Abstract
The objectives of this study were to evaluate dosimetric quality and acute toxicity of volumetric-modulated arc therapy (VMAT) and daily image guidance in high-risk prostate cancer patients. A total of 100 consecutive high-risk prostate cancer patients treated with definitive VMAT with prophylactic whole-pelvic radiotherapy (WPRT) were enrolled. All patients were treated with a double-arc VMAT plan delivering 52 Gy to the prostate planning target volume (PTV), while simultaneously delivering 46.8 Gy to the pelvic nodal PTV in 26 fractions, followed by a single-arc VMAT plan delivering 26 Gy to the prostate PTV in 13 fractions. Image-guided RT was performed with daily cone-beam computed tomography. Dose–volume parameters for the PTV and the organs at risk (OARs), total number of monitor units (MUs) and treatment time were evaluated. Acute toxicity was assessed using the Common Terminology Criteria for Adverse Events, version 4.0. All dosimetric parameters met the present plan acceptance criteria. Mean MU and treatment time were 471 and 146 s for double-arc VMAT, respectively, and were 520 and 76 s for single-arc VMAT, respectively. No Grade 3 or higher acute toxicity was reported. Acute Grade 2 proctitis, diarrhea, and genitourinary toxicity occurred in 12 patients (12%), 6 patients (6%) and 13 patients (13%), respectively. The present study demonstrated that VMAT for WPRT in prostate cancer results in favorable PTV coverage and OAR sparing with short treatment time and an acceptable rate of acute toxicity. These findings support the use of VMAT for delivering WPRT to high-risk prostate cancer patients.
Keywords: prostate cancer, whole-pelvic radiotherapy, volumetric-modulated arc therapy, treatment planning, image-guided radiotherapy, acute toxicity
INTRODUCTION
Whole-pelvic radiotherapy (WPRT) is theoretically a valid option for treatment of high-risk prostate cancer, given the potential risk of lymph node metastasis [1, 2]. However, its significance remains unclear because its effect on survival rate has not yet been clarified. The Radiation Therapy Oncology Group (RTOG) 9413 trial demonstrated improved progression-free survival (PFS) for high-risk prostate cancer patients treated with WPRT compared with prostate-only RT (PORT) [3]. The updated analysis of RTOG 9413 continued to show an advantage of WPRT with neoadjuvant hormone therapy with regards to prostate-specific antigen (PSA) control and PFS at 10 years [4]. On the other hand, the GETUG-01 trial, from the Groupe D'Etude des Tumeurs UroGenitales, showed no difference in PFS and overall survival between WPRT and PORT [5]. However, the GETUG-01 trial included only 45% of patients with a risk of lymph node (LN) involvement >15%, and their field sizes were smaller than those defined in the RTOG 9413 trial.
Several randomized controlled trials have demonstrated that escalation of radiation dose beyond 70 Gy improves PSA control [6, 7]. In contrast, WPRT results in an increased dose to the organs at risk (OARs) and therefore the prostate dose is generally restricted to ∼70 Gy with conventional RT [3, 5, 8–10]. Given this background, intensity-modulated radiotherapy (IMRT) has been used at some facilities in the context of dose escalation to the prostate with prophylactic WPRT. Planning studies comparing IMRT with conventional radiation techniques for WPRT have shown that IMRT provides superior OAR sparing [11] and target coverage [12]. In addition, early clinical results confirmed the potential of WP-IMRT with acceptable rates of acute toxicity [13–17]. Furthermore, a recently reported study demonstrated that adding an image-guided (IG) technique to WP-IMRT results in lower acute rectal and bladder toxicities [18].
Volumetric-modulated arc therapy (VMAT) is a relatively new rotational radiation therapy technique based on the idea of delivering IMRT with continuous dynamic modulation of the dose rate, field aperture, and gantry speed. Although planning studies on dosimetric comparison in the prostate only or in the prostate with seminal vesicles found that VMAT achieved equal or better target coverage and normal tissue sparing over IMRT [19–22], planning studies that have been focused on larger and more irregularly shaped pelvic target volumes, including the prostate, seminal vesicles, and pelvic lymph nodes, have shown inconsistent dosimetric results [23–26]. The report of Pesce et al. [27] is one of the limited studies reporting the toxicity of VMAT in the treatment of prostate cancer; however, this study was designed to evaluate PO-VMAT, not WP-VMAT. Thus, there remain several areas to be clarified in WP-VMAT.
The current study was undertaken in order to evaluate dosimetric quality and acute toxicity of WP-VMAT and image-guided RT (IGRT) in 100 consecutive patients with high-risk prostate cancer.
MATERIALS AND METHODS
Patients
This analysis is based on the first 100 high-risk prostate cancer patients treated with WP-VMAT with daily image-guidance between July 2011 and December 2013. High-risk was defined as cT3/4 N0 M0 and/or a Gleason score of 8, 9 or 10 and/or a pretreatment PSA concentration of ≥20 ng/ml. Relevant patient characteristics are shown in Table 1. Of the 100 patients, 96 received androgen deprivation therapy (ADT), which typically started 3–6 months before RT and continued for a total duration of ≥24 months. Written informed consent was obtained from all patients, and the institutional ethics committee approved the study.
Table 1.
Patient characteristics (n = 100)
| Characteristic | Value |
|---|---|
| Age (y) | |
| Median | 72 |
| Range | 53–79 |
| Gleason score | |
| 5–6 | 5 |
| 7 | 28 |
| 8–10 | 67 |
| PSA (ng/ml) | |
| Median | 28.4 |
| Range | 4.8–445.0 |
| Clinical stage | |
| T1 | 12 |
| T2 | 30 |
| T3 | 52 |
| T4 | 6 |
| Diabetes (%) | 17 |
| Anticoagulants (%) | 17 |
| Androgen deprivation (%) | 96 |
PSA = prostate-specific antigen.
Simulation, organ contouring, and planning
Computed tomography (CT) was acquired in the supine position, with 1.25-mm thick slices from the upper abdomen to 5 cm below the ischial tuberosities after immobilization with a vacuum-based device (VacLocTM, CIVCO Medical Solutions, Kalona, IA). The patients were instructed to have a comfortably filled bladder and an empty rectum at CT acquisition and for each treatment. The CT data set was transferred to the Eclipse ver. 10.0 treatment planning system (Varian Medical Systems, Palo Alto, CA). The prostate clinical target volume (CTV) was defined as the entire prostate with an area 1.5 cm proximal to the seminal vesicles, and any visible tumor extension. The prostate planning target volume (PTV) was generated by adding an 8-mm margin to the prostate CTV in all dimensions, except posteriorly, where a 5-mm margin was used. Pelvic lymph node volumes were standardized based on the consensus recommendations of the RTOG [28]. The nodal CTV consisted of a 0.7-cm expansion volume on the obturator vessels in addition to the common, external and internal iliac vessels, while excluding adjacent bone, muscle, bowel and bladder. The nodal CTV commenced at the L5 to S1 interspace, with the external iliac nodal volumes stopping at the top of the femoral head and the obturator nodal volumes stopping at the top of pubic symphysis. Differing from the RTOG consensus, the presacral nodes were not included in the nodal CTV. The nodal PTV was defined by a 0.5-cm expansion of the nodal CTV. Contouring of the OAR was defined according to the RTOG pelvic normal tissue contouring guidelines [29]. The OAR were contoured and considered as solid organs. The rectum was segmented from the level of the ischial tuberosities to the rectosigmoid flexure, and the entire bladder was contoured from its apex to the dome. The femoral heads were delineated to the level of the ischial tuberosities. The bowel bag was contoured as the entire volume of peritoneal space to within 1 cm of the cranial margin of the nodal PTV.
Treatment was prescribed such that the prostate PTV received 78 Gy in 39 fractions, while the nodal PTV received 46.8 Gy in 26 fractions, by use of 10-MV photons. In all cases, dose normalization was set to the mean dose to the prostate PTV, while keeping the mean dose to the nodal PTV as close to 46.8 Gy as possible. The VMAT plan was given in two phases. In the first phase, the nodal PTV and the prostate PTV received 46.8 Gy and 52 Gy, respectively, both in 26 fractions using double-arc VMAT with a simultaneous integrated boost (SIB) technique. In the second phase, the prostate PTV received 26 Gy in 13 fractions using single-arc VMAT. In general, double-arc VMAT can achieve higher conformity and homogeneity compared with a single-arc plan. Owing to the complexity of a WP-VMAT plan and the poor results obtained with single-arc, double-arc was used to optimize the WP-VMAT. Plans were defined as acceptable when ≥95% of the PTV received ≥95% of the prescribed dose. The OAR dose volume constraints were: volume receiving a minimal dose of 70 Gy (V70Gy) less than 20% and V50Gy less than 45% for the rectum; and V70Gy less than 25% and V50Gy less than 50% for the bladder. For the femoral heads, the dose objective was minimal dose to 2% (D2%) less than 50 Gy. The bowel bag was limited to D2% less than 50 Gy and V45Gy less than 195 ml. The dose calculation was performed using the anisotropic analytic algorithm (AAA, version 10.0.28) and a voxel size of 0.25 × 0.25 × 0.25 cm3.
Online image-guided radiotherapy
Patients were treated with a Novalis Tx treatment unit (Varian Medical Systems, and BrainLAB AG, Feldkirchen, Germany). Daily image guidance with ExacTrac and cone-beam CT (CBCT) was performed in all patients. After the patients were immobilized in the vacuum-based device, the skin marks on the patient were used for initial setup. Orthogonal kilovoltage radiographs of the patients were then obtained using the ExacTrac and registered to the reference digitally reconstructed radiographs generated from the planning CT. Once the bone registration was satisfactory, CBCT images were also obtained and then used to accomplish the target/soft tissue registration.
Dosimetric analysis
Dose–volume histograms (DVHs) were constructed for the prostate PTV, nodal PTV, rectum, bladder, femoral heads, and bowel bag in each plan. DVHs were averaged among 100 subjects. Parameters chosen for measuring dosimetric quality of treatments were D2% and D95% for the prostate PTV and mean dose and D95% for the nodal PTV. For the rectum and bladder, the analysis included the mean dose, D2%, V70Gy, V50Gy and V30Gy. D2% of each femoral head and both D2% and V45Gy of bowel bag were also scored. The total number of monitor units (MUs) per fraction and the beam-on time were used to evaluate the efficiency of treatment delivery.
Acute toxicity
Gastrointestinal (GI) and genitourinary (GU) toxicities were prospectively scored for all patients, using the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 adverse event scoring system. Patients were monitored weekly during RT and again at 2 weeks and 3 months after the end of RT. Toxicity occurring within 90 days of the end of RT was classified as acute toxicity. The dosimetric data for those patients experiencing Grade 1 or less acute toxicity associated with each normal structure (rectum, bladder, or bowel bag) was compared with the data for patients with Grade 2 toxicity.
Statistical analysis
An unpaired Student's t test was used to compare mean values of each dose metric after confirming that each dataset was normally distributed (using the Kolmogorov–Smirnov test) and the variances of two datasets to compare were equal (using the F-test). Significance was defined at P < 0.05. All reported P-values are two-tailed.
RESULTS
All patients completed RT and follow-up for 3 months after RT. No interruption of RT related to acute toxicity was observed.
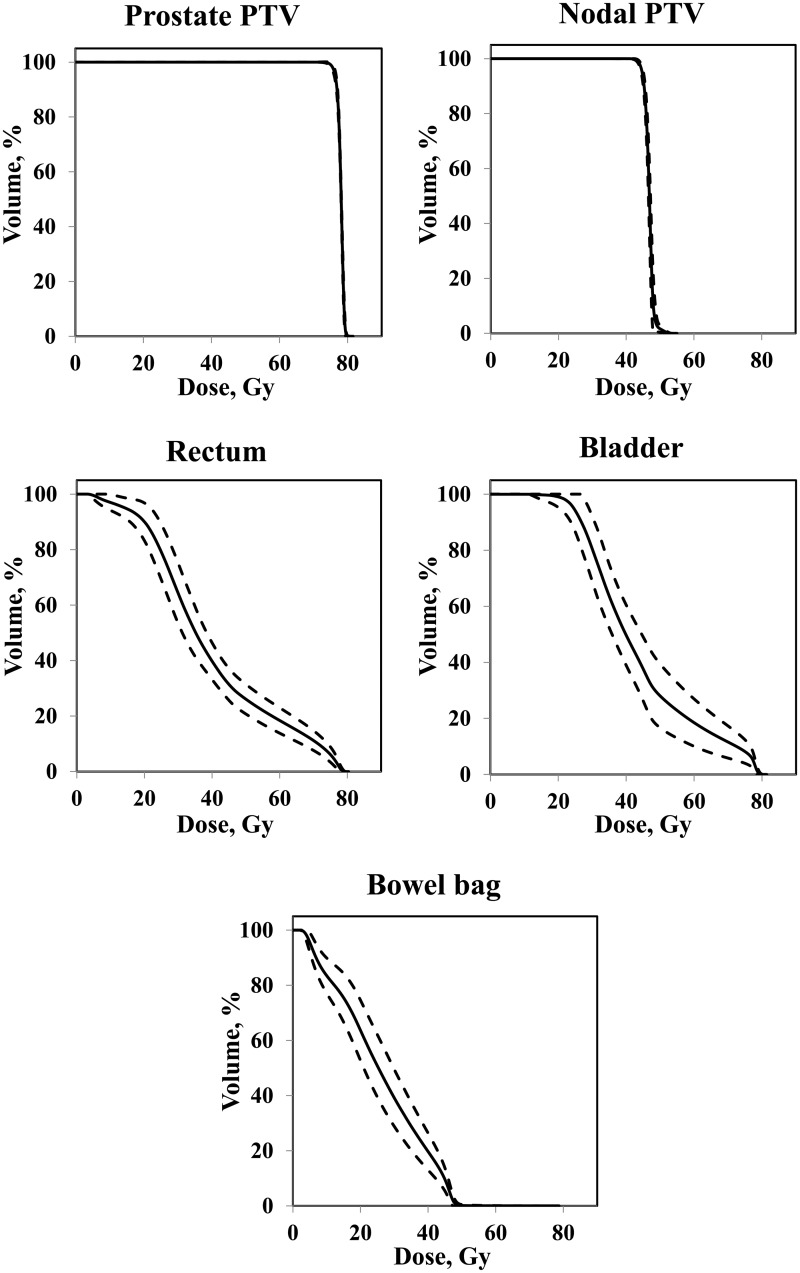
Representative axial and coronal dose distributions for the composite plans are shown in Fig. 1. The mean value of the dosimetric parameters of the PTVs and the OARs are shown in Table 2. All dose–volume constraints were satisfactorily met for all treatment plans. Figure 2 shows the average DVH for the prostate PTV, nodal PTV, rectum, bladder, and bowel bag. Mean MUs and beam-on time were 471 ± 45 and 146 ± 1 s for double-arc VMAT, respectively, and 520 ± 59 and 76 ± 2 s for single-arc VMAT, respectively.
Fig. 1.
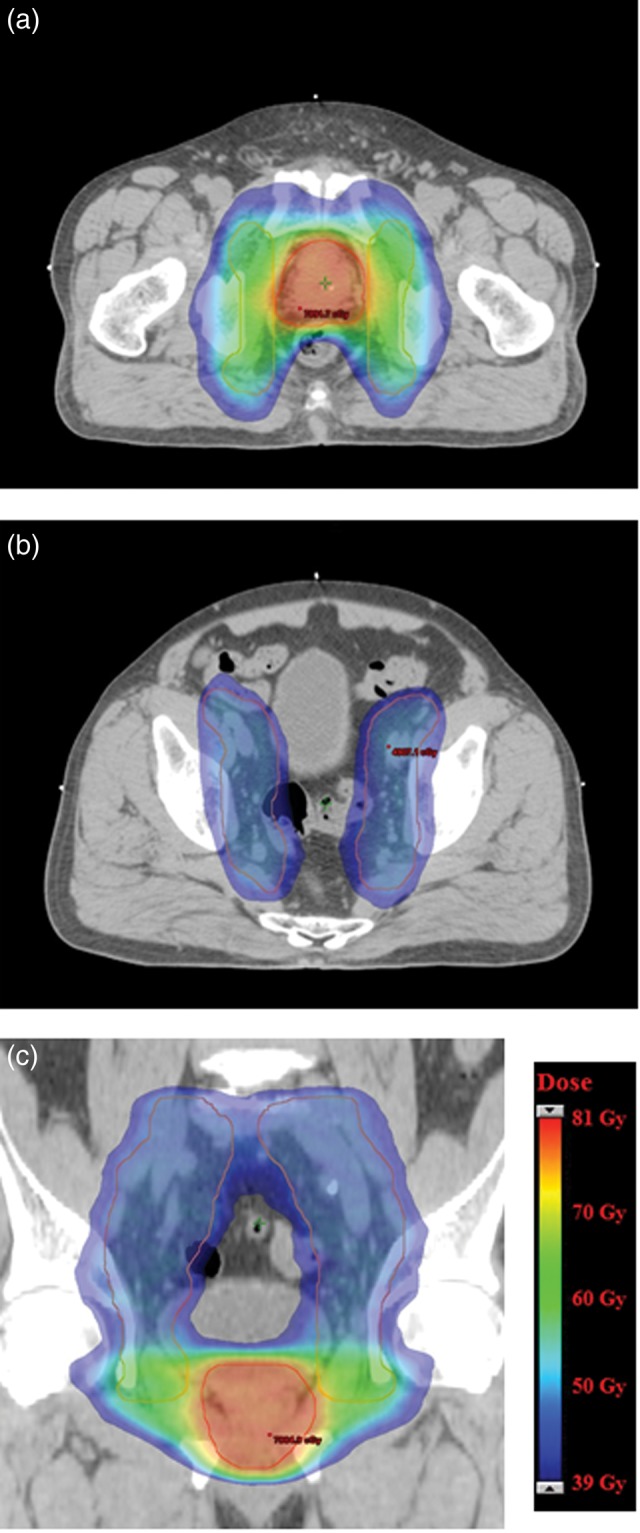
Representative dose distributions for the composite plan. Axial computed tomography (CT) images of the pelvis at the level of the prostate gland (a) and the pelvic lymph nodes (b). Coronal CT showing the prostate gland and the pelvic lymph nodes (c). Prostate planning target volumes (PTVs) are shown in red and nodal PTVs are shown in pink. Dose color wash is from 39 Gy (dark blue) to ∼81 Gy (red).
Table 2.
Dosimetric parameters for PTV and OAR
| Parameter |
Value | ||
|---|---|---|---|
| (mean ± SD) | |||
| Prostate PTV | Volume | (ml) | 92.4 ± 23.0 |
| D2% | (Gy) | 79.3 ± 0.2 | |
| Dmean | (Gy) | 78 | |
| D95% | (Gy) | 76.4 ± 0.3 | |
| Nodal PTV | Volume | (ml) | 774.6 ± 130.6 |
| Dmean | (Gy) | 46.8 ± 0.4 | |
| D95% | (Gy) | 44.8 ± 0.4 | |
| Rectum | Volume | (ml) | 61.1 ± 15.8 |
| Dmean | (Gy) | 39.8 ± 2.6 | |
| D2% | (Gy) | 77.1 ± 1.1 | |
| V70Gy | (%) | 11.3 ± 3.5 | |
| V50Gy | (%) | 26.3 ± 4.9 | |
| V30Gy | (%) | 64.4 ± 10.7 | |
| Bladder | Volume | (ml) | 193.6 ± 89.8 |
| Dmean | (Gy) | 43.9 ± 4.1 | |
| D2% | (Gy) | 78.0 ± 0.8 | |
| V70Gy | (%) | 11.6 ± 5.7 | |
| V50Gy | (%) | 28.1 ± 11.4 | |
| V30Gy | (%) | 80.5 ± 11.6 | |
| Left femoral head | D2% | (Gy) | 43.1 ± 2.9 |
| Right femoral head | D2% | (Gy) | 42.2 ± 2.9 |
| Bowel bag | D2% | (Gy) | 47.2 ± 0.7 |
| V45Gy | (ml) | 76.7 ± 30.3 | |
PTV = planning target volume, OAR = organ at risk, Dn% = minimal dose to n% of the structure, VnGy = absolute or percentage structure volume receiving ≥ n Gy.
Fig. 2.
Average (solid line) and 1 standard deviation (dashed line) dose–volume histograms (DVHs) for the prostate planning target volume (prostate PTV), nodal PTV, rectum, bladder, and bowel bag.
No Grade 3 or higher acute toxicity was observed. Maximal acute toxicity is detailed in Table 3. Acute Grade 2 GI and GU toxicity occurred in 16 patients (16%) and 13 patients (13%), respectively. Despite the use of WPRT, only six patients (6%) experienced acute Grade 2 diarrhea. Urinary frequency was the most frequent acute Grade 2 GU toxicity.
Table 3.
Maximal acute toxicity
| Toxicity | Grade |
||
|---|---|---|---|
| 0 | 1 | 2 | |
| GI | 37 (37%) | 47 (47%) | 16 (16%) |
| Proctitis | 73 (73%) | 15 (15%) | 12 (12%) |
| Diarrhea | 47 (47%) | 47 (47%) | 6 (6%) |
| GU | 8 (8%) | 79 (79%) | 13 (13%) |
| Frequency | 16 (16%) | 72 (72%) | 12 (12%) |
| Incontinence | 94 (94%) | 6 (6%) | 0 |
| Retention | 92 (92%) | 7 (7%) | 1 (1%) |
| Urinary tract pain | 70 (70%) | 30 (30%) | 0 |
| Urgency | 60 (60%) | 40 (40%) | 0 |
GI = gastrointestinal, GU = genitourinary.
Point prevalence of Grade 1–2 and Grade 2 toxicities during RT and at early follow-up (2 weeks and 3 months after the end of RT) are illustrated in Fig. 3a and 3b, respectively. Figure 3b indicates that the highest incidence of acute Grade 2 diarrhea was reached around Week 5 (corresponding to the end of WPRT), whereas the highest incidence of acute Grade 2 proctitis and GU toxicity was reached around Week 8, corresponding to the end of the whole course of RT. Three months after treatment, most acute diarrhea, proctitis, and Grade 2 GU toxicity had resolved, whereas Grade 1 GU toxicity was observed in 30% of patients.
Fig. 3.
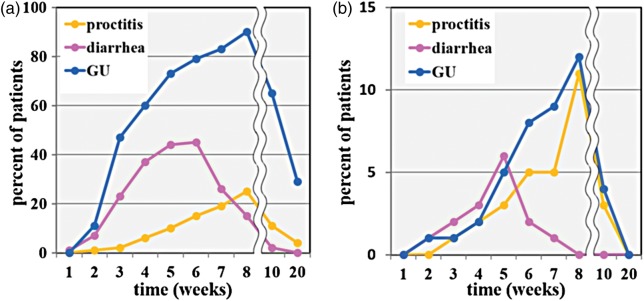
Point prevalence of (a) Grade 1–2 toxicity and (b) Grade 2 toxicity. The horizontal axis shows the number of weeks from start of radiation therapy. The line is cut between the eighth and tenth weeks because different timescales are applied to the first and the latter periods. GU = genitourinary.
The doses to the rectum, bladder, and bowel bag were compared between patients experiencing Grade 2 toxicity and those experiencing Grade 1 or no toxicity (Table 4). The analysis included the volume, mean dose, D2%, V70Gy, V50Gy and V30Gy for the rectum and bladder; and D2% and V45Gy for the bowel bag. As a result, no dosimetric values correlated with acute rectal, bladder, or bowel bag toxicity.
Table 4.
Comparison of rectum, bladder, and bowel bag dose statistics stratified by toxicity grade
| Parameter |
Grade 2 | Grade ≤1 | P* | |
|---|---|---|---|---|
| (mean ± SD) | (mean ± SD) | |||
| Rectum | ||||
| Volume | (cm3) | 64.5 ± 17.6 | 60.6 ± 15.6 | 0.52 |
| Dmean | (Gy) | 38.7 ± 2.5 | 39.9 ± 2.6 | 0.22 |
| D2% | (Gy) | 77.0 ± 1.4 | 77.1 ± 1.0 | 0.84 |
| V70Gy | (%) | 10.9 ± 4.6 | 11.4 ± 3.3 | 0.44 |
| V50Gy | (%) | 25.2 ± 4.2 | 26.4 ± 5.0 | 0.47 |
| V30Gy | (%) | 59.7 ± 8.5 | 65.0 ± 11.0 | 0.16 |
| Bladder | ||||
| Volume | (cm3) | 162.8 ± 69.2 | 198.2 ± 92.9 | 0.26 |
| Dmean | (Gy) | 46.1 ± 3.2 | 43.6 ± 4.2 | 0.07 |
| D2% | (Gy) | 78.3 ± 0.7 | 78.0 ± 0.8 | 0.19 |
| V70Gy | (%) | 14.3 ± 6.4 | 11.2 ± 5.6 | 0.08 |
| V50Gy | (%) | 32.5 ± 11.3 | 27.4 ± 11.4 | 0.13 |
| V30Gy | (%) | 82.8 ± 11.0 | 80.2 ± 11.7 | 0.50 |
| Bowel bag | ||||
| D2% | (Gy) | 47.9 ± 0.5 | 47.2 ± 0.7 | 0.22 |
| V45Gy | (cm3) | 88.0 ± 34.6 | 76.0 ± 30.0 | 0.37 |
Dn% = minimal dose to n% of the structure, VnGy = percentage or absolute structure volume receiving ≥ n Gy. Asterisk indicates unpaired Student's t test
DISCUSSION
In RT for patients with prostate cancer, VMAT is a relatively new approach [19–27, 30]. To the best of our knowledge, this is the first report of prostate cancer patients treated with WP-VMAT and IGRT. All dose–volume constraints were met satisfactorily for all treatment plans with a short treatment time. Clinical outcomes observed were also promising, with low rates of acute GI and GU toxicity.
In patients with high-risk prostate cancer, the use of WPRT is currently controversial. Whereas the RTOG 9413 trial reported a benefit of WPRT [3], the GETUG-01 trial showed no difference between PORT and WPRT [5]. One limitation of these trials is that the total dose administered to the prostate was < 72 Gy. Therefore, it remains an unsettled question whether WPRT is necessary when high-dose radiation is delivered to the prostate. RTOG has recently launched a larger Phase 3 trial (RTOG 0924) in order to address the question of the impact of WPRT with dose escalation to the prostate on overall survival. Traditionally, WPRT for high-risk prostate cancer is delivered in two consecutive phases: the first phase encompasses the whole pelvis to treat the prostate, seminal vesicles, and PLN at risk, and the second phase boosts the prostate alone. Combining WPRT with hypofractionated prostate radiation using the SIB technique is a new approach that can be used to treat patients with high-risk prostate cancer. However, there is little evidence on the long-term toxicity of this approach and no evidence on efficacy [31]. Therefore, the present study was designed to maintain the standard dose fractionation and use the two-phase treatment in the design of this study, as well as the RTOG 0924 trial [32].
IMRT generates concave dose distributions and has the potential to deliver radical doses to the pelvic nodes and prostate gland while reducing the dose to surrounding normal tissues. Several authors have described that WP-IMRT provides superior OAR sparing [11] and target coverage [12] over 3D conformal radiotherapy (3D-CRT). The DVH data in the present study demonstrate that VMAT can produce equivalent quality of dose distributions when compared with IMRT as reported in other series (Table 5). In addition, the bowel bag DVH parameters met the Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC) dose recommendations, in which the bowel bag volume receiving >45 Gy should be < 195 ml [33]. Thus, the present study confirms that WP-VMAT is dosimetrically feasible.
Table 5.
Comparison of bladder and rectum dosimetric parameters between recent studies using dose-escalated IMRT and VMAT for treatment of the whole pelvis
| Study | Technique | Patients (n) | Total dose (Gy)/
No. of fractions |
Bladder |
Rectum |
|||
|---|---|---|---|---|---|---|---|---|
| Prostate | Whole pelvis | V70Gy (%) | V50Gy (%) | V70Gy (%) | V50Gy (%) | |||
| Deville et al. 2010 [36] | IMRT | 30 | 79.2/44 | 45/25 | 9.3 | 44.3 | 15.7 | 51.0 |
| RTOG 0924a [32] | IMRT | 79.2/44 | 45/25 | 20 | N/A | 35 | N/A | |
| Current study | VMAT | 100 | 78/39 | 46.8/26 | 11.6 | 28.1 | 11.3 | 26.3 |
IMRT = intensity-modulated radiotherapy, VMAT = volumetric-modulated arc therapy, VnGy = percentage structure volume receiving ≥n Gy, N/A = not applicable. aDose constraints used in RTOG 0924 trial.
A major advantage of VMAT over IMRT originates from the superior delivery efficiency. The decreased treatment time of VMAT has the potential to reduce the effects of intrafractional prostate motion [34, 35]. Moreover, this time saving could be used to increase patient throughput on a treatment unit, which then provides additional time for on-line image guidance without increasing the overall treatment time. Considering the delivery efficiency as well as the acceptable plan quality of VMAT compared with that of IMRT, VMAT may be the preferred modality for treating high-risk prostate cancer in the context of WPRT.
There are limited data reporting the acute toxicity of WP-IMRT in the setting of dose escalation to the prostate. Additionally, despite several favorable dosimetric studies [23, 24, 26], clinical outcome data reporting toxicity in prostate cancer patients treated with WP-VMAT in the literature are lacking. The acute toxicity rates reported in the current study compare favorably with those reported in other series that employed WP-IMRT (Table 6). Therefore, it seems reasonable to say that WP-VMAT is a useful and valuable way to treat patients with high-risk prostate cancer. There have been some reports of an association between acute toxicity and development of subsequent late complications [37–39]. Therefore, the acceptably low incidence of acute toxicity in the current study might contribute to the lower frequency and lower severity of late toxicity. In the present study, as also observed by others [13, 40, 41], no correlation was found between acute GI and GU toxicities and dosimetric parameters. The low dosimetric parameters and low frequencies of the severe acute toxicities may have led to the lack of correlation between toxicity and dosimetric variables.
Table 6.
Comparison of acute toxicity between recent studies using dose-escalated IMRT and VMAT for treatment of the whole pelvis
| Study | Technique | Patients (n) | Total dose (Gy)/
No. of fractions |
Acute GI toxicity (%) |
Acute GU toxicity (%) |
|||
|---|---|---|---|---|---|---|---|---|
| Prostate | Whole pelvis | Grade 2 | Grade 3 | Grade 2 | Grade 3 | |||
| Sanguineti et al. 2008 [14] | IMRT | 87 | 76/38 | 54/30 | 43.7 | 5.7 | 43.7 | 8 |
| Bayley et al. 2010 [17] | IMRT | 103 | 79.8/42 | 55.1/29 | 31.1 | 1.9 | 43.7 | 2.9 |
| Deville et al. 2010 [36] | IMRT | 30 | 79.2/44 | 45/25 | 50 | 0 | 50 | 3 |
| Current study | VMAT | 100 | 78/39 | 46.8/26 | 16 | 0 | 13 | 0 |
IMRT = intensity-modulated radiotherapy, VMAT = volumetric-modulated arc therapy, GI = gastrointestinal, GU = genitourinary.
IGRT is becoming increasingly popular in combination with IMRT. In this study, daily image guidance with ExacTrac and CBCT was practiced in all patients. WPRT by IG-IMRT is challenging because the pelvic nodes move independently of the prostate. Ferjani et al. [42] demonstrated that for the concurrent treatment of the prostate and PLN, with a planning margin to the prostate of 6–8 mm posterior and a planning margin of 5 mm to the PLN, aligning to the prostate soft tissue on daily CBCT was an effective strategy, and aligning to the pelvic bone would result in underdosing to the prostate in one-third of fractions. The results of Ferjani et al. justify the present IG methods and planning margins. Further, IGRT has been shown to be associated with reductions in toxicity. Chung et al. [18] reported that whole-pelvic IG-IMRT permits the use of smaller margins and corresponding lower acute bladder and rectal toxicities compared with IMRT in prostate cancer patients. Although the current study performed IG-VMAT using daily CBCT-based soft tissue registration without intraprostatic fiducial markers (FMs), the incidence of acute toxicity were comparable with those of the Chung et al. [18], who reported that acute Grade 2 proctitis and GU toxicity were both 13% with IG-IMRT using daily target localization with intraprostatic FMs. In addition, Zelefsky et al. [43] have reported that the enhanced accuracy associated with IGRT for the treatment of prostate cancer results in a significant reduction in late urinary toxicity. These data suggest that the use of daily IGRT contributed to the low incidence of acute toxicity in the current study.
The present study has some limitations. First, ADT was not prescribed uniformly. However, 96% of patients received long-term ADT. Second, the follow-up period is still short; hence long-term toxicity has not yet been determined. Although a lower incidence of acute toxicity may decrease the development of subsequent late toxicity, it is possible that WPRT has the potential for worse late toxicity because of the larger field size compared with PORT. Therefore, longer follow-up for assessment of late toxicity is needed.
In conclusion, the present study demonstrated that VMAT provided feasible PTV coverage and OAR sparing with short treatment time in 100 patients. Acute GI and GU toxicity was acceptably low. These findings support the use of VMAT with daily CBCT to deliver WPRT in high-risk prostate cancer. Further study is needed to assess late toxicity and disease control.
FUNDING
Funding to pay the Open Access publication charges for this article was provided by K.I. (Tane General Hospital).
ACKNOWLEDGEMENTS
An analysis of results for the first 51 consecutive patients (‘A treatment planning and acute toxicity of VMAT in the treatment of high-risk prostate cancer with whole pelvic radiotherapy’) was presented at ASTRO's 55th Annual Meeting, Atlanta, 22–25 September 2013. The results for the first 100 patients (‘A treatment planning and acute toxicity of whole-pelvis volumetric-modulated arc therapy in the first 100 consecutive patients with high-risk prostate cancer’) was presented at JRS's 73th Annual Meeting, Yokohama, 10–13 April 2014.
REFERENCES
- 1.Roach M, III, Marquez C, You HS, et al. Predicting the risk of lymph node involvement using the pre-treatment prostate specific antigen and Gleason score in men with clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 1994;28:33–7. doi: 10.1016/0360-3016(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 2.Roberts T, Roach M., III The evolving role of pelvic radiation therapy. Semin Radiat Oncol. 2003;13:109–20. doi: 10.1053/srao.2003.50014. [DOI] [PubMed] [Google Scholar]
- 3.Roach M, III, DeSilvio M, Lawton C, et al. Phase III trial comparing whole-pelvis versus prostate-only radiotherapy and neoadjuvant versus adjuvant combined androgen suppression: Radiation Therapy Oncology Group 9413. J Clin Oncol. 2003;21:1904–11. doi: 10.1200/JCO.2003.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Lawton CA, DeSilvio M, Roach M, III, et al. An update of the phase III trial comparing whole pelvic to prostate only radiotherapy and neoadjuvant to adjuvant total androgen suppression: updated analysis of RTOG 94-13, with emphasis on unexpected hormone/radiation interactions. Int J Radiat Oncol Biol Phys. 2007;69:646–55. doi: 10.1016/j.ijrobp.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pommier P, Chabaud S, Lagrange JL, et al. Is there a role for pelvic irradiation in localized prostate adenocarcinoma? Preliminary results of GETUG-01. J Clin Oncol. 2007;25:5366–73. doi: 10.1200/JCO.2006.10.5171. [DOI] [PubMed] [Google Scholar]
- 6.Zietman AL, DeSilvio ML, Slater JD, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA. 2005;294:1233–9. doi: 10.1001/jama.294.10.1233. [DOI] [PubMed] [Google Scholar]
- 7.Kuban DA, Tucker SL, Dong L, et al. Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:67–74. doi: 10.1016/j.ijrobp.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 8.Pilepich MV, Winter K, Lawton CA, et al. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma—long-term results of phase III RTOG 85-31. Int J Radiat Oncol Biol Phys. 2005;61:1285–90. doi: 10.1016/j.ijrobp.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 9.Pilepich MV, Winter K, John MJ, et al. Phase III radiation therapy oncology group (RTOG) trial 86-10 of androgen deprivation adjuvant to definitive radiotherapy in locally advanced carcinoma of the prostate. Int J Radiat Oncol Biol Phys. 2001;50:1243–52. doi: 10.1016/s0360-3016(01)01579-6. [DOI] [PubMed] [Google Scholar]
- 10.Hanks GE, Pajak TF, Porter A, et al. Phase III trial of long-term adjuvant androgen deprivation after neoadjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate: the Radiation Therapy Oncology Group Protocol 92-02. J Clin Oncol. 2003;21:3972–8. doi: 10.1200/JCO.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 11.Sanguineti G, Cavey ML, Endres EJ, et al. Does treatment of the pelvic nodes with IMRT increase late rectal toxicity over conformal prostate-only radiotherapy to 76 Gy? Strahlenther Onkol. 2006;182:543–9. doi: 10.1007/s00066-006-1586-9. [DOI] [PubMed] [Google Scholar]
- 12.Wang-Chesebro A, Xia P, Coleman J, et al. Intensity-modulated radiotherapy improves lymph node coverage and dose to critical structures compared with three-dimensional conformal radiation therapy in clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2006;66:654–62. doi: 10.1016/j.ijrobp.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 13.Arcangeli S, Saracino B, Grazia M, et al. Analysis of toxicity in patients with high risk prostate cancer treated with intensity-modulated pelvic radiation therapy and simultaneous integrated dose escalation to prostate area. Radiother Oncol. 2007;84:148–55. doi: 10.1016/j.radonc.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 14.Sanguineti G, Endres EJ, Parker BC, et al. Acute toxicity of whole-pelvis IMRT in 87 patients with localized prostate cancer. Acta Oncol. 2008;47:301–10. doi: 10.1080/02841860701558849. [DOI] [PubMed] [Google Scholar]
- 15.Muren LP, Wasbø E, Helle SI, et al. Intensity-modulated radiotherapy of pelvic lymph nodes in locally advanced prostate cancer: planning procedures and early experiences. Int J Radiat Oncol Biol Phys. 2008;71:1034–41. doi: 10.1016/j.ijrobp.2007.11.060. [DOI] [PubMed] [Google Scholar]
- 16.Pervez N, Small C, MacKenzie M, et al. Acute toxicity in high-risk prostate cancer patients treated with androgen suppression and hypofractionated intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:57–64. doi: 10.1016/j.ijrobp.2009.01.048. [DOI] [PubMed] [Google Scholar]
- 17.Bayley A, Rosewall T, Craig T, et al. Clinical application of high-dose, image-guided intensity-modulated radiotherapy in high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2010;77:477–83. doi: 10.1016/j.ijrobp.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Chung HT, Xia P, Chan LW, et al. Does image-guided radiotherapy improve toxicity profile in whole pelvic-treated high-risk prostate cancer? Comparison between IG-IMRT and IMRT. Int J Radiat Oncol Biol Phys. 2009;73:53–60. doi: 10.1016/j.ijrobp.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 19.Palma D, Vollans E, James K, et al. Volumetric modulated arc therapy for delivery of prostate radiotherapy: comparison with intensity-modulated radiotherapy and three dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72:996–1001. doi: 10.1016/j.ijrobp.2008.02.047. [DOI] [PubMed] [Google Scholar]
- 20.Zhang P, Happersett L, Hunt M, et al. Volumetric modulated arc therapy: planning and evaluation for prostate cancer cases. Int J Radiat Oncol Biol Phys. 2010;76:1456–62. doi: 10.1016/j.ijrobp.2009.03.033. [DOI] [PubMed] [Google Scholar]
- 21.Aznar MC, Petersen PM, Logadottir A, et al. Rotational radiotherapy for prostate cancer in clinical practice. Radiother Oncol. 2010;97:480–4. doi: 10.1016/j.radonc.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 22.Kopp RW, Duff M, Catalfamo F, et al. VMAT vs. 7-field-IMRT: assessing the dosimetric parameters of prostate cancer treatment with a 292-patient sample. Med Dosim. 2011;36:365–72. doi: 10.1016/j.meddos.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Yoo S, Wu J, Lee R, et al. Radiotherapy treatment plans with RapidArc for prostate cancer involving seminal vesicles and lymph nodes. Int J Radiat Oncol Biol Phys. 2010;76:935–42. doi: 10.1016/j.ijrobp.2009.07.1677. [DOI] [PubMed] [Google Scholar]
- 24.Davidson MTM, Blake S, Batchelar DL, et al. Assessing the role of volumetric modulated arc therapy (VMAT) relative to IMRT and helical tomotherapy in the management of localized, locally advanced, and post-operative prostate cancer. Int J Radiat Oncol Biol Phys. 2011;80:1550–8. doi: 10.1016/j.ijrobp.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 25.Myerhaug S, Chan G, Graig T, et al. A treatment planning and acute toxicity comparison of two pelvic nodal volume delineation techniques and delivery comparison of intensity-modulated radiotherapy versus volumetric modulated arc therapy for hypofractionated high-risk prostate cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2012;82:e657–62. doi: 10.1016/j.ijrobp.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 26.Fontenot JD, King ML, Johnson SA, et al. Single-arc volumetric-modulated arc therapy can provide dose distributions equivalent to fixed-beam intensity-modulated radiation therapy for prostatic irradiation with seminal vesicle and/or lymph node involvement. Br J Radiol. 2012;85:231–6. doi: 10.1259/bjr/94843998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pesce GA, Clivio A, Cozzi L, et al. Early clinical experience of radiotherapy of prostate cancer with volumetric modulated arc therapy. Radiat Oncol. 2010;5:54. doi: 10.1186/1748-717X-5-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lawton CAF, Michalski J, El-Naqa I, et al. RTOG GU radiation oncology specialists reach consensus on pelvic lymph node volumes for high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2009;74:383–7. doi: 10.1016/j.ijrobp.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gay HA, Barthold HJ, O'Meara E, et al. Pelvic normal tissue contouring guidelines for radiation therapy: a Radiation Therapy Oncology Group consensus panel atlas. Int J Radiat Oncol Biol Phys. 2012;83:e353–62. doi: 10.1016/j.ijrobp.2012.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Otto K. Volumetric modulated arc therapy: IMRT in a single gantry arc. Med Phys. 2008;35:310–7. doi: 10.1118/1.2818738. [DOI] [PubMed] [Google Scholar]
- 31.Kaidar-Person O, Roach M, III, Crehange G. Whole-pelvic nodal radiation therapy in the context of hypofractionation for high-risk prostate cancer patients: a step forward. Int J Radiat Oncol Biol Phys. 2013;86:600–5. doi: 10.1016/j.ijrobp.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 32.Radiation Therapy Oncology Group RTOG 0924. Androgen deprivation therapy and high dose radiotherapy with or without whole-pelvic radiotherapy in unfavorable intermediate or favorable high risk prostate cancer: a phase III randomized trial. Available from: http://www.rtog.org/ClinicalTrials/ProtocolTable.aspx. (1 June 2014, data last accessed) [Google Scholar]
- 33.Kavanagh BD, Pan CC, Dawson LA, et al. Radiation dose–volume effects in the stomach and small bowel. Int J Radiat Oncol Biol Phys. 2010;76:s101–7. doi: 10.1016/j.ijrobp.2009.05.071. [DOI] [PubMed] [Google Scholar]
- 34.Langen KM, Willoughby TR, Meeks SL, et al. Observations on real-time prostate gland motion using electromagnetic tracking. Int J Radiat Oncol Biol Phys. 2008;71:1084–90. doi: 10.1016/j.ijrobp.2007.11.054. [DOI] [PubMed] [Google Scholar]
- 35.Li JS, Lin MH, Buyyounouski MK, et al. Reduction of prostate intrafractional motion from shortening the treatment time. Phys Med Biol. 2013;58:4921–32. doi: 10.1088/0031-9155/58/14/4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deville C, Both S, Hwang WT, et al. Clinical toxicities and dosimetric parameters after whole-pelvis versus prostate-only intensity-modulated radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2010;78:763–72. doi: 10.1016/j.ijrobp.2009.08.043. [DOI] [PubMed] [Google Scholar]
- 37.Vargas C, Martinez A, Kestin LL, et al. Dose–volume analysis of predictors for chronic rectal toxicity after treatment of prostate cancer with adaptive image-guided radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62:1297–308. doi: 10.1016/j.ijrobp.2004.12.052. [DOI] [PubMed] [Google Scholar]
- 38.Zelefsky MJ, Levin EJ, Hunt M, et al. Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:1124–9. doi: 10.1016/j.ijrobp.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 39.Michalski JM, Yan Y, Watkins-Bruner, et al. Preliminary toxicity analysis of 3-dimensional conformal radiation therapy versus intensity modulated radiation therapy on the high-dose arm of the Radiation Therapy Oncology Group 0126 prostate cancer trial. Int J Radiat Oncol Biol Phys. 2013;87:932–8. doi: 10.1016/j.ijrobp.2013.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Storey MR, Pollack A, Zagars G, et al. Complications from radiotherapy dose escalation in prostate cancer: preliminary results of randomized trial. Int J Radiat Oncol Biol Phys. 2000;48:635–42. doi: 10.1016/s0360-3016(00)00700-8. [DOI] [PubMed] [Google Scholar]
- 41.De Meerleer G, Vakaet L, Meersschout S, et al. Intensity-modulated radiotherapy as primary treatment for prostate cancer: acute toxicity in 114 patients. Int J Radiat Oncol Biol Phys. 2004;60:777–87. doi: 10.1016/j.ijrobp.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 42.Ferjani S, Huang G, Shang Q, et al. Alignment focus of daily image guidance for concurrent treatment of prostate and pelvic lymph nodes. Int J Radiat Oncol Biol Phys. 2013;87:383–9. doi: 10.1016/j.ijrobp.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 43.Zelefsky MJ, Kollmeier M, Cox B, et al. Improved clinical outcomes with high-dose image guided radiotherapy compared with non-IGRT for the treatment of clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2012;84:125–9. doi: 10.1016/j.ijrobp.2011.11.047. [DOI] [PubMed] [Google Scholar]