Abstract
Surgical treatment remains the only curative treatment for gallbladder cancer. However, even after liver resection, locoregional failure seems to be a significant problem. While there is no Level I evidence, multiple studies have shown benefit for adjuvant radiation in high-risk patients. After extensive liver resection, tolerance to conventional chemoradiation may be limited by potential liver toxicity. Stereotactic body radiotherapy has been used safely and effectively in hepatobiliary malignancies. We present a case report, highlighting the potential therapeutic role of adjuvant stereotactic body radiotherapy (SBRT) for gallbladder cancer.
Keywords: gallbladder cancer, adjuvant, sbrt
Introduction
Gallbladder cancer (GBC) is a rare malignancy. Its incidence varies widely geographically with an age-standardized incidence rate of 1.7 in the US to as high as 10.4 in Chile [1]. In 2014, there were 10,650 gallbladder and extrahepatic bile duct cancers in the US with 3,630 deaths [2]. Surgical resection offers the only curative treatment for gall bladder cancer. While gallbladder cancers can be incidentally detected after cholecystectomy, the majority of patients present with invasive liver disease [3]. Even after organ-confined disease, local recurrence, in up to 65% of patients, is a significant cause of morbidity and mortality [4]. This has led to the use of liver resections to improve outcome in gallbladder confined disease [5] and the use of adjuvant chemotherapy and chemoradiation [6-8].
Despite the potential role of postoperative RT [9-11], its limitation remains radiation-induced liver disease, which develops in 5% to 10% of patients exposed to an excess of 30 to 35 Gy of radiation, depending on irradiated liver volume and hepatic functional reserve [12]. This is particularly true after hepatic resection with limited reserve. Very little data exists describing the risk of hepatobiliary toxicity for lesions near the central hepatobiliary area around the porta hepatis [13].
Stereotactic body radiotherapy (SBRT) enables the delivery of effective doses of radiation to tumors with steep dose gradients minimizing doses to the surrounding normal tissues. It has been successfully used in hepatobiliary and pancreatic malignancies. Moreover, short courses of SBRT avoids delays or interruption of systemic therapy [14-16].
We present a report of two cases where SBRT was used as postoperative radiation for gallbladder cancer in the adjuvant setting.
Case presentation
This study was reviewed and approved by the Dana-Farber Harvard Cancer Center Institutional Review Board (DFHCC 09-451). Informed patient consent was obtained from all patients in this report.
Case 1
A 70-year-old female presented with a one-month history of right upper quadrant abdominal pain. She noted a 20-pound weight loss and significant loss of appetite over the previous three months. On physical examination, the liver was palpable 4 cm below the right costal margin and was firm and tender. She underwent an ultrasound of the liver that demonstrated a large mass in the right lobe of the liver that was heterogeneous, predominantly hypoechoic, with internal vascularity and measuring 10.5 x 7.4 x 12.2 cm. There was no intra- or extrahepatic biliary ductal dilatation. She then underwent an MRI of the abdomen that demonstrated a mass within the anterior segments of the right lobe of the liver measuring 7.1 x 4.6 x 5.2 cm that had heterogeneous decreased signal T1 images with slightly increased signal on T2 imaging. She underwent an upper GI endoscopy and colonoscopy that demonstrated no evidence of primary tumors. Her liver function tests revealed an AST 23, ALT 24, alkaline phosphatase 169, total bilirubin 0.5, CEA less than 0.5, and CA 19-9 34.
The patient received induction gemcitabine and capecitabine chemotherapy and portal vein embolization to induce left lobe hypertrophy. Restaging scans showed no evidence of metastasis and significant response with decreasing size of the lesion. She then underwent a right hepatic resection of the gallbladder fossa and the involved liver. Fiducial seeds were placed in the liver bed. It is our institutional practice to place fiducials in the liver tumor resection bed in all liver tumor resections at the time of surgery. The pathology showed that the patient had a poorly differentiated adenocarcinoma in the gallbladder with secondary involvement of the liver making this T3 disease. The cauterized liver margin was involved with invasive carcinoma; perineural invasion was absent. None of the lymph nodes was involved. She was thought to be a candidate for adjuvant postoperative radiation therapy since she had high-risk factors of a positive margin, T3 stage, and high grade. Because she had little residual liver, SBRT was advised.
The planning target volume was a 1.5 cm deep segment of the liver resection margin, which included the fiducial seeds. She received 24 Gy in three consecutive fractions to the liver resection bed. She completed the therapy with minimal side-effects, including fatigue and tiredness for a week and was well at her last follow-up 24 months later.
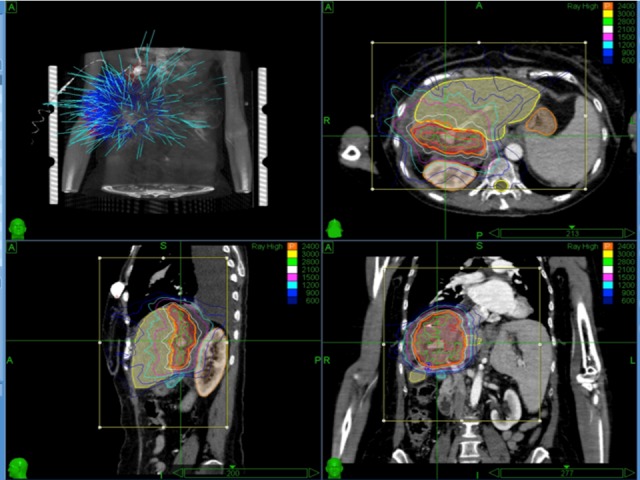
A representative treatment plan is shown in Figure 1. The treatment prescribed to the 79% isodose line using the CyberKnifeTM technique with Synchrony respiratory tracking. The max dose was 30.4 Gy and the V21 (volume of residual liver receiving 21 Gy) and V15 (volume of residual normal liver receiving 15 Gy) was 11% and 21%, respectively. The maximum dose received by the < 1 cc of bowel was 17 Gy in three fractions.
Figure 1. Representative Treatment Plan.
Case 2
A 77-year-old gentleman, known to have bladder cancer along with cardiac disease, had staging surveillance scans by his urologist. The CT showed focal gallbladder wall thickening in the fundus with enhancement consistent with probably what was thought to be a 2.3 x 1.6 x 2.6 cm tumor mass suspicious for primary gallbladder cancer. The tumor marker CA19-9 was elevated at 287. He had a cholecystectomy with partial hepatectomy and resection of segment IVB and V as well as the removal of portal nodes. Fiducial seeds were placed in the tumor bed during surgery. The pathology showed a moderately differentiated carcinoma of 2.8 cm in the gallbladder invading the perimuscular connective tissues. There was no evidence of regional metastasis. There was a perineural invasion, but no evidence of any venous invasion. He developed cardiac toxicity with adjuvant capecitabine. In view of his high-risk disease, age, and co-morbidities, he was advised to undergo adjuvant radiation with SBRT followed by gemcitabine chemotherapy. At his last follow-up 74 months after surgery, he was still free of disease.
The planning target volume and the treatment technique was similar to the previous patient. He received 24 Gy in three fractions to the 68% isodose line with a maximum dose of 35.3 and the V21 and V15 - 9% and 17%. The maximum dose to < 1 cc of bowel was 10 Gy in three fractions.
Discussion
Complete surgical resection remains the only potential curative treatment for GBC [17]. Additional liver resections improve outcomes in incidentally detected cancer and in organ-confined and locally advanced disease [4, 18-19]. However, even after organ-confined disease, locoregional recurrence is a significant problem [4, 20]. The high incidence of locoregional recurrence in patients with GBC makes postoperative radiotherapy (RT) a rational option. The benefit of adjuvant RT or chemoradiation has not been tested in randomized studies. However, attempts to define the role of adjuvant RT have been made by single and multi-institutional studies [9, 11, 21-22] and analysis of national databases. Mojica, et al. analyzed 3,187 patients from the SEER (Surveillance, Epidemiology and End Results of the National Cancer Institute) population database of whom 17% were treated with adjuvant radiotherapy [23]. For patients presenting with locally advanced disease and lymph node involvement, an increase in median survival with adjuvant treatment (14 versus 8 months, p < 0.001) was observed. Hyder, et al. analyzed a sample of 5,011 patients (18% received adjuvant RT) and reported that RT was associated with an improved short-term survival but the benefit of RT seemed to dissipate after 12 months because there was no difference in five-year survival [24]. This highlights the role for systemic therapy in controlling metastatic disease [8, 25]. It should be noted that in neither of the studies was margin status evaluated. A recent systematic review and meta-analysis on biliary tract cancers included six studies addressing the role of adjuvant treatments in GBC. However, due to the rarity of this disease, there is no Class I evidence from randomized clinical trials supporting the role of adjuvant radiation or chemoradiation. The National Comprehensive Cancer Network Guidelines for GBC suggest adjuvant chemoradiation or chemotherapy alone, recognizing, however, that limited data exist to define a standard regimen.
Several investigators have used multivariate analysis to determine useful prognostic factors for recurrence of gallbladder carcinoma after surgical resection to identify patients who might benefit from adjuvant therapy. Margin positive (R1 resection) patients derived the clearest survival benefit from the use of adjuvant therapies [26-27]. It would also appear to benefit patients with high T stage and positive nodes [28]. Others have developed multivariate predictive models [25] and nomograms [29] for risk assessment. By these reports, other potentially significant factors include tumor grade and perineural invasion.
In the present report, our patients carried high-risk features of a positive margin, liver invasion, and high grade and were in a subgroup where studies favored adjuvant treatment. However, they had small residual liver after their liver resections. This places them at a high risk of radiation-induced liver injury. It is known that radiation-induced liver disease develops in 6% to 66% of patients exposed to an excess of 30 to 35 Gy of radiation, depending on irradiated liver volume and hepatic functional reserve [28]. These patients may face RILD (radiation-induced liver damage) starting with the classic triad of ascites, hepatomegaly, and elevated liver enzymes. The majority of patients recover completely in three to five months, while some progress towards a chronic stage, with worsening liver fibrosis and failure, developing fulminant hepatic failure [12].
To reduce the radiation to remnant liver and the risk of RILD, SBRT was proposed as adjuvant treatment in our patients. SBRT has been reported to be used effectively and safely for the treatment of hepatobiliary and pancreatic cancers in the setting of both primary and adjuvant treatment [14-16]. Effective radiation therapy can be delivered to the tolerance of the adjacent critical normal tissue structures. A report from Korea also addressed the use of SBRT in unresectable gallbladder cancer in a case series that included four GBC patients [30].
Conclusions
SBRT can potentially be safely delivered to a planned postopertaive liver resection target volume as decribed. This first report of use of SBRT in the postoperative adjuvant setting may pave the way of safe and effective adjuvant radiation for gallbladder cancer in future studies.
Appendices
CONSENT
Written informed consent was obtained from the patients for publication of their treatment with accompanying images accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this Journal.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS CONTRIBUTION:
AM treated the patients and was involved in drafting the manuscript. ND was involved in drafting and editing the manuscript. All authors read and approved the manuscript. JFT, KK and EV were involved in the surgical amangement of the patients.
ACKNOWLEDGEMENTS: None
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Dana Farber Harvard Cancer Center issued approval DFHCC 09-451
References
- 1.Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Int J Cancer. 2015;136:0–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Cancer statistics 2014. Siegel R, Ma J, Zou Z, Jemal A. CA Cancer J Clin. 2014 Jan;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.The impact of tumor extent (T stage) and lymph node involvement (N stage) on survival after surgical resection for gallbladder adenocarcinoma. Zaydfudim V, Feurer ID, Wright JK, Pinson CW. HPB (Oxford) 2008;10:420–427. doi: 10.1080/13651820802320057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.A 21-year analysis of stage I gallbladder carcinoma: is cholecystectomy alone adequate? Hari DM, Howard JH, Leung AM, Chui CG, Sim MS, Bilchik AJ. HPB (Oxford) 2013;15:40–48. doi: 10.1111/j.1477-2574.2012.00559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Combined resection and radiofrequency ablation for advanced hepatic malignancies: results in 172 patients. Pawlik TM, Izzo F, Cohen DS, Morris JS, Curley SA. Ann Surg Oncol. 2003;10:1059–1069. doi: 10.1245/ASO.2003.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. Horgan AM, Amir E, Walter T, Knox JJ. J Clin Oncol. 2012;30:1934–1940. doi: 10.1200/JCO.2011.40.5381. [DOI] [PubMed] [Google Scholar]
- 7.Review article: surgical, neo-adjuvant and adjuvant management strategies in biliary tract cancer. Skipworth JR, Olde Damink SW, Imber C, Bridgewater J, Pereira SP, Malagó M. Aliment Pharmacol Ther. 2011;34:1063–1078. doi: 10.1111/j.1365-2036.2011.04851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Recent advances in systemic therapies and radiotherapy for gallbladder cancer. Caldow Pilgrim CH, Groeschl RT, Quebbeman EJ, Gamblin TC. Surg Oncol. 2013;22:61–67. doi: 10.1016/j.suronc.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 9. Postoperative radiotherapy for gallbladder cancer. Jeong Y, Park JH, Lee YJ, Park KM, Hwang S, Chang HM, Kim KP, Yoon SM, Jung NH, Kim JH. Anticancer Res. 2014;34:5621–5629. [PubMed] [Google Scholar]
- 10.A review of recent data in the treatment of gallbladder cancer: what we know, what we do, and what should be done. Müller BG, De Aretxabala X, González Domingo M. Am Soc Clin Oncol Educ Book. 2014:0–70. doi: 10.14694/EdBook_AM.2014.34.e165. [DOI] [PubMed] [Google Scholar]
- 11.Magnitude of combination therapy of radical resection and external beam radiotherapy for patients with carcinomas of the extrahepatic bile duct and gallbladder. Itoh H, Nishijima K, Kurosaka Y, Takegawa S, Kiriyama M, Dohba S, Kojima Y, Saitoh Y. Dig Dis Sci. 2005;50:2231–2242. doi: 10.1007/s10620-005-3040-8. [DOI] [PubMed] [Google Scholar]
- 12. Partial irradiation of the liver. Dawson LA, Ten Haken RK, Lawrence TS. Semin Radiat Oncol. 2001;11:240–246. doi: 10.1053/srao.2001.23485. [DOI] [PubMed] [Google Scholar]
- 13.Evolution of surgical treatment for perihilar cholangiocarcinoma: a single-center 34-year review of 574 consecutive resections. Nagino M, Ebata T, Yokoyama Y, Igami T, Sugawara G, Takahashi Y, Nimura Y. Ann Surg. 2013;258:129–140. doi: 10.1097/SLA.0b013e3182708b57. [DOI] [PubMed] [Google Scholar]
- 14.Induction gemcitabine and stereotactic body radiotherapy for locally advanced nonmetastatic pancreas cancer. Mahadevan A, Miksad R, Goldstein M, Sullivan R, Bullock A, Buchbinder E, Pleskow D, Sawhney M, Kent T, Vollmer C, Callery M. Int J Radiat Oncol Biol Phys. 2011;81:0–22. doi: 10.1016/j.ijrobp.2011.04.045. [DOI] [PubMed] [Google Scholar]
- 15.Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas. Chang DT, Schellenberg D, Shen J, Kim J, Goodman KA, Fisher GA, Ford JM, Desser T, Quon A, Koong AC. Cancer. 2009;115:665–672. doi: 10.1002/cncr.24059. [DOI] [PubMed] [Google Scholar]
- 16.Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. Rusthoven KE, Kavanagh BD, Cardenes H, Stieber VW, Burri SH, Feigenberg SJ, Chidel MA, Pugh TJ, Franklin W, Kane M, Gaspar LE, Schefter TE. J Clin Oncol. 2009;27:1572–1578. doi: 10.1200/JCO.2008.19.6329. [DOI] [PubMed] [Google Scholar]
- 17.Gallbladder carcinoma. Miller G, Jarnagin WR. Eur J Surg Oncol. 2008;34:306–312. doi: 10.1016/j.ejso.2007.07.206. [DOI] [PubMed] [Google Scholar]
- 18.Incidence of finding residual disease for incidental gallbladder carcinoma: implications for re-resection. Pawlik TM, Gleisner AL, Vigano L, Kooby DA, Bauer TW, Frilling A, Adams RB, Staley CA, Trindade EN, Schulick RD, Choti MA, Capussotti L. J Gastrointest Surg. 2007;11:1478–1487. doi: 10.1007/s11605-007-0309-6. [DOI] [PubMed] [Google Scholar]
- 19.Gallbladder cancer: Defining the indications for primary radical resection and radical re-resection. Foster JM, Hoshi H, Gibbs JF, Iyer R, Javle M, Chu Q, Kuvshinoff B. Ann Surg Oncol. 2007;14:833–840. doi: 10.1245/s10434-006-9097-6. [DOI] [PubMed] [Google Scholar]
- 20. Incidental gall bladder cancers: Are they truly incidental? Rammohan A, Cherukuri SD, Sathyanesan J, Palaniappan R, Govindan M. World J Gastrointest Oncol. 2014;6:441–443. doi: 10.4251/wjgo.v6.i12.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Extrahepatic bile duct cancers: surgery alone versus surgery plus postoperative radiation therapy. Gwak HK, Kim WC, Kim HJ, Park JH. Int J Radiat Oncol Biol Phys. 2010;78:194–198. doi: 10.1016/j.ijrobp.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Adjuvant external beam radiation therapy with concurrent chemotherapy in the management of gallbladder carcinoma. Kresl JJ, Schild SE, Henning GT, Gunderson LL, Donohue J, Pitot H, Haddock MG, Nagorney D. Int J Radiat Oncol Biol Phys. 2002;52:167–175. doi: 10.1016/s0360-3016(01)01764-3. [DOI] [PubMed] [Google Scholar]
- 23.Adjuvant radiation therapy is associated with improved survival for gallbladder carcinoma with regional metastatic disease. Mojica P, Smith D, Ellenhorn J. J Surg Oncol. 2007;96:8–13. doi: 10.1002/jso.20831. [DOI] [PubMed] [Google Scholar]
- 24.Impact of adjuvant external beam radiotherapy on survival in surgically resected gallbladder adenocarcinoma: a propensity score-matched Surveillance, Epidemiology, and End Results analysis. Hyder O, Dodson RM, Sachs T, Weiss M, Mayo SC, Choti MA, Wolfgang CL, Herman JM, Pawlik TM. Surgery. 2014;155:85–93. doi: 10.1016/j.surg.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prediction model for estimating the survival benefit of adjuvant radiotherapy for gallbladder cancer. Wang SJ, Fuller CD, Kim JS, Sittig DF, Thomas CR Jr, Ravdin PM. J Clin Oncol. 2008;26:2112–2117. doi: 10.1200/JCO.2007.14.7934. [DOI] [PubMed] [Google Scholar]
- 26.Postoperative chemoradiotherapy for gallbladder cancer. Kim K, Chie EK, Jang JY, Kim SW, Han SW, Oh DY, Im SA, Kim TY, Bang YJ, Ha SW. Strahlenther Onkol. 2012;188:388–392. doi: 10.1007/s00066-012-0074-7. [DOI] [PubMed] [Google Scholar]
- 27.Prognostic factors in patients with gallbladder cancer after surgical resection: analysis of 279 operated patients. Lim H, Seo DW, Park do H, Lee SS, Lee SK, Kim MH, Hwang S. J Clin Gastroenterol. 2013;47:443–448. doi: 10.1097/MCG.0b013e3182703409. [DOI] [PubMed] [Google Scholar]
- 28.Radiation-induced liver disease. Khozouz RF, Huq SZ, Perry MC. J Clin Oncol. 2008;26:4844–4845. doi: 10.1200/JCO.2008.18.2931. [DOI] [PubMed] [Google Scholar]
- 29.Nomogram for predicting the benefit of adjuvant chemoradiotherapy for resected gallbladder cancer. Wang SJ, Lemieux A, Kalpathy-Cramer J, Ord CB, Walker GV, Fuller CD, Kim JS, Thomas CR Jr. J Clin Oncol. 2011;29:4627–4632. doi: 10.1200/JCO.2010.33.8020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.An experience of CyberKnife treatment in patients with advanced pancreaticobilliary malignancy (article in Korean) Jung YH, Choi HS, Cheon YK, Moon JH, Cho YD, Chang AR, Won JH. Korean J Gastroenterol. 2011;58:264–269. doi: 10.4166/kjg.2011.58.5.264. [DOI] [PubMed] [Google Scholar]


