Abstract
Lactobacillus spp. are associated with the maintenance of reproductive health, but obesity reduces fertility and is a risk factor for obstetric and neonatal complications. We assessed the association between obesity and the cervical Lactobacillus composition, which has not been examined previously. Pyrosequencing was performed using cervical swabs collected from 76 normal participants with negative results for cervical intraepithelial neoplasia (CIN) and 57 participants with CIN, based on histological examinations. Cluster analysis of nine Lactobacillus spp. was performed, and five cluster types were identified. The association between obesity and the Lactobacillus community was assessed by logistic regression analysis after adjustment for confounding factors. The proportion of Lactobacillus iners increased and that of Lactobacillus crispatus decreased according to body mass index (BMI) categories, i.e., underweight (BMI of <18.5 kg m−2), normal weight (BMI of 18.5 to 22.9 kg m−2), overweight (BMI of 23.0 to 24.9 kg m−2), and obese (BMI of ≥25 kg m−2). The L. iners-dominant type had a significant association with obesity (odds ratio [OR], 7.55 [95% confidence interval [CI], 1.18 to 48.2]), compared to the L. crispatus-dominant type. The group with high values for the ratio obtained by dividing the relative abundance of L. iners by that of L. crispatus had a significant association with obesity (OR, 6.54 [95% CI, 1.22 to 35.1]), compared to the low-ratio group. Associations between obesity and the L. iners/L. crispatus ratio were observed among young women (OR, 6.26 [95% CI, 1.15 to 33.9]) but not older women and in the normal group (OR, 6.97 [95% CI, 1.20 to 70.4]) but not the CIN group. Obesity was associated with cervical microflora dominated by L. iners in reproductive-age women without dysplasia.
INTRODUCTION
Dominance of the vaginal microbiota by Lactobacillus spp. is associated with the retention of a healthy reproductive status and with a relatively low pH (pH ≤ 4.5) of the genital tract (1). Lactobacillus spp. produce metabolic products, such as hydrogen peroxide (H2O2), lactic acid, and bacteriocin, that are thought to play important roles in maintaining a normal vaginal flora by preventing colonization by pathogens (2, 3). In a community of approximately 400 asymptomatic women, those with smaller proportions of lactobacilli showed higher Nugent scores and pH values, which are used to diagnose bacterial vaginosis (4). A lack of lactobacilli in the vaginal tract may disturb the balance of the microbial flora and can lead to bacterial vaginosis via overgrowth of anaerobic bacteria (5). Women whose genital microbial florae are dominated by lactobacilli also have lower risks of contracting sexually transmitted diseases, such as those caused by human immunodeficiency virus (HIV), herpes simplex virus type 2, human papillomavirus (HPV), and Chlamydia trachomatis (5–7). In addition, colonization by lactobacilli has been shown to be correlated with lower risks for pelvic inflammatory disease and pregnancy-related complications (1, 8).
The structure and composition of the Lactobacillus community in the vaginal ecosystem are affected by various factors such as age, menarche, menopause, menstrual cycle, pregnancy, infections, medications, and hygiene (9). In addition, obesity could be a contributing factor related to bacterial community structure and composition. Obese women have been shown to have different physiological characteristics that may lead to dysbiosis of the genital tract, including high plasma estrogen levels, systemic inflammation, and low immune function (10, 11). Furthermore, several previous studies showed that prepregnancy body mass index (BMI) and plasma leptin levels may influence the outcomes of pregnancies, such as preterm delivery, low-birth-weight infants, macrosomia, and delivery disorders (12, 13). These obstetric and neonatal disorders were also associated with Lactobacillus iners-dominant microbiota in early pregnancy and the presence of bacteria related to bacterial vaginosis, as detected by vaginal smears (8, 14–16). To the best of our knowledge, no study to date has examined the relationship between obesity and Lactobacillus composition in the reproductive tract.
In this study, we assessed the association between obesity and the composition of cervical lactobacilli in Korean women. In a previous study, we performed pyrosequencing of 16S rRNA with 120 cervical swab samples that either showed negative results (n = 50) or tested positive for cervical intraepithelial neoplasia (CIN) (n = 70) in histological examinations (17). In this study, we included the previous samples and 37 additional swabs; after excluding 24 samples that showed an absence of Lactobacilli or for which participant epidemiological data (age and BMI) were not available, we had a total of 133 samples with either negative CIN results (n = 76) or CIN of various grades (n = 57), based on histological examinations. The relative abundances of nine representative Lactobacillus spp., including Lactobacillus crispatus, L. iners, Lactobacillus fornicalis, Lactobacillus johnsonii, Lactobacillus gasseri, Lactobacillus jensenii, Lactobacillus acidophilus, Lactobacillus psittaci, and uncultured Lactobacillus species, and various epidemiological data were used for analysis.
MATERIALS AND METHODS
Study participants and design.
Women 18 to 65 years of age who participated in the Korean HPV cohort study, from 2006 to the present, were included in this study; details regarding the design of the baseline measures for the cohort have been described previously (18). All study participants provided written informed consent before participation. Women with a history of treatment for cervical intraepithelial neoplasia (CIN) within the past 18 months, pregnant women, and women with chronic diseases were excluded at the time of enrollment. Obesity is a known risk factor for chronic diseases, including cardiovascular disease, diabetes mellitus, high blood pressure, and stroke. However, none of our study participants had obesity and a chronic disease concurrently. Cervical swabs were collected for the Papanicolaou test, the oncogenic HPV DNA test, and pyrosequencing of cervical microbiota (Cervical Sampler, Digene Co., MD, USA). Colposcopy and histological verifications were performed at baseline. A total of 133 participants, including 76 with negative CIN results and 57 with positive CIN results at the baseline histological examination, were selected randomly from the enrolled women, and their baseline swab samples were used for this study. The procedure for collecting swab samples was described in our previous work (17). This study was approved by the institutional review board of the Korea National Cancer Center (IRB no. NCCNCS-06-062) and by the ethics committees of the Korea National Cancer Center and Korea University Guro Hospital.
High-risk HPV DNA detection.
The cervical cytological findings were classified according to the Bethesda system (19). HPV DNA detection was performed with the Digene Hybrid Capture II DNA test (Qiagen, Gaithersburg, MD, USA). A chemiluminescent HPV DNA test (scored in relative light units [RLU]) with a probe designed to detect 13 types of high-risk (HR) HPV was used. The test results were read as positive at concentrations of 1 pg/ml or levels greater than the RLU/cutoff ratio (RLU of the specimen/mean RLU of 2 positive controls).
DNA extraction and pyrosequencing analysis.
Metagenomic DNA samples were extracted using a FastDNA Spin extraction kit (MP Biomedicals, Santa Ana, CA, USA). The target fragments of the 16S rRNA gene corresponding to the V1 to V3 regions were amplified by using barcoded primers. Detailed information, including the amplification conditions and a description of the pyrosequencing procedure, was provided in a previous report (17). The pyrosequencing readings are available in the EMBL Sequence Read Archive (SRA) database (http://www.ebi.ac.uk/ena/data/view/PRJEB5760).
Statistical analysis.
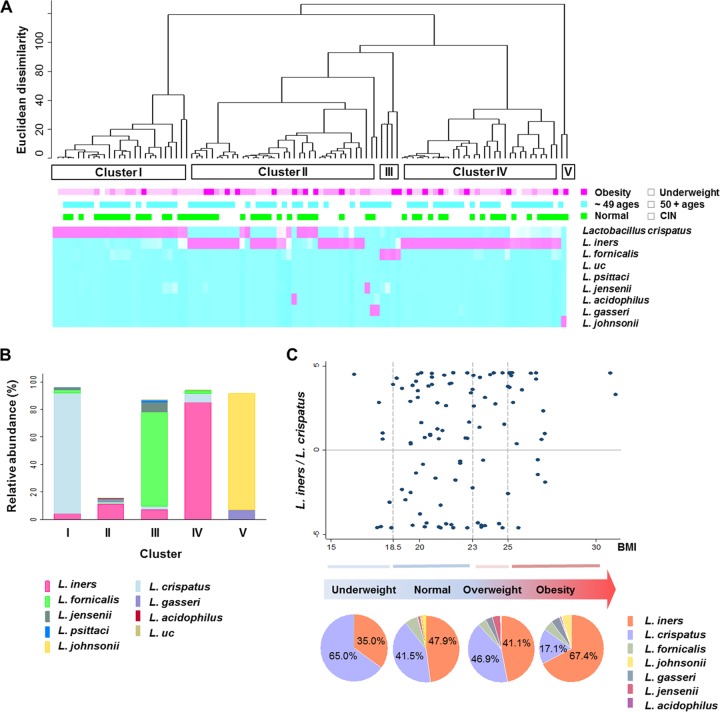
The chi-square test was used to analyze the differences in the distributions of categorical or general variables. Hierarchical clustering analysis, based on average linkage with Euclidean dissimilarity measurements for the relative abundances of nine Lactobacillus spp. (L. iners, L. crispatus, L. fornicalis, L. johnsonii, L. gasseri, L. jensenii, L. acidophilus, L. psittaci, and uncultured Lactobacillus species), was performed using Stata 12.0 software (Stata Corp., College Station, TX, USA). A dendrogram for the hierarchical cluster analysis was drawn to display only the top 100 branches, and subjects were grouped from cluster analysis performed using cluster analysis stopping rules for the number of clusters determined by default (Calinski-Harabasz pseudo-F index). A simple heat map for the relative abundances of nine Lactobacillus spp. was prepared using an R software package.
BMIs were categorized into four groups according to the Asia-Pacific standards, i.e., underweight (<18.5 kg m−2), normal weight (18.5 to 22.9 kg m−2), overweight (23.0 to 24.9 kg m−2), and obese (≥25 kg m−2) (20). The Wilcoxon rank-sum test was used to measure the median difference in the ratios obtained by dividing the relative abundance of L. iners by that of L. crispatus for nonobese (BMI of <25 kg m−2) and obese (BMI of ≥25 kg m−2) groups. The distribution of the L. iners/L. crispatus ratios and the percentage of each Lactobacillus species were depicted according to the BMIs or BMI categories, with a scatter plot and pie graphs, respectively. Lactobacillus cluster types and the L. iners/L. crispatus ratios were used for assessment of an association between obesity and the cervical Lactobacillus microflora. The multivariate odds ratios (ORs) and 95% confidence intervals (CIs) for the risk of obesity were estimated by using L. crispatus-dominant Lactobacillus cluster types or the low-ratio group as references. All variables were adjusted for age, menopausal status, HR-HPV infection, and histological grade as categorical variables. The relative abundances of the nine Lactobacillus species are provided in Table S1 in the supplemental material.
RESULTS
General characteristics of study participants.
The distributions of the general characteristics of all study participants (n = 133), with either normal or atypical squamous cells of undetermined significance (ASCUS) (n = 76) or CIN grade 1 (n = 57) results, grouped by nonobesity (BMI of <25 kg m−2) versus obesity (BMI of ≥25 kg m−2), were investigated. Significant differences were observed for age and menopausal status; the older and postmenopausal groups had larger proportions of obese women (P < 0.001 and P < 0.006, respectively), compared to the young and premenopausal groups, respectively. No differences were observed for the other variables.
Lactobacillus cluster types.
L. iners (50.9%) and L. crispatus (37.9%) were the most abundant Lactobacillus species among all participants, followed by L. fornicalis (5.3%), L. johnsonii (2.2%), L. gasseri (1.6%), L. jensenii (1.4%), L. acidophilus (0.3%), L. psittaci (0.2%), and uncultured Lactobacillus species (0.2%). Hierarchical clustering analysis of the relative abundances of the nine Lactobacillus spp. produced five Lactobacillus cluster types (Fig. 1A). Cluster type I (n = 30) was dominated by L. crispatus (approximately 90%), whereas cluster type IV (n = 37) was dominated by L. iners (approximately 90%) (Fig. 1B). Cluster type III (n = 4) was characterized by large proportions of L. fornicalis and L. jensenii, and cluster type V (n = 2) was characterized by large proportions of L. johnsonii and L. gasseri. Cluster type II (n = 60) was dominated by non-Lactobacillus species and was partially dominated by L. iners. Clusters I, II, and IV were the main community types in this study.
FIG 1.
Proportions of Lactobacillus iners and L. crispatus according to body mass index. (A) Hierarchical clustering of nine Lactobacillus species and five Lactobacillus cluster types. The relative abundances of the species are represented by colors (pink squares, large proportions; blue squares, small proportions). The heat map includes information on obesity, age, and cervical intraepithelial neoplasia (CIN). First line below the dendrogram: dark pink boxes, obesity; medium pink boxes, overweight; light pink boxes, normal weight; white spaces, underweight. Second line below the dendrogram: blue boxes, age of ≤49 years; white spaces, age of ≥50 years. Third line below the dendrogram: green boxes, healthy participants with negative CIN results; white spaces, participants with CIN. (B) Mean percentages of species in the five Lactobacillus cluster types. (C) Scatter plot of the log-transformed L. iners/L. crispatus ratios (dot chart) and the mean proportions of L. iners and L. crispatus according to body mass index (BMI) categories (pie charts). BMI values were categorized according to the Asia-Pacific standards, i.e., underweight, BMI of <18.5 kg m−2; normal weight, BMI of 18.5 to 22.9 kg m−2; overweight, BMI of 23 to 24.9 kg m−2; obesity, BMI of ≥25 kg m−2. L. uc, uncultured Lactobacillus species.
Proportions of L. iners and L. crispatus according to BMI.
Obese participants were more likely to be associated with cluster types II and IV than cluster type I, although this finding was not significant (P = 0.091, chi-square test for clusters I, II, and IV) (Fig. 1A). There was no difference in the distributions of young women (≤49 years of age) and older women (≥50 years of age) in the main cluster types (P = 0.161, chi-square test). All participants in cluster type III had CIN, but there was no difference in the distributions of normal and CIN participants in the main cluster types (P = 0.328, chi-square test).
L. iners and L. crispatus were the dominant species among the participants sampled in this study. The log-transformed ratio obtained by dividing the relative abundance of L. iners by that of L. crispatus was calculated using 105 samples with >1% relative abundance of either species. Twenty-eight participants were excluded from the calculation due to a lack of abundance of these species. The L. iners/L. crispatus ratios differed significantly between the participants with BMIs of <25 kg/m2 and those with BMIs of ≥25 kg/m2 (P < 0.038, Wilcoxon rank sum test). Most of the obese women had larger proportions of L. iners and smaller proportions of L. crispatus and thus had higher ratio values, whereas the nonobese women showed inconsistent variations in ratios (Fig. 1C, dot chart). The mean percentage of L. crispatus (65.0%) was higher than that of L. iners (35.0%) in the underweight group, while the mean percentage of L. crispatus (17.1%) was lower than that of L. iners (67.4%) in the obese group (Fig. 1C, pie charts). The mean percentages of these lactobacilli in the normal-weight and overweight groups were approximately equal.
Association between obesity and L. iners-dominant Lactobacillus cluster type.
Obesity was significantly associated with the L. iners-dominant cluster type (cluster type IV), compared to the L. crispatus-dominant cluster type (cluster type I) (multivariate OR, 7.55 [95% CI, 1.18 to 48.2]; P = 0.033) (Table 1). Additionally, obesity was associated with the group with high values for the L. iners/L. crispatus ratio (multivariate OR, 6.54 [95% CI, 1.22 to 35.1]; P = 0.022; P for trend = 0.022).
TABLE 1.
Association between obesity and Lactobacillus cluster IV, dominated by L. iners
| Cluster type and species ratio | No. obesea/no. nonobese | OR (95% CI)b | P | P for trendc |
|---|---|---|---|---|
| Lactobacillus type (main)d | ||||
| Cluster type I | 2/28 | 1 (reference) | ||
| Cluster type II | 14/46 | 5.10 (0.85–30.7) | 0.075 | |
| Cluster type IV | 10/27 | 7.55 (1.18–48.2) | 0.033 | |
| L. iners/L. crispatus ratioe | ||||
| Low | 4/31 | 1 (reference) | 0.022 | |
| Medium | 7/28 | 3.22 (0.55–18.8) | 0.194 | |
| High | 11/24 | 6.54 (1.22–35.1) | 0.029 |
Obesity was defined according to the Asia-Pacific standards, i.e., underweight, BMI of <18.5 kg m−2; normal weight, BMI of 18.5 to 22.9 25 kg m−2; overweight, BMI of 23 to 24.9 25 kg m−2; obese, BMI of ≥25 kg m−2.
The multivariate odds ratios (ORs) were estimated with the nonobese group as a reference, after adjustment for age, menopausal status, high-risk human papillomavirus infection, and histological grade as categorical variables.
The P value was calculated for the linear trend of multivariate ORs.
Cluster type I was dominated by L. crispatus, cluster type II was dominated by non-Lactobacillus species, and cluster type IV was dominated by L. iners. Cluster types III and V were excluded from the analysis because of the small numbers of subjects.
Only the 105 samples with a sufficient relative abundance (>1%) of either L. iners or L. crispatus were used to calculate L. iners/L. crispatus ratios. Subjects were divided automatically into three groups with equal sample sizes.
According to the general characteristics of the study participants, the distributions of BMI categories were different for young versus older participants. Furthermore, in our previous work we demonstrated the association between a specific cervical microbiome pattern and the risk of CIN (17). To control for the effects of age and cervical dysplasia on the relationship between obesity and the L. iners-dominant Lactobacillus cluster type, study participants were stratified according to age (young versus older) and histological result (normal versus CIN). Obesity was associated with the group with high values for the L. iners/L. crispatus ratio among young women (multivariate OR, 6.26 [95% CI, 1.15 to 33.9]; P = 0.033) but not among older women (Table 2). For normal participants with negative CIN results, the multivariate OR for the high-ratio group was 6.97 (95% CI, 1.20 to 70.4; P = 0.030). For participants with a CIN grade, however, there was no significant association between obesity and the L. iners/L. crispatus ratio.
TABLE 2.
Association between obesity and L. iners/L. crispatus ratios
| Group | Low L. iners/L. crispatus ratiosa |
High L. iners/L. crispatus ratios |
Pb | ||
|---|---|---|---|---|---|
| No. obese/no. nonobese | OR (95% CI) | No. obese/no. nonobese | OR (95% CI) | ||
| Age | |||||
| Young (≤49 yr) | 2/36 | 1 (reference) | 8/26 | 6.26 (1.15–33.9) | 0.033 |
| Older (≥50 yr) | 6/9 | 1 (reference) | 6/12 | 3.52 (0.38–32.4) | 0.266 |
| Menopausal status | |||||
| Premenopausal | 3/43 | 1 (reference) | 7/15 | 6.47 (1.46–28.7) | 0.014 |
| Postmenopausal | 8/14 | 1 (reference) | 4/9 | 2.32 (0.27–19.8) | 0.443 |
| Histological result | |||||
| Normal | 5/39 | 1 (reference) | 6/15 | 6.97 (1.20–70.4) | 0.030 |
| CIN | 9/27 | 1 (reference) | 8/11 | 1.57 (0.29–8.60) | 0.602 |
Only the 105 samples with a sufficient relative abundance (>1%) of either L. iners or L. crispatus were used to calculate L. iners/L. crispatus ratios. Subjects were divided automatically into two groups with equal sample sizes. The multivariate odds ratios (ORs) were estimated with the nonobese group as a reference, after adjustment for age, menopausal status, high-risk human papillomavirus infection, and histological grade as categorical variables.
P values for the regression coefficients.
In a Korean twin cohort study, postmenopausal women had smaller proportions of Lactobacillus spp., with greater diversity (21). Obesity was associated with the group with high values for the L. iners/L. crispatus ratio among premenopausal women (multivariate OR, 6.47 [95% CI, 1.46 to 28.7]; P = 0.014) but not among postmenopausal women (Table 2).
DISCUSSION
This study was the first to demonstrate that the cervical Lactobacillus microflora of obese women differed from that of nonobese women. Obesity (BMI of ≥25 kg/m2) was strongly associated with the cervical Lactobacillus community type characterized by a large proportion of L. iners and small proportions of L. crispatus, L. johnsonii, and L. jensenii. This significant association between obesity and an L. iners-dominant Lactobacillus community type was found for relatively young women but was not found for older women. This community type also was found for participants without CIN but was not found for participants with CIN. Taken together, these findings showed that obesity was associated with a cervical Lactobacillus community type dominated by L. iners among women of reproductive age without cervical dysplasia.
In this study, we showed that obesity may be associated with a higher L. iners/L. crispatus ratio for the cervical microbiome, although 28 women were excluded because of a lack of relative abundance values for the two species. L. crispatus and L. iners are the dominant species in the cervical microbiomes of Asian women, including our study participants. The predominance of L. crispatus was shown, in a longitudinal analysis, to promote the stability of the vaginal microbiota (22). However, new evidence suggests that these two species differ in their ecological functions in the reproductive tract (23). Hydrogen peroxide, an antimicrobial compound, is produced by 95% of L. crispatus isolates and 94% of L. jensenii isolates but only 9% of L. iners isolates (described as L. 1086V by Antonio et al. [1]). d-Lactic acid levels were higher in women whose vaginal flora was dominated by L. crispatus than in those whose flora was dominated by L. iners or Gardnerella species. L. crispatus and L. gasseri contain one or two copies of both l- and d-lactate dehydrogenases, whereas the gene coding for d-lactate dehydrogenase is absent from the genome of L. iners UPII 60-B (16).
The reason why obese women had larger proportions of L. iners in their cervical microflorae is unclear. L. iners is the dominant species in the vaginal flora when the flora is in a transitional stage between normal and abnormal, because of either treatment with antibiotics or physiological changes such as higher plasma estradiol levels (24). Estrogen may be the most probable factor contributing to a predominance of L. iners in the obese women of this study. Circulating estradiol levels are strongly associated with adiposity (25), and the effects of being overweight or obese on the risk of breast and endometrial cancer are largely mediated by this physiological factor (10). Although the estrogen levels of our study participants were not measured, the possibility of estrogen contributions to this association remains.
Recently, evidence from animal studies has shown that the composition of the intestinal flora differs between obese individuals and normal-weight individuals (26). Differences in the bacterial diversity of the gastrointestinal tract, either in composition or in quantity, are associated with obesity, cardiovascular disease, metabolic syndrome, and type 2 diabetes mellitus (26). One study examining male-to-female transsexual women showed that 79.5% of the women simultaneously harbored the same lactobacilli in both the neovagina and the rectum (27). This result suggests that the rectum may be associated with the colonization of vaginal Lactobacillus species. Thus, the gut microbiomes of obese women may have effects on the composition of their cervicovaginal microbiomes.
The atypical characteristics of L. iners also include a protein produced by the species. Clinical isolates of L. iners were shown to produce inerolysin, a cholesterol-dependent cytolysin, during growth in vitro, whereas this protein was absent in other Lactobacillus species (28). Inerolysin, a pore-forming toxic protein, activates p38 mitogen-activated protein kinase and allows the entry of molecules from the extracellular space into the cytoplasm of epithelial cells. Cytolysin may serve a defensive function by killing immune cells and may make new anatomical niches available by disrupting epithelial barriers (28). What is remarkable is that the distribution of cholesterol in the cell membranes may be important for cytolysin binding (29). Perfringolysin O, a thiol-activated cytolysin produced by Clostridium perfringens, selectively binds to cholesterol-rich lipid rafts where their cholesterol concentrations in the membrane are high (30). Insulin-resistant animals fed a high-fat diet demonstrated elevated cellular cholesterol levels, contributing to larger lipid rafts (31). Although it is difficult to ascertain the size of the effect of systemic cholesterol levels on local membrane cholesterol levels, physiological states such as dyslipidemia could facilitate the colonization of L. iners in the reproductive tract.
An abnormal vaginal microflora in pregnant women is a risk factor for preterm delivery, and the dominance of L. iners in vaginal smears from healthy women during early pregnancy is associated with preterm delivery (8, 14). Women with large proportions of L. crispatus had high Escherichia coli inhibitory activity, whereas women whose vaginal smears were dominated by L. iners had low E. coli inhibitory activity (15). Additionally, obesity is a proinflammatory disease state in which the serum levels of inflammatory modulators are associated with preterm birth (32). Several reports have demonstrated associations between the serum expression levels of proinflammatory cytokines or peripheral blood mononuclear cells and preterm birth (33–35). This pregnancy outcome is influenced by prepregnancy BMI, via high C-reactive protein levels, in Korean women (12). Obesity is closely associated with cardiovascular disease, diabetes, high blood pressure, and stroke, but it was difficult to verify these associations among the study participants due to the exclusion criteria used at the time of enrollment. Although direct or indirect evidence is lacking, it is reasonable to infer that there is a relationship between L. iners-dominant cervical microbiota in obese women of reproductive age and an increased risk of preterm birth. Additional research is needed to determine whether obesity is causally linked to a predominance of L. iners in the female reproductive tract and whether this interaction has an influence on obstetric and neonatal complications.
The association between obesity and the cervical Lactobacillus type dominated by L. iners was observed in relatively young women of reproductive age but not in older women. Lee et al. reported that the composition or diversity of the vaginal microbiota was influenced by genetic or physiological factors, such as menopause and estrogen levels, in Korean women (21). In addition, the relationship between obesity and L. iners-dominant Lactobacillus microflora was found only in normal participants and was not found in participants with CIN. Although lactobacilli significantly contribute to the maintenance of normal vaginal flora, it is highly probable that other pathogens may be associated with abnormal cell growth. Several studies, including our previous work, reported significant correlations between bacterial vaginosis, or the proportion of anaerobic bacteria related to bacterial vaginosis, and CIN (17, 36, 37). Taken together, the association between obesity and a cervical Lactobacillus microflora dominated by L. iners does not affect cervical dysplasia, but it is likely to be established in reproductive-age women and may influence obstetric and neonatal complications related to obesity among these women.
Our study has several limitations. Cross-sectional studies are limited by the fact that they are carried out at one point in time and give no indication of the sequence of events, i.e., whether exposure occurred before, after, or during the onset of the disease outcome (38). Therefore, it is impossible to infer causality between obesity and the Lactobacillus type dominated by L. iners in this study. The small sample size of this study limited the estimation of the association and led to a wide 95% confidence interval. Various factors that influence the vaginal Lactobacillus flora, such as menstrual cycle, estradiol levels, and C-reactive protein levels, were not assessed concurrently. A large-scale study that considers various potential factors will be important to replicate and to confirm our findings.
In conclusion, the results of this study showed that obesity was associated with a cervical Lactobacillus microflora dominated by L. iners in women of reproductive age. We suggest that obesity may promote the predominance of L. iners in the cervicovaginal ecosystem and that this state may increase the risk of obstetric and neonatal complications related to obesity, such as preterm birth, in Korean women of reproductive age. More evidence is needed to reveal the causal link between obesity and the composition of the microbiota and to explain the role of the cervicovaginal microbiota in the maintenance of a healthy reproductive tract.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the Korea National Cancer Center (grants 1110320 and 1310360).
We declare no conflicts of interest.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.01387-15.
REFERENCES
- 1.Antonio MA, Hawes SE, Hillier SL. 1999. The identification of vaginal Lactobacillus species and the demographic and microbiologic characteristics of women colonized by these species. J Infect Dis 180:1950–1956. doi: 10.1086/315109. [DOI] [PubMed] [Google Scholar]
- 2.Klebanoff SJ, Hillier SL, Eschenbach DA, Waltersdorph AM. 1991. Control of the microbial flora of the vagina by H2O2-generating lactobacilli. J Infect Dis 164:94–100. doi: 10.1093/infdis/164.1.94. [DOI] [PubMed] [Google Scholar]
- 3.Aroutcheva A, Gariti D, Simon M, Shott S, Faro J, Simoes JA, Gurguis A, Faro S. 2001. Defense factors of vaginal lactobacilli. Am J Obstet Gynecol 185:375–379. doi: 10.1067/mob.2001.115867. [DOI] [PubMed] [Google Scholar]
- 4.Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, Brotman RM, Davis CC, Ault K, Peralta L, Forney LJ. 2011. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A 108(Suppl 1):S4680–S4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danielsson D, Teigen PK, Moi H. 2011. The genital econiche: focus on microbiota and bacterial vaginosis. Ann N Y Acad Sci 1230:48–58. doi: 10.1111/j.1749-6632.2011.06041.x. [DOI] [PubMed] [Google Scholar]
- 6.Galvin SR, Cohen MS. 2004. The role of sexually transmitted diseases in HIV transmission. Nat Rev Microbiol 2:33–42. doi: 10.1038/nrmicro794. [DOI] [PubMed] [Google Scholar]
- 7.Cherpes TL, Melan MA, Kant JA, Cosentino LA, Meyn LA, Hillier SL. 2005. Genital tract shedding of herpes simplex virus type 2 in women: effects of hormonal contraception, bacterial vaginosis, and vaginal group B Streptococcus colonization. Clin Infect Dis 40:1422–1428. doi: 10.1086/429622. [DOI] [PubMed] [Google Scholar]
- 8.Petricevic L, Domig KJ, Nierscher FJ, Sandhofer MJ, Fidesser M, Krondorfer I, Husslein P, Kneifel W, Kiss H. 2014. Characterisation of the vaginal Lactobacillus microbiota associated with preterm delivery. Sci Rep 4:5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mastromarino P, Vitali B, Mosca L. 2013. Bacterial vaginosis: a review on clinical trials with probiotics. New Microbiol 36:229–238. [PubMed] [Google Scholar]
- 10.Calle EE, Kaaks R. 2004. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer 4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 11.Choi J, Joseph L, Pilote L. 2013. Obesity and C-reactive protein in various populations: a systematic review and meta-analysis. Obes Rev 14:232–244. doi: 10.1111/obr.12003. [DOI] [PubMed] [Google Scholar]
- 12.Han YS, Ha EH, Park HS, Kim YJ, Lee SS. 2011. Relationships between pregnancy outcomes, biochemical markers and pre-pregnancy body mass index. Int J Obes (Lond) 35:570–577. doi: 10.1038/ijo.2010.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wendremaire M, Mourtialon P, Goirand F, Lirussi F, Barrichon M, Hadi T, Garrido C, Le Ray I, Dumas M, Sagot P, Bardou M. 2013. Effects of leptin on lipopolysaccharide-induced remodeling in an in vitro model of human myometrial inflammation. Biol Reprod 88:45. doi: 10.1095/biolreprod.112.104844. [DOI] [PubMed] [Google Scholar]
- 14.Tamrakar R, Yamada T, Furuta I, Cho K, Morikawa M, Yamada H, Sakuragi N, Minakami H. 2007. Association between Lactobacillus species and bacterial vaginosis-related bacteria, and bacterial vaginosis scores in pregnant Japanese women. BMC Infect Dis 7:128. doi: 10.1186/1471-2334-7-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghartey JP, Smith BC, Chen Z, Buckley N, Lo Y, Ratner AJ, Herold BC, Burk RD. 2014. Lactobacillus crispatus dominant vaginal microbiome is associated with inhibitory activity of female genital tract secretions against Escherichia coli. PLoS One 9:e96659. doi: 10.1371/journal.pone.0096659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Witkin SS, Mendes-Soares H, Linhares IM, Jayaram A, Ledger WJ, Forney LJ. 2013. Influence of vaginal bacteria and d- and l-lactic acid isomers on vaginal extracellular matrix metalloproteinase inducer: implications for protection against upper genital tract infections. mBio 4:e00460–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oh HY, Kim BS, Seo SS, Kong JS, Lee JK, Park SY, Hong KM, Kim HK, Kim MK. 2015. The association of uterine cervical microbiota with an increased risk for cervical intraepithelial neoplasia in Korea. Clin Microbiol Infect 21:674.e1–674.e9. [DOI] [PubMed] [Google Scholar]
- 18.Hwang JH, Lee JK, Kim TJ, Kim MK. 2010. The association between fruit and vegetable consumption and HPV viral load in high-risk HPV-positive women with cervical intraepithelial neoplasia. Cancer Causes Control 21:51–59. doi: 10.1007/s10552-009-9433-9. [DOI] [PubMed] [Google Scholar]
- 19.Solomon D, Davey D, Kurman R, Moriarty A, O'Connor D, Prey M, Raab S, Sherman M, Wilbur D, Wright T Jr, Young N. 2002. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA 287:2114–2119. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. 2004. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 21.Lee JE, Lee S, Lee H, Song YM, Lee K, Han MJ, Sung J, Ko G. 2013. Association of the vaginal microbiota with human papillomavirus infection in a Korean twin cohort. PLoS One 8:e63514. doi: 10.1371/journal.pone.0063514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verstraelen H, Verhelst R, Claeys G, De Backer E, Temmerman M, Vaneechoutte M. 2009. Longitudinal analysis of the vaginal microflora in pregnancy suggests that L. crispatus promotes the stability of the normal vaginal microflora and that L. gasseri and/or L. iners are more conducive to the occurrence of abnormal vaginal microflora. BMC Microbiol 9:116. doi: 10.1186/1471-2180-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hummelen R, Fernandes AD, Macklaim JM, Dickson RJ, Changalucha J, Gloor GB, Reid G. 2010. Deep sequencing of the vaginal microbiota of women with HIV. PLoS One 5:e12078. doi: 10.1371/journal.pone.0012078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jakobsson T, Forsum U. 2007. Lactobacillus iners: a marker of changes in the vaginal flora? J Clin Microbiol 45:3145. doi: 10.1128/JCM.00558-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Emaus A, Espetvedt S, Veierod MB, Ballard-Barbash R, Furberg AS, Ellison PT, Jasienska G, Hjartaker A, Thune I. 2008. 17-β-Estradiol in relation to age at menarche and adult obesity in premenopausal women. Hum Reprod 23:919–927. doi: 10.1093/humrep/dem432. [DOI] [PubMed] [Google Scholar]
- 26.Tremaroli V, Backhed F. 2012. Functional interactions between the gut microbiota and host metabolism. Nature 489:242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 27.Petricevic L, Kaufmann U, Domig KJ, Kraler M, Marschalek J, Kneifel W, Kiss H. 2014. Rectal Lactobacillus species and their influence on the vaginal microflora: a model of male-to-female transsexual women. J Sex Med 11:2738–2743. doi: 10.1111/jsm.12671. [DOI] [PubMed] [Google Scholar]
- 28.Rampersaud R, Planet PJ, Randis TM, Kulkarni R, Aguilar JL, Lehrer RI, Ratner AJ. 2011. Inerolysin, a cholesterol-dependent cytolysin produced by Lactobacillus iners. J Bacteriol 193:1034–1041. doi: 10.1128/JB.00694-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tweten RK. 2005. Cholesterol-dependent cytolysins, a family of versatile pore-forming toxins. Infect Immun 73:6199–6209. doi: 10.1128/IAI.73.10.6199-6209.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waheed AA, Shimada Y, Heijnen HF, Nakamura M, Inomata M, Hayashi M, Iwashita S, Slot JW, Ohno-Iwashita Y. 2001. Selective binding of perfringolysin O derivative to cholesterol-rich membrane microdomains (rafts). Proc Natl Acad Sci U S A 98:4926–4931. doi: 10.1073/pnas.091090798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Habegger KM, Penque BA, Sealls W, Tackett L, Bell LN, Blue EK, Gallagher PJ, Sturek M, Alloosh MA, Steinberg HO, Considine RV, Elmendorf JS. 2012. Fat-induced membrane cholesterol accrual provokes cortical filamentous actin destabilisation and glucose transport dysfunction in skeletal muscle. Diabetologia 55:457–467. doi: 10.1007/s00125-011-2334-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sykes L, MacIntyre DA, Teoh TG, Bennett PR. 2014. Anti-inflammatory prostaglandins for the prevention of preterm labour. Reproduction 148:R29–R40. doi: 10.1530/REP-13-0587. [DOI] [PubMed] [Google Scholar]
- 33.Makhseed M, Raghupathy R, El-Shazly S, Azizieh F, Al-Harmi JA, Al-Azemi MM. 2003. Pro-inflammatory maternal cytokine profile in preterm delivery. Am J Reprod Immunol 49:308–318. doi: 10.1034/j.1600-0897.2003.00038.x. [DOI] [PubMed] [Google Scholar]
- 34.Curry AE, Vogel I, Drews C, Schendel D, Skogstrand K, Flanders WD, Hougaard D, Olsen J, Thorsen P. 2007. Mid-pregnancy maternal plasma levels of interleukin 2, 6, and 12, tumor necrosis factor-alpha, interferon-gamma, and granulocyte-macrophage colony-stimulating factor and spontaneous preterm delivery. Acta Obstet Gynecol Scand 86:1103–1110. doi: 10.1080/00016340701515423. [DOI] [PubMed] [Google Scholar]
- 35.Raghupathy R, Makhseed M, El-Shazly S, Azizieh F, Farhat R, Ashkanani L. 2001. Cytokine patterns in maternal blood after premature rupture of membranes. Obstet Gynecol 98:122–126. doi: 10.1016/S0029-7844(01)01408-9. [DOI] [PubMed] [Google Scholar]
- 36.Nam KH, Kim YT, Kim SR, Kim SW, Kim JW, Lee MK, Nam EJ, Jung YW. 2009. Association between bacterial vaginosis and cervical intraepithelial neoplasia. J Gynecol Oncol 20:39–43. doi: 10.3802/jgo.2009.20.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gillet E, Meys JF, Verstraelen H, Verhelst R, De Sutter P, Temmerman M, Vanden Broeck D. 2012. Association between bacterial vaginosis and cervical intraepithelial neoplasia: systematic review and meta-analysis. PLoS One 7:e45201. doi: 10.1371/journal.pone.0045201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bland M. 2001. An introduction to medical statistics, 3rd ed Oxford University Press, Oxford, United Kingdom. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.