Abstract
Existing therapies such as irradiation or sorafenib have limited success in the treatment of hepatocellular carcinoma (HCC) due to tumor recurrence and metastasis. Therefore, combination with other therapeutics is often considered. Macrophage inflammatory protein-1 alpha (MIP-1α) is a member of a family of chemoattractant cytokines that can induce the migration of monocytes, which in turn can play a role in fighting tumors. This study investigated whether intravenous injection of MIP-1α in conjunction with irradiation or sorafenib could enhance the antitumor effects on murine hepatoma. An HCa-I tumor was grown on the right thigh of each C3H/HeN mouse. Mice were then treated with 10 Gy of irradiation, sorafenib, or a combination of MIP-1α with either irradiation or sorafenib, and antitumor and antimetastatic effects were then investigated. To understand the mechanisms, changes in the level of immunological markers were also evaluated. Combination treatment of MIP-1α with irradiation or sorafenib resulted in a significant enhancement of antitumor effects, prevention of lung metastasis and increase in host survival. This was achieved by significantly increasing the levels of the immunological markers: Cluster Differentiation (CD) 8, CD107A and CD11C. We conclude that a combination treatment of MIP-1α with irradiation or sorafenib would be a useful strategy for management of hepatoma.
Keywords: hepatocellular carcinoma, metastasis, irradiation, sorafenib, MIP-1α
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most common cancer in men and seventh in women worldwide, and the global incidence of this malignancy is rising [1]. HCC is a devastating tumor that has a survival rate of < 5% over a 5-year period as a result of a high incidence of metastasis and recurrence [2, 3]. Close attention to metastasis to other organs is required in order to achieve effective HCC management, and surgery, chemotherapy and irradiation are widely used [4, 5]. However, the therapeutic outcome of these treatments remains unsatisfactory. In particular, irradiation and sorafenib, which are increasingly used in HCC, are not very effective at preventing metastasis [6, 7].
Irradiation uses high-energy beams to destroy cancer cells and is used as adjuvant therapy, either to prevent tumor recurrence after surgery or to remove a primary tumor [8, 9]. However, irradiation has to be used carefully in the treatment of HCC because liver tissues are highly susceptible to radiation damage [10, 11]. Moreover, some metastatic HCC can recur when cancer stem cells are resistant to irradiation. Recent studies have shown a survival benefit from sorafenib, making it the new standard therapy for patients with advanced HCC [12]. However, the survival benefit is only a few months. Additionally, many patients require dosage reduction or cessation of treatment because of adverse effects of the drug [13]. A recent study has even indicated that treatment with sorafenib contributes to increased invasiveness and metastatic potential in orthotopic HCC models and cell lines [14]. Therefore, combination treatments of existing therapies with novel chemotherapeutics are being investigated in an attempt to overcome these problems [15, 16].
Macrophage inflammatory protein-1 alpha (MIP-1α) is a member of a family of chemotactic cytokines. MIP-1α have been implicated in immune reactions. MIP-1α induces the migration of T cells and monocytes, and the monocytes can then differentiate into immune cells such as macrophages or dendritic cells (DCs). Additionally, MIP-1α-recruited DCs have therapeutic efficacy on metastatic tumors [17], and fission yeast-generated MIP-1α, now known as ECI-301, enhances the anti-tumor efficacy of irradiation via recruitment of CD8-positive T cells and NK cells in lung and colon cancers [18].
In this study, we investigated whether the combination of recombinant MIP-1α with irradiation or sorafenib treatment enhances antitumor effects and host survival in a system of murine lung metastatic hepatoma.
MATERIALS AND METHODS
Animals and tumors
The animal facilities were approved by the Association of Assessment and Accreditation of Laboratory Animal Care (AAALAC), and all experiments were performed under the institutional guidelines established by the Institutional Animal Care and Use Committee at Yonsei University (IACUC-2012-0177). Male C3H/HeN mice aged 6–7 weeks old were purchased from Orient (Seong-nam, South Korea). All mice were raised with free access to food and water under specific pathogen-free conditions in a room maintained on a 12-h light/dark cycle. HCa-Ι is a murine hepatoma was used for tumor growth. Heterotopic tumor models were generated by injecting 1 × 106/100 μl of tumor cells into the right thigh.
Compounds
Sorafenib [N-(3-trifluoromethyl-4-chlorophenyl)-N'-(4-(2-methylcarbamoyl pyridin-4-yl)oxyphenyl)urea] was synthesized at Bayer Corporation (West Haven, CT, USA). Sorafenib in 100% DMSO was mixed with an aqueous solution containing 8.75% ethanol and 12.5% Chremophor EL (30 mg/kg, peroral, Sigma, St Louis, MO). Human recombinant MIP-1α was purchased from Sigma (St Louis, MO, USA) and dissolved in phosphate-buffered saline (PBS, 2 μg/30 ml, intravenous), as described previously([19].
Irradiation
Tumor irradiation was accomplished by placing animals in an acrylic chamber that immobilized the right leg without the use of anesthesia. Lead shields were used to avoid irradiation of other body parts. Tumors were irradiated with an X-ray irradiator (X-RAD 320, Precision X-Ray) using 2.0-mm Al filtration (300 kVp) at a dose of 10 Gy.
Hematoxylin and eosin (H & E) staining
The tumors were harvested after euthanasia of mice and were fixed using a 4% paraformaldehyde solution. Histological evaluation was conducted by means of H & E staining. All of the stained tissue sections were analyzed using a virtual microscope (Olympus BX51, Japan) and Olyvia® software.
Lung metastasis assays
Mouse lungs were harvested at 20 days after first irradiation. All extractions were performed after euthanasia. Bouin's solution was prepared using 75 ml saturated aqueous solution of picric acid and 25 ml of 40% aqueous formalin (Sigma, St Louis, MO). Lungs were fixed by submersion in Bouin's solution for 6 h, and fixed lungs were transferred to 70% ethanol. The number and mean size of lung nodules were analyzed.
Immunofluorescence
Immunofluorescence was performed according to the previously published procedure [20]. Tumor tissues were collected from mice under deep anesthesia with pentobarbital (50 mg/kg, intraperitoneal, Sigma, St Louis, MO, USA). Tumor samples were fixed in 10% formalin and embedded in paraffin. The paraffin-embedded blocks were cut into 5-μm-thick sections. For immunofluorescent staining, deparaffinized samples were blocked with 10% normal horse serum for 1 h and then incubated with primary antibody against CD11c (ArHm mAb CD11c, 1:100, Abcam, CA, USA). Antigen retrieval was accomplished at 37°C by protease K solution. The samples were incubated with ArHm IgG antibody (Abcam, CA, USA). These were biotinylated and conjugated with streptavidin-HRP (DAKO code K0675; DAKO Corp., Carpinteria, CA, USA). Sections were incubated with anti-rabbit secondary biotinylated antibody and visualized with streptavidin conjugated to Fluorescein (460 nm) (Vector, Burlingame, CA, USA).
Western blot analysis
Tumor tissues were collected from mice under deep anesthesia, as mentioned above. Tumor tissues were chopped into 4-mm2 samples, and biopsies were homogenized in tissue protein lysis buffer (Pierce, Rockford, IL). Homogenates were centrifuged (13 000 rpm, 5 min), and protein supernatant was collected. Total protein concentration was determined using a BCA assay (Pierce, Rockford, IL). For western blots, 20 μg/30 μl protein extracts were separated by electrophoresis on 12% polyacrylamide gels and electrotransferred to nitrocellulose membranes (GE Life sciences, Pittsburgh, PA, USA). Membranes were blocked for 1 h in 5% skim milk at room temperature and incubated overnight at 4°C with the antibodies: Cluster Differentiation (CD) 8 (27 kDa) or CD107a (43 kDa) (1:500, Bioss, Woburn, MA). Membranes were washed three times with TBST and incubated for 1 h at room temperature in 1:1000 diluted goat anti-rabbit IgG HRP conjugate (Bioss, Woburn, MA, USA). After three washes, blots were developed using chemiluminescent peroxidase substrate (Sigma–Aldrich, St Louis, MO, USA) and exposed using X-ray film and developer (Agfa, Mortsel, Belgium). Computer-assisted analysis of the bands was performed with the ImageJ program (National Institutes of Health, USA).
Immunohistochemistry
We immunostained 5-µm sections of the tumor tissues embedded in paraffin using the ABC technique (Vector Elite Kit, Vector, Burlingame, CA). Sections were incubated over two nights at 4°C with monoclonal mouse anti-CD8, CD107A and proliferating cell nuclear antigen (PCNA) (1:1000, Chemicon, CA) and rinsed with 0.05M phosphate buffer saline (PBS) and 3% NGS. Then, sections were incubated with the secondary biotinylated antibody (1:200, Vector, Burlingame, CA) and reacted with 0.5 mg/ml 3,3'-diaminobenzidine tetrahydrochloride (DAB, Vector, Burlingame, CA). The DAB reactions showed under the light microscope, and the number of stained cells was quantified by stereological analysis using Image-J software (National Institutes of Health, USA).
Statistical analysis
All data are presented as mean ± standard error of the mean (SEM). Statistical significance was analyzed using Student's t-test or the Mann–Whitney rank sum test, depending on the normality of the data. A difference of P < 0.05 was considered to be statistically significant. All statistical analyses were carried out using Sigma Stat (ver. 3.5, Systat Software Inc., Chicago, IL).
RESULTS
Existing therapies produce antitumor effects in murine hepatoma but do not prevent lung metastasis
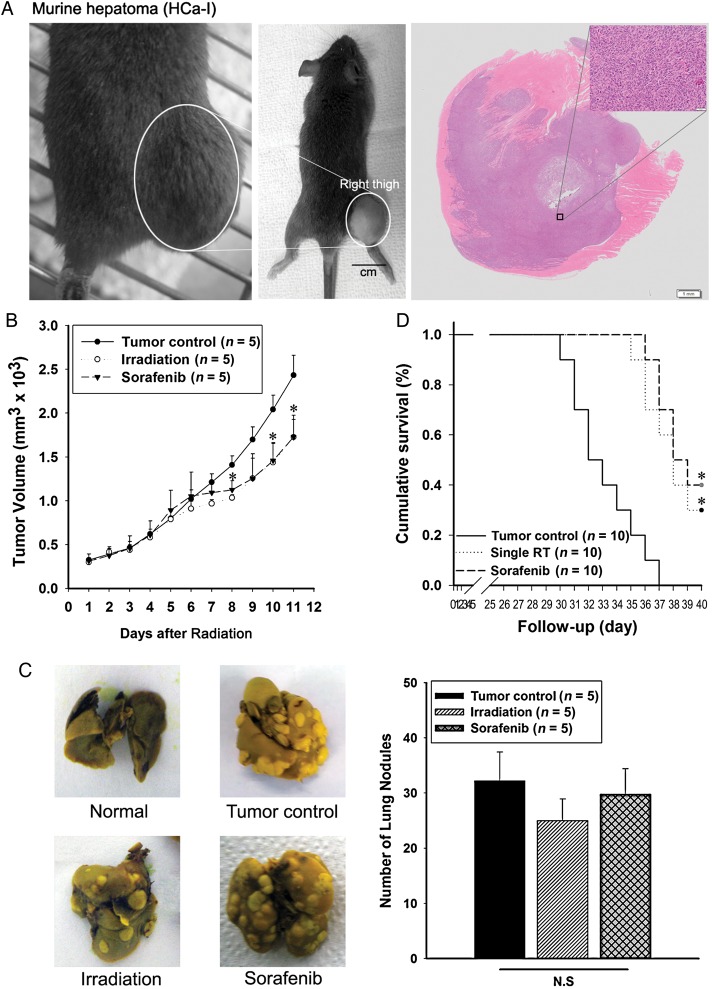
Heterotopic hepatomas grew on the right thigh of each mouse, and H & E staining indicated their malignancy (Fig. 1A). To evaluate the in vivo anticancer effects of existing therapies, we investigated tumor volume and host survival. The number of lung nodules was determined in order to investigate metastatic effects. Tumor volume was calculated as previously described [18]. Tumor growth was significantly inhibited by irradiation and also by sorafenib (Fig. 1B). However, lung nodules were not reduced by the existing therapies. There was no significant difference when compared with the tumor control group mice (Fig. 1C). When all mice in the tumor control group had died, 30% of mice still survived in the irradiation treatment group and 40% in the sorafenib treatment group (Fig. 1D).
Fig. 1.
Comparison of anti-tumor effects on murine hepatoma from irradiation or sorafenib. (A) Heterotopic growth of murine hepatoma (left) and H&E staining of tumor sections (right, 5 μm). (B) Treatment effect of irradiation or sorafenib on tumor growth. (C) Number of metastatic lung nodules visualized (left) and quantified (right graphs). (D) Survival rate was analyzed by a log-rank test based on the Kaplan–Meier method. A single asterisk indicates P < 0.05 compared with the tumor control. Results are presented as mean ± S.E.M.
Strategies for combining MIP-1α with existing therapies to treat murine hepatoma
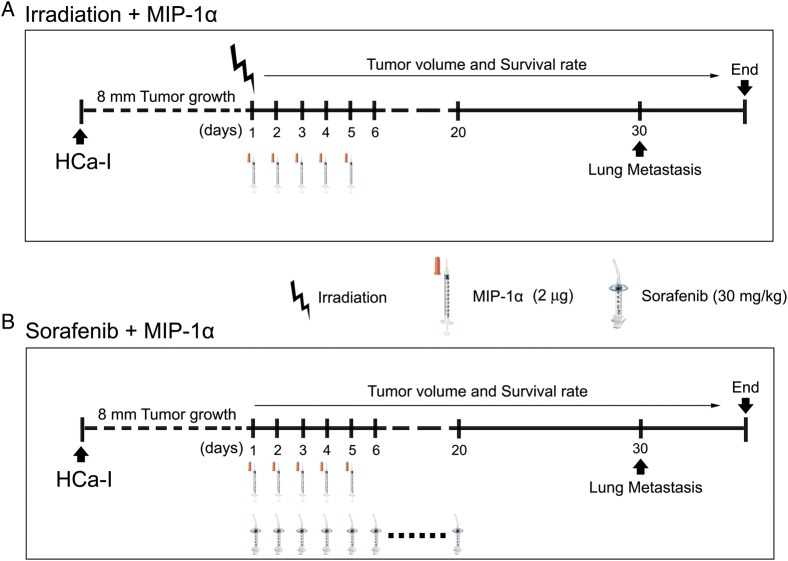
HCa-Ι cells (1 × 106/mouse) were injected into the right thigh of each mouse, and each treatment was performed to 8-mm size of HCa-Ι tumor (Fig. 2A and B). The tumor was irradiated with 10 Gy in a single dose on the first day of the experiment (Fig. 2A). Sorafenib was given perorally for 20 days from the first day of the experiment (Fig. 2B). MIP-1α was intravenously injected between the first and fifth days of the experiment (Fig. 2A and B). Tumor volume and survival rate were measured from the first day of the experiment until 3 days after all mice in the tumor control group had died. The lungs of the mice in each experimental group were harvested on experimental Day 30 for lung metastatic analysis (Fig. 2A and B).
Fig. 2.
Schematic representation of experimental scheme for the effect of combination treatment of MCP-1α with irradiation or sorafenib on murine hepatoma. (A) Combination treatment of irradiation with MIP-1α. (B) Combination treatment of sorafenib with MIP-1α.
Combination of MIP-1α with existing therapies increased antitumor efficacy, decreased lung metastasis and increased host survival
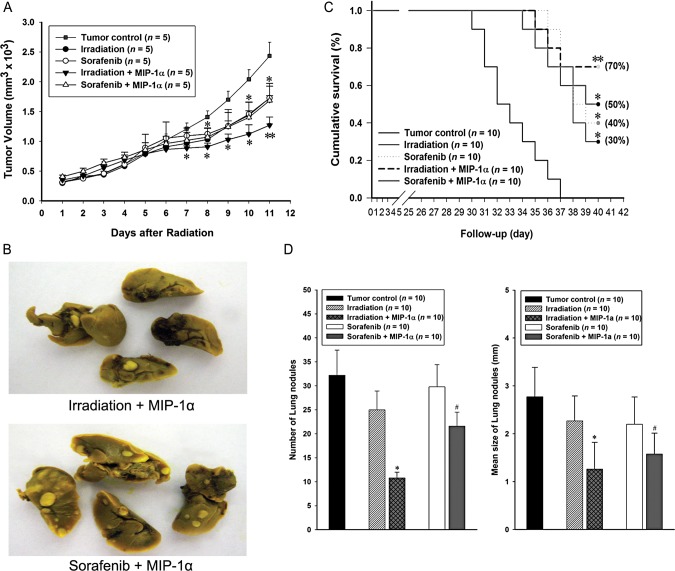
To assess the in vivo the antitumor effects of combination treatments, we investigated tumor number and volume and host survival. The number of lung nodules was determined in order to investigate metastatic effects. Using a combination treatment of sorafenib with MIP-1α (sorafenib + MIP-1α), the tumor volume significantly decreased by ∼35% compared with the tumor control group. And there was no significant difference, compared with a single treatment with sorafenib alone. The combination treatment of irradiation with MIP-1α (irradiation + MIP-1α) significantly suppressed tumor growth by ∼56% compared with the tumor control group and by ∼34% compared with a single treatment with irradiation alone. (Fig. 3A). Lung nodules were also significantly reduced by combination treatments. The number of lung nodules were reduced in the (irradiation + MIP-1α) and in the (sorafenib + MIP-1α) groups by ∼34% compared with the tumor control group (Fig. 3D, left). The mean size of lung nodules also decreased by ∼55% in the (irradiation + MIP-1α) group and by ∼43% in the (sorafenib + MIP-1α) group compared with the tumor control group (Fig. 3D, right). All lung nodule data indicated that combination treatment with MIP-1α produced a better outcome compared with usage of the existing therapies singly (Fig. 3D). When all mice in the tumor control were dead, surviving mice remained in the combined treatment groups. The final survival rate was 50% in the (sorafenib + MIP-1α) group, but the (irradiation + MIP-1α) group demonstrated the greatest rate of survival at 70% (Fig. 3C).
Fig. 3.
Comparison of antitumor effects on murine hepatoma from combination treatment of MCP-1α with irradiation or sorafenib. (A) Effect of combination treatment of MIP-1α with irradiation or sorafenib on tumor growth. (B) Visualization of metastatic lung nodules after combination treatments. (C) Survival rate was analyzed by a log-rank test based on the Kaplan–Meier method. A single asterisk indicates P < 0.05 compared with the tumor control; two asterisks indicate P < 0.01 comparing the tumor control group with the (sorafenib + MIP-1α) group. (D) The number (left graph) and mean size (right graph) of lung nodules were quantified. A single asterisk indicates P < 0.01 compared with the tumor control and the irradiation groups; hash indicates P < 0.01 compared with the tumor control and the sorafenib groups. Results are presented as mean ± S.E.M.
Combination treatment of MIP-1α with existing therapies increased dendritic cell infiltration in tumor
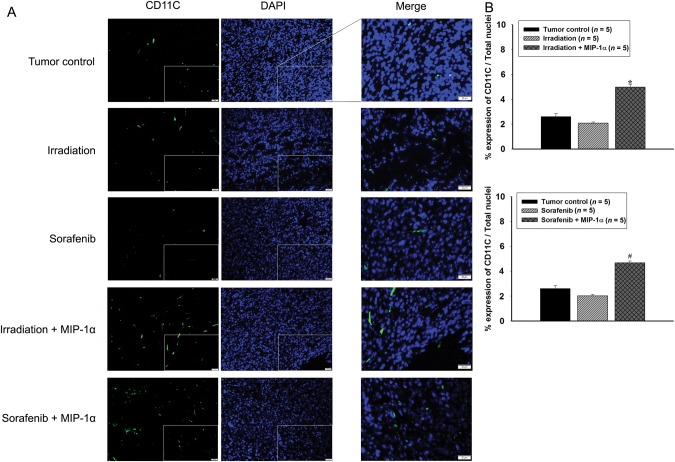
To investigate the chemoattractant effects of MIP-1α, we evaluated the infiltration of DCs into tumors by combination of MIP-1α treatment with existing therapies. DCs were confirmed by immunofluorescence staining using the CD11C marker (Fig. 4A). Quantitative analyses indicated that combination of MIP-1α treatment with irradiation or sorafenib significantly induced DC recruitment into tumors compared with the tumor control or single treatment with irradiation or sorafenib (Fig. 4B).
Fig. 4.
Comparison of dendritic cell infiltration into tumors. (A) Images at × 400 magnification. Square box in images indicates merged site. Scale bars, 20 μm. (B) Quantification analyses of CD11C expression. Single asterisk indicates P < 0.01 compared with the tumor control and the irradiation groups; hash indicates P < 0.01 compared with the tumor control and the sorafenib groups. Results are presented as mean ± S.E.M.
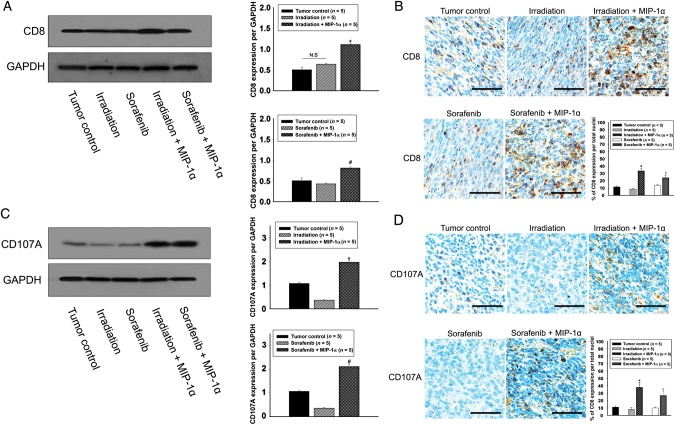
Combination of MIP-1α treatment with existing therapies increased CD8 and CD107A expression in tumors
To investigate the relative mechanism for the infiltration of DCs, western blot and immunohistochemical analyses were performed to investigate the expression of CD8 and CD107A in the tumor (Fig. 5). The single treatments with irradiation or sorafenib did not change the levels of CD8 protein or positive cells compared with the tumor control (Fig. 5A and B), however they decreased CD107A expression compared with the tumor control (Fig. 5C). The CD8 protein level and CD8-positive cells were significantly increased ∼2- and 3-fold after combination treatment of MIP-1α with irradiation or sorafenib compared with the tumor control (Fig. 5A and B). The CD107A protein level and CD107-positive cells were significantly increased ∼2- and 3.5-fold after combination treatment of MIP-1α with irradiation or sorafenib compared with the tumor control (Fig. 5C and D).
Fig. 5.
Determination of CD8 and CD107A expression. (A) Western blot analysis of CD8 expression in the tumor. (B) Immunohistochemical analysis of CD8-positive cells. Scale bars, 200 μm. (C) Western blot analysis of CD107A expression in the tumor. (D) Immunohistochemical analysis of CD107A-positive cells. Scale bars, 200 μm. Single asterisk indicates P < 0.01 compared with the tumor control and the irradiation groups; hash indicates P < 0.01 compared with the tumor control and the sorafenib groups. Results are presented as mean ± S.E.M.
DISCUSSION
Our results demonstrated that combination of MIP-1α treatment with irradiation or sorafenib enhanced the antitumor effects on murine hepatoma. Radiotherapy and chemotherapy are considered as helpful methods for management of human HCC [21, 22]. Irradiation is used treat a primary tumor or to prevent tumor recurrence after surgery [8, 9], while sorafenib has been widely recognized, among the various anticancer drugs, as a suitable management drug for HCC. Recent research has indicated the survival benefits of sorafenib [12].
Many patients with HCC in an advanced stage have been given radiation therapy [23]. It has been reported that some metastatic HCC can occur when cancer stem cells are resistant to irradiation [24, 25]. Sorafenib has been demonstrated to suppress tumor cell proliferation and angiogenesis by inhibiting serine/threonine kinases [26, 27]. However, sorafenib also influences human peripheral blood T-cell in vitro. Sorafenib brings down the immune response mediated by T-cells in rodents [28, 29]. These recent results clearly indicate that treatment with sorafenib contributes to increased invasiveness and metastatic potential in orthotopic HCC models and cell lines [14].
We investigated the disadvantages of existing therapies in a model of murine heterotopic hepatoma. HCa-Ι has radioresistant and lung metastatic features and was established from murine hepatoma grown spontaneously in C3H/HeN mice [30]. Although existing therapies suppress tumor growth and increase the survival rate, they have limitations in the management of HCC. In particular, lung metastasis is not inhibited by existing therapies, as indicated by our results.
MIP-1α is a chemokine in the CC subfamily of chemokines with the ability to induce a number of types of hematopoietic cells, particularly in those involved in adaptive immune responses such as macrophages, DCs and T lymphocytes [31]. In previous animal studies combining irradiation treatment with a recombinant MIP-1α variant (ECI301), antitumor effects on lung carcinoma (together with systemic effects) were demonstrated [18]. We developed these strategies using a combination treatment of MIP-1α with either irradiation or sorafenib and demonstrated improvement over existing therapies and increase in the antitumor effects in hepatoma.
Administration of MIP-1α effectively recruited DCs into the peripheral blood, and these recruited DC were able to generate sufficient cell numbers for the DC-based antitumor effects. MIP-1α-recruited DC can be used for eliminating tumors and preventing metastasis [17]. When DCs recognize an antigen, they stimulate T-cell responses via their potent antigen-presenting capacity [32].
Solid tumors are thought to contain cancer stem cells (CSCs) as a distinct population, and it is believed that these are responsible for tumor relapse and metastasis as a consequence of their abilities to self-renew, differentiate, and give rise to a new tumor in local or distant organs. CSCs have been identified in many tumor types, including HCC [33]. CSCs can escape the toxic effects of chemotherapy through an assortment of mechanisms. There are a number of signaling pathways that have been demonstrated to contribute to chemoresistance, such as the notch signaling pathway [34]. Based on findings that cancer cell clonogens exhibit stem cell features, it has been suggested that cancer stem-like cells are relatively radioresistant owing to a range of intrinsic and extrinsic factors, including enhanced DNA repair, or hypoxia and interaction with stromal elements [35]. Since DNA polymerase epsilon is involved in the resynthesis of excised damaged DNA strands during DNA repair, PCNA is important for both DNA synthesis and DNA repair. The combination treatments significantly decreased PCNA expression in the primary tumor (Supplementary Fig. 1). In addition, the increase in MIP-1α-recruited DCs could have a major role in triggering tumor-specific T-cell responses [17]. It is widely known that CD8-positive cytotoxic T-cells recognize and kill stem-like cells [36].
In our results, the combination of MIP-1α treatment with existing therapies significantly inhibited lung metastasis. The antitumor effect and host survival were also enhanced. We have reasserted that infiltration of DCs into the tumor is increased by MIP-1α. CD107a is a vesicle membrane protein that becomes transiently mobilized to the cell surface during this degranulation [37]. Confirmation of CD107a expression is utilized to distinguish and isolate functional tumor-reactive T-cellular telephones with high recognition efficiency directly of peripheral blood mononuclear cells of cancer patients. The expression of CD107a can be applied to isolate tumor-cytolytic T cells. CD107a moblization correlates well with the cytotoxic activity of CD8-positive T cells [38]. The expression of CD107a rather decreased in the tumor after single treatment with existing therapies, however CD107a significantly increased in the tumor after the combination of MIP-1α treatment with existing therapies. Activation of CD8-positive T lymphocytes stimulated by MIP-1α-recruited DCs would enable efficient recognition of stem-like cancer cells [39].
In conclusion, we demonstrated that the combination of MIP-1α treatment with existing therapies (such as irradiation or sorafenib) for hepatoma enhanced antitumor activity, particularly the inhibition of lung metastasis. These effects were brought about by cytotoxic CD8-positive T-cell activation resulting from the interaction of T-cells with DCs recruited by MIP-1α. Therefore, our findings indicate that the combination of MIP-1α treatment with existing therapies is a potential strategy for treating metastatic hepatoma.
SUPPLEMENTARY DATA
Supplementary data are available at the Journal of Radiation Research online.
FUNDING
Funding to pay the Open Access publication charges for this article was provided by the National Nuclear R&D Program through a National Research Foundation of Korea (NRF) grant (2010-001854) funded by the Ministry of Education, Science and Technology. This study was also supported by a grant from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A121982).
Supplementary Material
REFERENCES
- 1.Barazani Y, Hiatt JR, Tong MJ, et al. Chronic viral hepatitis and hepatocellular carcinoma. World J Surg. 2007;31:1243–8. doi: 10.1007/s00268-007-9041-3. [DOI] [PubMed] [Google Scholar]
- 2.Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000;232:10–24. doi: 10.1097/00000658-200007000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou X. Recurrence and metastasis of hepatocellular carcinoma: progress and prospects. Hepatobiliary Pancreat Dis Int. 2002;1:35–41. [PubMed] [Google Scholar]
- 4.Lin S, Hoffmann K, Schemmer P. Treatment of hepatocellular carcinoma: a systematic review. Liver Cancer. 2012;1:144–58. doi: 10.1159/000343828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dawson LA, Guha C. Hepatocellular carcinoma: radiation therapy. Cancer J. 2008;14:111–6. doi: 10.1097/PPO.0b013e31816a0e80. [DOI] [PubMed] [Google Scholar]
- 6.Keating GM, Santoro A. Sorafenib: a review of its use in advanced hepatocellular carcinoma. Drugs. 2009;69:223–40. doi: 10.2165/00003495-200969020-00006. [DOI] [PubMed] [Google Scholar]
- 7.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. New Engl J Med. 2008;359:378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 8.Bujold A, Dawson LA. Stereotactic radiation therapy and selective internal radiation therapy for hepatocellular carcinoma. Cancer Radiother. 2011;15:54–63. doi: 10.1016/j.canrad.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Huang WY, Jen YM, Lee MS, et al. Stereotactic body radiation therapy in recurrent hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2012;84:355–61. doi: 10.1016/j.ijrobp.2011.11.058. [DOI] [PubMed] [Google Scholar]
- 10.Stone HB, Coleman CN, Anscher MS, et al. Effects of radiation on normal tissue: consequences and mechanisms. Lancet Oncol. 2003;4:529–36. doi: 10.1016/s1470-2045(03)01191-4. [DOI] [PubMed] [Google Scholar]
- 11.Willemart S, Nicaise N, Struyven J, et al. Acute radiation-induced hepatic injury: evaluation by triphasic contrast enhanced helical CT. Br J Radiol. 2000;73:544–6. doi: 10.1259/bjr.73.869.10884753. [DOI] [PubMed] [Google Scholar]
- 12.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 13.Desar IM, Mulder SF, Stillebroer AB, et al. The reverse side of the victory: flare up of symptoms after discontinuation of sunitinib or sorafenib in renal cell cancer patients. A report of three cases. Acta Oncol. 2009;48:927–31. doi: 10.1080/02841860902974167. [DOI] [PubMed] [Google Scholar]
- 14.Zhang W, Sun HC, Wang WQ, et al. Sorafenib down-regulates expression of HTATIP2 to promote invasiveness and metastasis of orthotopic hepatocellular carcinoma tumors in mice. Gastroenterology. 2012;143:1641–9. doi: 10.1053/j.gastro.2012.08.032. e5. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Jin Y, Zhang Z. Radiotherapy and intra-arterial chemotherapy of locally advanced hepatocellular carcinoma. Analysis of prognostic factors. Cancer Radiother. 2000;4:191–6. doi: 10.1016/s1278-3218(00)89093-9. [DOI] [PubMed] [Google Scholar]
- 16.Kawashita Y, Ohtsuru A, Kaneda Y, et al. Regression of hepatocellular carcinoma in vitro and in vivo by radiosensitizing suicide gene therapy under the inducible and spatial control of radiation. Hum Gene Ther. 1999;10:1509–19. doi: 10.1089/10430349950017842. [DOI] [PubMed] [Google Scholar]
- 17.Cao Q, Jin Y, Jin M, et al. Therapeutic effect of MIP-1alpha-recruited dendritic cells on preestablished solid and metastatic tumors. Cancer Lett. 2010;295:17–26. doi: 10.1016/j.canlet.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 18.Shiraishi K, Ishiwata Y, Nakagawa K, et al. Enhancement of antitumor radiation efficacy and consistent induction of the abscopal effect in mice by ECI301, an active variant of macrophage inflammatory protein-1alpha. Clin Cancer Res. 2008;14:1159–66. doi: 10.1158/1078-0432.CCR-07-4485. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Yoneyama H, Wang Y, et al. Mobilization of dendritic cell precursors into the circulation by administration of MIP-1alpha in mice. J Natl Cancer Inst. 2004;96:201–9. doi: 10.1093/jnci/djh024. [DOI] [PubMed] [Google Scholar]
- 20.Baschong W, Suetterlin R, Laeng RH. Control of autofluorescence of archival formaldehyde-fixed, paraffin-embedded tissue in confocal laser scanning microscopy (CLSM) J Histochem Cytochem. 2001;49:1565–72. doi: 10.1177/002215540104901210. [DOI] [PubMed] [Google Scholar]
- 21.Matsuura M, Nakajima N, Arai K, et al. The usefulness of radiation therapy for hepatocellular carcinoma. Hepatogastroenterology. 1998;45:791–6. [PubMed] [Google Scholar]
- 22.Kelley RK, Venook AP. Sorafenib in hepatocellular carcinoma: separating the hype from the hope. J Clin Oncol. 2008;26:5845–8. doi: 10.1200/JCO.2008.19.7996. [DOI] [PubMed] [Google Scholar]
- 23.Ursino S, Greco C, Cartei F, et al. Radiotherapy and hepatocellular carcinoma: update and review of the literature. Eur Rev Med Pharmacol Sci. 2012;16:1599–604. [PubMed] [Google Scholar]
- 24.Vlashi E, McBride WH, Pajonk F. Radiation responses of cancer stem cells. J Cell Biochem. 2009;108:339–42. doi: 10.1002/jcb.22275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kickingereder P, Dorn F, Blau T, et al. Differentiation of local tumor recurrence from radiation-induced changes after stereotactic radiosurgery for treatment of brain metastasis: case report and review of the literature. Radiat Oncol. 2013;8:52. doi: 10.1186/1748-717X-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 27.Carlomagno F, Anaganti S, Guida T, et al. BAY 43-9006 inhibition of oncogenic RET mutants. J Natl Cancer Inst. 2006;98:326–34. doi: 10.1093/jnci/djj069. [DOI] [PubMed] [Google Scholar]
- 28.Zhao W, Gu YH, Song R, et al. Sorafenib inhibits activation of human peripheral blood T cells by targeting LCK phosphorylation. Leukemia. 2008;22:1226–33. doi: 10.1038/leu.2008.58. [DOI] [PubMed] [Google Scholar]
- 29.Hipp MM, Hilf N, Walter S, et al. Sorafenib, but not sunitinib, affects function of dendritic cells and induction of primary immune responses. Blood. 2008;111:5610–20. doi: 10.1182/blood-2007-02-075945. [DOI] [PubMed] [Google Scholar]
- 30.Kim W, Seong J, An JH, et al. Enhancement of tumor radioresponse by wortmannin in C3H/HeJ hepatocarcinoma. J Radiat Res. 2007;48:187–95. doi: 10.1269/jrr.06077. [DOI] [PubMed] [Google Scholar]
- 31.Schall TJ, Bacon K, Camp RD, et al. Human macrophage inflammatory protein alpha (MIP-1 alpha) and MIP-1 beta chemokines attract distinct populations of lymphocytes. J Exp Med. 1993;177:1821–6. doi: 10.1084/jem.177.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ni K, O'Neill HC. The role of dendritic cells in T cell activation. Immunol Cell Biol. 1997;75:223–30. doi: 10.1038/icb.1997.35. [DOI] [PubMed] [Google Scholar]
- 33.Ji J, Wang XW. Clinical implications of cancer stem cell biology in hepatocellular carcinoma. Semin Oncol. 2012;39:461–72. doi: 10.1053/j.seminoncol.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdullah LN, Chow EK. Mechanisms of chemoresistance in cancer stem cells. Clin Transl Med. 2013;2:3. doi: 10.1186/2001-1326-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hittelman WN, Liao Y, Wang L, et al. Are cancer stem cells radioresistant? Future Oncol. 2010;6:1563–76. doi: 10.2217/fon.10.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pang RW, Poon RT. Cancer stem cell as a potential therapeutic target in hepatocellular carcinoma. Curr Cancer Drug Targets. 2012;12:1081–94. doi: 10.2174/156800912803987995. [DOI] [PubMed] [Google Scholar]
- 37.Betts MR, Koup RA. Detection of T-cell degranulation: CD107a and b. Methods Cell Biol. 2004;75:497–512. doi: 10.1016/s0091-679x(04)75020-7. [DOI] [PubMed] [Google Scholar]
- 38.Aktas E, Kucuksezer UC, Bilgic S, et al. Relationship between CD107a expression and cytotoxic activity. Cell Immunol. 2009;254:149–54. doi: 10.1016/j.cellimm.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 39.Hirohashi Y, Torigoe T, Inoda S, et al. Cytotoxic T lymphocytes: sniping cancer stem cells. Oncoimmunology. 2012;1:123–5. doi: 10.4161/onci.1.1.18075. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.