Abstract
Background
Breast cancers aberrantly express gastrin-releasing peptide (GRP) hormone and its cognate receptor, gastrin-releasing peptide receptor (GRP-R). Experimental evidence suggests that bombesin (BBS), the pharmacological homologue of GRP, promotes breast cancer growth and progression. The contribution of GRP-R to other poor prognostic indicators in breast cancer, such as the expression of the EGF-R family of growth factors, and hormone insensitivity is unknown.
Materials and Methods
Two estrogen receptor (ER)-negative breast cancer cell lines were used. MDA-MB-231 overexpress both EGFR and GRPR, whereas SK-BR-3 cells express EGF-R but lack GRP-R. Cellular proliferation was assessed by Coulter counter. Chemotactic migration was performed using Transwell chambers and the migrated cells were quantified. Northern blot and real-time PCR were used to evaluate if pro-angiogenic factor interleukin-8 (IL-8) mRNA expression.
Results
In MDA-MB-231 cells, GRP-R and EGF-R synergize to regulate cell migration, IL-8 expression, but not cell proliferation. In SK-BR-3 cells, ectopic expression of GRP-R was sufficient to increase migration and IL-8 mRNA.
Conclusions
These data suggest relevant roles for GRP-R in ER-negative breast cancer progression. Future mechanistic studies to define the molecular role of GRP-R in breast cancer metastasis provide novel targets for the treatment of ER-negative breast cancers.
Keywords: gastrin-releasing peptide receptor, bombesin, breast cancer, migration, interleukin-8
INTRODUCTION
Among women, breast cancer is the most common malignancy in the United States and the second most frequent cause of cancer-related death. In 2008, 184,000 new cases and over 40,000 deaths are predicted to occur [1]. Breast cancers aberrantly express gastrin-releasing peptide (GRP) hormone and its cognate receptor, gastrin-releasing peptide receptor (GRP-R) [2, 3]. In vivo and in vitro experiments suggest that GRP, or bombesin (BBS), the pharmacological homologue of GRP derived from amphibians, promotes breast cancer growth and progression [4, 5]. Furthermore, among GRP-R expressing breast cancers with metastasis to regional lymph nodes, the metastatic deposit also maintains GRP-R expression [3]. The prevalence of these high-affinity receptors in breast cancer has led to the development of GRPR-based diagnostic tools [6, 7] as well as GRP-R-targeted therapeutics [8].
Ligand activation of GRP-R promotes the cancer phenotype by modulating cancer cell proliferation, cell migration, secretion, and specific gene expression [9]. For breast cancer, it is well established that poor prognostic features include hormone insensitivity, such as lack of estrogen receptor (ER), as well as overexpression of the epidermal growth factor (EGF) family of receptor tyrosine kinases, especially epidermal growth factor receptor (EGF-R, also, HER1 and erbB1) and HER2/neu (erbB2). The relationship between GRP-R and the EGF-R family of growth factors in ER-negative breast cancers is unknown. Here, we report that stimulation GRP-R and EGF-R synergistically stimulate cellular migration and production of pro-angiogenic factor interleukin -8 (IL-8) in an ER-negative breast cancer cell line, MDA-MB-231. Further, in another ER-negative cell line, SK-BR-3, ectopic expression of GRP-R was sufficient to increase cell migration and IL-8 mRNA.
MATERIALS AND METHODS
Reagents
Recombinant human EGF was purchased from R&D Biosystems (Minneapolis, MN), and BBS peptide was purchased from Bachem (Torrence, CA). Transfection reagents Lipofectamine 2000 and Geneticin (G418) were obtained from Invitrogen (Rockville, MD).
Cell Culture
Human breast cancer cell lines, MDA-MB-231 and SK-BR-3, were purchased from American Type Culture Collection (Manassas, VA). Cells were maintained at 37°C in a humidified atmosphere of 95% air and 5% CO2. MDA-MB-231 cells were grown in Leibowitz L-15 Medium and SK-BR-3 cells were grown in Mcoy 5A (Cellgro Mediatech, Inc. Herndon, VA). Media was supplemented with 10% Fetal Bovine Serum (FBS; HyClone, Thermo Fisher Scientific; Waltham, MA).
Transfection
Recombinant GRP-R or pCDNA.3 vector control was transfected into SKBR-3 breast cancer cell lines, as described previously [10]. Cells were grown in G418-free medium for three days and selection of stable clones were selected in the presence of G418 (800 μg/ml). G418-resistant colonies were then maintained in G418 (400 μg/ml) and pooled together for experiments.
Intracellular Ca2+ Measurements
Cells were plated and grown on 25-mm glass coverslips. Prior to stimulation, cells were washed with a physiological medium (KRH) containing NaCl (125 mM), KCl (5mM), KH2PO4 (1.2 mM), MgSO4 (1.2 mM), CaCl2 (2 mM), glucose (6 mM), HEPES (25 mM; pH 7.4), and loaded with 2 μM Fura-2AM (Molecular Probes, Eugene, OR) at 25°C for 50 min. The cells were treated with EGF (10 ng/ml) or BBS (100 nM), and single cell changes in the concentration of free intracellular Ca2+([Ca2+]i) were recorded with a Nikon Diaphot inverted microscope (Garden City, NY) and a CCD camera (Dage-MITI, Inc., Michigan City, IN). Data points were collected every 1-8 s from approximately 35 cells/coverslip and processed using ImageMaster software. Data are presented as the mean change in [Ca2+]i ± SEM.
Cellular proliferation assay
MDA-MB-231 cells (1×105) were plated in triplicate and serum starved overnight. The cells were then treated with BBS (10 nM), EGF (10 ng/ml), or both. Growth in media with 10%FCS served as a positive control. Cells were harvested by trypsinization and counted by Coulter counter (Beckman Coulter, Inc., Fullerton, CA).
Migration Assay
: 5×104 cells resuspended in serum-free media and 0.25% BSA were added to 6.5 mm Transwell chambers (8 μm pore size, Costar, Corning, NY) coated with rat tail Collagen I (Sigma-Aldrich, St. Louis, MO) and allowed to migrate toward the bottom chamber with EGF, BBS, both, or control media (Leibowitz L-15 and 0.25% BSA) at 37°C for 5 h in a humidified atmosphere of 95% air and 5% CO2. The migrated cells were fixed in 100% methanol, stained in 1% crystal violet, and quantified by visual counting using a 25 mm2 reticle (Upstate Technical Equipment Company, Inc., East Syracuse, NY). Kruskal-Wallis test and a post-test Dunn's Multiple Comparison test was used to assess significance between the groups, defined at p<0.05.
RNA isolation, Northern Blot Analysis and Real-Time PCR
Total RNA was extracted from breast cancer cells using Ultraspec reagent (Biotecx; Houston, TX). For Northern blot hybridization, 15 μg of total RNA was resolved on 1% agarose/formaldehyde gels by electrophoresis, transferred to Hybond=N+ membrane (Amersham Pharmacia Biotech, Buckinhamshire, England), aprobed with IL-8 cDNA labeled with [α-32P] dATP (Perkin-Elmer Life Sciences Inc., Boston, MA) using a random-priming DNA-labeling kit (Stratagene, Cedar Creek, TX). Prehybridization (30 min) and hybridization (3 h) were performed at 68°C in Express Hybridization solution (Clontech, Palo Alto, CA). Blots were washed in high-stringency solution containing 0.1 × SSC and 0.1% SDS for 30 min at 65°C. Specific hybridization was visualized by autoradiography using BioMas MR film (Kodak, Rochester, NY). To ensure RNA integrity and to confirm equal loading and transfer of RNA, the membranes were stripped and hybridized with a probe for 18S RNAse. Human IL-8 expression was quantified using real-time quantitative PCR (TaqMan; Applied Biosystems, Foster City, CA). The sequence of the primer/probe set was based on IL-8 mRNA sequence (GenBank NM_000584). 18S rRNA TaqMan assay reagent was used for internal control. One-step reverse transcription (RT)-PCR was performed with 40 ng of RNA for both target gene and endogenous controls. Duplicate CT values were analyzed in Microsoft Excel using the comparative CT (ΔΔCT) method as described by the manufacturer (Applied Biosystems). The amount of target (2-ΔΔCT) was obtained by normalizing to 18S (endogenous reference) and relative to a calibrator (one of the experimental samples, mRNA from SK-BR-3 parental cells).
Statistical Analysis
Statistical analysis was performed using Graphpad Prism version 4.0 (San Diego, CA). Statistical significance was assumed if p≤0.05.
RESULTS
Effects of BBS and EGF on MDA-MB-231 cell proliferation in vitro
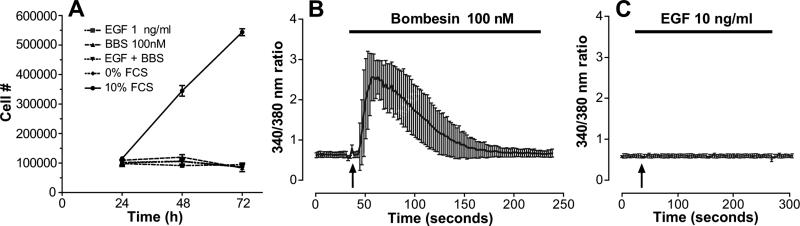
MDA-MB-231 cells are a metastatic line that lacks estrogen receptor, but over-expresses EGF-R (erbB1), Her-2-neu and GRP-R. Previous reports have demonstrated that MDA-MB-231 cells do not proliferate in response to EGF [11]. With regard to the mitogenic effects of BBS on MDA-MB-231 cells, conflicting results in the literature exist [2, 4, 12]. Whether these cells will increase cell proliferation with both EGF and BBS through a cooperative mechanism is unknown. We addressed this question by treating serum-starved MDA-MB-231 cells with BBS (10 nM), EGF (1 ng/ml) or both BBS and EGF together. As shown in Figure 1A, neither agonist, alone or in combination, increased cellular growth, compared with cells grown in unsupplemented Leibowitz L-15 medium alone.
Figure 1. Effects of EGF and/or BBS on cell growth in MDA-MB-231 cells.
A) MDA-MB-231 cells (1×105/well) were plated in quadruplicate in Leibowitz L-15 medium and 10% FBS supplement. After 18 h, the media was changed and the cells were serum starved overnight. Initial cells counts were performed to determine the plating efficiency (24-h time point). The cells were then treated daily with BBS (10 nM), EGF (1 ng/ml), or both. Growth in media with 10%FCS served as a positive control. The graphs represent the mean of two experiments; bars, ±SEM. B, C) MDA-MB-231 cells were loaded with the Ca2+ indicator dye, Fura-2, and then stimulated with either B) BBS (100 nM) or C) EGF (10 ng/ml) at the time indicated by the arrow. Single cell florescence image analysis recorded the agonist-induced changes in [Ca2+]i confirming the expression of functional GRP-R on MDA-MB-231 cells.
To verify the presence of functional GRP-R, these cells were stimulated with the ligand BBS, and a characteristic transient [Ca2+]i increase is observed, due to the interaction of G-proteins, activation of phospholipase Cβ, and the generation of second messenger inositol 1,4,5-triphosphate, and release of Ca2+ from the endoplasmic reticulum. As shown in Figure 1B, functional GRP-R is present on the cell surface, indicating that the lack of cell growth cannot be attributable to lack of functional cell surface receptors. Interestingly, stimulation of EGFR by EGF does not result in a [Ca2+]i (Figure 1C).
BBS and EGF increase migration of MDA-MB-231 cells
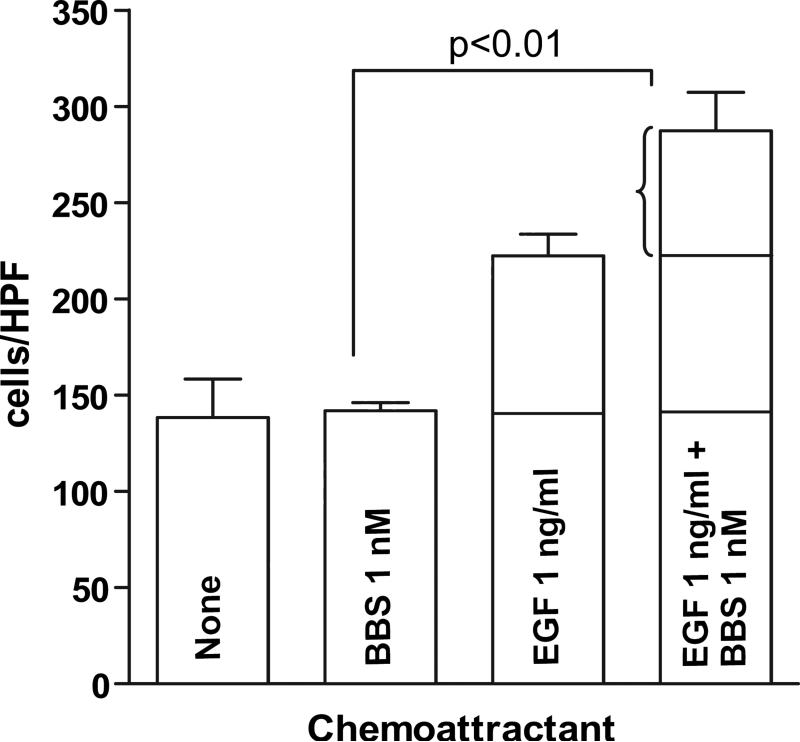
EGF is key mediator of cell motility, which is critical for cancer invasion and metastasis. MDA-MB-231 cells will chemotactically migrate toward EGF in a dose dependent manner [13]. BBS peptide has been shown to facilitate cell migration in colon cancer indirectly by increasing the transcription of cyclooxgenase-2 and release of prostaglandin E2 [14] and in prostate cancer directly by activation of RhoA and stress fiber formation [15]. To address the question of whether BBS can elicit a migratory response in breast cancer cells expressing GRP-R, we performed chemotactic assays using transwell chambers coated with Collagen I (15 μg/ml). MDA-MB-231 cells were allowed to migrate toward EGF (1 ng/ml), BBS (1 nM) or both EGF and BBS. After five hours, cells migrated toward EGF more than vehicle alone or BBS alone. Figure 2 demonstrates that a synergistic effect was achieved when cells migrated toward both EGF and BBS together (p<0.01).
Figure 2. EGF and BBS stimulate a synergistic migratory response in MDA-MB-231 cells.
Cells were allowed to migrate in chemotaxis chamber assays as described in “Materials and Methods”. The experiment was performed in triplicate and repeated three times. A one-way ANOVA (Kruskal-Wallis) test demonstrated significant differences between the groups (p<0.001). Dunn's Multiple Comparison test was used to assess significance between paired groups. Compared to the cells migrating toward control media (Leibowitz L-15 and 0.25% BSA) alone, cells migrating toward BBS or EGF alone were not statistically different at the doses of chemoattractant used. However, the combination of both EGF and BBS increased cell migration significantly over the sum of each chemoattractant alone, demonstrating synergy between EGF (1 ng/ml) and BBS (1 nM).
BBS and EGF synergistically upregulate IL-8 mRNA
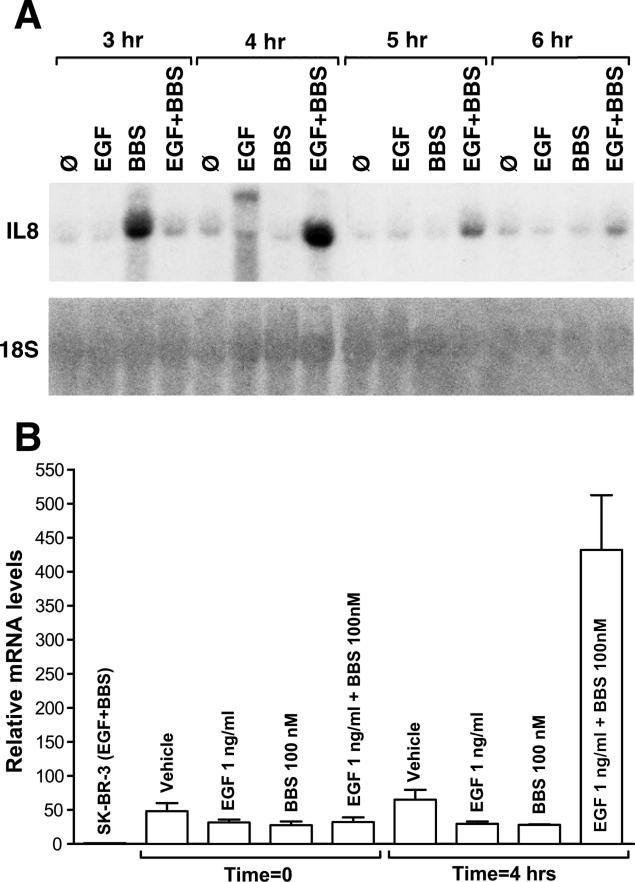
ER-negative breast cancer cells lines have been shown to over-express interleukin-8 (IL-8), a chemokine that contributes to growth and progression. Elevated IL-8 expression strongly correlates with ER-negative breast cancer cells as well as increased invasive potential, while ER-positive tumor cells do not secrete significant levels of IL-8 and demonstrate decreased invasive potential [16]. IL-8 is a potent chemoattractant stimulating cell migration. BBS has been shown to be coupled to the regulation of IL-8 gene expression in PC-3 prostate cancer cells [17]. To determine whether GRP-R and/or EGF-R mediates induction of pro-angiogenic factor IL-8, we treated MDA-MB-231 cells with EGF (1 ng/ml), BBS (100 nM), or both and measured steady state IL-8 mRNA expression levels at 3-4 h after treatment. IL-8 mRNA levels increased at 3 h with BBS alone, and was maximal at 4 h with both BBS and EGF (Figure 3A). The synergistic upregulation of IL-8 by BBS and EGF decreased after 4 h. To confirm this finding, relative IL-8 mRNA levels in MDA-MB-231 cells were compared before and after 4 h of stimulation with EGF and BBS, as before. All MDA-MB-231 samples were normalized to 18S as well as mRNA from the breast cancer cell line SK-BR-3, a negative control. SK-BR-3 cells, similar to MDA-MB-231 cells, are ER-negative, express EGF-R, Her-2-neu, but lack GRPR, have been shown to have insignificant levels of IL-8 compared to MDA-MB-231 cells [16].
Figure 3.
Time course of IL-8 mRNA induction by EGF and /or BBS. A) Autoradiography of Northern blots demonstrates steady state levels of IL-8 mRNA after treatment with EGF (1 ng/ml), BBS (100nM), or both EGF and BBS. Total RNA (15 μg/lane) was isolated from MDA-MB-231 cells at the indicated time points after treatment. RNA was resolved on a 1% agarose-formaldehyde gel, blotted onto a Hybond-N+ membrane, and hybridized with a [α32P]dATP-labeled cDNA probe. Equal loading and transfer was verified by rehybridizing the blot with a radiolabeled probe for 18S RNAse. B) Quantitative real-time PCR analysis of IL-8 mRNA levels showing synergistic increase at 4 h. As a negative control, mRNA from SK-BR-3 cells was used.
Effects of GRP-R expression in SK-BR-3 cells
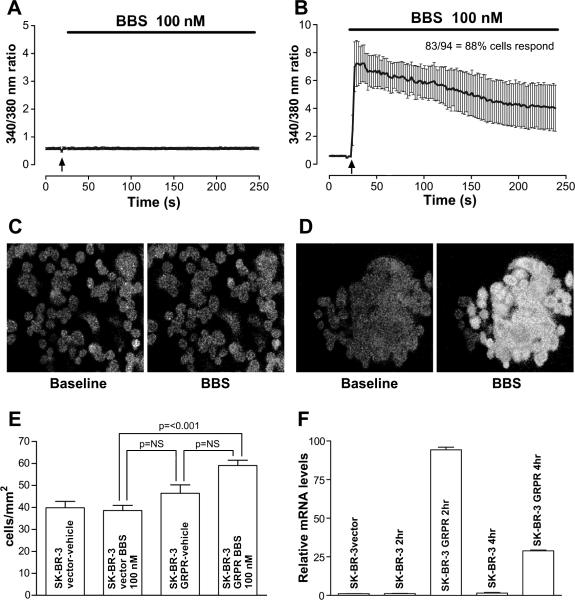
To determine if expression of GRP-R would be sufficient to couple the regulation of cellular migration and IL-8 gene expression in another metastatic human mammary cancer cell line, we assessed the effects of BBS treatment on SK-BR-3 cells ectopically expressing GRP-R. The presence of a BBS-mediated [Ca2+]i response verified expression of a functional GRP-R in the SK-BR-3-GRP-R transfectants (Figure 4B, D), unlike the SK-BR-3 transfected with empty vector (Figure 4A, C). With BBS (100 nM) as a chemoattractant, SK-BR-3 GRP-R transfected cells are more motile compared to the SK-BR-3 vector control cells (Figure 4E). Additionally, expression GRP-R is sufficient to confer BBS-inducible increase in IL-8 mRNA after 2-4 h in SK-BR-3 cells (Figure 4F).
Figure 4.
Effects of BBS on SK-BR-3 cells transfected with GRP-R. A, C) Time-course of the BBS-induced change in [Ca2+]i in SK-BR-3 cells transfected with empty vector. Psuedo-color image of cells loaded with Fura-2, a Ca2+sensitive dye, before and after treatment with BBS (100 nM) shown below. B, D) Time-course of the BBS-induced change in [Ca2+]i in SK-BR-3 cells stably transfected recombinant GRP-R. Psuedo-color image of cells loaded with Fura-2, demonstrating a BBS-stimulated response shown below. E) Cells were allowed to migrate in chemotaxis chamber assays as described in “Materials and Methods”. The experiment was performed in triplicate and repeated two times. A one-way ANOVA test demonstrated significant differences between the groups and Dunn's Multiple Comparison test was used to assess significance between paired groups. Cells expressing GRP-R demonstrated significantly more migration toward BBS in the lower chamber compared with either vector transfected cells or GRP-R transfected cells in the absence of chemoattractant. F) Time course of human IL-8 mRNA expression by SK-BR-3-GRPR cells stimulated with BBS (100 nM). IL-8 mRNA expression was induced after 2 h of treatment with BBS, and decreased by 4 h.
DISCUSSION
A mechanistic link between the expression of GRP-R and EGF-R was suggested in an early report by Liebow et al [18]. The authors showed that patient samples from many human cancers, including breast cancer, when subjected to in vitro treatment with BBS, EGF, and/or the GRPR antagonist, RC-3095, the BBS and EGF combination resulted in a synergistic increase in total phosphorylation of multiple proteins, which decreased in the presence of RC-3095. Their data demonstrated that BBS further stimulated the phosphorylation of the same substrates that were phosphorylated to a lesser degree by EGF-R alone, suggesting that BBS may augment the EGF-R tyrosine kinase response. BBS has been shown to mediate EGF-R transactivation via the triple membrane-passing signal (TMPS) mechanism [19]. When BBS binds to GRP-R, intracellular signalling pathways activate matrix metalloproteinases (MMPs), which in turn, cleave and release membrane-bound pro-EGF-like ligands. These soluble ligands interact with the ligand-binding domain of EGF-R, resulting in receptor dimerization. EGF-R is transactivated when it becomes tyrosine phosphorylated on distinct residues in its kinase domain [20]. The TMPS mechanism has been shown to mediate cell migration in the androgen-insensitive PC-3 prostate cancer cell line when the cells were treated with BBS [21].
In the PC-3 cell line, BBS treatment also stimulates nuclear factor-kappa B (NF-κB) activation and increases both gene expression and secretion of IL-8 [17]. IL-8 is an inflammatory cytokine and potent angiogenic activator. Its expression correlates with the metastatic potential of many cancers. Freund et al [16] reported that most ER-negative cell lines (5/7) expressed IL-8, whereas all seven ER-positive breast cancer cells lines tested did not have any measurable levels of IL-8. Interesting, the SK-BR-3 cell line, although ER-negative, did not have detectable levels of IL-8, in agreement with our findings. High levels of secreted IL-8, as well as mRNA expression, correlated with the invasive potential of the breast cancer cell lines [16, 22]. Our data demonstrates that one mediator of IL-8 mRNA in ER-negative breast cancer cells is BBS-activated GRP-R. Furthermore, expression of recombinant GRP-R was sufficient to induce IL-8 mRNA in SK-BR-3 cells.
A correlation between the biologically aggressive behavior of endocrine-resistant cancers and aberrant or overexpression BBS/GRP-R has been reported for androgen-resistant prostate cancer cells [17] and metastatic ovarian cancer [23]. Interestingly, Gugger et al [3] have shown that when GRP-R expressing breast cancers spread to regional lymph nodes, GRP-R expression is evident in the metastatic deposit. We have shown that GRP-R contributes to a metastatic phenotype in breast cancer by increasing cellular migration and IL-8 expression in two different ER-negative cell lines. In MDA-MB-231 cells, GPR-R cooperates with EGF-R, whereas in SK-BR-3 cells, which are GRP-R-deficient, expression of recombinant GRP-R alone was sufficient to increase cell migration and IL-8 expression. These data suggest relevant roles for GPR-R in the metastatic process, particularly in ER-negative breast cancer, which are a subgroup that clinically presents significant therapeutic challenges [24]. Future studies to define the molecular role of GRP-R in breast cancer tumor progression and metastasis will provide novel targets for anticancer drug discovery [25].
ACKNOWLEDGMENTS
We thank Dr. Javier Navarro for the plasmids containing the IL-8 cDNA, and Eileen Figueroa and Steve Schuenke for their assistance with the preparation of this manuscript. Dr. Chao is supported by a research career development award (K12HD052023: Building Interdisciplinary Research Careers in Women's Health Program-BIRCWH) from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD); the National Institute of Allergy and Infectious Diseases (NIAID); and the Office of the Director (OD), National Institutes of Health. The content is solely the responsibility of the authors and does not represent the official views of these Institutes or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Giacchetti S, Gauville C, de Cremoux P, et al. Characterization, in some human breast cancer cell lines, of gastrin-releasing peptide-like receptors which are absent in normal breast epithelial cells. Int J Cancer. 1990;46:293. doi: 10.1002/ijc.2910460226. [DOI] [PubMed] [Google Scholar]
- 3.Gugger M, Reubi JC. Gastrin-releasing peptide receptors in non-neoplastic and neoplastic human breast. Am J Pathol. 1999;155:2067. doi: 10.1016/S0002-9440(10)65525-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bold RJ, Ishizuka J, Yao CZ, Townsend CM, Jr., Thompson JC. Bombesin stimulates in vitro growth of human breast cancer independent of estrogen receptors status. Anticancer Res. 1998;18:4051. [PubMed] [Google Scholar]
- 5.Miyazaki M, Lamharzi N, Schally AV, et al. Inhibition of growth of MDA-MB-231 human breast cancer xenografts in nude mice by bombesin/gastrin-releasing peptide (GRP) antagonists RC-3940-II and RC-3095. Eur J Cancer. 1998;34:710. doi: 10.1016/s0959-8049(97)10123-x. [DOI] [PubMed] [Google Scholar]
- 6.Scopinaro F, Di Santo GP, Tofani A, et al. Fast cancer uptake of 99mTc-labelled bombesin (99mTc BN1). In Vivo. 2005;19:1071. [PubMed] [Google Scholar]
- 7.Parry JJ, Andrews R, Rogers BE. MicroPET imaging of breast cancer using radiolabeled bombesin analogs targeting the gastrin-releasing peptide receptor. Breast Cancer Res Treat. 2007;101:175. doi: 10.1007/s10549-006-9287-8. [DOI] [PubMed] [Google Scholar]
- 8.Schwartsmann G, DiLeone LP, Horowitz M, et al. A phase I trial of the bombesin/gastrin-releasing peptide (BN/GRP) antagonist RC3095 in patients with advanced solid malignancies. Invest New Drugs. 2006;24:403. doi: 10.1007/s10637-006-6886-5. [DOI] [PubMed] [Google Scholar]
- 9.Patel O, Shulkes A, Baldwin GS. Gastrin-releasing peptide and cancer. Biochim Biophys Acta. 2006;1766:23. doi: 10.1016/j.bbcan.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Guo YS, Hellmich MR, Wen XD, Townsend CM., Jr. Activator protein-1 transcription factor mediates bombesin-stimulated cyclooxygenase-2 expression in intestinal epithelial cells. J Biol Chem. 2001;276:22941. doi: 10.1074/jbc.M101801200. [DOI] [PubMed] [Google Scholar]
- 11.Davidson NE, Gelmann EP, Lippman ME, Dickson RB. Epidermal growth factor receptor gene expression in estrogen receptor-positive and negative human breast cancer cell lines. Mol Endocrinol. 1987;1:216. doi: 10.1210/mend-1-3-216. [DOI] [PubMed] [Google Scholar]
- 12.Yano T, Pinski J, Groot K, Schally AV. Stimulation by bombesin and inhibition by bombesin/gastrin-releasing peptide antagonist RC-3095 of growth of human breast cancer cell lines. Cancer Res. 1992;52:4545. [PubMed] [Google Scholar]
- 13.Price JT, Tiganis T, Agarwal A, Djakiew D, Thompson EW. Epidermal growth factor promotes MDA-MB-231 breast cancer cell migration through a phosphatidylinositol 3′-kinase and phospholipase C-dependent mechanism. Cancer Res. 1999;59:5475. [PubMed] [Google Scholar]
- 14.Corral RS, Iniguez MA, Duque J, Lopez-Perez R, Fresno M. Bombesin induces cyclooxygenase-2 expression through the activation of the nuclear factor of activated T cells and enhances cell migration in Caco-2 colon carcinoma cells. Oncogene. 2007;26:958. doi: 10.1038/sj.onc.1209856. [DOI] [PubMed] [Google Scholar]
- 15.Zheng R, Iwase A, Shen R, et al. Neuropeptide-stimulated cell migration in prostate cancer cells is mediated by RhoA kinase signaling and inhibited by neutral endopeptidase. Oncogene. 2006;25:5942. doi: 10.1038/sj.onc.1209586. [DOI] [PubMed] [Google Scholar]
- 16.Freund A, Chauveau C, Brouillet JP, et al. IL-8 expression and its possible relationship with estrogen-receptor-negative status of breast cancer cells. Oncogene. 2003;22:256. doi: 10.1038/sj.onc.1206113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levine L, Lucci JA, 3rd, Pazdrak B, et al. Bombesin stimulates nuclear factor kappa B activation and expression of proangiogenic factors in prostate cancer cells. Cancer Res. 2003;63:3495. [PubMed] [Google Scholar]
- 18.Liebow C, Crean DH, Lee MT, Kamer AR, Mang TS, Schally AV. Synergistic effects of bombesin and epidermal growth factor on cancers. Proc Natl Acad Sci U S A. 1994;91:3804. doi: 10.1073/pnas.91.9.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prenzel N, Zwick E, Leserer M, Ullrich A. Tyrosine kinase signalling in breast cancer. Epidermal growth factor receptor: convergence point for signal integration and diversification. Breast Cancer Res. 2000;2:184. doi: 10.1186/bcr52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prenzel N, Zwick E, Daub H, et al. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402:884. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- 21.Madarame J, Higashiyama S, Kiyota H, et al. Transactivation of epidermal growth factor receptor after heparin-binding epidermal growth factor-like growth factor shedding in the migration of prostate cancer cells promoted by bombesin. Prostate. 2003;57:187. doi: 10.1002/pros.10295. [DOI] [PubMed] [Google Scholar]
- 22.De Larco JE, Wuertz BR, Rosner KA, et al. A potential role for interleukin-8 in the metastatic phenotype of breast carcinoma cells. Am J Pathol. 2001;158:639. doi: 10.1016/S0002-9440(10)64005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun B, Schally AV, Halmos G. The presence of receptors for bombesin/GRP and mRNA for three receptor subtypes in human ovarian epithelial cancers. Regul Pept. 2000;90:77. doi: 10.1016/s0167-0115(00)00114-2. [DOI] [PubMed] [Google Scholar]
- 24.Engel JB, Schally AV, Halmos G, Baker B, Nagy A, Keller G. Targeted cytotoxic bombesin analog AN-215 effectively inhibits experimental human breast cancers with a low induction of multi-drug resistance proteins. Endocr Relat Cancer. 2005;12:999. doi: 10.1677/erc.1.01022. [DOI] [PubMed] [Google Scholar]
- 25.Cornelio DB, Roesler R, Schwartsmann G. Gastrin-releasing peptide receptor as a molecular target in experimental anticancer therapy. Ann Oncol. 2007;18:1457. doi: 10.1093/annonc/mdm058. [DOI] [PubMed] [Google Scholar]