Abstract
Alveolar echinococcosis (AE), caused by the Echinococcus multilocularis metacestode, represents one of the most frequently fatal zoonoses. Early diagnosis significantly reduces morbidity and mortality associated with AE. Diagnosis of AE largely depends on a combination of imaging and serological tests due to its minimal clinical manifestations. Several antigens derived from the whole worm and protoscolex have been targeted for AE serodiagnosis, while the antigenic properties of E. multilocularis hydatid fluid (EmHF) are unclear. We observed two AE-specific 6- and 8-kDa antigen proteoforms through an immunoproteome array of the EmHF. We identified these proteins as representing an E. multilocularis antigen B3 (EmAgB3) isoform, and the proteins were shown to be encoded by the same gene. We cloned the gene and expressed the recombinant EmAgB3 protein (rEmAgB3) in Escherichia coli. rEmAgB3 exhibited sensitivity of 90.9% (80/88 cases) and specificity of 98.5% (597/606 samples) by immunoblotting. The positive and negative predictive values were 89.9% and 98.6%, respectively. The protein did not show antibody responses to 33 AE sera collected during posttreatment follow-up monitoring. Mouse sera experimentally infected with AE protoscoleces began to demonstrate specific antibody responses to native and recombinant EmAgB3 6 months after infection. At that stage, fully mature metacestode vesicles that harbored the brood capsule, primary cell, and protoscolex were observed within an AE mass(es). The response declined along with worm degeneration. Our results demonstrate that the immune responses to this EmAgB3 isoform were highly correlated with worm viability accompanied with AE progression. rEmAgB3 is a promising biomarker for serological assessment of AE patients.
INTRODUCTION
Alveolar echinococcosis (AE), an infection caused by Echinococcus multilocularis metacestodes, is one of the most frequently chronic and fatal zoonoses (1–3). Epidemiological evidence has demonstrated that 0.5 to 6 of 100,000 inhabitants are infected in areas of endemicity of Europe and Central Asia (4, 5). In the Tibetan and Qinghai plateaus in China, the population at risk of infection is estimated to encompass 60 million (6).
Two kinds of hosts are intimately involved in the life cycle of E. multilocularis. Domestic and wild canids (definitive hosts) harbor the adult tapeworm in their intestines. The worms produce numerous eggs, which pass out of the host with gravid segments. When wild rodents and humans (intermediate hosts) take in the eggs, oncospheres are activated in the small intestine; they then penetrate the intestinal wall and enter the circulation. They primarily lodge in the liver and grow slowly into multivesiculated metacestode vesicles, in which brood capsules, primary cells, and protoscoleces develop, resulting in AE (1, 7). AE usually presents with a complex of nonspecific liver manifestations that mimic cystadenoma, hepatocellular carcinoma, and liver cirrhosis (8, 9).
Diagnosis of AE largely depends on a combination of imaging and serological tests, since most patients complain of only minimal or vague clinical symptoms and signs (10, 11). For both diagnosis and follow-up monitoring of AE, detection of AE-specific DNA fragments by PCR is also specific and sensitive. However, PCR detection has limitations, because the use of PCR is not feasible unless AE-derived parasitic materials are available (10–12). Imaging diagnosis also requires a differential diagnosis due to the atypical finding of an AE mass(es) (13). Serological tests, such as immunoblotting and enzyme-linked immunosorbent assay (ELISA), may provide additional data for detection and disease course characterization of AE, but these require better detection antigens unique to AE.
Identification of antigens that can effectively detect AE is a challenging issue. A host of studies have focused on the development of AE serodiagnosis. A 54-kDa Em2 antigen was purified from whole E. multilocularis metacestode through immunoaffinity chromatography by absorption of cross-reactive components (14). Em2, a mucin-containing glycoprotein, is tightly associated with the laminated layer (15). Its immunodominant epitope comprises a trisaccharide (Galα1-4Galβ1-3GalNAc) which is widely distributed in several trematodes and which shows cross-reactivity (16, 17). A membrane-bound 53-kDa alkaline phosphatase of E. multilocularis showing strong antigenicity also shares a common epitope with Em2 (18, 19).
In another study, screening of a cDNA library using pooled AE patient sera yielded two recombinant proteins of 31 and 34 kDa (20). By employing antisera against these proteins, a full-length cDNA that encoded a 65-kDa Em10 protein (EmII/3) was isolated (21). Immunoblotting revealed two proteins at 52 and 65 kDa. The 52-kDa protein was a degradation product of the 65-kDa protein. In addition, 16- and 18-kDa proteins (Em16/18) were identified from the protoscolex using isoelectric focusing (22). Native and recombinant forms of these proteins have been investigated for targeted AE serodiagnosis and have demonstrated reliable diagnostic performance (10, 11). However, these proteins are components/proteolytic fragments of ezrin-like protein (ELP), which belongs to the ezrin-radexin-moesin (ERM) family of eukaryotes (23, 24). These proteins bear the same epitope derived from a single ELP molecule, which might hamper detection of the diverse immune responses of infected patients according to individual immune status and/or progression of AE. The proteins cross-react with sera from cystic echinococcosis (CE), cysticercosis, and other trematodiases, including fascioliasis and schistosomiasis (10, 11).
Characterization of a novel serodiagnostic biomarker(s) may be beneficial in the practical diagnosis of AE in various clinical settings. In this study, two proteoforms (6 and 8 kDa) in E. multilocularis hydatid fluid (EmHF) exhibited specific antibody responses to AE patient sera. We identified each of these proteoforms as a B3 antigen (EmAgB3). The two B3 antigens might be encoded by the same gene (E. multilocularis GeneDB accession number EmuJ_000381600). We expressed EmAgB3 and provided evidence that the bacterially expressed recombinant protein is a promising candidate for serological assessment of AE. We also analyzed the correlation between the elevation of levels of specific antibodies against EmAgB3 and histopathological states of AE in an experimental mouse AE model.
MATERIALS AND METHODS
Parasites.
Kunming mice (Lanzhou Institute of Biological Products, China) (40 6-week-old mice) were intraperitoneally infected with 1,000 viable protoscoleces collected from naturally infected voles (Microtus fuscus) for 9 months. The voles had been caught in an area of AE endemicity in Qinghai Province, China. EmHF was drawn from intact AE cysts in the presence of protease inhibitor cocktail (Roche, Penzberg, Germany) (10 ml/half tablet). EmHF was centrifuged at 20,000 × g for 1 h at 4°C, concentrated by lyophilization, and stored at −80°C until use. Echinococcus granulosus HF (EgHF) was collected from a single fertile ovine CE2 cyst at a local abattoir in Xining, Qinghai Province, China (25).
Mouse infection sera and pathological specimens.
Another 40 Kunming mice were infected with protoscoleces as described above. Mice (5 to 7 per group) were sacrificed serially at 1, 3, 6, 9, and 14 months after infection under ether anesthesia and bled. Sera were collected by centrifugation at 3,000 × g for 10 min and stored at −80°C. Infection patency was confirmed in individual mice by macroscopic observation of an AE lesion(s). To observe the histological status of the AE mass, lesions were dissected, fixed in 10% neutral formalin, and embedded in paraffin. Sections (4 μm thick) were cut and subjected to periodic acid-Shiff (PAS) staining or hematoxylin-eosin (HE) staining. The slides were photographed using TissueFAXSi8 Plus (TissueGnostics, Vienna, Austria).
Patient sera.
Sera of 88 hepatic AE patients were tested. The patients were diagnosed by typical ultrasonographic (US) findings and were categorized as early-stage patients (P1 and P2; n = 48) or advanced-stage patients (P3 and P4; n = 40) according to the tumor node metastasis (PNM) classification (26). Table 1 summarizes the demographic, radiological, and clinical features of the enrolled AE patients. Thirty-three AE sera were additionally collected during follow-up. The patients were treated with albendazole (15 mg/kg of body weight bis in die [b.i.d.]) for between 6 months and 1 year according to patient accessibility. After the end of the medication course, the patients were monitored by US for between 6 months and 2 years. Sera were collected during follow-up (see listings in Table 2). Sera from patients with other helminthic diseases (419 samples), including CE (n = 101), neurocysticercosis (n = 100), sparganosis (n = 50), clonorchiasis (n = 87), fascioliasis (n = 30), schistosomiasis japonicum (n = 21), and paragonimiasis (n = 30), were used. Sera from patients with other pathologically and/or radiologically proven hepatic diseases (84 cases), which included cirrhosis (n = 23), simple cyst (n = 23), carcinoma (n = 18), and the presence of masses (n = 20), were tested. Sera from healthy individuals who declared no possibility of exposure to protozoan and helminthic infections (n = 103) were used (Table 2). Informed consent was obtained from individual patients. Consent was also obtained in written form from parents and/or local health authorities in cases involving children or verbally in cases of illiterate patients. All protocols for use of patient sera and animal infections were approved by the Institutional Review Board (IRB). Animal experiments were conducted in the Laboratory Animal Research Center of Qinghai Province Institute for Endemic Disease Prevention and Control (protocol 2013-7-22).
TABLE 1.
Demographic, radiological, and clinical features of AE patients in this study
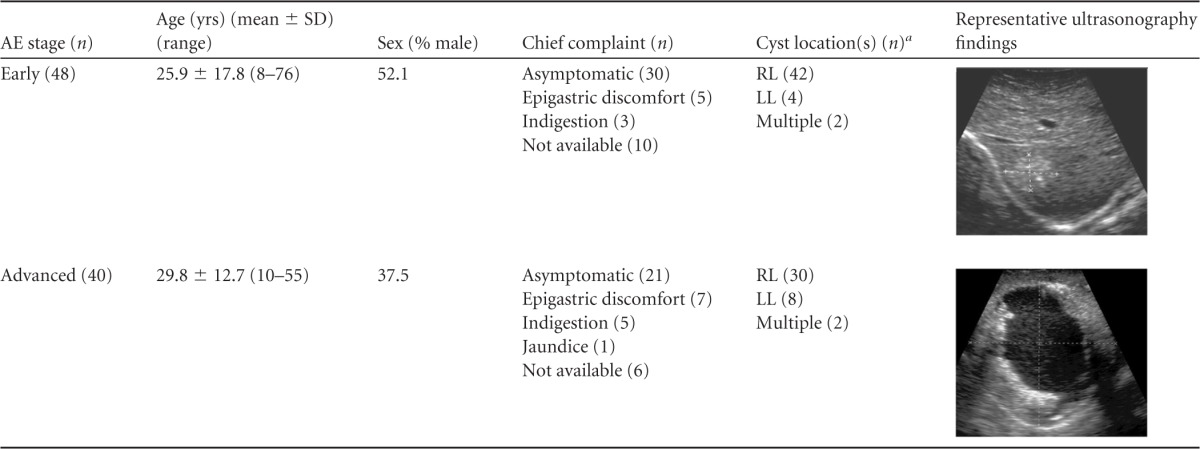
RL, right hepatic lobe; LL, left hepatic lobe.
TABLE 2.
Diagnostic performance of recombinant EmAgB3 (EmuJ_000381600)
| Disease category | No. positive/no. tested | % sensitivity | % specificity |
|---|---|---|---|
| AE | |||
| Early | 44/48 | 91.7 | |
| Advanced | 36/40 | 90 | |
| Follow-up | 0/33 | 100 | |
| >1 yrs | 0/13 | 100 | |
| 1–2 yrs | 0/20 | 100 | |
| CE | |||
| Stage 1 | 1/23 | 95.7 | |
| Stage 2 | 3/16 | 81.4 | |
| Stage 3 | 2/27 | 92.6 | |
| Stage 4 | 1/21 | 95.2 | |
| Stage 5 | 1/14 | 92.9 | |
| Neurocysticercosis | 0/100 | 100 | |
| Sparganosis | 0/50 | 100 | |
| Clonorchiasis | 1/87 | 98.9 | |
| Fascioliasis | 0/30 | 100 | |
| Schistosomiasis japonicum | 0/21 | 100 | |
| Paragonimiasis | 0/30 | 100 | |
| Liver cirrhosis | 0/23 | 100 | |
| Liver cyst | 0/23 | 100 | |
| Hepatocellular carcinoma | 0/18 | 100 | |
| Liver mass | 0/20 | 100 | |
| Normal control | 0/103 | 100 | |
| Overalla | 80/88 | 90.9 | |
| 9/606 | 98.5 |
Overall sensitivity and specificity were calculated employing 88 active-stage-AE sera and 606 other sera, respectively.
2-DE and protein identification.
EmHF (200 μg) samples were electrofocused with immobilized pH gradient (IPG) strips (GE Healthcare, Piscataway, NJ) (13 cm in length, pH 6 to 11) for 25 kVh, further separated by 15% SDS-PAGE (gels, 160 by 160 by 1.5 mm) and two-dimensional electrophoresis (2-DE), and stained with Coomassie brilliant blue G-250 (CBB). Spots were in-gel tryptic digested and subjected to Ab Sciex matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) (4800 Plus System; Ab Sciex, Framingham, MA). Internal standards were tryptic autodigestion peaks (m/z = 842.5099 and 2211.1046). Monoisotopic peptides (600 to 3,500 Da) were detected. The proteins were identified by peptide mass fingerprinting (PMF) using Matrix Science Mascot. Mass tolerance was ± 50 ppm, and one missed cleavage site was allowed. Identification was accepted when PMF revealed ≥2 identified peptides (>99% probability). Cysteine carbamidomethylation and methionine oxidation were considered. Searches were conducted for files in both the NCBInr database (DB) (http://www.ncbi.nlm.nih.gov) and the Sanger E. multilocularis GeneDB (http://www.genedb.org/Homepage/Emultilocularis).
Cloning and expression of recombinant protein.
We designed gene-specific primers tagged with BamHI (forward; 5′-CGCGGATCCGATGATGATGAAGTGACC-3′) and HindIII (reverse; 5′-CGCAAGCTTCTACTCATCCTCTTTAAT-3′) to clone the AE-specific EmAgB3 gene (EmuJ_000381600). Primers were used in reverse transcription (RT)-PCR with total RNA (200 ng) extracted from the whole AE mass and an RT-PCR Premix kit (iNtRON; Seongnam, South Korea). The thermal cycler profile included 30 min at 45°C and 3 min at 95°C followed by 35 cycles of 1 min at 95°C, 45 s at 60°C, and 1 min at 72°C and a final extension for 5 min at 72°C. The amplicon was ligated to the pGEX-6p-1 vector (Novagen, Cambridge, MA) and transformed into Escherichia coli BL21(DE3). Recombinant protein (rEmAgB3) was induced with IPTG (1 mM isopropyl–d-thiogalactopyranoside) for 4 h at 37°C. Glutathione transferase (GST)-tagged rEmAgB3 was purified using a glutathione affinity column (GE Healthcare). GST tags were cleaved by the use of a Thrombin CleanCleave kit (Sigma-Aldrich, St. Louis, MO).
Generation of monospecific antibodies.
Mouse polyclonal monospecific antibodies were generated in specific-pathogen-free BALB/c mice. rEmAgB3 (50 μg/mouse) was intraperitoneally immunized with Freund's adjuvants (Sigma-Aldrich) at 2-week intervals. Two weeks after the third immunization, rEmAgB3 (10 μg) was administered through the tail vein. One week later, blood was collected by heart puncture and centrifuged at 3,000 × g for 5 min. The antiserum was stored at −80°C. Immunization protocols were approved by the IRB and conducted in the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)-approved Laboratory Animal Research Center of Sungkyunkwan University (protocol 2014-12).
Immunoblotting.
EmHF and EgHF samples separated by the use of 10% Tricine SDS-PAGE or 2-DE were electroblotted to nitrocellulose membranes (Santa Cruz, Dallas, TX) for 1 to 4 h at 4°C and blocked with Tris-buffered saline (pH 8.0) containing 0.05% Tween 20 and 3% skim milk. Membranes were incubated with pooled patient serum (1:1,000 dilution) or mouse-specific antibodies (1:2,000 dilution) overnight at 4°C, followed by incubation with horseradish peroxidase (HRP)-conjugated host-specific antibodies (Cappel, Huntington, CA) (1:4,000 dilution) for 2 h, and visualized with SuperSignal chemiluminescence (ECL; GE Healthcare) after a 2-min exposure. rEmAgB3 was resolved by 12% SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA). The membranes were cut into strips and probed with a panel of sera (1:200 dilution) overnight. The strips were further incubated with HRP-conjugated anti-human IgG (Cappel) (1:1,000 dilution) for 4 h and developed with 4-chloro-1-naphthol (4C1N) for 10 min. Sensitivity and specificity were calculated as follows: sensitivity = number of true positives/(number of true positives + number of false negatives) × 100; specificity = number of true negatives/(number of false positives + number of true negatives) × 100. Positive predictive and negative predictive values were calculated as follows: positive predictive value = number of true positives/(number of true positives + number of false positives) × 100; negative predictive value = number of true negatives/(number of true negatives + number of false negatives) × 100.
RESULTS
Clinical characteristics of AE cases.
We categorized AE case subjects in this study as being in the early stage (n = 48) or the advanced stage (n = 40) of the disease according to their US findings (26). The early-stage patients constituted 25 males and 23 females (mean age, 25.9 ± 17.8 years; range, 8 to 76 years). These patients demonstrated clusters of multiple echogenic small cysts with an indistinct margin (multiple small hailstorm-like cysts without a solid component). Small calcifications were noticed in three P2 patients. Patients (15 males and 25 females) who revealed an irregular cystic area associated with central necrosis surrounded by peripheral nonhomogenous echogenicity were classified as in the advanced stage of disease. The mean of the ages of the members of this group was 29.8 ± 12.7 years (range, 10 to 55 years). Regardless of the differential imaging findings, large proportions (62.5% and 52.5% for the early and advanced stages, respectively) of the patients did not present any discernible or specific symptoms. Some patients complained of epigastric discomfort (12 patients) and indigestion (8 patients). Clinical manifestations were not evident in 16 patients (18.2%). Involvement of the right hepatic lobe was frequently detected (87.5%). Multiple lesions were observed in four cases (4.6%) (Table 1). Of 33 follow-up patients, 3 complained of mild indigestion (9.1%). The other 30 patients did not present any clinical manifestations.
Identification of EmHF proteins with specific immunoreactivity to AE sera.
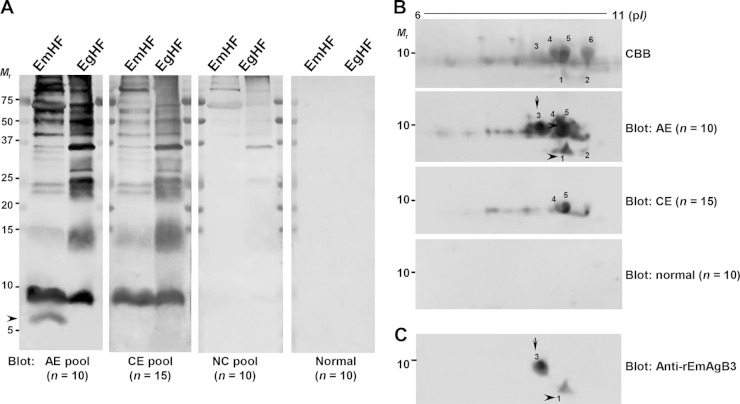
We determined the global immunoreactivity profiles of EmHF and EgHF employing pooled sera from patients with AE, CE, and neurocysticercosis and from normal controls. Several protein bands, including those corresponding to 8, 15, 21, 23, 28, and >35 kDa, showed concomitant positive reactions with AE and CE sera (Fig. 1A). Neurocysticercosis sera exhibited cross-reactions with >35-kDa proteins. Interestingly, the 6-kDa protein of EmHF displayed a specific antibody response to AE sera (arrowhead), demonstrating that at least one molecule in EmHF invoked a specific response to AE sera.
FIG 1.
Identification of specific proteins of Echinococcus multilocularis hydatid fluid (EmHF) that are immunoreactive to sera of patients with alveolar echinococcosis (AE). (A) EmHF and E. granulosus hydatid fluid (EgHF) were separated by 10% Tricine SDS-PAGE, transferred to PVDF membranes, and probed with pooled serum from patients with AE, cystic echinococcosis (CE), and neurocysticercosis (NC) and from healthy controls. The blots were further incubated with HRP-conjugated anti-human IgG and developed with 4C1N. (B) EmHF (200 μg proteins) was electrofocused with IPG strips (pH 6 to 11), resolved by 15% SDS-PAGE, transferred to nitrocellulose membranes, and probed with the respective serum samples. The blots were developed with ECL. Spots 1 to 6 were subjected to protein identification by MALDI-TOF MS. The identified proteins are listed in Table S1 in the supplemental material. CBB, Coomassie brilliant blue G-250-stained 2-DE gel; Mr, relative molecular weights; pI, isoelectric point. (C) A 2-DE blot incubated with mouse antibodies specific to rEmAgB3 was developed with ECL. AE-specific reactive bands and spots are indicated by arrows and arrowheads, respectively.
We analyzed the subproteome of the EmHF low-molecular-mass proteins (5 to 15 kDa). Numerous proteoforms were superimposed at approximately 8 kDa with different isoelectric points (pI) between 6.5 and 9.8 (Fig. 1B). When we probed these blots with the pooled sera of AE and CE patients, spot 1 (6 kDa with pI 9.5) and spot 3 (8 kDa with pI 9.2) revealed a strong and specific response to pooled AE serum (arrow and arrowhead). Spots 4 and 5 showed cross-reactions with CE sera. We conducted MALDI-TOF MS analysis. More than 21 sequences putatively coding for EmAgB isoforms registered in the GenBank database (http://www.ncbi.nlm.nih.gov) were annotated through a Mascot search. However, most of them were partial or were variants that contained one to four different amino acid sequences (data not shown).
We retrieved EmAgB gene data in the Sanger E. multilocularis GeneDB (http://www.genedb.org/Homepage/Emultilocularis) and observed that three different genes had identity with EmAgB3 isoforms. Spots 1 and 3 showed strong matches with two EmAgB3 genes (EmuJ_000381600 and EmuJ_000381700). Spot 2 (6 kDa with pI 9.8), which exhibited minimal antibody responses, represented another EmAgB3 isoform (EmuJ_000381500). Spots 5 and 6 were recognized as EmAgB1 and EmAgB2 (EmuJ_000381200 and EmuJ_000381100). The identified proteins are listed in Table S1 in the supplemental material. The anti-rEmAgB3 antibodies also demonstrated specific antibody responses to spots 1 and 3 but not to other EmAgB isoforms, including spot 2 (Fig. 1C). We analyzed these sequences. EmuJ_000381600 and EmuJ_000381700 were identical, including their chromosomal structures, but EmuJ_000381500 harbored different sequences and showed a different exon-intron boundary structure (data not shown). These two genes displayed 93% identity at the protein level. These results indicated that spots 1 and 3 are encoded by the same gene and that the molecule induces AE-specific immunoreactivity.
Cloning and expression of recombinant protein.
We cloned the EmuJ_000381600 gene and expressed the mature domain (amino acids 22 to 86) in fusion with GST in an E. coli cell. Recombinant protein (rEmAgB3) migrated to ca. 34 kDa due to its GST tag. When the GST domain was cleaved, it was found to be positioned at 8 kDa, in agreement with the deduced molecular mass (7,525 Da). We determined the antibody reactivity of these proteins. Both the 34-kDa rEmAgB3-GST fusion protein and 8-kDa rEmAgB3 reacted strongly with pooled sera from AE patients or anti-rEmAgB3 antibodies, while the 26-kDa GST tag revealed no reaction (see Fig. S1 in the supplemental material; results of immunoblotting probed with anti-rEmAgB3 antibodies and normal controls are not shown).
Assessment of diagnostic reliability of rEmAgB3.
We evaluated the diagnostic applicability of rEmAgB3 by immunoblotting. When blots containing rEmAgB3 were probed with AE sera (48 early stage and 40 advanced stage), positive antibody reactions were evident in 80 patients, irrespectively of AE status (sensitivity, 90.9%). In contrast, the blots probed with 33 follow-up AE sera did not show positive reactions (Fig. 2A). Among 101 CE sera, eight cases showed weak positive reactions (Fig. 2B). Sera from other hepatic lesions (cirrhosis, primary carcinoma, and cyst/mass), other liver invasive helminthiases (clonorchiasis, fascioliasis, and schistosomiasis japonicum), and normal controls demonstrated no reaction, except for one sample from a patient with clonorchiasis, which showed a weak cross-reaction (Fig. 2C and D). The diagnostic specificity was 98.5% (597/606 samples). Positive and negative predictive values were 89.9% and 98.6%, respectively. Table 2 summarizes the diagnostic performance of rEmAgB3.
FIG 2.
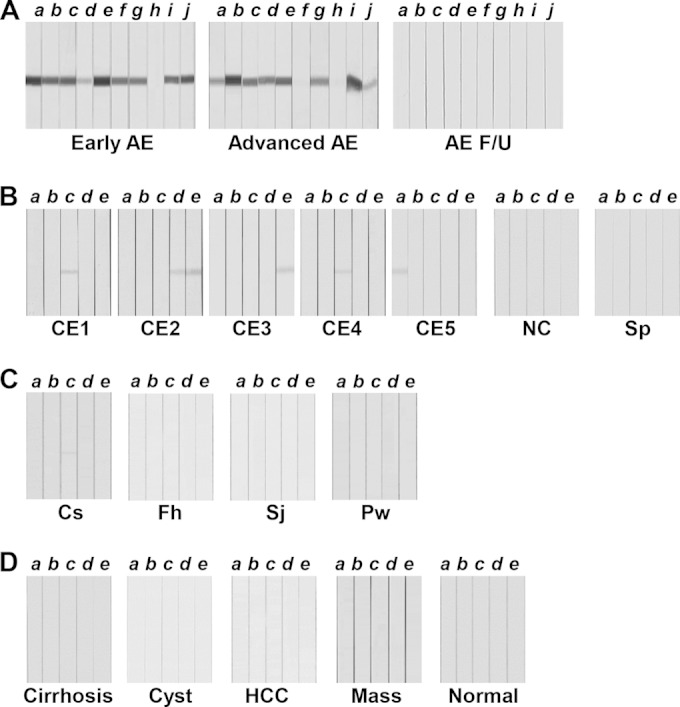
Assessment of the diagnostic reliability of recombinant EmAgB3 (rEmAgB3). rEmAgB3 protein separated by 12% SDS-PAGE was transferred to PVDF membranes. The membranes were cut into strips and incubated with individual patient sera. The strips were subsequently incubated with HRP-conjugated anti-human IgG and developed with 4C1N. (A) Immunoreactions of sera from patients with alveolar echinococcosis (AE). AE F/U, AE sera collected during follow-up monitoring. (B) Blots containing rEmAgB3 were probed with sera from patients with larval cestodiases, such as those seen in cases of cystic echinococcosis (CE; stages CE1 to CE5 are indicated), neurocysticercosis (NC), and sparganosis (Sp). (C) Sera from patients with clonorchiasis (Cs), fascioliasis (Fh), schistosomiasis japonicum (Sj), and paragonimiasis (Pw) were reacted with rEmAgB3. (D) Sera of patients with hepatic lesions, such as those with liver cirrhosis (Cirrhosis), simple liver cyst (Cyst), primary hepatocellular carcinoma (HCC), and liver mass (Mass), and sera from healthy controls (Normal) were incubated with rEmAgB3. Letters a to j indicate individual serum samples.
Correlation between appearance of specific antibodies against EmAgB3 and histopathological features of AE.
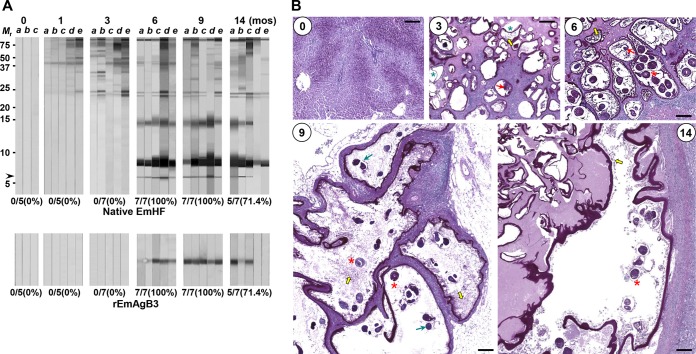
AE sera collected from the follow-up monitoring did not show antibody responses to native EmAgB3 or rEmAgB3. This result suggested that the presence of anti-EmAgB3 might be related to the pathological states of AE in accordance with disease progression. To determine whether EmAgB3 immunoreactivity was correlated with worm viability, we analyzed the antibody responses of the EmHF proteins probed with mouse sera chronologically collected after experimental infection. One month after infection, proteins of >35 kDa revealed positive reactions. Antibody responses became stronger over time. Several proteins, including those corresponding to molecular masses of 21 and 23 kDa, which showed prominent reactions (27) exhibited antibody responses 3 months postinfection. However, these proteins were cross-reactive with CE sera (Fig. 1A). Specific antibodies against 8-kDa EmAgB isoforms were detected from 6 months postinfection. Specifically, 6-kDa EmAgB3 reactions (7/7 cases, 100%) were evident (arrow). This response became regressive at 14 months postinfection (5/7 samples, 71.4%; upper panel, Fig. 3A). Immunoblot outcomes employing rEmAgB3 matched well with the findings observed with native EmHF (lower panel, Fig. 3A).
FIG 3.
Induction profile of specific antibody responses to native and recombinant EmAgB3 and histopathological alteration of AE masses in experimental murine AE. Mice were infected with 1,000 AE protoscoleces. Sera and tissue specimens were collected as indicated. (A) Chronological changes in the antibody responses of experimental mouse sera (each with n = 5 to 7) to EmHF. EmHF was separated by 10% Tricine SDS-PAGE and transferred to PVDF membranes. The strips were incubated with individual mouse sera, further reacted with HRP-conjugated anti-mouse IgG, and developed with 4C1N. Letters a to e indicate the serum samples. The numbers (percentages) of cases showing seropositivity for native 6-kDa EmAgB3 and rEmAgB3 are indicated. Mr, relative molecular weights. mos, months postinfection. (B) Characteristic histopathological features of AE at 3, 6, 9, and 14 months after experimental infection in mouse. The slides were observed with periodic acid-Shiff (PAS) staining. Newly developing primary vesicles seen as septa (green asterisks), brood capsules (red arrows), primary cells (yellow arrows), immature protoscoleces (green arrows), and mature protoscoleces (red asterisks) are marked. Bar, 200 μm. Detailed descriptions with magnified views are also presented in Fig. S2A in the supplemental material.
We subsequently observed the histopathological alterations of AE as infection time increased. Three months postinfection, numerous newly developing metacestode vesicles, seen as septa, were observed (panel 3, Fig. 3B). After 6 months of worm growth, mature metacestode vesicles, which contained brood capsules, primary cells, and protoscoleces, were recognized (panel 6, Fig. 3; see also Fig. S2A in the supplemental material). Nine months postinfection, intact metacestode vesicles showing significant growth were surrounded by thick granulomas. The protoscoleces, primary cells, and brood capsules were also detected, but their numbers were decreased compared to those seen at 6 months (panel 9, Fig. 3B). The AE mass at 14 months postinfection displayed various histological features of involution; some lesions showed lamellated masses with diffuse and extensive necrosis, while other lesions simultaneously demonstrated degenerating masses with disrupted and thickened laminated layers with few protoscoleces. Advanced granuloma walls were recognized (panel 14, Fig. 3B). In contrast, a pathological liver specimen from a mouse whose serum did not show positive reactions to EmAgB3 revealed an extensive degeneration pattern, with only empty metacestode vesicles observed (see Fig. S2B in the supplemental material). Sera from these mice also exhibited relatively weak antibody responses to EmHF (Fig. 3A).
DISCUSSION
In this study, we compared the immunoreactivity profiles of EmHF and EgHF, employing sera from patients with AE, CE, and other diseases. Two EmAgB3 proteoforms exhibited AE-specific immune responses. Proteomic and genomic analyses demonstrated that the same gene encoded these proteins. A previous report indicated that this gene was actively transcribed during the metacestode stage (28). We also observed expression of the mRNA transcript of the EmAgB3 gene by real-time RT-PCR (unpublished observation). We cloned the gene and expressed the recombinant protein. Bacterially expressed rEmAgB3 indeed demonstrated a specific and sensitive reaction to sera from patients with early-stage or advanced-stage AE. Moreover, it showed no response to AE patient sera collected after treatment. These results convincingly indicate that the antibody responses culminating in this EmAgB3 response disappeared rapidly after parasitostatic chemotherapy and further suggested that EmAgB3 might be applicable for serodiagnosis and follow-up surveillance of AE. The use of rEmAgB3, with easy applicability and high reproducibility, might be appropriate for bedside diagnosis for individual patients and for large-scale epidemiological surveys in areas of endemicity.
We do not have information regarding the time at which the levels of specific antibodies against EmAgB3 become elevated after infection and how long the antibodies persist in infected patients. Therefore, we observed a correlation between the presence of specific anti-EmAgB3 antibodies and histopathological alterations of the lesions in experimental murine AE, although the patterns of AE establishment and growth might differ in human hosts. We surmised that the positive reaction of the 6-kDa protein was an indicator of a genuine EmAgB3 antibody response, since at least three EmAgB isoforms were superimposed at 8 kDa (Fig. 3A). Specific antibody responses to EmAgB3 were detected at from 6 to 14 months postinfection until termination of the experiment, although the detection rate decreased somewhat at 14 months postinfection. In the period during which all mouse sera examined showed positive reactions to both native and recombinant EmAgB3 (14/14 samples; Fig. 3A), mature metacestode vesicles harboring brood capsules, primary cells, and protoscoleces were clearly recognizable in pathological specimens. Fourteen months postinfection, AE masses demonstrated mixed patterns of proliferative and degenerative changes. At that stage, decreased specific antibody responses to EmAgB3 were observed (5/7 samples; 71.4%).
The lowered sensitivity of detection of specific anti-EmAgB3 antibodies in mouse sera at 14 months postinfection may argue against the high reliability of the EmAgB3 as an antigen specific for active-stage AE. However, the two mice that did not show serum antibody responses to EmAgB3 (panel 14, Fig. 3A) had only degenerated small lesions in their liver by macroscopic examination. A histopathological specimen also demonstrated highly degenerated empty metacestode vesicles. The typical internal organs, such as brood capsules, primary cells, and protoscoleces, could not be observed (see Fig. S2B in the supplemental material). This result suggests that infection patency and immune response following the infection course may vary according to the infected mouse, possibly due to different immune states of individual mice (29). This result also implies that EmAgB3 may have a relatively short half-life, as it may be related to the worm's viability. In human cases, AE lesions usually appear as progressively proliferating masses, as they concurrently proliferate peripherally and degenerate centrally for a long period (1). Therefore, specific anti-EmAgB3 antibodies could be detected for a considerable period, as long as the AE metacestode retained its viability in the hosts. Seronegative patients in this study might have had disease in the very early and far-advanced stages. In cases of CE that lead to false-positive results, simultaneous monitoring of immunoproteome profiles and imaging diagnostics might be helpful to properly diagnose AE patients (25).
Several protein molecules have been studied for the feasibility of their use in AE serodiagnosis. Em2, EmII/3, and Em18 proteins revealed sensitivity and specificity of 82% to 97% and 73% to 98%, respectively (11). However, the diagnostic applicability of EmHF has not been fully addressed due to the difficulty of collecting hydatid fluid from an AE mass(es). When the antigenic properties of EmHF cultured in vitro were investigated, some contradictions were observed. There was low antigenicity of EmHF obtained from the in vitro culture, and there was no reaction for low-molecular-mass proteins of <15 kDa (27). In contrast, an immunoblotting study revealed relatively strong antibody responses to <20-kDa proteins, although whether or not EmAgB3 was in those fractions was not determined (29).
A recent study demonstrated that the metacestode vesicles cultured in vitro did not secrete EmAgB isoforms (30). This result strongly suggests that EmAgB3 might be synthesized during the late developmental stage of the metacestode vesicle or that it may start being synthesized upon receiving specific stimuli from the host. The metacestodes grown in vitro underwent both proliferation and differentiation, as they produced the brood capsule, primary cell, and protoscolex (7, 31, 32). However, despite its histologically normal phenotype, its functional relevance might differ from the relevance of those cultured in vivo due possibly to a lack of host-parasite interactions. EmHF samples obtained from in vitro culture in the presence or absence of fetal calf serum and feeder cells indeed displayed different antibody responses (33). Analyses of EmHF derived from different cultures may be crucial in determining the proteins possibly involved in the host-parasite interplay and their inherent immunological and biological roles.
rEmAgB1 was previously shown to induce high immune responses to sera from both AE and CE patients (34). Our 2-DE/immunoblot analyses also exhibited strong antigenicity of EmAgB1 in both AE and CE patient sera (spot 5; Fig. 1B). In contrast, EmAgB3 revealed only AE-specific immunoreactivity. We subsequently demonstrated the potential value of rEmAgB3 for serodiagnosis and follow-up monitoring of AE. The antigenic specificity displayed by this molecule among several EmAgB isoforms is difficult to explain. However, for example, subunits of the Taenia solium metacestode 120- and 150-kDa macromolecules, which share functional and structural similarities, exhibit significantly different antibody responses to homologous infection sera (35, 36). An antigen (EpC1) used for CE serodiagnosis with high (82%) sequence identity to the T. solium homolog also showed minimal cross-reactivity with neurocysticercosis sera (37). This was also true for the EmAgB3 isoform. The reason for the differing pI and Mr values of the two distinct proteoforms is not clear. Proteolytic cleavage of the 8-kDa molecule during secretion into EmHF might result in a small, 6-kDa fragment, as previously reported (21, 24).
It is noteworthy that EgAgB3, an ortholog of EmAgB3, showed high sequence identity with EmAgB3 at both the amino acid level (94.2%) and the nucleotide level (96.2%) and yet exhibits negligible antibody responses to CE patient sera (25). In E. granulosus, EgAgB3 was abundantly transcribed in the protoscolex (38), but secretion into the hydatid fluid was minimal at the protein level (25, 39). The differential expression patterns of AgB3 in phylogenetically close neighbors imply that an as-yet-unknown transcriptional regulatory mechanism operates in different manners during the maturation of EmAgB3 and EgAgB3. Further studies addressing the molecular mechanisms underlying expression regulation of the gene and protein are warranted.
In conclusion, through immunoproteome analysis, we identified an AE-specific immunoreactive EmAgB3 proteoform whose antibody responses were sensitive and specific when AE metacestodes conserved their viability within the hosts. rEmAgB3 offers easy applicability for serodiagnosis and follow-up surveillance of AE with minimal cross-reactivity to CE. Bacterially expressed rEmAgB3 might be suitable for ELISA formats because rEmAgB3 did not harbor putative glycosylation sites (data not shown). Preliminary observations employing AE serum samples have demonstrated encouraging results. rEmAgB3 may also prove useful in generating a platform of chimeric/cocktail antigens for AE serodiagnosis by compiling different epitope specificities (35).
Supplementary Material
ACKNOWLEDGMENTS
We are sincerely grateful to the local health workers of Qinghai Province Institute for Endemic Diseases Prevention and Control for their help in surveying the patients.
This work was supported by the Samsung Biomedical Research Institute (SMX1150651).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.01362-15.
REFERENCES
- 1.Eckert J, Deplazes P. 2004. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev 17:107–135. doi: 10.1128/CMR.17.1.107-135.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keutgens A, Simoni P, Detrembleur N, Frippiat F, Giot JB, Spirlet F, Aghazarian S, Descy J, Meex C, Huynen P, Melin P, Müller N, Gottstein B, Carlier Y, Hayette MP. 2013. Fatal alveolar echinococcosis of the lumbar spine. J Clin Microbiol 51:688–691. doi: 10.1128/JCM.01906-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bresson-Hadni S, Vuitton DA, Bartholomot B, Heyd B, Godart D, Meyer JP, Hrusovsky S, Becker MC, Mantion G, Lenys D, Miguet JP. 2000. A twenty-year history of alveolar echinococcosis: analysis of a series of 117 patients from eastern France. Eur J Gastroenterol Hepatol 12:327–336. doi: 10.1097/00042737-200012030-00011. [DOI] [PubMed] [Google Scholar]
- 4.Davidson RK, Romig T, Jenkins E, Tryland M, Robertson LJ. 2012. The impact of globalisation on the distribution of Echinococcus multilocularis. Trends Parasitol 28:239–247. doi: 10.1016/j.pt.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Piarroux M, Piarroux R, Knapp J, Bardonnet K, Dumortier J, Watelet J, Gerard A, Beytout J, Abergel A, Bresson-Hadni S, Gaudart J, FrancEchino Surveillance Network. 2013. Populations at risk for alveolar echinococcosis, France. Emerg Infect Dis 19:721–728. doi: 10.3201/eid1905.120867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang W, Zhang Z, Wu W, Shi B, Li J, Zhou X, Wen H, McManus DP. 2015. Epidemiology and control of echinococcosis in central Asia, with particular reference to the People's Republic of China. Acta Trop 141:235–243. doi: 10.1016/j.actatropica.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 7.Spiliotis M, Lechner S, Tappe D, Scheller C, Krohne G, Brehm K. 2008. Transient transfection of Echinococcus multilocularis primary cells and complete in vitro regeneration of metacestode vesicles. Int J Parasitol 38:1025–1039. doi: 10.1016/j.ijpara.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Kern P. 2010. Clinical features and treatment of alveolar echinococcosis. Curr Opin Infect Dis 23:505–512. doi: 10.1097/QCO.0b013e32833d7516. [DOI] [PubMed] [Google Scholar]
- 9.Piarroux M, Piarroux R, Giorgi R, Knapp J, Bardonnet K, Sudre B, Watelet J, Dumortier J, Gérard A, Beytout J, Abergel A, Mantion G, Vuitton DA, Bresson-Hadni S. 2011. Clinical features and evolution of alveolar echinococcosis in France from 1982 to 2007: results of a survey in 387 patients. J Hepatol 55:1025–1033. doi: 10.1016/j.jhep.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 10.Siles-Lucas MM, Gottstein BB. 2001. Molecular tools for the diagnosis of cystic and alveolar echinococcosis. Trop Med Int Health 6:463–475. doi: 10.1046/j.1365-3156.2001.00732.x. [DOI] [PubMed] [Google Scholar]
- 11.Carmena D, Benito A, Eraso E. 2007. The immunodiagnosis of Echinococcus multilocularis infection. Clin Microbiol Infect 13:460–475. doi: 10.1111/j.1469-0691.2007.01665.x. [DOI] [PubMed] [Google Scholar]
- 12.Kern P, Frosch P, Helbig M, Wechsler JG, Usadel S, Beckh K, Kunz R, Lucius R, Frosch M. 1995. Diagnosis of Echinococcus multilocularis infection by reverse-transcription polymerase chain reaction. Gastroenterology 109:596–600. doi: 10.1016/0016-5085(95)90350-X. [DOI] [PubMed] [Google Scholar]
- 13.Kantarci M, Bayraktutan U, Karabulut N, Aydinli B, Ogul H, Yuce I, Calik M, Eren S, Atamanalp SS, Oto A. 2012. Alveolar echinococcosis: spectrum of findings at cross-sectional imaging. RadioGraphics 32:2053–2070. doi: 10.1148/rg.327125708. [DOI] [PubMed] [Google Scholar]
- 14.Deplazes P, Gottstein B. 1991. A monoclonal antibody against Echinococcus multilocularis Em2 antigen. Parasitology 103:41–49. doi: 10.1017/S0031182000059278. [DOI] [PubMed] [Google Scholar]
- 15.Hülsmeier AJ, Gehrig PM, Geyer R, Sack R, Gottstein B, Deplazes P, Köhler P. 2002. A major Echinococcus multilocularis antigen is a mucin-type glycoprotein. J Biol Chem 277:5742–5748. doi: 10.1074/jbc.M107161200. [DOI] [PubMed] [Google Scholar]
- 16.Yamano K, Goto A, Nakamura-Uchiyama F, Nawa Y, Hada N, Takeda T. 2009. Galβ1-6Gal, antigenic epitope which accounts for serological cross-reaction in diagnosis of Echinococcus multilocularis infection. Parasite Immunol 31:481–487. doi: 10.1111/j.1365-3024.2009.01129.x. [DOI] [PubMed] [Google Scholar]
- 17.Yamano K, Koizumi A, Takeda T, Kiuchi F, Hada N. 2012. Galα1-4Galβ1-3GalNAc is the dominant epitope of Em2 antigen, the mucin-type glycoprotein from Echinococcus multilocularis. Parasitol Res 111:795–805. doi: 10.1007/s00436-012-2902-1. [DOI] [PubMed] [Google Scholar]
- 18.Sarciron EM, Bresson-Hadni S, Mercier M, Lawton P, Duranton C, Lenys D, Petavy AF, Vuitton DA. 1997. Antibodies against Echinococcus multilocularis alkaline phosphatase as markers for the specific diagnosis and the serological monitoring of alveolar echinococcosis. Parasite Immunol 19:61–68. doi: 10.1046/j.1365-3024.1997.d01-183.x. [DOI] [PubMed] [Google Scholar]
- 19.Lawton P, Hemphill A, Deplazes P, Gottstein B, Sarciron ME. 1997. Echinococcus multilocularis metacestodes: immunological and immunocytochemical analysis of the relationships between alkaline phosphatase and the Em2 antigen. Exp Parasitol 87:142–149. doi: 10.1006/expr.1997.4190. [DOI] [PubMed] [Google Scholar]
- 20.Vogel M, Gottstein B, Müller N, Seebeck T. 1988. Production of a recombinant antigen of Echinococcus multilocularis with high immunodiagnostic sensitivity and specificity. Mol Biochem Parasitol 31:117–125. doi: 10.1016/0166-6851(88)90162-4. [DOI] [PubMed] [Google Scholar]
- 21.Frosch PM, Frosch M, Pfister T, Schaad V, Bitter-Suermann D. 1991. Cloning and characterisation of an immunodominant major surface antigen of Echinococcus multilocularis. Mol Biochem Parasitol 48:121–130. doi: 10.1016/0166-6851(91)90108-I. [DOI] [PubMed] [Google Scholar]
- 22.Ito A, Ma L, Schantz PM, Gottstein B, Liu YH, Chai JJ, Abdel-Hafez SK, Altintas N, Joshi DD, Lightowlers MW, Pawlowski ZS. 1999. Differential serodiagnosis for cystic and alveolar echinococcosis using fractions of Echinococcus granulosus cyst fluid (antigen B) and E. multilocularis protoscolex (EM18). Am J Trop Med Hyg 60:188–192. [DOI] [PubMed] [Google Scholar]
- 23.Brehm K, Jensen K, Frosch P, Frosch M. 1999. Characterization of the genomic locus expressing the ERM-like protein of Echinococcus multilocularis. Mol Biochem Parasitol 100:147–152. doi: 10.1016/S0166-6851(99)00051-1. [DOI] [PubMed] [Google Scholar]
- 24.Sako Y, Nakao M, Nakaya K, Yamasaki H, Gottstein B, Lightowers MW, Schantz PM, Ito A. 2002. Alveolar echinococcosis: characterization of diagnostic antigen Em18 and serological evaluation of recombinant Em18. J Clin Microbiol 40:2760–2765. doi: 10.1128/JCM.40.8.2760-2765.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahn CS, Han X, Bae YA, Ma X, Kim JT, Cai H, Yang HJ, Kang I, Wang H, Kong Y. 2015. Alteration of immunoproteome profile of Echinococcus granulosus hydatid fluid with progression of cystic echinococcosis. Parasit Vectors 8:10. doi: 10.1186/s13071-014-0610-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kern P, Wen H, Sato N, Vuitton DA, Gruener B, Shao Y, Delabrousse E, Kratzer W, Bresson-Hadni S. 2006. WHO classification of alveolar echinococcosis: principles and application. Parasitol Int 55:S283–S287. doi: 10.1016/j.parint.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 27.Müller N, Frei E, Nuñez S, Gottstein B. 2007. Improved serodiagnosis of alveolar echinococcosis of humans using an in vitro-produced Echinococcus multilocularis antigen. Parasitology 134:879–888. doi: 10.1017/S0031182006002083. [DOI] [PubMed] [Google Scholar]
- 28.Mamuti W, Sako Y, Xiao N, Nakaya K, Nakao M, Yamasaki H, Lightowlers MW, Craig PS, Ito A. 2006. Echinococcus multilocularis: developmental stage-specific expression of antigen B 8-kDa-subunits. Exp Parasitol 113:75–82. doi: 10.1016/j.exppara.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 29.Matsumoto J, Kouguchi H, Oku Y, Yagi K. 2010. Primary alveolar echinococcosis: course of larval development and antibody responses in intermediate host rodents with different genetic backgrounds after oral infection with eggs of Echinococcus multilocularis. Parasitol Int 59:435–444. doi: 10.1016/j.parint.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Nono JK, Pletinckx K, Lutz MB, Brehm K. 2012. Excretory/secretory-products of Echinococcus multilocularis larvae induce apoptosis and tolerogenic properties in dendritic cells in vitro. PLoS Negl Trop Dis 6:e1516. doi: 10.1371/journal.pntd.0001516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jura H, Bader A, Hartmann M, Maschek H, Frosch M. 1996. Hepatic tissue culture model for study of host-parasite interactions in alveolar echinococcosis. Infect Immun 64:3484–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hemphill A, Stettler M, Walker M, Siles-Lucas M, Fink R, Gottstein B. 2002. Culture of Echinococcus multilocularis metacestodes: an alternative to animal use. Trends Parasitol 18:445–451. doi: 10.1016/S1471-4922(02)02346-2. [DOI] [PubMed] [Google Scholar]
- 33.Hemphill A, Gottstein B. 1995. Immunology and morphology studies on the proliferation of in vitro cultivated Echinococcus multilocularis metacestode. Parasitol Res 81:605–614. doi: 10.1007/BF00932028. [DOI] [PubMed] [Google Scholar]
- 34.Mamuti W, Yamasaki H, Sako Y, Nakao M, Xiao N, Nakaya K, Sato N, Vuitton DA, Piarroux R, Lightowlers MW, Craig PS, Ito A. 2004. Molecular cloning, expression, and serological evaluation of an 8-kilodalton subunit of antigen B from Echinococcus multilocularis. J Clin Microbiol 42:1082–1088. doi: 10.1128/JCM.42.3.1082-1088.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bae YA, Jeong YT, Chung JY, Kim SH, Mahanta J, Feng Z, Chong CK, Kim TS, Kong Y. 2008. A recombinant chimeric antigen toward a standardized serodiagnosis of Taenia solium neurocysticercosis. Proteomics Clin Appl 2:1596–1610. doi: 10.1002/prca.200800084. [DOI] [PubMed] [Google Scholar]
- 36.Kim SH, Bae YA, Yang Y, Hong ST, Kong Y. 2011. Paralogous proteins comprising the 150 kDa hydrophobic-ligand-binding-protein complex of the Taenia solium metacestode have evolved non-overlapped binding affinities toward fatty acid analogs. Int J Parasitol 41:1207–1215. doi: 10.1016/j.ijpara.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 37.Li J, Zhang WB, Wilson M, Ito A, McManus DP. 2003. A novel recombinant antigen for immunodiagnosis of human cystic echinococcosis. J Infect Dis 188:1951–1960. doi: 10.1086/379976. [DOI] [PubMed] [Google Scholar]
- 38.Zhang W, Li J, Jones MK, Zhang Z, Zhao L, Blair D, McManus DP. 2010. The Echinococcus granulosus antigen B gene family comprises at least 10 unique genes in five subclasses which are differentially expressed. PLoS Negl Trop Dis 4:e784. doi: 10.1371/journal.pntd.0000784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Virginio VG, Monteiro KM, Drumond F, de Carvalho MO, Vargas DM, Zaha A, Ferreira HB. 2012. Excretory/secretory products from in vitro-cultured Echinococcus granulosus protoscoleces. Mol Biochem Parasitol 183:15–22. doi: 10.1016/j.molbiopara.2012.01.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.