Abstract
Molecular typing of Mycoplasma pneumoniae is an important tool for identifying grouped cases and investigating outbreaks. In the present study, we developed a new genotyping method based on single nucleotide polymorphisms (SNPs) selected from the whole-genome sequencing of eight M. pneumoniae strains, using the SNaPshot minisequencing assay. Eight SNPs, localized in housekeeping genes, predicted lipoproteins, and adhesin P1 genes were selected for genotyping. These SNPs were evaluated on 140 M. pneumoniae clinical isolates previously genotyped by multilocus variable-number tandem-repeat analysis (MLVA-5) and adhesin P1 typing. This method was also adapted for direct use with clinical samples and evaluated on 51 clinical specimens. The analysis of the clinical isolates using the SNP typing method showed nine distinct SNP types with a Hunter and Gaston diversity index (HGDI) of 0.836, which is higher than the HGDI of 0.583 retrieved for the MLVA-4 typing method, where the nonstable Mpn1 marker was removed. A strong correlation with the P1 adhesin gene typing results was observed. The congruence was poor between MLVA-5 and SNP typing, indicating distinct genotyping schemes. Combining the results increased the discriminatory power. This new typing method based on SNPs and the SNaPshot technology is a method for rapid M. pneumoniae typing directly from clinical specimens, which does not require any sequencing step. This method is based on stable markers and provides information distinct from but complementary to MLVA typing. The combined use of SNPs and MLVA typing provides powerful discrimination of strains.
INTRODUCTION
Mycoplasma pneumoniae is the second leading cause of community-acquired pneumonia behind Streptococcus pneumoniae. This bacterium affects both the upper and lower respiratory tracts of individuals of all age groups, and infections occur endemically and epidemically worldwide (1, 2). Many outbreaks of M. pneumoniae respiratory infections have been reported in the community and in closed or semiclosed settings, such as military bases, hospitals, religious communities, schools, and institutions for the mentally disabled and may be associated with considerable morbidity (1, 3–6). Since 2010, a substantial increased incidence of M. pneumoniae infections has been reported in several countries (7–9). Molecular typing methods for M. pneumoniae have been developed for the identification of grouped cases and investigation of outbreaks (10). Until recently, the most common typing methods for M. pneumoniae were based on the analysis of single nucleotide polymorphisms (SNPs) within the gene encoding the major immunogenic protein P1, which is involved in adhesion to host cells. Several methodologies were used, including PCR-restriction fragment length polymorphism (11), amplification and gene sequencing (12), real-time PCR with high-resolution melt analysis (13), and pyrosequencing (14). However, due to the homogeneity of the M. pneumoniae species, few polymorphisms have been identified, and P1-based typing methods allow the discrimination of only two types and a few variants related to each type. A multilocus variable-number tandem-repeat (VNTR) analysis (MLVA-5) based on the study of five repeated sequence loci of the genome was developed (15). This method is the most discriminatory typing technique as it allows the distribution of M. pneumoniae strains into >60 types. However, the rapid evolution of VNTRs and the recently reported lack of stability of the marker Mpn1 (16), the most discriminant VNTR of the five VNTRs used in this method, are limitations of this technique. Recently, an amendment of the MLVA-5 nomenclature system, which eliminates the unstable Mpn1 marker and bases the typing method on the remaining 4 VNTRs (named the MLVA-4 typing method here), has been proposed (17). With MLVA-4, M. pneumoniae strains were separated into 25 types. Consequently, in addition to identifying new VNTRs, a typing method, also based on the study of several loci of the genome, but less subject to a rapid evolution, is needed.
The data provided by whole-genome sequencing (WGS) have significantly contributed to genotyping by identifying sequence deletions, sequence insertions, including sequence duplications, such as VNTRs, and SNPs, which are less prone to distortion resulting from selective pressure, than VNTRs (18). Regarding the M. pneumoniae species, molecular typing is hindered by the fact that this species is genetically homogeneous. Previous attempts to type M. pneumoniae using SNPs located within housekeeping genes were uninformative (19). Conversely, the SNP-based analysis was successfully applied to define relationships among isolates of diverse pathogens of homogeneous species, such as Mycobacterium tuberculosis (20), Bacillus anthracis (21), and Salmonella typhi (22). Thus, we aimed to identify SNPs in the M. pneumoniae genome, which might be used for genotyping purpose, by sequencing and comparing the whole genome of several M. pneumoniae strains.
The analysis of several bacterial SNPs is generally based on DNA sequencing, which is time consuming and expensive. In recent years, many SNP genotyping technologies, including fully integrated commercial solutions, have been developed (23, 24), representing an easier alternative to sequencing. Among the various SNP typing methodologies currently available, the SNaPshot minisequencing-based approach (Applied Biosystems) is remarkable because of its high multiplexing capacity, robustness, and high sensitivity (24). This approach is based on the single-base extension (SBE) of an unlabeled minisequencing primer that anneals one base upstream of the relevant SNP using a fluorochrome-labeled dideoxynucleotide (ddNTP). The allelic state is subsequently determined after separation of the extension products and detection of their fluorescence and size using capillary electrophoresis (25–28).
The aim of the present study was to develop a new genotyping method based on SNPs selected from the data provided by WGS of eight M. pneumoniae strains and using the SNaPshot minisequencing assay. This new typing scheme was evaluated on a collection of 140 M. pneumoniae clinical isolates and adapted to be performed directly on 51 M. pneumoniae-positive clinical specimens. The results were compared with the results of P1 adhesin gene and MLVA-4 and MLVA-5 typing methods.
MATERIALS AND METHODS
Strains and clinical specimens.
Seven epidemiologically unrelated clinical isolates of M. pneumoniae and the reference strain FH (ATCC 15531) were selected for complete genome sequencing. Those strains belong to six different MLVA-5 types, covering several geographical areas, years of isolation, types of specimens, and susceptibility or resistance to macrolides (Table 1).
TABLE 1.
Characteristics of the eight M. pneumoniae clinical isolates and strains selected for whole-genome sequencing
| Isolate/strain | Year of isolation | Specimen | Susceptibility to macrolidesa | Geographical origin | MLVA-5 typeb | P1 gene type |
|---|---|---|---|---|---|---|
| B1145 | 1994 | Cerebrospinal fluid | WT | Brest/France | P | 1 |
| B3996 | 2005 | Respiratory tract | R | Bordeaux/France | N | 1 |
| B4560 | 2007 | Respiratory tract | WT | Bayonne/France | E | 1 |
| M2285 | 1995 | Pericardial fluid | WT | Madrid/Spain | E | 1 |
| J382 | 2000-2003 | Respiratory tract | R | Japan | P | 1 |
| F59 | Unknown | Respiratory tract | WT | Germany | V | 2a |
| B3896 | 2005 | Respiratory tract | WT | Bordeaux/France | S | 2-related variantc |
| FH (ATCC 15531) | 1954 | Respiratory tract | WT | Boston, MA/USA | T | 2 |
The SNP-based typing method was evaluated on 140 M. pneumoniae clinical isolates (Table 2) collected between 1962 and 2012 from various geographical origins (France [n = 102], Belgium [n = 8], Germany [n = 6], Denmark [n = 11], Tunisia [n = 7], and Japan [n = 6]) and all from respiratory tract specimens, except for one strain from a sternal wound specimen. All of the isolates were single patient isolates belonging to 29 MLVA-5 types initially characterized according to Dégrange et al. (15), corresponding to 11 MLVA-4 types when the marker Mpn1 was removed. Ninety-nine isolates were adhesin P1 type 1 and 41 isolates were type 2 or type 2 variants 2a, 2b, or 2c (Table 2). The collection included 129 macrolide-susceptible and 11 macrolide-resistant isolates. The reference strain M129 (ATCC 29342) was also included in the study. DNA extraction was performed using the MagNA Pure LC DNA isolation kit I (Roche Diagnostics) according to the manufacturer's instructions.
TABLE 2.
Characteristics of the 149 M. pneumoniae isolates analyzed using the SNP typing method
| Straina | Year of isolation | Macrolide resistanceb | Geographical origin | SNP type | MLVA-4 profilec | MLVA-5 type | P1 gene type | Reference or source |
|---|---|---|---|---|---|---|---|---|
| M129 | Unknown | USA | SNP5 | 4572 | P | 1 | ATCC 29342 | |
| B1145d | 1994 | Brest/France | SNP9 | 4572 | P | 1 | 15 | |
| B3996d | 2005 | R | Bordeaux/France | SNP2 | 3572 | N | 1 | 15 |
| B4560d | 2007 | Bayonne/France | SNP1 | 4572 | E | 1 | 15 | |
| M2285d | 1995 | Madrid/Spain | SNP3 | 4572 | E | 1 | 15 | |
| J382d | 2000-2003 | R | Japan | SNP4 | 4572 | P | 1 | 15 |
| F59d | Unknown | Germany | SNP6 | 3562 | V | 2a | 15 | |
| B3896d | 2005 | Bordeaux/France | SNP7 | 3562 | S | 2-related variant | 48 | |
| FHd | 1954 | Boston, MA/USA | SNP8 | 3662 | T | 2 | ATCC 15531 | |
| B5767 | 2011 | Bordeaux/France | SNP9 | 4572 | J | 1 | This study | |
| B5596 | 2009 | Bordeaux/France | SNP1 | 4572 | J | 1 | This study | |
| B5612 | 2009 | Bordeaux/France | SNP2 | 4572 | E | 1 | This study | |
| B5029 | 2009 | Bordeaux/France | SNP8 | 3562 | M | 2 | This study | |
| B4997 | 2008 | Bordeaux/France | SNP9 | 4572 | X | 1 | This study | |
| J376 | 2000-2003 | R | Japan | SNP8 | 3662 | C | 2 | 15 |
| J380 | 2000-2003 | R | Japan | SNP4 | 4572 | J | 1 | 15 |
| J377 | 2000-2003 | R | Japan | SNP8 | 3662 | C | 2 | 15 |
| B3737 | 2004 | Bordeaux/France | SNP1 | 4572 | X | 1 | 15 | |
| B4692 | 2007 | Bordeaux/France | SNP9 | 4472 | 31e | 1 | 15 | |
| B4879 | 2008 | Bordeaux/France | SNP9 | 4472 | 31e | 1 | This study | |
| B5475 | 2009 | Bordeaux/France | SNP6 | 3562 | M | 2 | This study | |
| B4709 | 2007 | Bordeaux/France | SNP9 | 4572 | J | 1 | 15 | |
| B3766 | 2004 | Bordeaux/France | SNP2 | 4572 | U | 1 | 15 | |
| L3 | 1995 | Lyon/France | SNP9 | 4572 | X | 1 | 15 | |
| L19 | 1997 | Lyon/France | SNP8 | 2662 | L | 2 | 15 | |
| FG9 | 1997 | St-Etienne/France | SNP1 | 4572 | P | 1 | 15 | |
| FG8 | 1997 | St-Etienne/France | SNP3 | 4572 | E | 1 | 15 | |
| J22 | 1993 | Germany | SNP1 | 4572 | Z | 1 | 15 | |
| B4578 | 2007 | Bordeaux/France | SNP9 | 4572 | E | 1 | 15 | |
| J17 | 1992 | Germany | SNP3 | 4572 | P | 1 | 15 | |
| J11 | 1992 | Germany | SNP1 | 4572 | X | 1 | 15 | |
| B4112 | 2006 | Bordeaux/France | SNP6 | 3562 | M | 2 | 15 | |
| B2892 | 1999 | Bordeaux/France | SNP8 | 3662 | O | 2 | 15 | |
| B936 | 1994 | Bordeaux/France | SNP1 | 4572 | J | 1 | 15 | |
| B4602 | 2007 | Bayonne/France | SNP1 | 4572 | J | 1 | 15 | |
| Pn22 | 1992 | Bordeaux/France | SNP1 | 3572 | R | 1 | 15 | |
| L59 | 2000 | Lyon/France | SNP8 | 3572 | N | 2 | 15 | |
| B3885 | 2005 | Bordeaux/France | SNP1 | 4572 | P | 1 | 15 | |
| B4223 | 2006 | Bordeaux/France | SNP1 | 4572 | J | 1 | 15 | |
| B3119 | 2001 | Bordeaux/France | SNP6 | 3562 | V | 2 | 15 | |
| B3163 | 2001 | Bordeaux/France | SNP8 | 3662 | C | 2 | 15 | |
| B3448 | 2003 | Bordeaux/France | SNP8 | 3662 | C | 2 | 15 | |
| Pn15 | 1992 | Bordeaux/France | SNP9 | 4572 | J | 1 | 15 | |
| Pn21 | 1992 | Bordeaux/France | SNP9 | 4572 | U | 1 | 15 | |
| N35 | 1986 | Nantes/France | SNP6 | 3562 | V | 2 | 15 | |
| FG10 | 1997 | St-Etienne/France | SNP9 | 4572 | U | 1 | 15 | |
| 79692 | 2000 | Germany | SNP6 | 3562 | M | 2a | 15 | |
| Sta | 1995 | Germany | SNP6 | 3562 | M | 2a | 15 | |
| C83 | 2006 | Caen/France | SNP8 | 3662 | T | 2 | 15 | |
| C71 | 2005 | Caen/France | SNP1 | 4572 | Z | 1 | 15 | |
| B3869 | 2005 | Bordeaux/France | SNP2 | 4572 | U | 1 | 15 | |
| B4100 | 2006 | Bordeaux/France | SNP9 | 4572 | E | 1 | 15 | |
| B2829 | 1999 | Bordeaux/France | SNP1 | 4572 | U | 1 | 15 | |
| AV3 | 1992 | Anvers/Belgium | SNP9 | 4572 | P | 1 | 15 | |
| FG12 | 1997 | St-Etienne/France | SNP9 | 4572 | J | 1 | 15 | |
| Pn19 | 1992 | Bordeaux/France | SNP3 | 4572 | E | 1 | 15 | |
| J16 | 1992 | Germany | SNP4 | 4572 | P | 1 | 15 | |
| L37 | 1997 | Lyon/France | SNP9 | 4572 | U | 1 | 15 | |
| B3722 | 2004 | Bordeaux/France | SNP2 | 4572 | U | 1 | 15 | |
| B6254 | 2012 | Bordeaux/France | SNP1 | 4572 | P | 1 | This study | |
| B6199 | 2012 | Bordeaux/France | SNP1 | 4572 | U | 1 | This study | |
| B6205 | 2012 | Bordeaux/France | SNP5 | 4672 | 37e | 1 | This study | |
| B6303 | 2012 | Bordeaux/France | SNP2 | 4572 | P | 1 | This study | |
| B6203 | 2012 | Bordeaux/France | SNP1 | 4572 | U | 1 | This study | |
| C6 | 2011 | Caen/France | SNP1 | 4572 | P | 1 | This study | |
| C43 | 2011 | Caen/France | SNP8 | 3662 | T | 2b | This study | |
| C2 | 2011 | Caen/France | SNP1 | 4572 | P | 1 | This study | |
| C17 | 2011 | Caen/France | SNP1 | 4572 | P | 1 | This study | |
| C40 | 2011 | Caen/France | SNP9 | 4572 | J | 1 | This study | |
| B6094 | 2012 | Bordeaux/France | SNP9 | 4572 | J | 1 | This study | |
| B6102 | 2012 | Bordeaux/France | SNP9 | 4572 | E | 1 | This study | |
| B6148 | 2012 | Bordeaux/France | SNP9 | 4572 | E | 1 | This study | |
| B6074 | 2012 | Bordeaux/France | SNP9 | 4572 | E | 1 | This study | |
| B6085 | 2012 | Bordeaux/France | SNP1 | 3572 | 39e | 1 | This study | |
| B6128 | 2012 | Bordeaux/France | SNP9 | 4572 | U | 1 | This study | |
| B5973 | 2011 | Bordeaux/France | SNP6 | 3562 | G | 2b | This study | |
| B5927 | 2011 | Bordeaux/France | SNP9 | 4572 | P | 1 | This study | |
| B6009 | 2011 | Bordeaux/France | SNP6 | 3562 | V | 2a | This study | |
| B6052 | 2011 | Bordeaux/France | SNP9 | 4572 | P | 1 | This study | |
| Mpnlim | 2011 | R | Limoges/France | SNP1 | 3672 | I | 1 | This study |
| B6318 | 2012 | Bordeaux/France | SNP8 | 3662 | T | 2b | This study | |
| B5674 | 2010 | Bordeaux/France | SNP5 | 4572 | P | 1 | This study | |
| B5959 | 2011 | Bordeaux/France | SNP9 | 4672 | 29e | 1 | This study | |
| B3568 | 2003 | Bordeaux/France | SNP1 | 4572 | A | 1 | 15 | |
| B3487 | 2003 | Bordeaux/France | SNP1 | 4572 | A | 1 | 15 | |
| B718 | 1993 | Bordeaux/France | SNP3 | 4562 | D | 1 | 15 | |
| L15 | 1997 | Lyon/France | SNP8 | 3662 | W | 2 | 15 | |
| B2085 | 1996 | Bordeaux/France | SNP8 | 3662 | W | 2 | 15 | |
| L10 | 1996 | Lyon/France | SNP1 | 4571 | Q | 1 | 15 | |
| B2918 | 2000 | Bordeaux/France | SNP1 | 4571 | Q | 1 | 15 | |
| C76 | 2006 | Caen/France | SNP8 | 3662 | W | 2 | 15 | |
| J173 | Unknown | R | Japan | SNP1 | 4572 | A | 1 | 15 |
| J186 | Unknown | R | Japan | SNP1 | 4572 | A | 1 | 15 |
| J381 | 2000-2003 | R | Japan | SNP5 | 4562 | K | 1 | 15 |
| B3098 | 2000 | Argenteuil/France | SNP9 | 4582 | F | 1 | 15 | |
| B4466 | 2007 | Bordeaux/France | SNP6 | 3562 | S | 2 | 15 | |
| B3836 | 2004 | Bordeaux/France | SNP8 | 3662 | C | 2 | 15 | |
| B4079 | 2005 | Bordeaux/France | SNP8 | 3662 | W | 2 | This study | |
| B6048 | 2012 | Bordeaux/France | SNP9 | 4572 | U | 1 | This study | |
| B6056 | 2012 | Bordeaux/France | SNP5 | 4572 | X | 1 | This study | |
| B6021 | 2011 | Bordeaux/France | SNP1 | 4572 | U | 1 | This study | |
| B6096 | 2012 | Bordeaux/France | SNP1 | 4572 | P | 1 | This study | |
| B6028 | 2012 | Bordeaux/France | SNP9 | 4572 | J | 1 | This study | |
| B5938 | 2011 | Bordeaux/France | SNP6 | 3562 | G | 2c | This study | |
| B5904 | 2011 | Bordeaux/France | SNP9 | 4572 | J | 1 | This study | |
| B5847 | 2011 | Bordeaux/France | SNP1 | 4572 | X | 1 | This study | |
| B5837 | 2011 | Bordeaux/France | SNP1 | 4572 | X | 1 | This study | |
| B5817 | 2011 | Bordeaux/France | SNP1 | 4572 | U | 1 | This study | |
| B6312 | 2012 | Bordeaux/France | SNP6 | 3562 | G | 2a | This study | |
| B5336 | 2009 | Bordeaux/France | SNP9 | 4672 | 29e | 1 | This study | |
| B5719 | 2010 | Bordeaux/France | SNP9 | 4572 | P | 1 | This study | |
| B4747 | 2008 | Bordeaux/France | SNP6 | 3562 | B | 2 | This study | |
| B5563 | 2010 | Bordeaux/France | SNP9 | 4572 | E | 1 | This study | |
| B4972 | 2008 | R | Bordeaux/France | SNP8 | 4572 | Z | 2 | This study |
| B5776 | 2011 | Bordeaux/France | SNP9 | 4572 | J | 1 | This study | |
| B5954 | 2011 | R | Bordeaux/France | SNP2 | 4572 | P | 1 | This study |
| B6329 | 2012 | R | Bordeaux/France | SNP8 | 3662 | C | 2 | This study |
| C95 | 2007 | Caen/France | SNP8 | 3662 | H | 2 | 15 | |
| C98 | 2007 | Caen/France | SNP8 | 3662 | H | 2 | 15 | |
| C89 | 2006 | Caen/France | SNP8 | 3662 | H | 2 | 15 | |
| L8 | 1996 | Lyon/France | SNP8 | 3562 | B | 2 | 15 | |
| L62 | 2000 | Lyon/France | SNP8 | 3562 | M | 2 | 15 | |
| B4561 | 2007 | Bayonne/France | SNP6 | 3562 | S | 2 | 15 | |
| L18 | 1997 | Lyon/France | SNP8 | 3662 | C | 2 | 15 | |
| AV1 | 1992 | Anvers/Belgium | SNP8 | 3662 | C | 2 | 15 | |
| AV2 | 1992 | Anvers/Belgium | SNP3 | 4572 | E | 1 | 15 | |
| AV4 | 1992 | Anvers/Belgium | SNP3 | 4572 | E | 1 | 15 | |
| AV8 | 1993 | Anvers/Belgium | SNP3 | 4572 | E | 1 | 15 | |
| AV9 | 1993 | Anvers/Belgium | SNP3 | 4572 | E | 1 | 15 | |
| AV6 | 1993 | Anvers/Belgium | SNP1 | 4572 | J | 1 | 15 | |
| AV7 | 1993 | Anvers/Belgium | SNP9 | 4572 | J | 1 | 15 | |
| A131 | 2006 | Tunisia | SNP5 | 4572 | P | 1 | 15 | |
| A167 | 2006 | Tunisia | SNP5 | 4572 | P | 1 | 15 | |
| A168 | 2006 | Tunisia | SNP5 | 4572 | P | 1 | 15 | |
| A195 | 2006 | Tunisia | SNP5 | 4572 | P | 1 | 15 | |
| A251 | 2007 | Tunisia | SNP5 | 4572 | P | 1 | 15 | |
| A340 | 2008 | Tunisia | SNP5 | 4572 | P | 1 | 15 | |
| A355 | 2008 | Tunisia | SNP5 | 4572 | P | 1 | 15 | |
| M5 | 1962 | Denmark | SNP5 | 4572 | P | 1 | 15 | |
| M40 | 1963 | Denmark | SNP5 | 4572 | P | 1 | 15 | |
| M38 | 1963 | Denmark | SNP5 | 4572 | Z | 1 | 15 | |
| M62 | 1964 | Denmark | SNP5 | 4572 | X | 1 | 15 | |
| M547 | 1967 | Denmark | SNP8 | 3662 | T | 2 | 15 | |
| M1121 | 1977 | Denmark | SNP4 | 4572 | J | 1 | 15 | |
| M1873 | 1987 | Denmark | SNP8 | 3662 | C | 2 | 15 | |
| M2018 | 1988 | R | Denmark | SNP4 | 4572 | P | 1 | 15 |
| M2155 | 1990 | Denmark | SNP9 | 4572 | P | 1 | 15 | |
| M4350 | 1991 | Denmark | SNP8 | 3562 | V | 2 | 15 | |
| 4817 | 1993 | Denmark | SNP1 | 4572 | J | 1a | 15 |
All strains were isolated from respiratory tract specimens (sputum samples, bronchoalveolar lavage [BAL] fluid samples, throat swabs, nasopharyngeal aspirates, and tracheal aspirates) except for strains B1145, M2285, and B3163 isolated from cerebrospinal fluid, pericardial fluid, and sternal wound specimens, respectively.
Only macrolide-resistant isolates are indicated by R. All other isolates were susceptible to macrolides.
Order of VNTRs: Mpn13, Mpn14, Mpn15, Mpn16.
Isolates submitted to whole-genome sequencing.
MLVA-5 types are named according to reference 15 and MLVA profiles of MLVA-5 types 29, 31, 37, and 39 are 24672, 84472, 54672, and 63572, respectively.
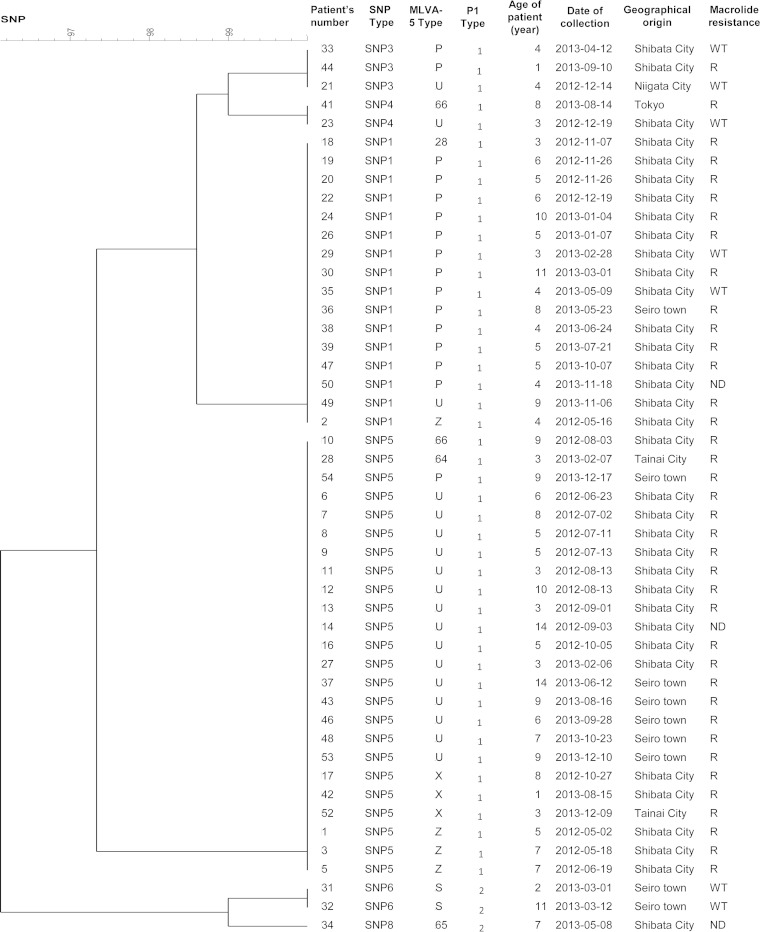
Fifty-one throat swabs collected from several cities in Niigata prefecture (Niigata city, Seiro town, Shibata city, Tainai city, and Tokyo), Japan, between May 2012 and December 2013, from children (25 female and 26 male) aged 1 to 14 years old were used. These patients were diagnosed with pneumonia at the department of pediatrics in the Niigata University Medical and Dental Hospital, Niigata, Japan. All of the specimens were confirmed positive for M. pneumoniae using the Loopamp Mycoplasma P detection kit (Eiken Chemical). Thirty-nine out of the 51 specimens were resistant to macrolides, nine specimens had a wild-type genotype, and the remaining specimens could not be amplified. The specimens belonged to nine different MLVA-5 types with two major types, MLVA-5 type P or 44572 (n = 18) and MLVA-5 type U or 54572 (n = 19), and the majority of the samples were adhesin P1 type 1 (47/51) (Fig. 1). DNA was extracted using the QIAamp DNA minikit (Qiagen) in accordance with the manufacturer's instructions and stored at −20°C until further use.
FIG 1.
Clustering dendrogram of the 48 M. pneumoniae-positive Japanese clinical specimens based on SNP profiles at eight genomic loci. The dendrogram was constructed using the categorical coefficient and UPMGA clustering method (BioNumerics v.7.1). The MLVA profiles of MLVA-5 types 28, 64, 65, and 66 are 64573, 74473, 43262, and 24571, respectively. R, presence of macrolide resistance-associated mutation (A2058G or A2059G); WT, wild type; ND, not determined.
Whole-genome sequencing and analysis.
The genomes of the eight strains described above were sequenced using Solexa (Illumina) technology (single reads, 36 bp) with a Genome Analyzer IIx in the CEA/Genoscope (Centre National de Séquençage, Evry, France). Raw sequencing data were incorporated into a bioinformatics pipeline called EvolScope based on SSAHA2 (Sequence Search and Alignment by Hashing Algorithm) alignment software v.2.5.1 (29). This pipeline was part of the PALOMA (Polymorphism Analyses in Light Of MAssive DNA sequencing) platform designed for evolution projects and allows the detection of small variations (SNPs and indels) between the reads of the sequenced genomes and the sequence of the reference M. pneumoniae strain M129 (GenBank accession number NC_000912) (30). The PALOMA tool was accessible through MicroScope v.2.5.5 (http://www.genoscope.cns.fr/agc/microscope/home/), which is a web-based platform for microbial comparative genome analysis and manual functional annotation.
The read quality was checked using, for each base, an internal Q score, ranging between 0 and 40, which takes into account the frequency of a given base at a particular location and the base coverage, i.e., the number of reads mapping this given location. Only bases with a Q score of >25 were retained for further alignments. Based on the characteristics of the read alignments onto the reference sequence, a file containing the lists of all possible SNPs was generated. Each event was subsequently scored to retain only significant SNPs. This EvolScope score was a two-component score, ranging from 0 to 1, considering both biological (allele rate in the studied population) and technical (ratio of reads mapped on forward and reverse strands) aspects of the analysis. The EvolScope pipeline was not adapted to analyze intergenic sequences, and reads containing repeated regions could not be mapped, which decreased the number of potential SNPs. A second approach, complementary to the previous method, was used to detect SNPs. A reference-based assembly was realized using the Power Assembler module of BioNumerics software v.7.1 (Applied Maths) with the raw sequencing data of the eight studied strains. The sequences were independently assembled using the M129 sequence as a reference. After assembly, polymorphic sites were detected, using a homemade script generated using the Python script editor (a programming language used to manually create specific modules for BioNumerics), by comparison to the genome of the reference strain M129. The sequence of the strain 309 (type 2a; GenBank accession number AP012303) (31) was included in this analysis.
Criteria for the SNP selection.
Based on the list of SNPs predicted using both EvolScope and BioNumerics, the criteria for the selection of SNPs for our typing method were an EvolScope score of ≥0.5, a distribution throughout the genome, and a unique SNP profile per isolate. All putative SNPs were verified in the whole-genome-sequenced strains by sequencing the relevant gene region in both directions using the Sanger method.
Amplification of the SNPs selected for the genotyping assay.
Target SNPs selected for the genotyping method were amplified using the primers listed in Table 3. The primers were designed using Primer3 software (v.0.4.0) (http://bioinfo.ut.ee/primer3-0.4.0/primer3/), and the properties of these oligonucleotides were verified using OligoCalc software (32). A Basic Local Alignment Search Tool (BLAST) analysis of each primer was also performed to assess the primer specificity. All primers were tested in singleplex reactions before optimization for multiplex PCR.
TABLE 3.
PCR primers used for the amplification of the eight gene regions encompassing the SNPs of interest
| Mnemonic (M. pneumoniae M129) | Gene name | Product | Orientationa | Sequence 5′→3′ | Amplicon size (bp) |
|---|---|---|---|---|---|
| MPN004 | gyrA | DNA gyrase subunit A | F | AAAGTCAGCACGGATTGTCG | 364 |
| R | GAGACGGAATGGAAGTGGAC | ||||
| MPN582 | —b | Hypothetical lipoprotein | F | ATGATCAGCCCTTTGTTTCC | 323 |
| R | AACACTTCGCGGTTTTCAGC | ||||
| MPN246 | gmk | Guanylate kinase | F | TTCACAACGGTGGCAACTTG | 269 |
| R | TGCTTGTAACTCCGCTAAGG | ||||
| MPN050 | glpK | Glycerol kinase | F | TTAGCTACGATGCAAAGTGC | 375 |
| R | TACATGCATCTTACCACCCG | ||||
| MPN516 | rpoB | DNA-directed RNA polymerase subunit beta | F | ATACTCACCCACTGCTACCC | 407 |
| R | TCCCGTTGACAATAAAGACC | ||||
| MPN442 | — | Hypothetical lipoprotein | F | GATGAGCAATACAACCAAGC | 347 |
| R | TGTATTCCGCCCATTGATCG | ||||
| MPN168 | rplB | 50S ribosomal protein L2 | F | GATCCACCACTCCACGGTAA | 353 |
| R | TAATGTCAATCGGGTGTTCG | ||||
| MPN141 | P1 adhesin gene | P1 adhesin | F | ACGATGATTACAGGCGGTTC | 274 |
| R | AGTTGGTGGCCTCTTGTTGA |
F, forward; R, reverse.
—, no gene name.
The multiplex PCR of the eight selected loci was performed using the Qiagen multiplex PCR kit (Qiagen) as recommended by the manufacturer in an Eppendorf Mastercycler ep gradient S thermocycler (Eppendorf), with the following conditions: initial heat activation at 95°C for 15 min, then 35 cycles of denaturation at 95°C for 30 s, annealing at 58°C for 90 s, and primer extension at 72°C at 60 s, and a final extension at 60°C for 30 min. Each 50-μl multiplex PCR mix contained 0.2 μM PCR primers (Eurogentec) for the amplification of all the loci and 1 μl of template DNA. When clinical specimens were tested, the amplification program comprised 40 cycles instead of 35, and 5 μl of template DNA was used. To remove unincorporated deoxynucleoside triphosphates (dNTPs) and PCR primers, the amplification products were purified using the Wizard PCR Preps DNA System (Promega) according to the manufacturer's instructions and stored at −20°C until processed in the multiplex minisequencing reaction.
Design and validation of SBE primers.
The SBE primers (Table 4) were designed to anneal to the nucleotide immediately adjacent to the SNP investigated. The length of the primer might be modified through the addition of nonhomologous polynucleotides at the 5′ end [poly(dT), poly(dA), poly(dC), or poly(dGACT) tail], which are predicted to have minimal secondary structures. The following conditions were required: (i) primers must be longer than 20 nucleotides, (ii) primers must differ significantly in length (4 to 8 nucleotides) to avoid overlap between the different SNaPshot products in a multiplex reaction, (iii) the 3′ end of the primer must be hybridized just before the target SNP; (iv) the primer can be designed either on a positive or negative DNA strand, and (v) the melting temperature of the annealing region of the primers should be at least 50°C. All SBE primers (Eurogentec) were PAGE purified and tested in singleplex reactions. A BLAST search was performed to assess for cross-reaction with other species phylogenetically related to M. pneumoniae.
TABLE 4.
Minisequencing primers used for the SNaPshot reaction
| SBE primer namea | SNPb | Sequence 5′→3′c | Orientationd | Primer size (nt)e |
|---|---|---|---|---|
| gyrA446 | A/G | AATTAGCGGGGGAACTGTTACGTG | F | 24/24 |
| glpK360 | G/A | gactgactTGTGTGATAAGTTAAACCAAGA | F | 22/30 |
| rpoB168 | G/A | actgactgactgactCTGGAAAACCTGATTGCTGCTTA | F | 23/38 |
| rplB234 | C/A | ctgactgactgactgactCTTTAAACGAACGCACTATGACAA | F | 24/42 |
| gmk578 | A/G | ctgactgactgactgactgactgactgactAAAGAAGCGTAATGACGAGG | F | 20/50 |
| MPN442376 | C/A | ctgactgactgactgactgactgactgactgactGCTAGCCGAAACAAAAACCTCTTT | F | 24/58 |
| MPN5821013 | G/A | ctgactgactgactgactgactgactgactgactgactgactGCGATTAAACGGAAAGTTCTTG | R | 22/64 |
| P12774 | G/A | actgactgactgactgactgactgactgactgactgactgactgactgactCGCGGAGGTACCTGATTGT | R | 19/70 |
The name of the single base extension (SBE) primer corresponds to the name or the mnemonic of the gene containing the SNP and the position of the SNP (superscript number) in the gene in M. pneumoniae M129 genome.
The two possible alleles are indicated for each SNP.
The sequences in lowercase represent the nonhomologous polynucleotides used to modify the length of the primers.
F, forward; R, reverse.
The primer size represents the size of the annealing sequence (bold numbers)/the size of the whole SBE primer (including the nonhomologous tail). nt, nucleotides.
SNaPshot multiplex minisequencing assay for SNP detection.
The SBE analysis was performed using the SNaPshot multiplex kit (Applied Biosystems) for the eight examined SNPs in a final volume of 5 μl containing 1.5 μl of purified multiplex PCR product, 0.2 μM mixture of SBE primers, and 2.5 μl of SNaPshot multiplex ready reaction mix containing fluorescent ddNTPs. Positive and negative controls were included in each set of reactions. The reaction mixture was subjected to 25 cycles of denaturation at 96°C for 10 s, primer annealing at 50°C for 5 s, and primer extension at 60°C at 30 s. To remove the unincorporated PCR primers and fluorescent ddNTPs, 1 U of shrimp alkaline phosphatase (SAP) (USB Products) was added to the multiplex extension product and incubated at 37°C for 60 min, followed by incubation for 15 min at 70°C to inactivate the enzyme.
After purification, 0.5 μl of each multiplex minisequencing product was mixed with 9 μl of Hi-Di Formamide (Applied Biosystems) and 0.7 μl of GeneScan 120 LIZ Size Standard (Applied Biosystems). The samples were denatured at 95°C for 5 min and subjected to capillary electrophoresis using the ABI 3500xL Dx genetic analyzer (Applied Biosystems). Each DNA sample was tested in five independent experiments to assess reproducibility. The data analysis was performed using GeneMapper software v.4.1 (Applied Biosystems) in the SNaPshot default analysis method. Each peak was identified according to color and size. The data from all the peaks were concatenated to produce an 8-position SNP profile for each isolate tested.
Marker stability determination.
Five M. pneumoniae isolates, including the M129 and FH reference strains, were selected for stability analysis. Each strain was passaged 10 times in Hayflick-modified broth medium supplemented with glucose to determine the stability of each SNP before and after 10 passages.
Data analysis.
An SNP profile corresponding to the concatenated alleles, converted into numerical values (A = 1, C = 2, G = 3, T = 4), was assigned to each isolate and specimen. SNP profiles were entered into BioNumerics software package v.7.1 (Applied Maths) as character values, and a dendrogram and a minimum spanning tree (MST) were constructed to visualize the relationships between the clinical isolates or specimens using the categorical coefficient and unweighted pair-group method with arithmetic mean (UPGMA) clustering. The creation of hypothetical types was allowed for the MST with a priority rule consisting of the highest number of single-locus variants.
The discriminatory power of the different typing schemes was calculated using Simpson's index of diversity (Hunter and Gaston diversity index [HGDI]) (33), which expresses the probability of assigning a different type to two unrelated strains randomly sampled and obtained from the population of a given species; 95% confidence intervals (95% CI) were determined as previously described (34). Adjusted Rand and adjusted Wallace coefficients and the 95% CI for these indices were also calculated using a BioNumerics script downloaded from http://darwin.phyloviz.net/ComparingPartitions/index.php?link=Downloads (35) to measure the concordance between group assignments obtained by SNP typing and MLVA and P1 gene analyses. The adjusted Rand index represents the proportion of agreement for both matches (number of isolate pairs that are clustered together in the two typing schemes) and mismatches (number of pairs that are found in different groups) and is corrected to consider the presence of chance agreement. It is a symmetric, nondirectional coefficient. The adjusted Wallace coefficients are calculated considering one typing method as the standard and estimate the probability that two isolates grouped together using a typing method are also in the same cluster under another method and vice versa (36).
RESULTS
Selection of SNP markers.
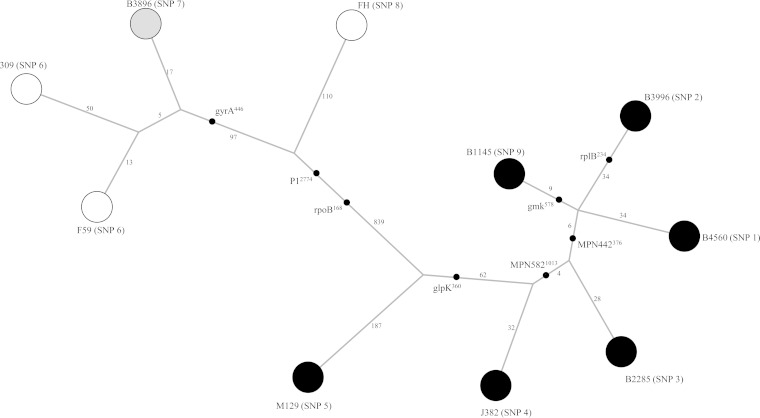
The sequence data obtained using Illumina/Solexa technology allowed the identification of 1,762 SNPs based on a comparison of eight genome sequences to the M129 sequence. An MST was constructed based on the SNPs detected, the branch lengths indicating the number of distinct SNPs attributed to a given branch (Fig. 2). The MST shows a clear separation between isolates belonging to P1 adhesin type 1 (isolates B1145, B3996, B4560, M2285, J382, and M129) and those belonging to P1 adhesin type 2 (isolates 309, F59, B3896, and FH). We observed that in each group, the reference strain was on a separate branch from the one harboring clinical isolates of the same group but shared the same node. Isolates 309 and F59, which are both P1 type 2a variants, were very close on the MST.
FIG 2.
Minimum spanning tree representing the totality of the SNPs detected in the 8 sequenced genomes of M. pneumoniae and the M. pneumoniae 309 genome (GenBank accession number AP012303) in comparison to that of the M. pneumoniae M129 genome. Each circle represents a genome-sequenced strain, and its SNP type is given in parentheses. The length of the branches reflects the number of distinct SNPs between nodes and is shown in logarithmic scale. The black dots indicate the position on the branch of the 8 SNPs used in our genotyping assay. The shade of the circles indicates the P1 adhesin type: black for type 1, white for type 2, and gray for the type 2-related variant (48).
Among the 1,762 SNPs predicted using the EvolScope pipeline, the nondiscriminant SNPs, i.e., those common to the eight sequenced strains compared with M129 (n = 158), were eliminated. The remaining SNPs (n = 1,604) were analyzed in silico to select a set of SNPs matching the following criteria: EvolScope score ≥0.5, distribution over the genome, and a unique SNP profile per isolate. Finally, 113 SNPs were selected as potential markers for genotyping. For each SNP, PCR primers were designed to amplify and sequence each genome fragment containing the selected SNP in the eight strains studied and M. pneumoniae M129. SNPs that were not confirmed by sequencing were discarded. The SNPs which were nondiscriminant for typing were also excluded, as the aim of the present study was to identify the minimal number of SNP loci required to resolve the studied strains. Within the confirmed SNPs, eight SNPs were retained, five SNPs were located in housekeeping genes (gyrA, MPN004; gplK, MPN050; rpoB, MPN516; rplB, MPN168; and gmk, MPN246), one SNP was located in the P1 gene (MPN141), and two SNPs were located in hypothetical lipoprotein genes (MPN582 and MPN442) (Table 5). The eight SNPs were concatenated, resulting in a total of nine distinct SNP profiles. The stability of the eight selected SNPs was assessed for five strains before and after 10 passages in Hayflick modified medium. The analysis of the five strains before and after passages resulted in identical SNP profiles.
TABLE 5.
Overview of the SNP profiles obtained for the eight selected SNPs markers in the eight whole genome-sequenced strains and the reference strain M. pneumoniae M129
| Strain/isolate | SNP namea |
SNP profile | SNP type | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| gyrA446 | glpK360 | rpoB168 | rplB234 | gmk578 | MPN442376 | MPN5821013 | P12774 | |||
| B4560 | A | A | G | C | A | A | A | G | AAGCAAAG | SNP1 |
| B3996 | A | A | G | A | A | A | A | G | AAGAAAAG | SNP2 |
| M2285 | A | A | G | C | A | C | A | G | AAGCACAG | SNP3 |
| J382 | A | A | G | C | A | C | G | G | AAGCACGG | SNP4 |
| M129 | A | G | G | C | A | C | G | G | AGGCACGG | SNP5 |
| F59 | G | G | A | C | A | C | G | A | GGACACGA | SNP6 |
| B3896 | G | G | A | C | A | C | G | G | GGACACGG | SNP7 |
| FH | A | G | A | C | A | C | G | A | AGACACGA | SNP8 |
| B1145 | A | A | G | C | G | A | A | G | AAGCGAAG | SNP9 |
The SNP name corresponds to the name or the mnemonic of the gene containing the SNP and the position (superscript number) of the SNP in the gene of M. pneumoniae M129 genome.
Validation of the SNaPshot assay.
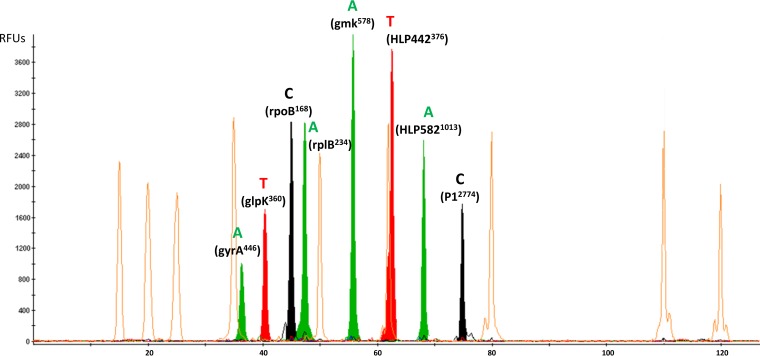
To automate the detection of the eight SNPs and avoid sequencing the gene fragments, we used the SNaPshot multiplex minisequencing assay. SNP-specific primers, named SBE primers, were designed for simultaneous annealing and single-base extension of the eight SNP loci (Table 4). All of the primers, except gyrA446, were designed with 5′-nonhomologous polynucleotide tails of different lengths to facilitate the differentiation of ddNTP-incorporated primers based on size. The SBE primer sizes ranged from 24 to 70 bp. There was no similarity of the SNP primers with those for other species phylogenetically related to M. pneumoniae based on the BLAST analysis. Singleplex minisequencing reactions were first applied to the PCR products obtained with the eight genome-sequenced strains and M. pneumoniae M129. Each reaction was repeated five times to confirm the reproducibility. The results showed the presence of a single peak with the expected color and position in all samples, confirming the specificity of each primer. No false-positive results were observed in control samples without polymorphisms. In addition, repeated assays demonstrated the reproducibility of this method. Subsequently, a multiplex minisequencing reaction was performed using a mixture of the eight SBE primers without requiring any other modification. Because each SBE primer was of a known size and extended by one dideoxynucleotide, it was possible to distinguish each SNP in the multiplex reaction by matching the migration position of each SBE primer and the color of the fluorescent dye specific to each base (Fig. 3).
FIG 3.
Example of electropherogram obtained for a M. pneumoniae isolate (SNP type 2) using the eight-plex SNaPshot minisequencing assay (Applied Biosystems). The x axis represents the size (in nucleotides) of the minisequencing products relative to the GeneScan-120 LIZ internal size standard represented with the orange peaks (Applied Biosystems), whereas the y axis represents the relative fluorescence units (RFUs). Each plot was obtained using GeneMapper software (v.4.1; Applied Biosystems). For SNPs glpK360, rpoB168, and HLP442376, the genes were localized on the negative (−) DNA strand, but the SBE primers were designed on the positive (+) DNA strand. For SNP P12774, the gene is localized on the positive (+) DNA strand, but the SBE primer was designed on the negative (−) DNA strand.
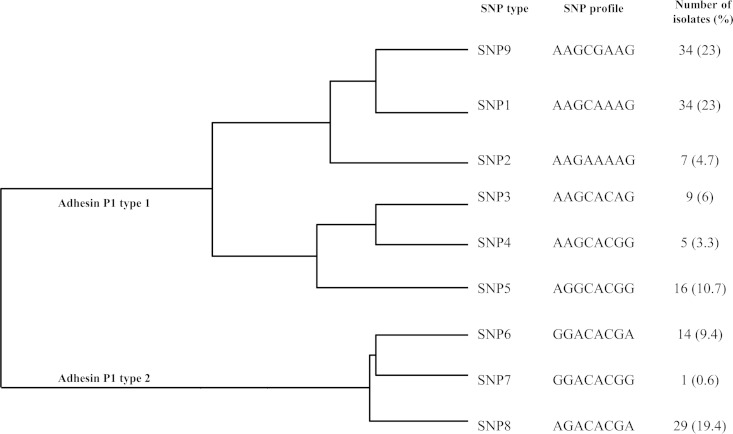
SNP typing of 140 M. pneumoniae isolates using SNaPshot.
The SNP-based typing assay was evaluated using 140 M. pneumoniae clinical isolates belonging to two adhesin P1 types and 29 MLVA-5 types corresponding to 11 MLVA-4 types when the Mpn1 marker was removed (Table 2). The analysis of the polymorphisms of the eight SNPs using SNaPshot showed a distribution into the nine SNP types described above (SNP1 to SNP9). No new profile was observed. The relationships and frequencies of the SNP types were displayed in a clustering dendrogram based on the concatenated SNPs (Fig. 4). No clustering was emphasized based on the geographical locations of the tested isolates, except for those of SNP type 2, which were all from Bordeaux, France. Moreover, no link was established between SNP typing and macrolide resistance, year of isolation, specimen origin, and MLVA typing. Interestingly, isolates from a clonal spread in a school in 2011 (5) showed the same SNP type 9. Moreover, 14 isolates collected in Bordeaux during the worldwide epidemic of M. pneumoniae infections in 2011 (8) belonged to only four SNP types, namely, SNP1, SNP2, SNP9, and SNP6, instead of eight MLVA-5 types, confirming the polyclonality of the M. pneumoniae surge. Notably, SNP1, SNP2, and SNP9 types are close according to the clustering dendrogram (Fig. 4).
FIG 4.
Clustering dendrogram of M. pneumoniae clinical isolates based on SNP profiles at eight SNP loci. The dendrogram was constructed using the categorical coefficient and UPMGA clustering method (BioNumerics v.7.1) and included 140 clinical isolates, the eight genome-sequenced strains, and the reference strain M. pneumoniae M129. Each branch represents a unique SNP profile. The genotype frequencies are indicated in parentheses. The relationship with the P1 type is also represented.
Comparison of the typing methods.
The SNP scheme was compared with the MLVA and P1-gene typing methods and with the combination of the MLVA and SNP methods for the analysis of 140 clinical isolates of M. pneumoniae. The HGDIs were 0.836 (95% CI, 0.813 to 0.859) for the SNP method, 0.92 (95% CI, 0.896 to 0.940) for the MLVA-5 method, 0.583 (95% CI, 0.500 to 0.665) for the MLVA-4 method, and 0.422 (95% CI, 0.352 to 0.483) for the P1 gene typing with 9, 29, 11, and 2 different types, respectively. When the results of SNP and MLVA-5 typing were combined, the diversity was increased, as 50 different types were obtained with a HGDI of 0.972 (95% CI, 0.966 to 0.979). The combination of SNPs and MLVA-4 typing results also increased the diversity with a HGDI of 0.882 and 22 different types identified.
The congruence between typing methods was calculated using the adjusted Rand index, which measures the overall agreement between two typing methods, adjusted for chance agreement. Bidirectional Wallace coefficients were also calculated. When SNP typing was compared with P1 gene typing, the concordance of the two methods was low, based on the calculation of the adjusted Rand coefficient (0.237; 95% CI, 0.171 to 0.301). However, the adjusted Wallace coefficient SNP → P1 was 0.963 (95% CI, 0.895 to 1.000), indicating that the SNP typing scheme highly predicts the P1 gene type of a given isolate. Indeed, all isolates belonging to SNP types 1 to 5 and 9 were adhesin P1 type 1, whereas isolates of SNP type 6 to 8 belonged to adhesin P1 type 2 (Fig. 4). When SNP typing was compared with MLVA-5, the adjusted Rand coefficient was very low (0.13; 95% CI, 0.121 to 0.233), indicating a poor overall match between the two typing systems. The adjusted Wallace coefficient SNP → MLVA-5 was 0.095 (95% CI, 0.053 to 0.137), whereas the adjusted Wallace MLVA-5 → SNP was 0.208 (95% CI, 0.142 to 0.275), suggesting that when two strains are clustered together by SNP, these strains only have a 9.5% chance of belonging to the same MLVA-5 type, while conversely this chance is 21%. When the SNP typing method was compared with the MLVA-4 method, the overall match between both methods remained poor, with an adjusted Rand coefficient value of 0.218 (95% CI, 0.139 to 0.295) and an adjusted Wallace coefficient SNP → MLVA-4 of 0.508 (95% CI, 0.336 to 0.680). These results implied that the SNP type does not predict the MLVA type.
SNP typing of 51 M. pneumoniae-positive Japanese clinical specimens using SNaPshot.
Our SNP-based SNaPshot assay was adapted to be performed directly on clinical specimens using an increase in the template volume and number of PCR cycles. Forty-eight out of the 51 Japanese respiratory specimens were successfully genotyped, yielding a clinical sensibility of 94%. For the remaining three specimens, incomplete SNP profiles were obtained. Six SNP types were obtained with a predominance of two types, 1 and 5, detected in 16 and 24 specimens, respectively (Fig. 1). As observed for the M. pneumoniae isolates, the distribution of the SNP types was correlated with the P1 adhesin gene typing results. Interestingly, the majority of specimens belonging to MLVA-5 type P (44572) and U (54572) were of SNP type 1 and SNP type 5, respectively. The majority of these specimens (n = 39) were resistant to macrolides. However, no clustering according to macrolide resistance, date of isolation, geographical location, and age of children was observed.
DISCUSSION
SNP analysis is becoming increasingly useful for studies of drug resistance, evolution, and molecular epidemiology, particularly with the increased accessibility to these markers through high-throughput WGS. In the present study, we described a new typing scheme for M. pneumoniae based on SNP markers selected through the comparison of eight M. pneumoniae genomes with the genome of the reference strain M129. From the 1,762 predicted SNPs, 113 SNPs were selected as potential markers. After verification by PCR and sequencing, eight SNPs were retained, five SNPs were located in housekeeping genes, one SNP was located in the P1 adhesin gene, and two SNPs were located in hypothetical lipoprotein genes. In classical multilocus sequence typing (MLST), only housekeeping genes are investigated, and although this approach has been successfully employed for the characterization of several mycoplasmal species, such as M. hyopneumoniae, M. agalactiae, and M. bovis (37–39), this typing method was uninformative for M. pneumoniae (19). In this species, MLST afforded insufficient resolution for precise epidemiological investigations because housekeeping genes showed limited variability. The additional analysis of nonhousekeeping genes and of noncoding sequences increased the variability in M. pneumoniae, as in the case of multilocus sequence analysis (MLSA) in ruminant mycoplasmas (37). Thus, we also selected SNPs located in hypothetical genes and in genes implicated in virulence, such as the adhesin P1 gene. The stability of the selected SNP markers was confirmed after repeated passages in broth medium. Nevertheless, the stability should be evaluated prospectively over time in a variety of scenarios because in vitro passages in medium are not entirely reflective of what would occur in nature as strains pass among individuals with immune pressure along with a variety of other confounding factors (underlying medical conditions, coinfections, etc.). When concatenated, the eight selected SNPs resulted in nine distinct SNP profiles (SNP1 to SNP9). These nine profiles were also retrieved among the 140 clinical isolates of M. pneumoniae, confirming that all loci were present in all genomes and may be used for SNP genotyping. In this collection, no clustering was established based on macrolide resistance, collection year, specimen origin, MLVA-5 typing, and geographical location, except for isolates of SNP type 2, which were all obtained from Bordeaux, France. However, a strong correlation was observed with the P1 gene typing results. Moreover, a clonal spread of M. pneumoniae in a primary school in 2011 was attributed to the single SNP type 9, validating the epidemiological consistency of the typing method presented herein.
In the present study, we investigated the use of a novel SNP genotyping technology called SNaPshot based on the SBE of primers rather than on standard time-consuming DNA sequencing. The SNaPshot assay is currently widely used in forensic and population genetics (26, 28) and has recently raised interest in microbiological research (25, 27). A major advantage of this technology is that the eight SNP sites can be simultaneously analyzed, significantly reducing the cost and analysis time, as the automated fluorescent capillary electrophoresis analysis of minisequencing products requires only 30 min. A recent study described the simultaneous investigation of 16 SNPs in a single 16-plex SNaPshot assay (40), supporting the fact that SNaPshot assays can be readily multiplexed to a level higher than suggested, as the manufacturer recommends a limit of 10 SNPs per assay. Thus, this flexibility facilitates updating the data set with additional new SNPs. Moreover, the data interpretation of the peak patterns using GeneMapper software is simple and automatable, providing precise base identity determination. The results are easily comparable between laboratories. The SNaPshot method was robust and easily amenable to high-throughput analysis, as the assay was performed in 96-well plates using the same equipment as that used for automatized MLVA typing, i.e., a thermal cycler and a genetic analyzer. Overall, starting from DNA extracts, this method has a global turnaround time of 7 h with a mean reagent cost of 4.2€ per reaction, excluding PCR, which is comparable to the cost of MLVA analysis. Moreover, we demonstrated that the SNP SNaPshot typing method was directly applicable to clinical specimens, which is particularly useful, considering the fastidious growth of the M. pneumoniae species.
In the comparison of M. pneumoniae typing systems, the MLVA-5 assay was the most discriminatory method, with the highest HGDI, followed by the SNP typing, the MLVA-4 typing, and the adhesin P1 typing methods. To compare different methods of genotyping, the degree of congruence between the resulting clustering has to be determined (41). The overall congruence between SNP and MLVA-5 methods was poor, 0.13 and 0.095, according to the adjusted Rand index and adjusted Wallace coefficient, respectively. Although these results might vary according to the size of the sample (41), they suggest that these genotyping schemes are noncorrelated, i.e., the information given by one typing method is independent of or unrelated to the information given by another method (34, 41). Indeed, SNP and VNTR markers differ in mutation rates and mechanisms, with independent evolution processes. SNPs typically mutate slowly through changes in single base-pair identities (two segregating alleles) that may take many years to occur and might not offer enough polymorphisms to unravel recently acting evolutionary mechanisms, whereas some VNTRs might mutate faster through the addition or subtraction of repeats, producing greater levels of variation and often providing more discriminatory power per marker (more than two different alleles). SNP markers are robust phylogenic markers, less prone to distortion via selective pressure, as is the case for repetitive sequences (18). As a result of these different mutational dynamics, the simultaneous consideration of polymorphism patterns at SNPs and VNTRs conveys complementary information (42). MLVA is more suitable for short-term epidemiological analysis (microepidemiology), whereas SNP typing is ideally suited to long-term or global epidemiological (macroepidemiology) investigations (43). Although this SNP-based typing scheme might someday supplant the existing MLVA method, it was previously shown in mycoplasmal species that the joint application of SNPs and VNTRs can provide biological insight, which is not available from investigations of each marker type alone (44–46). In M. pneumoniae, the combined hierarchical use of the two typing methods may be recommended. Strains may be first characterized by SNP typing, followed by the fine-scale analysis of strains sharing identical SNP types with MLVA. In M. pneumoniae, among the 140 tested clinical isolates, the combination of SNPs and MLVA-5 increased the number of different types up to 50 and increased the HGDI up to 0.972, showing a highly discriminatory power. However, the described instability of Mpn1, one of the five VNTRs constituting MLVA-5, is a limitation of this typing method (16). Deprived of the most discriminatory marker, the resulting MLVA-4 assay dramatically lost discrimination power with an HGDI of only 0.583, which was lower than the SNP HGDI of 0.836. This observation emphasizes that the identification of additional targets for MLVA should be urgently investigated. Nevertheless, the combination of SNPs and MLVA-4 typing still yielded a good HGDI of 0.882 among the 140 clinical M. pneumoniae isolates of our study. Interestingly, in recent years, several laboratories reported a large polyclonality when MLVA-5 was applied to large populations during an epidemic phenomenon with numerous circulating types (8, 47). Notably, this finding suggested that the large polyclonality was mainly due to the high discrimination of the unstable marker Mpn1, which can have more than seven alleles (15). The use of our SNP typing method on these populations might reduce the large polyclonality and reveal the spread of a lower number of clones. In addition, it allows faster tracking and identification of M. pneumoniae outbreaks. Indeed, it is essential to rapidly identify grouped cases due to the risk of potentially severe outbreaks.
In conclusion, the typing method based on SNPs and the SNaPshot technology is a rapid method for M. pneumoniae typing, which can simultaneously detect eight SNPs without any sequencing step in a large-scale format. This SNP typing scheme is based on stable markers, showing higher strain discrimination than the MLVA-4 analysis, where the nonstable Mpn1 marker was removed. This typing method provides independent information complementary to that of MLVA, which may be associated with SNP typing results when higher strain discrimination is required.
ACKNOWLEDGMENTS
We thank Brigitte Tauzin at the Plateforme Technique de Biologie Moléculaire, Bordeaux University Hospital, Bordeaux, France, for valuable assistance during the development of the SNaPshot assay and Caroline Rooryck at the Service de Génétique Médicale, Bordeaux University Hospital, Bordeaux, France, for helpful discussions of the SNaPshot technology. We also thank Valérie Barbe, Claudine Medigue, and Stéphane Cruveiller from the CEA/Genoscope, Evry, France, for providing the raw data of the genome sequences and for assistance with the EvolScope pipeline and help with the SNP analysis.
This work was financially supported through internal funding. A. Touati was awarded a postdoctoral fellowship from the AXA Research Fund.
REFERENCES
- 1.Atkinson TP, Balish MF, Waites KB. 2008. Epidemiology, clinical manifestations, pathogenesis and laboratory detection of Mycoplasma pneumoniae infections. FEMS Microbiol Rev 32:956–973. doi: 10.1111/j.1574-6976.2008.00129.x. [DOI] [PubMed] [Google Scholar]
- 2.Korppi M, Heiskanen-Kosma T, Kleemola M. 2004. Incidence of community-acquired pneumonia in children caused by Mycoplasma pneumoniae: serological results of a prospective, population-based study in primary health care. Respirology 9:109–114. doi: 10.1111/j.1440-1843.2003.00522.x. [DOI] [PubMed] [Google Scholar]
- 3.Klement E, Talkington DF, Wasserzug O, Kayouf R, Davidovitch N, Dumke R, Bar-Zeev Y, Ron M, Boxman J, Lanier Thacker W, Wolf D, Lazarovich T, Shemer-Avni Y, Glikman D, Jacobs E, Grotto I, Block C, Nir-Paz R. 2006. Identification of risk factors for infection in an outbreak of Mycoplasma pneumoniae respiratory tract disease. Clin Infect Dis 43:1239–1245. doi: 10.1086/508458. [DOI] [PubMed] [Google Scholar]
- 4.Walter ND, Grant GB, Bandy U, Alexander NE, Winchell JM, Jordan HT, Sejvar JJ, Hicks LA, Gifford DR, Alexander NT, Thurman KA, Schwartz SB, Dennehy PH, Khetsuriani N, Fields BS, Dillon MT, Erdman DD, Whitney CG, Moore MR. 2008. Community outbreak of Mycoplasma pneumoniae infection: school-based cluster of neurologic disease associated with household transmission of respiratory illness. J Infect Dis 198:1365–1374. doi: 10.1086/592281. [DOI] [PubMed] [Google Scholar]
- 5.Pereyre S, Renaudin H, Charron A, Bébéar C. 2012. Clonal spread of Mycoplasma pneumoniae in primary school, Bordeaux, France. Emerg Infect Dis 18:343–345. doi: 10.3201/eid1802.111379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waller JL, Diaz MH, Petrone BL, Benitez AJ, Wolff BJ, Edison L, Tobin-D'Angelo M, Moore A, Martyn A, Dishman H, Drenzek CL, Turner K, Hicks LA, Winchell JM. 2014. Detection and characterization of Mycoplasma pneumoniae during an outbreak of respiratory illness at a university. J Clin Microbiol 52:849–853. doi: 10.1128/JCM.02810-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nir-Paz R, Abutbul A, Moses AE, Block C, Hidalgo-Grass C. 2012. Ongoing epidemic of Mycoplasma pneumoniae infection in Jerusalem, Israel, 2010 to 2012. Euro Surveill 17(8):pii:20095 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20095. [PubMed] [Google Scholar]
- 8.Pereyre S, Touati A, Petitjean-Lecherbonnier J, Charron A, Vabret A, Bébéar C. 2013. The increased incidence of Mycoplasma pneumoniae in France in 2011 was polyclonal, mainly involving M. pneumoniae type 1 strains. Clin Microbiol Infect 19:E212–E217. doi: 10.1111/1469-0691.12107. [DOI] [PubMed] [Google Scholar]
- 9.Lenglet A, Herrador Z, Magiorakos AP, Leitmeyer K, Coulombier D. 2012. Surveillance status and recent data for Mycoplasma pneumoniae infections in the European Union and European Economic Area, January 2012. Euro Surveill 17(5):pii:20075 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20075. [DOI] [PubMed] [Google Scholar]
- 10.Touati A, Cazanave C, Bébéar C. 2013. Strain typing of Mycoplasma pneumoniae and its value in epidemiology. Curr Pediatr Rev 9:334–342. doi: 10.2174/157339630904131223111632. [DOI] [Google Scholar]
- 11.Cousin-Allery A, Charron A, de Barbeyrac B, Fremy G, Jensen JS, Renaudin H, Bébéar C. 2000. Molecular typing of Mycoplasma pneumoniae strains by PCR-based methods and pulsed-field gel electrophoresis. Application to French and Danish isolates. Epidemiol Infect 124:103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dumke R, Luck PC, Noppen C, Schaefer C, von Baum H, Marre R, Jacobs E. 2006. Culture-independent molecular subtyping of Mycoplasma pneumoniae in clinical samples. J Clin Microbiol 44:2567–2570. doi: 10.1128/JCM.00495-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz SB, Thurman KA, Mitchell SL, Wolff BJ, Winchell JM. 2009. Genotyping of Mycoplasma pneumoniae isolates using real-time PCR and high-resolution melt analysis. Clin Microbiol Infect 15:756–762. doi: 10.1111/j.1469-0691.2009.02814.x. [DOI] [PubMed] [Google Scholar]
- 14.Spuesens EB, Hoogenboezem T, Sluijter M, Hartwig NG, van Rossum AM, Vink C. 2010. Macrolide resistance determination and molecular typing of Mycoplasma pneumoniae by pyrosequencing. J Microbiol Methods 82:214–222. doi: 10.1016/j.mimet.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Dégrange S, Cazanave C, Charron A, Renaudin H, Bébéar C, Bébéar CM. 2009. Development of multiple-locus variable-number tandem-repeat analysis for molecular typing of Mycoplasma pneumoniae. J Clin Microbiol 47:914–923. doi: 10.1128/JCM.01935-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benitez AJ, Diaz MH, Wolff BJ, Pimentel G, Njenga MK, Estevez A, Winchell JM. 2012. Multilocus variable-number tandem-repeat analysis of Mycoplasma pneumoniae clinical isolates from 1962 to the present: a retrospective study. J Clin Microbiol 50:3620–3626. doi: 10.1128/JCM.01755-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun H, Xue G, Yan C, Li S, Cao L, Yuan Y, Zhao H, Feng Y, Wang L, Fan Z. 2013. Multiple-locus variable-number tandem-repeat analysis of mycoplasma pneumoniae clinical specimens and proposal for amendment of MLVA nomenclature. PLoS One 8:e64607. doi: 10.1371/journal.pone.0064607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schork NJ, Fallin D, Lanchbury JS. 2000. Single nucleotide polymorphisms and the future of genetic epidemiology. Clin Genet 58:250–264. [DOI] [PubMed] [Google Scholar]
- 19.Dumke R, Catrein I, Pirkil E, Herrmann R, Jacobs E. 2003. Subtyping of Mycoplasma pneumoniae isolates based on extended genome sequencing and on expression profiles. Int J Med Microbiol 292:513–525. doi: 10.1078/1438-4221-00231. [DOI] [PubMed] [Google Scholar]
- 20.Filliol I, Motiwala AS, Cavatore M, Qi W, Hazbon MH, Bobadilla del Valle M, Fyfe J, Garcia-Garcia L, Rastogi N, Sola C, Zozio T, Guerrero MI, Leon CI, Crabtree J, Angiuoli S, Eisenach KD, Durmaz R, Joloba ML, Rendon A, Sifuentes-Osornio J, Ponce de Leon A, Cave MD, Fleischmann R, Whittam TS, Alland D. 2006. Global phylogeny of Mycobacterium tuberculosis based on single nucleotide polymorphism (SNP) analysis: insights into tuberculosis evolution, phylogenetic accuracy of other DNA fingerprinting systems, and recommendations for a minimal standard SNP set. J Bacteriol 188:759–772. doi: 10.1128/JB.188.2.759-772.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Ert MN, Easterday WR, Simonson TS, U'Ren JM, Pearson T, Kenefic LJ, Busch JD, Huynh LY, Dukerich M, Trim CB, Beaudry J, Welty-Bernard A, Read T, Fraser CM, Ravel J, Keim P. 2007. Strain-specific single-nucleotide polymorphism assays for the Bacillus anthracis Ames strain. J Clin Microbiol 45:47–53. doi: 10.1128/JCM.01233-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holt KE, Parkhill J, Mazzoni CJ, Roumagnac P, Weill FX, Goodhead I, Rance R, Baker S, Maskell DJ, Wain J, Dolecek C, Achtman M, Dougan G. 2008. High-throughput sequencing provides insights into genome variation and evolution in Salmonella Typhi. Nat Genet 40:987–993. doi: 10.1038/ng.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ragoussis J. 2009. Genotyping technologies for genetic research. Annu Rev Genomics Hum Genet 10:117–133. doi: 10.1146/annurev-genom-082908-150116. [DOI] [PubMed] [Google Scholar]
- 24.Sobrino B, Brion M, Carracedo A. 2005. SNPs in forensic genetics: a review on SNP typing methodologies. Forensic Sci Int 154:181–194. doi: 10.1016/j.forsciint.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 25.Bouakaze C, Keyser C, de Martino SJ, Sougakoff W, Veziris N, Dabernat H, Ludes B. 2010. Identification and genotyping of Mycobacterium tuberculosis complex species by use of a SNaPshot minisequencing-based assay. J Clin Microbiol 48:1758–1766. doi: 10.1128/JCM.02255-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grignani P, Peloso G, Achilli A, Turchi C, Tagliabracci A, Alu M, Beduschi G, Ricci U, Giunti L, Robino C, Gino S, Previdere C. 2006. Subtyping mtDNA haplogroup H by SNaPshot minisequencing and its application in forensic individual identification. Int J Legal Med 120:151–156. doi: 10.1007/s00414-005-0059-5. [DOI] [PubMed] [Google Scholar]
- 27.Huang CH, Chang MT, Huang MC, Lee FL. 2011. Rapid identification of Lactobacillus plantarum group using the SNaPshot minisequencing assay. Syst Appl Microbiol 34:586–589. doi: 10.1016/j.syapm.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Lou C, Cong B, Li S, Fu L, Zhang X, Feng T, Su S, Ma C, Yu F, Ye J, Pei L. 2011. A SNaPshot assay for genotyping 44 individual identification single nucleotide polymorphisms. Electrophoresis 32:368–378. doi: 10.1002/elps.201000426. [DOI] [PubMed] [Google Scholar]
- 29.Ning Z, Cox AJ, Mullikin JC. 2001. SSAHA: a fast search method for large DNA databases. Genome Res 11:1725–1729. doi: 10.1101/gr.194201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dandekar T, Huynen M, Regula JT, Ueberle B, Zimmermann CU, Andrade MA, Doerks T, Sanchez-Pulido L, Snel B, Suyama M, Yuan YP, Herrmann R, Bork P. 2000. Re-annotating the Mycoplasma pneumoniae genome sequence: adding value, function and reading frames. Nucleic Acids Res 28:3278–3288. doi: 10.1093/nar/28.17.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kenri T, Horino A, Matsui M, Sasaki Y, Suzuki S, Narita M, Ohya H, Okazaki N, Shibayama K. 2012. Complete genome sequence of Mycoplasma pneumoniae type 2a strain 309, isolated in Japan. J Bacteriol 194:1253–1254. doi: 10.1128/JB.06553-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kibbe WA. 2007. OligoCalc: an online oligonucleotide properties calculator. Nucleic Acids Res 35:W43–W46. doi: 10.1093/nar/gkm234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hunter PR, Gaston MA. 1988. Numerical index of the discriminatory ability of typing systems: an application of Simpson's index of diversity. J Clin Microbiol 26:2465–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grundmann H, Hori S, Tanner G. 2001. Determining confidence intervals when measuring genetic diversity and the discriminatory abilities of typing methods for microorganisms. J Clin Microbiol 39:4190–4192. doi: 10.1128/JCM.39.11.4190-4192.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carriço JA, Silva-Costa C, Melo-Cristino J, Pinto FR, de Lencastre H, Almeida JS, Ramirez M. 2006. Illustration of a common framework for relating multiple typing methods by application to macrolide-resistant Streptococcus pyogenes. J Clin Microbiol 44:2524–2532. doi: 10.1128/JCM.02536-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Severiano A, Pinto FR, Ramirez M, Carrico JA. 2011. Adjusted Wallace coefficient as a measure of congruence between typing methods. J Clin Microbiol 49:3997–4000. doi: 10.1128/JCM.00624-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manso-Silván L, Dupuy V, Lysnyansky I, Ozdemir U, Thiaucourt F. 2012. Phylogeny and molecular typing of Mycoplasma agalactiae and Mycoplasma bovis by multilocus sequencing. Vet Microbiol 161:104–112. doi: 10.1016/j.vetmic.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 38.Mayor D, Jores J, Korczak BM, Kuhnert P. 2008. Multilocus sequence typing (MLST) of Mycoplasma hyopneumoniae: a diverse pathogen with limited clonality. Vet Microbiol 127:63–72. doi: 10.1016/j.vetmic.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 39.McAuliffe L, Gosney F, Hlusek M, de Garnica ML, Spergser J, Kargl M, Rosengarten R, Ayling RD, Nicholas RA, Ellis RJ. 2011. Multilocus sequence typing of Mycoplasma agalactiae. J Med Microbiol 60:803–811. doi: 10.1099/jmm.0.028159-0. [DOI] [PubMed] [Google Scholar]
- 40.Bouakaze C, Keyser C, Gonzalez A, Sougakoff W, Veziris N, Dabernat H, Jaulhac B, Ludes B. 2011. Matrix-assisted laser desorption ionization-time of flight mass spectrometry-based single nucleotide polymorphism genotyping assay using iPLEX gold technology for identification of Mycobacterium tuberculosis complex species and lineages. J Clin Microbiol 49:3292–3299. doi: 10.1128/JCM.00744-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pinto FR, Melo-Cristino J, Ramirez M. 2008. A confidence interval for the wallace coefficient of concordance and its application to microbial typing methods. PLoS One 3:e3696. doi: 10.1371/journal.pone.0003696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Payseur BA, Cutter AD. 2006. Integrating patterns of polymorphism at SNPs and STRs. Trends Genet 22:424–429. doi: 10.1016/j.tig.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 43.Whiley DM, Goire N, Rahimi F, Lahra MM, Limnios AE, Nissen MD, Sloots TP. 2013. Real-time PCR genotyping of Neisseria gonorrhoeae isolates using 14 informative single nucleotide polymorphisms on gonococcal housekeeping genes. J Antimicrob Chemother 68:322–328. doi: 10.1093/jac/dks381. [DOI] [PubMed] [Google Scholar]
- 44.Ma L, Taylor S, Jensen JS, Myers L, Lillis R, Martin DH. 2008. Short tandem repeat sequences in the Mycoplasma genitalium genome and their use in a multilocus genotyping system. BMC Microbiol 8:130. doi: 10.1186/1471-2180-8-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cazanave C, Charron A, Renaudin H, Bébéar C. 2012. Method comparison for molecular typing of French and Tunisian Mycoplasma genitalium-positive specimens. J Med Microbiol 61:500–506. doi: 10.1099/jmm.0.037721-0. [DOI] [PubMed] [Google Scholar]
- 46.Sulyok KM, Kreizinger Z, Fekete L, Janosi S, Schweitzer N, Turcsanyi I, Makrai L, Erdelyi K, Gyuranecz M. 2014. Phylogeny of Mycoplasma bovis isolates from Hungary based on multi locus sequence typing and multiple-locus variable-number tandem repeat analysis. BMC Vet Res 10:108. doi: 10.1186/1746-6148-10-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chalker V, Stocki T, Litt D, Bermingham A, Watson J, Fleming D, Harrison T. 2012. Increased detection of Mycoplasma pneumoniae infection in children in England and Wales, October 2011 to January 2012. Euro Surveill 17(6):pii=20081 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20081. [PubMed] [Google Scholar]
- 48.Pereyre S, Charron A, Renaudin H, Bébéar C, Bébéar CM. 2007. First report of macrolide-resistant strains and description of a novel nucleotide sequence variation in the P1 adhesin gene in Mycoplasma pneumoniae clinical strains isolated in France over 12 years. J Clin Microbiol 45:3534–3539. doi: 10.1128/JCM.01345-07. [DOI] [PMC free article] [PubMed] [Google Scholar]