Abstract
Background
Pretreating renal formulas with medications to lower the potassium and phosphorus content is common in clinical practice; however, the effect of this treatment on other nutrients is relatively unstudied. We examine whether nutrient composition is affected by pretreating renal formulas with sodium polystyrene sulfonate (SPS) suspension and sevelamer carbonate.
Methods
Fixed medication doses and treatment times were utilized to determine changes in the nutrient composition of Suplena® and Similac® PM 60/40. The effect of simultaneously adding both medications (co-administration) to the formula on the nutrient composition of Suplena® was also evaluated.
Results
Pretreatment of Suplena® with SPS reduced the concentrations of calcium (11–38 %), copper (3–11 %), manganese (3–16 %), phosphorus (0–7 %), potassium (6–34 %), and zinc (5–20 %) and increased those of iron (9–34 %), sodium (89–260 %), and sulfur (19–45 %) and the pH (0.20–0.50 units). Pretreatment of Similac® PM 60/40 with SPS reduced the concentrations of calcium (8–29 %), copper (5–19 %), magnesium (3–26 %), and potassium (33–63 %) and increased those of iron (13–87 %) and sodium (86–247 %) and the pH (0.40–0.81 units). Pretreatment of both formulas with the SPS suspension led to significant increases in the aluminum concentration in both formulas (507–3957 %). No differences in potassium concentration were observed between treatment times. Unexpectedly, the levels of neither phosphorus nor potassium were effectively reduced in Suplena® pretreated with sevelamer carbonate alone or when co-administered with SPS.
Conclusions
Pretreating formula with medications alters nutrients other than the intended target(s). Future studies should be aimed at predicting the loss of these nutrients or identifying alternative methods for managing serum potassium and phosphorus levels in formula-fed infants. The safety of pretreating formula with SPS suspension should also be examined.
Keywords: Renal nutrition, Drug–nutrient interactions, Sevelamer, Sodium polystyrene sulfonate, Hyperkalemia, Hyperphosphatemia, Pretreatment
Introduction
In 2012, approximately 7500 children in the USA suffered from end-stage renal disease [1]. Hyperkalemia and hyperphosphatemia are common complications in infants and young children with kidney disease and occur more frequently as kidney function declines [2]. These nutrient imbalances require dietary restriction of potassium and phosphorus, and, if unsuccessful, concurrent use of medications. Specialized renal formulas that are low in potassium and phosphorus are often prescribed for infants and young children, yet even these formulas may fail to resolve elevated serum potassium and phosphorus levels. In such cases, renal formulas are commonly treated with medications, such as sodium polystyrene sulfonate (SPS) and sevelamer carbonate, to further reduce the potassium and phosphorus content of formula, respectively. Unfortunately, pretreating formulas also reduces the levels of other essential nutrients, including calcium and magnesium [3–6]. It is important for clinicians prescribing such formulas to be aware of which essential nutrients can be altered in order to avoid the iatrogenic effects of the pretreated formula, especially since infants and children with kidney disease are already at high risk of malnutrition and growth impairment [7].
A variety of formulas, treatment times, and medication doses have been tested; however, no single, standardized pretreatment method has been determined best for clinical practice [3–6, 8–12]. Published studies have also examined a limited number of nutrients [3–6, 8–12]. The goal of this study was to evaluate the effects of controlled treatment times, medication doses, and co-administration of medications in renal-specific formulas on a broader range of essential and nonessential minerals and formula pH.
Methods
Experimental design
Three experiments were conducted with the following objectives: (1) to lower the potassium content of formula (Low K); (2) to lower the phosphorus content of formula (Low P); (3) to lower the potassium and phosphorus content of formula simultaneously (Low KP). The experimental protocols with doses and treatment times were based on previous work with SPS and sevelamer (Table 1) [3–6, 8–12]. The goals of this study were: (1) to evaluate whether nutrients other than potassium and phosphorus are affected in formulas pretreated with SPS and sevelamer carbonate, respectively; (2) to determine whether treating formula with SPS for 60 min causes a significant reduction in potassium concentration compared to a 30 min treatment; (3) to determine whether the co-administration of SPS and sevelamer carbonate in Suplena® is effective at reducing potassium and phosphorus simultaneously. Approval from Children’s Mercy Hospital’s IRB committee was completed prior to study initiation (IRB #13120398). Human subjects were not involved in this study.
Table 1.
Study design with controlled doses and treatment times for all study protocols
| Protocol | Objective | Medication(s) used | Formula(s) used | SPS dose (g/mEq K+)a |
Sevelamer carbonate dose (mg/100 mL formula)b |
Treatment time(s) |
|---|---|---|---|---|---|---|
| Low Kc | Decrease K+ content of formula | SPS | Suplena® | 0.25 | – | 30 minf |
| Similac® PM 60/40 | 0.50 | – | 60 minf | |||
| 1.0 | – | |||||
| Low Pd | Decrease P content of formula | Sevelamer carbonate | Suplena® | – | 400 | 10 ming |
| – | 800 | |||||
| – | 1600 | |||||
| – | 2400 | |||||
| – | 3200 | |||||
| Low KPe | Decrease K+ and P content of formula | SPS+ Sevelamer carbonate | Suplena® | 0.25 | 400 | 30 minf |
| 0.50 | 800 | 60 minf | ||||
| 1.0 | 1600 | |||||
| 2400 |
K+, Potassium; P, Phosphorus; SPS, Sodium polystyrene sulfonate
SPS doses are based on a previously established effective dose range for potassium (K+) removal in various formulas [3–5, 8, 9]
Sevelamer carbonate doses of 400–1600 mg /100 mL formula are based on a previously established dose range for phosphorus (P) removal in breast milk and various formulas using sevelamer [6, 12]. Additional dose levels of 2400 and 3200 mg /100 mL formula were selected to test for the effectiveness of higher doses in formula in the Low P protocol
For the Low K experiment, SPS was added to the formula using each dose and experimental treatment time combination listed
For the Low P experiment, sevelamer carbonate was added to the formula using each dose listed for the single treatment time listed
For the Low KP experiment, SPS and sevelamer carbonate were added to the formula using each dose combination and experimental treatment time listed. Medications were added simultaneously for ease of use for parents and nurses
Treatment times for protocols using SPS were based on previous studies demonstrating that pretreating the formula for ≤60 min is adequate for K+ removal and that longer treatment times do not result in more significant reductions in K+ [8, 9]
Treatment times for protocols using sevelamer carbonate were based on previous studies demonstrating that pretreating for 10 min is adequate for P removal and that longer treatment times do not result in a more significant reductions in P when using sevelamer [12]
Renal formula preparation
Cans of Suplena® (Abbott Laboratories, Abbott Park, North Chicago, IL), a ready-to-feed formula, were shaken thoroughly and combined into one large container. Similac® PM60/40 powder (Abbott Laboratories) was weighed and mixed with tap water according to the manufacturer’s recommendations to produce a 20 kcal/oz formula. Formulas were kept refrigerated at 3° C until used for each protocol.
Medication preparation
Low K protocol
Multiple doses of SPS (SPS® Suspension; Carolina Medical Products Co., Farmville, NC) were added to formula based on the potassium content of the formula stated on the manufacturer website. SPS was shaken prior to each administration and was measured by volume using a syringe. Dose volumes of SPS were added to achieve concentrations of 0.25, 0.5, and 1.0 g/mEq K+ and were selected based on previous studies [3–5, 8, 9]. Each dose–treatment time combination was repeated three times for the Low K protocol.
Low P protocol
Packets of sevelamer carbonate powder (Renvela®; Sanofi US, Bridgewater, NJ) were measured using a weight scale to achieve designated amounts of the active ingredient. All doses were per 100 mL formula, as previously described [6, 12]. Doses of 400, 800, and 1600 mg were selected based on prior studies demonstrating that sevelamer binds phosphorus effectively at doses of between 200 and 1600 mg in breast milk and cow’s milk, respectively [6, 12]. Additional doses of 2400 and 3200 mg were used to examine the effects of higher doses on phosphorus removal. Each dose–treatment time combination was repeated three times for the Low P protocol.
Low KP protocol
Dosing of SPS and sevelamer carbonate were identical to that of the Low K and Low P protocols, excluding the 2400 and 3200 mg sevelamer carbonate doses used in the Low P protocol. All possible combinations of dose and treatment time were used, and each combination was repeated three times for the Low KP protocol.
Pretreatment of formula
Untreated samples were drawn from the large batches of Suplena® and Similac® PM 60/40 prior to conducting each protocol. Formula was measured in 100-mL volumes and poured into containers. Medications were added directly to the formula and were shaken by hand for 60 s and then placed in a refrigerator at 3° C, undisturbed, for the allotted treatment times. Aliquots were drawn from the center of the sample. Formula pretreatment times of 30 and 60 min were selected for the Low K and Low KP protocols based on previous work by Thompson et al. [8] and Cameron et al. [9] demonstrating that pretreating formula with SPS for ≤60 min is adequate for potassium removal. A 10 min treatment time was selected for the Low P protocol based on previous work by Ferrera et al. [12] demonstrating that pretreating with sevelamer hydrochloride for 10 min is adequate for phosphorus removal.
Instrumentation and analytic methods
Samples were collected, and both treated and untreated samples were frozen at −20° C and sent to the Diagnostic Center for Population and Animal Health at Michigan State University for analysis. The samples were then thawed and prepared for radial inductively coupled plasma-optical emission spectrometry (ICP/OES) analysis by digesting 2 mL of each sample overnight at 95° C with 2 mL of concentrated nitric acid. Post-digest, samples were diluted to 10 mL and from that dilution a 1:10 dilution was prepared, resulting in a final 50-fold dilution of treated formula. ICP/OES analysis was conducted using a Vista-pro CCD simultaneous ICP-OES instrument (Agilent Technologies, Santa Clara, CA) to measure calcium, copper, iron, magnesium, manganese, phosphorus, potassium, sodium, sulfur, and zinc. pH was measured in thawed samples using a Corning pH meter 220 (Corning®, Corning, NY) with glass electrode.
Statistical analysis
A priori power analysis used results from a previous study in which Similac® PM60/40 was pretreated with SPS to observe changes in K+ and other select nutrients [4]. The results of that study indicated that potassium would be reduced by 0 mEq/L in untreated samples and by 12 mEq/L in samples treated with SPS at a dose of 1.0 g/mEq K+, respectively, with a standard deviation of zero. The sample size for the Low K and Low KP protocols was calculated based on this outcome variable at a two-sided level of significance of 0.025. Using a two sample t test, three samples per dose level gave us over 95 % power to detect the expected difference in potassium. For the Low P protocol, a power analysis was unable to be performed due to insufficient data available. Three samples per dose were used, as has previously been studied [6]. Multivariate analysis of variance was performed to determine whether differences in nutrient composition existed between untreated samples and samples treated with medications. If significant, pairwise comparisons were performed to determine whether changes in nutrient composition were due to medication dose or treatment times and adjusted using Tukey’s Honest Significant Difference test. A dose-by-treatment time interaction was also considered. A level of significance of p≤0.05 was considered to be statistically significant. Statistical analyses were performed with SPSS software (Release 2011. IBM SPSS Statistics for Windows, Version 20.0. IBM Corp., Armonk, NY).
Results
Nutrient content was examined in untreated formula and compared with the manufacturer’s specifications. All nutrients were found to be within the acceptable error limits allowed by the U.S. Food and Drug Administration (Title 21, Code of Federal Regulations, part 101.9) except for sodium (>20 % above manufacturer’s label). Manufacturer information on aluminum content was unavailable.
Low K protocol
Suplena®
Only the highest dose of SPS (1.0 g/mEq K+) reduced potassium concentration significantly compared to untreated samples. SPS also significantly reduced calcium, copper, manganese, phosphorus, and zinc levels at this dose concentration. Additionally, the levels of iron, sodium, and sulfur and pH increased in the formula, whereas magnesium was not affected (Table 2).
Table 2.
Nutrient composition of infant formula pretreated with sodium polystyrene sulfonate (SPS) in the Low K protocol
| Nutrient (mg/L) | SPS dose in formula (in g/mEq K+) | |||
|---|---|---|---|---|
| Untreated formula (no SPS added) |
0.25 | 0.50 | 1.0 | |
| Suplena® | ||||
| Nutrients reduced | ||||
| Calcium | 1145±20 | 1021±43 (−11 %) | 954±89 (−17 %)* | 706±53 (−38 %)*** |
| Copper | 2.1±0.1 | 2.1±0.1(−3%) | 2.1±0.1(−3%) | 1.9±0(−11 %)** |
| Manganese | 2.4±0.1 | 2.4±0.1(−3%) | 2.3±0.1(−7 %)* | 2.0±0.1(−16 %)*** |
| Phosphorus | 816±20 | 814±17 (0 %) | 830±8(+2 %) | 762±9(−7%)** |
| Potassium | 1069±23 | 976±96 (−9%) | 1002±155(−6%) | 710±61(−34 %)** |
| Zinc | 31.5±0.6 | 29.9±0.8(−5%) | 28.4±1(−10 %)** | 25.1±0.6(−20 %)*** |
| Nutrients increased | ||||
| Iron | 22±0.6 | 24±0.6(+9 %)** | 27±0(+25 %)*** | 29±0(+34 %)*** |
| Sodium | 673±17 | 1271±68 (+89 %)** | 1897±191(+182 %)*** | 2426±139(+260 %)*** |
| Sulfur | 438±9 | 521±21 (+19 %) | 636±76 (+45 %)** | 619±38 (+41 %)** |
| pH [H+] | 6.69 | 6.89*** | 7.03*** | 7.19*** |
| Nutrients unaffected | ||||
| Magnesium | 194±4 | 192±10 (−1 %) | 200±19 (+3 %) | 182±12 (−6%) |
| Similac® PM 60/40 | ||||
| Nutrients reduced | ||||
| Calcium | 456±29 | 420±39 (−8%) | 396±73(−13 %) | 323±31 (−29 %)* |
| Copper | 0.7±0 | 0.6±0.1(−19 %)* | 0.7±0.1(−5%) | 0.6±0(−14 %) |
| Magnesium | 48±0.6 | 35±2.9(−26 %)** | 40±5(−17 %) | 46±2(−3%) |
| Potassium | 588±7 | 395±36 (−33 %)*** | 338±50 (−42 %)*** | 218±9(−63 %)*** |
| Nutrients increased | ||||
| Iron | 5±0 | 6±0.6(+13 %) | 8±0.6(+53 %)** | 9±0.6(+87 %)*** |
| Sodium | 214±3 | 399±31 (+86 %)*** | 617±54 (+188 %)*** | 744±24 (+247 %)*** |
| pH [H+] | 7.13 | 7.53 *** | 7.71 *** | 7.94 *** |
| Nutrients unaffected | ||||
| Phosphorus | 206±10 | 214±18 (+4 %) | 221±27 (+7 %) | 208±13 (+6 %) |
| Sulfur | 227±3 | 224±16 (−1 %) | 296±95(+31 %) | 284±20(+25 %) |
| Zinc | 5.9±0.2 | 5.4±0.4(−8%) | 5.7±0.3(−2%) | 5.6±0.2(−4%) |
Significant differences were tested with pairwise comparison analysis (Tukey’s Honest Significant Difference) and compared to untreated samples:
p≤ 0.05,
p≤ 0.01,
p≤0.001
Data are given as the mean ± standard deviation (SD), with the percentage change given in parenthesis. Only results from the 30 min treatment times are shown due to non-significant differences being detected between treatment times
SPS, Sodium polystyrene sulfonate
Similac® PM 60/40
In contrast to Suplena, potassium concentration was significantly reduced with all doses of SPS compared to untreated samples (Table 2). Calcium, copper, and magnesium concentration were also reduced significantly for at least one dose concentration of SPS. Additionally, the levels of iron, sodium, and pH increased significantly, whereas no changes in phosphorus, sulfur, and zinc levels were observed.
Low P protocol
Only the highest dose of sevelamer carbonate (3200 mg/100 mL Suplena®) showed a significant reduction in the phosphorus content in Suplena®. Sevelamer carbonate also significantly reduced the concentrations of calcium, copper, magnesium, manganese, potassium, sulfur, and zinc and raised pH at this concentration. Iron and sodium concentrations were not affected (Table 3).
Table 3.
Nutrient composition of Suplena® pretreated with sevelamer carbonate in the Low P protocol
| Nutrients (mg/L) | Sevelamer carbonate dose (in mg/100 mL Suplena®) | |||||
|---|---|---|---|---|---|---|
| Untreated Suplena® (no sevelamer carbonate added) |
400 | 800 | 1600 | 2400 | 3200 | |
| Nutrients reduced | ||||||
| Calcium | 1145±20 | 1050 ±33(−8%) | 1118±111(−2%) | 1061±32 (−7%) | 1033±25 (−10 %) | 890±50 (−20 %)** |
| Copper | 2.1±0.1 | 2.0±0.1 (−5%) | 2.1±0.2 (−1%) | 2.0±0.1 (−5%) | 1.9±0.1(−10 %) | 1.7±0.1 (−19 %)* |
| Magnesium | 194±4 | 181±6(−7%) | 191±18 (−2%) | 180±5(−7%) | 179±3(−8%) | 154±8(−19 %)** |
| Manganese | 2.4±0.1 | 2.3±0.1 (+6 %) | 2.4±0.3 (+5 %) | 2.3±0.1 (0 %) | 2.2±0.1(−1%) | 1.9±0.2(−16 %)* |
| Phosphorus | 816±20 | 759±18(−7%) | 815±83 (0%) | 777±21 (−5%) | 757±16(−7%) | 651±36(−18%)* |
| Potassium | 1069 ±23 | 1022 ±64 (−4%) | 1071 ±76 (0%) | 1028 ±4(−4%) | 956±21(−11%) | 825±54 (−20 %)** |
| Sulfur | 438±9 | 416±21 (−5%) | 435±35 (−1%) | 414±5(−5%) | 394±10(−10 %) | 340±21 (−20 %)** |
| Zinc | 31.5 ±0.6 | 29.4±1.6 (−7%) | 30.8±2.7 (−2%) | 28.9 ±0.9 (−8%) | 28.4 ±0.6 (−10 %) | 24.6±1.4 (−20 %)** |
| Nutrients increased | ||||||
| pH [H+] | 6.69 | 7.88*** | 8.80*** | 9.23*** | 9.49*** | 9.62*** |
| Nutrients unaffected | ||||||
| Iron | 22±0.6 | 20±0.6 (−10 %) | 22±3.2 (0 %) | 20±1.5 (−5%) | 22±0.6(−3%) | 20±1.2(−8%) |
| Sodium | 673±17 | 632±47 (−6%) | 678±58 (+1 %) | 681±17 (+1 %) | 653±10 (−3%) | 613±35(−7%) |
Significant differences were tested with pairwise comparison analysis (Tukey’s Honest Significant Difference) and compared to untreated samples:
p≤ 0.05,
p≤ 0.01,
p≤0.001
Data are given as the mean ± (SD), with the percentage change given in parenthesis
Low KP protocol
Co-administration of SPS and sevelamer carbonate did not significantly reduce the level of either potassium or phosphorus for any of the dose combinations examined. However, co-administration of these drugs in Suplena® still significantly reduced the concentrations of calcium, manganese, and zinc and increased iron, magnesium, sodium, and sulfur and increased the pH of the formula (Electronic Supplementary Material Table 1).
Pretreatment times
Pretreatment of the formula for 60 min resulted in slightly higher reductions in potassium concentration in the samples compared to a pretreatment of 30 min; however, the difference was not significant. Treatment time did not affect the concentration of the other nutrients examined.
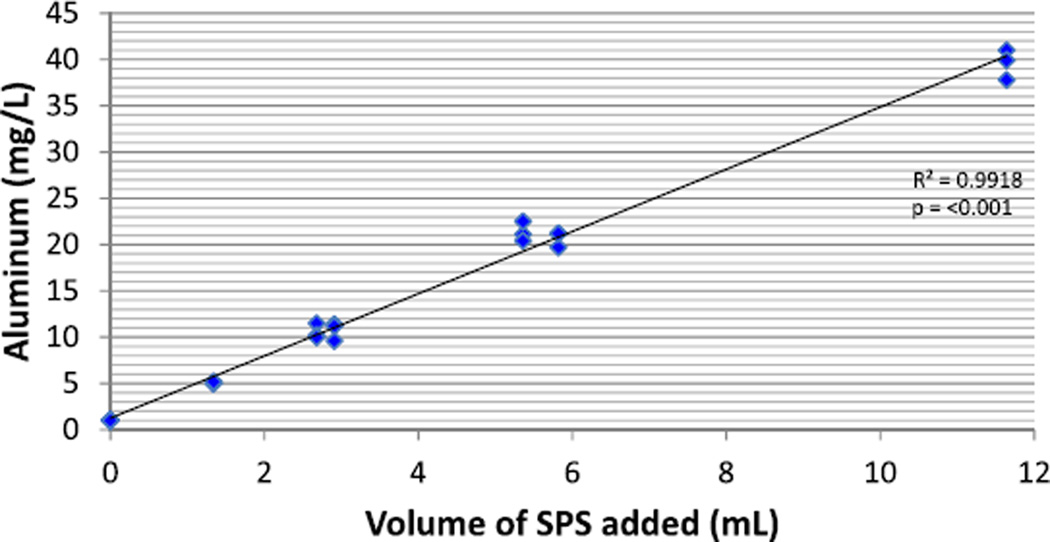
Aluminum
There was a positive linear association between SPS dose and aluminum concentration in both formulas (p< 0.001) in the Low K and Low KP protocols. Data from the Low K protocol are shown in Fig. 1. Aluminum concentration was not affected by sevelamer carbonate in the Low P protocol.
Fig. 1.
Aluminum concentration of formula pretreated with sodium polystyrene sulfonate (SPS) suspension in the Low K Protocol. Individual values of aluminum for each dose level of SPS suspension in the Low K protocol are included. Due to differing levels of potassium in the formulas being tested, dose was converted to the amount (mL) of SPS suspension added to formula to standardize the measurement. Dose equivalent is 1 g SPS=4 mL of SPS suspension
Discussion
Our findings demonstrate that SPS suspension not only significantly reduces the potassium concentration, but also significantly alters the concentrations of calcium, copper, iron, magnesium, manganese, phosphorus, potassium, sodium, sulfur, and zinc, as well as the pH in Suplena® and Similac® PM 60/40 while increasing aluminum concentration. Our results also illustrate that 60 min treatment times do not result in more significant reductions in potassium concentration than 30 min treatment times. Lastly, sevelamer carbonate as monotherapy or co-administration with SPS was ineffective in reducing either the phosphorus or potassium concentration of Suplena.
Similar to previous studies, we observed reductions in potassium concentration and a subsequent rise in the concentration of sodium in Similac® PM 60/40 pretreated with SPS, with the latter due to the sodium content of the SPS exchange resin added to the formula [3–5, 9, 10]. However, SPS pretreatment of Suplena®, a renal formula often used in children aged >1 year, was ineffective in reducing the potassium level of this formula. To our knowledge, this is the first time Suplena® has been tested in a study of this kind. The mechanism for the differences observed between these two formulas is unknown. Suplena® contains fiber and is more viscous than Similac® PM 60/40 due to its higher caloric density (53 vs. 20 kcal/oz, respectively). Both of these factors potentially contribute to reduced efficacy of pretreating Suplena® with SPS by inhibiting potassium binding or decreasing the amount of SPS that settles out after binding. It should be noted that doses of SPS were chosen based on previous studies of Similac® PM 60/40 and that these doses may be insufficient to reduce the level of potassium in Suplena®.
Consistent with previous studies, we observed reductions in calcium and magnesium in SPS-treated formula [3–5]. Loss of these nutrients is of particular concern in children with kidney disease since as early as stage 2 chronic kidney disease approximately 30 % of patients have bone mineralization defects; this percentage increases as kidney function declines [13]. Bone abnormalities in renal patients persist into adulthood and may affect long-term functional status [14]. The loss of calcium and magnesium, essential nutrients in bone mineralization, through the pretreatment of formula may contribute to worsened bone health in a group already at high risk for bone disease.
Due to the expanded nutrient profile examined in this study, we also observed increases in the concentrations of sulfur and iron and reductions in those of zinc with pretreatment of SPS. Previous work by Bunchman et al. [4] demonstrated that approximately 5 % of SPS, a sulfur-containing compound, remained in Similac® PM 60/40 after a 30 min treatment. Due to the limitations of our study, we were unable to demonstrate with certainty whether the observed increase is related to the exchange of sulfur from SPS or to the sulfur from residual SPS remaining in formula after treatment, these being the two most plausible circumstances. We also observed a positive linear relationship between iron concentration and SPS dose in both formulas tested. These findings are consistent with previous work by Rivard et al. [3] which demonstrated higher iron levels in a high protein adult formula pretreated with SPS at dose concentrations of 0.5 and 1 g/mEq K+. Given that many children with kidney failure require iron supplementation, physicians need to be aware that starting, stopping, or changing the dose of SPS in treated formula may affect the amount of iron supplementation that should be given to patients. Finally, in Suplena®, we observed a negative linear relationship between zinc concentration and SPS dose. Zinc deficiency is common in dialysis patients (40–78 %) due to dialysate losses and is associated with increased risk of cardiovascular disease, failure to thrive, dermatitis, inflammatory disease, and delayed sexual maturation [15–19]. Serum zinc levels should be routinely checked in children on dialysis, and when SPS-treated Suplena® is used, zinc supplementation and more frequent checks of serum zinc levels are recommended.
In addition to observing changes in the nutrient composition of the two formulas tested, we observed a significant rise in aluminum concentration in both formulas that was directly correlated to the volume of SPS suspension added during treatment. Suspension forms of SPS contain a magnesium aluminum silicate additive which resulted in the increased aluminum content in our samples (range 5–40 mg/L); these levels are significantly higher than that proposed by Burrell and Exley [20] to be of concern in infant formulas (0.2–0.7 mg/L). Aluminum is a neurotoxin filtered by the kidneys and exposure to high levels of the metal in infants, and subsequent incorporation into tissue, has been associated with cognitive impairment and aluminum-induced bone disease [21, 22]. No safe dose of aluminum has been identified and, consequently, intermittent or low dose use of aluminum–phosphate binders remains inadvisable in the pediatric population [23, 24]. SPS is also available in a powder form which does not contain aluminum additives, so use of SPS powder is advised.
Treatment times among published studies have been highly variable, ranging from 30 min to 24 h [3–5, 8–11]. The lack of standardized treatment times for pretreating formula with SPS makes comparisons among studies difficult. Similar to previous findings by Thompson et al. [8], our results indicate that pretreatment of >30 min provides little benefit in further reducing the potassium content of a formula. Reduction of treatment times to 30 min and standardizing methods for pretreating formula with SPS will not only allow for consistency among practitioners and centers, but also reduce the burden on nurses and families that longer treatment times confer.
This is the first study to pretreat Suplena® with sevelamer carbonate, both as monotherapy and in combination with SPS. Previous studies pretreating cow’s milk and tube feedings with sevelamer have shown that phosphorus content is reduced effectively at a dose of 800 mg/100 mL formula, while doses as low as 200 mg/100 mL are effective at reducing the phosphorus level in breast milk [6, 12]. In the Low P protocol, significant reductions of phosphorus in Suplena® were not observed until a sevelamer carbonate dose of 3200 mg/100 mL formula was added. These findings correspond with previous work from Raaijmakers et al. who demonstrated a decreased efficacy in phosphate binding (measured as mean percentage change) with increasing phosphate concentration in formulas [6]. Formulas tested in that study contained 56, 94, 438, and 625 mg/L of phosphate, with corresponding reductions of 87, 69, 25, and 22 %, respectively, when 800 mg sevelamer carbonate per 50 mL formula was tested. By comparison, in our study a 5 % decrease in phosphorus content was observed after pretreatment with Suplena® (higher phosphorus concentration 816 mg/L) with a similar dose (1600 mg sevelamer carbonate per 100 mL) of sevelamer carbonate. The highest dose of sevelamer carbonate we tested (3200 mg/100 mL formula) also resulted in significant changes in the concentrations of calcium, copper, magnesium, manganese, phosphorus, potassium, sulfur, and zinc. Sevelamer carbonate is an anion exchange resin and, similar to SPS, resulted in the binding of other ions. While the mechanism is unknown, SPS and sevelamer carbonate may not bind potassium and phosphorus, respectively, as selectively as desired. Additionally, the pH increased from 6.69 in untreated samples to much higher values of 7.88–9.32 in treated samples. Similar findings have been previously observed in various formulas pretreated with sevelamer carbonate and do not warrant safety concerns given that prolonged treatment of alkaline medications (sodium bicarbonate) have been previously shown to be safe in humans [6, 25]. Due to the large doses of sevelamer carbonate required, pretreating Suplena with sevelamer carbonate to reduce phosphorus content is ill-advised.
Finally, pretreating Suplena® with both SPS and sevelamer carbonate simultaneously resulted in the non-significant binding of potassium and phosphorus while still significantly altering other nutrients. Since pretreating Suplena® in the Low K and Low P protocols only showed significant reductions in potassium and phosphorus in the highest doses tested, the use of additional formulas where pretreating is more effective should be tested to determine whether using this method is valid. While no data have been reported, pretreating formula with SPS, decanting, and then pretreating with sevelamer carbonate may prove to be the better method.
Limitations of the study include limiting the dose of SPS to 1.0 g/mEq K+, testing only the suspension form of SPS, and only pretreating one formula (Suplena®) with sevelamer carbonate and both medications simultaneously. We limited SPS to 1.0 g/mEq K+ based on previous data from pretreating other products; however, effective dose ranges may vary between formulas. SPS is also available in a powder form which should be tested since it may alter nutrient composition differently than the suspension form used in this study. Also, pretreating additional formulas simultaneously with these medications may yield different results than when tested in Suplena® given that SPS and sevelamer carbonate bind potassium and phosphorus more effectively in other formulas [3–6, 8–12]. The strengths of this study include collecting all samples in triplicate and testing multiple medications, doses, and treatment times in formula. This is the first study to have examined the effects of pretreating formula with both SPS and sevelamer carbonate separately and together. Also, to date, this is the largest panel of nutrients examined in this type of study.
In summary, we demonstrate that pretreating Suplena® and Similac® PM 60/40 with SPS suspension significantly affected the nutrient profile and that pretreating Suplena® with sevelamer carbonate or with SPS suspension and sevelamer carbonate simultaneously was not effective in reducing either phosphorus or potassium and should be avoided. Our findings also demonstrate that other essential nutrients may be adversely affected by pretreatment, which should be considered to ensure that patients do not ingest deficient or overabundant amounts of these nutrients when pretreated formulas are used. Establishing standardized methods for pretreatment of formulas to be adopted by institutions will help achieve this goal. Future studies should be aimed at determining appropriate doses of SPS and sevelamer carbonate for pretreating various formulas as well as predicting changes in nutrient composition after treatment. Due to the additional aluminum in the SPS suspension, use of SPS powder is advised until studies examining the safety of SPS suspension can be further evaluated.
Supplementary Material
Acknowledgments
We thank our pharmacists, Chelsey Jensen and Valerie Smith, who provided assistance with the technical, dosage, and administration aspects of the pharmaceutical agents used in this study; Douglas Bittel and Patricia Cudmore for their assistance and training in the lab; Robin Carroll and the Patient Care Services Research staff for assisting in the approval and logistics of this study; Children’s Mercy Hospital and the University of Kansas Medical Center for their support and use of resources and space that was needed to complete this study.
Funding This work was supported by the Children’s Mercy Hospital Patient Care Services Research Fund and Department of Nutrition Services funding, KL2 TR000119-04.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00467-015-3115-5) contains supplementary material, which is available to authorized users.
Conflict of interest The authors declare that they have no conflict of interest.
Contributor Information
Jacob M. Taylor, Email: jacobtaylor.ms_rd@yahoo.com.
Leah Oladitan, Email: lmoladitan@cmh.edu.
Susan Carlson, Email: scarlson@kumc.edu.
Jill M. Hamilton-Reeves, Email: jhamilton-reeves@kumc.edu.
References
- 1.National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. U.S. Renal Data System. USRDS 2014 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2014. [Google Scholar]
- 2.Wong H, Mylrea K, Feber J, Drukker A, Filler G. Prevalence of complications in children with chronic kidney disease according to KDOQI. Kidney Int. 2006;70:585–590. doi: 10.1038/sj.ki.5001608. [DOI] [PubMed] [Google Scholar]
- 3.Rivard AL, Raup SM, Beilman GJ. Sodium polystyrene sulfonate used to reduce the potassium content of a high-protein enteral formula: a quantitative analysis. J Parenter Enteral Nutr. 2004;28:76–78. doi: 10.1177/014860710402800276. [DOI] [PubMed] [Google Scholar]
- 4.Bunchman TE, Wood EG, Schenck MH, Weaver KA, Klein BL, Lynch RE. Pretreatment of formula with sodium polystyrene sulfonate to reduce dietary potassium intake. Pediatr Nephrol. 1991;5:29–32. doi: 10.1007/BF00852836. [DOI] [PubMed] [Google Scholar]
- 5.Starbuck WC. Reduction of potassium and calcium in milk by sodium sulfonated polystyrene resins. Kidney Int. 1972;2:175–177. doi: 10.1038/ki.1972.88. [DOI] [PubMed] [Google Scholar]
- 6.Raaijmakers R, Houkes LM, Schröder CH, Willems JL, Monnens LA. Pre-treatment of dairy and breast milk with sevelamer hydrochloride and sevelamer carbonate to reduce phosphate. Perit Dial Int. 2013;33:565–572. doi: 10.3747/pdi.2012.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warady BA, Neu AM, Schaefer F. Optimal care of the infant, child, and adolescent on dialysis: 2014 update. Am J Kidney Dis. 2014;64:128–142. doi: 10.1053/j.ajkd.2014.01.430. [DOI] [PubMed] [Google Scholar]
- 8.Thompson K, Flynn J, Okamura D, Zhou L. Pretreatment of formula or expressed breast milk with sodium polystyrene sulfonate (kayexalate) as a treatment for hyperkalemia in infants with acute or chronic renal insufficiency. J Ren Nutr. 2013;23:333–339. doi: 10.1053/j.jrn.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Cameron JC, Kennedy D, Feber J, Wong E, Geier P, Vaillancourt R. Pretreatment of infant formula with sodium polystyrene sulfonate : focus on optimal amount and contact time. Paediatr Drugs. 2013;15:43–48. doi: 10.1007/s40272-012-0003-3. [DOI] [PubMed] [Google Scholar]
- 10.Fassinger N, Dabbagh S, Mukhopadhyay S, Lee DY. Mineral content of infant formula after treatment with sodium polystyrene sulfonate or calcium polystyrene sulfonate. Adv Perit Dial. 1998;14:274–277. [PubMed] [Google Scholar]
- 11.Bonnet L, Goudable J, Accominotti M, Fontaine D, Cochat P. Effect of ion exchange resins on the composition of milk. Nephrologie. 1997;18:287–289. [PubMed] [Google Scholar]
- 12.Ferrara E, Lemire J, Reznik VM, Grimm PC. Dietary phosphorus reduction by pretreatment of human breast milk with sevelamer. Pediatr Nephrol. 2004;19:775–779. doi: 10.1007/s00467-004-1448-6. [DOI] [PubMed] [Google Scholar]
- 13.Wesseling-Perry K. Bone disease in pediatric chronic kidney disease. Pediatr Nephrol. 2013;28:569–576. doi: 10.1007/s00467-012-2324-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartosh SM, Leverson G, Robillard D, Sollinger HW. Long-term outcomes in pediatric renal transplant recipients who survive into adulthood. Transplantation. 2003;76:1195–1200. doi: 10.1097/01.TP.0000092524.75807.84. [DOI] [PubMed] [Google Scholar]
- 15.Reina de la Torre ML, Navarro-Alarcon M, del Moral LM, Lopez GSH, Palomares-Bayo M, Oliveras Lopez MJ, Blanca Herrera RM, Agil A. Serum Zn levels and Cu/Zn ratios worsen in hemodialysis patients, implying increased cardiovascular risk: a 2-year longitudinal study. Biol Trace Elem Res. 2014;158:129–135. doi: 10.1007/s12011-014-9921-y. [DOI] [PubMed] [Google Scholar]
- 16.Roozbeh J, Sharifian M, Sagheb MM, Shabani S, Hamidian Jahromi A, Afshariani R, Pakfetrat M, Salehi O. Comment on: does zinc supplementation affect inflammatory markers in hemodialysis patients? Ren Fail. 2011;33:466–467. doi: 10.3109/0886022X.2011.568144. [DOI] [PubMed] [Google Scholar]
- 17.Filler G, Felder S. Trace elements in dialysis. Pediatr Nephrol. 2014;29:1329–1335. doi: 10.1007/s00467-013-2585-6. [DOI] [PubMed] [Google Scholar]
- 18.Bock DE, Prabhakaran V, Filler G. Picture of the month: severe zinc deficiency in infancy (acrodermatitis enteropathicalike picture) Arch Pediatr Adolesc Med. 2009;163:765–766. doi: 10.1001/archpediatrics.2009.126-a. [DOI] [PubMed] [Google Scholar]
- 19.American Academy of Pediatrics. Committee on Nutrition, Kleinman RE. Pediatric nutrition handbook. Elk Grove Village: American Academy of Pediatrics; 2004. [Google Scholar]
- 20.Burrell SA, Exley C. There is (still) too much aluminium in infant formulas. BMC Pediatr. 2010;10:63. doi: 10.1186/1471-2431-10-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Priest ND. The biological behaviour and bioavailability of aluminium in man, with special reference to studies employing aluminium-26 as a tracer: review and study update. J Environ Monit. 2004;6:375–403. doi: 10.1039/b314329p. [DOI] [PubMed] [Google Scholar]
- 22.Bishop NJ, Morley R, Day JP, Lucas A. Aluminum neurotoxicity in preterm infants receiving intravenous-feeding solutions. N Engl J Med. 1997;336:1557–1561. doi: 10.1056/NEJM199705293362203. [DOI] [PubMed] [Google Scholar]
- 23.Cannata-Andia JB. Reconsidering the importance of long-term low-level aluminum exposure in renal failure patients. Semin Dial. 2001;14:5–7. doi: 10.1046/j.1525-139x.2001.00002.x. [DOI] [PubMed] [Google Scholar]
- 24.Hutchison AJ, Smith CP, Brenchley PE. Pharmacology, efficacy and safety of oral phosphate binders. Nat Rev Nephrol. 2011;7:578–589. doi: 10.1038/nrneph.2011.112. [DOI] [PubMed] [Google Scholar]
- 25.Van Goidsenhoven GM, Gray OV, Price AV, Sanderson PH. The effect of prolonged administration of large doses of sodium bicarbonate in man. Clin Sci (Lond) 1954;13:383–401. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.