Abstract
This review gives a comparative evaluation of the radioprotective properties and the therapeutic index (TI) of radioprotectors from various pharmacological group in experiments on both small and large animals. It presents a hypothesis explaining the decrease in the TI of cystamine and 5-methoxytryptamine (mexamine), and the retention of that of α1-adrenomimetic indralin, and also compares the effects on large and small animals. The considerable differences in the therapeutic indices of catecholamines, serotonin and cystamine are a consequence of specific features of their mechanisms of radioprotective action. Radioprotectors acting via receptor mediation tend to provide a more expanded window of protection. The reduction in the TI of cystamine in larger animals, such as dogs, may be caused by the greater increase in toxicity of aminothiols in relation to the decrease in their optimal doses for radioprotective effect in going from mice to dogs, which is a consequence of the slower metabolic processes in larger animals. The somatogenic phase of intoxication by cystamine is significantly longer than the duration of its radioprotective effect, and increases with irradiation. The decrease in the radioprotective effect and the TI of mexamine in experiments with dogs may be caused by their lower sensitivity to the acute hypoxia induced by the mexamine. This is because of lower gradient in oxygen tension between tissue cells and blood capillaries under acute hypoxia that is determined by lower initial oxygen consumption in a large animal as compared with a small animal. Indralin likely provides optimal radioprotective effects and a higher TI for large animals via the increased specificity of its adrenergic effect on tissue respiration, which supports the development of acute hypoxia in the radiosensitive tissues of large animals. The stimulatory effect of indralin on early post-irradiation haematopoietic recovery cannot provide a high level of radioprotective action for large animals, but it may promote recovery.
Keywords: indralin, epinephrine, norepinephrine, serotonin, 5-methoxytryptamine, cystamine, therapeutic index
INTRODUCTION
The key aspects of radioprotective agents are their practicality for use in specific scenarios of radiation exposure and the corresponding tactical and technical requirements for medical preparations. At the present time, amifostine, a radioprotector from the aminothiol family, is used in clinical practice as a radioprotectant and a chemoprotectant during the radio–chemotherapy of patients with head and neck tumors, lung cancer and breast cancer, reducing the radiotoxicity and cytoxicity of therapies [1–3]. According to clinical data reported by Trog et al. [1] (on reduction of the symptoms of postradiation mucositis during radiotherapy treatment of head and neck cancer patients), dose reduction factor (DRF) for amifostine is equal to 1.37 [4]. The first clinical investigations of the radioprotective effect of radioprotector mexamine were reported by Votkevich and Palyga [5]. Mexamine is used as a mitigator to reduce the chemotoxicity of chemotherapy [6–8]. Indralin (B-190) is used as a radioprotective agent for the medical protection of personnel during emergency situations at nuclear power plants [9, 10].
In 1951, Zenon Bacq [11] discovered the phenomenon of ‘chemical protection’ against the damaging effects of ionizing radiation via the administration of cystamine prior to lethal doses of radiation. Cystamine gave complete protection under such conditions. Most of the known medications at the time of this discovery were screened for radioprotective properties [12, 13]. Biogenic amines including epinephrine, norepinephrine, dopamine, serotonin, tryptamine, 5-methoxytryptamine (mexamine), melatonin and histamine, i.e. essential components of the neurohumoral regulation of vital body functions, were also subjected to such testing.
Of the above-mentioned compounds, serotonin had the highest radioprotective effect, comparable with that of cystamine [14–16]. Later, similar protective properties were found in 5-methyl derivatives of serotonin—e.g. mexamine [17]. In the first experiments with catecholamines and histamine, the radioprotective effects were slight and did not exceed 10–40% [18–22]. In later studies, however, with high-dose-rate radiation exposure and reduction of the exposure time to a few minutes, these biogenic amines exhibited a pronounced radioprotective effect [23, 24]. According to Kulinskii et al. [25], the radioprotective effectiveness of epinephrine and norepinephrine is realized through their binding to α1-adrenergic receptors. Later, highly protective properties were observed in the α1-adrenergic agonists methoxamine, phenylephrine, naphazoline and indralin [26–33].
The radioprotective activity of biogenic amines is associated with a partial neutralization of the radiobiological ‘oxygen effect’ phenomenon: i.e. the fact that an increase in cellular oxygen tension permits more radiation damage to occur. Evidence for the hypoxic mechanism of the radioprotective effect of biogenic amines was first obtained by van der Meer and van Bekkum [23, 34], and confirmed by Konstantinova and Graevskii [22]. A close relationship between the radioprotective efficacy of biogenic amines and the local tissue hypoxia induced by their vasoactive actions has been established [35–41].
A similar correlation for sympathomimetics was not always quite so explicit [42]. Application of pharmacological antagonists eliminated the radioprotective effect of serotonin, histamine, epinephrine, norepinephrine, phenylephrine and indralin [25, 32, 35, 43, 44]. The same effect was observed with animal radiation exposure in an atmosphere of increased oxygen pressure [32, 45–47].
REVIEW
The window of radioprotection for biogenic amines and aminothiols: a comparative investigation
The therapeutic window for drugs, including radioprotectors, is their most important characteristic [13], and is closely associated with the selectivity and affinity of the drug in relation to the expressed cellular receptors responsible for its pharmacological action. The therapeutic window for radioprotectors can be estimated from the therapeutic index (TI), which is defined as the ratio of the drug LD50 (lethal dose, 50%) to the drug ED50 (effective dose, 50%). The LD50 and the ED50 is based on a probit analysis, typically using at least three drug doses that do not result in all-or-none mortality or drug effect [48]. The ED50/30 of a radioprotector is its average effective dose for 30-day survival when the animals are exposed to radiation LD90–100/30. The LD50/3 is the average lethal toxic dose of the radioprotector for 3-day survival.
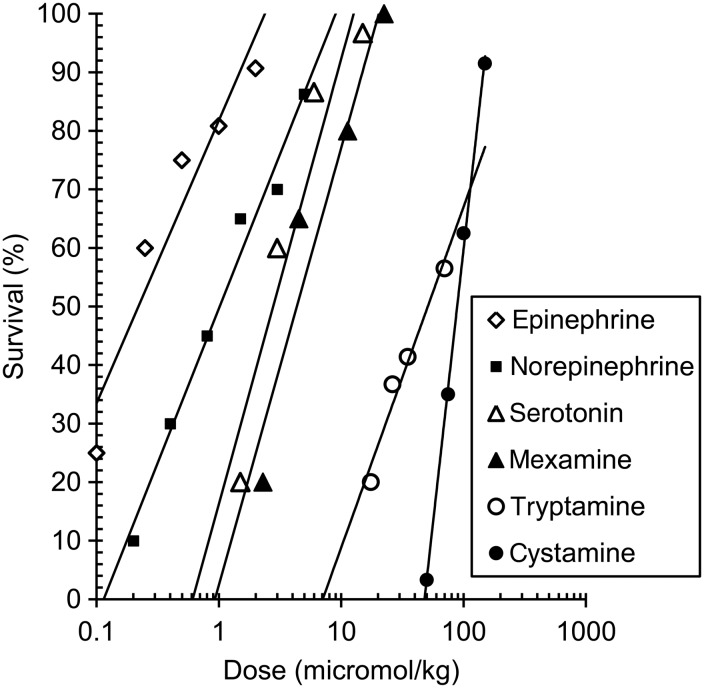
Figure 1 and Table 1 present comparative data for the dose–response of the radioprotectors and the TI of the following biogenic amines: epinephrine, norepinephrine, tryptamine, serotonin and mexamine; and the aminothiol cystamine, following intraperitoneal (IP) administration in mice. In small laboratory animals exposed to 9-Gy γ-radiation at a high dose rate (>1 Gy/min), epinephrine, norepinephrine, serotonin, mexamine and cystamine have been observed to have remarkable radioprotective properties [40, 49].
Fig. 1.
The dose–response radioprotective effect of epinephrine, norepinephrine, serotonin, mexamine, tryptamine and cystamine injected intraperitoneally to mice 5 min before 9 Gy (LD90–100/30) and >1 Gy/min γ-irradiation [41, 49].
Table 1.
The therapeutic index for the radioprotective effect of epinephrine, norepinephrine, serotonin, mexamine, tryptamine and cystamine injected IP to mice 5 min before 9 Gy (LD90–100) and >1 Gy/min γ-irradiation [41, 49]
| Compound | ED50/30 (mg/kg) | LD50/3 (mg/kg) | Therapeutic index |
|---|---|---|---|
| Epinephrine | 0.23 | 6.28 | 27.3 (14.6–52.0) |
| Norepinephrine | 0.90 | 29.2 | 32.4 (17.0–61.9) |
| Serotonin | 3.16 | 435.3 | 137.7 (91.8–206.6) |
| Mexamine | 3.46 | 186.4 | 53.9 (43.5–60.8) |
| Tryptamine | 90.4 | 288.8 | 3.08 (1.98–4.79) |
| Cystamine | 87.5 | 285.1 | 3.26 (2.82–3.7) |
Data shown are therapeutic index with confidence limits for the interval 95%
The window of radioprotection defined by the LD50/ED50 for epinephrine (27.3) and norepinephrine (32.4) in mice was very similar (Table 1). The TI of serotonin was 137.7, more than 2–3-fold higher than that of mexamine and the sympathomimetics (Table 1). The significantly lower radioprotective effectiveness of tryptamine resulted in its low TI. The tryptamine molecule differs from the serotonin molecule by the lack of a hydroxyl group in the fifth position of the indole ring, which predetermines a high binding affinity for the serotonin receptor. Therefore, there is a marked decrease in selectivity of the pharmacological and radioprotective action of tryptamine. As seen in Fig. 1, an ED50/30 of tryptamine is an 80-fold higher that of serotonin.
The TI of aminothiols, such as cystamine, was 10-fold lower than that of the biogenic amines (Table 1); this was also noted in early research [50–52]. The largest TI for sulphur-containing radioprotectors is 9–12 (for phosphorothioates) [13, 53–55], 10-fold lower than for serotonin. This distinction is caused by the different mechanism of action in the sulphur-containing radioprotectors compared with the biogenic amines. Biogenic amines exert their effect via specific cell receptors that initiate an amplification cascade and produce a vasoconstrictive reaction, inducing acute hypoxia in radiosensitive tissues and thus increasing body radioresistance. The dose–response effect of biogenic amines can be described by the Clark–Ariëns relation. Sulphur-containing radioprotectors produce their effect via immediate participation in the primary radiochemical and biochemical processes that develop in the cell during irradiation.
As is known, the damage to body tissues by radiation is induced by the development of free radical processes in cells, resulting in DNA radical among other radical molecules. Aminothiols can take part in competitive radical oxidation/reduction reactions via OH scavenging and by ‘chemical repair’ (H donation from SH groups). Radical scavenging by aminothiols is a first-order reaction. The mechanism of their radioprotective action is first of all to prevent interactions between DNA radicals and oxygen (which lead to DNA strand breakage and chromosome aberrations). The induction of increased cellular reducing equivalents [56, 57] and the change in tertiary DNA structure [58] by aminothiols contributes significantly to this process.
Finally, a common feature of the radioprotective action of biogenic amines and aminothiols is the neutralization of the ‘oxygen effect’, although the mechanisms differ for these compounds. Biogenic amines exert their effect through the neurohormonal receptor system, but sulphur-containing radioprotectors act directly on tissues. Biogenic amine molecules have some anti-radical activity, but compared with aminothiols, their contribution to cell redox potential is limited due to the extremely low drug doses used for radioprotection (Fig. 1).
As a result of these different mechanisms, there is a distinction in the dose–response function in terms of DRF: a linear relationship for sulphur-containing radioprotectors [49, 59–61] and a logarithmic relationship for biogenic amines [62]. This translates to the larger protective effect of low doses of biogenic amines, compared with sulphur-containing radioprotectors, increasing the therapeutic window of the former. In Fig. 1, the higher specificity of the radioprotective action of biogenic amines compared with cystamine is seen by the 100-fold lower requirement in concentration. This scheme explaining the radioprotective effect of biogenic amines and aminothiols rather oversimplifies the true situation [13, 63], but as noted above, the character of their dose–response curves confirms the basic mechanisms of their modes of action.
The window of radioprotection for indralin, mexamine and cystamine in small and large animals: a comparative analysis
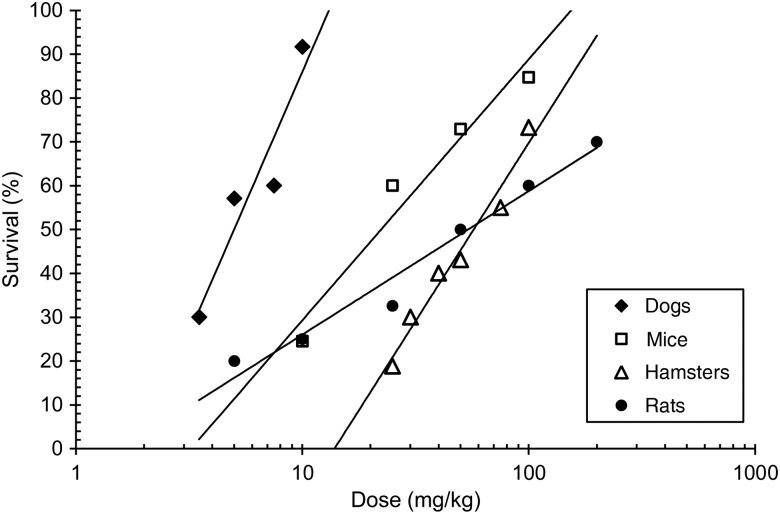
To compare the windows of radioprotection for radioprotectors in small and large animals, the following drugs were chosen: indralin (α1-adrenomimetic), mexamine (serotonin derivative) and cystamine (a sulphur-containing radioprotector). This choice was based on the availability of published data from experiments with large animals (dogs and monkeys). Figure 2 shows the dose–response relationship of indralin in experiments with mice, rats, hamsters and dogs [64]. As seen, optimal radioprotective doses of indralin for dogs are appreciably lower than those for small animals. Table 2 presents the comparative radioprotective efficacies of indralin and mexamine in experiments with dogs. A 5–30 mg/kg dose of indralin protects 90–100% of dogs exposed to lethal doses of γ-radiation. Mexamine at doses that are effective in small animals is ineffective at similar doses in dogs under similar irradiation conditions.
Fig. 2.
The dose–response radioprotective effect of indralin in various species of animals exposed to LD90/30–60 and >1 Gy/min γ-radiation [66].
Table 2.
The radioprotective effect of indralin and mexamine injected IM into dogs 5 min before γ-irradiation (47)
| Groups | Dose (Gy) | Dose rate (Gy/min) | Dose (mg/kg) | n | 60-day Survival (%) | MLS, (days) |
|---|---|---|---|---|---|---|
| Control | 3.8 | 2.3–2.8 | – | 14 | 14.3 | 17.0 |
| Indralin | 3.8 | 2.3–2.8 | 30.0 | 12 | 100.0* | – |
| 10.0 | 15 | 86.7* | 18.0 | |||
| 5.0 | 11 | 90.9* | 20.0 | |||
| Mexamine | 3.8 | 2.3–2.8 | 30.0 | 7 | 14.3 | 17.2 |
| 10.0 | 7 | 0 | 16.4 | |||
| 5.0 | 5 | 20.0 | 17.3 | |||
| Control | 4.0 | 0.1–0.11 | – | 27 | 14.8 | 17.3 |
| Indralin | 4.0 | 0.1–0.11 | 30.0 | 11 | 90.9* | 16.0 |
| Mexamine | 4.0 | 0.1–0.11 | 30.0 | 8 | 12.5 | 17.6 |
Statistically significant (P < 0.05 by two-tail Fisher exact test) difference between indralin and mexamine groups is indicated with an asterisk. MLS = mean of life span of deceased animals, n = number of animals.
This striking difference between indralin and mexamine provides evidence of the fact that the same decrease in blood flow in hematopoietic tissues (owing to the vasoconstrictor effects of mexamine and indralin) when used in experiments with dogs [47] does not exert a highly radioprotective effect, and that indralin has another mechanism of action for its protective properties.
Table 3 displays the windows of radioprotective action for indralin, mexamine and cystamine in various laboratory animals, including dogs. There is a fundamental difference between indralin versus mexamine or cystamine in the TI value for smaller and larger animals. Indralin, an α1-adrenergic agent [32, 44], had a TI typical for epinephrine and norepinephrine (Tables 1 and 3) and this TI remained the same for both smaller and larger animals. For example, the TI for indralin in mice, rats, hamsters, guinea pigs and dogs following an intramuscular (IM) injection of the drug was equal to 23.7, 16.9, 17.8, 25.6 and 31.1, respectively (Table 3). It is very important to have an expanded window of radiation protection for indralin when orally administered in large animals (when sulphur-containing radioprotectors are weak or ineffective) [54, 65]. TI for indralin given per os in dogs corresponds to 23.7 (Table 3). The large window of radioprotection for indralin is maintained for both dogs and non-human monkeys [66]. In contrast, the window of protection for cystamine was decreased in going from mice to rats and dogs, i.e. with increased size of animal. The TI of cystamine following parenteral injection of the drug in mice, rats and dogs was 3.3, 2.1 and 1.2, respectively (Table 3). A similar picture was observed for amifostine, whose window of protection was reduced in terms of TI from 12 to 3 in going from mice to dogs [53–55, 67–69].
Table 3.
The window for the radioprotective effect of indralin, mexamine and cystamine administrated by various routes to small and large animals 5 min before LD90–100/30 and >1 Gy/min γ-irradiation [66]
| Radioprotectors | Animal species | Administration | n | ED50/30 (mg/kg) | Therapeutic index |
|---|---|---|---|---|---|
| Indralin | mice | IP | 480 | 17.4 (13.5–22.4) | 19.3 (14.0–25.1) |
| IM | 240 | 21.9 (16.2–29.4) | 23.7 (15.3–36.5) | ||
| PO | 180 | 14.8 (12.4–17.6) | 59.6 (41.4–85.9) | ||
| rats | IP | 210 | 32.1 (25.8–38.5) | 8.4 (6.3–11.3) | |
| IM | 310 | 61.5 (39.2–96.6) | 16.9 (9.6–29.8) | ||
| PO | 110 | 70.0 | 18.2 | ||
| hamsters | IM | 522 | 50.7 (42.9–59.8) | 17.8 (14.8–24.1) | |
| PO | 90 | 124.4 (95.7–161.8) | 8.9 (5.5–14.5) | ||
| guinea pigs | IM | 35 | 28.8 (17.0–49.0) | 25.6 (13.7–47.4) | |
| dogs | IM | 96 | 6.0 (4.3–8.3) | 31.1 (20.6–47.3) | |
| Mexamine | mice | PO | 78 | 23.2 (20.7–25.9) | 23.7 |
| IP | 320 | 4.1 (3.0–5.5) | 53.9 (43.5–60.8) | ||
| rats | IP | 90 | 5.7 (4.3–7.6) | 23.6 (14.1–37.9) | |
| dogs | IM | 20 | 30.0 < ED50 | No | |
| Cystamine | mice | IP | 400 | 87.5 (77.0–98.0) | 3.3 (2.8–3.7) |
| rats | IP | 100 | 57.7 (45.1–73.9) | 2.1 (1.6–2.8) | |
| dogs | IV | 35 | 60.0 | 1.2 |
Data shown are the means and confidence limits for the means interval 95%. PO = oral administration, IV = intravenous injection.
The above fact may be explained by the more pronounced increase in aminothiol toxicity in going from mice to dogs compared with the change in the radioprotective effect of the doses. It is known that the somatogenic stage of cystamine toxicity is much longer than the duration of its radiation protective effect, and this toxicity is prolonged by radiation exposure [70]. One might expect aggravation of the above situation in dogs as a result of the slower metabolism of aminothiols in irradiated animals. Accumulation of the toxic effects of aminothiols decreases their radioprotective action [71–73]. The increase in the radioprotective effectiveness of cystamine with increasing drug dosage is limited by the maximum tolerated dose [49], which does not exceed the ED50 in dogs [66, 74]. The decrease in the toxicity of aminothiols by vitamins and other agents permits a possible increase in their radioprotective effect [13, 75–82].
Similar trends in reducing the window of protection were observed for mexamine, where TI decreased from 53.9 (43.5–60.8) for mice to 23.6 (14.1–37.9) for rats (Table 3); the radioprotective effect of mexamine did not exceed 50% in experiments with dogs [83]. In contrast to cystamine, a reduction in mexamine toxicity was observed following radiation exposure, a result of receptor desensitization under these conditions [84]. The decrease in the radioprotective effectiveness of mexamine is likely attributed to the weaker hypoxic response caused by this agent in larger animals. This effect is associated with the initial cellular oxygen consumption rate. If there is sufficient time for the cell to adapt to the lack of oxygen supply by reducing oxygen consumption, acute low cellular oxygen tension is averted. The reduced reactivity of dogs to acute hypoxia may be explained by the 2–5-fold lower initial oxygen consumption per unit body weight in large animals as compared with mice and rats. The acute stress reaction to hypoxia caused by inhalation of a hypoxic gas mixture that contains 5–7% oxygen, reflected by an increase in the succinate dehydrogenase activity in lymphocytes, has been shown to be lower in dogs than in rats [64, 85]. In contrast to the effects seen in small animals, the acute hypoxic hypoxia noted above protects less than 50% of dogs [64, 86].
Therefore, the decrease in blood flow in the dog's spleen and bone marrow (by up to 25% and 50% respectively) induced by mexamine [47] is not adequate for radioprotection. The low radioprotective effectiveness of acute hypoxia and mexamine has previously been shown in experiments in dogs and monkeys [81, 87–91].
A hypothesis for the difference in the protective effectiveness between indralin and mexamine or cystamine in large animals
The question arises about whether the radioprotective properties of indralin in experiments in dogs and monkeys [64, 92] are high compared with those of mexamine, with the same reduction of blood flow in hematopoietic tissues [47]. The mechanism for the high level of effectiveness of indralin remains elusive. We propose a hypothesis based on the scientific concept that metabolic activation in hypoxic tissues is initiated and sustained by the sympathetic nervous system, for which an adaptation–trophic role was discovered by Orbeli [93]; and excessive adrenergic stimulation may sharply increase cellular oxygen consumption. This can lead to acute ischemia in tissues in both small and large animals, with a concomitant increase in radioresistance if tissues lack an adequate oxygen supply. The vasoconstrictive effect of sympathomimetic drugs is inevitably associated with increased tissue oxygen consumption, in contrast to serotonin [94–101]. An increase in adrenaline leads to an increase in succinate-dependent ATP synthesis and Ca2+ accumulation in mitochondria, which is due to the known activation of succinate oxidation and oxygen consumption [102–105]. Adrenaline activates oxidative phosphorylation through α1-adrenoceptors [106]. Indralin increases oxygen consumption in bone marrow cells in vitro by up to 50% when tissue oxygen tension is lower than 10 µmol [107]. Myeloid multipotent progenitors and pluripotent stem cells [108] have α1-adrenergic receptors that realize a similar scenario.
The lack of blood flow in radiosensitive tissues caused by the vasoconstrictive effect of the α1-adrenergic agonist indralin, with its simultaneous stimulation of tissue respiration, may lead to more acute tissue hypoxia, which would be sufficient to explain the observed increase in tissue radioresistance.
The increase in cell radioresistance owing to acute low oxygen tension is a result of the considerable increase in cellular oxygen consumption previously discussed [64, 109]. The radioprotective effect of uncouplers of oxidative phosphorylation confirms such a possibility [110–112].
However, there is hitherto no direct proof of our proposed hypothesis. It is necessary to examine the other pharmacological properties of adrenergic agents that could potentially mitigate radiation damage and possibly influence their radioprotective effects.
Catecholaminergic neurotransmitters are known to be able to regulate the migration and repopulation of immature human CD34+ cells [113–116]. Norepinephrine increases DNA synthesis in bone marrow mesenchymal stem cells through α1-adrenergic receptors [117], which plays a significant part in early post-irradiation haematopoietic recovery [118]. This stimulatory effect is very likely accomplished via MAP kinase signaling cascades (MEK > ERK) as intracellular transducers of noradrenergic signals [119, 120]. Besides, acute adrenergic stimulation inhibits the proliferation of haematopoietic progenitor cells via p38/MAPK signalling [121], which could provide an opportunity for an extension of post-irradiation repair time and mitigation of radiation damage to myelopoiesis. Proinflammatory cytokine IL-6 gene expression, induced by α1-adrenergic agents through involving p38 MAPK and NF-κB pathways [122, 123], could potentially contribute to early processes of post-irradiation hematopoietic recovery [124–127]. ROS play a critical role in mediating the response to alpha1-adrenergic stimulation [128].
The importance of these effects of adrenomimetics for their complete protective action may be observed if radioprotectors are applied after radiation exposure. In such a situation, they fail to exert a protective effect as antagonists of the ‘oxygen effect’. Radioprotectors, such as serotonin, adrenaline, cystamine and 2-aminoethylisothiuronium bromide hydrobromide (AET) are known to have a small radioprotective effect if applied within 10 min after irradiation [129–132]. Under conditions of liver shielding in rats, Maisin et al. [133, 134] has detected a protective effect from cysteamine applied after exposure to lethal doses of whole-body radiation in cases where the radioprotector alone was not sufficient. The therapeutic effect of indralin saves up to 55% when it is applied to rats after whole-body irradiation with partial shielding of the upper quadrant of the abdomen [63]. Indralin used after carboplatin injection also reduces its hematoxicity [135, 136].
It is clear that the therapeutic action of indralin and other radioprotectors noted above is essentially lower than its preventive protective effect. Thus, neutralization of the ‘oxygen effect’ by these drugs is a key aspect of their radioprotective action. It is important to note that pharmacological modulation of gene expression by radioprotector action can't of itself achieve significant ‘chemical’ protection. Therefore, the favourable effects of indralin on early post-irradiation hematopoietic recovery do not in themselves constitute a high radioprotective action.
In summary, indralin is likely to provide a high therapeutic index in large animals via the specificity of its adrenergic effect on tissue respiration, promoting the development of acute hypoxia in radiosensitive tissues (aggravated by its vasoconstrictive effect), and also partly via the therapeutic potential of its influence on early post-irradiation hematopoietic recovery.
CONCLUSION
The radioprotective properties of biogenic amines and aminothiols have attracted investigators' attention for more than six decades. This review provides a comparative study of the window of radioprotection for biogenic amines and aminothiols based on personal and literary databases. Comparative analysis of the window of radioprotection for biogenic amines and their derivates and the aminothiol cystamine indicates that catecholamines, serotonin and mexamine have a more than 10-fold greater TI relative to cystamine in experiments with small animals. The TI of tryptamine, which lacks a hydroxyl-group in the fifth position of the indole ring, is deprived of serotonin selectivity and does not differ from that of cystamine. The considerable differences in TI between catecholamines, serotonin and cystamine are caused by the differences in the pharmacology, toxicology of radioprotectors and mechanisms of their radioprotective action. Receptor-mediated radioprotective agents have greater preferences over aminothiols and thus provide an expanded window of protection.
We propose a hypothesis explaining why the window of radioprotection for cystamine and mexamine is reduced, and that for the α1-adrenomimetic indralin is not essentially changed in moving from small to large animals. The reduction in the TI of cystamine in larger animals, such as dogs, may be caused by the greater increase in toxicity of aminothiols in relation to the decrease in their optimal doses for radioprotective effect in going from mice to dogs, which is a consequence of the slower metabolic processes in larger animals. The somatogenic phase of intoxication by cystamine is significantly longer than the duration of its radioprotective effect, and increases with irradiation [70]. The protective action of cystamine is limited by the maximum tolerated dose. Antioxidants lower the toxicity of aminothiols and increase the maximum tolerated dose and thus the corresponding radioprotective effect.
The decrease in the radioprotective effect and TI of mexamine in experiments with dogs may be caused by their lower sensitivity to the acute hypoxia induced by mexamine (because of a decrease in the oxygen tension gradient in tissues under conditions of lower initial oxygen consumption in a large animal as compared with a small animal).
Indralin, owing to its high radiation protective effect as observed in experiments on dogs and monkeys, would not provide that via only a vasoconstrictor action for reasons similar to that noted above for mexamine. The stimulatory effect of indralin on early post-irradiation haematopoietic recovery cannot provide high radioprotective action, but may only promote recovery. Thus, indralin is likely to provide optimal radioprotective effects and a high TI in large animals because of the specificity of its adrenergic effect on tissue respiration, promoting acute hypoxia in radiosensitive tissues when the tissues lack an adequate oxygen supply because of the development of pharmacological vasoconstriction.
ACKNOWLEDGEMENTS
The research cited in this article was from publications through July 2014. The opinions contained in this paper are the views of the authors.
REFERENCES
- 1.Trog D, Bank P, Wendt TG, et al. Daily amifostine given concomitantly to chemoradiation in head and neck cancer. A pilot study. Strahlenther Oncol. 1999;175:444–9. doi: 10.1007/s000660050034. [DOI] [PubMed] [Google Scholar]
- 2.Feng M, Smith DE, Normolle DP, et al. A phase I clinical and pharmacology study using amifostine as a radioprotector in dose-escalated whole liver radiation therapy. Int J Radiat Oncol Biol Phys. 2012;83:1441–7. doi: 10.1016/j.ijrobp.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koukourakis MI, Panteliadou M, Abatzoglou IM, et al. Postmastectomy hypofractionated and accelerated radiation therapy with (and without) subcutaneous amifostine cytoprotection. Int J Radiat Oncol Biol Phys. 2013;85:e7–13. doi: 10.1016/j.ijrobp.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 4.Vasin MV. Comments to the mechanism of protective and pharmacological action of radioprotectors from the family of aminothiols. J Radioprot Res. 2014;2:15–36. [Google Scholar]
- 5.Voĭtkevich ND, Palyga GF. Antiradiation effect of mexamine. Med Radiol (Mosk) 1974;19:74–86. (in Russian) [PubMed] [Google Scholar]
- 6.Lissoni P, Malugani F, Bukovec R, et al. Reduction of cisplatin-induced anemia by the pineal indole 5-methoxytryptamine in metastatic lung cancer patients. Neuro Endocrinol Lett. 2003;24:83–5. [PubMed] [Google Scholar]
- 7.Lissoni P. Biochemotherapy with immunomodulating pineal hormones other than melatonin: 5-methoxytryptamine as a new oncostatic pineal agent. Pathol Biol (Paris) 2007;55:198–200. doi: 10.1016/j.patbio.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Lissoni P, Messina G, Rovelli F. Cancer as the main aging factor for humans: the fundamental role of 5-methoxy-tryptamine in reversal of cancer-induced aging processes in metabolic and immune reactions by non-melatonin pineal hormones. Curr Aging Sci. 2012;5:231–5. doi: 10.2174/1874609811205030010. [DOI] [PubMed] [Google Scholar]
- 9.Ilyin LA, Ushakov IB, Vasin MV. Radioprotective drugs in the system of radiation protection of exposed radiation workers and population in the case of radiation accidents. Med Radiol Radiat Safety. 2012;57:26–31. (in Russian) [Google Scholar]
- 10.Ilyin LA, Ushakov IB, Vasin MV. Radioprotective drugs in the system of radiation protection of exposed radiation workers and population in the case of nuclear accidents, The IRPA13 Abstract Book, Posters Session, P09.06.pdf., 2013. www/irpa.net/members/IRPA13-abstract-USB-FINAL.pdf. (22 February 2013, date last accessed).
- 11.Bacq ZM, Herve A, Lecomte J, et al. Protection contre le rayonnement X par la beta-mercaptoethylamine. Arch Int Physiol. 1951;59:442–7. doi: 10.3109/13813455109150836. [DOI] [PubMed] [Google Scholar]
- 12.Tiunov LA, Vasil'yev GA, Val'dshteyn EA. Antiradiation Agents. Moscow: Nauka; 1964. http://handle.dtic.mil/100.2/AD683060. (28 July 2008, date last accessed) [Google Scholar]
- 13.Weiss JF, Landauer MR. History and development of radiation-protective agents. Int J Radiat Biol. 2009;85:539–73. doi: 10.1080/09553000902985144. [DOI] [PubMed] [Google Scholar]
- 14.Gray JL, Tew JT, Jensen H. Protective effect of serotonin and of paraaminopropiophenone against lethal doses of X-irradiation. Proc Soc Exp Biol Med. 1952;80:604–7. doi: 10.3181/00379727-80-19706. [DOI] [PubMed] [Google Scholar]
- 15.van den Brenk H, Eliott K. Radioprotective action of 5-hydroxytryptamine. Nature. 1958;182:1506–7. doi: 10.1038/1821506b0. [DOI] [PubMed] [Google Scholar]
- 16.Langendorff H, Melching HJ, Ladner H. 5-Hydroxytryptamine as a radiation protective substance in animals. Int J Radiat Biol. 1959;1:24–31. [Google Scholar]
- 17.Krasnykh IG, Zherebchenko PG, Murashova VS, et al. Radioprotective action of 5-methoxytryptamine and other alkoxytryptamines. Radiobiologiia. 1962;2:156–60. (in Russian) [PubMed] [Google Scholar]
- 18.Bacq ZM, Herve A. The protective effect of amines against x-irradiation. J Physiol. 1952;118:24P–25P. [PubMed] [Google Scholar]
- 19.Gray JL, Moulden EJ, Tew JT, et al. Protective effect of pitressin and of epinephrine against total body X-irradiation. Proc Soc Exp Biol Med. 1952;79:384–7. doi: 10.3181/00379727-79-19388. [DOI] [PubMed] [Google Scholar]
- 20.Bacq ZM. The amines and particularly cysteamine as protectors against roentgen rays. Acta Radiol. 1954;41:47–55. doi: 10.3109/00016925409175832. [DOI] [PubMed] [Google Scholar]
- 21.Semenov LF, Prokudina EA. Combination of adrenalin and acetylcholine in prevention of radiation sickness. Med Radiol. 1957;2:35–40. (in Russian) [PubMed] [Google Scholar]
- 22.Konstantinova MM, Graevskii EY. Tissue hypoxia as the mechanism of radioprotective action of epinephrine, heroin and morphin. Dokl Akad Nauk. 1960;133:1427–30. (in Russian) [Google Scholar]
- 23.van der Meer C, van Bekkum DW. The mechanism of radiation protection by hystamine and other biological amines. Int J Radiat Biol. 1959;1:5–12. [Google Scholar]
- 24.Ovakimov VG, Airapetian GM, Ivanov VN, et al. Protective effect of biogenic amines in intestinal radiation syndrome. Radiobiologiia. 1970;10:561–5. (in Russian) [PubMed] [Google Scholar]
- 25.Kulinskii VI, Klimova AD, Iashunskil VG, et al. Mechanism of the radioprotective effect of catecholamine receptor agonists. Inclusion in the radioprotective effect of both subtypes of alpha-adrenoreceptors. Radiobiologiia. 1986;26:11–6. (in Russian) [PubMed] [Google Scholar]
- 26.Smith AD, Ashwood-Smith MJ, Lowman D. Radioprotective action of methoxamine. Nature. 1959;184(Suppl. 22):1729–30. doi: 10.1038/1841729a0. [DOI] [PubMed] [Google Scholar]
- 27.Nakatsuka H, Shakudo Y, Fujino M. Modification of the acute lethality in mice following whole body x-irradiation by several vasoconstrictor agents. Nihon Igaku Hoshasen Gakkai Zasshi. 1966;26:437–45. [PubMed] [Google Scholar]
- 28.Kulinskii VI. Role of catecholamine depots and alpha-adrenoreceptors in the radiation-protective effect of hydroxyphenylethanolamines. Radiobiologiia. 1970;10:887–91. (in Russian) [PubMed] [Google Scholar]
- 29.Mourret A, Agnius-Delord C, Rinaldi R. Etude de l'efficacité de trois hétérocycles azotés radioprotecteurs sur des souris C3H irradiées au cobalt 60. C R Acad Sci Hebd Seances Acad Sci D. 1972;275:1575–8. [PubMed] [Google Scholar]
- 30.Mourret A, Agnius-Delord C, Martinet C, et al. Etude de l'influence de divers hétérocycles azotés sur la survie de souris soumises á une dose létale de rayons gamma. C R Acad Sci Hebd Seances Acad Sci D. 1974;279:1963–6. [PubMed] [Google Scholar]
- 31.Caravel JP, Luu Duc C. Sur les doses de radioprotecteurs utilisés dans les essais de radioprotection: a propos de l'imidazole et de la naphtazoline. Farmaco Prat. 1981;36:49–57. [PubMed] [Google Scholar]
- 32.Vasin MV, Chernov GA, Koroleva LV, et al. Mechanism of the radiation-protective effect of indralin. Radiats Biol Radioecol. 1996;36:36–46. (in Russian) [PubMed] [Google Scholar]
- 33.Prouillac C, Célariès B, Vicendo P, et al. Evaluation, in vitro, of the radioprotection of DNA from gamma-rays by naphazoline. C R Biol. 2006;329:196–9. doi: 10.1016/j.crvi.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 34.van der Meer C, van Bekkum D. A study on the mechanism of radiation protection by 5-hydroxytryptamine and tryptamine. Int J Radiat Biol. 1961;4:105–10. doi: 10.1080/09553006114550991. [DOI] [PubMed] [Google Scholar]
- 35.van den Brenk H, Moore R. Effect of high oxygen pressure on the protective action of cystamine and 5-hydroxytryptamine in irradiated rats. Nature. 1959;183:1530–1. doi: 10.1038/1831530a0. [DOI] [PubMed] [Google Scholar]
- 36.Zherebchenko PG, Suvorov NN. On the relation between the radioprotective and vasoconstrictive action of indolylalkylamines. Radiobiologiia. 1963;3:595–602. (in Russian) [PubMed] [Google Scholar]
- 37.Hasegawa AT, Landahl HD. Studies on spleen oxygen tension and radioprotection in mice with hypoxia, serotonin and p-aminopropiophenone. Radiat Res. 1967;31:389–99. [PubMed] [Google Scholar]
- 38.Iarmonenko SP, Rampan IuI, Karochkin BB, et al. Oxygen tension kinetic in critical organ under mexamine influence in comparison with its radiation protective effect. Radiobiologiia. 1970;10:700–5. (in Russian) [PubMed] [Google Scholar]
- 39.Prewitt RL, Musacchia XJ. Mechanisms of radio-protection by catecholamines in the hamster (Mesocricetus auratus) Int J Radiat Biol. 1975;27:181–91. doi: 10.1080/09553007514550181. [DOI] [PubMed] [Google Scholar]
- 40.Vasin MV, Antipov VV, Suvorov NN, et al. Characteristics of the role of the hydroxyl group in serotonin in the pharmacological and antiradiation effect of serotonin. Radiobiologiia. 1984;24:411–4. (in Russian) [PubMed] [Google Scholar]
- 41.Vasin MV, Suvorov NN, Abramov MM, et al. Changes in the therapeutic spectrum with respect to the pharmacological and radioprotective activity after O-alkylation of serotonin and 5(2-hydroxyethoxytryptamine) Radiobiologiia. 1987;27:700–3. (in Russian) [PubMed] [Google Scholar]
- 42.Kulinskii VI, Zolochevskaia LI. The absence of correlation between the effects of sympathomimetics on blood flow in internal organs, oxygen tension, and the viability of irradiated animals. Radiobiologiia. 1973;13:373–6. (in Russian) [PubMed] [Google Scholar]
- 43.van den Brenk H, Haas M. Studies on the mechanisms of chemical radiation protection in vivo. 1. 5-hydroxytryptamine in relation to effect of antimetabolites, antagonists and releasing agents. Int J Radiat Biol. 1961;3:73–94. doi: 10.1080/09553006114550081. [DOI] [PubMed] [Google Scholar]
- 44.Vasin MV, Ushakov IB, Semenova LA, et al. Pharmacologic analysis of the radiation-protecting effect of indraline. Radiats Biol Radioecol. 2001;41:307–9. (in Russian) [PubMed] [Google Scholar]
- 45.van den Brenk H, Jamieson D. Studies of mechanisms of chemical radiation protection in vivo. II. Effect of pressure oxygen on radioprotection in vivo and its relation to “Oxygen poisoning”. Int J Radiat Biol. 1962;4:379–402. doi: 10.1080/09553006214550191. [DOI] [PubMed] [Google Scholar]
- 46.Vasin MV, L'vova TS, Antipov VV, et al. Radiosensitivity of animals irradiated in an altered gaseous environment. 1. The effect of breathing normobaric pure oxygen during irradiation on body radioresistance and the antiradiation effectiveness of radioprotectors. Radiobiologiia. 1979;19:712–5. (in Russian) [PubMed] [Google Scholar]
- 47.Vasin MV, Antipov VV, Chernov GA, et al. The role of the vasoconstrictor effect in realizing the radioprotective properties of indralin in experiments on dogs. Radiats Biol Radioecol. 1997;37:46–55. (in Russian) [PubMed] [Google Scholar]
- 48.Finney DJ. Statistical Methods in Biological Assay. New York: Hafner Publishing Co; 1964. [Google Scholar]
- 49.Vasin MV, Saksonov PP, Shashkov VS, et al. Relation between the anti-radiation activity of aminothiols (cystamine), the dose of the preparation and the duration of its use under various conditions of gamma-irradiation. Radiobiologiia. 1970;10:380–5. (in Russian) [PubMed] [Google Scholar]
- 50.Strelkov RB, Semenov LF. On the relationship between the antiradiation effect and the doses of the radioprotectors administered. Radiobiologiia. 1967;7:562–4. (in Russian) [PubMed] [Google Scholar]
- 51.Kulinskii VI, Kovtun VIu, Klimova AD, et al. Significance of ED50 and therapeutic indexes of various radiation-protective agent groups. Radiobiologiia. 1992;32:896–900. (in Russian) [PubMed] [Google Scholar]
- 52.Kulinskii VI, Kovtun VIu. Choice of quantitative characteristics of a radiation modifier affinity and the range of its pharmacological action (minireview) Radiobiologiia. 1992;32:901–3. (in Russian) [PubMed] [Google Scholar]
- 53.Terekhov AV, Besedina LN, Zherebchenko PG, et al. Toxicity and antiradiation activity of aminopropyl aminoethyl thiophosphate. Radiobiologiia. 1976;16:249–52. (in Russian) [PubMed] [Google Scholar]
- 54.Davidson DE, Grenan MM, Sweeney TR. Biological characteristics of some improved radioprotectors. In: Brady LW, editor. Radiation Sensitizers. Their Use in the Clinical Management of Cancer. New York: Masson Publishing; 1980. pp. 309–20. [Google Scholar]
- 55.Vladimirov VG, Strel'nikov IuE, Smirnova SM, et al. The radioprotective efficacy and pharmacological properties of S,beta-amidinoethylthiophosphate. Radiobiologiia. 1993;33:116–21. (in Russian) [PubMed] [Google Scholar]
- 56.Wardman P, Dennis MF, Stratford MR, et al. Extracellular: intracellular and subcellular concentration gradients of thiols. Int J Radiat Oncol Biol Phys. 1992;22:751–4. doi: 10.1016/0360-3016(92)90517-l. [DOI] [PubMed] [Google Scholar]
- 57.Newton GL, Aguilera JA, Ward JF, et al. Binding of radioprotective thiols and disulfides in Chinese hamster V 79 cell nuclei. Radiat Res. 1996;146:298–305. [PubMed] [Google Scholar]
- 58.Savoye C, Swenberg C, Hugot S, et al. Thiol WR-1065 and disulphide WR-33278, two metabolites of the drug Ethyol (WR-2721), protect DNA against fast neutron-induced strand breakage. Int J Radiat Biol. 1997;71:193–202. doi: 10.1080/095530097144319. [DOI] [PubMed] [Google Scholar]
- 59.Doherty DG. Chemical protection to mammals against ionizing radiation. In: Hollaender A, editor. Radiation Protection and Recovery. New York: Pergamon Press; 1960. pp. 45–86. [Google Scholar]
- 60.Koch R. Untersuchungen über einen biologischen Strahlenschutz. 80 Mitteilung: Über quantitative Beziehungen des chemischen Strahlenschutzes zu seinen Wirkungsmechanismus. Strahlentherapie. 1967;134:102–6. [PubMed] [Google Scholar]
- 61.Hasegawa AT, Landahl HD. Dose-reduction factor for radiation lethality in mice as function of dose of mercaptoethylamine. Radiat Res. 1970;44:738–47. [PubMed] [Google Scholar]
- 62.Kazymbetov P. Radioprotective action of mexamine in fractionated irradiation. Med Radiol. 1988;33:57–60. (in Russian) [PubMed] [Google Scholar]
- 63.Vasin MV, Ushakov IB, Kovtun VIu, et al. Radioprotective properties of a radioprotector of emergency action indralin at its administration after irradiation in conditions of local shielding of a rat abdomen. Radiats Biol Radioecol. 2008;48:199–202. (in Russian) [PubMed] [Google Scholar]
- 64.Vasin MV, Ushakov IB, Koroleva LV, et al. The role of cell hypoxia in the effect of radiation protectors. Radiats Biol Radioecol. 1999;39:238–48. (in Russian) [PubMed] [Google Scholar]
- 65.Znamenskii VV, Zherebchenko PG, Terekhov AV, et al. Radioprotective effectiveness of intragastric administration of cystaphos to monkeys. Radiobiologiia. 1975;15:79–82. (in Russian) [PubMed] [Google Scholar]
- 66.Vasin MV, Chernov GA, Antipov VV. Width of radiation protective effects of indralin in comparative studies using different animal species. Radiats Biol Radioecol. 1997;37:896–904. (in Russian) [PubMed] [Google Scholar]
- 67.Wagner M, Sedlmeier H, Metzger E, et al. Untersuchungen zu toxizität und strahlenschutz effect der chemischen strahlenschutzsubstanz WR-2721 bei beagle-hunden. Teil II: Strahlenschutzeffekt des WR-2721. Strahlentherapie. 1980;156:655–62. [PubMed] [Google Scholar]
- 68.Wagner M, Sedlmeier H, Wustrow T, et al. [Investigations with beagles about toxicity and radioprotective effect of the clinical radioprotection substance WR 2721. Part I. Toxicity of WR 2721 (author's transl)] Strahlentherapie. 1980;156:486–91. [PubMed] [Google Scholar]
- 69.Palmer TE, Glaza SM, Dickie BC, et al. Toxicity studies on the radioprotective agent WR-2721 in CDF1 mice and beagle dogs. Toxocol Path. 1985;13:58–65. doi: 10.1177/019262338501300108. [DOI] [PubMed] [Google Scholar]
- 70.Vasin MV, Davydov BI, Antipov VV. Comparative elimination of radiation-protective and toxic effects of cystamine. Radiobiologiia. 1971;11:517–21. (in Russian) [PubMed] [Google Scholar]
- 71.Wang RI, Hasegawa AT. Value of chemical mixture in multiple supralethal X-irradiation of mice. Radiat Res. 1968;36:254–60. [PubMed] [Google Scholar]
- 72.Vasin MV, Chernov IuN, Semenova LA. Antiradiation properties of radioprotectors, immunomodulators and agents affecting tissue metabolism in fractionated irradiation. Radiobiologiia. 1991;31:271–5. (in Russian) [PubMed] [Google Scholar]
- 73.Vasin MV, Ushakov IB, Kovtun VIu, et al. Effect of melatonin, ascorbic acid, and succinic acid on the cumulative toxic effect of repeated treatment with gammafos (amifostine) Bull Exp Biol Med. 2004;137:450–2. doi: 10.1023/b:bebm.0000038150.18659.3b. [DOI] [PubMed] [Google Scholar]
- 74.Mozzhukhin AS, Makhalova OK, Sokolova EN. The influence of cystamine to survival of dogs suffering from pancytopenia syndrome of acute radiation illness. Radiobiologiia. 1965;5:621–3. (in Russian) [Google Scholar]
- 75.Belaia VE, Vasil'ev PV, Saksonov PP. Data on comparative pharmacological characteristics of various salts of mercamine. Farmakol Toksikol. 1960;23:450–5. (in Russian) [PubMed] [Google Scholar]
- 76.Vasin MV, Saksonov PP, Shashkov VS. Combined use of radioprotective compounds (review of literature) Farmakol Toksikol. 1970;33:501–7. (in Russian) [PubMed] [Google Scholar]
- 77.Weiss JF, Hoover RL, Kumar KS. Selenium pretreatment enhances the radioprotective effect and reduces the lethal toxicity of WR-2721. Free Radic Res Commun. 1987;3:33–8. doi: 10.3109/10715768709069767. [DOI] [PubMed] [Google Scholar]
- 78.Weiss JF, Kumar KS, Walden TL, et al. Advances in radioprotection through the use of combined agent regimens. Int J Radiat Biol. 1990;57:709–22. doi: 10.1080/09553009014550881. [DOI] [PubMed] [Google Scholar]
- 79.Srinivasan V, Weiss JF. Radioprotection by vitamin E: injectable vitamin E administered alone or with WR-3689 enhances survival of irradiated mice. Int J Radiat Oncol Biol Phys. 1992;23:841–5. doi: 10.1016/0360-3016(92)90657-4. [DOI] [PubMed] [Google Scholar]
- 80.Ganasoundari A, Devi PU, Rao BS. Enhancement of bone marrow radioprotection and reduction of WR-2721 toxicity by Ocimum sanctum. Mutat Res. 1998;397:303–12. doi: 10.1016/s0027-5107(97)00230-3. [DOI] [PubMed] [Google Scholar]
- 81.Grachev SA, Sverdlov AG, Nikanorova NG, et al. Increased efficacy of radiation protection against fission neutrons using unithiol. Radiats Biol Radioecol. 1999;39:258–60. (in Russian) [PubMed] [Google Scholar]
- 82.Simone CB, II, Simone NL, Simone V, et al. Antioxidants and other nutrients do not interfere with chemotherapy or radiation therapy and can increase kill and increase survival, Part 2. Altern Ther Health Med. 2007;13:40–7. [PubMed] [Google Scholar]
- 83.Chernov GA, Trushina MN, Suvorov NN. The radioprotective effectiveness of oral administration of mexamine in dogs. Radiobiologiia. 1973;13:464–6. (in Russian) [PubMed] [Google Scholar]
- 84.Vasin MV. Effect of preliminary application of mexamine on its toxicity during repeated administration to irradiated and intact animals. Radiobiologiia. 1973;13:129–31. (in Russian) [PubMed] [Google Scholar]
- 85.Antipov VV, Vasin MV, Gaidamakin AN. Species-specific reactions of succinate dehydrogenase of lymphocytes in animals to acute hypoxic hypoxia and its relation to the radiation resistance of the body. Kosm Biol Aviakosm Med. 1989;23:63–6. (in Russian) [PubMed] [Google Scholar]
- 86.Vasin MV. Comparative characteristic of modification of radiosensitivity of mice and rats by hypoxic hypoxia. Radiobiologiia. 1986;26:563–5. (in Russian) [PubMed] [Google Scholar]
- 87.Krasnykh IG, Zherebchenko PG, Semenov LF, et al. Prevention of radiation injury in monkeys with the aid of 5-methoxytryptamine. Radiobiologiia. 1963;3:259–61. (in Russian) [PubMed] [Google Scholar]
- 88.Stork EJ, Gass AE, Melville GS. Indolylalkylamines as radioprotectors. Tech Rep SAM-TR. 1969. SAM-TR-68-144 Aug:1–6. [PubMed]
- 89.Trushina MN, Znamenskii VV, Chernov GA, et al. Radioprotective effect of mexamine on oral administration to monkeys. Radiobiologiia. 1973;13:719–22. (in Russian) [PubMed] [Google Scholar]
- 90.Semenov LF, Lapin BA, Strelkov RB, et al. [Comparative study of radiation-protective effectiveness of mexamine and gas hypoxic mixture in experiments on rhesus monkeys] Vestn Akad Med Nauk SSSR. 1978. pp. 83–8. (in Russian) [PubMed]
- 91.Strelkov RB, Chizhov AIa, Kucherenko NG, et al. Radioprotective effectiveness of gas hypoxic mixture GHM-10 in experiments on dogs. Radiobiologiia. 1984;24:264–6. (in Russian) [PubMed] [Google Scholar]
- 92.Vasin MV, Semenov LF, Suvorov NN, et al. Protective effect and the therapeutic index of indralin in juvenile rhesus monkeys. J Radiat Res, 10.1093/jrr/rru046. [DOI] [PMC free article] [PubMed]
- 93.Orbeli LA. Adaptation-trophic role of the sympathetic nervous system and of the cerebellum. Fiziol Zh SSSR im I M Sechenova. 1949;35:594–5. (in Russian) [PubMed] [Google Scholar]
- 94.Grubb B, Folk GE., Jr The role of adrenoceptors in norepinephrine-stimulated VO2 in muscle. Eur J Pharmacol. 1977;43:217–23. doi: 10.1016/0014-2999(77)90020-6. [DOI] [PubMed] [Google Scholar]
- 95.Quinlan PT, Halestrap AP. The mechanism of the hormonal activation of respiration in isolated hepatocytes and its importance in the regulation of gluconeogenesis. Biochem J. 1986;236:789–800. doi: 10.1042/bj2360789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shiota M, Masumi S. Effect of norepinephrine on consumption of oxygen in perfused skeletal muscle from cold-exposed rats. Am J Physiol. 1988;254:E482–9. doi: 10.1152/ajpendo.1988.254.4.E482. [DOI] [PubMed] [Google Scholar]
- 97.Dora KA, Richards SM, Rottigan S, et al. Serotonin and norepinephrine vasoconstriction in rat hindlimb have different oxygen requirements. Am J Physiol. 1992;262:H698–703. doi: 10.1152/ajpheart.1992.262.3.H698. [DOI] [PubMed] [Google Scholar]
- 98.Clark MG, Colquhoun EQ, Rattigan S, et al. Vascular and endocrine control of muscle metabolism. Am J Physiol. 1995;268:E797–812. doi: 10.1152/ajpendo.1995.268.5.E797. [DOI] [PubMed] [Google Scholar]
- 99.Ye JM, Clark MG, Colquhoun EQ. Constant-pressure perfusion of rat hindlimb shows alpha- and beta-adrenergic stimulation of oxygen consumption. Am J Physiol. 1995;269:E960–8. doi: 10.1152/ajpendo.1995.269.5.E960. [DOI] [PubMed] [Google Scholar]
- 100.Hall JL, Ye JM, Clark MG, et al. Sympathetic stimulation elicits increased or decreased VO2 in the perfused rat hindlimb via alpha 1-adrenoceptors. Am J Physiol. 1997;272:H2146–53. doi: 10.1152/ajpheart.1997.272.5.H2146. [DOI] [PubMed] [Google Scholar]
- 101.Newman JM, Clark MG. Stimulation and inhibition of resting muscle thermogenesis by vasoconstrictors in perfused rat hind limb. Can J Physiol Pharmacol. 1998;76:867–72. doi: 10.1139/cjpp-76-9-867. [DOI] [PubMed] [Google Scholar]
- 102.Jouaville LS, Pinton P, Bastianutto C, et al. Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc Natl Acad Sci U S A. 1999;96:13807–12. doi: 10.1073/pnas.96.24.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Saakyan IR, Saakyan SG, Kondrashova MN. Activation and inhibition of succinate-dependent Ca2+ transport in liver mitochondria during adaptation. Biochemistry (Moscow) 2001;66:795–802. doi: 10.1023/a:1010272914835. [DOI] [PubMed] [Google Scholar]
- 104.Vasin MV, Ushakov IB, Koroleva LV, et al. In vitro response of mitochondrial succinate oxidase system to epinephrine in human blood lymphocytes from health individuals and patients with neurocirculatory dystonia. Bull Exp Biol Med. 2002;134:393–6. doi: 10.1023/a:1021976718941. [DOI] [PubMed] [Google Scholar]
- 105.Szabadkai G, Simoni AM, Rizzuto R. Mitochondrial Ca2+ uptake requires sustained Ca2+ release from the endoplasmic reticulum. J Biol Chem. 2003;278:15153–61. doi: 10.1074/jbc.M300180200. [DOI] [PubMed] [Google Scholar]
- 106.Breton L, Clot JP, Bouriannes J, et al. Adrenaline activates oxidative phosphorylation of rat liver mitochondria through alpha 1-receptors. C R Seances Soc Biol Fil. 1987;181:242–8. (in French) [PubMed] [Google Scholar]
- 107.Vasin MV, Ushakov IB, Korovkina EP, et al. Effect of α1-adrenomimetic indralin on oxygen consumption by bone marrow cells in vitro. Bull Exp Biol Med. 2013;155:360–2. doi: 10.1007/s10517-013-2153-x. [DOI] [PubMed] [Google Scholar]
- 108.Muthu K, Iyer S, He LK, et al. Murine hematopoietic stem cells and progenitors express adrenergic receptors. J Neuroimmunol. 2007;186:27–36. doi: 10.1016/j.jneuroim.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Korystov IuN. Role of changes in oxygen concentration during modification of cell reproductive death in vitro. 2. Modification of radiosensitivity during changes in the rate of oxygen consumption by cells. Radiobiologiia. 1983;23:200–4. (in Russian) [PubMed] [Google Scholar]
- 110.Praslicka M, Hill M, Novak L. Protective action of 2,4-dinitrophenol against x-radiation injury. Radioprotective effect of 2,4-dinitrophenol. Int J Radiat Biol. 1962;4:567–79. doi: 10.1080/09553006214550381. [DOI] [PubMed] [Google Scholar]
- 111.Vacek A, Rotkovska D. On the protective effect of 2,4-dinitrophenol. Int J Radiat Biol. 1964;8:285–9. doi: 10.1080/09553006414550301. [DOI] [PubMed] [Google Scholar]
- 112.Michel S, Laval F. Protease inhibitors suppress the survival increase mediated by uncouplers in X-irradiated mammalian cells. Biochimie. 1982;64:753–5. doi: 10.1016/s0300-9084(82)80124-7. [DOI] [PubMed] [Google Scholar]
- 113.Katayama Y, Battista M, Kao WM, et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–21. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 114.Spiegel A, Shivtiel S, Kalinkovich A, et al. Catecholaminergic neurotransmitters regulate migration and repopulation of immature human CD34+ cells through Wnt signaling. Nat Immunol. 2007;8:1123–31. doi: 10.1038/ni1509. [DOI] [PubMed] [Google Scholar]
- 115.Kalinkovich A, Spiegel A, Shivtiel S, et al. Blood-forming stem cells are nervous: direct and indirect regulation of immature human CD34+ cells by the nervous system. Brain Behav Immun. 2009;23:1059–65. doi: 10.1016/j.bbi.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 116.Dar A, Schajnovitz A, Lapid K, et al. Rapid mobilization of hematopoietic progenitors by AMD3100 and catecholamines is mediated by CXCR4-dependent SDF-1 release from bone marrow stromal cells. Leukemia. 2011;25:1286–96. doi: 10.1038/leu.2011.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Han J, Zou Z, Zhu C, et al. DNA synthesis of rat bone marrow mesenchymal stem cells through alpha1-adrenergic receptors. Arch Biochem Biophys. 2009;490:96–102. doi: 10.1016/j.abb.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 118.Lange C, Brunswig-Spickenheier B, Cappallo-Obermann H, et al. Radiation rescue: mesenchymal stromal cells protect from lethal irradiation. PLoS One. 2011;6:e14486. doi: 10.1371/journal.pone.0014486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Waldrop BA, Mastalerz D, Piascik MT, et al. α1B- and α1D-adrenergic receptors exhibit different requirements for agonist and mitogen-activated protein kinase activation to regulate growth responses in rat 1 fibroblasts. J Pharmacol Exp Ther. 2002;300:83–90. doi: 10.1124/jpet.300.1.83. [DOI] [PubMed] [Google Scholar]
- 120.Nishiura T, Abe K. Alpha1-adrenergic receptor stimulation induces the expression of receptor activator of nuclear factor kappaB ligand gene via protein kinase C and extracellular signal-regulated kinase pathways in MC3T3-E1 osteoblast-like cells. Arch Oral Biol. 2007;52:778–85. doi: 10.1016/j.archoralbio.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 121.Schraml E, Fuchs R, Kotzbeck P, et al. Acute adrenergic stress inhibits proliferation of murine hematopoietic progenitor cells via p38/MAPK signaling. Stem Cells Dev. 2009;18:215–27. doi: 10.1089/scd.2008.0072. [DOI] [PubMed] [Google Scholar]
- 122.Sancho-Bru P, Bataller R, Colmenero J, et al. Norepinephrine induces calcium spikes and proinflammatory actions in human hepatic stellate cells. Am J Physiol. 2006;291:G877–84. doi: 10.1152/ajpgi.00537.2005. [DOI] [PubMed] [Google Scholar]
- 123.Perez DM, Papay RS, Shi T. α1-adrenergic receptor stimulates interleukin-6 expression and secretion through both mRNA stability and transcriptional regulation: involvement of p38 mitogen-activated protein kinase and nuclear factor-κB. Mol Pharmacol. 2009;76:144–52. doi: 10.1124/mol.108.054320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Michalevicz R, Lifshitz D, Revel M. Interferon beta 2/interleukin-6 and interleukin-3 synergize in stimulating proliferation of human early hematopoietic progenitor cells. Scanning Microsc. 1989;3:1143–9. [PubMed] [Google Scholar]
- 125.Kishimoto T, Akira S, Narazaki M, et al. Interleukin-6 family of cytokines and gp130. Blood. 1995;86:1243–54. [PubMed] [Google Scholar]
- 126.Sun L, Liu X, Qiu L, et al. Administration of plasmid DNA expressing human interleukin-6 significantly improves thrombocytopoiesis in irradiated mice. Ann Hematol. 2001;80:567–72. doi: 10.1007/s002770100345. [DOI] [PubMed] [Google Scholar]
- 127.Mouthon MA, Vandamme M, van der Meeren A, et al. Inflammatory response to abdominal irradiation stimulates hemopoiesis. Int J Radiat Biol. 2001;77:95–103. doi: 10.1080/0955300010000782. [DOI] [PubMed] [Google Scholar]
- 128.Amin JK, Xiao L, Pimental DR, et al. Reactive oxygen species mediate alpha-adrenergic receptor-stimulated hypertrophy in adult rat ventricular myocytes. J Mol Cell Cardiol. 2001;33:131–9. doi: 10.1006/jmcc.2000.1285. [DOI] [PubMed] [Google Scholar]
- 129.Rixon EH, Baird KM. The therapeutic effect of serotonin on the survival of x-irradiated rats. Radiat Res. 1968;33:395–402. [PubMed] [Google Scholar]
- 130.Shashlov VS, Anashkin OD, Suvorov NN, et al. Effectiveness of serotonin, mexamine, AET and cystamine during multiple administration following gamma-irradiation. Radiobiologiia. 1971;11:621–3. (in Russian) [PubMed] [Google Scholar]
- 131.Smirnova IB, Dontsova GV, Konstantinova MM, et al. Radiomodifying effect of serotonin on the cells of the hematopoietic system. Radiobiologiia. 1984;24:236–40. (in Russian) [PubMed] [Google Scholar]
- 132.Smirnova IB, Dontsova GV, Konstantinova MM, et al. Characteristic of the post-radiation reaction of hematopoietic tissue with adrenaline use. Radiobiologiia. 1984;24:545–8. (in Russian) [PubMed] [Google Scholar]
- 133.Maisin JH, Lambert G, Mandart M, et al. Therapeutic action of glutathione and beta-mercaptoethylamine against a lethal dose of x-rays. Nature. 1953;171:971. doi: 10.1038/171971a0. [DOI] [PubMed] [Google Scholar]
- 134.Maisin J, Mandart M, Lambert G, et al. Curative action of beta-mercapto-ethylamine in the rat irradiated with the liver protected. C R Seances Soc Biol Fil. 1953;147:362–4. (in French) [PubMed] [Google Scholar]
- 135.Vasin MV, Ushakov IB, Kovtun VIu, et al. Effect of radioprotector indralin on carboplatinum hemotoxicity. Bull Exp Biol Med. 2006;141:437–9. doi: 10.1007/s10517-006-0193-1. [DOI] [PubMed] [Google Scholar]
- 136.Vasin MV, Kovtun VIu, Komarova SN, et al. Combined effect of quercetin and indralin (B-190) in alleviating carboplatin hematologic toxicity. Vopr Onkol. 2012;58:77–80. (in Russian) [PubMed] [Google Scholar]