Abstract
Reactions of edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one) with deoxyguanosine monophosphate (dGMP) hydroxyl radical adducts were investigated by pulse radiolysis technique. Edaravone was found to reduce the dGMP hydroxyl radical adducts through electron transfer reactions. The rate constants of the reactions were greater than 4 × 108 dm3 mol−1 s−1 and similar to those of the reactions of ascorbic acid, which is a representative antioxidant. Yields of single-strand breaks, base lesions, and abasic sites produced in pUC18 plasmid DNA by gamma ray irradiation in the presence of low concentrations (10–1000 μmol dm−3) of edaravone were also quantified, and the chemical repair activity of edaravone was estimated by a method recently developed by the authors. By comparing suppression efficiencies to the induction of each DNA lesion, it was found that base lesions and abasic sites were suppressed by the chemical repair activity of edaravone, although the suppression of single-strand breaks was not very effective. This phenomenon was attributed to the chemical repair activity of edaravone toward base lesions and abasic sites. However, the chemical repair activity of edaravone for base lesions was lower than that of ascorbic acid.
Keywords: antioxidant, edaravone (Radicut®), chemical repair, pulse radiolysis
INTRODUCTION
Ionizing radiation causes irreversible molecular changes (i.e. damage) to DNA. Damage is caused by direct ionization or reactions of hydroxyl radicals (•OH) from water radiolysis, and includes strand breaks and base lesions. Double-strand breaks (DSBs), consisting of two or more single-strand breaks (SSBs) in close proximity produced in genomic DNA, are thought to be harmful lesions that can cause serious biological effects, such as cell death. Moreover, base lesions can cause cell mutations that may contribute to carcinogenesis.
Production of these DNA lesions can be suppressed by some chemical compounds. Dimethyl sulfoxide (DMSO), which is used to limit the formation of DNA lesions under irradiation, acts as an •OH scavenger [1, 2]. Antioxidants, such as catechins and ascorbic acid, also reduce the formation of DNA lesions. High reactivity between antioxidants and oxidative radicals has been reported by several researchers [3–10]. In addition to acting as radical scavengers, some antioxidants are reported to repair the precursors of DNA lesions (DNA radicals) by chemical reactions [11–16]. This repair process is called ‘chemical repair’. As DNA radicals generally have longer lifetimes than •OH in a cell or under cell-mimetic conditions [17–19], chemical repair should be a more efficient radioprotection process than radical scavenging. In a previous report, the authors investigated the chemical repair activity of ascorbic acid and found that the precursors of abasic sites (AP sites) and other base lesions were more easily repaired than those of strand breaks [16]. Approximately 50–60% of these lesions were chemically repaired by ascorbic acid. On the other hand, <10% of •OH produced from radiolysis was scavenged by the ascorbic acid concentrations under the experimental conditions. The results showed that the chemical repair activity of ascorbic acid was more efficient than its •OH scavenging activity. It was also suggested that ascorbic acid might be suitable as an agent to reduce mutations.
Edaravone (Radicut®, 3-methyl-1-phenyl-2-pyrazolin-5-one; Fig. 1), which is an artificial antioxidant with low toxicity, is generally known as a neuroprotective drug, and it has been developed and clinically used in Japan since 2001. Its medical and pharmacological effects on brain infarctions and cardiovascular diseases have been confirmed by numerous in vivo experiments [20–27]. The pharmacological effects of edaravone have mainly been attributed to its reactivity with free radicals, such as •OH and peroxide radicals [28–31]. Because edaravone has recently received much attention for use as a radioprotector [32, 33], the authors have investigated its reactions with •OH and other oxidative radicals by pulse radiolysis and determined a pattern of reaction for edaravone as an antioxidant, together with the rate constants of the reactions [29, 34]. Much information about the radical scavenging properties of edaravone was revealed by these experiments. However, to understand the radioprotective effects of edaravone, other effective repair processes, such as chemical repair properties, must also be investigated. In the present study, pulse radiolysis using a DNA model compound, deoxyguanosine monophosphate (dGMP), was conducted to observe the basic reactions of chemical repair and reactivity, and was compared with that of ascorbic acid. Furthermore, to investigate the chemical repair activity of edaravone on DNA lesions, yields of lesions on plasmid DNA in gamma ray-irradiated aqueous solutions containing several concentrations of edaravone were measured by a method recently developed by the authors [16].
Fig. 1.
Chemical structure of edaravone in water.
MATERIALS AND METHODS
Samples
Edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one), Tris, boric acid, ethylenediaminetetraacetic acid (EDTA)·2Na·2H2O, sodium hydrate (NaOH), 1% bromophenol blue, Orange G, NaH2PO4 and Na2HPO4 were purchased from Wako Pure Chemical Industries, Ltd (Osaka, Japan). Ascorbic acid and deoxyguanosine monophosphate (dGMP) were purchased from Sigma–Aldrich (St Louis, MO). Formamidopyrimidine-DNA glycosylase (Fpg), endonuclease III (Nth), endonuclease IV (Nfo), and reaction buffer solutions used for the treatment of these enzymes were purchased from New England BioLabs Inc. (Ipswich, MA). Stock solutions of the enzymes (concentrations of 10 U μl−1 for Nth and Nfo, and 8 U μl−1 for Fpg) and the reaction buffers were stored at −20°C. Nth protein mainly excises ring-saturated pyrimidines (e.g. 5,6-dihydrothymine [DHT]), thymine glycol, and AP sites. Fpg protein excises mainly 2,6-diamino-4-hydroxy-5-N-methyl formamidopyrimidine, 7,8-dihydro-8-oxo-2′-deoxyguanine (8-oxoGua) and AP sites. Nfo protein excises various AP sites [35, 36]. Water (>18 MΩ) purified by Milli-Q system (Millipore, Billerica, MA) was used to make all aqueous solutions.
Phosphate buffer solutions (pH 6.9) were prepared from NaH2PO4. 2H2O and Na2HPO4. 12H2O. TBE buffer solutions consisted of 8.9 × 10−2 mol dm−3 Tris, 8.9 × 10−2 mol dm−3 boric acid, and 2 × 10−3 mol dm−3 EDTA. The loading buffer solutions for electrophoresis consisted of 2% (w/v) g dm−1, bromophenol blue, 2% (w/v) Orange G, 30% (w/v) glycerol, and 1 × 10−2 mol dm−3 EDTA. The pH of the TBE buffer and the loading buffer were adjusted to 8·0 with 1N NaOH. The deionized water and buffer solutions were sterilized at 120°C for 20 min before sample preparation.
Pulse radiolysis
Pulse radiolysis experiments were carried out at The University of Tokyo using an electron linear accelerator (energy: 35 MeV; pulse width: 10 ns). Details of the experimental setup are described elsewhere [29, 34]. All irradiated solutions were adjusted to pH 6.9 by 1.0 × 10−2 mol dm−3 phosphate buffer. Since the sample solutions containing antioxidants could be spontaneously oxidized in an aerated solution, they were freshly prepared and quickly deaerated using N2O gas for 15 min. Dosimetry was carried out using an N2O-saturated 1 × 10−2 mol dm−3 KSCN aqueous solution, taking Gϵ as 5.2 × 10−4 m2 J−1 at 475 nm [37]. An absorbed dose of 9 Gy per pulse was used in this experiment.
Electron beam irradiation causes water radiolysis, generating many kinds of reactive species. To observe the reactions of chemicals with •OH, other radical species need to be quenched. N2O gas is often used to scavenge hydrated electrons, one of the main products of water radiolysis, and convert them to •OH [38]. The scheme for water radiolysis and the reaction of N2O have been described previously [34]. The conversion of hydrated electrons to •OH is completed within 10 ns. Under these experimental conditions, hydrated electrons react predominantly with N2O because the concentration and the rate constant of N2O are both much higher than those of the antioxidants [39]. The yield of •OH has been estimated at 0.59 μmol J−1 [40].
Gamma ray irradiation of DNA solutions
Plasmid DNA pUC18 (2686 bp) was obtained from E. coli JM109 by Qiagen HiSpeed Plasmid Kit (Qiagen, Hilden, Germany) and purified by dialysis using a nitrocellulose membrane of pore size 0.025 μm (Millipore, Billerica, MA). After dialysis, it was confirmed by agarose electrophoresis that >90% of the plasmids were intact (closed circular form). Plasmids were stored at −20°C at a concentration of 1.88 × 10−1 g dm−3 dissolved in 2.0 × 10−2 mol dm−3 phosphate buffer solution (pH 6.9).
DNA stock solutions were diluted with 2.0 × 10−2 mol dm−3 phosphate buffer solution (pH 6.9) to a concentration of 1.0 × 10−2 g dm−3 for irradiation samples. Edaravone was added to the solutions just before irradiation. The concentrations of edaravone were adjusted to 0, 10, 100 and 1000 μmol dm−3. As the number of electrons of the solutes was less than that of water in a unit volume, over 99% of the radiation energy was believed to be deposited onto the water molecules under these conditions. Thus, DNA damage was predominantly induced by free radicals, such as •OH, produced from water radiolysis.
Irradiation conditions were the same as in the previous experiment [16]. For irradiation, 100 μdm3 samples were placed into 1.5-ml polypropylene tubes (Eppendorf, Hamburg, Germany). Each tube was placed into 20 ml of icy water maintained at 4°C during irradiation and exposed to 60Co gamma rays under aerobic conditions using a gamma ray apparatus (The University of Tokyo, Tokyo, Japan). The dose rate, measured by a Fricke dosimeter, ranged from 0.05–3.2 Gy min−1, depending on the position of samples. Each sample was irradiated at least three times.
After irradiation the lesions were detected as strand breaks by electrophoresis. To detect base lesions and AP sites, irradiated samples were treated with Nth, Fpg, and Nfo before electrophoresis. The details have been described previously [16]. The dose response of the residual closed-circular DNA was determined from the logarithmic loss. A D37 value, which represents the dose of radiation required to give on average one SSB per plasmid molecule, was calculated from the slope of the response curve. The chemical yield of the SSBs (Gssb) is represented using the value of D37 as follows:
| (1) |
where [plasmid] is the molar concentration of plasmid DNA molecules in an irradiated solution, and ρ is the density of the solution (∼1.0 kg dm−3). Yields of base lesions revealed by the enzymatic treatments, Genzyme (= Nth, Fpg or Nfo) were determined by subtracting the yield of SSBs in the incubated samples without the enzyme, Genz−, from that in the solution incubated with the enzyme, Genz+.
| (2) |
RESULTS
Reactivity of antioxidants with dGMP radicals
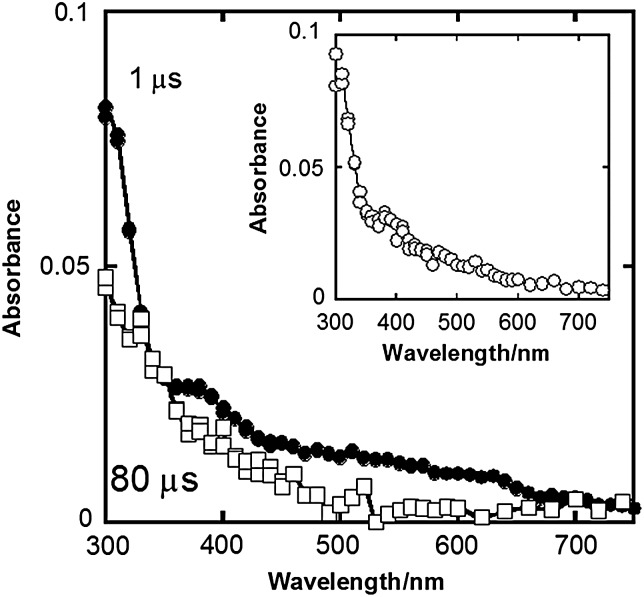
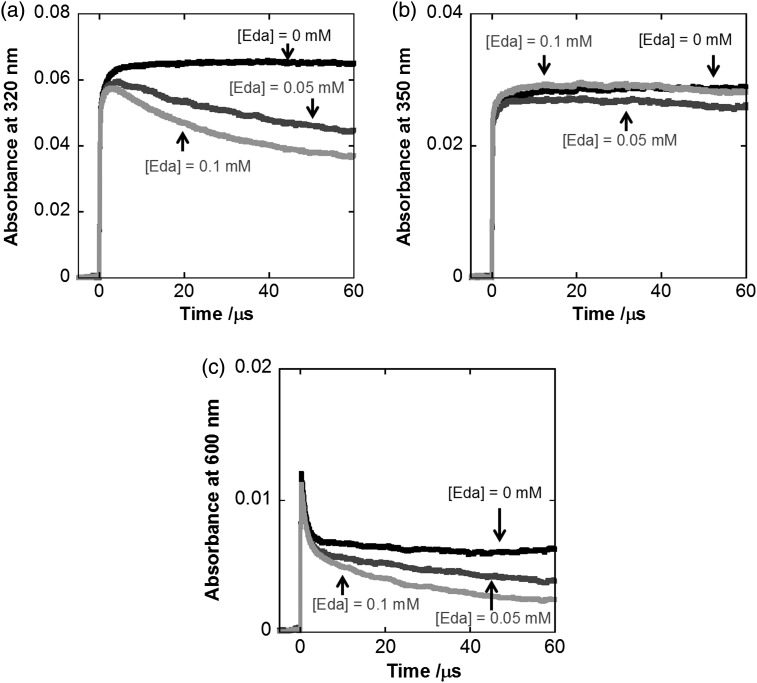
Transient absorption spectra (Fig. 2) were obtained by pulse radiolysis of N2O-saturated solution containing 2.0 × 10−3 mol dm−3 dGMP and 1.0 × 10−4 mol dm−3 edaravone at pH 6.9. Under these conditions, it is calculated that 94% of •OH was scavenged by dGMP based on a competition reaction between dGMP [41] and edaravone [29]. Therefore the spectrum obtained 1 μs after pulse irradiation is due to the absorption spectrum of dGMP hydroxyl radical adducts [42]. According to a previous report, this broad spectrum consists of absorption bands of two different dGMP hydroxyl radical adducts, G•8-OH (∼300 nm) and G•4-OH (∼600 nm) [43]. Absorption spectrum of dGMP hydroxyl radical adducts obtained 80 μs after the pulse irradiation by pulse radiolysis of the N2O-saturated solution of 2.0 × 10−3 mol dm−3 dGMP without edaravone is also shown in the inset of Fig. 2. This spectrum showed that these dGMP radicals were relatively stable in the solution without edaravone. From the time profiles in Fig. 3 and the spectrum in the inset of Fig. 2, it is clear that the decay rate of absorbance at 320 and 600 nm depended on the concentrations of edaravone. These profiles show that these dGMP hydroxyl radical adducts were scavenged by edaravone. Rate constants of the reactions were found to be 4.1 × 108 dm3 mol−1 s−1 and 4.3 × 108 dm3 mol−1 s−1 from the absorbance decay at 320 and 600 nm. On the other hand, the absorbance at 350 nm almost remained unchanged by the addition of edaravone (Fig. 3b). It seems that the edaravone radical, the absorption peak of which is ∼350 nm, was produced by these reactions (see Discussion).
Fig. 2.
Transient absorption spectra obtained 1 μs (closed circles) and 80 μs (open squares) after irradiation by pulse radiolysis of a N2O-saturated aqueous solution containing 2 × 10−3 mol dm−3 dGMP, 1.0 × 10−4 mol dm−3 edaravone, and 2.0 × 10−2 mol dm−3 phosphate buffer at pH 6.9. Inset: the transient spectrum obtained 80 μs after irradiation by pulse radiolysis of a N2O-saturated aqueous solution containing 2 × 10−3 mol dm−3 dGMP.
Fig. 3.
Time profiles of absorbance at (a) 320, (b) 350 and (c) 600 nm of the solutions containing several concentrations of edaravone and 2 × 10−3 mol dm−3 dGMP at pH 6.9.
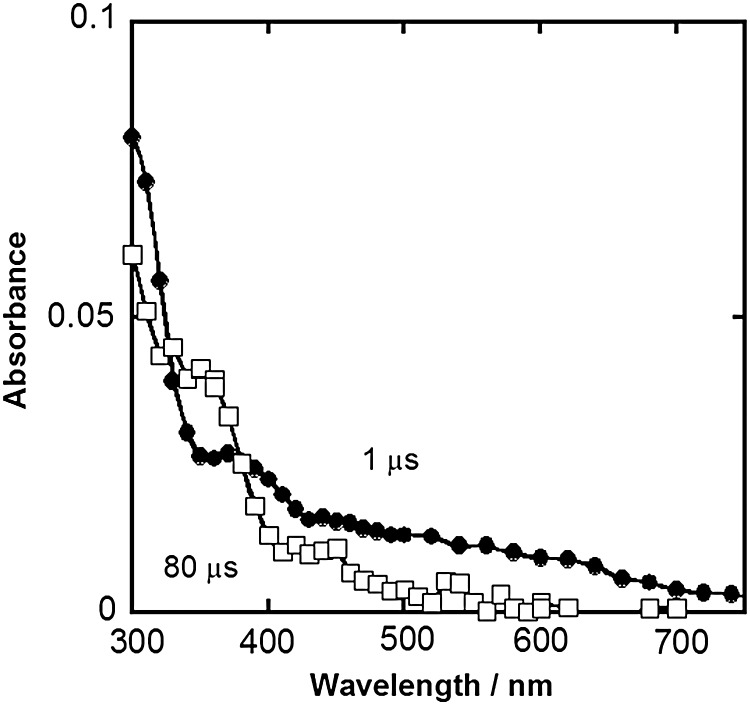
Transient absorption spectra of a N2O-saturated solution containing 2.0 × 10−3 mol dm−3 dGMP and 1.0 × 10−4 mol dm−3 ascorbic acid determined 1 and 80 μs after the pulse irradiation were also obtained to compare with that of edaravone (Fig. 4). Under the experimental conditions, the absorption spectrum of dGMP hydroxyl radical adducts was observed 1 μs after pulse irradiation because it was calculated that >97% of •OH was scavenged by dGMP based on a competition reaction between dGMP and ascorbic acid. The rate constant of the reaction between ascorbic acid and •OH, (4.5 ± 0.3) × 109 dm3 mol−1 s−1, which was determined in this study by measuring the buildup rate of ascorbate radical after pulse irradiation of N2O-saturated solutions containing 0.2–1.0 × 10−3 mol dm−3 ascorbic acid at pH 6.9, was used for the competition reaction. The absorbance derived from dGMP hydroxyl radical adducts decreased, with an increase in a strong absorption band at 360 nm. This spectral alteration, which was also reported previously by O'Neill [42], is believed to be due to electron transfer reactions of dGMP hydroxyl radical adducts and ascorbic acid, resulting in the production of an ascorbate radical. The rate constants of the decay at 320 and 600 nm were found to be 4.1 × 108 dm3 mol−1 s−1 and 4.2 × 108 dm3 mol−1 s−1, respectively. These values are similar to the reported value measured by O'Neill, (4.8 ± 0.5) × 108 dm3 mol−1 s−1 and are almost the same as those of the reactions of edaravone and dGMP hydroxyl radical adducts.
Fig. 4.
Transient absorption spectra obtained 1 μs (closed circles) and 80 μs (open squares) after irradiation by pulse radiolysis of a N2O-saturated aqueous solution containing 2 × 10−3 mol dm−3 dGMP, 1.0 × 10−4 mol dm−3 ascorbic acid, and 2.0 × 10−2 mol dm−3 phosphate buffer at pH 6.9.
Gamma ray-irradiation of plasmid DNA solutions containing edaravone
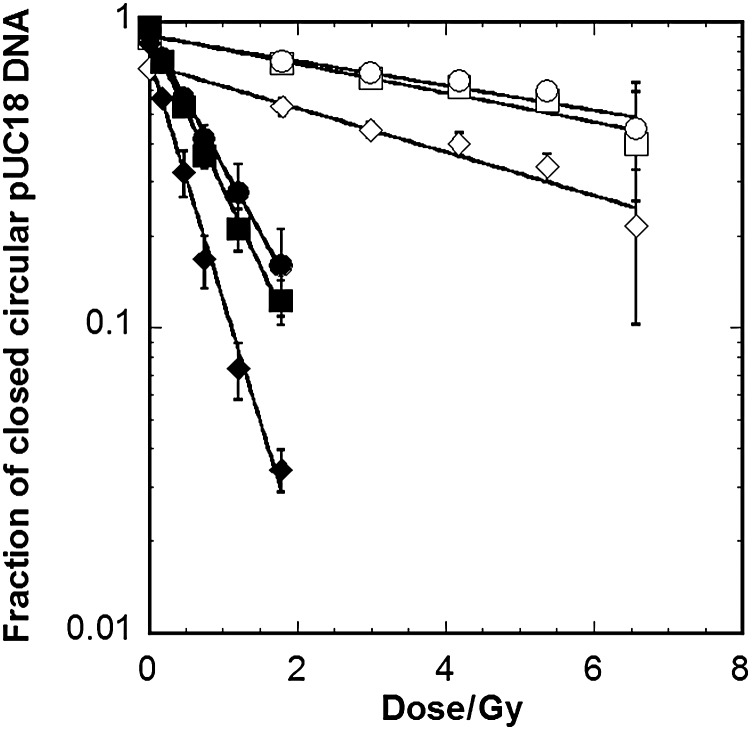
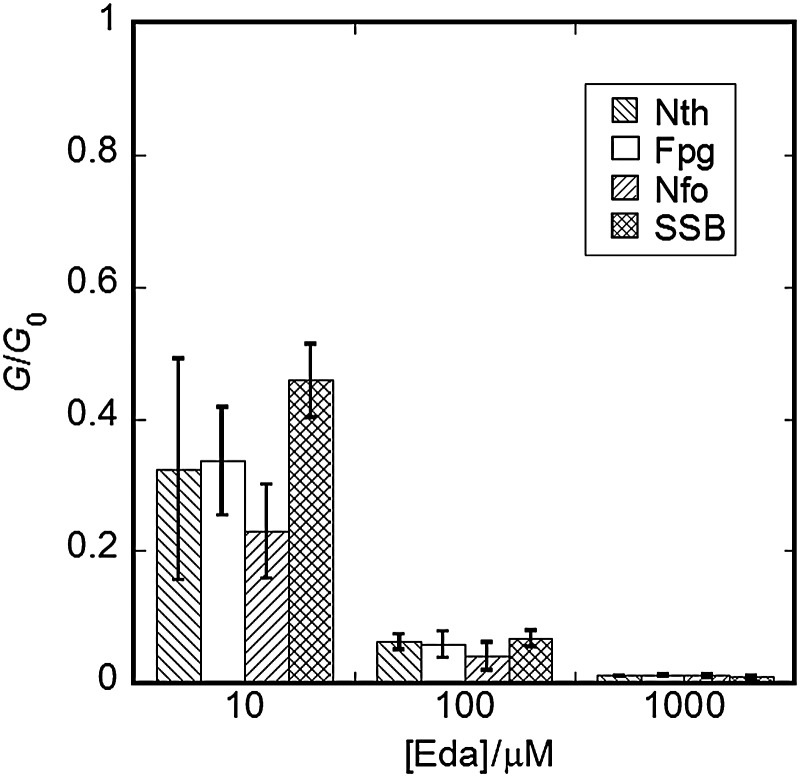
The semi-log plots of the dose–response curves of fractions of closed-circular forms in gamma ray-irradiated pUC18 plasmid DNA (1.0 × 10−2 g dm−3) aqueous solutions, with or without 100 μmol dm−3 edaravone, are shown in Fig. 5. The fractions of closed circular forms of DNA in the solutions incubated at 37°C for 1 h, with or without Nth, are also plotted. The dose–response curve of closed-circular forms in DNA incubated without Nth was slightly steeper than that of closed-circular forms in DNA without incubation, regardless of whether ascorbic acid was added (see the circle and square symbols in Fig. 5). This indicates that heat treatment during incubation induced damage in heat labile sites [44]. On the other hand, closed-circular forms in DNA incubated with Nth decreased drastically. This indicates that a lot of Nth-sensitive sites are produced in irradiated DNA. The results were similar to the results of the previous experiments in DNA solutions containing ascorbic acid [16]. Nth-sensitive lesions and strand breaks, which are produced by irradiation, were suppressed by edaravone. The chemical yields of prompt SSBs and Nth-, Fpg- and Nfo-sensitive sites (GSSB, GNth, GFpg, GNfo) in the absence and presence of 10, 100 and 1000 μmol dm−3 edaravone were determined (Table 1). The ratio of the yield of each lesion produced in the presence of edaravone to the yield produced in the absence of edaravone (G0) was calculated for each concentration (shown in Fig. 6 as ‘G/G0’). The G/G0 ratio decreased with increasing concentrations of edaravone. At the lower concentrations (10 and 100 μmol dm−3), the production of Nfo-sensitive sites (AP sites) has a tendency to be more suppressed than that of SSBs. The Nth- and Fpg-sensitive sites (AP sites and other base lesions) also had lower ratios than those of prompt SSBs, though the margin of error for the experimental data was large.
Fig. 5.
Dose–response curves of the remaining fraction of closed circular plasmid DNA in 2.0 × 10−2 mol dm−3 phosphate buffer (pH 7) aqueous solution of 1.0 × 10−2 g dm−3 pUC18 plasmid DNA with 100 μmol dm−3 edaravone (open symbols). The results of our previous study in DNA solution without the addition of antioxidants are also plotted (closed symbols) [11]. Circles: DNA directly analyzed without any chemical treatments after irradiation; diamonds: DNA incubated with Nth treatment after irradiation; squares: DNA incubated without Nth treatment after irradiation.
Table 1.
Chemical yields (G values) of strand breaks and of Nth-, Fpg- and Nfo-sensitive sites, and the fraction of repairable precursors of DNA lesions (p)
| [Eda] (μmol dm−3) |
p | |||||
|---|---|---|---|---|---|---|
| 0 | 10 | 100 | 1000 | |||
| G (10−3 μmol J−1) | Nth-sensitive lesions | 4.7 ± 1.2 | 1.5 ± 0.8 | 0.29 ± 0.05 | 0.056 ± 0.003 | 0.36 ± 0.13 |
| Fpg-sensitive lesions | 7.3 ± 2.2 | 2.5 ± 0.6 | 0.43 ± 0.15 | 0.090 ± 0.014 | 0.35 ± 0.13 | |
| Nfo-sensitive lesions | 2.9 ± 0.5 | 0.67 ± 0.21 | 0.12 ± 0.06 | 0.031 ± 0.007 | 0.56 ± 13 | |
| SSBs | 6.4 ± 2.2 | 2.9 ± 0.3 | 4.3 ± 0.8 | 0.055 ± 0.013 | 0.13 ± 0.09 | |
Fig. 6.
Ratios of the chemical yields of prompt SSBs and of Nth-, Fpg- and Nfo-sensitive sites obtained in the presence of edaravone at several concentrations (G) to the yields obtained without edaravone (G0).
According to our previous paper on ascorbic acid, the enzyme-sensitive lesions with lower G/G0 ratios indicate a higher level of chemical repair activity of ascorbic acid at AP sites and other base lesions. The chemical repair efficiency was estimated by the parameter, p, which represents the fraction of chemically repairable yields in all detected lesions, obtained by a simple model of a competition reaction (16). The value of p represents the proportion of lesions potentially repairable by an antioxidant. Enzyme-sensitive lesions were more easily repaired by edaravone than SSBs (Fig. 6), although the error margins of the G/G0 ratios of Fpg- and Nth-sensitive lesions were rather large. The value of p for edaravone was also determined for each lesion (Table 1). The value of p for Nfo-sensitive sites was 0.56 ± 0.13, indicating that about half of the precursors of AP sites could be chemically repaired by edaravone. On the other hand, the p values for edaravone to Nth- and Fpg-sensitive sites were ∼0.3, showing that ∼30% of the precursors of these lesions could be chemically repaired. Thus, edaravone can chemically repair precursors of AP sites and other base lesions more easily than those of SSBs. As these enzyme-sensitive lesions can cause cell mutations, this result suggests that edaravone may have a potentially beneficial role in the suppression of cell mutations under irradiation.
As mentioned above, two types of dGMP hydroxyl radical adducts, G•8-OH and G•4-OH, are mainly produced through the reaction of guanine and •OH. These radicals are known to be precursors of 8oxoG, which is an Fpg-sensitive lesion. In this gamma ray irradiation experiment, the inhibition of Fpg-sensitive lesions was observed by adding edaravone. This result was consistent with the results of our pulse radiolysis experiments, in which G•8-OH and G•4-OH were scavenged by the antioxidants.
DISCUSSION
Reduction reaction of edaravone
The decay rate of absorbance at 320 and 600 nm depends on the concentrations of edaravone (Fig. 3), which indicates that the decay of the dGMP hydroxyl radical adducts is attributable to the reactions with edaravone. However, unlike the result for ascorbic acid (Fig. 4), the definite absorption bands of edaravone radicals that should be produced as a result of the reactions were not observed. In our previous report, one-electron oxidized edaravone radicals, the molecular coefficient of which at 345 nm was (2.60 ± 0.10) × 103 dm3 mol−1 cm−1 [29], were produced by reactions of edaravone with oxidizing radicals. The molecular coefficient of the edaravone radicals is almost the same as that of dGMP hydroxyl radical adducts, which is calculated from Fig. 2 using the yield of •OH in N2O-saturated solutions, 0.59 μmol J−1 [40], as the yield of dGMP hydroxyl radical adducts. Therefore, it was presumed these dGMP hydroxyl radical adducts were reduced by edaravone through electron transfer reactions, from which a one-electron oxidized edaravone radical was produced. This pattern of reaction is similar to that of ascorbic acid. Pulse radiolysis experiments showed that edaravone can reduce dGMP hydroxyl radical adducts through electron transfer reactions with high rate constants in the same manner as ascorbic acid.
Comparison of chemical repair activity of edaravone with that of ascorbic acid
In our previous report, 50–60% of the precursors of the enzyme-sensitive lesions were chemically repaired by ascorbic acid [16]. The results of the present experiment show that the chemical repair activity of edaravone on enzyme-sensitive lesions is lower than that of ascorbic acid. On the other hand, the values of p for both edaravone and ascorbic acid to SSBs were almost zero, indicating that SSBs are seldom repaired by either edaravone or ascorbic acid.
As discussed above, the pattern of reaction of edaravone to dGMP hydroxyl radical adducts and the rate constants were similar to those of ascorbic acid. However, the chemical repair activity of edaravone against the precursors of Fpg-sensitive lesions, which include 8oxoG, was relatively low. Several reasons can be suggested to explain this difference. First, the reactivity of edaravone with other precursors of Fpg-sensitive lesions might be different from that of ascorbic acid. This could be verified by pulse radiolysis experiments using other DNA model compounds, such as those used in previous experiments performed by Shi et al. [45]. Another reason could be the difference in accessibility to the precursors of lesions on DNA, which is attributable to the structures of the antioxidants. Measuring the chemical repair activity of derivatives of antioxidants might be a useful way to investigate their accessibility in terms of structure–activity relationships.
Some sulfhydryl compounds, such as glutathione, are also known to show chemical repair activity via hydrogen donation reactions [12, 41]. Applying the methods used in this study to sulfhydryl compounds might help improve our understanding of their chemical repair activity and provide some information about the differences observed in the chemical repair activities between antioxidants and sulfhydryl compounds.
CONCLUSIONS
The conclusions obtained from the pulse radiolysis experiment in dGMP solutions and the gamma ray irradiation experiment in plasmid DNA solutions are summarized as follows.
(i) Edaravone reduced dGMP hydroxyl radical adducts through electron transfer reactions, from which a one-electron oxidized edaravone radical was produced. The pattern of reaction and the rate constants were similar to those of ascorbic acid.
(ii) By adding edaravone into plasmid DNA solutions before gamma ray irradiation, Nth-, Fpg- and Nfo-sensitive lesions were suppressed. This phenomenon was attributed to the chemical repair reactions of edaravone. However, the suppression of SSBs was not effective.
(iii) The efficiency of the chemical repair reaction of edaravone on Nth- and Fpg-sensitive lesions was relatively lower than that of ascorbic acid. Further studies will be required to elucidate the reasons for this difference.
FUNDING
Funding to pay the Open Access publication charges for this article was provided by The University of Tokyo.
ACKNOWLEDGEMENTS
The authors are grateful to Mr Hiroishi of The University of Tokyo for assistance in operating the 60Co gamma ray equipment. The authors also thank Dr Fujii of the Japan Atomic Energy Agency for technical support to prepare the DNA samples.
REFERENCES
- 1.Ward JF, Blakely WF, Joner EI. Mammalian cells are not killed by DNA single-strand breaks caused by hydroxyl radicals from hydrogen peroxide. Radiat Res. 1985;103:383–92. [PubMed] [Google Scholar]
- 2.Hirayama R, Ito A, Tomita M, et al. Contributions of direct and indirect actions in cell killing by high-LET radiations. Radiat Res. 2009;171:212–8. doi: 10.1667/RR1490.1. [DOI] [PubMed] [Google Scholar]
- 3.Cabelli DE, Bleiski BHJ. Kinetics and mechanism for the oxidation of ascorbic acid/ascorbate by HO2/O2− radicals. A pulse radiolysis and stopped-flow photolysis study. J Phys Chem. 1983;87:1809–12. [Google Scholar]
- 4.Davies MJ, Forni LG, Willson RL. Vitamin E analogue Trolox C. E.s.r. and pulse-radiolysis studies of free-radical reactions. Biochem J. 1988;255:513–22. [PMC free article] [PubMed] [Google Scholar]
- 5.Priyadarsini KI, Maity DK, Naik GH, et al. Role of phenolic O-H and methylene hydrogen on the free radical reactions and antioxidant activity of curcumin. Free Radic Biol Med. 2003;35:475–84. doi: 10.1016/s0891-5849(03)00325-3. [DOI] [PubMed] [Google Scholar]
- 6.Bonifacic M, Ljubenkov I, Eckertmaksic M. One-electron oxidation and reduction reactions of vitamin C derivatives: 6-bromo and 6-chloro-6-deoxy-ascorbic acid. Int J Radiat Biol Relat Stud Phys Chem Med. 1994;66:123–31. doi: 10.1080/09553009414551021. [DOI] [PubMed] [Google Scholar]
- 7.Bors W, Michel C. Antioxidant capacity of flavanols and gallate esters: pulse radiolysis studies. Free Radic Biol Med. 1999;27:1413–26. doi: 10.1016/s0891-5849(99)00187-2. [DOI] [PubMed] [Google Scholar]
- 8.Erben-Russ M, Bors W, Saran M. Reactions of linoleic acid peroxyl radicals with phenolic antioxidants: a pulse radiolysis study. Int J Radiat Biol Relat Stud Phys Chem Med. 1987;52:393–412. doi: 10.1080/09553008714551871. [DOI] [PubMed] [Google Scholar]
- 9.Jovanovic SV, Hara Y, Steenken S, et al. Antioxidant potential of gallocatechins. A pulse radiolysis and laser photolysis study. J Am Chem Soc. 1995;117:9881–8. [Google Scholar]
- 10.Jovanovic SV, Steenken S, Tosic M, et al. Flavonoids as antioxidants. J Am Chem Soc. 1994;116:4846–51. [Google Scholar]
- 11.Lafleur MVM, Woldhuis J, Loman H. Effects of sulphydryl compounds on the radiation damage in biologically active DNA. Int J Radiat Biol Relat Stud Phys Chem Med. 1980;37:493–8. doi: 10.1080/09553008014550611. [DOI] [PubMed] [Google Scholar]
- 12.von Sonntag C. A Chemical Perspective. Berlin: Springer; 2006. Free-radical-induced DNA damage and its Repair. [Google Scholar]
- 13.Anderson RF, Amarasinghe C, Fisher LJ, et al. Reduction in free-radical-induced DNA strand breaks and base damage through fast chemical repair by flavonoids. Free Radic Res. 2000;33:91–103. doi: 10.1080/10715760000300651. [DOI] [PubMed] [Google Scholar]
- 14.Anderson RF, Fisher LJ, Hara Y, et al. Green tea catechins partially protect DNA from •OH radical-induced strand breaks and base damage through fast chemical repair of DNA radicals. Carcinogenesis. 2001;22:1189–93. doi: 10.1093/carcin/22.8.1189. [DOI] [PubMed] [Google Scholar]
- 15.Anderson RF, Fisher LJ, Harris T. The ‘pivotal antioxidant’ hypothesis for the role of flavonoids in their reduction of HO• radical-induced damage on DNA. Redox Rep. 2001;6:197–9. doi: 10.1179/135100001101536201. [DOI] [PubMed] [Google Scholar]
- 16.Hata K, Urushibara A, Yamashita S, et al. Chemical repair of base lesions, AP-sites, and strand breaks on plasmid DNA in dilute aqueous solution by ascorbic acid. Biochem Biophys Res Commun. 2013;434:341–5. doi: 10.1016/j.bbrc.2013.03.075. [DOI] [PubMed] [Google Scholar]
- 17.Hildenbrand K, SchulteFrohlinde D. Time-resolved EPR studies on the reaction rates of peroxyl radicals of poly(acrylic acid) and of calf thymus DNA with glutathione. Re-examination of a rate constant for DNA. Int J Radiat Biol Relat Stud Phys Chem Med. 1997;71:377–85. doi: 10.1080/095530097143996. [DOI] [PubMed] [Google Scholar]
- 18.Roots R, Okada S. Protection of DNA molecules of cultured mammalian cells from radiation-induced single-strand scissions by various alcohols and SH compounds. Int J Radiat Biol Relat Stud Phys Chem Med. 1972;21:329–42. doi: 10.1080/09553007214550401. [DOI] [PubMed] [Google Scholar]
- 19.Schultefrohlinde D, Behrens G, Onal A. Lifetime of peroxyl radicals of poly(U), poly(A) and single-stranded and double-stranded DNA and the rate of their reaction with thiols. Int J Radiat Biol Relat Stud Phys Chem Med. 1986;50:103–10. doi: 10.1080/09553008614550481. [DOI] [PubMed] [Google Scholar]
- 20.Amemiya S, Kamiya T, Nito C, et al. Anti-apoptotic and neuroprotective effects of edaravone following transient focal ischemia in rats. Eur J Pharmacol. 2005;516:125–30. doi: 10.1016/j.ejphar.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 21.Minhaz U, Tanaka M, Tsukamoto H, et al. Effect of MCI-186 on postischemic reperfusion injury in isolated rat heart. Free Radic Res. 1996;24:361–7. doi: 10.3109/10715769609088034. [DOI] [PubMed] [Google Scholar]
- 22.Mizuno A, Umemura K, Nakashima M. Inhibitory effect of MCI-186, a free radical scavenger, on cerebral ischemia following rat middle cerebral artery occlusion. Gen Pharmacol Vasc Syst. 1998;30:575–8. doi: 10.1016/s0306-3623(97)00311-x. [DOI] [PubMed] [Google Scholar]
- 23.Otomo E, Tohgi H, Kogure K, et al. Effect of a novel free radical scavenger, edaravone (MCI-186), on acute brain infarction. Randomized, placebo-controlled, double-blind study at multicenters. Cerebrovasc Dis. 2003;15:222–9. doi: 10.1159/000069318. [DOI] [PubMed] [Google Scholar]
- 24.Shichinohe H, Kuroda S, Yasuda H, et al. Neuroprotective effects of the free radical scavenger Edaravone (MCI-186) in mice permanent focal brain ischemia. Brain Res. 2004;1029:200–6. doi: 10.1016/j.brainres.2004.09.055. [DOI] [PubMed] [Google Scholar]
- 25.Tsujita K, Shimomura H, Kawano H, et al. Effects of edaravone on reperfusion injury in patients with acute myocardial infarction. Am J Cardiol. 2004;94:481–4. doi: 10.1016/j.amjcard.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto T, Yuki S, Watanabe T, et al. Delayed neuronal death prevented by inhibition of increased hydroxyl radical formation in a transient cerebral ischemia. Brain Res. 1997;762:240–2. doi: 10.1016/s0006-8993(97)00490-3. [DOI] [PubMed] [Google Scholar]
- 27.Zhang N, Komine-Kobayashi M, Tanaka R, et al. Edaravone reduces early accumulation of oxidative products and sequential inflammatory responses after transient focal ischemia in mice brain. Stroke. 2005;36:2220–5. doi: 10.1161/01.STR.0000182241.07096.06. [DOI] [PubMed] [Google Scholar]
- 28.Abe S, Kirima K, Tsuchiya K, et al. The reaction rate of edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one (MCI-186)) with hydroxyl radical. Chem Pharm Bull. 2004;52:186–91. doi: 10.1248/cpb.52.186. [DOI] [PubMed] [Google Scholar]
- 29.Lin M-Z, Katsumura Y, Hata K, et al. Pulse radiolysis study on free radical scavenger edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one) J Photochem Photobiol B. 2007;89:36–43. doi: 10.1016/j.jphotobiol.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Wang L-F, Zhang H-Y. A theoretical investigation on DPPH radical-scavenging mechanism of edaravone. Bioorg Med Chem Lett. 2003;13:3789–92. doi: 10.1016/j.bmcl.2003.07.016. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto Y, Kuwahara T, Watanabe K, et al. Antioxidant activity of 3-methyl-1-phenyl-2-pyrazolin-5-one. Redox Rep. 1996;2:333–8. doi: 10.1080/13510002.1996.11747069. [DOI] [PubMed] [Google Scholar]
- 32.Anzai K, Furuse M, Yoshida A, et al. In vivo radioprotection of mice by 3-methyl-1-phenyl-2-pyrazolin-5-one (Edaravone; Radicut®), a clinical drug. J Radiat Res. 2004;45:319–323. doi: 10.1269/jrr.45.319. [DOI] [PubMed] [Google Scholar]
- 33.Sasano N, Enomoto A, Hosoi Y, et al. Free radical scavenger edaravone suppresses X-ray-induced apoptosis through p53 inhibition in MOLT-4 cells. J Radiat Res. 2007;48:495–503. doi: 10.1269/jrr.07061. [DOI] [PubMed] [Google Scholar]
- 34.Hata K, Lin M-Z, Katsumura Y, et al. Pulse radiolysis study on free radical scavenger edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one). 2: A comparative study on edaravone derivatives. J Radiat Res. 2011;52:15–23. doi: 10.1269/jrr.10060. [DOI] [PubMed] [Google Scholar]
- 35.David SS, Wiliams SD. Chemistry of glycosylases and endonucleases involved in base-excision repair. Chem Rev. 1998;98:1221–61. doi: 10.1021/cr980321h. [DOI] [PubMed] [Google Scholar]
- 36.Haring M, Rudiger H, Demple B, et al. Recognition of oxidized abasic sites by repair endonucleases. Nucleic Acids Res. 1994;22:2010–5. doi: 10.1093/nar/22.11.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buxton GV, Stuart CR. Reevaluation of the thiocyanate dosimeter for pulse radiolysis. J Chem Soc Faraday Trans. 1995;91:279–81. [Google Scholar]
- 38.Spinks JWT, Woods RJ. Introduction to Radiation Chemistry. New York: John Wiley & Sons Inc; 1990. [Google Scholar]
- 39.Janata E, Schuler RH. Rate constant for scavenging eaq− in N2O-saturated solutions. J Phys Chem. 1982;86:2078–84. [Google Scholar]
- 40.Schuler RH, Patterson LK, Janata E. Yield for the scavenging of OH radicals in the radiolysis of N2O-saturated aqueous solutions. J Phys Chem. 1980;84:2088–9. [Google Scholar]
- 41.Willson RL, Wardman P, Asmus K-D. Interaction of dGMP radical with cysteamine and promethazine as possible model of DNA repair. Nature. 1974;252:323–4. doi: 10.1038/252323a0. [DOI] [PubMed] [Google Scholar]
- 42.O'Neill P. Pulse radiolytic study of the interaction of thiols and ascorbate with OH adducts of dGMP and dG: implications for DNA repair processes. Radiat Res. 1983;96:198–210. [PubMed] [Google Scholar]
- 43.Candeias LP, Steenken S. Reaction of HO• with guanine derivatives in aqueous solution: formation of two different redox-active OH-adduct radicals and their unimolecular transformation reactions. Properties of G(-H)•. Chem Eur J. 2000;6:475–84. doi: 10.1002/(sici)1521-3765(20000204)6:3<475::aid-chem475>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 44.Jones GDD, Boswell TV, Ward JF. Effects of postirradiation temperature on the yields of radiation-induced single-strand and double-strand breakage in SV40 DNA. Radiat Res. 1994;138:291–6. [PubMed] [Google Scholar]
- 45.Shi Y, Kang J, Lin W, et al. Fast repair of deoxynucleotide radical cations by phenylpropanoid glycosides (PPGs) and their analogs. Biochim Biophys Acta. 1999;1472:279–89. doi: 10.1016/s0304-4165(99)00133-6. [DOI] [PubMed] [Google Scholar]