Abstract
Giardia duodenalis is a major cause of infectious gastroenteritis worldwide, and it is diversified into eight genetic assemblages (A to H), which are distinguishable only by molecular typing. There is some evidence that the assemblages infecting humans (assemblages A and B) may have different transmission routes, but systematically acquired data, combining epidemiological and molecular findings, are required. We undertook a case-control study with Giardia genotyping in North West England, to determine general and parasite assemblage-specific risk factors. For people without a history of foreign travel, swimming in swimming pools and changing diapers were the most important risk factors for the disease. People infected with assemblage B reported a greater number of symptoms and higher frequencies of vomiting, abdominal pain, swollen stomach, and loss of appetite, compared with people infected with assemblage A. More importantly, keeping a dog was associated only with assemblage A infections, suggesting the presence of a potential zoonotic reservoir for this assemblage. This is the first case-control study to combine epidemiological data with Giardia genotyping, and it shows the importance of integrating these two levels of information for better understanding of the epidemiology of this pathogen.
INTRODUCTION
Giardia duodenalis is a leading but neglected cause of infectious gastroenteritis worldwide (1); it is transmitted either through contaminated water or food or from person to person. In the United Kingdom, giardiasis has received little public health attention, probably because it is widely assumed to be an infection predominantly acquired through foreign travel. Between 2000 and 2009, a total of 33,317 Giardia cases were reported in England and Wales (2). However, the Second Study of Infectious Intestinal Disease in the community (IID2 Study) estimated 52,434 cases of “reported” disease in 2008 and 2009 in the United Kingdom (3), suggesting a greatly underestimated disease burden. In the United Kingdom, not all community diarrheal samples are routinely tested for Giardia, with testing being based on criteria (most commonly a foreign travel history or infancy) that vary between laboratories (4). Following the introduction of a Giardia immunoassay for testing of all community diarrheal samples in Central Lancashire, the rates of disease significantly increased (from 10.1 cases/100,000 population in 2002 to 33.6 cases/100,000 population in 2006), compared to the rates reported for an area in which samples were selectively screened using microscopy (4).
Little is known about Giardia infections in the United Kingdom, particularly those not associated with foreign travel. The risk factors for sporadic giardiasis in this country have been investigated previously in only three case-control studies. Traveling to developing countries, camping, and caravanning (traveling in a trailer) were confirmed to be independent risk factors for the disease (5) but, for patients without a history of foreign travel, giardiasis was significantly associated with being exposed to recreational fresh water, drinking additional glasses of tap water, and eating lettuce (6). A study in a rural district of East Anglia reported a significant association between the risk of giardiasis and having contact with pets or farm animals (7).
Previous case-control studies did not take into account the genetic diversity of G. duodenalis. In fact, this parasite is diversified into eight morphologically identical genetic assemblages (A to H) or cryptic species, which infect different hosts (8). The parasite assemblages are currently distinguishable only by PCR and sequencing of appropriate genes. Humans are infected with assemblages A and B, with the latter being predominant worldwide (8). Three major subassemblages have been identified within assemblage A, i.e., AI, AII, and AIII. According to molecular epidemiological surveys, humans are primarily infected with AII parasites, whereas animals (including pets, livestock, and wildlife) are infected mostly by parasites belonging to subassemblages AI and AIII (9).
There is a lack of epidemiological evidence to show whether the two Giardia assemblages infecting humans are associated with different transmission routes. To date, only one study, from a community in Malaysia, has suggested the presence of assemblage-specific risk factors, with the results indicating infection with assemblage A to be associated with contact with pets in the household and infection with assemblage B to be associated with the presence of children and other family members with Giardia in the household (10). However, more data are needed to verify whether these epidemiological differences can be confirmed in a country with low levels of endemicity, such as the United Kingdom. We report the results of the first case-control study with Giardia genotyping conducted in the North West region of England, which was designed to study parasite assemblage-specific risk factors.
MATERIALS AND METHODS
General study design, hypotheses, and sample size.
The study involved data collection within North West England, namely, in Central and East Lancashire and Central Manchester, from February 2012 to August 2013. Ethical approval for the study was obtained through the National Research Ethics Service, Committee North West. The major hypotheses that were tested in this study were that sporadic giardiasis is associated with traveling abroad, swimming in a swimming pool, having contact with animals (pets, livestock or wildlife), drinking unboiled water from public supplies or the environment, and having contact with children or other people with diarrhea in the household. The hypothesis that the two parasite assemblages are associated with different transmission routes was also tested.
The study sample size was estimated using generalized linear interactive modeling (GLIM) (11). Between 90 (minimum) and 140 (maximum) cases would need to be recruited to detect an odds ratio (OR) of at least 2 for a range of factors with different prevalences, with 80% power and a 5% significance level, with a case/control ratio of 1:3.
Case patient and control subject recruitment and data collection.
Case patients were defined as residents within the study areas who presented with gastroenteritis and a Giardia infection identified using the fecal antigen detection method, as described in a previous study (4). The presence of Cryptosporidium, Salmonella, Shigella, Campylobacter, Escherichia coli O157, or Vibrio spp. was excluded. Control subjects were defined as residents within the study areas who had not experienced diarrhea in the previous 2 weeks.
All laboratory-confirmed cases were reported by the hospitals' microbiology departments to their respective area health protection units, which then transmitted the details about the patient's gender and age to the Cumbria and Lancashire and Greater Manchester health protection units (for the Central and East Lancashire and Central Manchester areas, respectively) for control selection. Control subjects were selected at random, using the randomize function in Microsoft Access (Microsoft Corp., Redmond, WA), from the respective areas' general practitioner (GP) lists by the information departments holding the GP registration databases. Controls were frequency-matched to cases according to gender and age (within the age ranges of 0 to 4, 5 to 14, 15 to 44, 45 to 64, and ≥65 years). In order to take into account potential dropouts, five controls were recruited for each case.
Participants were sent the study questionnaire by mail, with an invitation letter, an information leaflet, and a consent form. Both the study questionnaire and the information leaflet were reviewed by the Liverpool Medicines for Children Research Network, in order to make it comprehensible and child-friendly. The study questionnaire included questions about various sociodemographic, occupational, and self-reported clinical details and a range of exposures experienced in the 3 weeks prior to either illness onset (for cases) or completion of the questionnaire (for controls). Exposures included foreign travel, outdoor recreational activities, water and food consumption, pet ownership, and contact with animals, as well as household composition. Cases who did not respond to the initial invitation within 1 week were approached via telephone or via personal contact by either an environmental health officer (Central and East Lancashire) or a health protection practitioner (Central Manchester); if they did not respond after 2 weeks, then they were not contacted again. Controls who did not respond to the initial invitation within 2 weeks were sent a reminder letter and a new copy of the questionnaire, with accompanying documents. Controls who did not reply to the reminder within 2 weeks were not contacted again.
Giardia genotyping.
Fecal specimens from the case patients were sent to the Department of Infection Biology at the University of Liverpool for parasite genotyping. Whole fecal DNA was extracted from the case specimens using a commercial kit (QIAamp DNA Stool minikit; Qiagen Ltd.), according to the manufacturer's instructions.
Four parasite genes, namely, the small-subunit rRNA (12), β-giardin, triose phosphate isomerase, and glutamate dehydrogenase genes (13), were amplified to ensure typing success. PCR products were purified (QIAquick PCR purification kit; Qiagen Ltd.) and sequenced in both directions using an Applied Biosystems 3730 DNA analyzer, at the Core Genomic Facility, Medical School, Sheffield University (United Kingdom). The sequences obtained were then edited (BioEdit version 7.0.9), and compared to homologous sequences (>99% similarity) in GenBank using BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi), to determine the Giardia assemblage.
Data analysis.
Questionnaire information was manually entered into a database created with EpiData 3.1 (EpiData Association, Odense, Denmark). Data were entered at both Liverpool University and the Central Lancashire Health Protection Unit and were compared for detection of data entry mistakes, which were then corrected. No multiple imputation of missing data was performed.
Statistical analyses were performed using IBM SPSS Statistics 20. Variables were first assessed singly as potential risk factors by calculation of their odds ratio (OR) estimates and 95% confidence intervals (CIs). Cross-tabulations were produced, and two-sided Pearson's χ2 test (or Fisher's exact test for sparse data) was used to test associations. Categorical variables with more than two categories and dose-response variables were analyzed using univariable logistic regression. The Mann-Whitney U test was used for continuous variables. P values of less than 5% were considered statistically significant.
Multivariable binary logistic regression modeling was used to determine which variables were independently associated with symptomatic giardiasis. Variables returning P values of ≤0.2 in univariable analysis were selected for inclusion in the models and were entered individually, and the importance of each factor was assessed by its effect on the overall model fit, using likelihood ratio tests. Variables without a statistically significant effect on the model fit were dropped. The only variables that were retained in the final model regardless of their significance were gender and age, and interaction terms for these two variables were also tested. Only cases and controls with complete variable data (e.g., without missing data for any of the variables retained in the model) were included in the final models. Confounding or effect-measure modifications by ethnicity, type of living area (city, town, or village), study area (Central or East Lancashire or Central Manchester), and season for the variables retained in the final model were also tested. Separate models were built for general risk factors (including all records), indigenous risk factors (excluding participants with a history of foreign travel during the exposure window), and Giardia assemblage-specific risk factors (two models, including either assemblage A or assemblage B cases).
RESULTS
Study groups.
Overall, 236 notified case patients and 1,180 control subjects were invited to take part in the study; 123 case patients (response rate of 52.1%) and 253 control subjects (response rate of 21.4%) returned the questionnaire (Fig. 1). Five cases were excluded either because they were asymptomatic or they were mistakenly sent a wrong questionnaire, and 27 controls were excluded because they had experienced diarrhea in the 2 weeks prior to completing the questionnaire. The proportions of male subjects were similar, i.e., 55.1% of cases and 51.3% of controls (P = 0.508). The majority of cases were adults (15 to 44 years, 37.3%; 45 to 64 years, 22.9%; ≥65 years, 21.2%; 0 to 4 years, 13.5%; 5 to 14 years, 5.1%), and the age distributions did not differ between cases and controls (P = 0.272).
FIG 1.
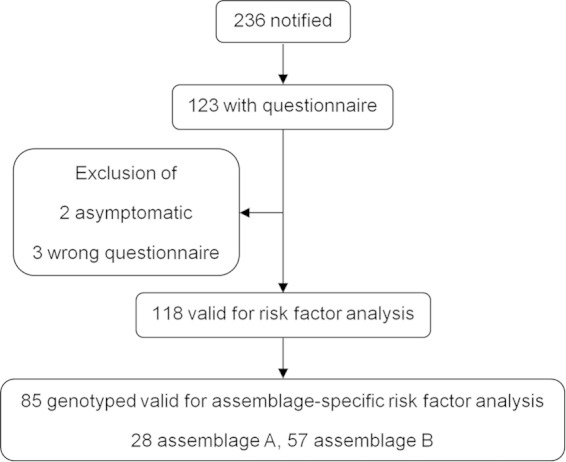
Case-control study case recruitment and inclusion in the analysis.
A total of 192 cases had a fecal specimen available for DNA extraction, and 150 (78%) were successfully genotyped for at least one of the loci tested; 46 (31%) were infected with assemblage A, 101 (67%) with assemblage B, and three (2%) with both. Twenty-eight cases with assemblage A and 57 with assemblage B also returned the study questionnaire.
Risk factors for giardiasis.
The results from the univariable analyses for significant variables, including all participants and only participants without a history of foreign travel, are reported in Tables 1 and 2, respectively. Nonsignificant variables are reported in Tables S1 and S2 in the supplemental material. The final multivariable models for the general and indigenous risk factors for giardiasis are shown in Tables 3 and 4, respectively.
TABLE 1.
Significant (P < 0.05) variables in univariable analyses for all eligible 118 cases and 226 controls
| Variable | No. (%)a |
OR (95% CI)b | P | |
|---|---|---|---|---|
| Case | Control | |||
| Traveling abroad (outside UK) | 29 (25.2) | 19 (8.4) | 3.67 (1.95–6.90) | <0.001 |
| Traveling abroad to at-risk destination | <0.001 | |||
| No travel abroad | 86 (74.8) | 207 (91.6) | Reference | |
| Travel abroad to non-at-risk destinations | 10 (8.7) | 17 (7.5) | 1.41 (0.62–3.22) | |
| Travel abroad to at-risk destinationsc | 19 (16.5) | 2 (0.9) | 22.87 (5.21–100.31) | |
| Swimming in swimming pool | 40 (34.8) | 49 (21.7) | 1.93 (1.17–3.17) | 0.009 |
| Doing gardening | 28 (26.2) | 105 (48.4) | 0.38 (0.23–0.63) | <0.001 |
| Touching any pet (own or other people's) | 52 (48.6) | 130 (62.8) | 0.56 (0.35–0.90) | 0.016 |
| Visiting or working at wildlife park or zoo | 10 (8.6) | 8 (3.6) | 2.56 (0.98–6.67) | 0.048 |
| Eating salads or raw vegetables | 92 (81.4) | 201 (91.4) | 0.41 (0.21–0.81) | 0.008 |
| No. of times per week eating salads or raw vegetables | 0.038 | |||
| 0 | 22 (19.8) | 34 (15.6) | Reference | |
| 1 or 2 | 41 (36.9) | 71 (32.6) | 0.89 (0.46–1.73) | |
| 3 or 4 | 28 (25.2) | 54 (24.8) | 0.80 (0.39–1.62) | |
| ≥5 | 20 (18) | 59 (27.1) | 0.52 (0.25–1.10) | |
| No. of children (<16 yr) in household | 0.013 | |||
| 0 | 60 (51.7) | 134 (59.3) | Reference | |
| 1 | 19 (16.4) | 43 (19) | 0.99 (0.53–1.83) | |
| 2 | 19 (16.4) | 34 (15) | 1.25 (0.66–2.36) | |
| 3 | 10 (8.6) | 11 (4.9) | 2.03 (0.82–5.04) | |
| ≥4 | 8 (6.9) | 4 (1.8) | 4.47 (1.29–15.41) | |
Percentages refer to the proportions among participants who answered the question.
OR, odds ratio; CI, confidence interval.
Asia, Africa, Central America, or South America.
TABLE 2.
Significant (P < 0.05) variables in univariable analyses for subset of 86 cases and 207 controls who did not report traveling abroad
| Variable | No. (%)a |
OR (95% CI)b | P | |
|---|---|---|---|---|
| Case | Control | |||
| Swimming in swimming pool | 28 (32.6) | 39 (18.8) | 2.08 (1.18–3.68) | 0.011 |
| Doing gardening | 25 (30.9) | 93 (46.7) | 0.51 (0.29–0.88) | 0.015 |
| Eating salads or raw vegetables | 67 (80.7) | 183 (91) | 0.41 (0.20–0.85) | 0.015 |
| No. of times per week eating salads or raw vegetables | 0.026 | |||
| 0 | 18 (22.2) | 32 (16.1) | Reference | |
| 1 or 2 | 31 (38.3) | 68 (34.2) | 0.81 (0.40–1.66) | |
| 3 or 4 | 19 (23.5) | 51 (25.6) | 0.66 (0.30–1.45) | |
| ≥5 | 13 (16) | 48 (24.1) | 0.48 (0.21–1.12) | |
| No. of children (<16 yr) in household | 0.014 | |||
| 0 | 42 (48.8) | 121 (58.5) | Reference | |
| 1 | 16 (18.6) | 40 (19.3) | 1.15 (0.58–2.27) | |
| 2 | 14 (16.3) | 31 (15) | 1.30 (0.63–2.68) | |
| 3 | 7 (8.1) | 11 (5.3) | 1.83 (0.67–5.04) | |
| ≥4 | 7 (8.1) | 4 (1.9) | 5.04 (1.40–18.09) | |
| Children at nursery/playgroup | 0.038 | |||
| No children | 42 (49.4) | 121 (58.5) | Reference | |
| Children not at nursery/playgroup | 15 (17.6) | 47 (22.7) | 0.92 (0.47–1.81) | |
| At least one child at nursery/playgroup | 28 (32.9) | 39 (18.8) | 2.07 (1.14–3.76) | |
| Changing diapers | 0.029 | |||
| No children or children not in diapers | 61 (71.8) | 170 (82.9) | Reference | |
| Children in diapers but not changing diapers | 6 (7.1) | 16 (7.8) | 1.04 (0.39–2.79) | |
| Children in diapers and changing diapers | 18 (21.2) | 19 (9.3) | 2.64 (1.30–5.36) | |
Percentages refer to the proportions among participants who answered the question.
OR, odds ratio; CI, confidence interval.
TABLE 3.
Final multivariable model for giardiasis, based on data for 99 cases and 204 controlsa
| Variables included | Adjusted OR (95% CI)b | P |
|---|---|---|
| Traveling abroad (outside UK) | 9.59 (2.75–33.51) | 0.001 |
| Swimming in swimming pool | 2.79 (1.40–5.54) | 0.002 |
| Doing gardening | 0.21 (0.09–0.54) | 0.001 |
| Weekly frequency of raw fruit consumption (per event) | 0.85 (0.78–0.93) | 0.001 |
| Eating salad or raw vegetables | 0.35 (0.16–0.79) | 0.009 |
| Male | 0.78 (0.38–1.58) | 0.539 |
| Age | 0.179 | |
| 0–4 yr | Reference | |
| 5–14 yr | 0.74 (0.18–3.03) | |
| 15–44 yr | 2.32 (0.88–6.12) | |
| 45–64 yr | 1.22 (0.45–3.32) | |
| ≥65 yr | 1.79 (0.59–5.42) |
The numbers of cases and controls refer to those with complete variable data (e.g., without missing data for any of the variables retained in the model). Model statistics were as follows: model χ212 = 60.49, P < 0.001; Nagelkerke R2 = 0.25; Hosmer-Lemeshow χ28 = 13.24, P = 0.104.
OR, odds ratio; CI, confidence interval.
TABLE 4.
Final multivariable model for indigenous giardiasis, based on data for 69 cases and 172 controls without history of foreign travela
| Variables included | Adjusted OR (95% CI)b | P |
|---|---|---|
| Swimming in swimming pool | 2.67 (1.14–6.25) | 0.023 |
| Changing diapers | ||
| No children or children not in diapers | Reference | 0.051 |
| Children in diapers but not changing diapers | 1.14 (0.22–6.07) | 0.874 |
| Changing diapers | 3.38 (1.25–9.16) | 0.016 |
| Reporting irritable bowel syndrome | 3.66 (1.18–11.37) | 0.025 |
| Drinking unboiled water straight from tap | 8.17 (1.45–46.03) | 0.017 |
| Weekly frequency of raw fruit consumption (per event) | 0.77 (0.68–0.87) | <0.001 |
| Practicing field sports | 0.21 (0.05–0.89) | 0.035 |
| Visiting animal premises other than farm, wildlife park, or zoo (e.g., friends' or relatives' houses) | 0.10 (0.01–0.89) | 0.040 |
| Male | 1.90 (0.98–3.69) | 0.057 |
| Age | 0.813 | |
| 0–4 yr | Reference | |
| 5–14 yr | 0.60 (0.08–4.23) | |
| 15–44 yr | 0.84 (0.21–3.37) | |
| 45–64 yr | 0.5 (0.13–2.33) | |
| ≥65 yr | 0.60 (0.13–2.82) |
The numbers of cases and controls refer to those with complete variable data (e.g., without missing data for any of the variables retained in the model). Model statistics were as follows: model χ213 = 55.18, P < 0.001; Nagelkerke R2 = 0.293; Hosmer-Lemeshow χ28 = 5.66, P = 0.686.
OR, odds ratio; CI, confidence interval.
Traveling abroad and swimming in a swimming pool were both significantly associated with increased risk for giardiasis. Conversely, negative associations with the disease were found for doing gardening, eating salad or raw vegetables during the exposure window, and the weekly frequency of raw fruit consumption. By considering only the participants without a history of travel abroad, multivariable analysis confirmed that giardiasis was positively associated with swimming in a swimming pool, as well as with changing diapers. Positive associations with disease were also seen for reporting irritable bowel syndrome (IBS) and drinking unboiled water straight from the tap. The weekly frequency of consumption of raw fruit, practicing field sports, and visiting animal premises other than a farm, wildlife park, or zoo were all negatively associated with giardiasis.
Sociodemographic characteristics, clinical outcomes, and risk factors according to assemblage.
Among 147 cases with single-assemblage infections, there was no difference in the gender distributions for the two assemblages (Pearson's χ2 test, P = 0.952). The median age of assemblage A cases (47.5 years [range, 1 to 94 years]) was higher than that of assemblage B cases (37 years [range, 1 to 79 years]), but the difference was only marginally significant (Mann-Whitney U test, P = 0.055) (Fig. 2). Among the 85 genotyped cases with study questionnaires, cases with assemblage B significantly more frequently reported vomiting (64%, compared with 36.4% for patients with assemblage A; P = 0.030), abdominal pain (85% versus 64%; P = 0.041), swollen stomach (65% versus 33.3%; P = 0.012), and loss of appetite (83% versus 58%; P = 0.020), with a greater number of symptoms (assemblage B, median of 7 symptoms [range, 1 to 9 symptoms]; assemblage A, median of 5 symptoms [range, 1 to 9 symptoms]) (Mann-Whitney U test, P = 0.001).
FIG 2.
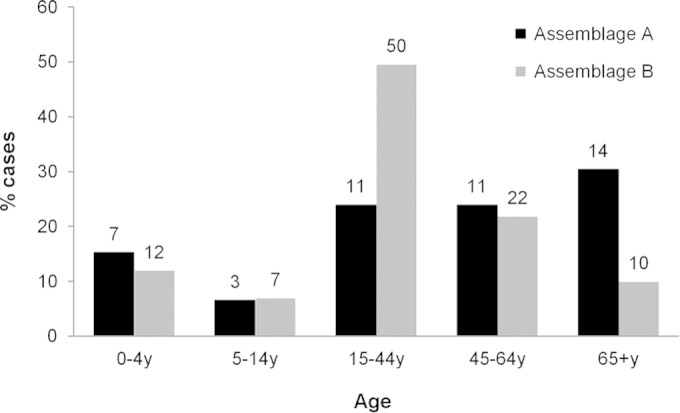
Age distributions of Giardia assemblages A and B among 147 cases who were successfully genotyped and had single-assemblage infections. The raw numbers of cases are reported above the bars.
The variables that were significantly associated with assemblage A or B infection in univariable analysis are listed in Table 5, and the multivariable models for the Giardia assemblage-specific risk factors are shown in Table 6. In multivariable analysis, keeping a dog and swimming in a swimming pool were confirmed to be positively associated with assemblage A infection, whereas walking in the countryside showed a negative association. In contrast, assemblage B infection was associated with taking medicines for indigestion, using a Jacuzzi or hot tub, and reporting the tap water being discolored.
TABLE 5.
Variables significantly associated (P < 0.05) with assemblage A or B infections in univariable analyses
| Assemblage and variable | No. (%)a |
OR (95% CI)b | P | |
|---|---|---|---|---|
| Case | Control | |||
| Assemblage A | ||||
| Keeping dog | 11 (40.7) | 49 (21.8) | 2.47 (1.08–5.66) | 0.029 |
| Swimming in swimming pool | 12 (44.4) | 49 (21.7) | 2.89 (1.27–6.58) | 0.009 |
| Walking in countryside | 5 (20) | 93 (42.1) | 0.34 (0.12–0.95) | 0.033 |
| Assemblage B | ||||
| Age | 0.017 | |||
| 0–4 yr | 8 (14) | 26 (11.5) | Reference | |
| 5–14 yr | 2 (3.5) | 12 (5.3) | 0.54 (0.10–2.94) | |
| 15–44 yr | 28 (49.1) | 62 (27.4) | 1.47 (0.59–3.64) | |
| 45–64 yr | 13 (22.8) | 75 (33.2) | 0.56 (0.21–1.51) | |
| ≥65 yr | 6 (10.5) | 51 (22.6) | 0.38 (0.12–1.22) | |
| Keeping any pet | 17 (30.4) | 106 (46.9) | 0.49 (0.26–0.92) | 0.025 |
| Touching any pet | 23 (44.2) | 130 (62.8) | 0.47 0.25–0.87 | 0.015 |
| Visiting or working at zoo or wildlife park | 6 (10.7) | 8 (3.6) | 3.25 (1.08–9.80) | 0.039 |
| Traveling abroadc | 12 (21.4) | 19 (8.4) | 2.97 (1.34–6.56) | 0.005 |
| Swimming in swimming pool | 21 (38.2) | 49 (21.7) | 2.23 (1.19–4.19) | 0.011 |
| Using Jacuzzi or hot tub | 12 (24.5) | 14 (6.5) | 4.70 (2.02–10.97) | <0.001 |
| Doing gardening | 13 (25.5) | 105 (48.4) | 0.36 (0.18–0.72) | 0.003 |
| Reporting water from tap with unusual taste | 4 (7.3) | 3 (1.4) | 5.70 (1.24–26.26) | 0.031 |
| Reporting water from tap being discolored | 5 (9.1) | 4 (1.8) | 5.42 (1.41–20.93) | 0.018 |
| Children at nursery/playgroup | 0.002 | |||
| No children | 26 (48.1) | 134 (59.3) | Reference | |
| Children not at nursery/playgroup | 6 (11.1) | 50 (22.1) | 0.62 (0.24–1.59) | |
| At least one child at nursery/playgroup | 22 (40.7) | 42 (18.6) | 2.70 (1.39–5.25) | |
| Changing diapers | 0.034 | |||
| No children or children not in diapers | 38 (71.7) | 186 (83.4) | Reference | |
| Children in diapers but not changing diapers | 3 (5.7) | 17 (7.6) | 0.86 (0.24–3.09) | |
| Children in diapers and changing diapers | 12 (22.6) | 20 (9) | 2.93 (1.32–6.51) | |
| Another person with diarrhea in household | 11 (21.2) | 23 (10.9) | 2.19 (0.99–4.85) | 0.048 |
Percentages refer to the proportions among participants who answered the question.
OR, odds ratio; CI, confidence interval.
All assemblage B cases traveled to at-risk destinations (e.g., Asia, Africa, Central America, or South America).
TABLE 6.
Final multivariable models for Giardia assemblage A infections (23 cases and 220 controls) and assemblage B infections (44 cases and 213 controls)a
| Assemblage and variables included | Adjusted OR (95% CI)b | P |
|---|---|---|
| Assemblage Ac | ||
| Keeping dog | 3.57 (1.34–9.52) | 0.011 |
| Swimming in swimming pool | 4.36 (1.24–15.36) | 0.022 |
| Walking in countryside | 0.22 (0.07–0.69) | 0.010 |
| Traveling abroad (outside UK) | 2.12 (0.59–7.63) | 0.250 |
| Male | 1.30 (0.49–3.49) | 0.598 |
| Age | 0.662 | |
| 0–4 yr | Reference | |
| 5–14 yr | 1.32 (0.18–9.49) | |
| 15–44 yr | 0.80 (0.16–4.09) | |
| 45–64 yr | 0.99 (0.21–4.67) | |
| ≥65 yr | 2.20 (0.41–11.66) | |
| Assemblage Bd | ||
| Taking medicines for indigestion | 3.40 (1.46–7.90) | 0.005 |
| Using Jacuzzi or hot tub | 4.12 (1.46–11.67) | 0.008 |
| Reporting water from tap being discolored | 5.13 (0.84–31.35) | 0.077 |
| Children at nursery/playgroup | ||
| No children | Reference | 0.055 |
| Children not at nursery/playgroup | 0.49 (0.15–1.61) | 0.238 |
| At least one child at nursery/playgroup | 2.17 (0.80–5.92) | 0.129 |
| Traveling abroad (outside UK) | 2.42 (0.81–7.25) | 0.114 |
| Male | 1.21 (0.57–2.56) | 0.624 |
| Age | 0.045 | |
| 0–4 yr | Reference | |
| 5–14 yr | NAe | |
| 15–44 yr | 2.15 (0.59–7.81) | |
| 45–64 yr | 0.67 (0.15–2.89) | |
| ≥65 yr | 0.46 (0.08–2.70) |
The numbers of cases and controls refer to those with complete variable data (e.g., without missing data for any of the variables retained in the model).
OR, odds ratio; CI, confidence interval.
Model statistics were as follows: model χ212 = 23.82, P = 0.005; Nagelkerke R2 = 0.20; Hosmer-Lemeshow χ28 = 7.03, P = 0.533.
Model statistics were as follows: model χ211 = 48.79, P < 0.001; Nagelkerke R2 = 0.29; Hosmer-Lemeshow χ28 = 12.88, P = 0.116.
NA, not applicable; the odds ratio was not calculated because no variation was present in the data.
DISCUSSION
We have investigated for the first time the risk factors for sporadic giardiasis in North West England; through the integration of epidemiological and molecular information, we found that the two assemblages of Giardia infecting humans may differ in their preferential transmission routes. Although the presence of epidemiological differences for assemblages A and B has been hypothesized for a long time (8, 9), our study was the first to test this hypothesis using a case-control design in a developed country.
Overall, assemblage B cases were younger than assemblage A cases; this age-related pattern was due to a greater prevalence of assemblage B in adults 15 to 44 years of age and a peak of assemblage A in older people (≥65 years of age). The age prevalence of Giardia assemblages had not been thoroughly investigated previously. Although in a previous study from England (14) both assemblages peaked in adults and in children, assemblage B was more prevalent than assemblage A in children and adults in their thirties, whereas the opposite was seen in people in their forties or older. Whether this pattern is related to age-related differences in either the frequency of exposure to the assemblages or the development of immunity to one or the other requires further investigation.
Analysis of the clinical outcomes suggested that assemblage B causes more-severe illness than assemblage A. Assemblage-related differences in patients' clinical outcomes were suggested in several studies previously, but results were not consistent (8). Previously, assemblage B was found to be associated with higher frequencies of diarrhea, flatulence, and abdominal pain in Cuban children (15) and flatulence in Swedish children (16). If assemblage B causes more-severe illness, then people infected with this assemblage present more frequently to general practitioners, representing the majority of notified cases. This could also explain the greater prevalence of assemblage B infections, compared to assemblage A infections, reported for clinical patients from countries with low levels of endemicity. However, it is not possible to infer clinical differences between the parasite assemblages by comparing populations from countries with low versus high levels of endemicity, due to the different levels of exposure to the parasite and the potential development of immunity. More data from countries with low levels of endemicity are needed to verify whether assemblage A commonly occurs in people who are asymptomatic or presenting relatively mild illness.
Our most important finding was that, in our sample, dog ownership was significantly associated only with assemblage A infection. Our results are the first from a developed country setting, and they correspond to those from a previous study in Malaysia, where close contact with pet dogs and cats was identified as the only significant predictor of assemblage A infection (10). This finding confirms the zoonotic potential of this assemblage on an epidemiological level, a hypothesis supported by the fact that assemblage A is also the most frequent non-host-specific assemblage and is common in pets and wildlife (8). However, all of the case patients in our sample were infected with subassemblage AII and not with subassemblage AI. Although subassemblage AI is usually the most frequently reported for dogs, reports of the presence of AII parasites in these pets are not uncommon (9). Due to logistical constraints, we could not collect fecal samples from the pets of the case patients; therefore, we could not confirm the presence of a shared Giardia genotype between the dogs and their owners, to confirm zoonotic transmission. More data on the subassemblage diversity of Giardia parasites in dogs and other owned pets in the United Kingdom are needed. Due to the reduced sample size and the dangers of subgroup analysis performed with small numbers, we were not able to determine whether the different age distributions observed for the two assemblages were due to differences in underlying risk factors. Larger studies with more genotyped cases are needed to elucidate age-specific exposures, which may shed more light on the epidemiological differences between the two parasite assemblages.
Exposures related to contact with young children (including changing diapers) or other people with diarrhea were consistently and exclusively associated with assemblage B. Overall, our results suggest that children may be a major reservoir for assemblage B infection. As shown for the zoonotic potential of assemblage A, these findings are in line with those on the association between assemblage B infection and the presence of children and other infected family members (10). During an outbreak in a nursery in Wales, all of the children, child care workers, and parents who were successfully genotyped were indeed infected with assemblage B (17).
In our study, traveling abroad during the exposure period was the most important risk factor for giardiasis overall, confirming the findings of previous case-control studies from the United Kingdom (5) and other developed countries such as New Zealand (18) and Canada (19). The strength of the association was even stronger (data not shown) when we analyzed specifically traveling to countries that were considered high risk for infection in previous studies (18, 20), including countries in Africa, Asia, and Central and South America.
Swimming in a swimming pool was strongly associated with giardiasis in both travel-associated and non-travel-associated cases, suggesting that transmission in swimming pool settings occurs in North West England. Our findings contrasted with those from a previous study from England, which found that exposure to recreational unchlorinated water was more important than exposure to chlorinated water (6). Interestingly, in our sample, swimming in lakes, ponds, or rivers was not associated with the disease, although the number of case patients who reported these exposures was small. Sporadic outbreaks of giardiasis in swimming pools in the United Kingdom (21) and the United States (22) have been reported. We did not detect any swimming pool-related outbreaks during the study period; however, such outbreaks may remain unnoticed since cases can be asymptomatic, as previously observed among people infected during a swim class (22).
Drinking unboiled tap water was also a risk factor for giardiasis among people who did not travel abroad. The association between Giardia infection and drinking water from the tap has been inconsistent in the literature, but it is not new for the United Kingdom (6). In contrast to the aforementioned study, however, we did not observe any dose-response relationship with respect to the daily number of glasses. Previously in the United Kingdom, a private water source was involved in a Giardia outbreak (21) and a water main contamination was the likely cause in another outbreak (23). No waterborne outbreak was reported during our study, and nearly all cases were supplied by a water main system (data not shown). More data on the characteristics and provenience of different water main supplies are needed in order to determine whether they may be associated with different levels of risk for waterborne giardiasis. Drinking unsafe surface water directly from the environment (e.g., water taken from a lake, pond, or river) is a well-known risk factor for giardiasis; however, no evidence of this route of transmission was found in our study. This was likely due to a lack of statistical power, since only two cases and controls reported this exposure.
In our study, changing diapers was also independently associated with increased risk of giardiasis in non-travel-associated cases. Although a previous study in the United Kingdom failed to detect it (6), the significance of changing diapers for giardiasis has been reported in other studies (18, 19, 24).
In our sample, irritable bowel syndrome (IBS) was reported significantly more often for cases than controls. The clinical presentation of chronic giardiasis closely resembles IBS (25). The association between IBS and giardiasis in our sample could be spurious and due to the misclassification of patients with IBS for whom Giardia was diagnosed by chance. Furthermore, IBS was self-reported by the case patients, and we could not verify whether it had been confirmed by a general practitioner using the appropriate diagnostic criteria.
The apparent protective effect given by certain exposures cannot be easily explained. In particular, the consumption of salad and raw vegetables and the frequency of consumption of raw fruit were negatively associated with giardiasis. Although eating fresh products was reported previously as a risk factor for giardiasis (6, 20), a similar negative association with disease was reported for cryptosporidiosis (26, 27). It has been suggested that repeated exposure to the pathogen via contaminated fresh products could lead to immunity (26). This protective effect would be particularly evident among people consuming fresh products more frequently, but more data are needed to support this hypothesis.
Our study was not without limitations. The recruitment suffered from low response rates, particularly for controls. The reduced study power might have affected our ability to detect additional risk factors for giardiasis. Furthermore, the analysis of the assemblage-specific risk factors was based on a relative small number of cases, which might have had an impact on our ability to detect other significant exposures associated with the two assemblages.
In summary, we have shown that overseas travel is the principle risk factor for sporadic giardiasis in North West England, but human-to-human transmission (e.g., through diaper changing) and transmission in swimming pools play major roles in the acquisition of indigenous giardiasis. Our results suggest that assemblages A and B may have preferential reservoirs (animal for the former and human for the latter). Our finding of an association between assemblage A infections and dog ownership demonstrates the importance of considering the genotypic diversity of the parasite to highlight its zoonotic risk. The presence of assemblage-specific risk factors has important implications for the surveillance and control of Giardia. Using the assemblage information for surveillance will facilitate investigation of clusters and transmission routes and will enable detailed clinicoepidemiological studies to explore whether the clinical courses of infections truly differ between the parasite assemblages.
Supplementary Material
ACKNOWLEDGMENTS
We thank Ruth White, Pamela Henderson, and Andrew Dodgson for providing the fecal samples from case patients, Lorraine Lighton for the notification and recruitment of case patients, and Ruth Park and Maggie Elsley for the selection and recruitment of control subjects. We thank environmental health officers from Preston, Chorley, South Ribble, Burnley, Pendle, Rossendale, Blackburn with Darwen, Hyndburn, and Ribble Valley Councils for completion of case questionnaires.
This study was funded by Public Health England (formerly the Health Protection Agency).
John Cheesbrough is retired.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.00715-15.
REFERENCES
- 1.Savioli L, Smith H, Thompson A. 2006. Giardia and Cryptosporidium join the ‘Neglected Diseases Initiative’. Trends Parasitol 22:203–208. doi: 10.1016/j.pt.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Health Protection Agency. 2011. Giardia lamblia laboratory reports. http://webarchive.nationalarchives.gov.uk/20120528152708/ http://www.hpa.org.uk/Topics/InfectiousDiseases/InfectionsAZ/Giardia/EpidemiologicalData/gairDataEwAgeRates/.
- 3.Tam CC, Rodrigues LC, Viviani L, Dodds JP, Evans MR, Hunter PR, Gray JJ, Letley LH, Rait G, Tompkins DS, O'Brien SJ, IID2 Study Executive Committee. 2012. Longitudinal study of infectious intestinal disease in the UK (IID2 study): incidence in the community and presenting to general practice. Gut 61:69–77. doi: 10.1136/gut.2011.238386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellam H, Verlander NQ, Lamden K, Cheesbrough JS, Durband CA, James S. 2008. Surveillance of giardiasis in Northwest England 1996–2006: impact of an enzyme immunoassay test. Euro Surveill 13(37):pii=18977 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=18977. [DOI] [PubMed] [Google Scholar]
- 5.Gray SF, Gunnell DJ, Peters TJ. 1994. Risk factors for giardiasis: a case-control study in Avon and Somerset. Epidemiol Infect 113:95–102. doi: 10.1017/S0950268800051505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stuart JM, Orr HJ, Warburton FG, Jeyakanth S, Pugh C, Morris I, Sarangi J, Nichols G. 2003. Risk factors for sporadic giardiasis: a case-control study in southwestern England. Emerg Infect Dis 9:229–233. doi: 10.3201/eid0902.010488. [DOI] [PubMed] [Google Scholar]
- 7.Warburton AR, Jones PH, Bruce J. 1994. Zoonotic transmission of giardiasis: a case control study. Commun Dis Rep CDR Rev 4:R32–R36. [PubMed] [Google Scholar]
- 8.Feng Y, Xiao L. 2011. Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clin Microbiol Rev 24:110–140. doi: 10.1128/CMR.00033-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryan U, Cacciò SM. 2013. Zoonotic potential of Giardia. Int J Parasitol 43:943–956. doi: 10.1016/j.ijpara.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Anuar TS, Nor Azreen S, Salleh FM, Moktar N. 2014. Molecular epidemiology of giardiasis among Orang Asli in Malaysia: application of the triosephosphate isomerase gene. BMC Infect Dis 14:78. doi: 10.1186/1471-2334-14-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Francis B, Green M, Payne C (ed). 1993. The GLIM system release 4 manual. Clarendon Press, Oxford, United Kingdom. [Google Scholar]
- 12.Hopkins RM, Meloni BP, Groth DM, Wetherall JD, Reynoldson JA, Thompson RCA. 1997. Ribosomal RNA sequencing reveals differences between the genotypes of Giardia isolates recovered from humans and dogs living in the same locality. J Parasitol 83:44–51. doi: 10.2307/3284315. [DOI] [PubMed] [Google Scholar]
- 13.Geurden T, Levecke B, Cacció SM, Visser A, De Groote G, Casaert S, Vercruysse J, Claerebout E. 2009. Multilocus genotyping of Cryptosporidium and Giardia in non-outbreak related cases of diarrhoea in human patients in Belgium. Parasitology 136:1161–1168. doi: 10.1017/S0031182009990436. [DOI] [PubMed] [Google Scholar]
- 14.Breathnach AS, McHugh TD, Butcher PD. 2010. Prevalence and clinical correlations of genetic subtypes of Giardia lamblia in an urban setting. Epidemiol Infect 138:1459–1467. doi: 10.1017/S0950268810000208. [DOI] [PubMed] [Google Scholar]
- 15.Puebla LJ, Núñez FA, Fernández YA, Fraga J, Rivero LR, Millán IA, Valdés LA, Silva IM. 2014. Correlation of Giardia duodenalis assemblages with clinical and epidemiological data in Cuban children. Infect Genet Evol 23:7–12. doi: 10.1016/j.meegid.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Lebbad M, Petersson I, Karlsson L, Botero-Kleiven S, Andersson JO, Svenungsson B, Svärd SG. 2011. Multilocus genotyping of human Giardia isolates suggests limited zoonotic transmission and association between assemblage B and flatulence in children. PLoS Negl Trop Dis 5:e1262. doi: 10.1371/journal.pntd.0001262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amar CFL, Dear PH, Pedraza-Díaz S, Looker N, Linnane E, McLauchlin J. 2002. Sensitive PCR-restriction fragment length polymorphism assay for detection and genotyping of Giardia duodenalis in human feces. J Clin Microbiol 40:446–452. doi: 10.1128/JCM.40.2.446-452.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoque ME, Hope VT, Kjellström T, Scragg R, Lay-Yee R. 2002. Risk of giardiasis in Aucklanders: a case-control study. Int J Infect Dis 6:191–197. doi: 10.1016/S1201-9712(02)90110-4. [DOI] [PubMed] [Google Scholar]
- 19.Gagnon F, Duchesne JF, Lévesque B, Gingras S, Chartrand J. 2006. Risk of giardiasis associated with water supply in an endemic context. Int J Environ Health Res 16:349–359. doi: 10.1080/09603120600869265. [DOI] [PubMed] [Google Scholar]
- 20.Espelage W, an der Heiden M, Stark K, Alpers K. 2010. Characteristics and risk factors for symptomatic Giardia lamblia infections in Germany. BMC Public Health 10:41. doi: 10.1186/1471-2458-10-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith A, Reacher M, Smerdon W, Adak GK, Nichols G, Chalmers RM. 2006. Outbreaks of waterborne infectious intestinal disease in England and Wales, 1992–2003. Epidemiol Infect 134:1141–1149. doi: 10.1017/S0950268806006406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harter L, Frost F, Grunenfelder G, Perkins-Jones K, Libby J. 1984. Giardiasis in an infant and toddler swim class. Am J Public Health 74:155–156. doi: 10.2105/AJPH.74.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jephcott AE, Begg NT, Baker IA. 1986. Outbreak of giardiasis associated with mains water in the United Kingdom. Lancet 327:730–732. doi: 10.1016/S0140-6736(86)91114-1. [DOI] [PubMed] [Google Scholar]
- 24.Hoque ME, Hope VT, Scragg R, Kjellstrom T, Lay-Yee R. 2001. Nappy handling and risk of giardiasis. Lancet 357:1017–1018. doi: 10.1016/S0140-6736(00)04251-3. [DOI] [PubMed] [Google Scholar]
- 25.Stark D, van Hal S, Marriott D, Ellis J, Harkness J. 2007. Irritable bowel syndrome: a review on the role of intestinal protozoa and the importance of their detection and diagnosis. Int J Parasitol 37:11–20. doi: 10.1016/j.ijpara.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 26.Hunter PR, Hughes S, Woodhouse S, Syed Q, Verlander NQ, Chalmers RM, Morgan K, Nichols G, Beeching N, Osborn K. 2004. Sporadic cryptosporidiosis case-control study with genotyping. Emerg Infect Dis 10:1241–1249. doi: 10.3201/eid1007.030582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robertson B, Sinclair MI, Forbes AB, Veitch M, Kirk M, Cunliffe D, Willis J, Fairley CK. 2002. Case-control studies of sporadic crytosporidiosis in Melbourne and Adelaide, Australia. Epidemiol Infect 128:419–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


