Abstract
Blood donor samples (1,007) were assessed for anti-phenolic glycolipid 1 (PGL-1) IgM antibodies and Mycobacterium leprae DNA presence, which had 3.8% and 0.3% positivity, respectively. After a 5-year follow-up period, six individuals with positive markers developed leprosy, raising the hypothesis that asymptomatic infection among blood donors may be an undisclosed mode of leprosy transmission via transfusion.
TEXT
Leprosy is one of the oldest infectious diseases known to affect humans and remains a public health issue, particularly in Brazil, which accounts for almost all new cases detected in the Americas (1).
Untreated leprosy patients are considered the main source of transmission. However, the dichotomy between the highly effective treatment and the occurrence of new cases among people without previous contact with patients indicates that other infectious sources must be investigated (2).
The majority of exposed individuals will not develop the disease, although cumulative evidence demonstrates widespread dissemination of bacilli in regions where leprosy is endemic, strengthening the hypothesis that asymptomatic individuals are involved in the Mycobacterium leprae chain of transmission (3).
Serological reactivity to M. leprae antigens has been used as an immunological marker for exposure and infection (4). Seropositivity for antibodies against phenolic glycolipid 1 (PGL-1), M. leprae-specific cell surface antigenic molecule has been correlated to a greater chance for later onset of leprosy among household contacts of leprosy patients (5).
Studies regarding the detection of M. leprae DNA in peripheral blood samples are scarce. It has recently been suggested that whole-blood nested-PCR amplification could be used for early diagnosis of leprosy (6) and that the presence of M. leprae DNA in peripheral blood may be associated with bacillary migration and a high risk for disease onset (7).
There are no published data on the assessment of randomly selected healthy blood donors for any kind of M. leprae marker. Therefore, this is the first epidemiological study to assess donor blood samples for the presence of antibodies against M. leprae and bacillary DNA through enzyme-linked immunosorbent assay (ELISA) and real-time PCR, respectively.
Subjects were screened from a population of 1,035 blood donors at the regional blood bank of Uberlandia, Minas Gerais, Brazil. All subjects had normal dermatoneurological clinical examinations, and 28 people who reported prior contact with leprosy patients were excluded from the study. The reported new-case detection rate in the Uberlandia region was 11 per 100,000 population.
A total of 1,007 peripheral blood samples were assessed for the presence of anti-PGL-1 antibodies and M. leprae DNA. Indirect ELISA against M. leprae native PGL-1 molecule was applied to detect specific IgM antibodies in serum samples, according to previously described methodology (4). Detection of DNA was performed with a species-specific TaqMan primer/probe assay targeting the repetitive region RLEP element of dispersed repeats in the M. leprae genome, as previously described (8).
For specificity analysis, DNA templates from microbial cultures and clinical samples (Mycobacterium tuberculosis, M. gordonae, M. fortuitum, M. avium, Plasmodium falciparum, P. vivax, Leishmania braziliensis, L. amazonensis, Trypanosoma cruzi, Paracoccidioides braziliensis, and newborn human blood) were evaluated for PCR cross-reactions. Basic Local Alignment Search Tool (www.ncbi.nlm.nih.gov/BLAST/) was employed to confirm that primers were M. leprae specific. A sample with a known concentration of M. leprae DNA and amplification of human NRAMP1 (Homo sapiens solute carrier family 11 member 1, SLC11A1 [NG_012128.1]) gene was used as the PCR positive control. In order to avoid false-positive results, positive samples from healthy donors were confirmed using a second primer set targeting another M. leprae- specific fragment of 130 bp in the repetitive region RLEP3, as previously described (9). No cross-reactivity was observed with any other organism assessed, and BLAST results for the PCR primers confirmed species-specific alignment to only the M. leprae genome.
Thirty-eight individuals (3.8%) presented positive results for anti-PGL-1 ELISA, and PCR positivity among blood donors was 0.3% (3/1,007). Individuals with positive results were informed that they did not have the disease, but they were invited to participate in a 5-year follow-up, with collection of new samples and a clinical dermatoneurological examination at least once a year. The confirmed positive blood units were not used for transfusions and were discarded. When participants presented more than one positive result during follow-up, they underwent slit-skin smear sampling for M. leprae detection.
Five out of nine donors that were continuously seropositive for anti-PGL-1 and one with a positive PCR that became seropositive developed leprosy during follow-up (6/41 [14.6%]), but only one was positive for both tests during follow-up (Table 1). Out of the five individuals who initially presented positivity to anti-PGL-1 and later became ill, two showed little variation in the anti-PGL-1 titers throughout the follow-up, while three tended to increase the ELISA titration (Fig. 1).These six new cases were subjected to other confirmatory tests and presented positivity for M. leprae DNA in slit-skin smears and skin biopsy specimens, providing strong evidence that subclinical infection progressed to disease. Four out of the six new cases presented abnormalities in electroneuromyography that indicated sensory nerve damage, leading to the clinical classification tuberculoid leprosy (T) (1) or borderline-tuberculoid leprosy (BT) (3), and two did not present any abnormalities and were classified indeterminate (I).
TABLE 1.
Anti-PGL-1 serology (ELISA) and Mycobacterium leprae DNA detection (PCR) in 41 blood donors who presented positive markers in 2009 and were followed up for at least 5 years (2009 to 2014)
| Donor no. | ELISA follow-up (yearly) |
PCR (blood) | PCR slit-skin smeara | Mitsuda testf (mm) | Disease onset (clinical manifestationb) | ||||
|---|---|---|---|---|---|---|---|---|---|
| 2009 | 2010 | 2011 | 2012 | 2013 | 2009 | ||||
| 01 | + | + | − | − | − | − | − | 8 | No |
| 02 | + | − | − | − | NDd | − | ND | 9 | No |
| 03 | + | + | + | + | +e | − | + | 7 | Yes (I) |
| 04 | + | + | + | + | +e | − | + | 10 | Yes (T) |
| 05c | + | ND | ND | ND | ND | − | ND | ND | No |
| 06 | + | ND | ND | ND | + | − | − | 10 | No |
| 07 | + | + | + | − | − | − | − | 10 | No |
| 08 | + | ND | − | − | − | − | ND | 8 | No |
| 09 | + | + | ND | ND | ND | − | − | 5 | No |
| 10 | + | + | + | + | + | − | − | 5 | No |
| 11 | + | ND | ND | ND | ND | − | ND | 4 | No |
| 12 | + | + | + | + | − | − | − | 5 | No |
| 13 | + | + | + | + | + | − | − | 7 | No |
| 14 | + | ND | ND | ND | − | − | ND | 12 | No |
| 15 | + | ND | ND | ND | − | − | ND | 0 | No |
| 16 | + | + | − | − | − | − | − | 7 | No |
| 17 | + | − | − | ND | ND | − | ND | 8 | No |
| 18c | + | ND | ND | ND | ND | − | ND | ND | No |
| 19 | + | ND | ND | ND | + | − | − | 7 | N |
| 20 | + | + | + | + | +e | − | + | 11 | Yes (BT) |
| 21 | + | + | + | + | + | − | − | 9 | N |
| 22 | + | + | + | + | + | − | − | 9 | No |
| 23 | + | − | + | − | + | − | − | 7 | No |
| 24c | + | ND | ND | ND | ND | − | ND | ND | No |
| 25 | + | + | − | + | + | − | − | 10 | No |
| 26 | + | ND | ND | + | + | − | − | 10 | No |
| 27 | + | ND | ND | − | − | − | ND | 9 | No |
| 28 | + | + | + | + | + | − | − | 10 | No |
| 29c | + | ND | ND | ND | ND | − | ND | ND | No |
| 30 | + | + | + | − | + | − | − | 9 | No |
| 31 | + | ND | ND | ND | ND | − | − | 0 | No |
| 32 | + | − | + | − | − | − | − | 8 | No |
| 33 | + | ND | ND | ND | ND | − | ND | 13 | No |
| 34 | + | + | + | + | +e | − | + | 6 | Yes (BT) |
| 35 | + | + | + | + | +e | − | + | 0 | Yes (BT) |
| 36c | + | ND | ND | ND | ND | − | ND | ND | No |
| 37 | + | − | − | − | − | − | ND | 10 | No |
| 38 | + | ND | ND | ND | ND | − | ND | 10 | No |
| 39 | − | − | − | − | +e | + | + | 13 | Yes (I) |
| 40 | − | − | − | − | − | + | ND | 9 | No |
| 41c | − | ND | ND | ND | ND | + | ND | ND | No |
Criteria for PCR in slit-skin smears: PCR was performed in individuals positive for both ELISA and PCR or with two positive ELISA results.
Clinical manifestation: T, tuberculoid leprosy; BT, borderline-tuberculoid leprosy; I, indeterminate leprosy.
Blood donors who did not return for analysis.
ND, test not done due to participant's decision.
Disease onset.
The Mitsuda test involves an intradermal injection of a heat-killed M. leprae suspension. As the cell-mediated immunity is closely related to the clinical manifestation of leprosy, the response elicited against M. leprae antigens can be visually observed 28 days later by the formation of a nodular epithelioid granuloma at the site of injection. Multibacillary (MB) patients often do not show any reaction, and at the opposite end of the spectrum, paucibacillary (PB) patients usually display strong positive reaction. For healthy individuals, a positive response to the Mitsuda test is associated with greater chance of protection against disease.
FIG 1.
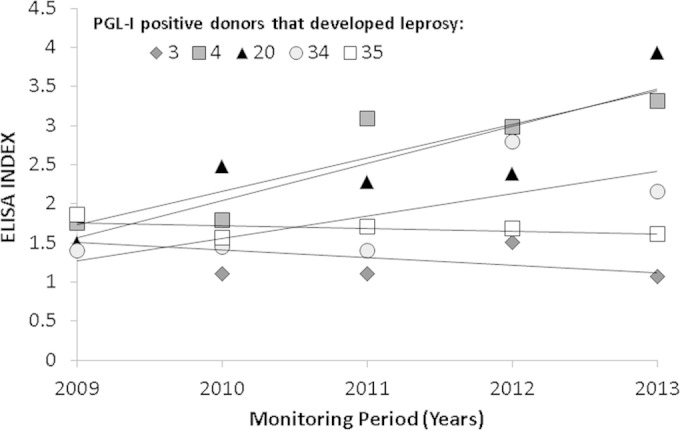
Anti-PGL-1 serology (ELISA index [EI]) behavior during follow-up of 5 blood donors who developed leprosy and presented seropositivity in 2009. The antibody titers were expressed as the EI according to the following formula: EI = ODsample/ODcutoff, where OD is optical density, as described previously (4). EI values of >1.1 were considered positive.
During the 5-year follow-up period, we performed an extensive search in the database of the notified cases of leprosy in Brazil (National System of Diseases Notifications, Ministry of Health) for donors who presented negative results in the initial evaluation. We identified only one donor who was diagnosed with leprosy among those with negative results (1/966 [0.1%]).
The test performance results for the ELISA anti-PGL-1 were sensitivity of 0.83 (95% confidence interval [CI], 0.36 to 0. 97), specificity of 0.97 (95% CI, 0.95 to 0.98), positive likelihood ratio of 25.20 (95% CI, 15.43 to 41.16), and odds ratio of 146.21 (95% CI, 16.61 to 1,286.9; P < 0.0001). The test performance results for PCR in blood samples were sensitivity of 0.5 (95% CI, 0.08 to 0.91), specificity of 0.998 (95%CI, 0.993 to 0.999), positive likelihood ratio of 242.0 (95% CI, 34.12 to 1,716.3), and odds ratio of 482.5 (95% CI, 21.74 to 10,707.02; P < 0.0001).
The seropositivity observed in our study was slightly higher than that observed in a recent study sampling healthy controls (1/35), which assessed serum samples for antibodies against a PGL-1 synthetic antigen (ND-O-BSA) (6). It is important to emphasize that the case detection rate in the Uberlandia region was 10-fold higher than that in Yunnan province, and the absolute number of new leprosy cases detected in Brazil was 30-fold higher than the number detected in China (1). Additionally, our blood donor population was more representative and does not present any specific subgroups, such as health care professionals or food handlers (6). Discrepancies may also be the result of our larger sampling. The same study did not report PCR positive results among the healthy controls, although household contacts presented a PCR positivity of 6.25% (6/96) (6). Not surprising, in addition to living in the same dwelling, household contacts often share similar conditions of nutrition, social status, hygiene, and, in some cases, consanguinity.
Compared with the prevalence of other diseases with mandatory notification at blood centers, our findings presented rates similar to those observed for hepatitis C virus (HCV) and hepatitis B virus (HBV) and higher than the rates observed for human immunodeficiency virus (HIV) and Chagas disease (10).
Blood donors are usually individuals with above-average health, and, as expected, most of those exposed to M. leprae do not develop disease. Once M. leprae has successfully entered the bloodstream and before the appearance of leprosy signs or symptoms, it remains a subclinical infection, which can spontaneously heal or progress to disease. In this period, it is plausible that these healthy carriers play a role in the chain of transmission and contribute to the maintenance of bacillary burden in regions where leprosy is endemic (3).
Potentially infective viable bacilli have been isolated from peripheral blood samples of untreated leprosy patients (11), and our findings draw attention to the potential transmission of bacilli via transfusions. Although probably uncommon, blood transmission should be considered, particularly when considering that transfusion recipients are almost always hospitalized individuals who require great care and are vulnerable to infections.
There are no reports of M. leprae blood transmission, but there are several reports of leprosy manifestation in individuals after they have undergone organ transplantation (12–18). However, these studies did not investigate the origin and quality of the blood received by these patients during the surgical procedure nor the origin of the transplanted organs, i.e., whether the donors were from regions where leprosy is endemic.
Our findings emphasize the importance of serological and DNA-based techniques for the assessment and confirmation of diagnosis in suspected and early cases of leprosy. The anti-PGL-1 detection was the trigger for the investigation of skin smear samples for the presence of M. leprae DNA by PCR. This has led to early diagnosis and treatment, thus preventing nerve damage and interrupting the transmission. Diagnosis was determined by a committee of medical specialists for leprosy based on clinical and laboratory evaluations.
We advise that in countries where leprosy remains a public health problem, these tests be performed in the screening of all samples collected at blood banks, particularly in regions where the disease is highly endemic. The success of a leprosy control program must be supported by prevention, early diagnosis, treatment, and interruption of transmission. Hence, we underscore our previous recommendation for the adoption of chemoprophylaxis as a disease prevention strategy and to promote clearance of bacilli in close contacts of leprosy patients and in those with asymptomatic infection, consequently disrupting transmission of the disease (3, 5, 7).
ACKNOWLEDGMENTS
We are grateful to the staffs of the National Reference Center for Sanitary Dermatology and Leprosy and the Hemominas – Regional Blood Bank of Uberlandia, Minas Gerais, Brazil.
This study was supported by grants from the Foundation for Research Support of the State of Minas Gerais (FAPEMIG), Brazilian National Council for Scientific and Technological Development (CNPq), Brazilian Coordination for Improvement of Higher Education Personnel (CAPES), and National Fund for Health/Brazilian Ministry of Health.
We declare that we have no conflicts of interest.
The research protocol was approved by two independent research ethics committees at the Federal University of Uberlandia (261/05) and the Hemominas Foundation (127/05). All participants were informed about the research, voluntarily agreed to take part in this study without any financial incentive, and signed consent forms. Individuals had the right to refuse or stop participation at any time without explanation.
I.M.B.G., S.A., and L.R.G. conceived and designed the study. A.B. and P.H.R.D.P. implemented the study and carried out the clinical assessment. I.M.B.G., S.A., and L.R.G. carried out the analysis, data interpretation, and writing of the manuscript. All authors read and approved the final manuscript.
REFERENCES
- 1.WHO. 2014. Global leprosy update, 2013: reducing disease burden. Wkly Epidemiol Rec 89:389–400. [PubMed] [Google Scholar]
- 2.Matsuoka M, Zhang L, Budiawan T, Saeki K, Izumi S. 2004. Genotyping of Mycobacterium leprae on the basis of the polymorphism of TTC repeats for analysis of leprosy transmission. J Clin Microbiol 42:741–745. doi: 10.1128/JCM.42.2.741-745.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Araujo S, Lobato J, Reis Ede M, Souza DO, Goncalves MA, Costa AV, Goulart LR, Goulart IMB. 2012. Unveiling healthy carriers and subclinical infections among household contacts of leprosy patients who play potential roles in the disease chain of transmission. Mem Inst Oswaldo Cruz 107(Suppl 1):55–59. doi: 10.1590/S0074-02762012000900010. [DOI] [PubMed] [Google Scholar]
- 4.Lobato J, Costa MP, Reis Ede M, Goncalves MA, Spencer JS, Brennan PJ, Goulart LR, Goulart IMB. 2011. Comparison of three immunological tests for leprosy diagnosis and detection of subclinical infection. Lepr Rev 82:389–401. [PubMed] [Google Scholar]
- 5.Goulart IMB, Bernardes Souza DO, Marques CR, Pimenta VL, Goncalves MA, Goulart LR. 2008. Risk and protective factors for leprosy development determined by epidemiological surveillance of household contacts. Clin Vaccine Immunol 15:101–105. doi: 10.1128/CVI.00372-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wen Y, Xing Y, Yuan LC, Liu J, Zhang Y, Li HY. 2013. Whole-blood nested-PCR amplification of M. leprae-specific DNA for early diagnosis of leprosy. Am J Trop Med Hyg 88:918–922. doi: 10.4269/ajtmh.11-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reis EM, Araujo S, Lobato J, Neves AF, Costa AV, Goncalves MA, Goulart LR, Goulart IMB. 2014. Mycobacterium leprae DNA in peripheral blood may indicate a bacilli migration route and high-risk for leprosy onset. Clin Microbiol Infect 20:447–452. doi: 10.1111/1469-0691.12349. [DOI] [PubMed] [Google Scholar]
- 8.Truman RW, Andrews PK, Robbins NY, Adams LB, Krahenbuhl JL, Gillis TP. 2008. Enumeration of Mycobacterium leprae using real-time PCR. PLoS Negl Trop Dis 2:e328. doi: 10.1371/journal.pntd.0000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goulart IMB, Cardoso AM, Santos MS, Goncalves MA, Pereira JE, Goulart LR. 2007. Detection of Mycobacterium leprae DNA in skin lesions of leprosy patients by PCR may be affected by amplicon size. Arch Dermatol Res 299:267–271. doi: 10.1007/s00403-007-0758-5. [DOI] [PubMed] [Google Scholar]
- 10.Borelli SD, Mazzola JC, Matta AC, Takemoto AY, Bertoli M. 2013. Blood discard rate and the prevalence of infectious and contagious diseases in blood donors from provincial towns of the state of Parana, Brazil. Rev Bras Hematol Hemoter 35:395–399. doi: 10.5581/1516-8484.20130126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lane JE, Balagon MV, Dela Cruz EC, Abalos RM, Tan EV, Cellona RV, Sadaya PG, Walsh GP, Walsh DS. 2006. Mycobacterium leprae in untreated lepromatous leprosy: more than skin deep. Clin Exp Dermatol 31:469–470. doi: 10.1111/j.1365-2230.2006.02075.x. [DOI] [PubMed] [Google Scholar]
- 12.Modi K, Mancini M, Joyce MP. 2003. Lepromatous leprosy in a heart transplant recipient. Am J Transplant 3:1600–1603. doi: 10.1046/j.1600-6135.2003.00284.x. [DOI] [PubMed] [Google Scholar]
- 13.Launius BK, Brown PA, Cush E, Mancini MC. 2004. A case study in Hansen's disease acquired after heart transplant. Crit Care Nurs Q 27:87–91. doi: 10.1097/00002727-200401000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Shih HC, Hung TW, Lian JD, Tsao SM, Hsieh NK, Yang JH. 2005. Leprosy in a renal transplant recipient: a case report and literature review. J Dermatol 32:661–666. doi: 10.1111/j.1346-8138.2005.tb00818.x. [DOI] [PubMed] [Google Scholar]
- 15.Gasink LB, Seymour C, Blumberg EA, Goldberg LR, Fishman NO. 2006. An uncommon presentation of an uncommon disease: leprosy in a heart transplant recipient. J Heart Lung Transplant 25:854–856. doi: 10.1016/j.healun.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 16.Guditi S, Ram R, Ismal KM, Sahay M, Dakshinamurthy KV, Girish N, Prasad N. 2009. Leprosy in a renal transplant recipient: review of the literature. Transpl Infect Dis 11:557–562. doi: 10.1111/j.1399-3062.2009.00428.x. [DOI] [PubMed] [Google Scholar]
- 17.Trindade MA, Palermo ML, Pagliari C, Valente N, Naafs B, Massarollo PC, D'Albuquerque LA, Benard G. 2011. Leprosy in transplant recipients: report of a case after liver transplantation and review of the literature. Transpl Infect Dis 13:63–69. doi: 10.1111/j.1399-3062.2010.00549.x. [DOI] [PubMed] [Google Scholar]
- 18.Ardalan M, Ghaffari A, Ghabili K, Shoja MM. 2011. Lepromatous leprosy in a kidney transplant recipient: a case report. Exp Clin Transplant 9:203–206. [PubMed] [Google Scholar]


