Abstract
The refinement of innervation is a common developmental mechanism that serves to increase the specificity of connections following initial innervation. In the peripheral gustatory system, the extent to which innervation is refined and how refinement might be regulated is unclear. The initial innervation of taste buds is controlled by brain-derived neurotrophic factor (BDNF). Following initial innervation, taste receptor cells are added and become newly innervated. The connections between the taste receptor cells and nerve fibers are likely to be specific in order to retain peripheral coding mechanisms. Here, we explored the possibility that the down-regulation of BDNF regulates the refinement of taste bud innervation during postnatal development. An analysis of BDNF expression in BdnflacZ/+ mice and real-time reverse transcription polymerase chain reaction (RT-PCR) revealed that BDNF was down-regulated between postnatal day (P) 5 and P10. This reduction in BDNF expression was due to a loss of precursor/progenitor cells that express BDNF, while the expression of BDNF in the subpopulations of taste receptor cells did not change. Gustatory innervation, which was identified by P2X3 immunohistochemistry, was lost around the perimeter where most progenitor/precursor cells are located. In addition, the density of innervation in the taste bud was reduced between P5 and P10, because taste buds increase in size without increasing innervation. This reduction of innervation density was blocked by the overexpression of BDNF in the precursor/progenitor population of taste bud cells. Together these findings indicate that the process of BDNF restriction to a subpopulation of taste receptor cells between P5 and P10, results in a refinement of gustatory innervation. We speculate that this refinement results in an increased specificity of connections between neurons and taste receptor cells during development.
Keywords: precursor/progenitor taste cells, taste receptor cells, innervation density, fungiform taste buds, BDNF overexpression, β-galactosidase
Introduction
Neural circuits reorganize throughout development. For example, individual muscle fibers are temporarily innervated by multiple motor axons; the postnatal elimination of axonal branches reduces the number of target cells innervated by each axon (Walsh and Lichtman, 2003). Innervation within the taste bud also may reorganize during development. Developing taste buds are localized to specific epithelial structures called papillae, and taste fibers are directed to these taste buds during initial embryonic targeting (Lopez and Krimm, 2006b; Mbiene and Mistretta, 1997). Remodeling is not required for neurons to make initial connections with taste buds. However, following initial innervation, taste buds continue to grow and differentiate postnatally (Bigiani et al., 2002; Hosley and Oakley, 1987; Kinnamon et al., 2005; Ohtubo et al., 2012; Zhang et al., 2008), resulting in an adult taste bud that has multiple different cell types based on anatomy, function, and expression (Clapp et al., 2006; Clapp et al., 2004; Delay et al., 1986; Finger, 2005; Kataoka et al., 2008; Murray and Murray, 1967; Murray et al., 1969; Yang et al., 2000a; Yang et al., 2000b; Yee et al., 2001). Therefore, it would not be surprising if some developmental remodeling were required for taste receptor cells to be innervated by the appropriate nerve fibers. Consistent with this idea, taste innervation is reduced postnatally in the rat and sheep in a manner that suggests reorganization (Kinnamon et al., 2005; Mistretta et al., 1988; Nagai et al., 1988).
Although it is well established that synaptic reorganization can be driven by afferent activity (Erzurumlu and Kind, 2001; Espinosa and Stryker, 2012; Kirkby et al., 2013), developmental factors that regulate survival, axon guidance, and targeting can also regulate postnatal synaptic reorganization and the specificity of connections (Gonzalez et al., 1999; Nguyen et al., 1998; Pfeiffenberger et al., 2005). For example, the neurotrophin, brain-derived neurotrophic factor (BDNF), regulates the critical period in which eye-specific input is segregated in the visual cortex (Cabelli et al., 1997; Huang et al., 1999). BDNF also is an important guidance factor for peripheral taste neurons that allows taste afferents to find and innervate taste buds during embryonic development (Lopez and Krimm, 2006a; Ma et al., 2009). Interestingly, this process occurs during a critical period, after which BDNF is no longer required to innervate a taste bud (Ma et al., 2009). Yet, BDNF is still expressed in postnatal and adult taste buds (Huang and Krimm, 2010; Yee et al., 2003). Therefore, as with other systems BDNF could have a role remodeling innervation within taste buds during postnatal development.
Our goal was to examine the role of BDNF during postnatal development. During embryonic development, the expression patterns of BDNF in the peripheral taste system correlate with their roles in neuronal survival and target innervation (Huang and Krimm, 2010). Therefore, we wanted to determine when BDNF is expressed, and in which types of taste cells during various stages of postnatal development. We also wanted to determine the potential involvement of BDNF in the postnatal remodeling of innervation within the taste bud. We found that a reduction of BDNF in progenitor/precursor cells between postnatal day 5 and 10 mediates the postnatal refinement of innervation within the taste bud.
Materials and methods
Animals
BdnfLacZ/+ mice, in which the Bdnf coding sequence at one allele is replaced by the E. coli galactosidase (LacZ) sequence, were used to determine the localization of BDNF expression (Jones et al., 1994). K14-Bdnf-OE mice, in which the expression of BDNF is driven by the keratin-14 promoter, were used to overexpress BDNF in the tongue epithelial progenitor cells, including those of the taste buds (Krimm et al., 2001; LeMaster et al., 1999; Lopez and Krimm, 2006a). Mice were analyzed at birth and postnatal days (P) 5, 10, 20, and 60 (adult). All animals were cared for and studied in accordance with the guidelines set by the U.S. Public Health Service Policy on the Humane Care and Use of Laboratory Animals and the NIH Guide for the Care and Use of Laboratory Animals.
Laser capture microdissection and RNA extraction
Taste buds and geniculate ganglion cells were isolated from newborn and postnatal mice using laser capture microdissection (LCM) using the protocols described previously (Huang and Krimm, 2010). Briefly, the anterior tongue and circumvallate (CV) were dissected and then sectioned (10-μm) and processed to visualize the taste buds. The identified taste buds were captured onto CapSure Macro LCM Caps (Molecular Devices, Sunnyvale, CA, USA). For each animal, all captured samples were stored for RNA isolation.
Total RNA was extracted from the taste buds using an RNeasy micro kit according to the manufacturer's instructions (#74004; Qiagen, Germantown, MD, USA). DNase I treatment was applied to eliminate traces of DNA during the procedure. Following isolation, the RNA quality was analyzed using a Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA, USA). The RNA Integrity Number (RIN) and 28S/18S ratio were used to estimate the RNA quality. Only RNA samples with a 260/280 ratio ≥ 1.80 and RIN ≥ 8.0 were used.
Real-time reverse transcription polymerase chain reaction
Taste bud cDNA was synthesized from total RNA using random primers (Invitrogen, Carlsbad, CA, USA). The cDNA was quantified by real-time reverse transcription polymerase chain reaction (RT-PCR) using a TaqMan Universal PCR kit (#4304437; Applied Biosystems, Waltham, MA, USA). The real-time RT-PCR reactions were conducted using 10 μl total volume with 300 nM primers. We used the same primers reported in a previous study (Huang and Krimm, 2010). For the normalization of the cDNA loading, all samples were run in parallel with the 18S ribosomal RNA housekeeping gene. Real-time RT-PCR was performed with ABI PRISM/7900HT Sequence detection systems (Applied Biosystems). Each assay was conducted in triplicate. The RT-PCR conditions were an initial incubation of 50°C for 2 min and 95°C for 15 min, followed by 40 cycles of 94°C for 15 s, 58°C for 30 s, and 72°C for 30 s.
β-galactosidase (X-Gal) staining
To detect β-galactosidase, X-Gal staining was performed on the tissues dissected from BdfnLacZ/+ mice using procedures described in a previous study (Huang and Krimm, 2010). Briefly, newborn and postnatal mice were perfused and post-fixed with 0.5% glutaraldehyde, and rinsed in PBS/MgCl2. The anterior tongue, CV, and geniculate ganglia were dissected and frozen in OCT. These tissues were sectioned (16 μm) and stained using β-galactosidase (β-Gal) staining solution (InvivoGen, San Diego, CA, USA). Images of the β-Gal staining in the taste buds and geniculate ganglion were taken using a Retiga 1300 digital camera (QImaging, Surrey, BC, Canada) mounted to a DMLB Leica microscope.
Immunohistochemistry
Newborn and postnatal mice were perfused with 4% paraformaldehyde. The anterior tongue and CV were dissected and post-fixed overnight, cryoprotected in 30% sucrose, and frozen in OCT. For immunohistochemistry, the anterior tongue was cut at a thickness of 50 μm, and the sections were collected into 0.1M PB and rinsed. After blocking with 3% normal serum in 0.1M PB containing 0.5% Triton X-100, the tissues were incubated with primary antibodies (Table 1) for 5-7 days at 4°C. Monovalent Fab fragment antibody (#711-007-003, Jackson ImmunoResearch Laboratories, West Grove, PA, USA) was used in multiple labeling experiments when the primary antibodies were from the same species (rabbit). After the samples were incubated with primary antibodies and rinsed, appropriate secondary antibodies (Jackson ImmunoResearch Laboratories), including Alexa Fluor 488 (#711-545-152; #705-545-147; #712-545-153), Alexa Fluor 647 (#712-605-153), and Cyanine Cy3 (#711-165-152), were applied overnight. The tissues then were washed and mounted with aqueous mounting medium (Fluoromount-G; SouthernBiotech, Birmingham, AL, USA). Serial optical sections were captured every 1 μm in labeled whole taste buds using a confocal microscope (FV1200; Olympus) under a 60× lens at 3.5 zoom. Each label was collected separately with single wavelength excitation and then merged to produce the composite image. At least 5 (for taste innervation) or 10 (for the co-expression of BDNF with taste bud cell markers) taste buds from each animal were captured for further analyses.
Table 1. The antibodies used in the study.
| Antibody | Host | Concentration | Company | Cat. # |
|---|---|---|---|---|
| 5-HT | Rabbit | 1:500 | ImmunoStar | 20080 |
| β-Gal | Rabbit | 1:1000 | MP Biomedicals | 55976 |
| Car4 | Goat | 1:500 | R&D Systems | AF2414 |
| Keratin-8 | Rat | 1:50 | Developmental Studies Hybridoma Bank | Troma-1 |
| NTPDase2 | Rabbit | 1:500 | CHUL | mN2-35L; mN2-36L |
| P2X3 | Rabbit | 1:500 | Millipore | AB5895 |
| PCLβ2 | Rabbit | 1:500 | Santa Cruz | sc-206 |
| SNAP25 | Rabbit | 1:500 | Millipore | AB1762 |
BDNF expression in taste buds decreases between postnatal days 5 and 10 BDNF is reduced specifically from progenitor cells and not taste receptor cells Innervation density in the taste bud is reduced between postnatal days 5 and 10 BDNF overexpression blocks the refinement of innervation
Data analysis
Results are expressed as the mean ± SEM. For real-time RT-PCR, the comparative 2-ΔΔCT method was used to determine the target gene expression levels (Livak and Schmittgen, 2001). For the co-expression of BDNF with taste bud cell markers, we first counted the total cell number (DAPI) within the taste bud, the borders of which were defined by keratin-8. Individual cells were followed in serial optical sections so that each cell was counted only once. Cells immunoreactive for specific markers were counted similarly, and because we could not be sure all the taste buds were whole, these numbers were expressed relative to the total taste bud number.
We examined innervation to the taste bud across age using two different approaches (supplementary Fig. 1), each of which has different advantages and disadvantages. In the first approach the areas occupied by keratin-8 and P2X3 were examined in each optical section and summed to get total volumes (supplementary Fig. 4 A,B,E,F). Specifically, each 1-μm optical section was traced, and the area measured using Neurolucida imaging software (MicroBrightField, Williston, VT, USA). The area of each 1-μm optical section within the taste bud was summed to yield the taste bud volume. The volume of anti-P2X3 within the taste bud was measured using MicroBrightField ImageJ software (ImageJ 1.47). The ImageJ software sets an unbiased threshold automatically, and the pixels were analyzed for each 1-μm optical section and summed across optical sections to obtain the total volume of P2X3-labeled pixels within one taste bud. The percentage of the taste bud occupied by innervation was obtained by dividing the P2X3 volume by the taste bud volume and multiplying by 100. In the second method we used Imaris software to create a mask that enveloped all the innervation in the 3-D taste bud and measured the volume in this region (supplementary Fig, 1 C,D,G,H). Specifically, the taste bud, as defined by cytokeratin-8, was outlined in Imaris software (Bitplane, http://www.bitplane.com/contact). The masking feature available in Imaris was used to mask all signal from the 546 channel (representative of P2X3-labeled nerve fibers) within the boundaries of the taste bud. This mask included red signal only within the taste bud and excluded red signal outside the taste bud surface (Figure 1. C,G). A surface of P2X3 innervation within the taste bud was created based on this mask, and the volume within this surface was calculated by the software to measure total innervation to the taste bud. This method has the advantage of reducing the impact of patchy label because poorly labeled pixels between bright pixels would be included, but it also could overestimate innervation for the same reason.
Figure 1.
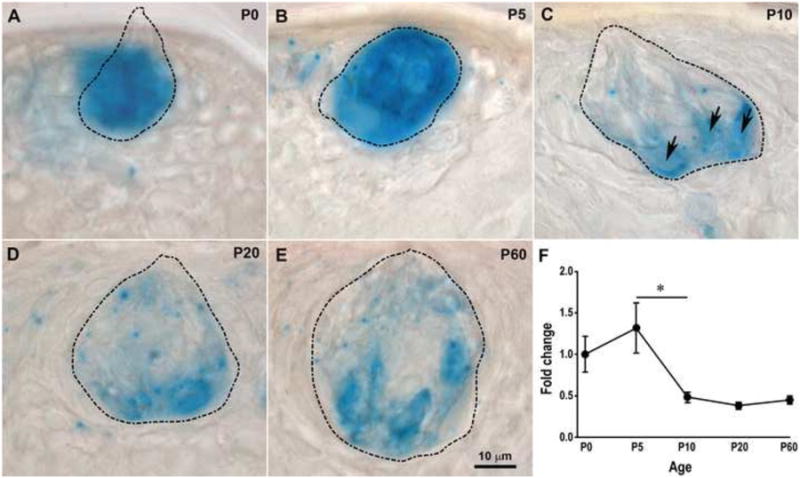
BDNF expression decreases in fungiform taste buds between P5 and P10. (A-E) Enzyme histochemical detection of β-galactosidase (β-Gal) activity in BdnfLacZ/+ mouse taste buds from birth to adulthood. The taste bud borders (dashed lines) were determined by reducing the numerical aperture of the condenser and focusing continuously up and down through the section. At P0 (A) and P5 (B), β-Gal staining appeared to stain the entire taste bud. However, the staining was clearly restricted to a few taste bud cells (arrows) by P10 (C), and this restricted staining pattern continued through P20 (D) and P60 (E). (F) BDNF expression, as measured by RT-PCR, decreased within fungiform taste buds. BDNF expression was normalized to 18s rRNA and then reported as a fold of P0 BDNF levels. The expression of BDNF in fungiform taste buds was not different between birth and P5, but was significantly reduced from P5 to P10 (p = 0.03). BDNF expression was maintained at constant levels after P10. (A-E) Taste buds are outlined by a dashed line. The scale bar in E applies to panels A-E. The error bars in F represent the standard error.
A one-way ANOVA was used to analyze BDNF expression levels and the percentage of the taste buds occupied by P2X3 in wild-type mice across different ages. A two-way ANOVA followed by Bonferroni-corrected post-hoc t-tests were used to compare the percentage of the taste bud occupied by P2X3 between the wild-type and K14-BDNF-OE mice at P5 and P10. The co-expression of BDNF with taste bud markers was analyzed using t-tests. Six mice per group were used for the RT-PCR experiments and 3 mice per group were used for the immunohistochemistry experiments. The significance level was set at p<0.05 for all statistical comparisons.
Results
BDNF is down-regulated in taste buds between P5 and P10
BDNF is expressed throughout the entire taste bud at birth (Huang and Krimm, 2010) and is restricted to subpopulations of taste bud cells by adulthood (Yee et al., 2003). These results imply that BDNF expression within the taste bud decreases during postnatal development. To verify that this is the case and determine the timing of this decrease, we performed β-Gal staining on the tongue sections dissected from BdnfLacZ/+ mice at various postnatal ages. The intensity and distribution of β-Gal staining in the taste buds was not different between P0 and P5 (Fig. 1 A,B). However, β-Gal staining was limited to some, but not all, taste bud cells by P10 (Fig. 1C); this decreased β-Gal staining continued from P10 through adulthood. Control tissue from littermate wild type mice of the same ages, processed simultaneously, never showed staining (Supplementary Fig. 2, A-C). To confirm the timing of BDNF reduction in fungiform taste buds, we examined Bdnf expression using RT-PCR in taste buds captured by laser capture microdissection. There was a significant reduction in BDNF expression in fungiform taste buds across postnatal ages (F(4,14) = 4.01, p = 0.023). Consistent with the β-Gal staining results, Bdnf expression was not different between P0 and P5 but significantly down-regulated from P5 to P10 (p = 0.03), after which the expression of Bdnf remained the same until adulthood (Fig. 1D-F). In addition, we examined the expression of Nt4 and TrkB in fungiform taste buds using real-time RT-PCR. The expression of Nt4 and TrkB in fungiform taste buds was almost undetectable in newborn mice and did not increase at later postnatal ages or adulthood, which was consistent with the results from a previous study (Huang and Krimm, 2010). Thus, BDNF is reduced in fungiform taste buds during a very specific time frame (between P5 and P10) of postnatal development, with no changes in NT4 or the BDNF/NT4 receptor, TrkB.
We speculated that the decline in BDNF expression might be related to the timing of postnatal differentiation and maturation of the taste buds. Taste buds develop much later in the circumvallate papillae than in the fungiform papillae (Hosley and Oakley, 1987; Zhang et al., 2008). Therefore, if decreases in BDNF are due to developmental changes within the taste bud, then the timing of this reduction would be later in circumvallate taste buds compared with fungiform taste buds. To test this idea we also analyzed β-Gal labeling in the circumvallate papilla of BdnflacZ/+ mice during postnatal development. β-Gal staining decreased in the circumvallate papillae during postnatal development, but the change in expression was delayed compared with that in the fungiform papillae such that the staining was not limited to specific taste bud cells until P20 (Fig. 2A-E). Real-time RT-PCR analysis demonstrated that Bdnf expression in circumvallate taste buds was statistically reduced during postnatal development (F(4,10) = 5.73, p = 0.012). However, this reduction did not reach statistical significance until P20 (p = 0.02) (Fig. 2G), which was consistent with the β-Gal staining results. Thus, the expression of BDNF in circumvallate taste buds decreases during postnatal development, but this decrease is delayed compared with that in fungiform taste buds. This result implies that the down-regulation of BDNF occurs as part of postnatal taste bud maturation.
Figure 2.
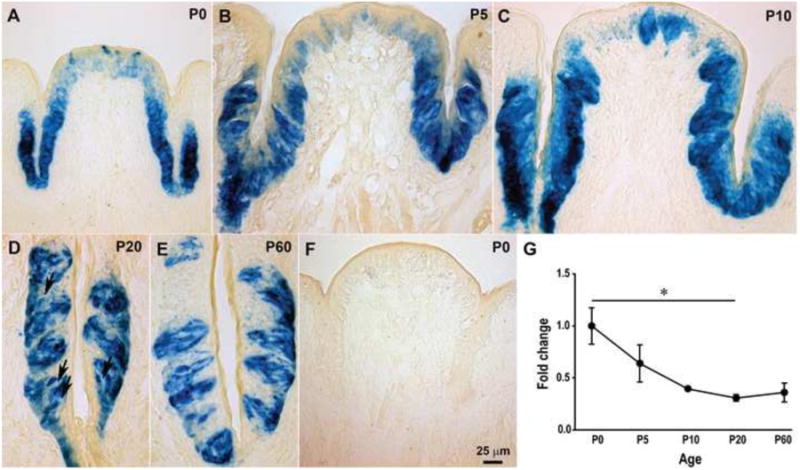
BDNF expression is reduced in circumvallate taste buds during late postnatal development. β-Gal activity was detected in the circumvallate (CV) taste buds of BdnfLacZ/+ mice from birth to adulthood (A-E), but not in wild-type mice (F). β-Gal staining was observed in the entire circumvallate trench wall from P0 to P10 (A-C). However, the staining was restricted within taste buds by P20 (D) and P60 (E), and some taste bud cells were negative for β-Gal (arrows). (G) RT-PCR confirmed a decrease in BDNF expression in the circumvallate papilla epithelium across postnatal ages. The expression of BDNF in circumvallate taste buds was not different between birth and P10, but BDNF expression levels gradually decreased from P0 to P20 (p = 0.02). There was no difference in BDNF expression between P20 and P60. The scale bar in F applies to panels A-F. The error bars in G represent the standard error, and the single asterisk represents p ≤ 0.05.
Taste buds lose BDNF-expressing taste progenitor and/or precursor cells during postnatal development
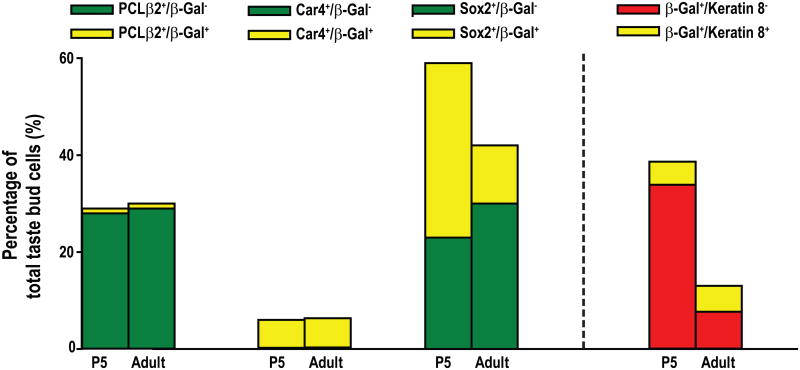
It is possible that the level of BDNF expression is reduced in all taste cells. Alternatively, the number of taste cells in which BDNF is expressed may be reduced. To distinguish between these possibilities, the total number of taste bud cells in each section was determined by counting the number of DAPI-stained nuclei present in each taste bud, the borders of which were defined by keratin-8. BDNF+ cells were represented by β-Gal (Fig. 3A-H). We found that there were more β-Gal immunoreactive cells at P5 than adulthood (7.41 ± 1.18 versus 5.16 ± 0.50, p<0.05), even though the taste bud size and total taste bud cell number were greatly increased from P5 (20.9 ± 3.08) to adulthood (41.3 ± 5.21). Both changes led to a large decrease in the percentage of β-Gal immunoreactive cells within the taste buds from P5 (39 ± 2%) to adulthood (13 ± 1%) (Fig. 3I). Therefore, BDNF expression is reduced in the taste bud because the percentage of taste bud cells that express BDNF decreases during postnatal development.
Figure 3.
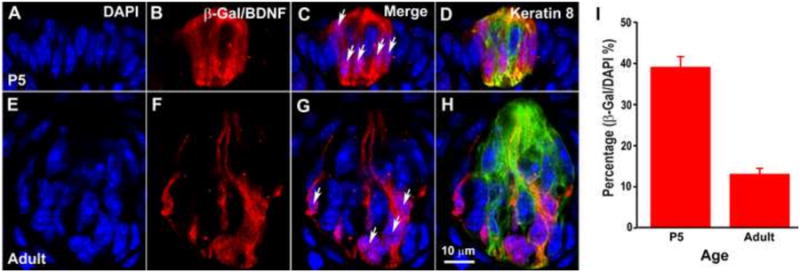
The percentage of β-Gal+ cells decreases postnatally in fungiform taste buds. Taste buds were triple-labeled with anti-β-Gal (red), DAPI (blue), and anti-keratin-8 (green) at P5 (A-D) and adulthood (E-H). Taste bud cells were quantified by counting the number of DAPI nuclei within the keratin-8-defined taste buds (A and E), and nuclei surrounded by β-Gal-stained cytoplasm were counted as β-Gal+ (arrows in B and D). The percentage of β-Gal+ cells within the taste buds was significantly reduced between P5 and adulthood (p<0.001) (I). The scale bar in H applies to panels A-H.
Taste buds contain a variety of cell types that differ based on structure, function, and expression characteristics (Clapp et al., 2006; Clapp et al., 2004; Delay et al., 1986; Finger, 2005; Kataoka et al., 2008; Murray and Murray, 1967; Murray et al., 1969; Yang et al., 2000a; Yang et al., 2000b; Yee et al., 2001). To determine if BDNF is reduced in a specific taste cell type during postnatal development, immunohistochemistry was performed using antibodies against β-Gal and other markers for different taste bud cell types. Previous studies have shown that the synaptic protein, SNAP25, is co-expressed with BDNF in adulthood (Yee et al., 2003). We were able to replicate those earlier findings (Supplementary Fig. 3), but also noticed that numerous fibers were labeled with SNAP25 and the label was weak in the taste cells, which made quantification of labeled taste cells difficult in poorly differentiated P5 taste buds. Therefore, we sought an alternate marker for this cell type. SNAP25+ taste cells have been described as “type III” cells, and have been reported to express polycystic kidney disease 2-like 1 protein (PKD2L1), carbonic anhydrase (Car4), and 5-HT and likely transduce sour taste (Chandrashekar et al., 2009; Huang et al., 2006; Kataoka et al., 2008; Yee et al., 2001). Consistent with these previous results, we found that 100% of SNAP25+ cells also expressed Car4 (Supplementary Fig. 4, A-D) and 5-HT (Supplementary Fig. 4, E-H). No Car4+ cells expressed phospholipase Cβ2 (PLCβ2), which is a signal downstream of the G-protein-coupled receptors for sweet, bitter, and umami taste (Supplementary Fig. 3 I-L); this result is also consistent with previous results (Clapp et al., 2004; Zhang et al., 2003).
We began our developmental analysis of BDNF reduction with mature taste receptor cells that transduce sweet, umami, and bitter taste (PLCβ2+) and those that transduce sour taste (Car4+). Most (95%) of the adult fungiform Car4+ (type III) taste cells exhibited β-Gal immunoreactivity, whereas only 11% of the PCLβ2+ (type II) taste bud cells exhibited β-Gal immunoreactivity (Fig. 4A-P), which is consistent with previous findings (Yee et al., 2003). The co-expression of β-Gal with Car4 but not PLCβ2 was also observed within fungiform taste buds at P5 (Fig. 4A-P). Although the total number of Car4+ and PLCβ2+ taste cells increases during postnatal development, the percentage of PLCβ2+ or Car4+ cells remains the same between P5 and adulthood. More importantly, the co-expression of these markers with β-Gal (BDNF) did not change from P5 to adulthood (Fig. 5). Therefore, PLCβ2+ cells never express much BDNF, and Car4+ cells express BDNF throughout development. Thus, the reduction in BDNF expression was not due to changes in either of these cell types.
Figure 4.
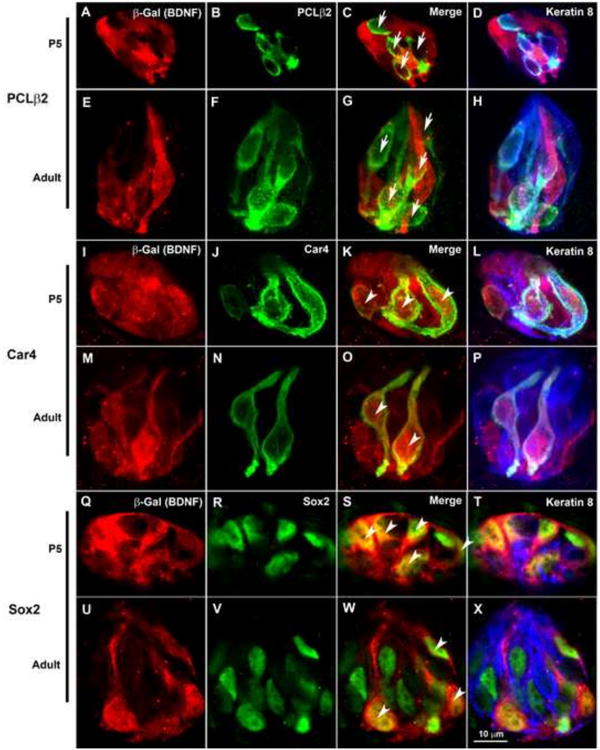
BDNF is expressed in subsets of postnatal fungiform taste cells. Taste buds were labeled with anti-β-Gal (red), taste cell specific antibodies (green), and kertatin-8 (blue). (A-H) β-Gal was not found in most PLCβ2+ taste cells (arrows in C and G, respectively) at either P5 or adulthood. (I-P) However, all Car4+ taste cells were co-labeled with β-Gal (arrowheads in K and O) at both P5 and adulthood. In both P5 and adult fungiform taste buds, many Sox2+ cells co-express β-Gal (arrowheads in S and W). The scale bar in X applies to all the panels.
Figure 5.
The percentage of Sox2+/keratin-8- (K8-) taste bud cells that co-express β-Gal decreases in fungiform taste buds during postnatal development. Labeled taste bud cells are plotted as a percentage of the total number of cells, which were defined by the number of DAPI-stained nuclei. Each label is divided into those cells that also show β-Gal immunoreactivity (yellow) and those that do not (green). The percentage of PLCβ2+ and Car4+ cells was not different between P5 and adulthood, and there were no changes for those cells co-labeled with β-Gal. However, the percentage of Sox2+/K8-cells was significantly reduced from P5 to adulthood (p = 0.01), which was caused by a reduction of Sox2+/K8- cells that were co-labeled with β-Gal (p = 0.003). The percentage of Sox2+/K8- cells that do not co-label with β-Gal does not change during postnatal development (p = 0.16). The percentage of β-Gal+ cells that are Keratin-8- is also reduced across ages (p = 0.0002), while the percentage of keratin-8+ β-Gal+ cells remains the same throughout development.
Because ATP functions as a transmitter in the taste system, many taste bud cells also express nucleoside triphosphate diphohydrolase (NTPDase2) for hydrolyzing ATP to ADP (Finger et al., 2005; Vandenbeuch et al., 2013). NTPDase2 co-localizes with glial glutamate/aspartate transporter (GLAST) but not 5-HT or PLCβ2, and so NTPDase2 may be expressed primarily in supporting cells (Bartel et al., 2006). To determine whether this taste cell subtype also expressed BDNF, we performed a co-labeling with anti-β-Gal and anti-NTPDase2. Because NTPDase2 was expressed in the membranes of many taste cells as well as the nerve fibers, it was not possible to quantify NTPDase2+ taste cells. We found that some β-Gal+ taste cells were also NTPDase2+ at both P5 and adulthood (Supplementary Fig. 5, A-H) without a noticeable decrease in the numbers of these cells across age.
New cells supply the taste bud from a progenitor population that expresses Sox2 and keratins 5 and 14 (Okubo et al., 2009) and a precursor population that expresses Sox2 and Shh (Miura et al., 2014; Okubo et al., 2006). To determine whether fewer progenitor/precursor cells express BDNF in adulthood compared with development, we labeled taste buds with anti-Sox2. However, because some mature taste cells express Sox2 (Okubo et al., 2006), we limited our quantification to cells that were Sox2+ but keratin-8−. Many β-Gal+ cells expressed Sox2 in both P5 and adult fungiform taste buds (Fig. 4Q-X). Our results indicated that the percentage of Sox2+/keratin-8− taste bud cells co-labeled with anti-β-Gal was greatly reduced in adulthood compared to P5 (Fig.5, 35.8 ± 3.47% versus 11.7 ± 0.98%, p<0.01). Because keratin-8 expression is limited to the more mature columnar cells of the taste bud, we also compared percentages of β-Gal+ cells that were keratin-8+ with those that were keratin-8-. Consistent with the idea that BDNF reduction is specific to the precursor population there was a substantial decrease in the percentage β-Gal+ cells that lacked keratin-8 (Fig 5. red bars); however, the percentage β-Gal+ cells that were keratin-8+ did not change during postnatal development. Thus, BDNF is reduced in the taste bud because the percentage of BDNF-expressing progenitor/precursor cells decreases between P5 and adulthood.
Taken together, β-Gal+ (BDNF) cells decrease in fungiform taste buds from P5 to adulthood. This decrease is not due to changes in BDNF expression within taste receptor cells or supporting cells, some of which express BDNF and some of which do not. Instead, the decrease is due to a reduction in the number of Sox2+/keratin-8− cells (precursor/progenitor cells) that co-express BDNF.
Postnatal changes in BDNF expression refine taste bud innervation
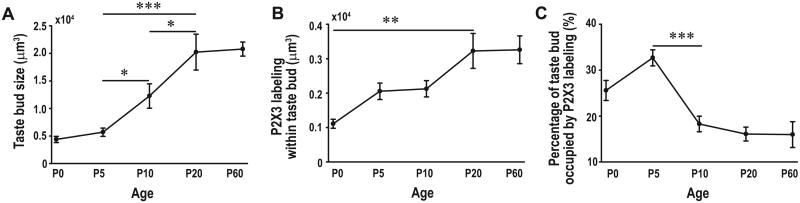
BDNF in taste placodes attracts and promotes taste innervation during embryonic development (Ma et al., 2009). Therefore, it is possible that a reduction in BDNF alters the development of taste bud innervation during postnatal development. If so, this change in innervation ought to occur between P5 and P10. Therefore, we examined the innervation within the taste buds from birth to adulthood. Anti-keratin-8 and anti-P2X3 were used to label fungiform taste buds and gustatory nerve fibers, respectively (Fig. 6 A-O). The density of innervation within the taste bud appears to decrease between P5 and P10. Because P2X3-label is patchy in the taste bud and there is no ideal way to measure innervation, we used two different methods. The results were the same regardless of the method used (see methods for details). We found that total innervation remained constant between P5 and P10 even though the taste bud was substantially increased in size (Fig 7. A, B, p ≤ 0.05). Because taste buds substantially increase in size during postnatal development, we compared the percentage of the taste bud occupied by innervation (i.e., the density, which was calculated as the volume of P2X3 label divided by the volume of the taste bud) across ages. We found that the density of innervation was not different between birth and P5. However, the density of innervation significantly decreased from P5 to P10 (p ≤ 0.001) (Fig. 7 C). After P10, the innervation remained proportional to the increase in taste bud size through adulthood. Because taste buds add taste cells between P5 and P10 of development, and amount of innervation is unchanged the number of fibers innervating each taste cell would decrease. In addition to the measured innervation there appeared to be more innervation immediately outside the keratin-8 staining at P0 and P5 compared with later postnatal ages (Fig. 6). These results indicate that taste innervation remodeling occurs during postnatal development, and the remodeling is correlated with a reduction in BDNF-expressing progenitor/precursor cells within the taste bud.
Figure 6.
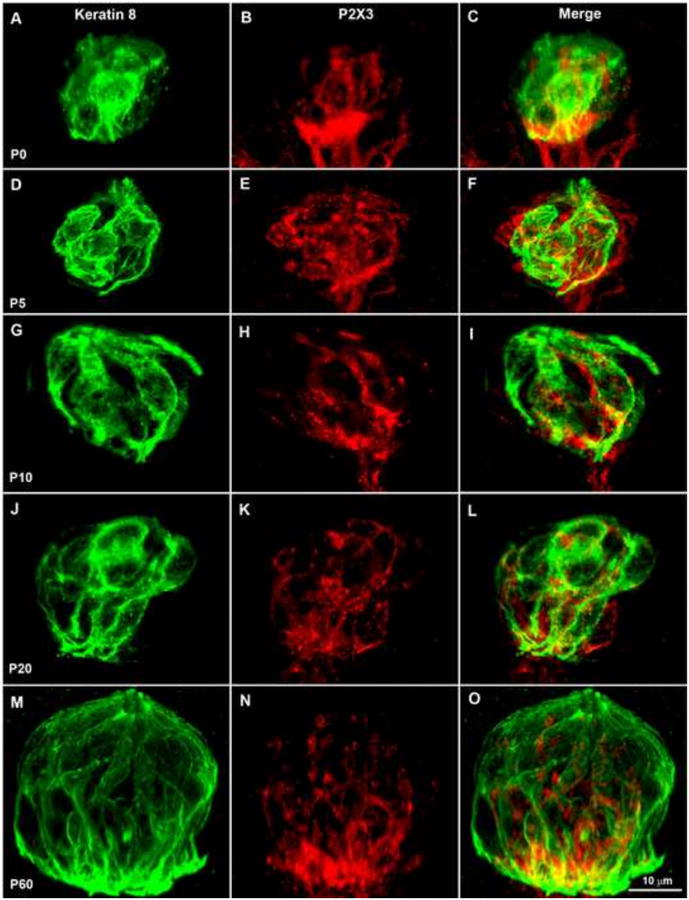
The percentage of fungiform taste buds labeled by P2X3 (innervation density) decreases between P5 and P10. Double-labeling of P2X3 (red) and keratin-8 (green) in postnatal fungiform taste buds (A-O). At all ages, P2X3-labeling was observed inside the keratin-8 borders but the density appears to decrease between P5 and P10. In addition, at P0 (A-C), P2X3 labeling was observed at the base of the taste bud, and at both P0 and P5 (D-F) P2X3 immunoreactivity was observed around the edges of the taste bud and immediately outside the region defined by anti-keratin-8. From P10 to P60 (G-O), the P2X3 label was contained within the perimeter of the anti-keratin label. After P10, the P2X3 label continued to occupy a constant small percentage of the taste bud. The scale bar in O applies to panels A-O.
Figure 7.
Innervation and taste bud volume were measured across ages within the taste bud using Imaris software. While taste buds increase in size between P5 and 10 (A), the amount of innervation within the taste bud stays roughly the same between these ages (B). This results in a substantial decrease in the proportion of the taste bud occupied by innervation (C). Following this sudden decrease, the proportion of the taste bud occupied by innervating fibers remains constant even though the taste bud continues to grow between P10 and P20. The single asterisks represents p ≤ 0.05, while the double asterisks represents p ≤ 0.01, triple asterisks represents p ≤ 0.001.
It is possible that the postnatal reduction in both BDNF expression and innervation density to the taste bud is simply a coincidence. To directly test the role of BDNF in this process, we sought to determine whether the overexpression of BDNF would prevent taste innervation from being reduced during postnatal development. We took advantage of mice that overexpress BDNF under the control of a keratin-14 promoter (K14-Bdnf-OE) (Krimm et al., 2001; LeMaster et al., 1999) so that BDNF would be overexpressed in taste progenitor cells (Okubo et al., 2009), the taste cell population in which BDNF reduction normally occurs during postnatal development. The density of taste bud innervation was compared between wild-type and K14-Bdnf-OE mice at P5 and P10 (Fig. 8 A-L). Since the two methods for measuring innervation produce equivalent results across ages, we limited this analysis to one method and measured the volume of pixels in each optical section in wild type and K14Bdnf-OE. We found that the density of innervation was reduced between P5 and P10 in wild-type mice (p < 0.05) but not in K14-Bdnf-OE mice. As a result, K14-Bdnf-OE mice had more innervation than wild-type mice by P10 (p ≤ 0.01). As during development, some additional innervation appeared at the perimeter of the taste bud (compare Fig 8 C, F, and L to I) where progenitor cells would be located, this additional perimeter innervation appeared to be lost by P10 in wild-type mice but retained in K14-Bdnf-OE mice. Thus, a reduction in BDNF in the progenitor/precursor cells of the taste buds during development results in a loss of innervation to the perimeter and a loss of density within the taste bud, which is blocked by BDNF overexpression in taste progenitors.
Figure 8.
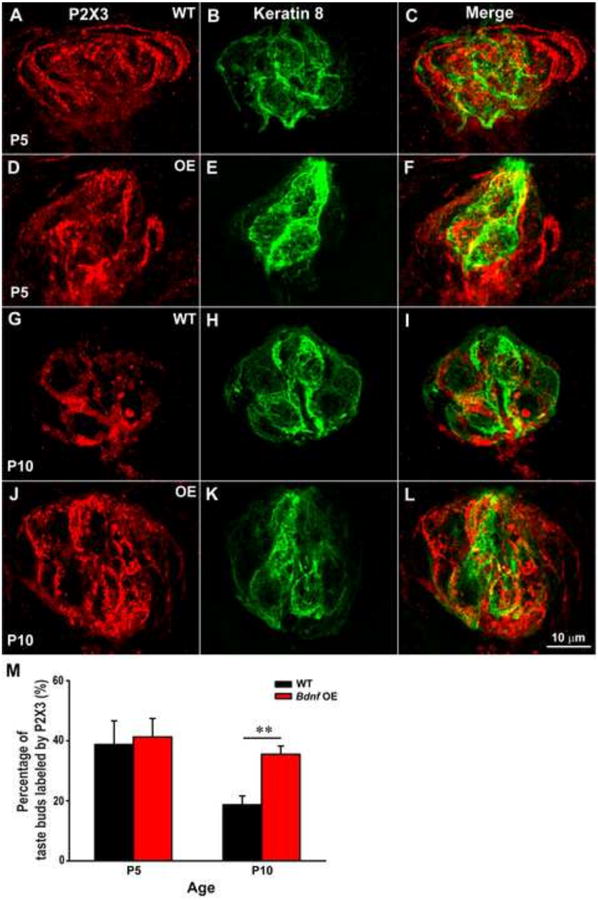
The overexpression of BDNF in K14+ progenitor/precursor cells eliminates the decrease in fungiform taste bud innervation during postnatal development. Double-labeling of P2X3 (red) and keratin-8 (green) in fungiform taste buds from wild-type and K14-Bdnf-OE mice (A-L). At P5, P2X3 immunoreactivity was robust around the outside edge of the taste buds in both wild-type and K14-Bdnf-OE mice (C and F, respectively). This innervation pattern was also observed in K14-Bdnf-OE mice at P10 (L). However, in wild-type mice, P2X3 labeling appeared to decrease and becomes limited to the region of the taste bud defined by anti-keratin-8 immunohistochemistry at P10 (I). (M) The percentage of the taste bud occupied by P2X3 label was not different between wild-type and K14-Bdnf-OE mice at P5, and decreased in wild-type mice but not K14-Bdnf-OE mice by P10 (p < 0.01). A two-way ANOVA revealed a significant age x genotype interaction (p < 0.05). The scale bar in L applies to panels A-L.
Discussion
Given that taste receptor cells and gustatory neurons have been reported to have similar responses to taste stimuli (Barretto et al., 2015; Yoshida and Ninomiya, 2010; Yoshida et al., 2006), a developmental mechanism likely coordinates the formation of specific connections between the two cell types. As with many other systems (Erzurumlu and Kind, 2001; Espinosa and Stryker, 2012; Marks et al., 2006; Walsh and Lichtman, 2003), postnatal remodeling is likely required for the formation of a mature taste bud with appropriate connections between taste receptor cells and gustatory neurons (Kinnamon et al., 2005; Mistretta et al., 1988; Nagai et al., 1988; Yuan and Yankner, 2000). BDNF regulates this remodeling in other systems (Cabelli et al., 1997; Gonzalez et al., 1999; Kaneko et al., 2008), and in the gustatory system is expressed in postnatal and adult taste buds (Huang and Krimm, 2010). Therefore, we speculated that BDNF could be involved in the postnatal remodeling of innervation within the taste bud. We found that BDNF was reduced in taste buds between P5 and P10 due to a reduction in BDNF-expressing cells. Specifically, the precursor/progenitor cells (which provide renewable sources of taste bud cells) that express BDNF were decreased in number. The timing of the BDNF reduction within the taste bud corresponds to a reduction in innervation density, indicating that the reduction of BDNF could orchestrate postnatal nerve fiber refinement. Consistently, we found that overexpression of BDNF in the progenitor cell population blocked this refinement in innervation which is likely why more geniculate neurons branch to innervate multiple taste buds by adulthood in K14-Bdnf-OE mice (Zaidi et al., 2007). Therefore, during normal development reduction in BDNF regulates refinement of innervation during a specific developmental window (P5-P10). We speculate that the loss of innervation density in the postnatal taste bud increases the specificity of connections between neurons and taste receptor cells during development.
The role of BDNF changes between embryonic and postnatal development. During embryonic development, BDNF is expressed in developing fungiform placodes before their innervation by nerve fibers (Nosrat et al., 1996; Nosrat and Olson, 1995). At this stage, there are no differentiated taste receptor cells in the placode. Therefore, it is not surprising that we found many BDNF-expressing progenitor/precursor cells at early postnatal ages. BDNF in this population of taste cells is both necessary and sufficient for taste fibers to reach their target during embryonic development (Lopez and Krimm, 2006a; Ma et al., 2009; Ringstedt et al., 1999). However, this role for BDNF is restricted to a critical period. After E14.5, BDNF is no longer needed for targeting; however, BDNF may be required for neuron survival for a limited period following target innervation (Ma et al., 2009). Typically, sensory neurons lose their dependency on neurotrophins for survival during development (Lindsay, 1988; Putcha et al., 2000), and geniculate neurons do not depend on BDNF for survival much past the embryonic development stage (Hoshino et al., 2010). Thus, by the time that BDNF is reduced in precursor/progenitor and supporting taste cells, BDNF is no longer required for either target innervation or neuron survival.
Taste bud development is a long process that spans the embryonic and postnatal period. The end result is a complicated sensory end organ with multiple taste cell types (Clapp et al., 2006; Clapp et al., 2004; Delay et al., 1986; Finger, 2005; Kataoka et al., 2008; Murray and Murray, 1967; Murray et al., 1969; Yang et al., 2000a; Yang et al., 2000b; Yee et al., 2001). The number of taste bud cells doubles between P5 and adulthood. Interestingly, the number of receptor cells as defined by PLCβ2 and Car4 staining increases proportionately as the taste bud grows. This result is consistent with a previous study in which IP3R3 cells (another taste receptor cell marker) increase in parallel with the total number of taste bud cells during postnatal development (Ohtubo et al., 2012). However, the proportion of Sox2+/Keratin-8- cells decreases during development. It is not clear whether fewer Sox2+/Keratin-8− cells express BDNF because they down-regulate BDNF expression or because this population in general decreases relative to the size of the taste bud. It is likely that both processes are occurring. The proportion of Sox2+/Keratin-8- taste cells that do not express BDNF increases during development. Therefore, some of the decrease in BDNF is likely due to a down-regulation of BDNF.
Taste buds increase tremendously in size throughout postnatal development due to both an increase in the number of taste cells, which almost triples, and increases in taste cell size. As a result innervation should also increase to accommodate the larger number of taste receptor cells. In this changing environment, innervation density decreases between P5 and P10, but the total innervation to the taste bud is unchanged. It could be argued that as the taste bud grows in size, some of which is due to an increase in the size of individual cells, the total amount of P2X3 label per unit area would naturally decline. While we agree that this ought to occur to some degree, it is also the case that innervation extends the full depth of the taste bud, so as taste buds become taller, innervation should increase. In addition, the taste bud primarily grows by adding new taste receptor cells, as new cells are added they need to be innervated. Since no new innervation is added between P5 and P10, the number of fibers innervating each cell would need to be reduced during this time if each taste receptor cell is to contact a nerve fiber. This is remodeling. Admittedly our rather crude measure does not directly measure innervation to single taste cells. The number of fibers innervating a single taste bud cannot be measured with current techniques. However, techniques allowing better labeling of nerve fibers or electron microscopy may eventually allow the number of nerve fibers innervating single taste cells to be quantified at different ages.
As BDNF is reduced postnatally, there is a concurrent loss of innervation density to the taste bud, which indicates that peripheral taste innervation undergoes remodeling during postnatal development. The remodeling of innervation is a common developmental phenomenon that typically results in increased specificity of connections (Erzurumlu and Kind, 2001; Espinosa and Stryker, 2012; Walsh and Lichtman, 2003). Although this process is well established for the central projections of gustatory neurons (King and Hill, 1991; Mangold and Hill, 2007; May and Hill, 2006; Sollars et al., 2006), only a few studies have attempted to study remodeling in the peripheral taste system. In sheep, the receptive fields of individual chorda tympani axons are reduced during postnatal development, demonstrating that branching between individual fungiform papillae is reduced (Mistretta et al., 1988; Nagai et al., 1988). In rat, circumvallate taste buds are initially hyper-innervated, and the nerve plexus contains neuro-neuronal synapses (Kinnamon et al., 2005). This plexus decreases in size and neuro-neuronal synapses are lost during postnatal development, which implies reorganization. This finding of decreased innervation within circumvallate taste buds is similar to our observation in fungiform taste buds that the density of innervation decreases. Chorda tympani axons likely branch both between and within taste buds, and remodeling could reduce branching in both locations, while individual branches widen and lengthen. A previous study has observed that the overexpression of BDNF in the precursor/progenitor subpopulation increases the number of gustatory neurons that innervate multiple taste buds (Zaidi et al., 2007). Because the same number of chorda tympani neurons innervate the tongue in wild-type and K14-Bdnf-OE mice (Sun et al., 2015), the increased in innervation to taste buds must be due to increased branching. Since innervation density is similar in the taste buds of P5 wild-type and K14-Bdnf-OE mice, the increased branching in adult K14-Bdnf-OE mice (Zaidi et al., 2007) could be due to a blockade of normal refinement between P5 and P10. Since BDNF is not reduced in these mice, it is also possible that branches continue to be added to the taste bud as new taste cells are added between P5 and P10. These possibilities are not mutually exclusive.
The reduction in innervation density could result in the formation of more specific connections between innervating fibers and the developing taste receptor cell population, as nerve fibers withdraw from one cell to innervate another. Interestingly, few PLCβ2+ taste receptor cells, which are important for sweet, umami, and/or bitter tastes, express BDNF. In contrast, Car4+ cells, which may be important for sour taste, continue to express BDNF throughout the lifetime of the animal. It is possible that the reduction in postnatal BDNF and innervation density in the taste bud may specifically reduce innervation to the PLCβ2 population of taste receptor cells because they lack BDNF. The overexpression of BDNF under the control of the α-gustducin promoter (which results in BDNF expression in half of the PLCβ2 population) also increases innervation to the taste bud (Nosrat et al., 2012). Therefore, the overexpression of BDNF in this location may also prevent the postnatal refinement of innervation within the taste bud. The functional consequences of BDNF overexpression in the precursor/progenitor subpopulation are increased sucrose responses relative to other taste stimuli (Sun et al., 2015). One potential explanation for this finding is that these sweet-transducing taste cells are innervated by nerve fibers whose innervation would normally be eliminated if BDNF had not been overexpressed.
BDNF continues to be expressed in a subpopulation of taste cells in adulthood, which suggests that BDNF has a role in mature taste cells. There is a constant turnover of taste cells even in adulthood, so one possibility is that new taste receptor cells need to attract innervation to form new connections. If so, then BDNF would attract innervation to Car4+ but not PLCβ2+ taste cells. Under this scenario, a subpopulation of nerve fibers that are not responsive to BDNF in adulthood would preferentially innervate PLCβ2+ cells. Even during development, only approximately half of the gustatory neurons are dependent on BDNF (Patel and Krimm, 2010), and there is a subpopulation of taste neurons that do not express the BDNF receptor, TrkB, and are not dependent on TrkB during development (Fei and Krimm, 2013). Therefore, BDNF could help to coordinate the innervation of a specific subpopulation of taste bud cells. Another possibility is that BDNF is involved in synapse formation. BDNF has been shown to be important for synapse formation (Chen et al., 2011; Rico et al., 2002), and so it may play this role in the adult taste bud. Consistently, we found that Car4+ cells co-express SNAP25, a synapse associated protein (Yang et al., 2000a), and so new Car4 cells would likely need to continuously form synapses. Lastly, BDNF in this location could be important for synaptic function (Poo, 2001; Schinder and Poo, 2000; Zhang and Poo, 2002).
In addition to being expressed by Car4+ taste receptor cells, we found that BDNF is present in another adult taste cell population. Because we found almost complete overlap between Car4 and SNAP25 in taste cells, this additional population of BDNF-expressing taste cells lacks SNAP25 and synapses. These additional BDNF+ taste cells may co-label for NTPDase2, since we observed some taste cells double-labeled with NTPDase2 and β-Gal in adult taste buds. In fungiform taste buds, NTPDase2 co-localizes with GLAST, and so has been described as a type 1 taste bud cell marker (Bartel et al., 2006) and so it could be in supporting cells. Alternatively, if BDNF has a functional role or coordinates innervation to taste receptor cells it is likely that this is a poorly defined taste cell type that transduces one or more taste stimuli.
Supplementary Material
Supplementary Figure 1. Images illustrating two methods of quantification of innervation for P5 (A-D) and P10 (E-H) taste buds. In method one, the keratin-8 staining was used to draw a border (A,E) and then the area occupied by P2X3 pixels was measured in each one micron optical section (B,F) and summed to give the volume in the entire taste bud. In method two, the taste bud was examined in 3-dimensions and keratin-8 labeling defined the region of interest while labeling outside this border was eliminated (C, G). All P2x3 labeling within the region of interest was identified and “masked” and the volume within the masked region was calculated.
Supplementary Figure 2. Tongue sections from wild type mice were typically conducted alongside sections from BdnflacZ, and we never observed staining in mice that lacked the lacZ gene. Example taste buds are shown at P0 (A), P5 (B), P20 (C).
Supplementary Figure 3. The co-expression of β-Gal with 5-HT and SNAP25 within the fungiform taste buds. (A-D) Double-labeling of β-Gal (red) and 5-HT (green). All 5-HT taste bud cells were β-Gal+ (arrows), but not all β-Gal+ cells were 5-HT+ (arrow heads). (E-H) Similarly, SNAP25+ cells were also β-Gal+ (arrows). The scale bar in H equal 10μm and applies to all the panels.
Supplementary Figure 4. The co-expression of Car4 with taste bud cell markers in the fungiform taste buds. (A-D) The double-labeling of Car4 (green) and PLCβ2 (red) indicated that there was almost no overlap between Car4 and PLCβ2 in fungiform taste buds (labeled by keratin-8, blue). However, Car4+ cells were labeled by antibodies against “type III” taste bud cell markers: 5-HT (E-H) and SNAP25 (I-L). The scale bar in L applies to A-L.
Supplementary Figure 5. The number of taste bud cells that co-express β-Gal and NTPDase2 decreases in fungiform taste buds during postnatal development. Double-labeling of β-Gal (red) with NTPDase2 (green) and keratin-8 (blue) within the fungiform taste buds. Fungiform taste buds co-labeled with β-Gal and NTPDase2 were observed at both P5 (A-D) and adulthood (E-H) (arrows); cells negative for β-Gal also were observed (arrow heads). (I) The proportion of cells that co-express NTPDase2 and β-Gal was significantly reduced between P5 and adulthood. The scale bar in H applies to all the panels.
Acknowledgments
This work was supported by National Institutes of Health Grants DC007176 (R.F.K). Thanks to Brad Biggs for his technical support with the animal colony.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting galley proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
References
- Barretto RP, Gillis-Smith S, Chandrashekar J, Yarmolinsky DA, Schnitzer MJ, Ryba NJ, Zuker CS. The neural representation of taste quality at the periphery. Nature. 2015;517:373–376. doi: 10.1038/nature13873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DL, Sullivan SL, Lavoie EG, Sevigny J, Finger TE. Nucleoside triphosphate diphosphohydrolase-2 is the ecto-ATPase of type I cells in taste buds. The Journal of comparative neurology. 2006;497:1–12. doi: 10.1002/cne.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigiani A, Cristiani R, Fieni F, Ghiaroni V, Bagnoli P, Pietra P. Postnatal development of membrane excitability in taste cells of the mouse vallate papilla. J Neurosci. 2002;22:493–504. doi: 10.1523/JNEUROSCI.22-02-00493.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabelli RJ, Shelton DL, Segal RA, Shatz CJ. Blockade of endogenous ligands of trkB inhibits formation of ocular dominance columns. Neuron. 1997;19:63–76. doi: 10.1016/s0896-6273(00)80348-7. [DOI] [PubMed] [Google Scholar]
- Chandrashekar J, Yarmolinsky D, von Buchholtz L, Oka Y, Sly W, Ryba NJ, Zuker CS. The taste of carbonation. Science. 2009;326:443–445. doi: 10.1126/science.1174601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AI, Nguyen CN, Copenhagen DR, Badurek S, Minichiello L, Ranscht B, Reichardt LF. TrkB (tropomyosin-related kinase B) controls the assembly and maintenance of GABAergic synapses in the cerebellar cortex. J Neurosci. 2011;31:2769–2780. doi: 10.1523/JNEUROSCI.4991-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp TR, Medler KF, Damak S, Margolskee RF, Kinnamon SC. Mouse taste cells with G protein-coupled taste receptors lack voltage-gated calcium channels and SNAP-25. BMC biology. 2006;4:7. doi: 10.1186/1741-7007-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp TR, Yang R, Stoick CL, Kinnamon SC, Kinnamon JC. Morphologic characterization of rat taste receptor cells that express components of the phospholipase C signaling pathway. The Journal of comparative neurology. 2004;468:311–321. doi: 10.1002/cne.10963. [DOI] [PubMed] [Google Scholar]
- Delay RJ, Kinnamon JC, Roper SD. Ultrastructure of mouse vallate taste buds: II. Cell types and cell lineage. The Journal of comparative neurology. 1986;253:242–252. doi: 10.1002/cne.902530210. [DOI] [PubMed] [Google Scholar]
- Erzurumlu RS, Kind PC. Neural activity: sculptor of ‘barrels’ in the neocortex. Trends Neurosci. 2001;24:589–595. doi: 10.1016/s0166-2236(00)01958-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa JS, Stryker MP. Development and plasticity of the primary visual cortex. Neuron. 2012;75:230–249. doi: 10.1016/j.neuron.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei D, Krimm RF. Taste Neurons Consist of Both a Large TrkB-Receptor-Dependent and a Small TrkB-Receptor-Independent Subpopulation. PLoS One. 2013;8:e83460. doi: 10.1371/journal.pone.0083460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger TE. Cell types and lineages in taste buds. Chem Senses. 2005;30(Suppl 1):i54–55. doi: 10.1093/chemse/bjh110. [DOI] [PubMed] [Google Scholar]
- Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310:1495–1499. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- Gonzalez M, Ruggiero FP, Chang Q, Shi YJ, Rich MM, Kraner S, Balice-Gordon RJ. Disruption of Trkb-mediated signaling induces disassembly of postsynaptic receptor clusters at neuromuscular junctions. Neuron. 1999;24:567–583. doi: 10.1016/s0896-6273(00)81113-7. [DOI] [PubMed] [Google Scholar]
- Hoshino N, Vatterott P, Egwiekhor A, Rochlin MW. Brain-derived neurotrophic factor attracts geniculate ganglion neurites during embryonic targeting. Developmental neuroscience. 2010;32:184–196. doi: 10.1159/000313902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosley MA, Oakley B. Postnatal development of the vallate papilla and taste buds in rats. Anat Rec. 1987;218:216–222. doi: 10.1002/ar.1092180217. [DOI] [PubMed] [Google Scholar]
- Huang AL, Chen X, Hoon MA, Chandrashekar J, Guo W, Trankner D, Ryba NJ, Zuker CS. The cells and logic for mammalian sour taste detection. Nature. 2006;442:934–938. doi: 10.1038/nature05084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T, Krimm RF. Developmental expression of Bdnf, Ntf4/5, and TrkB in the mouse peripheral taste system. Developmental dynamics : an official publication of the American Association of Anatomists. 2010;239:2637–2646. doi: 10.1002/dvdy.22412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- Jones KR, Farinas I, Backus C, Reichardt LF. Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell. 1994;76:989–999. doi: 10.1016/0092-8674(94)90377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M, Hanover JL, England PM, Stryker MP. TrkB kinase is required for recovery, but not loss, of cortical responses following monocular deprivation. Nat Neurosci. 2008;11:497–504. doi: 10.1038/nn2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka S, Yang R, Ishimaru Y, Matsunami H, Sevigny J, Kinnamon JC, Finger TE. The candidate sour taste receptor, PKD2L1, is expressed by type III taste cells in the mouse. Chem Senses. 2008;33:243–254. doi: 10.1093/chemse/bjm083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CT, Hill DL. Dietary sodium chloride deprivation throughout development selectively influences the terminal field organization of gustatory afferent fibers projecting to the rat nucleus of the solitary tract. The Journal of comparative neurology. 1991;303:159–169. doi: 10.1002/cne.903030114. [DOI] [PubMed] [Google Scholar]
- Kinnamon JC, Dunlap M, Yang R. Synaptic connections in developing and adult rat taste buds. Chem Senses. 2005;30(Suppl 1):i60–i61. doi: 10.1093/chemse/bjh113. [DOI] [PubMed] [Google Scholar]
- Kirkby LA, Sack GS, Firl A, Feller MB. A role for correlated spontaneous activity in the assembly of neural circuits. Neuron. 2013;80:1129–1144. doi: 10.1016/j.neuron.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krimm RF, Miller KK, Kitzman PH, Davis BM, Albers KM. Epithelial overexpression of BDNF or NT4 disrupts targeting of taste neurons that innervate the anterior tongue. Developmental biology. 2001;232:508–521. doi: 10.1006/dbio.2001.0190. [DOI] [PubMed] [Google Scholar]
- LeMaster AM, Krimm RF, Davis BM, Noel T, Forbes ME, Johnson JE, Albers KM. Overexpression of brain-derived neurotrophic factor enhances sensory innervation and selectively increases neuron number. J Neurosci. 1999;19:5919–5931. doi: 10.1523/JNEUROSCI.19-14-05919.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay RM. Nerve growth factors (NGF, BDNF) enhance axonal regeneration but are not required for survival of adult sensory neurons. J Neurosci. 1988;8:2394–2405. doi: 10.1523/JNEUROSCI.08-07-02394.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using realtime quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lopez GF, Krimm RF. Epithelial overexpression of BDNF and NT4 produces distinct gustatory axon morphologies that disrupt initial targeting. Developmental biology. 2006a;292:457–468. doi: 10.1016/j.ydbio.2006.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez GF, Krimm RF. Refinement of innervation accuracy following initial targeting of peripheral gustatory fibers. J Neurobiol. 2006b;66:1033–1043. doi: 10.1002/neu.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Lopez GF, Krimm RF. Epithelial-derived brain-derived neurotrophic factor is required for gustatory neuron targeting during a critical developmental period. J Neurosci. 2009;29:3354–3364. doi: 10.1523/JNEUROSCI.3970-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangold JE, Hill DL. Extensive reorganization of primary afferent projections into the gustatory brainstem induced by feeding a sodium-restricted diet during development: less is more. J Neurosci. 2007;27:4650–4662. doi: 10.1523/JNEUROSCI.4518-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks CA, Cheng K, Cummings DM, Belluscio L. Activity-dependent plasticity in the olfactory intrabulbar map. J Neurosci. 2006;26:11257–11266. doi: 10.1523/JNEUROSCI.2805-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May OL, Hill DL. Gustatory terminal field organization and developmental plasticity in the nucleus of the solitary tract revealed through triple-fluorescence labeling. The Journal of comparative neurology. 2006;497:658–669. doi: 10.1002/cne.21023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbiene JP, Mistretta CM. Initial innervation of embryonic rat tongue and developing taste papillae: nerves follow distinctive and spatially restricted pathways. Acta Anat (Basel) 1997;160:139–158. doi: 10.1159/000148006. [DOI] [PubMed] [Google Scholar]
- Mistretta CM, Gurkan S, Bradley RM. Morphology of chorda tympani fiber receptive fields and proposed neural rearrangements during development. J Neurosci. 1988;8:73–78. doi: 10.1523/JNEUROSCI.08-01-00073.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura H, Scott JK, Harada S, Barlow LA. Sonic hedgehog-expressing basal cells are general post-mitotic precursors of functional taste receptor cells. Developmental dynamics : an official publication of the American Association of Anatomists. 2014;243:1286–1297. doi: 10.1002/dvdy.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RG, Murray A. Fine structure of taste buds of rabbit foliate papillae. Journal of ultrastructure research. 1967;19:327–353. doi: 10.1016/s0022-5320(67)80224-7. [DOI] [PubMed] [Google Scholar]
- Murray RG, Murray A, Fujimoto S. Fine structure of gustatory cells in rabbit taste buds. Journal of ultrastructure research. 1969;27:444–461. doi: 10.1016/s0022-5320(69)80043-2. [DOI] [PubMed] [Google Scholar]
- Nagai T, Mistretta CM, Bradley RM. Developmental decrease in size of peripheral receptive fields of single chorda tympani nerve fibers and relation to increasing NaCl taste sensitivity. J Neurosci. 1988;8:64–72. doi: 10.1523/JNEUROSCI.08-01-00064.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen QT, Parsadanian AS, Snider WD, Lichtman JW. Hyperinnervation of neuromuscular junctions caused by GDNF overexpression in muscle. Science. 1998;279:1725–1729. doi: 10.1126/science.279.5357.1725. [DOI] [PubMed] [Google Scholar]
- Nosrat CA, Ebendal T, Olson L. Differential expression of brain-derived neurotrophic factor and neurotrophin 3 mRNA in lingual papillae and taste buds indicates roles in gustatory and somatosensory innervation. The Journal of comparative neurology. 1996;376:587–602. doi: 10.1002/(SICI)1096-9861(19961223)376:4<587::AID-CNE7>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Nosrat CA, Olson L. Brain-derived neurotrophic factor mRNA is expressed in the developing taste bud-bearing tongue papillae of rat. The Journal of comparative neurology. 1995;360:698–704. doi: 10.1002/cne.903600413. [DOI] [PubMed] [Google Scholar]
- Nosrat IV, Margolskee RF, Nosrat CA. Targeted taste cell-specific overexpression of brain-derived neurotrophic factor in adult taste buds elevates phosphorylated TrkB protein levels in taste cells, increases taste bud size, and promotes gustatory innervation. J Biol Chem. 2012;287:16791–16800. doi: 10.1074/jbc.M111.328476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtubo Y, Iwamoto M, Yoshii K. Subtype-dependent postnatal development of taste receptor cells in mouse fungiform taste buds. Eur J Neurosci. 2012;35:1661–1671. doi: 10.1111/j.1460-9568.2012.08068.x. [DOI] [PubMed] [Google Scholar]
- Okubo T, Clark C, Hogan BL. Cell lineage mapping of taste bud cells and keratinocytes in the mouse tongue and soft palate. Stem Cells. 2009;27:442–450. doi: 10.1634/stemcells.2008-0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo T, Pevny LH, Hogan BL. Sox2 is required for development of taste bud sensory cells. Genes Dev. 2006;20:2654–2659. doi: 10.1101/gad.1457106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AV, Krimm RF. BDNF is required for the survival of differentiated geniculate ganglion neurons. Developmental biology. 2010;340:419–429. doi: 10.1016/j.ydbio.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffenberger C, Cutforth T, Woods G, Yamada J, Renteria RC, Copenhagen DR, Flanagan JG, Feldheim DA. Ephrin-As and neural activity are required for eye-specific patterning during retinogeniculate mapping. Nat Neurosci. 2005;8:1022–1027. doi: 10.1038/nn1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- Putcha GV, Deshmukh M, Johnson EM., Jr Inhibition of apoptotic signaling cascades causes loss of trophic factor dependence during neuronal maturation. J Cell Biol. 2000;149:1011–1018. doi: 10.1083/jcb.149.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico B, Xu B, Reichardt LF. TrkB receptor signaling is required for establishment of GABAergic synapses in the cerebellum. Nat Neurosci. 2002;5:225–233. doi: 10.1038/nn808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringstedt T, Ibanez CF, Nosrat CA. Role of brain-derived neurotrophic factor in target invasion in the gustatory system. J Neurosci. 1999;19:3507–3518. doi: 10.1523/JNEUROSCI.19-09-03507.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinder AF, Poo M. The neurotrophin hypothesis for synaptic plasticity. Trends Neurosci. 2000;23:639–645. doi: 10.1016/s0166-2236(00)01672-6. [DOI] [PubMed] [Google Scholar]
- Sollars SI, Walker BR, Thaw AK, Hill DL. Age-related decrease of the chorda tympani nerve terminal field in the nucleus of the solitary tract is prevented by dietary sodium restriction during development. Neuroscience. 2006;137:1229–1236. doi: 10.1016/j.neuroscience.2005.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Dayal A, Hill DL. Expanded terminal fields of gustatory nerves accompany embryonic BDNF overexpression in mouse oral epithelia. J Neurosci. 2015;35:409–421. doi: 10.1523/JNEUROSCI.2381-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbeuch A, Anderson CB, Parnes J, Enjyoji K, Robson SC, Finger TE, Kinnamon SC. Role of the ectonucleotidase NTPDase2 in taste bud function. Proc Natl Acad Sci U S A. 2013;110:14789–14794. doi: 10.1073/pnas.1309468110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh MK, Lichtman JW. In vivo time-lapse imaging of synaptic takeover associated with naturally occurring synapse elimination. Neuron. 2003;37:67–73. doi: 10.1016/s0896-6273(02)01142-x. [DOI] [PubMed] [Google Scholar]
- Yang R, Crowley HH, Rock ME, Kinnamon JC. Taste cells with synapses in rat circumvallate papillae display SNAP-25-like immunoreactivity. The Journal of comparative neurology. 2000a;424:205–215. doi: 10.1002/1096-9861(20000821)424:2<205::aid-cne2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Yang R, Tabata S, Crowley HH, Margolskee RF, Kinnamon JC. Ultrastructural localization of gustducin immunoreactivity in microvilli of type II taste cells in the rat. The Journal of comparative neurology. 2000b;425:139–151. doi: 10.1002/1096-9861(20000911)425:1<139::aid-cne12>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Yee CL, Jones KR, Finger TE. Brain-derived neurotrophic factor is present in adult mouse taste cells with synapses. The Journal of comparative neurology. 2003;459:15–24. doi: 10.1002/cne.10589. [DOI] [PubMed] [Google Scholar]
- Yee CL, Yang R, Bottger B, Finger TE, Kinnamon JC. “Type III” cells of rat taste buds: immunohistochemical and ultrastructural studies of neuron-specific enolase, protein gene product 9.5, and serotonin. The Journal of comparative neurology. 2001;440:97–108. doi: 10.1002/cne.1372. [DOI] [PubMed] [Google Scholar]
- Yoshida R, Ninomiya Y. New insights into the signal transmission from taste cells to gustatory nerve fibers. International review of cell and molecular biology. 2010;279:101–134. doi: 10.1016/S1937-6448(10)79004-3. [DOI] [PubMed] [Google Scholar]
- Yoshida R, Yasumatsu K, Shigemura N, Ninomiya Y. Coding channels for taste perception: information transmission from taste cells to gustatory nerve fibers. Arch Histol Cytol. 2006;69:233–242. doi: 10.1679/aohc.69.233. [DOI] [PubMed] [Google Scholar]
- Yuan J, Yankner BA. Apoptosis in the nervous system. Nature. 2000;407:802–809. doi: 10.1038/35037739. [DOI] [PubMed] [Google Scholar]
- Zaidi FN, Krimm RF, Whitehead MC. Exuberant neuronal convergence onto reduced taste bud targets with preservation of neural specificity in mice overexpressing neurotrophin in the tongue epithelium. J Neurosci. 2007;27:13875–13881. doi: 10.1523/JNEUROSCI.2517-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GH, Zhang HY, Deng SP, Qin YM, Wang TH. Quantitative study of taste bud distribution within the oral cavity of the postnatal mouse. Arch Oral Biol. 2008;53:583–589. doi: 10.1016/j.archoralbio.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Zhang X, Poo MM. Localized synaptic potentiation by BDNF requires local protein synthesis in the developing axon. Neuron. 2002;36:675–688. doi: 10.1016/s0896-6273(02)01023-1. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Images illustrating two methods of quantification of innervation for P5 (A-D) and P10 (E-H) taste buds. In method one, the keratin-8 staining was used to draw a border (A,E) and then the area occupied by P2X3 pixels was measured in each one micron optical section (B,F) and summed to give the volume in the entire taste bud. In method two, the taste bud was examined in 3-dimensions and keratin-8 labeling defined the region of interest while labeling outside this border was eliminated (C, G). All P2x3 labeling within the region of interest was identified and “masked” and the volume within the masked region was calculated.
Supplementary Figure 2. Tongue sections from wild type mice were typically conducted alongside sections from BdnflacZ, and we never observed staining in mice that lacked the lacZ gene. Example taste buds are shown at P0 (A), P5 (B), P20 (C).
Supplementary Figure 3. The co-expression of β-Gal with 5-HT and SNAP25 within the fungiform taste buds. (A-D) Double-labeling of β-Gal (red) and 5-HT (green). All 5-HT taste bud cells were β-Gal+ (arrows), but not all β-Gal+ cells were 5-HT+ (arrow heads). (E-H) Similarly, SNAP25+ cells were also β-Gal+ (arrows). The scale bar in H equal 10μm and applies to all the panels.
Supplementary Figure 4. The co-expression of Car4 with taste bud cell markers in the fungiform taste buds. (A-D) The double-labeling of Car4 (green) and PLCβ2 (red) indicated that there was almost no overlap between Car4 and PLCβ2 in fungiform taste buds (labeled by keratin-8, blue). However, Car4+ cells were labeled by antibodies against “type III” taste bud cell markers: 5-HT (E-H) and SNAP25 (I-L). The scale bar in L applies to A-L.
Supplementary Figure 5. The number of taste bud cells that co-express β-Gal and NTPDase2 decreases in fungiform taste buds during postnatal development. Double-labeling of β-Gal (red) with NTPDase2 (green) and keratin-8 (blue) within the fungiform taste buds. Fungiform taste buds co-labeled with β-Gal and NTPDase2 were observed at both P5 (A-D) and adulthood (E-H) (arrows); cells negative for β-Gal also were observed (arrow heads). (I) The proportion of cells that co-express NTPDase2 and β-Gal was significantly reduced between P5 and adulthood. The scale bar in H applies to all the panels.