Abstract
AIM: To elucidate the mechanism by which somatostatin and its analogue exert the influence on liver fibrosis, and to investigate the mRNA expression of somatostatin receptors subtypes (SSTRs) and the distribution of somatostatin analogue octreotide in rat hepatic stellate cells (HSCs).
METHODS: HSCs were isolated from Sprague Dawley (SD) rats by in situ perfusion and density gradient centrifugation. After several passages, the mRNA expression of 5 subtypes of SSTRs were assessed by reverse transcription-polymerase chain reaction (RT-PCR). HSCs were planted on coverslip and co-cultured with octreotide tagged by FITC. Then the distribution of FITC fluorescence was observed under laser scanning confocal microscope (LSCM) in 12-24 h.
RESULTS: There were mRNA expression of SSTR2, SSTR3 and SSTR5 but not SSTR1 and SSTR4 in SD rat HSCs. The mRNA expression level of SSTR2 was significantly higher than that of other subtypes (P < 0.01). FITC fluorescence of octreotide was clearly observed on the surface and in the cytoplasm, but not in the nuclei of HSCs under LSCM.
CONCLUSION: The effect exerted by somatostatin and its analogues on HSCs may mainly depend on the expression of SSTR2, SSTR3 and SSTR5. Octreotide can perfectly combine with HSCs, and thereby exerts its biological activity on regulating the characters of active HSCs. This provides a potential prevention and management against liver fibrosis.
INTRODUCTION
In recent years, it has become clear that HSCs play an important role during occurence and progress of hepatic fibrosis and portal hypertension[1,2]. HSCs are located in a perisinusoidal orientation within the sinusoid and encircle vascular endothelial cells. They can also contract or relax in response to various vasoactive mediators. All of these suggest that these cells may have the capacity to modulate intrahepatic vascular resistance and blood flow at the sinusoidal level[3]. When HSCs are activated and proliferated abundantly, they can excrete many kinds of cytokine related to fibrosis to promote synthesis and deposition of extracellular matrix (ECM)[4,5]. Therefore, how to reduce and inhibit activation and proliferation of HSCs has become a hot spot in the area of liver fibrosis research[6].
Somatostatin and its analogues have comprehensive inhibitory actions on proliferation of many kinds of cells and the capacity to induce apoptosis, so they have been applied in cancer biotherapy at the present time[7,8]. The biological base by which somatostatin exerts its actions is the existence of somatostatin receptors (SSTRs). SSTRs belong to G-protein-coupled receptors family and are located on cell membrane. Only after somatostatin combines with SSTRs, could it exert the actions of anti-proliferation. Up to date, five different SSTR subtypes, termed SSTR1 to SSTR5, have been cloned and characterized[9].
In this research, we studied the mRNA expression and distribution of somatostatin receptors in hepatic stellate cells (HSCs) of rats to elucidate the mechanism by which somatostatin and its analogue exert the influence on liver fibrosis.
MATERIALS AND METHODS
Materials
Male Sprague Dawley rats, weighing (450 ± 50) g, were purchased from Experimental Animal Center of the Chinese Academy of Medical Science, Beijing, China. Nycodenz, fluorescein isothiacyanate (FITC) and collagenase IV were purchased from Sigma Co., U.S.A. Fetal bovine serum (FBS), Dulbecco MEM (DMEM) culture medium, Trizol and superscript II reverse transcriptase were produced by Gibco Co., U.S.A. Taq DNA polymerase, dNTPs, Oligo (dT) and RNasin were products of Promega Co. Octreotide (0.1 g/mL) was provided by Novartis Co. Mouse anti-human desmin antibody and rabbit anti-cow glial fibrillary acidic protein (GFAP) antibody were purchased from Genetech Co. Primers were synthesized by Shanghai Sangon Co.
Isolation and culture of hepatic stellate cells (HSC)
Adult male Sprague Dawley rats (450 ± 50) g were treated in accordance with the institution’s guidelines for the care and use of laboratory animals in research. All procedures were performed with animals under ether anesthesia. According to the method of Geerts and Weng et al[10,11], HSCs were isolated by in situ perfusion and density gradient centrifugation with nycodenz. Firstly, the trochar was inserted into the rat’s portal vein and the liver was perfused with pre-perfusion liquid. Then the liver was removed from the body and continuously perfused with perfusion liquid containing collagenase IV to digest the extracellular matrix. After centrifugation with 150 g/L nycodenze, the cells located in the interface were extracted and cultured in DMEM containing 200 mL/L FBS. HSC were fully stick to the wall within 48 h. Then the culture medium was exchanged in every 2-3 d. The identification of HSC was performed by observation of ultraviolet-excited fluorescence of vitamine A lipid droplet in HSC and immunocytochemical stain for desmin and GFAP.
Isolation of total RNA and semiquantitative RT-PCR
Total RNA was isolated from HSC with Trizol by phenol-chloroform extraction and isopropanol precipitation following manufacturer’s instructions. Reverse transcription of total RNA was carried out according to the instructions of the RT kit. Design of specific primers against rat SSTRs sequences was refered to the references[12] and verified in NCBI Blast. They are shown in Table 1. The PCR system (total 25 μL) contained 40 pmo1/L primers of SSTRs or β-actin, 0.5 μL of 10 mmol/L dNTPs, 1.5 μL of 50 mmol/L MgCl2, 2 μL of cDNA, 2.5 μL of 10 × PCR buffer, 2.5 μL of Taq DNA polymerase. The PCR conditions included an initial denaturation for 5 min at 94 °C; 40 amplification cycles consisting of denaturation at 94 °C for 1 min, primer annealing at 60 °C for 1 min and elongation at 72 °C for 1 min; and a final extension at 72 °C for 7 min. The PCR products or DNA marker mixed with 2 μL of loading buffer were electrophoresed on a 15 g/L agarose gel and visualized under ultraviolet light excitation. Then the images were taken and anlaylized by Kodak digital camera and science software (Kodak, EDAS290, U.S.A). The expression of SSTRs was calculated by determining the ratio of SSTRs relative to β-actin.
Table 1.
Primer sequence and expected PCR product length
| Primer | designation | Sequence | Product length |
| SSTR1 | upstream | 5’-CAC GCA CCG CAG CCA ACA-3’ | 390 bp |
| downstream | 5’-GGA AGC CGT AGA GTA TGG GGT T-3’ | ||
| SSTR2 | upstream | 5’-CCG GAG CAA CCA GTG GGG-3’ | 390 bp |
| downstream | 5’-GCG TAC AGG ATG GGG TTG GC-3’ | ||
| SSTR3 | upstream | 5’- CCC GGG GCA TGA GCA CGT-3’ | 415 bp |
| downstream | 5’-AAG CCG TAG AGG ATG GGG TTT GC-3’ | ||
| SSTR4 | upstream | 5’-TCG TGG GGG TGA GGC AGT AG-3’ | 365 bp |
| downstream | 5’-CAT AGA GAA TCG GGT TGG CAC AG-3’ | ||
| SSTR5 | upstream | 5’-ATG GAG CCC CTC TCT CTG G-3’ | 250 bp |
| downstream | 5’-CGT CAG CCA CGG CCA GGT T-3’ | ||
| β-actin | upstream | 5’-TGG GAC GAT ATG GAG AAG AT–3’ | 522 bp |
| downstream | 5’-ATT GCC GAT AGT GAT GAC CT –3’ |
The observation of combination of octreotide with HSC and distribution in HSC
After 3-4 passages, HSCs were planted on coverslip and culture medium was exchanged after sticking to the walls. Then octreotide tagged by FITC was added into the culture medium (10 μg/mL). After 24 h, the culture medium was removed and the coverslips were rinsed three times with PBS, whereafter the distribution of FITC fluorescence in live HSCs was observed by laser scanning confocal microscope (LSCM).
Statistical methods
The results of electrophoresis of PCR products were expressed as mean ± SD and analyzed by t-test and analysis of variance.
RESULTS
Expression of mRNA of somatostatin receptor subtypes
The mRNA expression of SSTR2, SSTR3 and SSTR5 were observed, whereas those of SSTR1 and SSTR4 were not observed in rat HSCs. Moreover the mRNA expression level of SSTR2 was significantly higher than those of other subtypes (4 and 5.64 times higher than that of SSTR3 and SSTR5, respectively, P < 0.01). There was no significant difference between the mRNA expression of SSTR3 and SSTR5 (Figures 1, Figures 2, and Figures 3, Table 2).
Figure 1.
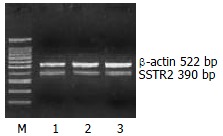
Expression of SSTR2 mRNA. M: 100 bp Marker; Lanes1-3: three groups of samples.
Figure 2.
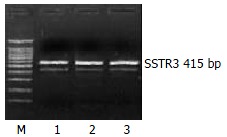
Expression of SSTR3 mRNA. M: 100 bp Marker; Lanes 1-3: three groups of samples.
Figure 3.
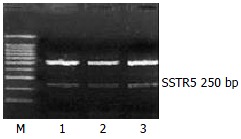
Expression of SSTR5 mRNA. M: 100 bp Marker; Lanes 1-3: three groups of samples.
Table 2.
Expression of SSTRs mRNA in quantity (mean ± SD)
| Subtypes | n | Expression of mRNA |
| SSTR2 | 3 | 0.8213 ± 0.1210 |
| SSTR3 | 3 | 0.2041 ± 0.1662b |
| SSTR5 | 3 | 0.1457 ± 0.1981d |
P < 0.01 vs SSTR2,
P < 0.01 vs SSTR2.
Distribution of octreotide in HSCs
The green fluorescence of FITC had an extensive distribution on the surface of HSCs and also in cytoplasm, but not in nucleus (Figure 4).
Figure 4.
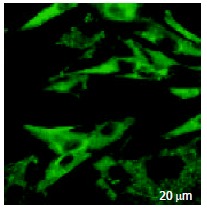
Distribution of the green fluorescence of octreotide tagged by FITC in live HSCs. FITC fluorescence of octreotide was clearly observed on the surface and in the cytoplasm, but not in the nuclei of HSCs under LSCM.
DISCUSSION
Several studies have demonstrated that somatostatin could restrain and decrease the process of hepatic fibrosis of rat liver cirrhosis models[14-16]. In addition, researchers studied the anti-fibrosis mechanisms of somatostatin. Reynaert et al[13] proved that somatostatin could suppress rat hepatic stellate cell contraction induced by endothelin-1. Chatterjee et al[17] found that in rat liver cirrhosis models of schistosomiasis, somatostatin might have the direct anti-fibrosis effects through modulating the synthesis and expression of collagen I, III and smooth muscle actin (SMA) of rat HSCs. All of these findings suggest that there are SSTRs on the surface of HSCs, thereby somatostatin could exert its actions on HSCs.
Whether SSTRs exist and what kind of SSTRs subtypes are expressed in rat HSCs We observed mRNA expression of SSTR2, SSTR3 and SSTR5, but not of SSTR1 and SSTR4 in Sprague Dawley rat HSCs. Our results slightly differed from Reynaert et al[13] that SSTR1, SSTR2 and SSTR3 were expressed, but not SSTR4 and SSTR5 in Wistar rat HSCs. Besides, we found that the mRNA level of SSTR2 was significantly higher than those of other subtypes in quantity. These results suggest that theoretically, somatostatin may be available to exert its inhibitive and pre-appoptosis actions on active HSCs by means of SSTRs (especially SSTR2) and consequently has the ability of anti-fibrotic action. Therefore, if the proper somatostatin analogues that have strong affinity to SSTR2, 3 and 5 are chosen, they will be able to exert better effect on active HSCs.
Taking advantage of the strong appetency by which somatostatin could combine with SSTRs, some researchers have applied the somatostatin analogues tagged by radioactive isotope to study the expression and distribution of SSTRs in cancer tissues and consequently the locational diagnosis of cancers. This kind of method has been proved to be satisfactory. Since our results showed that SSTR2, 3 and 5 were expressed and other studies reported that octreotide had the special affinity to SSTR2 and SSTR5[7,9], we treated HSCs with octreotide tagged by FITC. By means of the characteristics that hormones can specially bind to its receptors, we observed the green fluorescence on the surface of live HSCs, which reflected the distribution of SSTRs. This proves that octreotide has strong affinity with HSCs and thus the existence of SSTRs is supported. Meanwhile, we also observed the distribution of octreotide in the cytoplasm, but not in the nuclei of HSCs. In general, octreotide contains 8 peptides, so it can’t pass through the cell membrane without other pathways were introduced. Thereby, we presume that the endocytosis conducted by the receptors may works. But whether octreotide in cytoplasm is able to exert its biological actions by a certain mechanism independent of SSTRs remains to be confirmed in the future.
In conclusion, we demonstrates that SSTR2, 3 and 5 are obviously expressed in SD rat HSCs. In addition, octreotide, a somatostatin analogue, is able to combine perfectly with HSCs and thereby exerts its biological actions on liver fibrosis in clinical practice.
Footnotes
Supported by National Nature Science Foundation of China, No. 30271270
Edited by Kumar M and Xu FM
References
- 1.Wu J, Zern MA. Hepatic stellate cells: a target for the treatment of liver fibrosis. J Gastroenterol. 2000;35:665–672. doi: 10.1007/s005350070045. [DOI] [PubMed] [Google Scholar]
- 2.Pinzani M, Gentilini P. Biology of hepatic stellate cells and their possible relevance in the pathogenesis of portal hypertension in cirrhosis. Semin Liver Dis. 1999;19:397–410. doi: 10.1055/s-2007-1007128. [DOI] [PubMed] [Google Scholar]
- 3.Rockey DC. Hepatic blood flow regulation by stellate cells in normal and injured liver. Semin Liver Dis. 2001;21:337–349. doi: 10.1055/s-2001-17551. [DOI] [PubMed] [Google Scholar]
- 4.Shen H, Huang GJ, Gong YW. Effect of transforming growth factor beta and bone morphogenetic proteins on rat hepatic stellate cell proliferation and trans-differentiation. World J Gastroenterol. 2003;9:784–787. doi: 10.3748/wjg.v9.i4.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinzani M, Marra F. Cytokine receptors and signaling in hepatic stellate cells. Semin Liver Dis. 2001;21:397–416. doi: 10.1055/s-2001-17554. [DOI] [PubMed] [Google Scholar]
- 6.Liu XJ, Yang L, Mao YQ, Wang Q, Huang MH, Wang YP, Wu HB. Effects of the tyrosine protein kinase inhibitor genistein on the proliferation, activation of cultured rat hepatic stellate cells. World J Gastroenterol. 2002;8:739–745. doi: 10.3748/wjg.v8.i4.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pawlikowski M, Melen-Mucha G. Perspectives of new potential therapeutic applications of somatostatin analogs. Neuro Endocrinol Lett. 2003;24:21–27. [PubMed] [Google Scholar]
- 8.Wang CH, Tang CW, Liu CL, Tang LP. Inhibitory effect of octreotide on gastric cancer growth via MAPK pathway. World J Gastroenterol. 2003;9:1904–1908. doi: 10.3748/wjg.v9.i9.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel YC. Somatostatin and its receptor family. Front Neuroendocrinol. 1999;20:157–198. doi: 10.1006/frne.1999.0183. [DOI] [PubMed] [Google Scholar]
- 10.Geerts A, Niki T, Hellemans K, De Craemer D, Van Den Berg K, Lazou JM, Stange G, Van De Winkel M, De Bleser P. Purification of rat hepatic stellate cells by side scatter-activated cell sorting. Hepatology. 1998;27:590–598. doi: 10.1002/hep.510270238. [DOI] [PubMed] [Google Scholar]
- 11.Weng SG, Leng XS, Wei YH, Peng JR, Zheng ET, Cheng JH, Zham YB, Lu JF, Du RY. An improved method of isolating and identifying rat hepatic stellate cells. Beijing Daxue Xuebao. 2001;1:83–86. [Google Scholar]
- 12.Zhong ZH, Zhu JY, Leng XS, Du RY. Study on mRNA expression of subtypes of somatostatin receptors in the liver tissue of liver cirrhosis rats. Zhonghua Shiyan Waike Zazhi. 2000;5:439–441. [Google Scholar]
- 13.Reynaert H, Vaeyens F, Qin H, Hellemans K, Chatterjee N, Winand D, Quartier E, Schuit F, Urbain D, Kumar U, et al. Somatostatin suppresses endothelin-1-induced rat hepatic stellate cell contraction via somatostatin receptor subtype 1. Gastroenterology. 2001;121:915–930. doi: 10.1053/gast.2001.27971. [DOI] [PubMed] [Google Scholar]
- 14.Karalis K, Mastorakos G, Chrousos GP, Tolis G. Somatostatin analogues suppress the inflammatory reaction in vivo. J Clin Invest. 1994;93:2000–2006. doi: 10.1172/JCI117193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fort J, Oberti F, Pilette C, Veal N, Gallois Y, Douay O, Rousselet MC, Rosenbaum J, Calès P. Antifibrotic and hemodynamic effects of the early and chronic administration of octreotide in two models of liver fibrosis in rats. Hepatology. 1998;28:1525–1531. doi: 10.1002/hep.510280612. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Gong H, Wang Y. Experimental study on the preventive effect of octreotide on liver fibrosis. Zhonghua Gandan Waike Zazhi. 2003;2:100–102. [Google Scholar]
- 17.Chatterjee S, Van Marck E. The role of somatostatin in schistosomiasis: a basis for immunomodulation in host-parasite interactions. Trop Med Int Health. 2001;6:578–581. doi: 10.1046/j.1365-3156.2001.00758.x. [DOI] [PubMed] [Google Scholar]


