Abstract
AIM: To evaluate the safety and feasibility of laparoscopic abdominoperineal resection compared with the open procedure in multimodality management of rectal cancer.
METHODS: A total of 106 rectal cancer patients who underwent open abdominoperineal resection (OAPR) were matched with 106 patients who underwent laparoscopic abdominoperineal resection (LAPR) in a 1 to 1 fashion, between 2009 and 2013 at Fudan University Shanghai Cancer Center. Propensity score matching was carried out based on age, gender, pathological staging of the disease and administration of neoadjuvant chemoradiation. Data regarding preoperative staging, surgical technique, pathological results, postoperative recovery and complications were reviewed and compared between the LAPR and OAPR groups. Perineal closure around the stoma and pelvic floor reconstruction were performed only in OAPR, not in LAPR. Therefore, abdominoperineal resection procedure-specific surgical complications including parastomal hernia and perineal wound complications were compared between the open and laparoscopic procedure. Regular surveillance of the two cohorts was carried out to gather prognostic data. Disease-free survival was analyzed using Kaplan-Meier estimate and log-rank test. Subgroup analysis was performed in patients with locally advanced disease treated with preoperative chemoradiation followed by surgical resection.
RESULTS: No significant difference was found between the LAPR group and the OAPR group in terms of clinicopathological features. The operation time (180.8 ± 47.8 min vs 172.1 ± 49.2 min, P = 0.190), operative blood loss (93.9 ± 60.0 mL vs 88.4 ± 55.2 mL, P = 0.494), total number of retrieved lymph nodes (12.9 ± 6.9 vs 12.9 ± 5.4, P = 0.974), surgical complications (12.3% vs 15.1%, P = 0.549) and pathological characteristics were comparable between the LAPR and OAPR group, respectively. Compared with OAPR patients, LAPR patients showed significantly shorter postoperative analgesia (2.4 ± 0.7 d vs 2.7 ± 0.6 d, P < 0.001), earlier first flatus (57.3 ± 7.9 h vs 63.5 ± 9.2 h, P < 0.001), shorter urinary drainage time (6.5 ± 3.4 d vs 7.8 ± 1.3 d, P < 0.001), and shorter postoperative admission (11.2 ± 4.7 d vs 12.6 ± 4.0 d, P = 0.014). With regard to APR-specific complications (perineal wound complications and parastomal hernia), there were no significant differences between the two groups. Similar results were found in the 26 pairs of patients administered neoadjuvant chemoradiation in subgroup analysis. During the follow-up period, no port site recurrences were observed.
CONCLUSION: Laparoscopic abdominoperineal resection for multidisciplinary management of rectal cancer is safe, and is associated with earlier recovery and shorter admission time in combination with neoadjuvant chemoradiation.
Keywords: Abdominoperineal resection, Laparoscopy, Rectal cancer, Total mesorectal excision, Neoadjuvant chemoradiation
Core tip: This retrospective, case-matched study demonstrated that, for abdominoperineal resection of low rectal cancer, laparoscopy improved postoperative recovery, reduced admission time without jeopardizing clear circumferential resection margin, lymph node yield and surgical complications. In particular, the risks of abdominoperineal resection-specific surgical complications, including perineal wound reintervention and parastomal hernia, were comparable between the laparoscopy and open procedure groups. No significant differences regarding local recurrence and metachronous metastasis were detected. Laparoscopy in combination with neoadjuvant chemoradiation for multidisciplinary management of low rectal cancer is safe, and associated with earlier recovery.
INTRODUCTION
Oncological outcomes of patients with rectal cancer have been improved by multimodal treatment with adjuvant/neoadjuvant chemoradiation and total mesorectal excision (TME) over the last decades[1,2]. With the introduction and popularization of TME by Heald and coworkers since 1982[3], the rate of local recurrence and positive circumferential resection margin (CRM) has been greatly reduced[4,5]. Improvement in local control via neoadjuvant chemoradiation (neoCRT) has also been supported by high level evidence from randomized clinical trials with long-term follow-up, especially in patients with involved nodes[6-10]. Laparoscopic TME as a minimally invasive approach for the treatment of rectal cancer and has been proven to be technically feasible and safe with fewer complications and faster postoperative recovery than open TME[11-16]. The long-term oncologic equivalence of laparoscopic vs open surgery for rectal cancer has also been confirmed by several randomized clinical trials[17-20] and a meta-analysis[21].
Nevertheless, surgical resection plays a central role in curative treatment[22]. As one of the standard operations for low rectal cancer, abdominoperineal resection (APR), once known as Miles operation, was introduced in the late nineteenth century[23]. In spite of an increase in sphincter-preserving operations combined with neoCRT[24], APR remains the only option for 30% or more patients[25]. Tumors involving the levator ani muscle or the external anal sphincter, and inability to guarantee negative distal margin via sphincter-preserving operation, are still clear indications for APR[24].
Most of the studies mentioned above, enrolled patients who underwent low anterior resection (LAR) together with those underwent APR. However, APR is a completely different procedure to LAR in terms of approach, incision, field, area, digestive tract reconstruction, complications and difficulty[23,26,27]. There are few investigations to compare the short-term clinicopathological outcomes and long-term oncological equivalence between the laparoscopic and conventional open approach for APR in rectal cancer of all stages, especially in the era of multimodality. Therefore, the present study was conducted to evaluate the safety and feasibility of laparoscopic abdominoperineal resection (LAPR) with open abdominoperineal resection (OAPR) in the multidisciplinary treatment of rectal cancer with/without neoCRT.
MATERIALS AND METHODS
A total of 106 patients with rectal cancer who underwent LAPR in Fudan University Shanghai Cancer Center between March 2009 and December 2013 were enrolled in this study. According to the principle of intent-to-treat, patients who received conversional open procedure were included in the LAPR group. The diagnosis of adenocarcinoma, mucinous adenocarcinoma or signet-ring carcinoma of the rectum (≤ 10 cm from the anal verge) was confirmed pathologically. Exclusion criteria were patients with other gastrointestinal disease that required surgical intervention; previous malignant tumors; previous laparotomies; graded ASA IV or V.
In order to minimize the confounding effect of selection bias, 1:1 propensity score matching was performed to select a control group of 106 patients who underwent conventional OAPR during the same period from a cohort of 466 patients. Age, sex, BMI, ASA score, neoCRT, distance from tumor to anal verge and postoperative pathological staging were selected as covariates in the logistic regression model to estimate the propensity scores. These covariates were matched between the OAPR and LAPR group as they may influence short-term clinical outcomes.
Before surgery, all patients were assessed systemically by physical examination, biochemical analysis, colorectal cancer marker panel (carcinoembryonic antigen, carbohydrate antigen 19-9, cancer antigen 724, cancer antigen 242 and cancer antigen 125 for females) assay. Computed tomography of the chest, and abdominal, pelvic or whole body positron emission tomography (PET) were performed to detect distant metastasis. Magnetic resonance imaging or endorectal ultrasound was performed to determine the preoperative cT category and cN category of the primary tumor. Patients preoperatively staged at cT3/4 or cN+ with any cT were administered neoCRT in the absence of contraindications.
An interval time of 6-8 wk between surgery and preoperative chemoradiation was ensured. All open or laparoscopic abdominoperineal procedures performed followed the standard TME principles as described previously[16]. TME consists of maintaining the integrity of the mesorectal fascia and preservation of the autonomic nervous system with emphasis on sharp dissection under direct visualization. With regard to the extension of en bloc resection of the distal rectum, the conventional method instead of the extralevator approach was adopted in all cases. All operations in this study were performed by the same surgical team (Li XX, Ye X and Cai GX) with experience of more than 100 laparoscopic and open colorectal cancer procedures annually at the Department of Colorectal Surgery of Fudan University Shanghai Cancer Center.
Routine follow-up was carried out for all enrolled patients 2 wk after surgery, every 3 mo for the first year, every 6 mo for the second year and every year thereafter. Adjuvant chemoradiation was administered adhering to the NCCN guideline to treat rectal cancer based on TNM staging[28].
Data regarding demographics, preoperative evaluation and staging, surgical technique, pathological results, postoperative recovery and complications were obtained by chart review. The laparoscopic rectal cancer surgery program of our institution was approved by the Ethical Committee of Shanghai Cancer Center, Fudan University. Informed consent was obtained from all patients.
Statistical analysis
Parametric variables are shown as mean ± SD. The Student’s t test was used to assess differences in continuous variables. The χ2 test or Fisher’s exact test was performed to compare proportions between the two groups when appropriate. Difference in disease-free survival was evaluated using log-rank test with survival curves generated by the Kaplan-Meier method. A two-sided P < 0.05 was considered statistically significant. SPSS version 20.0 software package for Windows (SPSS Inc., Chicago, IL, United States) was used to conduct all statistical analyses. This study was reviewed and approved by the ethics committee of Fudan University Shanghai Cancer Center, and informed consent was acquired from each patient enrolled in the study.
The statistical methods used in this study were reviewed by the Center of Medical Biostatistics of Fudan University Shanghai Cancer Center.
RESULTS
Patient demographics
In the present study, 106 patients underwent LAPR and were matched with 106 patients who underwent OAPR. The proportion of patients administered neoCRT was 24.5% (26/106) in both groups. Patients’ demographics and preoperative evaluation are summarized in Table 1. 55 (51.9%), and 51 (48.1%) patients in each group were male, respectively (P = 0.583). The mean ages were 58.01 and 56.78 years in the LAPR and OAPR group, respectively (P = 0.454). The mean body mass index (BMI) were comparable between the two groups (23.13 vs 22.97, P = 0.690). The mean distance from the primary tumor to the anal verge was 3.87 and 3.58 in the LAPR and OAPR group, respectively (P = 0.099).
Table 1.
Clinicopathological features of the included patients
|
LAPR |
OAPR (%) |
P value | |||
| Mean, n | SD (%) | Mean, n | SD (%) | ||
| Age (yr) | 58.01 | 11.64 | 56.78 | 12.28 | 0.454 |
| BMI | 23.13 | 3.01 | 22.97 | 2.85 | 0.690 |
| Gender | 0.583 | ||||
| Male | 55 | 51.9 | 51 | 48.1 | |
| Female | 51 | 48.1 | 55 | 51.9 | |
| ASA score | 0.783 | ||||
| I | 54 | 50.9 | 52 | 49.1 | |
| II | 51 | 48.1 | 53 | 50.0 | |
| III | 1 | 0.9 | 1 | 0.9 | |
| Neoadjuvant therapy | 1.000 | ||||
| No | 80 | 75.5 | 80 | 75.5 | |
| Yes | 26 | 24.5 | 26 | 24.5 | |
| Histology | 1.000 | ||||
| Adenocarcinoma | 97 | 91.5 | 98 | 92.5 | |
| Mucinous adenocarcinoma | 9 | 8.5 | 8 | 7.5 | |
| Pathological stage | 1.000 | ||||
| pCR | 2 | 1.9 | 3 | 2.8 | |
| I | 43 | 40.6 | 41 | 38.7 | |
| II | 24 | 22.6 | 24 | 22.6 | |
| III | 25 | 23.6 | 25 | 23.6 | |
| IV | 12 | 11.3 | 13 | 12.3 | |
| Lymphovascular invasion | 0.846 | ||||
| No | 91 | 85.8 | 90 | 84.9 | |
| Yes | 15 | 14.2 | 16 | 15.1 | |
| Perineural invasion | 0.366 | ||||
| No | 85 | 80.2 | 90 | 84.9 | |
| Yes | 21 | 19.8 | 16 | 15.1 | |
| Grade | 0.451 | ||||
| NA | 25 | 23.6 | 18 | 17.0 | |
| III/IV | 19 | 17.9 | 23 | 21.7 | |
| I/II | 62 | 58.5 | 65 | 61.3 | |
| pT category | 0.612 | ||||
| pT0 | 4 | 3.8 | 4 | 3.8 | |
| pT1 | 12 | 11.3 | 15 | 14.2 | |
| pT2 | 40 | 37.7 | 31 | 29.2 | |
| pT3 | 50 | 47.2 | 56 | 52.8 | |
| pN category | 0.543 | ||||
| pN0 | 74 | 69.8 | 70 | 66.0 | |
| pN1 | 23 | 21.7 | 22 | 20.8 | |
| pN2 | 9 | 8.5 | 14 | 13.2 | |
| Total examined nodes | 12.88 | 6.93 | 12.91 | 5.40 | 0.974 |
| Distance (cm) | 3.87 | 1.34 | 3.58 | 1.28 | 0.099 |
LAPR: Laparoscopic abdominoperineal resection; OAPR: Open abdominoperineal resection; BMI: Body mass index; ASA: American Society of Anesthesiologists; pCR: Pathological complete remission.
Quality of surgery
Positive margin was defined as the presence of tumor cells at or within 1 mm from the margin[29]. In the present study, negative circumferential, distal and proximal margins were confirmed microscopically in all cases. The resections were considered curative (R0 resection) in all patients, except those with preoperatively confirmed isochronous metastatic disease. Conversion to open procedure was required in 3 cases (3/106, 2.83%). The reasons for conversion were massive adhesion of intestine and the peritoneum in two cases and severe invasion of the primary tumor to the bladder and seminal vesicle in one case.
Intraoperative blood loss was greater (93.87 mL vs 88.44 mL, P = 0.494) and operating time was longer (180.83 min vs 172.07 min, P = 0.190) in the LAPR group compared with the OAPR group, however, the difference was not statistically significant (Table 2).
Table 2.
Postoperative recovery
|
LAPR |
OAPR |
P value | |||
| Mean | SD | Mean | SD | ||
| Intraoperative blood loss (mL) | 93.87 | 60.04 | 88.44 | 55.15 | 0.494 |
| Operation time (min) | 180.83 | 47.83 | 172.07 | 49.16 | 0.190 |
| Time to first pass of flatus (h) | 57.31 | 7.91 | 63.51 | 9.20 | 0.000 |
| Postoperative analgesia (d) | 2.35 | 0.65 | 2.66 | 0.63 | 0.000 |
| Urinary drainage (d) | 6.45 | 3.40 | 7.75 | 1.30 | 0.000 |
| Postoperative hospital stay (d) | 11.15 | 4.72 | 12.63 | 3.96 | 0.014 |
LAPR: Laparoscopic abdominoperineal resection; OAPR: Open abdominoperineal resection.
Pathological results and postoperative recovery
Pathological staging was comparable between the groups (P = 1.000). No statistical differences were detected between the LAPR and OAPR groups regarding mucinous adenocarcinoma (8.5% vs 7.5%, P = 1.000), poorly differentiated (23.5% vs 26.1%, P = 0.687), perineural invasion (19.8% vs 15.1%, P = 0.366), lymphovascular invasion (14.2% vs 15.2%, P = 0.846), mean tumor size (3.08 cm vs 3.48 cm, P = 0.055) and mean number of retrieved lymph nodes (12.88 vs 12.91, P = 0.974, Table 1).
Patients in the LAPR group experienced a significantly shorter time to first pass of flatus (P < 0.001), time of postoperative analgesia (P < 0.001), duration of urinary drainage (P < 0.001) and postoperative admission days (P = 0.014, Table 2).
Surgical complications
Nine (84.9%) and 13 (12.3%) patients suffered postoperative complications in the LAPR and OAPR group, respectively (P = 0.500). Abdominal wound infection occurred in 3 and 5 cases and postoperative bowel obstruction was detected in 1 and 2 patients in the LAPR and OAPR group, respectively. One patient in each group experienced ureter damage. 1 case of urethral damage occurred in the LAPR group. In particular, two APR-specific surgical complications, perineal wound complication requiring reintervention (1 case vs 2 cases) and parastomal hernia (1 case vs none) were comparable between the two groups. No perioperative mortality, readmissions or relaparotomies were observed. The distribution of complications was not statistically different (P = 0.616; Table 3).
Table 3.
Summary of complications
| LAPR | OAPR | P value | |
| n | n | ||
| Abdominal wound infection | 3 | 5 | 0.721 |
| Perineal wound reintervention | 1 | 2 | 1.000 |
| Bowel obstruction | 1 | 2 | 1.000 |
| Urinary retention | 2 | 3 | 1.000 |
| Ureter damage | 1 | 1 | 1.000 |
| Urethra damage | 1 | 0 | 1.000 |
| Parastomal hernia | 1 | 0 | 1.000 |
| Total | 9 | 13 | 0.500 |
LAPR: Laparoscopic abdominoperineal resection; OAPR: Open abdominoperineal resection.
Follow-up
Short-term follow-up was carried out in all enrolled patients with a median follow-up time of 16 mo (range: 1-67 mo). Metachronous metastasis was detected in 15 cases (9 lung, 5 liver, 2 bone, 1 inguinal lymph node and 2 peritoneum). 4 cases were diagnosed with local recurrence. 2 and 3 deaths occurred in the LAPR and OAPR group, respectively, resulting from cachexia caused by metastatic disease. The number of metachronous metastases was comparable between the LAPR and OAPR groups (3 cases vs 12 cases, P = 0.275). Similarly, the number of local recurrences was not significantly different between the LAPR and OAPR groups (3 vs 1, P = 0.337). 3-year disease-free survival rate was 88.1% and 71.9% in the LAPR and OAPR group, respectively (P = 0.317, Figure 1). No port site recurrences were observed during the follow-up period.
Figure 1.
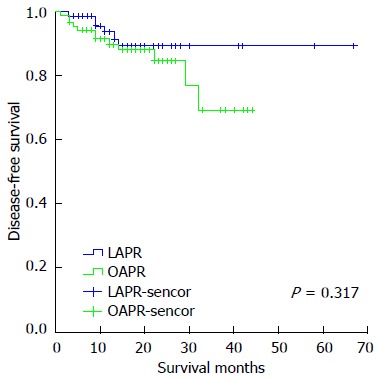
Survival curves of 161 patients with non-metastatic low rectal cancer using log-rank test and Kaplan-Meier estimate. The 3-year disease-free survival in the LAPR and OAPR group was 88.1% and 71.9%, respectively (P = 0.317). LAPR: Laparoscopic abdominoperineal resection; OAPR: Open abdominoperineal resection.
Neoadjuvant chemoradiation
Sub-group analysis was carried out by dividing the enrolled patients by preoperative chemoradiation. 24.5% (26/80) of patients in the LAPR and OAPR group were administered preoperative chemoradiation. Among the patients with preoperative chemoradiation, 2 cases in the LAPR group (7.7%) and 3 cases in the OAPR group (11.5%) achieved complete pathological remission.
The demographics of the patients, clinicopathological features of the tumors and surgical complications were comparable between the LAPR and OAPR group among patients with or without neoCRT (Table 4). Mean lymph node yield was significantly lower in patients with neoCRT (8 vs 14, P < 0.001). Among locally advanced patients treated with neoCRT, the intraoperative blood loss (P = 0.451), operation time (P = 0.301), postoperative analgesia (P = 0.094) and postoperative hospital stay were comparable in the LAPR and OAPR groups, while time to first pass of flatus (P < 0.001) and duration of urinary drainage (P < 0.001) was significantly shorter in the LAPR group compared with the OAPR group. Detailed comparative data are summarized in Tables 5 and 6.
Table 4.
Clinicopathological features according to neoadjuvant therapy
|
No neoCRT |
neoCRT |
|||||||||
|
LAPR |
OAPR |
P value |
LAPR |
OAPR |
P value | |||||
| Mean, n | SD (%) | Mean, n | SD (%) | Mean, n | SD (%) | Mean, n | SD (%) | |||
| Age (yr) | 58.41 | 11.98 | 57.44 | 12.86 | 0.623 | 56.81 | 10.64 | 54.77 | 10.23 | 0.483 |
| BMI | 23.21 | 3.00 | 22.84 | 2.89 | 0.431 | 22.90 | 3.08 | 23.37 | 2.74 | 0.561 |
| Gender | 0.113 | 0.087 | ||||||||
| Male | 42 | 52.5% | 32 | 40.0% | 13 | 50.0% | 19 | 73.1% | ||
| Female | 38 | 47.5% | 48 | 60.0% | 13 | 50.0% | 7 | 26.9% | ||
| ASA score | 0.429 | 0.696 | ||||||||
| I | 42 | 52.5% | 37 | 46.3% | 12 | 46.2% | 15 | 57.7% | ||
| II | 38 | 47.5% | 43 | 53.8% | 13 | 50.0% | 10 | 38.5% | ||
| III | 0 | 0.0% | 0 | 0.0% | 1 | 3.8% | 1 | 3.8% | ||
| Histology | 1.000 | 1.000 | ||||||||
| Adenocarcinoma | 57 | 80.3% | 56 | 73.7% | 5 | 50.0% | 9 | 75.0% | ||
| Mucinous adenocarcinoma | 6 | 7.5% | 6 | 7.5% | 3 | 11.5% | 2 | 7.7% | ||
| Pathological stage | 0.884 | 0.970 | ||||||||
| pCR | NA | NA | 2 | 7.7% | 3 | 11.5% | ||||
| I | 33 | 41.3% | 33 | 41.3% | 10 | 38.5% | 8 | 30.8% | ||
| II | 20 | 25.0% | 20 | 25.0% | 4 | 15.4% | 4 | 15.4% | ||
| III | 19 | 23.8% | 19 | 23.8% | 6 | 23.1% | 6 | 23.1% | ||
| IV | 8 | 10.0% | 8 | 10.0% | 4 | 15.4% | 5 | 19.2% | ||
| Lymphovascular invasion | 0.685 | 1.000 | ||||||||
| No | 66 | 82.5% | 64 | 80.0% | 25 | 96.2% | 26 | 100.0% | ||
| Yes | 14 | 17.5% | 16 | 20.0% | 1 | 3.8% | 0 | 0.0% | ||
| Perineural invasion | 0.151 | 0.701 | ||||||||
| No | 62 | 77.5% | 69 | 86.3% | 23 | 88.5% | 21 | 80.8% | ||
| Yes | 18 | 22.5% | 11 | 13.8% | 3 | 11.5% | 5 | 19.2% | ||
| Grade | 0.224 | 0.411 | ||||||||
| NA | 9 | 11.2% | 4 | 16 | 61.5% | 14 | 53.8% | |||
| III/IV | 14 | 17.5% | 20 | 25.0% | 5 | 19.2% | 3 | 11.5% | ||
| I/II | 57 | 71.2% | 56 | 70.0% | 5 | 19.2% | 9 | 34.6% | ||
| pT category | 0.816 | 0.196 | ||||||||
| pT0 | 0 | 0.0% | 0 | 0.0% | 4 | 15.4% | 4 | 15.4% | ||
| pT1 | 11 | 13.8% | 5 | 12.5% | 1 | 3.8% | 5 | 19.2% | ||
| pT2 | 27 | 33.8% | 7 | 30.0% | 13 | 50.0% | 7 | 26.9% | ||
| pT3 | 42 | 52.5% | 10 | 57.7% | 8 | 30.8% | 10 | 38.5% | ||
| pN category | 0.337 | 0.723 | ||||||||
| pN0 | 56 | 70.0% | 53 | 66.3% | 18 | 69.2% | 17 | 65.4% | ||
| pN1 | 17 | 21.3% | 14 | 17.5% | 6 | 23.1% | 8 | 30.8% | ||
| pN2 | 7 | 8.8% | 13 | 16.3% | 2 | 7.7% | 1 | 3.8% | ||
| Total retrieved nodes | 14.58 | 6.70 | 14.11 | 5.35 | 0.630 | 7.65 | 4.73 | 9.19 | 3.59 | 0.193 |
| Distance (cm) | 3.73 | 1.25 | 3.55 | 1.26 | 0.380 | 4.33 | 1.50 | 3.65 | 1.35 | 0.095 |
LAPR: Laparoscopic abdominoperineal resection; OAPR: Open abdominoperineal resection; BMI: Body mass index; ASA: American Society of Anesthesiologists; pCR: Pathological complete remission; neoCRT: Neoadjuvant chemoradiation.
Table 5.
Postoperative recovery according to neoadjuvant therapy
|
No neoCRT |
neoCRT |
|||||||||
|
LAPR |
OAPR |
P value |
LAPR |
OAPR |
P value | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| Intraoperative blood loss (mL) | 91.88 | 63.87 | 87.94 | 57.55 | 0.683 | 100.00 | 46.90 | 90.00 | 48.00 | 0.451 |
| Operation time (min) | 176.48 | 48.47 | 170.25 | 41.73 | 0.385 | 194.23 | 44.00 | 177.65 | 67.85 | 0.301 |
| Time to first pass of flatus (h) | 57.55 | 7.56 | 62.93 | 9.33 | 0.000 | 56.58 | 9.03 | 65.31 | 8.68 | 0.001 |
| Postoperative analgesia (d) | 2.39 | 0.63 | 2.70 | 0.64 | 0.001 | 2.23 | 0.71 | 2.54 | 0.58 | 0.094 |
| Urinary drainage (d) | 6.63 | 3.75 | 7.70 | 1.07 | 0.016 | 5.92 | 1.96 | 7.88 | 1.86 | 0.001 |
| Postoperative hospital stay (d) | 11.41 | 5.24 | 12.80 | 3.94 | 0.060 | 10.35 | 2.48 | 12.12 | 4.07 | 0.064 |
LAPR: Laparoscopic abdominoperineal resection; OAPR: Open abdominoperineal resection; neoCRT: Neoadjuvant chemoradiation.
Table 6.
Summary of complications according to neoadjuvant therapy
|
No neoCRT |
neoCRT |
|||||
| LAPR | OAPR | P value | LAPR | OAPR | P value | |
| n | n | n | n | |||
| Abdominal wound infection | 3 | 3 | 1.000 | 0 | 2 | 0.497 |
| Perineal wound reintervention | 0 | 1 | 1 | 1 | ||
| Bowel obstruction | 1 | 1 | 0 | 1 | ||
| Urinary retention | 1 | 3 | 1 | 0 | ||
| Ureter damage | 1 | 0 | 0 | 1 | ||
| Urethra damage | 1 | 0 | 0 | 0 | ||
| Parastomal hernia | 1 | 0 | 0 | 0 | ||
| Total | 7 | 8 | 1.000 | 2 | 5 | 0.191 |
LAPR: Laparoscopic abdominoperineal resection; OAPR: Open abdominoperineal resection; neoCRT: Neoadjuvant chemoradiation.
DISCUSSION
Laparoscopy has been used in colorectal surgery for more than 20 years. While waiting for long-term outcomes to confirm its oncological equivalence, according to a meta-analysis of short-term results from multiple non-randomized and randomized trials, laparoscopic resection of rectal cancer has been proved to be feasible, efficacious and safe with reduced risks of postoperative morbidity and mortality[30,31]. However, it is worth noting that none of these trials or meta-analyses provided a subgroup analysis to address this issue among patients who underwent the APR procedure, nor did they report the sphincter-preserving rate. Taking into account that the APR procedure is different from the sphincter-preserving procedure resulting in perineal wound, end-sigmoid-colostomy, prolonged postoperative recovery, increased incidence and wide spectrum of surgical complications, the effect of laparoscopy on the APR procedure could be overemphasized or underestimated according to the available evidence.
As shown in the present case-matched study, low rectal cancer patients who underwent LAPR, performed by extensively experienced surgeons, demonstrated improved postoperative recovery, similar risks of complications and retrieved lymph nodes compared with patients who underwent OAPR. All of the non-metastatic patients were radically resected with negative CRM. No readmissions, reoperations, perioperative mortality, port site or local recurrences were observed in both groups, indicating the safety, feasibility and oncological equivalence of LAPR. Furthermore, in patients who received neoCRT, LAPR decreased the time to first flatus pass and duration of urinary drainage, while maintaining similar risks of complications and comparable retrieved lymph nodes. During short-term follow-up, the rate of metachronous metastasis was comparable between LAPR and OAPR.
Meta-analyses of trials including both AR and APR since the 2000s have shown that patients benefit from laparoscopic rectal surgery with respect to improved postoperative recovery, improved abdominal cosmesis, reduced surgical complications, fewer abdominal wound infections and ventral hernias[32]. Similarly, as demonstrated in previous studies including only APR, laparoscopic APR reduced blood loss[33], reduced postoperative pain, shortened postoperative ileus, and resulted in earlier return of bowel function and earlier mobilization[34] which was in accordance with our findings. Greater magnification and illumination of the surgical field by laparoscopy allows better exposure and protection of the autonomic nerves, which probably results in shorter urinary drainage. The minimally invasive approach with a smaller abdominal wound and less blood loss may be the reason for reduced analgesic requirement and improved recovery.
In the case of surgical quality and oncological radicality reflected by CRM and lymph node yield, respectively, short-term results of the major multicenter randomized trials, CLASICC[35] and COLOR[19], comparing conventional open and laparoscopic rectal surgery showed that the rate of positive CRM and lymph node yield were not statistically different. A meta-analysis of randomized clinical trials by Aziz et al[14], Anderson et al[36] and Huang et al[21] also indicated a comparable rate of positive CRM and lymph node yield between the open and laparoscopic procedure for resection of rectal cancer which supported the results from the present study. In our study, lymph node yield was 8 and 14 in patients with or without neoCRT, respectively, which was comparable to that reported by previous studies.
APR performed in a number of studies was found to have greater risks of postoperative morbidity, prolonged recovery, worse survival and local control than sphincter-saving procedures[26,27,37-39]. Despite patient- and tumor-related factors such as older age and higher tumor stage[40,41], the procedure itself also contributed to worse short-term outcomes in APR due to the extensive surgical field leading to perineal wound complications and a greater chance of tumor perforation, involved resection margin and damage to adjacent structures such as the urethra, and stoma-related complications resulting from the inevitable permanent end colostomy. Intensified treatment of lower rectal cancer with neoCRT has been found to increase the chance of perineal wound complications[42,43]. However, a detailed comparison of the equivalence between laparoscopy and conventional open procedure regarding perineal wound and parastomal problems is lacking in most studies. In the present study, we compared the number of cases who suffered from perineal wound dehiscence and deep pelvic abscess resulting in reintervention of the perineal wound, and the number of parastomal hernias. The results showed that LAPR and OAPR had a similar chance of perineal wound reintervention and parastomal hernia. Although LAPR avoids a midline incision, LAPR patients showed only a slightly decreased chance of abdominal wound infection. The underlying reason could be the inability to detect differences due to a low event rate.
Toxicity due to chemotherapeutic agents and radiation effects results in increased operative blood loss and operation time, greater risk of surgical complications which exert a negative influence on postoperative recovery and surgical complications[44,45]. Intensified treatment of lower rectal cancer with neoCRT has been found to increase the chance of perineal wound complications[42,43]. According to our results, in patients who received neoadjuvant treatment, laparoscopy showed improved postoperative recovery of bowel and bladder function, and a comparable rate of APR-specific complications was observed. The intraoperative blood loss, operation time, postoperative admission day and APR-specific complications were comparable between the LAPR and OAPR groups.
There were several limitations in our study. Firstly, due to the limited sample size, only 26 patients received neoCRT in the OAPR and LAPR groups, which may have diminished the ability to distinguish the potential difference in postoperative recovery and complication risks. Secondly, due to the retrospective design of our study, selection bias could have interfered with interpretation of the statistical results. The majority of analyzed patients were ASA score I and II resulting in a lower postoperative complication rate. Also, in each group, almost 60% of patients were pathological stage I and II. The ability of laparoscopy to improve postoperative recovery and morbidity in late stage rectal cancer needs to be examined in a future study with a larger sample size in the subgroup. Lastly, since the median follow-up time was much shorter than 5 years, only short-term, but not long-term results were reported in this study. The equivalence of laparoscopy in the multidisciplinary treatment of rectal cancer requires further study.
In conclusion, our single-center, retrospective, case-matched study demonstrated that, among patients with low rectal cancer requiring abdominal perineal resection of primary tumor, laparoscopy improved postoperative recovery without jeopardizing a clear circumferential resection margin, lymph node yield and surgical complications. In particular, risks of the APR-specific surgical complications, perineal wound reintervention and parastomal hernia, were comparable in the LAPR and OAPR group.
COMMENTS
Background
Optimal perioperative chemoradiation, standard total mesorectal excision (TME) as well as multidisciplinary treatment have significantly contributed to reducing local recurrence of rectal cancer. Abdominoperineal resection (APR) is a highly complex procedure and the associated surgical complications and mortality rate are high. Nevertheless, APR remains the only curative option for more than 30% of patients with low rectal cancer.
Research frontiers
Laparoscopy as a minimally invasive procedure, is the gold standard in the treatment of cholecystitis and has gained tremendous value in the resection of colon cancer. More recently, high-level evidence suggested equivalent oncological outcomes between laparoscopic and open TME of rectal cancer, as well as improved postoperative recovery following laparoscopic TME.
Innovations and breakthroughs
The present study demonstrated improved postoperative recovery and shorter admission time after laparoscopic APR, compared with the conventional open procedure. Laparoscopic APR is safe and equal in terms of surgical and oncological outcome. Moreover, APR-specific complications including perineal wound reintervention and parastomal hernia are comparable between laparoscopy and open surgery.
Applications
In the era of individualized and multidisciplinary oncology, the increased application of laparoscopy to perform APR is another milestone and will benefit more patients with low rectal cancer.
Terminology
APR, also called Miles operation, involves surgical removal of the anus, the rectum and part of the distal pelvic colon together with the mesocolon and lymph nodes, and specimens are removed through the perineal incision and end-sigmoidostomy. Laparoscopy, a minimally invasive procedure, avoids a midline incision, and provides greater magnification and illumination of the surgical field.
Peer-review
This article is well written and well documented. The number of patients is important, and the team has good surgical results. Laparoscopy offers clear advantages found in large series and in your study. But the authors must not forget that the oncological outcome prevails. Laparoscopy and open surgery gives similar oncologic results. The surgeon must perform the procedure using the technique he mastered the best (even if open surgery is chosen).
Footnotes
Institutional review board statement: The study was reviewed and approved by the Ethical Committee and Institutional Review Board of Fudan University Shanghai Cancer Center.
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: All of the authors declared no conflict-of-interest.
Data sharing statement: Technical appendix, statistical code, and dataset available from the corresponding author at lxx1149@163.com. Consent was not obtained, but the presented data are anonymized and the risk of identification is low.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: March 20, 2015
First decision: April 23, 2015
Article in press: July 15, 2015
P- Reviewer: Falletto E, Kotzampassakis N S- Editor: Yu J L- Editor: Webster JR E- Editor: Zhang DN
References
- 1.Cervantes A, Chirivella I, Rodriguez-Braun E, Campos S, Navarro S, García Granero E. A multimodality approach to localized rectal cancer. Ann Oncol. 2006;17 Suppl 10:x129–x134. doi: 10.1093/annonc/mdl250. [DOI] [PubMed] [Google Scholar]
- 2.Allaix ME, Fichera A. Modern rectal cancer multidisciplinary treatment: the role of radiation and surgery. Ann Surg Oncol. 2013;20:2921–2928. doi: 10.1245/s10434-013-2966-x. [DOI] [PubMed] [Google Scholar]
- 3.Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery--the clue to pelvic recurrence? Br J Surg. 1982;69:613–616. doi: 10.1002/bjs.1800691019. [DOI] [PubMed] [Google Scholar]
- 4.Bach SP, Hill J, Monson JR, Simson JN, Lane L, Merrie A, Warren B, Mortensen NJ. A predictive model for local recurrence after transanal endoscopic microsurgery for rectal cancer. Br J Surg. 2009;96:280–290. doi: 10.1002/bjs.6456. [DOI] [PubMed] [Google Scholar]
- 5.Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986;1:1479–1482. doi: 10.1016/s0140-6736(86)91510-2. [DOI] [PubMed] [Google Scholar]
- 6.Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, Rutten HJ, Pahlman L, Glimelius B, van Krieken JH, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–646. doi: 10.1056/NEJMoa010580. [DOI] [PubMed] [Google Scholar]
- 7.van Gijn W, Marijnen CA, Nagtegaal ID, Kranenbarg EM, Putter H, Wiggers T, Rutten HJ, Påhlman L, Glimelius B, van de Velde CJ. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12:575–582. doi: 10.1016/S1470-2045(11)70097-3. [DOI] [PubMed] [Google Scholar]
- 8.Sebag-Montefiore D, Stephens RJ, Steele R, Monson J, Grieve R, Khanna S, Quirke P, Couture J, de Metz C, Myint AS, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet. 2009;373:811–820. doi: 10.1016/S0140-6736(09)60484-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, Becker H, Raab HR, Villanueva MT, Witzigmann H, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30:1926–1933. doi: 10.1200/JCO.2011.40.1836. [DOI] [PubMed] [Google Scholar]
- 10.Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 11.Leroy J, Jamali F, Forbes L, Smith M, Rubino F, Mutter D, Marescaux J. Laparoscopic total mesorectal excision (TME) for rectal cancer surgery: long-term outcomes. Surg Endosc. 2004;18:281–289. doi: 10.1007/s00464-002-8877-8. [DOI] [PubMed] [Google Scholar]
- 12.Staudacher C, Di Palo S, Tamburini A, Vignali A, Orsenigo E. Total mesorectal excision (TME) with laparoscopic approach: 226 consecutive cases. Surg Oncol. 2007;16 Suppl 1:S113–S116. doi: 10.1016/j.suronc.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 13.Kim SH, Park IJ, Joh YG, Hahn KY. Laparoscopic resection for rectal cancer: a prospective analysis of thirty-month follow-up outcomes in 312 patients. Surg Endosc. 2006;20:1197–1202. doi: 10.1007/s00464-005-0599-2. [DOI] [PubMed] [Google Scholar]
- 14.Aziz O, Constantinides V, Tekkis PP, Athanasiou T, Purkayastha S, Paraskeva P, Darzi AW, Heriot AG. Laparoscopic versus open surgery for rectal cancer: a meta-analysis. Ann Surg Oncol. 2006;13:413–424. doi: 10.1245/ASO.2006.05.045. [DOI] [PubMed] [Google Scholar]
- 15.Breukink S, Pierie J, Wiggers T. Laparoscopic versus open total mesorectal excision for rectal cancer. Cochrane Database Syst Rev. 2006;(4):CD005200. doi: 10.1002/14651858.CD005200.pub2. [DOI] [PubMed] [Google Scholar]
- 16.Gong J, Shi DB, Li XX, Cai SJ, Guan ZQ, Xu Y. Short-term outcomes of laparoscopic total mesorectal excision compared to open surgery. World J Gastroenterol. 2012;18:7308–7313. doi: 10.3748/wjg.v18.i48.7308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green BL, Marshall HC, Collinson F, Quirke P, Guillou P, Jayne DG, Brown JM. Long-term follow-up of the Medical Research Council CLASICC trial of conventional versus laparoscopically assisted resection in colorectal cancer. Br J Surg. 2013;100:75–82. doi: 10.1002/bjs.8945. [DOI] [PubMed] [Google Scholar]
- 18.Jeong SY, Park JW, Nam BH, Kim S, Kang SB, Lim SB, Choi HS, Kim DW, Chang HJ, Kim DY, et al. Open versus laparoscopic surgery for mid-rectal or low-rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): survival outcomes of an open-label, non-inferiority, randomised controlled trial. Lancet Oncol. 2014;15:767–774. doi: 10.1016/S1470-2045(14)70205-0. [DOI] [PubMed] [Google Scholar]
- 19.van der Pas MH, Haglind E, Cuesta MA, Fürst A, Lacy AM, Hop WC, Bonjer HJ. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013;14:210–218. doi: 10.1016/S1470-2045(13)70016-0. [DOI] [PubMed] [Google Scholar]
- 20.Bonjer HJ, Deijen CL, Abis GA, Cuesta MA, van der Pas MH, de Lange-de Klerk ES, Lacy AM, Bemelman WA, Andersson J, Angenete E, et al. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med. 2015;372:1324–1332. doi: 10.1056/NEJMoa1414882. [DOI] [PubMed] [Google Scholar]
- 21.Huang MJ, Liang JL, Wang H, Kang L, Deng YH, Wang JP. Laparoscopic-assisted versus open surgery for rectal cancer: a meta-analysis of randomized controlled trials on oncologic adequacy of resection and long-term oncologic outcomes. Int J Colorectal Dis. 2011;26:415–421. doi: 10.1007/s00384-010-1091-6. [DOI] [PubMed] [Google Scholar]
- 22.Lindsetmo RO, Joh YG, Delaney CP. Surgical treatment for rectal cancer: an international perspective on what the medical gastroenterologist needs to know. World J Gastroenterol. 2008;14:3281–3289. doi: 10.3748/wjg.14.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miles WE. A method of performing abdomino-perineal excision for carcinoma of the rectum and of the terminal portion of the pelvic colon (1908) CA Cancer J Clin. 1971;21:361–364. doi: 10.3322/canjclin.21.6.361. [DOI] [PubMed] [Google Scholar]
- 24.Tilney HS, Tekkis PP. Extending the horizons of restorative rectal surgery: intersphincteric resection for low rectal cancer. Colorectal Dis. 2008;10:3–15; discussion 15-16. doi: 10.1111/j.1463-1318.2007.01226.x. [DOI] [PubMed] [Google Scholar]
- 25.Ptok H, Marusch F, Kuhn R, Gastinger I, Lippert H. Influence of hospital volume on the frequency of abdominoperineal resection and long-term oncological outcomes in low rectal cancer. Eur J Surg Oncol. 2007;33:854–861. doi: 10.1016/j.ejso.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 26.Liang JT, Cheng JC, Huang KC, Lai HS, Sun CT. Comparison of tumor recurrence between laparoscopic total mesorectal excision with sphincter preservation and laparoscopic abdominoperineal resection for low rectal cancer. Surg Endosc. 2013;27:3452–3464. doi: 10.1007/s00464-013-2898-3. [DOI] [PubMed] [Google Scholar]
- 27.Kim JS, Hur H, Kim NK, Kim YW, Cho SY, Kim JY, Min BS, Ahn JB, Keum KC, Kim H, et al. Oncologic outcomes after radical surgery following preoperative chemoradiotherapy for locally advanced lower rectal cancer: abdominoperineal resection versus sphincter-preserving procedure. Ann Surg Oncol. 2009;16:1266–1273. doi: 10.1245/s10434-009-0338-3. [DOI] [PubMed] [Google Scholar]
- 28.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Rectal Cancer. Accessed September 12, 2014. Available from: http://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. [DOI] [PubMed]
- 29.Adam IJ, Mohamdee MO, Martin IG, Scott N, Finan PJ, Johnston D, Dixon MF, Quirke P. Role of circumferential margin involvement in the local recurrence of rectal cancer. Lancet. 1994;344:707–711. doi: 10.1016/s0140-6736(94)92206-3. [DOI] [PubMed] [Google Scholar]
- 30.Arezzo A, Passera R, Scozzari G, Verra M, Morino M. Laparoscopy for extraperitoneal rectal cancer reduces short-term morbidity: Results of a systematic review and meta-analysis. United European Gastroenterol J. 2013;1:32–47. doi: 10.1177/2050640612473753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arezzo A, Passera R, Salvai A, Arolfo S, Allaix ME, Schwarzer G, Morino M. Laparoscopy for rectal cancer is oncologically adequate: a systematic review and meta-analysis of the literature. Surg Endosc. 2015;29:334–348. doi: 10.1007/s00464-014-3686-4. [DOI] [PubMed] [Google Scholar]
- 32.Duepree HJ, Senagore AJ, Delaney CP, Fazio VW. Does means of access affect the incidence of small bowel obstruction and ventral hernia after bowel resection? Laparoscopy versus laparotomy. J Am Coll Surg. 2003;197:177–181. doi: 10.1016/S1072-7515(03)00232-1. [DOI] [PubMed] [Google Scholar]
- 33.Inada R, Yamamoto S, Oshiro T, Takawa M, Fujita S, Akasu T. Case-matched comparison of the short-term outcomes between laparoscopic and open abdominoperineal resection for rectal cancer. Surg Today. 2014;44:640–645. doi: 10.1007/s00595-013-0611-8. [DOI] [PubMed] [Google Scholar]
- 34.Ng SS, Leung KL, Lee JF, Yiu RY, Li JC, Teoh AY, Leung WW. Laparoscopic-assisted versus open abdominoperineal resection for low rectal cancer: a prospective randomized trial. Ann Surg Oncol. 2008;15:2418–2425. doi: 10.1245/s10434-008-9895-0. [DOI] [PubMed] [Google Scholar]
- 35.Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, Heath RM, Brown JM. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365:1718–1726. doi: 10.1016/S0140-6736(05)66545-2. [DOI] [PubMed] [Google Scholar]
- 36.Anderson C, Uman G, Pigazzi A. Oncologic outcomes of laparoscopic surgery for rectal cancer: a systematic review and meta-analysis of the literature. Eur J Surg Oncol. 2008;34:1135–1142. doi: 10.1016/j.ejso.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 37.Stelzner S, Koehler C, Stelzer J, Sims A, Witzigmann H. Extended abdominoperineal excision vs. standard abdominoperineal excision in rectal cancer--a systematic overview. Int J Colorectal Dis. 2011;26:1227–1240. doi: 10.1007/s00384-011-1235-3. [DOI] [PubMed] [Google Scholar]
- 38.Marr R, Birbeck K, Garvican J, Macklin CP, Tiffin NJ, Parsons WJ, Dixon MF, Mapstone NP, Sebag-Montefiore D, Scott N, et al. The modern abdominoperineal excision: the next challenge after total mesorectal excision. Ann Surg. 2005;242:74–82. doi: 10.1097/01.sla.0000167926.60908.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shihab OC, Brown G, Daniels IR, Heald RJ, Quirke P, Moran BJ. Patients with low rectal cancer treated by abdominoperineal excision have worse tumors and higher involved margin rates compared with patients treated by anterior resection. Dis Colon Rectum. 2010;53:53–56. doi: 10.1007/DCR.0b013e3181c70465. [DOI] [PubMed] [Google Scholar]
- 40.Wibe A, Syse A, Andersen E, Tretli S, Myrvold HE, Søreide O. Oncological outcomes after total mesorectal excision for cure for cancer of the lower rectum: anterior vs. abdominoperineal resection. Dis Colon Rectum. 2004;47:48–58. doi: 10.1007/s10350-003-0012-y. [DOI] [PubMed] [Google Scholar]
- 41.Reshef A, Lavery I, Kiran RP. Factors associated with oncologic outcomes after abdominoperineal resection compared with restorative resection for low rectal cancer: patient- and tumor-related or technical factors only? Dis Colon Rectum. 2012;55:51–58. doi: 10.1097/DCR.0b013e3182351c1f. [DOI] [PubMed] [Google Scholar]
- 42.Bullard KM, Trudel JL, Baxter NN, Rothenberger DA. Primary perineal wound closure after preoperative radiotherapy and abdominoperineal resection has a high incidence of wound failure. Dis Colon Rectum. 2005;48:438–443. doi: 10.1007/s10350-004-0827-1. [DOI] [PubMed] [Google Scholar]
- 43.Bobkiewicz A, Banasiewicz T, Krokowicz L, Paszkowski J, Hermann J, Malinger S, Drews M. Perineal wound healing after abdominoperineal resection for rectal cancer: a systematic review and meta-analysis. Dis Colon Rectum. 2015;58:e18. doi: 10.1097/DCR.0000000000000303. [DOI] [PubMed] [Google Scholar]
- 44.Simunovic M, Sexton R, Rempel E, Moran BJ, Heald RJ. Optimal preoperative assessment and surgery for rectal cancer may greatly limit the need for radiotherapy. Br J Surg. 2003;90:999–1003. doi: 10.1002/bjs.4210. [DOI] [PubMed] [Google Scholar]
- 45.Marijnen CA, Kapiteijn E, van de Velde CJ, Martijn H, Steup WH, Wiggers T, Kranenbarg EK, Leer JW. Acute side effects and complications after short-term preoperative radiotherapy combined with total mesorectal excision in primary rectal cancer: report of a multicenter randomized trial. J Clin Oncol. 2002;20:817–825. doi: 10.1200/JCO.2002.20.3.817. [DOI] [PubMed] [Google Scholar]


