Abstract
AIM: To analyze the structure and expressions of the protein encoded by an HCC-associated novel gene, lysosome-associated protein transmembrane 4 β (LAPTM4B).
METHODS: Primary structure and fundamental characteristics of LAPTM4B protein were analysed with bioinformatics. Expressions of LAPTM4B in HCC tissues and various cell lines were detected using polyclonal antibodies and Western blot.
RESULTS: LAPTM4B encoded two isoforms of proteins with molecular masses 35-ku and 24-ku, respectively. The expression level of LAPTM4B-35 protein in HCC tissues was dramatically upregulated and related to the differentiation status of HCC tissues, and it was also high in some cancer cell lines. Computer analysis showed LAPTM4B was an integral membrane protein with four transmembrane domains. LAPTM4B showed relatively high homology to LAPTM4A and LAPTM5 in various species.
CONCLUSION: LAPTM4B gene encoded two isoforms of tetratransmembrane proteins, LAPTM4B-35 and LAPTM4B-24. The expression of LAPTM4B-35 protein is upregulated and associated with poor differentiation in human HCC tissues, and also at high levels in some cancer cell lines. LAPTM4B is an original and conserved protein.
INTRODUCTION
We have previously reported the cloning and identification of a novel gene, which was designated by the International Nomenclature Committee as lysosomal associated protein transmembrane 4 β (LAPTM4B)[1-4]. LAPTM4B was overexpressed in 87.3% human hepatocellular carcinomas (HCC) at mRNA level performed by Northern blot[2]. BLAST program analysis showed that the LAPTM4B gene was mapped to chromosome 8 q22.1, spanning approximately at least 50 kb. It is composed of seven exons separated by six introns. The cloned full-length cDNA sequence (GenBank accession number: AY057051) of LAPTM4B is 2.2 kb and contains two ATGs at the 5’end with an interval of 273 bp and two polyadenylation signals: aataaa and aattaaa[2].
In this study, primary structures of proteins encoded by LAPTM4B gene were analyzed using bioinformatics. Specific polyclonal antibodies directing against two epitopes of the 10-peptides localized at the N-terminus (28-37aa) and in between the 3rd and 4th transmembrane domains (232-241aa) of LAPTM4B, respectively, were prepared. The expressions of LAPTM4B proteins in HCC, paired noncancerous liver (PNL), normal liver (NL) tissues and various cancer cell lines were detected by Western blot. The functions of LAPTM4B protein was discussed.
MATERIALS AND METHODS
Specimens
Fresh HCC and paired noncancerous liver specimens were obtained during operations on patients with HCC, normal liver tissues were obtained during operations on patients with hepatohemangioma.
Cell lines and culture
Human hepatoma cell lines BEL-7402, QGY-7701, SMMC-7721, HLE and human hepatic cell line L-02, human cervical cancer cell line HeLa, human prostate carcinoma cell lines PC3M and PC3, human pulmonary giant cell carcinoma cell lines BE1 and LH7, and human melanoma cell lines WM451 and WM983A were maintained in RPMI 1640 (GIBco BRL) medium supplemented with 100 mL/L fetal bovine serum.
Protein extraction[5-7]
The tissues stored at -80 °C or living cells were homogenized and extracted with lysis buffer (pH7.6) containing 10 mmol/L Tris, 150 mmol/L NaCl, 1 mmol/L EDTA, 5 g/L NP40, 1 mmol/L PMSF, 1 μg/mL aprotinin, 1 μg/mL pepstatin A. After centrifugation at 12000 g for 10 min at 4 °C, the supernatants were collected and the proteins were quantitated by Bradford microassay.
Peptide synthesis and antibody preparation[8-10]
Two KLH-conjugated 10 peptides (KLH- Ala-Lys-Gly-Thr-Asp-Pro-Ala-Glu-Ala-Arg and KLH-Pro-Tyr-Arg-Asp-Asp-Val-Met-Ser-Val-Asn) were synthesized by Invitrogen Company. Rabbits were immunized subcutaneously with 1 mg of KLH-peptide conjugates emulsified in Freund’s complete adjuvant (GIBco BRL). The immunization was repeated with the antigens in Freund’s incomplete adjuvant (GIBco BRL) 4 wk after the first injection and 2 wk after the second injection. The anti-sera were designated as LAPTM4B-N28-37-pAb and LAPTM4B-EC2-pAb, respectively. The titers of these two sera were both above 1 × 105 detected by ELISA with peptide-coated plates. The pre-immune sera did not show any reactivity to the peptides.
Western blotting
Proteins in cell lysates were fractionated by 100 g/L SDS-PAGE and then electrotransferred onto a nitrocellulose filter (Bio-Rad). The filters were blocked at 4 °C overnight with blocking buffer (pH7.6) containing 50 g/L fat free dry milk. Then the filters were incubated with indicated antibody for 2 h at room temperature. After washed with TBST (pH6.0), the filters were incubated at room temperature for 1 h with horseradish peroxidase conjugated goat anti-rabbit IgG at 1:2000 dilution in TBS-5 g/L fat free dry milk. Immunoreactive bands were visualized using ECL detection reagents (Santa Cruz).
Bioinformatics analysis tool
The Bioinformatics analysis tool included BLAST and FASTA for basic local alignment of sequences, PCGENE software for predicting primary structure and fundamental characteristics of the protein, Kyte&Doolittle hydrophobicity index for calculating hydrophobicity value, ClustalW software for multiple sequence alignment and DNAMAN software for displaying phylogenic tree.
RESULTS
Bioinformatics analysis of primary structure of LAPTM4B protein
The primary structure of LAPTM4B is shown in Figure 1. The LAPTM4B cDNA may encode two putative proteins with 35 ku (317 aa) and 24 ku (226 aa). Computer analysis (PCGENE) showed that LAPTM4B was an integral membrane protein with four highly conserved hydrophobic transmembrane domains, forming two extracellular loops (EC1 and EC2), a cytoplasmic loop and intracellular amino- and carboxyl tails. The transmembrane regions were located at 117-133, 163-179, 200-216, and 243-259 aa, respectively. The full amino acid sequence contained one N-glycosylation site, eight phosphorylation sites, and four N-myristoylation sites. A tyrosine phosphorylation site was predicted at 285 aa at C-terminal region, which depending on phosphorylation might form a binding site for specific SH2 domain in some signaling proteins. It was also predicted that LAPTM4B containing proline-rich motif (PXXP) at N-terminal region would be a SH3 domain binding protein. Moreover, LAPTM4B contained typical lysosomal targeting signals (3 YXXΦmotifs)[11] at C-terminal region. Human LAPTM4B shared 92% homology with mouse LAPTM4B (GenBank accession number: AAH1912)[12] at amino acid level, suggesting that LAPTM4B was highly conserved within vertebrate species. The region from 140 aa to 317 aa of LAPTM4B was highly conserved compared with mouse LAPTM4B. But the N-terminal region from 1 aa to 91 aa (between the first ATG and the second ATG) of LAPTM4B-35 was highly specific and no homological sequence was found using BLAST in nr database. LAPTM4B also showed 46% homology to a lysosomal tetra-transmembrane protein, LAPTM4A, at amino acid level. The orthodog of LAPTM4A in murine was a nucleoside transporter in intracellular membrane-bound compartments, and mediated a multidrug resistance phenotype in drug-sensitive strains of S. cerevisiae[12-14].
Figure 1.

Putative amino-acid sequence of LAPTM4B.
The four transmembrane domains are shadowed with gray color. Two translation initiation sites are thick black. Putative SH3 ligand is indicated by thick underlining. The sequence containing 10 amino acid residues (28aa-37aa or 232aa-241aa) and used as immunogen is wavy underlined. PKC-P, cAMP-P, cGMP-P, CK2-P, Tyr-P: various phosphorylation sites; G: N-glycosylation site; M: myristylation site.
The phylogenic tree (Figure 2) was constructed according to multiple sequence alignment results. It showed that LAPTM4B, LAPTM4A and LAPTM5 proteins were distributed in clusters. Human LAPTM4B showed the most close homology to Mus musculus and also had certain homology to Dannio rerio and Fugu rubripes, as well as Drosophila melanogaster, but was far-away from Yeast. In addition, LAPTM4B showed relatively high homology to LAPTM4A and LAPTM5 in various species to a certain extent, and LAPTM4B was closer to LAPTM4A than LAPTM5. These results indicated that LAPTM4B was an original and conserved protein.
Figure 2.
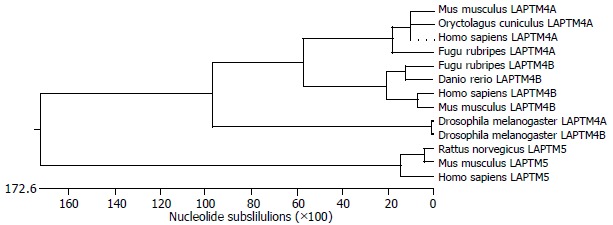
Phylogenic tree of LAPTM4B.
Expression of LAPTM4B protein in HCC tissues
As shown in Figure 3, 2 proteins with molecular masses 35-ku and 24-ku both reacted with LAPTM4B-EC2-pAb were identified by Western blot in HCC, PNL and NL tissues, and designated as LAPTM4B-35 and LAPTM4B-24, respectively. Nevertheless, only one band at the 35 ku position appeared when LAPTM4B-N28-37-pAb was used for Western blot in HCC tissues, indicating that LAPTM4B-35 was translated from the whole ORF and initiated from the first ATG, whereas LAPTM4B-24 was translated from the second ATG. Furthermore, LAPTM4B proteins were significantly overexpressed in HCC tissues than in PNL and NL tissues. Notably, the expression levels of LAPTM4B-35 were significantly related to the differentiation status of HCC tissues, they were higher in poorly differentiated HCCs than in moderately and well differentiated HCCs (Figure 4)[4]. In addition, the ratio of LAPTM4B-35 to LAPTM4B-24 was remarkably higher in HCC than in PNL and NL (Table 1). However, the ratio of LAPTM4B-35 to LAPTM4B-24 in PNL was kept at the same level as in NL, even though LAPTM4B-35 and LAPTM4B-24 were both slightly increased. Thus the development of HCC might be associated with the raised ratio of LAPTM4B-35 to LAPTM4B-24.
Figure 3.
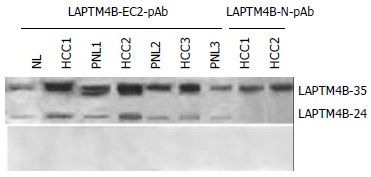
Expressions of LAPTM4B proteins in HCC tissues identified by Western blot with various antibodies. Top: Western Blot profiles performed with anti serum. LAPTM4B-EC2-pAb: polyclonal antibody directing against the epitope localized at the EC2 domain between the 3rd and 4th transmembrane regions of LAPTM4B. LAPTM4B-N28-37-pAb: polyclonal antibody directing against the epitope localized at the N-terminal region of LAPTM4B. Bottom: Western blot profiles performed with pre-immune serum.
Figure 4.
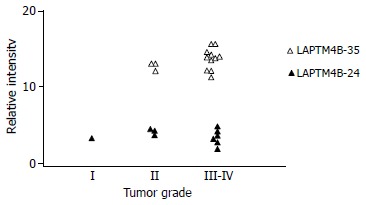
Correlation between LAPTM4B-35 protein expression and pathological grade of HCC.
Table 1.
Relative intensity of LAPTM4B-35 and LAPTM4B-24 (mean ± SD)
| HCC | PNL | NL | |
| LAPTM4B-35 | 13.32 ± 1.98b | 4.58 ± 1.31 | 2.78 ± 0.11 |
| LAPTM4B-24 | 3.59 ± 1.78b | 1.76 ± 1.24 | 1.00 ± 0.02 |
| LAPTM4B-35/LAPTM4B-24 (ratio) | 3.71 | 2.60 | 2.78 |
P < 0.01 vs PNL and NL.
Expression of LAPTM4B proteins in various cell lines
LAPTM4B proteins in 12 cell lines were detected by Western blot with LAPTM4B-EC2-pAb (Figure 5). With exception of HLE cells, LAPTM4B proteins were remarkably expressed in all of HCC-derived cell lines including BEL-7402, QGY-7701 and SMMC-7721, and human hepatic cell line L-02 cells. The ratio of LAPTM4B-35 to LAPTM4B-24 was higher in HCC cell lines than in immortal hepatic cell line L-02 (Table 2), suggesting that disturbance of LAPTM4B-35 to LAPTM4B-24 equilibrium in expressions might cause the malignant transformation in hepatocarcinogenesis. Moreover, the expressions of LAPTM4B-35 were also higher in highly metastatic cell lines[15-17], including prostate carcinoma PC-3M and pulmonary giant cell carcinoma BE1 cell lines than in the syngenic low metastatic cell lines, including PC-3 and LH7 cell lines. Therefore, the overexpression of LAPTM4B-35 was likely related to cancer cell invasion and metastasis. However, LAPTM4B was not detected in human cervical carcinoma cell line HeLa and human melanoma cell lines WM451 and WM983A[15], indicating that overexpression of LAPTM4B-35 was somehow specific for some carcinomas.
Figure 5.

Expression of LAPTM4B proteins in various cell lines shown via Western blot with LAPTM4B-EC2-pAb. Top: Western blot profiles performed with LAPTM4B-EC2-pAb. Bottom: Western blot profiles performed with pre-immune serum.
Table 2.
Relative intensity of LAPTM4B-35 and LAPTM4B-24 in various cell lines
| Cell line | LAPTM4B-35 | LAPTM4B-24 | LAPTM4B-35/-24 (ratio) |
| BEL-7402 | 3.98 | 2.09 | 1.91 |
| QGY-7701 | 3.21 | 2.20 | 1.45 |
| SMMC-7721 | 2.98 | - | - |
| HLE | 2.24 | - | - |
| L-02 | 3.32 | 2.56 | 1.29 |
| Hela | - | - | - |
| PC3M | 2.96 | - | - |
| PC3 | - | - | - |
| BE1 | 1.47 | - | - |
| LH7 | - | - | - |
| WM451 | - | - | - |
| WM983A | - | - | - |
DISCUSSION
HCC is one of the most common cancers world-wide, and is the major cause of malignant deaths in Asia and Africa[18-20]. The pathogenesis of HCC is particularly complex. The current model for HCC carcinogenesis postulated a multistage progression involving an accumulation of genetic alterations[21,22], including activation of oncogenes N-ras, H-ras, K-ras, c-erbA, c-met, and c-myc etc.[23,24], repression or mutation of tumor suppressor genes[25], besides the transcriptional activation of c-jun and nuclear factor NF-κB by HBV and HCV[26,27]. But the roles of these genes in cell proliferation, differentiation, and premalignant status in liver malignancy are far from elucidated.
We previously reported[2] that LAPTM4B was a novel gene associated with HCC, and significantly overexpressed in HCC as shown via Northern blot and in situ hybridization. LAPTM4B is also widely expressed in human tissues with a relatively high level in the testis, heart and skeletal muscles, and moderately in the ovary, kidney and pancreas, and poorly in the liver, spleen, and thymus, and lowest in the lung and peripheral leukocytes. It was remarkably overexpressed in 87.3% (48/55 cases) HCC tissues when compared with PNL and NL tissues. Furthermore, the LAPTM4B mRNA expression levels were significantly related to the differentiation status of HCC tissues. They were highest in poorly differentiated HCCs, higher in moderately differentiated HCCs, and relatively low in well differentiated HCCs.
Here we report the expressions of LAPTM4B proteins in HCC, PNL, NL and a number of cell lines via Western blot analysis using two antibodies, LAPTM4B-N28-37-pAb or LAPTM4B-EC2-pAb, which were prepared by immunization with two KLH-conjugated 10-peptides whose sequences are localized at either the second extracellular loop (EC2) between the third and fourth transmembrane regions or the N-terminal region in the cytoplasma. These two 10-peptides used as immunogens were highly specific, i.e. having very low homology compared to known proteins of Homo sapiens. By using PCGENE software, the 10-peptides should have characteristics of high hydrophilicity and no residues of putative glycosylation and phosphorylation. KLH was conjugated with the N-termini of 10-peptides in order to increase immunogenicity. Two isoforms of LAPTM4B, LAPTM4B-35 and LAPTM4B-24, whose mass was coincident with the predicted products translated from the first and second ATG in ORF, were identified. Expressions of LAPTM4B-35 proteins were highly upregulated in HCC with poor differentiation. This result was coincident with expression of LAPTM4B mRNA in HCC tissues. LAPTM4B expressions were also high in human HCC cell lines and some other cancer cell lines. Transient transfection of murine BHK cells was performed with two plasmids, pcDNA3/LAPTM4B-AE containing a full-length cDNA of LAPTM4B ORF and pcDNA3/LAPTM4B-BE containing the same ORF sequence but with 273 nucleotides deleted at 5’ end, respectively. The transfectants in AE-series expressed mainly LAPTM4B-35 and slightly LAPTM4B-24, and proliferated very rapidly and formed colonies powerfully. However, the transfectants in BE-series expressed only LAPTM4B-24, and lost potentials of long term survival and growth. Therefore, it is suggested that LAPTM4B-24 may play an antagonistic role in cell survival and proliferation, and the equilibrium of LAPTM4B-35 and LAPTM4B-24 in expression is involved in controlling cell survival/proliferation and differentiation. The disequilibrium in expressions of LAPTM4B-35 and LAPTM4B-24 may be involved in malignant transformation of hepatocytes and carcinogenesis.
Footnotes
Supported by the 248 Major R&D Program of Beijing, No. H020220020310 and Special Fund for Promotion of Education, Ministry of Education, China
Edited by Zhu LH and Wang XL Proofread by Xu FM
References
- 1.Liu J, Zhou R, Zhang N, Rui J, Jin C. Biological function of a novel gene overexpressed in human hepatocellular carcinoma. Chin Med J (Engl) 2000;113:881–885. [PubMed] [Google Scholar]
- 2.Shao GZ, Zhou RL, Zhang QY, Zhang Y, Liu JJ, Rui JA, Wei X, Ye DX. Molecular cloning and characterization of LAPTM4B, a novel gene upregulated in hepatocellular carcinoma. Oncogene. 2003;22:5060–5069. doi: 10.1038/sj.onc.1206832. [DOI] [PubMed] [Google Scholar]
- 3.He J, Shao G, Zhou R. [Effects of the novel gene, LAPTM4B, highly expression in hepatocellular carcinoma on cell proliferation and tumorigenesis of NIH3T3 cells] Beijing Da Daxue Xuebao. 2003;35:348–352. [PubMed] [Google Scholar]
- 4.Liu X, Zhou R, Zhang Q, Zhang Y, Shao G, Jin Y, Zhang S, Lin M, Rui J, Ye D. [Identification and characterization of LAPTM4B encoded by a human hepatocellular carcinoma-associated novel gene] Beijing Daxue Xuebao. 2003;35:340–347. [PubMed] [Google Scholar]
- 5.Schuck S, Honsho M, Ekroos K, Shevchenko A, Simons K. Resistance of cell membranes to different detergents. Proc Natl Acad Sci USA. 2003;100:5795–5800. doi: 10.1073/pnas.0631579100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones MN. Surfactants in membrane solubilisation. Int J Pharm. 1999;177:137–159. doi: 10.1016/s0378-5173(98)00345-7. [DOI] [PubMed] [Google Scholar]
- 7.Shetty J, Diekman AB, Jayes FC, Sherman NE, Naaby-Hansen S, Flickinger CJ, Herr JC. Differential extraction and enrichment of human sperm surface proteins in a proteome: identification of immunocontraceptive candidates. Electrophoresis. 2001;22:3053–3066. doi: 10.1002/1522-2683(200108)22:14<3053::AID-ELPS3053>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 8.Shen L, Guo ZY, Chen Y, Liu LY, Feng YM. Expression, Purification, Characterization of Amphioxus Insulin-like Peptide and Preparation of Polyclonal Antibody to It. Shengwu Huaxue Yu Shengwu Wuli Xuebao (Shanghai) 2001;33:629–633. [PubMed] [Google Scholar]
- 9.Hadidi S, Yu K, Chen Z, Gorczynski RM. Preparation and functional properties of polyclonal and monoclonal antibodies to murine MD-1. Immunol Lett. 2001;77:97–103. doi: 10.1016/s0165-2478(01)00208-5. [DOI] [PubMed] [Google Scholar]
- 10.Missbichler A, Hawa G, Schmal N, Woloszczuk W. Sandwich ELISA for proANP 1-98 facilitates investigation of left ventricular dysfunction. Eur J Med Res. 2001;6:105–111. [PubMed] [Google Scholar]
- 11.Hogue DL, Nash C, Ling V, Hobman TC. Lysosome-associated protein transmembrane 4 alpha (LAPTM4 alpha) requires two tandemly arranged tyrosine-based signals for sorting to lysosomes. Biochem J. 2002;365:721–730. doi: 10.1042/BJ20020205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hogue DL, Ellison MJ, Young JD, Cass CE. Identification of a novel membrane transporter associated with intracellular membranes by phenotypic complementation in the yeast Saccharomyces cerevisiae. J Biol Chem. 1996;271:9801–9808. doi: 10.1074/jbc.271.16.9801. [DOI] [PubMed] [Google Scholar]
- 13.Cabrita MA, Hobman TC, Hogue DL, King KM, Cass CE. Mouse transporter protein, a membrane protein that regulates cellular multidrug resistance, is localized to lysosomes. Cancer Res. 1999;59:4890–4897. [PubMed] [Google Scholar]
- 14.Hogue DL, Kerby L, Ling V. A mammalian lysosomal membrane protein confers multidrug resistance upon expression in Saccharomyces cerevisiae. J Biol Chem. 1999;274:12877–12882. doi: 10.1074/jbc.274.18.12877. [DOI] [PubMed] [Google Scholar]
- 15.Li S, Fang W, Zhong H. [Expression of tumor metastasis suppressor gene KAI1/CD82 in human cancer cell lines with different metastasis potential] Zhonghua Yixue Zazhi. 1999;79:708–710. [PubMed] [Google Scholar]
- 16.Kim IY, Kim BC, Seong DH, Lee DK, Seo JM, Hong YJ, Kim HT, Morton RA, Kim SJ. Raloxifene, a mixed estrogen agonist/antagonist, induces apoptosis in androgen-independent human prostate cancer cell lines. Cancer Res. 2002;62:5365–5369. [PubMed] [Google Scholar]
- 17.Liu Y, Zheng J, Fang W, You J, Wang J, Cui X, Wu B. Identification of metastasis associated gene G3BP by differential display in human cancer cell sublines with different metastatic potentials G3BP as highly expressed in non-metastatic. Chin Med J (Engl) 2001;114:35–38. [PubMed] [Google Scholar]
- 18.Liu YH, Zhou RL, Rui JA. Detection of hepatoma cells in peripheral blood of HCC patients by nested RT-PCR. World J Gastroenterol. 1998;4:106–108. doi: 10.3748/wjg.v4.i2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bosch FX, Ribes J, Borràs J. Epidemiology of primary liver cancer. Semin Liver Dis. 1999;19:271–285. doi: 10.1055/s-2007-1007117. [DOI] [PubMed] [Google Scholar]
- 20.Rui JA, Wang SB, Chen SG, Zhou L. Right trisectionectomy for primary liver cancer. World J Gastroenterol. 2003;9:706–709. doi: 10.3748/wjg.v9.i4.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kondoh N, Wakatsuki T, Ryo A, Hada A, Aihara T, Horiuchi S, Goseki N, Matsubara O, Takenaka K, Shichita M, et al. Identification and characterization of genes associated with human hepatocellular carcinogenesis. Cancer Res. 1999;59:4990–4996. [PubMed] [Google Scholar]
- 22.Qin LX, Tang ZY. The prognostic molecular markers in hepatocellular carcinoma. World J Gastroenterol. 2002;8:385–392. doi: 10.3748/wjg.v8.i3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo D, Liu QF, Gove C, Naomov N, Su JJ, Williams R. Analysis of N-ras gene mutation and p53 gene expression in human hepatocellular carcinomas. World J Gastroenterol. 1998;4:97–99. doi: 10.3748/wjg.v4.i2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ueki T, Fujimoto J, Suzuki T, Yamamoto H, Okamoto E. Expression of hepatocyte growth factor and its receptor c-met proto-oncogene in hepatocellular carcinoma. Hepatology. 1997;25:862–866. doi: 10.1002/hep.510250413. [DOI] [PubMed] [Google Scholar]
- 25.Zhu AX. Hepatocellular carcinoma: are we making progress. Cancer Invest. 2003;21:418–428. doi: 10.1081/cnv-120018233. [DOI] [PubMed] [Google Scholar]
- 26.Henkler F, Waseem N, Golding MH, Alison MR, Koshy R. Mutant p53 but not hepatitis B virus X protein is present in hepatitis B virus-related human hepatocellular carcinoma. Cancer Res. 1995;55:6084–6091. [PubMed] [Google Scholar]
- 27.Sansonno D, Cornacchiulo V, Racanelli V, Dammacco F. In situ simultaneous detection of hepatitis C virus RNA and hepatitis C virus-related antigens in hepatocellular carcinoma. Cancer. 1997;80:22–33. [PubMed] [Google Scholar]


