Abstract
Impaired ocular blood flow is involved in the pathogenesis of numerous ocular diseases like glaucoma or AMD. The purpose of the present study was to introduce and validate a novel, microscope based, non invasive laser Doppler flowmeter (NILDF) for measurement of blood flow in the choroid. The custom made NI-LDF was compared with a commercial fiber optic based laser Doppler flowmeter (Perimed PF4000). Linearity and stability of the NI-LDF were assessed in a silastic tubing model (i.d. 0.3 mm) at different flow rates (range 0.4 – 3 ml/h). In a rabbit model continuous choroidal blood flow measurements were performed with both instruments simultaneously. During blood flow measurements ocular perfusion pressure was changed by manipulations of intraocular pressure via intravitreal saline infusions. The NILDF measurement correlated linearly to intraluminal flow rates in the perfused tubing model (r = 0.99, p<0.05) and remained stable during a 1 hour measurement at a constant flow rate. Rabbit choroidal blood flow measured by the PF4000 and the NI-LDF linearly correlated with each other over the entire measurement range (r = 0.99, y = x* 1,01 – 12,35 P.U., p < 0,001). In conclusion, the NI-LDF provides valid, semi quantitative measurements of capillary blood flow in comparison to an established LDF instrument and is suitable for measurements at the posterior pole of the eye.
Keywords: Laser Doppler Flowmetry, Blood Flow, Eye, Choroid
1. Introduction
Laser Doppler flowmetry (LDF1) provides a continuous measurement of capillary blood flow. Its validity has been verified in mechanical models and in vivo by comparison with other established methods (e.g. microspheres, hydrogen clearance, Xenon isotope washout, isolated organ perfusion etc.) in many tissues (Kvietys, Shepherd et al. 1985; Smits, Roman et al. 1986; Kastrup, Bulow et al. 1987; Shepherd, Riedel et al. 1987; Neufeld, Galante et al. 1988; Wheatley, Almond et al. 1993; Rival, Bance et al. 1995). In eye research, Laser Doppler velocimetry was introduced by Charles Riva and coworkers (Tanaka, Riva et al. 1974) and subsequently LDF has been successfully established as a validated method to measure blood flow in the choroid, optic nerve head, ciliary body and iris (Kiel and Shepherd 1992; Riva, Cranstoun et al. 1994b; Riva and Petrig 1995; Chamot, Movaffaghy et al. 1999; Reitsamer and Kiel 2003)For the assessment of choroidal blood flow in animal models, many investigators have used LDF instruments with fiber optic based designs and intraocular probe placements in different species. Depending on the size relation between the probe and the eye, this approach could potentially disturb biological parameters relevant to the experiment (e.g. intraocular pressure, inflammatory responses) or introduce unwanted variability in the experimental setup, i.e. the location of the probe might not be controlled precisely and changes in position or the shape and size of a fiber optic probe per se restrict the freedom of choosing a suitable measurement site. While this was not a major concern in the rabbit eye model used by the authors of this manuscript, where the volume of the probe introduced into the vitreous is roughly 0.04% of the total eye volume, it seems plausible, that in a rodent eye (rat: 3.5%, mouse: 11%) the displaced volume is much larger and consequently of more concern to disturb the environment.
Even though non-invasive designs for an LDF instrument have been developed, to our knowledge these instruments are based on the optical setup of a fundus camera (Riva, Cranstoun et al. 1994a). The optics of a fundus camera is designed for the human eye and precludes its use in some widely used (small) animal models.
Here we present the design and evaluation of a new, non-invasive laser Doppler flowmeter suitable for animal research.
2. Materials and Supplies
The NI-LDF presented is intended for the use in animal experiments, hence, an appropriate setup for the species under investigation is needed. A detailed description for the use in rabbit experiments is given in section 4.4. As the LDF technique is sensitive to all motion within the measurement site, regardless of its origin, a solid fixation of the animal is necessary. This is typically accomplished by a stereotaxic head holder.
The materials needed for the instrument itself are listed in the next section. A suitable recording setup, typically consisting of a personal computer and an A/D converter, is needed to provide a continuous recording of the measurement signal. The NI-LDF instrument delivers a voltage coded blood flow signal and a voltage coded signal for the CMBC (concentration of moving blood cells) parameter. To visualize the image of the built-in CCD camera, a monitor or frame grabber card for a PC is needed.
3. Detailed Methods
3.1 Laser Doppler Flowmetry
Laser Doppler flowmetry utilizes the frequency broadening of monochromatic light reflected by moving blood cells. The theoretical background as well as the implementation of LDF systems for the measurement of capillary blood flow and their evaluation has been extensively reviewed elsewhere (Shepherd and Öberg 1990; Arildsson, Nilsson et al. 1996). Hence, only a short overview is given here:
When laser light is directed into a tissue of interest, the light is scattered by the stationary tissue matrix and by moving objects in the blood vessels (mainly red blood cells). Light scattered by the tissue matrix retains its original frequency whereas light scattered by moving blood components is Doppler shifted in proportion to their velocity vectors. Spectral analysis of the power spectrum can determine the average frequency shift as an index of the average velocity of the blood cells and the extent of spectral broadening as an index of the number of moving blood cells generating Doppler events (designated “blood volume” or “concentration of moving blood cells”, CMBC, by commercial LDF manufacturers). The product of average velocity times the concentration of moving blood cells is the flux of blood within the sampling volume of the tissue. When the blood cells within the sampling volume primarily move in random fashion (i.e. capillary blood flow), changes in flux correlate linearly with changes in blood flow as assessed with other blood flow measuring techniques. However, this condition is violated in larger vessels, where most of the cells move in the same direction. Hence, large vessels are not suited for valid measurement of blood flow with LDF. It is important to note that LDF does not provide volumetric measurements of blood flow as e.g. ml/min.
3.2 NI-LDF System
3.2.1 Optical Setup
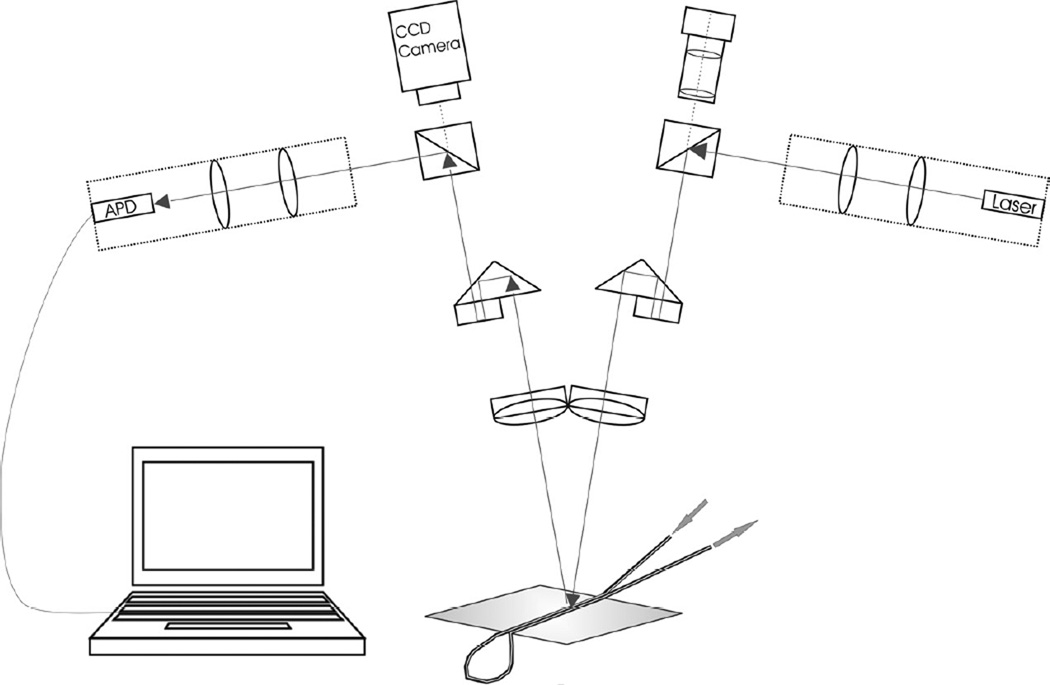
An infrared diode laser (811 nm) with integrated collimation optics (Micro Laser Systems, Garden Grove, CA) is used as the light source. The system delivers an output power of 1 mW at the corneal surface; the diameter of the beam at the fundus of the eye is determined by the optical setup and is also dependent on the optical system of the species investigated (approx. 300 µm in the tubing model approx. 150 µm at the rabbit fundus). An avalanche photodiode module (C5331-02, Hamamatsu Photonics, Hamamatsu City, Japan) is used as the detector. The laser and the detector are coupled into the optical paths of an adapted stereoscopic light microscope (SZ11 – STU2, Olympus, Vienna, Austria) using two beam splitters and a series of lenses (Fig. 1). Two axis positioners are used for correct alignment of the optical path. One eyepiece is removed and replaced by a CCD camera (LDH 0702/20, Philips, Vienna, Austria) sensitive to near infrared light to visualize the site of measurement. The detector output current is fed into an A/D converter (NI DAQ Card 6036E, National Instruments, Salzburg-Bergheim, Austria) and all further processing is done by software.
Figure 1. Schematic drawing of the NI-LDF setup.
A beam splitter is used to couple a diode laser into one optical path of a stereomicroscope (greenborough configuration); an avalanche photodiode as well as a CCD camera are coupled in the second optical path. The image captured by the camera is displayed on a monitor and used to direct the beam to the site of interest. The tube model shown is used for validation purposes (see section 4) and made of two parallel Silastic tubes glued on top of each other.
3.2.2 Software & Signal Analysis
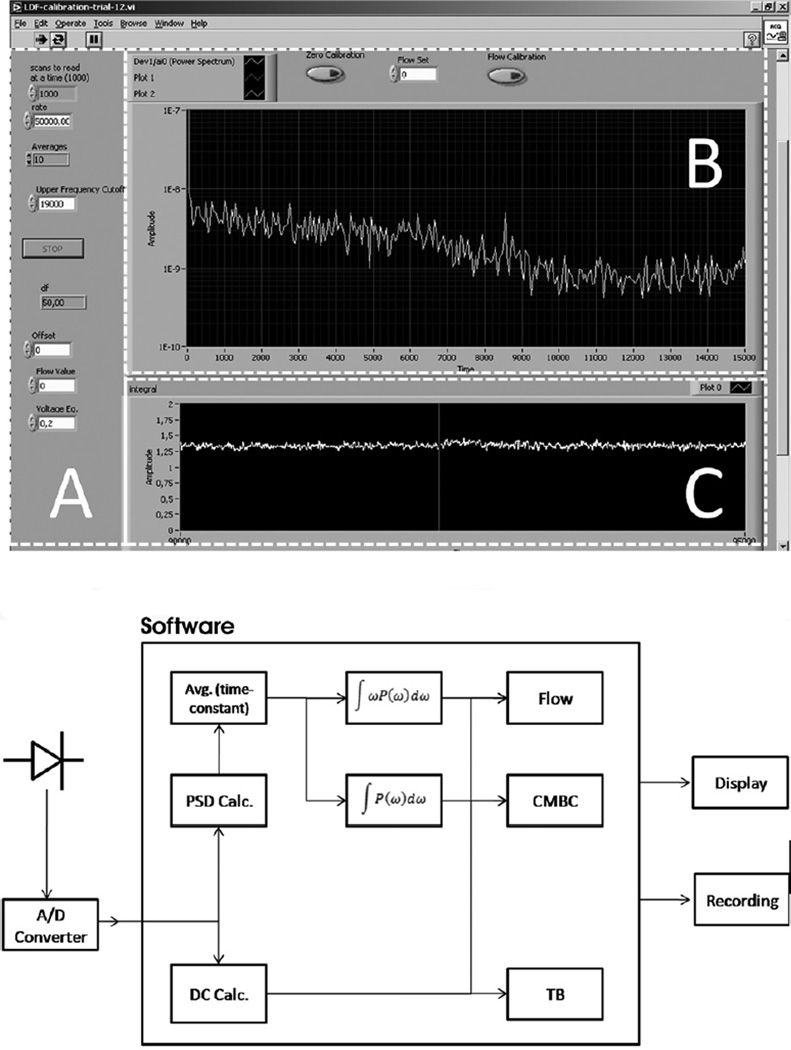
The signal analysis software was developed in the LabView graphical programming environment (version 7.1, National Instruments, Salzburg-Bergheim, Austria). A complete screenshot of the analysis algorithm is available online as supplementary material. The software allows the visualization of the following parameters: blood flow (flux), concentration of moving blood cells (CMBC), and total backscatter (TB, the amount of light returned to the detector) on the computer screen (Fig 2). The software also allows the user to input several parameters needed to optimize the calculations for the characteristics of the target tissue (e.g. upper and lower frequency cut-off and the averaging time constant).
Figure 2. Analysis software.
The top panel shows a screenshot of the user interface. Area A: The parameters for the calculation of the flux and CMBC are shown and can be set. Area B: The panel shows the power spectrum of the signal. Area C: Tracing of the flux value over a timeline. The panel shows the recording of the flow signal during a tube model experiment. The bottom panel shows the flow diagram of the software algorithm. The digitized signal of the detector is processed by software; the parameters are subsequently fed to a digital recording system.
The hemodynamic parameters are calculated according to the theory of Bonner and Nossal (Bonner and Nossal 1981). A more detailed theoretical discussion and validation of the algorithm is published elsewhere (Nilsson, Tenland et al. 1980; Nilsson 1984; Fredriksson, Fors et al. 2007). In short, the detector signal is processed by a Fast Fourier Transform (FFT) to obtain the frequency spectrum. To avoid artifacts due to breathing and movement, the default frequency range is set to 30 Hz at the lower end and to 19000 Hz at the higher end to cover the expected RBC speeds. The flow parameter is calculated as the integral of the frequency weighted power spectrum:
Perf denotes the perfusion index, ω the frequency after the FFT, P(ω) the power spectral density of the signal. nPerf(idc) denotes the noise of the detector dependent on the direct current component idc of the signal. The DC portion of the detector signal is proportional to the total backscattered light (TB). (□) indicates the averaged signal with a time constant set by the operator.
The concentration of moving blood cells (CMBC) is calculated as the natural logarithm of the zeroth moment of the power spectral density:
This parameter scales linearly to the moving scatterers only (i.e. if the flow stops, the CMBC parameters becomes zero, even if the blood volume remains unchanged).
3.3 Data Analysis and Statistics
All parameters were recorded through a PowerLab digital recording system (AD Instruments, Spechbach, Germany) on a standard PC running Chart 5.5.6 (AD Instruments, Spechbach, Germany).
LDF instruments typically deliver a continuous measurement signal in perfusion units that scales linearly to the blood flow in the target tissue. The assignment of perfusion units for the measurement signal is arbitrary, so we have given all results from the tube model experiments (see section 4.2) as the original voltage output of the instrument, without the subtraction of an offset, as the two instruments have different scales and the aim of the protocol is to assess linearity.
For the in-vivo protocol (section 4.4), the PF 4000 was calibrated according to the manufacturer’s instructions. The NI-LDF was zeroed using the same plastic block as in the Perimed procedure, the second point was set to match the mean of rabbit baseline data for PF4000 choroidal blood flow, i.e. to make both scales comparable during the decline of blood flow.
For the tube model experiments, at each set flow rate, the mean of the stable flow signal was calculated. Linear regression analysis was used to describe the averaged data points of the stability protocol and the linearity protocol. The standard error of the estimate (of the flux by linear regression) is given to characterize the fit of the linear model precisely.
In the rabbit model, the flow data were grouped and averaged in 5 mmHg bins for perfusion pressure and the flow values of the bins of both instruments were plotted against each other. Linear regression analysis was used to describe the data.
Unless specified differently, all data are given as mean ± standard error.
4. Pitfalls and Troubleshooting
4.1 Instrument design
LDF instruments can only provide valid measurements if the basic assumptions of the LDF theory are fulfilled (i.e. randomized velocity vectors of moving blood cells). Hence, the experimental setup has to ensure that this is the case.
The presented NI-LDF uses a CCD camera in the optical path of the detector that allows direct visual inspection and recording of the measurement site. This makes it possible to avoid large vessels within the sampling area, which would result in invalid measurements of blood flow. Since the procedure is not invasive, the NI-LDF measurement site can be changed without disturbing the experimental setup or causing further trauma to the tissue. The non-invasive design also makes it possible to make measurements in small animals (e.g. mouse and rat). The eyes of these animals have diameters between 3 and 6 mm and typical fiber optic probes would cause major trauma if placed inside such small eyes. The large rodent lens also precludes measurements with fiber optic probes of certain areas of interest (e.g. the optic nerve head) that are possible with the NI-LDF. While the instrument design per se enables studies in small animals, the species-dependent existence of a retinal circulation has to be taken into account in the interpretation of such experiments. Further studies are needed to validate the origin of the measurement signal in species with full retinal circulation.
A possible drawback of the current NI-LDF optics is the need for a cover slip to eliminate the refractive power of the cornea, as this might change the oxygen gradients in the anterior chamber (Reitsamer and Kiel 2007). Since we measured choroidal blood flow and since the choroid is the main source of oxygen for the posterior pole of the eye and has a low arterio-venous oxygen gradient (Alm and Bill 1972a; Alm and Bill 1972b), this factor is unlikely to interfere with measurements at the posterior pole of the eye.
4.2 Stability of the Measurement Signal
The LDF application is extremely sensitive to frequency changes of the light source as well as to its coherence length (Oberg 1990). Both can create artifacts in the measurement signal and have to be excluded before an LDF instrument can deliver reliable measurements.
We used a tubing model to check the stability (and linearity in the next chapter) and to compare the NI-LDF to a validated commercially available LDF instrument (Periflux 4000, Perimed, Uppsala, Sweden).
Our tube model is similar to that previously used by Nilsson et al. (Nilsson, Tenland et al. 1980; Nilsson 1984; Arildsson, Wardell et al. 1996). Two tubes (Silastic 508-001, i.d. 0.3 mm, o.d. 0.6 mm, Cole Parmer, Vernon Hills, IL) were mounted on a plate on top of each other using superglue (see Fig. 1) and perfused in opposite directions with diluted whole milk (1:2) using a syringe pump (sp100i, World Precision Instruments Germany, Berlin, Germany). The wall of the tubing provides a layer of static scattering matrix for the incident laser beam, in order to fulfill the basic assumptions of the theory of Bonner and Nossal, while the milk particles serve as moving objects. The PF4000 system uses glass fiber optic probes and provides 2 different laser wavelengths (632.8 nm and 780 nm). The near infrared (780 nm) laser and probe No. 411 with a fiber separation of 150 µm were used in the present experiments. Both instruments were directed perpendicular to the streaming milk in the tubing at the same time.
4.2.1. Stability protocol
The first goal of the stability protocol was to verify that the used diode laser is suitable for the application and does not generate relevant mode hopping. The second goal was to determine the signal-to- noise ratio of the measurement. To assess the stability of the signal the flow in the tube model was measured continuously at a flow rate of 0.3 ml/h for 1 hour. All measurements were made after a warm-up time of 30 minutes.
4.2.2. Instrument stability validation
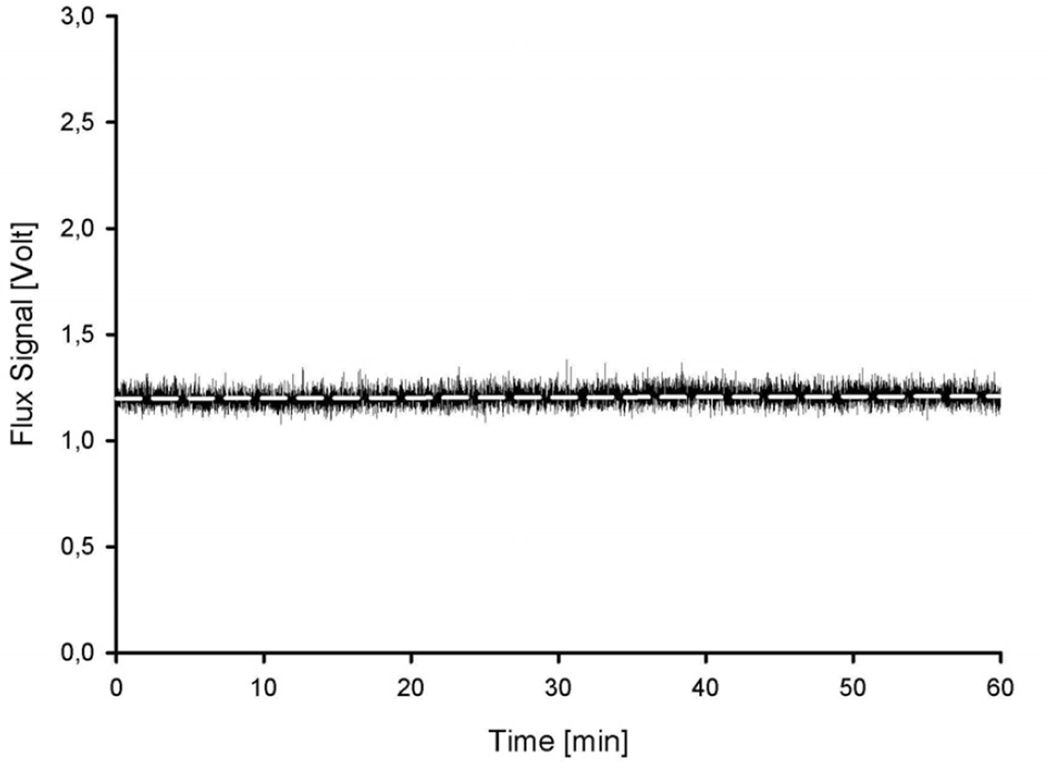
The stability protocol resulted in a stable signal over 1 hour (Fig. 3). The linear coefficient (slope) of the regression line was 8.06 * 10−6 (p < 0,001). The slope of the regression line is less than 10−5, indicating no relevant drift over 1 h. The variation coefficient (normalized standard deviation) is 3,5 %. This also indicates a stable signal and is within the range of previous reports from similar studies (Obeid 1993).
Figure 3. Stability protocol.
The measured flux (NI-LDF) in the tube model during stable flow conditions over 1 hour (0.3 ml/h). The regression line of the data (white dashed line) has a slope of 8.06 * 10−6. The coefficient of variation is 3,5 % of the flux value. Note that the offset was not subtracted.
4.3 Linearity of the Measurements
The instrument has to react linear to changes in blood flow in the tissue of interest in order to provide valid measurements. We used the same tubing model as in section 4.2 for the evaluation.
4.3.1 Linearity protocol
The goal of this protocol was to assess the relationship between the LDF signal and the flow rate of milk. Step increases of flow rates in the tube model were performed (Fig. 4 & Fig. 5, left panel), resulting in velocities that were previously reported for erythrocytes in the choriocapillary layer of monkeys, rabbits and rats (1 to 2 mm/s on average; (Braun, Dewhirst et al. 1997; Wajer, Taomoto et al. 2000; Flower, Peiretti et al. 2008)).
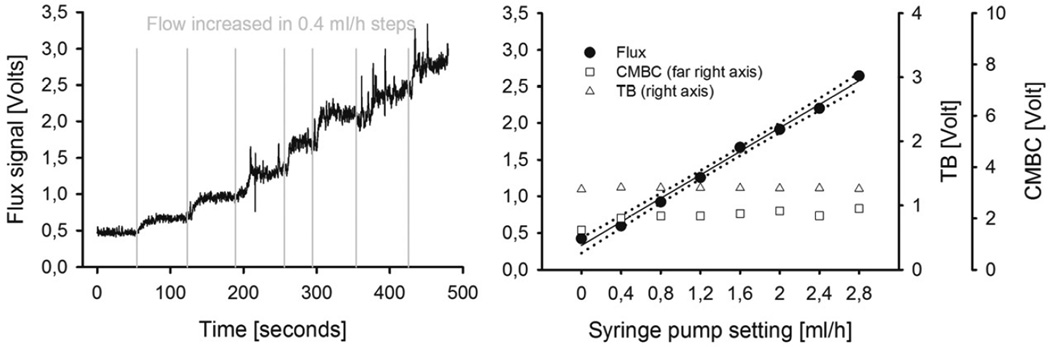
Figure 4. Periflux 4000.
Left panel: Tracing of the PF4000 Flux signal in response to stepwise increases of the flow in the tube model, provided by increased flow settings on the supplying syringe pump. Right panel: CMBC, TB and the Flux signal of the PF4000 plotted against the flow setting of the syringe pump. The Flux value correlates linearly and proportionally to the flow setting at the syringe pump. The CMBC parameter increases as the syringe pump is activated, and remains stable thereafter i.e. CMBC is independent of the flow rate. The TB parameter is also independent of the flow rate. (CMBC = concentration of moving blood cells, TB = total backscatter.)
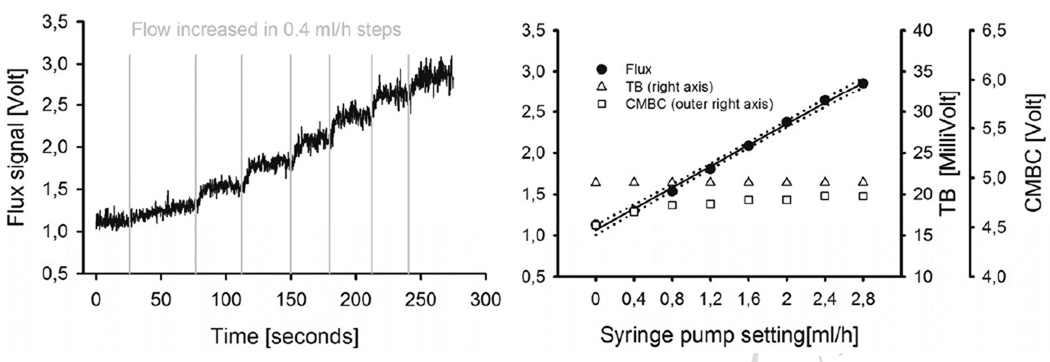
Figure 5. NI-LDF.
Left panel: Tracing of the NI-LDF Flux signal in response to stepwise increases of the flow in the tube model, provided by increased flow settings on the supplying syringe pump. Right panel: CMBC, TB and the Flux signal of the NI-LDF, plotted against the flow setting of the syringe pump. The response of the Flux parameter is linear, the total backscatter remains constant. The course of the CMBC parameter is similar to the PF4000 response, with a combined value for the offset (noise + Brownian motion) and a stable signal after the onset of the flow of the milk. Note that the scales of the two systems are dependent on instrumental factors (e.g. internal amplification etc.) and are not comparable directly. (CMBC = concentration of moving blood cells, TB = total backscatter.)
4.3.2 Instrument linearity validation
Figure 4 shows an experimental tracing of the linearity protocol for the PF4000 in the left panel and the Flow value, CMBC and TB plotted against the flow setting at the syringe pump in the right panel. The relationship between the preset flow and the PF4000 flow parameter were well fit by linear regression (y= 3308 *x + 0.3, r=0.99), the standard error of the estimate was 0.076 (approx. 3% of the total signal range). Figure 5 shows a tracing for the same linearity protocol for the NI-LDF in the left panel, and the corresponding Flow, CMBC and TB values in the right panel. The flow data were well fit by linear regression (y = 2.569 *x + 1.06, p<0,001, r = 0.99), the standard error of the estimate was 0.039 (approx. 1.5 % of the total signal range).
Since the first description of LDF to measure capillary blood flow by Stern, various algorithms have been proposed to calculate perfusion parameters (Stern 1975; Watkins and Holloway 1978; Bonner and Nossal 1981). Based on the theory of Bonner and Nossal the calculation of the flux as well as the average velocity proved to give valid measurements when compared to other methods in vivo as well as in bench tests (Shepherd and Öberg 1990). However, in previous studies the tube models used different tube diameter which has an important impact on the measurements. Under the assumption of constant and laminar flow, the cross sectional flow profile inside the tube exhibits its highest velocities in the middle of the tube, decreasing towards the periphery. Also the cross sectional velocity profile depends on the average velocity of the fluid within the tubing. Therefore, the validity of measurements depends crucially on the full coverage of the tube by the sampling volume of the LDF instrument. Moreover, with respect to a certain sampling bandwidth, the same bandwidth delivers linear measurements for a 5-fold velocity range if the tube diameter was increased 10 fold (280 µm to 3 mm) which has been shown elsewhere (Obeid 1993). As the exact measurement volume can only be estimated, this effect cannot be controlled for exactly. In order to minimize the error, the tube diameter in our model was chosen in a way that provided coverage of the full diameter by the laser, which was confirmed under visual control.
4.4 Suitability for in-vivo measurements
For the in vivo validation of the NI-LDF, an established animal model (rabbit) was used to measure mean arterial pressure (MAP), intraocular pressure (IOP), choroidal blood flow (ChorBF) and heart rate simultaneously (Reitsamer and Kiel 2002a; Reitsamer and Kiel 2002b; Reitsamer, Bogner et al. 2009). The Periflux 4000 (PF4000) was used as comparison with the red laser and probe No. 403 (fiber separation 250 µm), as previously published for this rabbit model (Reitsamer and Kiel 2002b).
The ocular perfusion pressure and thereby choroidal blood flow were changed by a continuous increase of intraocular pressure through saline infusion.
4.4.1 Animal preparation
All animal protocols were performed according to the EC Directive 86/609/EEC for animal experiments and were approved by the governmental animal experiments committee. New Zealand albino rabbits (n = 5, 2–3 kg; both sexes) were housed for 1 to 3 days in the vivarium with access to food and water ad libitum. The animals were anesthetized with pentobarbital sodium (30 mg/kg, intravenously, supplemented as needed) and paralyzed with gallamine triethiodide (1 mg/kg) to eliminate eye movement. The animals were intubated through a tracheotomy and ventilated with room air. Expired PCO2 was monitored (SurgiVet V9004 Capnograph, Smith Medical, Brunn am Gebirge, Austria) and maintained at 40 to 45 mm Hg. A heating pad was used to maintain normal body temperature (38 –39°C). All intravenous injections were given through cannulas placed in the marginal ear veins. The right eye was used in all experiments. To estimate the ocular arterial pressure (MAP) and ensure the adequacy of anesthesia, a catheter was inserted into the right ear artery and connected to a pressure transducer positioned at the same height above the heart as the eye. After the initial surgical preparation, the animals were mounted in a stereotaxic head holder, and the right eye was cannulated with a 23-gauge needle inserted into the vitreous cavity through the pars plana to measure the IOP with a pressure transducer. The eye was anesthetized topically with lidocaine before cannulation and care was taken not to disturb the cornea and anterior chamber.
4.4.2 Choroidal blood flow measurement
Choroidal blood flow was assessed by both instruments simultaneously at different measurement sites in the inferior choroid. As the peripheral retina (off the visual streak) in rabbits is supplied solely by the choroid, there is no influence of the retinal circulation to the blood flow signal. To eliminate the refractive power of the cornea for the measurement of choroidal blood flow by the NI-LDF, a cover slip was gently placed on the cornea on top of a liquid gel cushion. Choroidal PF4000 blood flow measurements were performed with the fiber-optic probe inserted through the pars plana with a micromanipulator so that the tip of the probe was positioned in the vitreous, close to the retinal surface over the posterior pole as described previously (Kiel and Shepherd 1992; Reitsamer and Kiel 2002b).
4.4.3 IOP protocol (n=5)
To manipulate the IOP, a second cannula was inserted in the vitreous. With the aid of a syringe pump (sp100i, World Precision Instruments Germany, Berlin, Germany), saline was infused at a rate of 60 µl/min until blood flow in the choroid ceased (Figure 6, left panel). To return to normal IOP, the additional volume was withdrawn by manual operation of the syringe pump (fast backward).
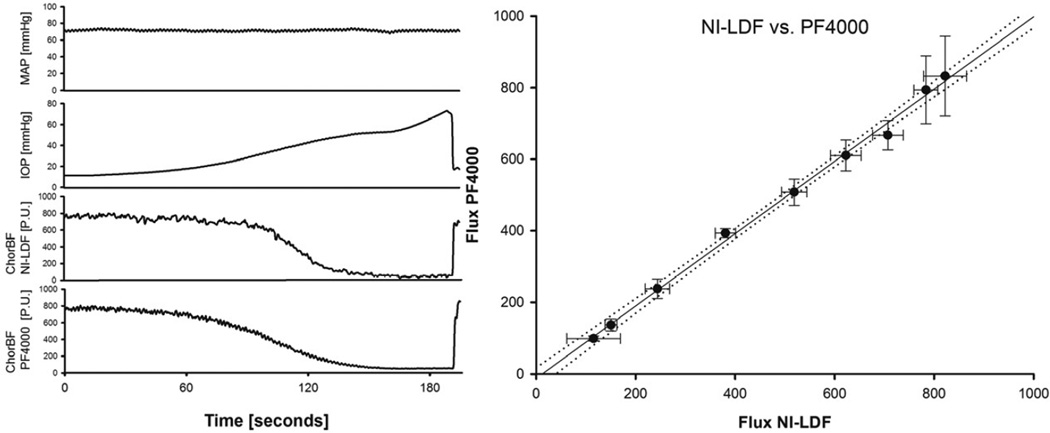
Figure 6. Comparison of both instruments in a rabbit model.
Left panel: Representative tracing of a single experiment of the IOP protocol. MAP, IOP and the flux signals of both instruments were recorded simultaneously. Note that the two instruments were directed at different measurement spots and utilize different wavelengths. The IOP was increased by infusion of saline to lower the ocular perfusion pressure (OPP = MAP – IOP), the MAP remained constant Right panel: Response of the NILDF plotted against the PF4000 during increase of IOP (n=5). Each data point represents the average flow (± standard deviation) over a 5 mmHg bin of perfusion pressure. The equation of the regression line is y=1,01*x −12,54 P.U., the correlation coefficient 0.99, the standard error of the estimate = 17,54 P.U. (2% of the total range).
4.4.4 Instrument in-vivo validation
The baseline values for the MAP, IOP and heart rate were 73,26 ± 2,27 mmHg, 13,85 ± 1,17 mmHg and 194,59 ± 6,10 min−1, respectively. Figure 6-right panel shows the correlation of both instruments resulting from the IOP protocol. The correlation can be fitted by a linear regression line (y=1,01*x – 12,35 P.U., p<0,001), the standard error of the estimate was 17,54 P.U. (2 % of the total range). The two instruments were directed at different measurement spots and utilize different laser wavelengths and are expected to have slightly different sampling volumes (Obeid, Dougherty et al. 1990). These facts might account for the slight difference in the choroidal flow tracings in Figure 6, left panel. As the absolute size of the sampling volume in the choroid of albino rabbits is not known, any estimation of the effect of different sampling volumes remains speculative. However, since choroidal blood flow goes to zero when IOP is elevated beyond MAP, it seems reasonable to assume that the LDF flux signal originates from blood flow within the eye only and does not include signals from vessels that are not under the control of intraocular perfusion pressure. It is important to note that these considerations are valid for measurements in rabbit eyes, which lack a full retinal circulation. Further studies are needed to validate the origin of the signal in species having a full retinal circulation.
Supplementary Material
A novel, non-invasive Laser Doppler Flowmeter for animal research is presented
In an in vitro model, it correlates linearly to different flow rates
In an animal model, it correlates well to a probe-based Laser Doppler instrument
The instrument enables non-invasive measurements of choroidal blood flow
Acknowledgments
Roles of the funding sources
Austrian Academy of Sciences: Provided financial support for the conduction of the study
PMU FFF: Provided financial support for the conduction of the study
Fuchs Foundation: Provided financial support for the conduction of the study
Adele Rabensteiner Foundation: Provided financial support for the conduction of the study
Lotte Schwarz Chair: Provided financial support for the conduction of the study
NEI grant EY09702: Provided financial support for the conduction of the study
Lion’s Club International: Provided financial support for the conduction of the study
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LDF: Laser Doppler Flowmetry, NI-LDF: Non Invasive LDF, MAP: Mean Arterial Pressure, ChorBF: Choroidal Blood Flow, IOP: Intraocular Pressure;
Disclosure Statement
C Strohmaier: no conflict of interest
RM Werkmeister: no conflict of interest
B Bogner: no conflict of interest
C Runge: no conflict of interest
F Schroedl: no conflict of interest
H Brandtner: no conflict of interest
W Radner: no conflict of interest
References
- Alm A, Bill A. The oxygen supply to the retina. I. Effects of changes in intraocular and arterial blood pressures, and in arterial P O2 and P CO2 on the oxygen tension in the vitreous body of the cat. Acta Physiol Scand. 1972a;84(2):261–274. doi: 10.1111/j.1748-1716.1972.tb05177.x. [DOI] [PubMed] [Google Scholar]
- Alm A, Bill A. The oxygen supply to the retina. II. Effects of high intraocular pressure and of increased arterial carbon dioxide tension on uveal and retinal blood flow in cats. A study with radioactively labelled microspheres including flow determinations in brain and some other tissues. Acta Physiol Scand. 1972b;84(3):306–319. doi: 10.1111/j.1748-1716.1972.tb05182.x. [DOI] [PubMed] [Google Scholar]
- Arildsson M, Nilsson GE, et al. Critical design parameters in laser Doppler perfusion imaging. Optical Diagnostics of Living Cells and Biofluids, Proceedings Of 2678. 1996:401–408. 560. [Google Scholar]
- Arildsson M, Wardell K, et al. Higher order moment processing of Laser Doppler Perfusion Signals. Journal of Biomedical Optics. 1996;2(4):358–363. doi: 10.1117/12.281499. [DOI] [PubMed] [Google Scholar]
- Bonner R, Nossal R. Model for laser Doppler measurements of blood flow in tissue. Appl. Opt. 1981;20(12):2097. doi: 10.1364/AO.20.002097. [DOI] [PubMed] [Google Scholar]
- Braun RD, Dewhirst MW, et al. Quantification of erythrocyte flow in the choroid of the albino rat. Am J Physiol. 1997;272(3 Pt 2):H1444–H1453. doi: 10.1152/ajpheart.1997.272.3.H1444. 6. [DOI] [PubMed] [Google Scholar]
- Chamot SR, Movaffaghy AM, et al. Blood flow in the human iris measured by laser Doppler flowmetry. Microvasc Res. 1999;57(2):153–161. doi: 10.1006/mvre.1998.2136. 6. [DOI] [PubMed] [Google Scholar]
- Flower R, Peiretti E, et al. Observation of erythrocyte dynamics in the retinal capillaries and choriocapillaris using ICG-loaded erythrocyte ghost cells. Invest Ophthalmol Vis Sci. 2008;49(12):5510–5516. doi: 10.1167/iovs.07-1504. [DOI] [PubMed] [Google Scholar]
- Fredriksson I, Fors C, et al. Department of Biomedical Engineering. Linköping University; 2007. Laser Doppler Flowmetry - a Theoretical Framework. 6: www.imt.liu.se/bit/ldf/ldfmain.html. [Google Scholar]
- Kastrup J, Bulow J, et al. A comparison between 133 Xenon washout technique and Laser Doppler flowmetry in the measurement of local vasoconstrictor effects on the microcirculation in subcutaneous tissue and skin. Clin Physiol. 1987;7(5):403–409. doi: 10.1111/j.1475-097x.1987.tb00182.x. [DOI] [PubMed] [Google Scholar]
- Kiel JW, Shepherd AP. Autoregulation of choroidal blood flow in the rabbit. Invest Ophthalmol Vis Sci. 1992;33(8):2399–2410. [PubMed] [Google Scholar]
- Kvietys PR, Shepherd AP, et al. Laser-Doppler, H2 clearance, and microsphere estimates of mucosal blood flow. Am J Physiol. 1985;249(2 Pt 1):G221–G227. doi: 10.1152/ajpgi.1985.249.2.G221. [DOI] [PubMed] [Google Scholar]
- Neufeld GR, Galante SR, et al. Skin blood flow from gas transport: helium xenon and laser Doppler compared. Microvasc Res. 1988;35(2):143–152. doi: 10.1016/0026-2862(88)90058-1. [DOI] [PubMed] [Google Scholar]
- Nilsson GE. Signal processor for laser Doppler tissue flowmeters. Med Biol Eng Comput. 1984;22(4):343–348. doi: 10.1007/BF02442104. 6. [DOI] [PubMed] [Google Scholar]
- Nilsson GE, Tenland T, et al. Evaluation of a Laser Doppler Flowmeter for Measurement of Tissue Blood Flow. Biomedical Engineering, IEEE Transactions on. 1980;27(10):597–604. doi: 10.1109/TBME.1980.326582. [DOI] [PubMed] [Google Scholar]
- Obeid AN. In vitro comparison of different signal processing algorithms used in laser Doppler flowmetry. Med Biol Eng Comput. 1993;31(1):43–52. doi: 10.1007/BF02446892. [DOI] [PubMed] [Google Scholar]
- Obeid AN, Dougherty G, et al. In vivo comparison of a twin wavelength laser Doppler flowmeter using He-Ne and laser diode sources. J Med Eng Technol. 1990;14(3):102–110. doi: 10.3109/03091909009015421. [DOI] [PubMed] [Google Scholar]
- Oberg PA. Laser-Doppler flowmetry. Crit Rev Biomed Eng. 1990;18(2):125–163. [PubMed] [Google Scholar]
- Reitsamer HA, Bogner B, et al. Effects of dorzolamide on choroidal blood flow, ciliary blood flow and aqueous production in rabbits. Invest Ophthalmol Vis Sci. 2009 doi: 10.1167/iovs.08-2468. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitsamer HA, Kiel JW. Effects of dopamine on ciliary blood flow, aqueous production, and intraocular pressure in rabbits. Invest Ophthalmol Vis Sci. 2002a;43(8):2697–2703. [PubMed] [Google Scholar]
- Reitsamer HA, Kiel JW. A rabbit model to study orbital venous pressure, intraocular pressure, and ocular hemodynamics simultaneously. Invest Ophthalmol Vis Sci. 2002b;43(12):3728–3734. [PubMed] [Google Scholar]
- Reitsamer HA, Kiel JW. Relationship between Ciliary Blood Flow and Aqueous Production in Rabbits. Invest Ophthal Vis Sci. 2003;44(9):3967–3971. doi: 10.1167/iovs.03-0088. [DOI] [PubMed] [Google Scholar]
- Reitsamer HA, Kiel JW. Oxygen Gradients in the Anterior Segment of the Eye. Invest. Ophthalmol. Vis. Sci. 2007;48 6: E-Abstract 6043. [Google Scholar]
- Riva CE, Cranstoun SD, et al. Choroidal blood flow in the foveal region of the human ocular fundus. Invest Ophthalmol Vis Sci. 1994a;35(13):4273–4281. [PubMed] [Google Scholar]
- Riva CE, Cranstoun SD, et al. Local choroidal blood flow in the cat by laser Doppler flowmetry. Invest Ophthalmol Vis Sci. 1994b;35(2):608–618. [PubMed] [Google Scholar]
- Riva CE, Petrig BL. Choroidal blood flow by laser Doppler flowmetry. Optical Engineering. 1995;34(3):746–752. [Google Scholar]
- Rival R, Bance M, et al. Comparison of laser Doppler flowmeter and radioactive microspheres in measuring blood flow in pig skin flaps. Laryngoscope. 1995;105(4 Pt 1):383–386. doi: 10.1288/00005537-199504000-00009. [DOI] [PubMed] [Google Scholar]
- Shepherd A, Öberg P. Laser-Doppler Blood Flowmetry. Norwell, MA: Kluwer Academic Publishers; 1990. [Google Scholar]
- Shepherd AP, Riedel GL, et al. Evaluation of an infrared laser-Doppler blood flowmeter. Am J Physiol. 1987;252(6 Pt 1):G832–G839. doi: 10.1152/ajpgi.1987.252.6.G832. [DOI] [PubMed] [Google Scholar]
- Smits GJ, Roman RJ, et al. Evaluation of laser-Doppler flowmetry as a measure of tissue blood flow. J Appl Physiol. 1986;61(2):666–672. doi: 10.1152/jappl.1986.61.2.666. [DOI] [PubMed] [Google Scholar]
- Stern MD. In vivo evaluation of microcirculation by coherent light scattering. Nature. 1975;254(5495):56–58. doi: 10.1038/254056a0. 6. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Riva C, et al. Blood velocity measurements in human retinal vessels. Science. 1974;186(4166):830–831. doi: 10.1126/science.186.4166.830. [DOI] [PubMed] [Google Scholar]
- Wajer SD, Taomoto M, et al. Velocity measurements of normal and sickle red blood cells in the rat retinal and choroidal vasculatures. Microvasc Res. 2000;60(3):281–293. doi: 10.1006/mvre.2000.2270. [DOI] [PubMed] [Google Scholar]
- Watkins D, Holloway GA., Jr An instrument to measure cutaneous blood flow using the Doppler shift of laser light. IEEE Trans Biomed Eng. 1978;25(1):28–33. doi: 10.1109/TBME.1978.326374. [DOI] [PubMed] [Google Scholar]
- Wheatley AM, Almond NE, et al. Interpretation of the laser Doppler flow signal from the liver of the rat. Microvasc Res. 1993;45(3):290–301. doi: 10.1006/mvre.1993.1025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.