individuals falling below Ctrough values of 45 nM were at the greatest risk of treatment failure, and thus PK targets for P1066 were updated to increase the desired C12hr. Thus, a target geometric mean C12hr of 75 nM for each cohort was used to determine appropriate doses of the GFS. This target, based on the geometric mean for the cohort, rather than a percentage of individual values being above 45 nM, was chosen to ensure a more robust interpretation of the data given the relatively small number of patients in each dosing Cohort. However, the totality of the data was considered when making dose recommendations for the GFS.
In the separate substudy designed to collect additional observed Ctrough data in children 2 to 6 years of age administered the chewable tablet, trough concentration data overall were similar to or higher than results observed in previous P1066 cohorts and from adult raltegravir studies. In P1066, the geometric mean C12hr in Cohorts IV and V (ages 4 weeks to <2 years receiving weight-based dosing of the oral GFS) were 108 and 117 nM, respectively. In Cohorts IIB and III (ages 2 to <12 years), where all subjects received weight-based dosing of the chewable tablets, the geometric mean C12hr was 130 and 71 nM, respectively. In Cohorts I and IIA (ages 12 to <19 years), where all subjects received the adult tablet (400 mg BID), the geometric mean C12hr for these groups were 333 and 246 nM, respectively. Thus, at a weight-based dose of approximately 6 mg/kg twice daily of the GFS in Cohorts IV and V, PK values met each of the above mentioned targets, with geometric mean C12hr values well above the 75 nM PK target and similar to those observed in older children administered the chewable tablet in Cohorts IIB and III. Additionally, only 1 of 20 patients in Cohorts IV and V fell below the 45 nM Ctrough threshold value identified form QDMRK, which is a similar frequency (5%) as observed in adults taking the 400 mg twice daily marketed dose of raltegravir (5–10% in multiple studies).
Based on prior modeling experience, information in the literature, and exploratory plots for clearance vs. covariates, it was recognized that several covariates are highly correlated with clearance and distribution volume. Therefore, following determination of the two compartment structural model, the covariate effects on the model parameters were examined prior to exploration of the random effects to avoid the inappropriate attribution of covariate effects to random effects. Allometric and physiologically based scaling with inclusion of enzyme maturation on clearance were explored. For allometric based scaling, continuous covariates were tested using an automated stepwise covariate modeling (SCM) routine in PsN where parameter θy in eq.1 was estimated and compared with fixing θy with an empirically determined power factor (0.75 for clearance and 1 for volume). After incorporating the selected continuous covariates (weight with a fixed power on clearance and volume), categorical covariates were subsequently screened by SCM. Inclusion of covariates was based on clinical relevance and statistical criteria (ΔOFV of 3.84, p<0.05 for 1 DF in forward inclusion and ΔOFV of 6.63 p<0.01 for backward exclusion). The exploratory plots at steady state show that the plasma concentrations of sparsely collected PK exhibit higher variability than that of intensive PK for both chewable tablet and GFS formulations. This difference was accounted for in the model as a difference in the magnitude of the inter-individual variability on the absorption rate constant (consistent with likely differences in food intake during intensive and sparse PK sampling, as administration of the chewable tablet with a high fat meal in P068 was found to slow the rate, but not impact the extent of absorption12) and different proportional error components of the residual variability for sparsely and intensively collected PK samples.
The final model was used to simulate the steady state PK of raltegravir in the pediatric population age 4 weeks to 2 years receiving 6 mg/kg of the GFS raltegravir formulation every 12 hours in the P1066 study. The model was used to estimate C12hr values for 9 patients in Cohorts IV and V, including 1 of 8 patients enrolled in Cohort IV (6 months to <2 years of age) and 8 of 11 patients enrolled in Cohort V (4 weeks to <6 months of age) that did not have an observed sample collected.
Based on results of both the non-model based and model-based analyses, PK parameters are consistent with those observed in prior adult studies and in older children in Cohorts I-III of P1066. Additionally, the chewable tablet and GFS formulations exhibit lower variability than the adult tablet, as indicated by both lower variability in the PK parameters and the construction of a population PK model, which to date has not been possible for the adult tablet due to the high observed variability.
In this study of raltegravir adult tablet, chewable tablet and GFS formulations in HIV-infected infants, toddlers, children and adolescents 4 weeks to 18 years of age, the targeted PK parameters were achieved in all cohorts with weight-based dosing of 6 mg/kg BID of the chewable tablet and GFS, and 400 mg BID dosing of the adult tablet, resulting in the dosing recommendations listed in Table 1. These recommended doses result in consistent and acceptable values of raltegravir AUC0-12hr and C12hr across the entire range of ages (4 weeks through 18 years) and weights (4 to 60 kg) studied in IMPAACT P1066. The PK data taken in context with the known PK/PD relationship for raltegravir demonstrate that the achieved range of exposure and trough concentrations are comparable across the pediatric age ranges and formulations, and are expected to result in similar efficacy and safety across all age groups.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge Edward Handelsman, MD (deceased), of the Division of AIDS, NIAID, NIH, for his contributions to the design and conduct of IMPAACT P1066.
Financial Support: Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC) and UM1AI106716 (IMPAACT LC), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH). This project has been funded in whole or in part with federal funds from the NIAID, NIH, Department of Health and Human Services, under Contract No. HHSN272200800014C. Support of the sites was provided by the National Institute of Allergy and Infectious Diseases (NIAID) and the NICHD International and Domestic Pediatric and Maternal HIV Clinical Trials Network funded by NICHD (contract number N01-DK-9-001/HHSN267200800001C), and by Merck & Co. Inc. Raltegravir supplies were provided by Merck & Co., Inc.
Potential Conflicts of Interest: C. Bennetto-Hood, B. Graham, C. Fry, C. Worrell, and B. Smith: no conflicts to report. S. Nachman, A. Wiznia, and E. Acosta: received research support for P1066 from Merck & Co., Inc. through a grant administered by Johns Hopkins University. M. Rizk, L. Du, L. Wenning, H. Teppler, and B. Homony: are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., and may own stock and/or stock options in the company.
APPENDIX
Institutional Review Boards/Ethical Review Committees
University of Medicine and Dentistry of New Jersey Medical School Office of IRB
UCLA Office of Protection of Research Subjects (OPRS)
Baylor College of Medicine IRB for Human Subjects Research
Children's Memorial Research Center IRB
Columbia University Medical Center Institutional Review Board
Medical Science IRB for Protection of Human Subjects in Research, Miller School of Medicine, University of Miami
UCSD Human Research Protections Program, LaJolla Village Professional Center
Duke University Health Systems IRB for Clinical Investigations
New York Medical College Office of Research Administration IRB
Columbia University Medical Center IRB
Committee on Clinical Investigation, Children's Hospital Boston
IRB Boston University Medical Center
NY University School of Medicine IRB Associates
Albert Einstein College of Medicine of Yeshiva University
Office for Protection of Human Subjects, Children's National Medical Center
Seattle Children's Hospital and Regional Medical Center IRB
All Children's Health Systems Inc. IRB
San Juan Hospital IRB
Stony Brook University Committee on Research Involving Human Subjects Office of Research Compliance
Human Investigations Committee, Wayne State University
Howard University IRB
Human Subjects Committee, LA Biomedical Research Institute at Harbor-UCLA Medical Center
USC Health Science Center IRB
IRB University of Florida
Colorado Multiple Institutional Review Board
North Broward Hospital District Institutional Review Board
Comite de Etica em Pesquisa Instituto de Puericultura e Pediatria Martagao Gesteira, Universidade Federal do Rio de Janeiro
Comite de Etica em Pesquisa em Seres Humanos do HSE, CONEP Ministerio da Saude Anexo
Comite de Etica em Pesquisa da Universidade Federal de Minas Gerais
Comite de Etica em Pesquisa do Hospital das Clinicas da Faculdade de Medicina de Ribeirao Preto da Universidade de Sao Paulo, CONEP – Ministerio da Saude, Esplanada dos Ministerios
Comite de Etica em Pesquisa do Instituto de Infectologia Emilio Ribas, Ministerio da Saude – CONEP – Esplanada dos Ministerios
Comision de Bioetica del Comite de Docencia e Investigacion Hospital General de Agudos JM Ramos Mejia
Cook County Hospital IRB
Committee on Clinical Investigations, Children's Hospital Los Angeles
Committee on Human Research, University of California San Francisco
Johns Hopkins Medicine IRB
MHS Research Council IRB
University of Maryland IRB
Tulane Office of Human Research Protections IRB
IRB University of Alabama at Birmingham
Comite de Etica em Pesquisa do Hospital Geral de Nova Iguacu, CONEP – Esplanada dos Ministerios
Comite de Etica em Pesquisa da Irmandade da Santa Casa de Misericordia de Porto Alegre
IRB St. Jude's Research Hospital
Comite de Derechos Humanos IRB
Children's Hospital of Philadelphia Committee for Protection of Human Subjects
IRB Bronx Lebanon Hospital Center
Committee for Protection of Human Subjects in Research, University of MA Medical School
University of the Witwatersrand, Johannesburg, Human Research Ethics Committee (Medical), Medicines Control Council
University of Stellenbosch Committee for Clinical Trials, Tygerberg Campus Medicines Control Council
Comite de Etica em Pesquisa do Hospital Nossa Senhora da Conceicao/GHC, CONEP Ministerio da Saude, Anexo
Health Research and Development Committee, Ministry of Health Botswana
Harvard School of Public Health IRB
Biomedical Research Ethics Administration, University of KwaZulu Natal
Footnotes
Publisher's Disclaimer: The views expressed in written conference materials or publications and by speakers and moderators at HHS-sponsored conferences, do not necessarily reflect the official policies of the Department of Health and Human Services; nor does mention of trade names, commercial practices, or organizations imply endorsement by the U.S. Government
This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record.
DISCLOSURES Medical writing and/or editorial assistance was provided by Kim Strohmaier and Karyn Davis, both of Merck Sharp & Dohme Corp.
REFERENCES
- 1.Lennox JL, DeJesus E, Lazzarin A, et al. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naïve patients with HIV-1 infection: a multicentre, double-blind randomized controlled trial. Lancet. 2009;374:796–806. doi: 10.1016/S0140-6736(09)60918-1. [DOI] [PubMed] [Google Scholar]
- 2.Merck & Co., Inc . Prescribing Information for ISENTRESS (raltegravir) 2014. [Google Scholar]
- 3.Steigbigel RT, Cooper DA, Kumar PN, et al. Raltegravir with optimized background therapy for resistant HIV-1 infection. N Engl J Med. 2008;359:339–354. doi: 10.1056/NEJMoa0708975. [DOI] [PubMed] [Google Scholar]
- 4.Nachman S, Zheng N, Acosta EP, et al. Pharmacokinetics, safety, and 48-week efficacy of oral raltegravir in HIV-1-infected children aged 2 through 18 years. Clin Infect Dis. 2014;58(3):413–22. doi: 10.1093/cid/cit696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eron JJ, Jr, Rockstroh JK, Reynes J, et al. Raltegravir once daily or twice daily in previously untreated patients with HIV-1: a randomised, active-controlled, phase 3 non-inferiority trial. Lancet Infect Dis. 2011;11:907–15. doi: 10.1016/S1473-3099(11)70196-7. [DOI] [PubMed] [Google Scholar]
- 6.Rizk ML, Hang Y, Luo W-L, et al. Pharmacokinetics and pharmacodynamics of once-daily versus twice-daily raltegravir in treatment-naïve HIV-infected patients. Antimicrob Agents Chemother. 2012;56(6):3101–6. doi: 10.1128/AAC.06417-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brainard DM, Friedman EJ, Jin B, et al. Effect of Low-, Moderate-, and High-Fat Meals on Raltegravir Pharmacokinetics. J Clin Pharmacol. 2011;51:422–427. doi: 10.1177/0091270010367652. [DOI] [PubMed] [Google Scholar]
- 8.Iwamoto M, Wenning LA, Petry AS, et al. Safety, tolerability, and pharmacokinetics of raltegravir after single and multiple doses in healthy subjects. Clin Pharmacol Ther. 2008;83:293–299. doi: 10.1038/sj.clpt.6100281. [DOI] [PubMed] [Google Scholar]
- 9.Wenning L, Nguyen BY, Sun X, et al. Pharmacokinetic/pharmacodynamic (PK/PD) analyses for raltegravir (RAL) in phase II and III studies in treatment experienced HIV-infected patients. Abstr; 9th International Workshop on Clin Pharmacol of HIV Therapy.2008. [Google Scholar]
- 10.Wang L, Soon GH, Seng KY, et al. Pharmacokinetic modeling of plasma and intracellular concentrations of raltegravir in healthy volunteers. Antimicrob Agents Chemother. 2011;55:4090–4095. doi: 10.1128/AAC.00593-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arab-Alameddine M, Mello A, Lubomirov R, et al. Population pharmacokinetics of raltegravir in HIV positive and healthy subjects. Abstr; 12th International Workshop on Clin Pharmacology of HIV Therapy.2011. [Google Scholar]
- 12.Rhee EG, Rizk ML, Brainard DM, et al. A pharmacokinetic comparison of adult and pediatric formulations of raltegravir in healthy adults. Antivir Ther. 2014 doi: 10.3851/IMP2765. doi: 10.3851/IMP2765. [DOI] [PubMed] [Google Scholar]
- 13.Merschman SA, Vallano PT, Wenning LA, et al. Determination of the HIV integrase inhibitor, MK-0518 (raltegravir), in human plasma using 96-well liquid-liquid extraction and HPLC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;857:15–24. doi: 10.1016/j.jchromb.2007.06.032. [DOI] [PubMed] [Google Scholar]
- 14.Long MC, Bennetto-Hood C, Acosta EP. A Sensitive HPLC-MS-MS method for the determination of raltegravir in human plasma, J Chromatogr B Analyt Technol Biomed Life Sci. 2008;867:165–171. doi: 10.1016/j.jchromb.2008.03.022. [DOI] [PubMed] [Google Scholar]