Abstract
Immunosuppressive effects of an intranasal challenge with non-cytopathic bovine viral diarrhea virus (BVDV) 2a (strain 1373) were assessed through acquired and innate immune system responses to ovalbumin (OVA). Concurrent BVDV infection was hypothesized to delay and reduce the humoral response to ovalbumin (administered on days 3 and 15 post-inoculation). Infected animals followed the expected clinical course. BVDV titers, and anti-BVDV antibodies confirmed the course of infection and were not affected by the administration of OVA. Both the T-helper (CD4+) and B-cell (CD20+) compartments were significantly (P < 0.05) reduced in infected animals, while the gamma-delta T-cell population (Workshop cluster 1+, WC1+) decreased slightly in numbers. Infection with BVDV delayed the increase in OVA IgG by approximately 3 d from day 12 through day 21 post-inoculation. Between days 25 and 37 post-inoculation following BVDV infection the IgM concentration in the BVDV− group decreased while the OVA IgM titer still was rising in the BVDV+ animals. Thus, active BVDV infection delays IgM and IgG responses to a novel, non-infectious antigen.
Résumé
Une infection aiguë par le BVDV-2 chez les veaux retarde les réponses humorales face à un test à l’aide d’un antigène non infectieux. Les effets immunosuppressifs d’une inoculation défin intranasale à l’aide du virus non cytopathogène de la diarrhée virale bovine (VBVD) 2a (souche 1373) ont été évalués par les réactions acquises et innées du système immunitaire à l’ovalbumine (OVA). On a émis l’hypothèse que l’infection concomitante par le VBVD retardait et réduisait la réaction humorale à l’ovalbumine (administrée aux jours 3 et 15 après l’inoculation). Les animaux infectés ont suivi le cheminement clinique prévu. Les titres de BVDV et les anticorps anti-BVDV ont confirmé le déroulement de l’infection et ils n’ont pas été affectés par l’administration d’OVA. Les compartiments des lymphocytes T auxiliaires (CD4+) et des cellules B (CD20+) étaient significativement réduits (P < 0,05) chez les animaux infectés, tandis que la numération de la population de cellules T gamma-delta (WC1+) a diminué légèrement. L’infection par le VBVD a retardé l’augmentation de l’OVA IgG d’environ 3 jours, à compter du jour 12 jusqu’au jour 21 après l’inoculation. Entre les jours 25 et 37 après l’inoculation suivant l’infection par le BVDV, la concentration d’IgM dans le groupe VBVD a diminué tandis que le titre d’OVA IgM augmentait toujours chez les animaux positifs pour le VBVD. Par conséquent, l’infection active par le VBVD retarde les réactions IgM et IgG face à un antigène non infectieux nouveau.
(Traduit par Isabelle Vallières)
Introduction
The link between bovine viral diarrhea virus (BVDV) and vulnerability to bovine respiratory disease (BRD) is well established (1). The presence of even a single, asymptomatic, persistently infected calf has demonstrable effects on growth performance and the need for antibiotic treatment for the entire pen (2). Bovine viral diarrhea viruses are members of the Flaviviridae family consisting of enveloped, positive-sense, single-stranded RNA viruses (3). These viruses are able to affect both innate and adaptive immune cells, including macrophages, granulocytes, antigen-presenting myeloid cells, CD4+ and CD8+ T-lymphocytes, and B-cells (4). Thus, there is evidence that BVDV potentiates vulnerability to BRD through effects on innate and adaptive immune responses (5).
The current study extended previous research efforts with 3 significant additions. i) The study was designed to closely mimic the specific seasonal effects and industry standards for fall-placed feedlot calves in Alberta. ii) Recent research into immune-system effects of BVDV has focussed on non-cytopathic BVDV-1 strains producing mild clinical symptoms between days 3 and 7 post-infection, with a rectal temperature spike on day 7, and complete clinical resolution by day 10 (5). In contrast, the current study used strain 1373, a non-cytopathic BVDV type 2a originating from an outbreak in Ontario, Canada during 1993–1995 (6). This strain is associated with more severe, and prolonged, acute infection. Experimentally it can be delivered intra-nasally (7), and necessitates a longer post-infection sampling interval. iii) The impact of BVDV infection on the humoral immune system was further assessed through a novel antigen challenge in the form of an intramuscular ovalbumin injection (8). Thus, calves in this experiment were exposed to more severe acute illness, under the variable temperature conditions that prevail in Alberta during the fall, while their humoral immune response to a non-infectious antigen was measured. We hypothesized that experimental BVDV-2 infection would both delay and reduce the humoral response to ovalbumin in calves, providing insight into the mechanisms through which BVDV could increase co-infection risk and BRD incidence.
Materials and methods
Research was conducted under University of Calgary Animal Care and Use Protocol #AC12-143. Biosecurity measures including physical separation of animals and restrictions on movement of staff and materials between pens were also approved and enforced for this project.
Animals and husbandry
Thirty-two recently weaned, infectious bovine rhinotracheitis virus and BVDV-free, unvaccinated, mixed-breed beef calves between 5 and 6 mo of age were obtained from a single source (Canadian Food Inspection Agency, Lethbridge, Alberta). The group consisted of 27 steers and 5 heifers with weights ranging from 155 to 311 kg and a herd median weight of 230 kg. Four days after arrival at the University of Calgary research facility on August 27, 2012, all animals received tulathromycin (Draxxin; Pfizer Animal Health, Kirkland, Quebec), 2.5 mg/kg body weight (BW), SC, and a multivalent clostridium vaccine (TasVax-8; Merck Animal Health Kirkland, Quebec). The calves were housed in outdoor pens with three-sided shelters that were bedded daily with straw or wood shavings. In 2 daily even feedings, a baled mix of alfalfa (20%), orchard grass (80%), and timothy hay plus a grain-based supplement of 0.75 kg/head per day, later increasing to 1.5 kg/head per day, were provided. All calves had unlimited access to calcium-phosphate trace minerals, vitamin/salt blocks, and tap water.
Study design
Calves (n = 32) were blocked by weight and gender, and randomly assigned to either the BVDV challenge (BVDV+) or control group (BVDV−) pen. Within these groups, 8 of the calves received an ovalbumin inoculation (OVA+), and 8 were sham-treated (OVA−), resulting in 4 treatment groups, each consisting of 8 animals: BVDV+/OVA+, BVDV+/OVA−, BVDV−/OVA+ and BVDV−/OVA−. The time course and activities associated with data collection began on September 11, 2012. Day post-inoculation will be abbreviated with “D.” All individuals were monitored daily for health, with direct veterinary oversight, beginning on D7 prior to challenge with BVDV or medium, and ending on D37 post-inoculation. Hands-on assessment, including individual identification, weight, temperature, and clinical signs occurred on D-7 and D-5, then daily from D0 to D15, and periodically thereafter through D37. Clinical signs were scored on a scale from 0 to 5, for each of respiratory, digestive, and disposition scores (9), which were summed to yield a global clinical score (GCS) for each individual (range: 0 to 15). Ethylenediamine tetraacetic acid (EDTA)-treated whole blood and serum (by jugular venipuncture) (Vacutainer; Becton Dickinson, Mississauga, Ontario), and nasal swabs (Dacron-tipped swabs; Roche, Branchburg, New Jersey, USA) were collected during hands-on assessment. Intra-nasal BVDV challenge (BVDV+) occurred on D0. Noncytopathic BVDV type 2a strain 1373 (6,10) was diluted in Dulbecco’s Modified Eagle’s Medium (DMEM; Lonza, Walkersville, Maryland, USA) to reach a final concentration of 107.2 Tissue Culture Infective Dose 50% (TCID50) per 4 mL inoculum (BVDV+). Syringes with spray nozzles (Zoetis, Kirkland, Quebec) were used to administer 2 mL of the inoculum into each nostril. Sham-inoculated calves (BVDV−) received DMEM only. The ovalbumin-challenged calves were inoculated intramuscularly on D3 and D15 with 2 mg ovalbumin (OVA; Sigma-Aldrich, Oakville, Ontario) solubilized in sterile water with incomplete Freund’s adjuvant (IFA; Sigma-Aldrich) in a final volume of 2 mL. OVA− animals received an injection of incomplete Freund’s adjuvant and sterile water only.
Processing of blood and nasal swab
Jugular venipuncture was performed to collect EDTA-treated whole blood and serum (Vacutainer). Serum was aliquoted and stored at −80°C. Serum used in the serum neutralization assay was inactivated by incubation at 56°C for 1 h and stored at −20°C. Nasal swab specimens were collected from each nostril using Dacron-tipped swabs and were stored at −80°C. The EDTA blood was used for a complete blood (cell) count (CBC) using a HemaTrue Veterinary Hematology Analyzer (Heska, Loveland, Colorado, USA).
Real-time quantitative polymerase chain reaction (qRT-PCR)
The BVDV RNA loads in serum were determined by real-time quantitative polymerase chain reaction (qRT-PCR). The RNA was extracted using the Mag-Bind Viral DNA/RNA kit (Omega Bio-Tek; Norcross, Georgia, USA) on a MagMAX Express-96 magnetic particle processor (Life Technologies, Burlington, Ontario). For PCR, the VetMAX-Gold BVDV detection kit (Life Technologies) was used on an Mx3005P qPCR system (Agilent Technologies Canada, Mississauga, Ontario). A serial dilution of RNA extracted from BVDV-infected cell culture supernatant with a known infectious titer served as an external reference for absolute quantification. Based on this standard, the qRT-PCR results are given as TCID50 equivalents per mL of sample (11–13). This does not imply a measurement of actual infectivity — the relationship between infectious titer and viral RNA content is highly variable (14) — but it allows comparisons of viral RNA content across different sample types and PCR runs.
Swabs were incubated with 1 mL of medium at room temperature (RT) for 30 min and then centrifuged at 400 × g for 5 min. The swabs were discarded and the supernatant was retained for RNA extraction and qRT-PCR as described. Due to the uncertainty of the amount of material extracted from the nostrils of the calves, the PCR results for swabs are only presented semi-quantitatively.
Anti-BVDV antibodies
Anti-BVDV antibody titers were determined on days 0, 6, 11, 15, 17, 19, 21, 23, and 37 for each calf using the IDEXX BVDV Total Antibody Test (IDEXX Laboratories, Westbrook Maine, USA) as per kit instructions. Serial two-fold dilutions from 1:5 to 1:320, at 25 μL, were tested for each serum sample. Optical density at 450 nm was read for each well. Sample-to-positive ratios were calculated against negative controls.
Serum neutralization assay
Sera collected on D-7 and days 7, 9, 11, 14, 17, 21, 26, and 34 were tested for neutralizing antibodies against BVDV. Duplicates of all samples were serially diluted 1:2 in DMEM with 5% horse serum (HS) and penicillin/streptomycin/amphotericin B (PSA) (all Life Technologies) to a final dilution of 1:4096, then 3 × 102 TCID50 of BVDV 1373 were added. Known negative and positive controls were included. After 1 h of incubation at 37°C, 4 × 103 Madin-Darby bovine kidney cells (MDBK; ATCC CCL-22, BVDV-free, maintained in DMEM + HS + PSA) were added to the serum-virus mixture. Following a 72-hour incubation at 37°C, the cells were washed with phosphate-buffered solution (PBS), fixed for 30 min in 4% w/v paraformaldehyde in PBS at room temperature (RT) and then washed with PBS-T. Fixed cells were permeabilized for 30 min at RT in permeabilization buffer (PBS with 0.1% w/v saponin and 0.1% w/v bovine serum albumin), incubated for 1 h at 37°C with an anti-E2 monoclonal antibody (348; VMRD, Pullman, Washington, USA) diluted 1:1000 in permeabilization buffer, followed by incubation with a fluorescent secondary antibody (Alexa Fluor 568 goat α-mouse IgG; A11004, Life Technologies) under the same conditions. Cells were washed 3 times with PBS-T after each incubation. Finally, cell nuclei were stained with 300 nM 4′,6-diamidino-2-phenylindole (DAPI; D3571, Life Technologies) in PBS for 5 min at RT in the dark, followed by another wash with PBS. Plates were analyzed using a fluorescence microscope (IX51; Olympus, Center Valley Pennsylvania, USA), and 50% neutralizing titers were calculated by the Spearman-Kärber formula.
Ovalbumin IgM and IgG ELISA
Flat-bottomed, 96-well medium-binding Enzyme-Linked ImmunoSorbent Assay (ELISA) plates (Corning, New York, New York, USA) were coated with 400 ng of ovalbumin per well (Sigma-Aldrich) in 0.05M carbonate/bi-carbonate buffer, pH 9.6 (Sigma-Aldrich). The plates were incubated overnight at 4°C, washed 3 times with PBS-T (0.1% v/v Tween® 20 in PBS), and incubated with a PBS-TSM buffer (PBS-T + 2% v/v skim milk) for 1 h at RT. In a 96-well polypropylene plate (VWR, Mississauga, Ontario), serum samples were prepared in 1:10 dilutions (IgM) or 1:1 dilutions (IgG) in a blocking buffer (10% v/v FBS in PBS-TSM), transferred to the ovalbumin-coated ELISA plate and incubated overnight at 4°C. Rabbit α-bovine IgG-HRP (1:1000) or IgM-HRP conjugate (1:3000) (Rockland Immunochemicals, Gilbertsville, Pennsylvania, USA) was added to each well and incubated for 1 h at 37°C. Between each step, the plates were washed 3 times with PBS-T. Substrate (ABTS; Sigma-Aldrich) was added and incubated in the dark at RT. Optical densities (OD) were measured after 20 min and 1 h using an iMark microplate absorbance reader (Bio-Rad, Mississauga, Ontario) at a wavelength of 405 nm. Titers are reported as the reciprocal of the highest dilution giving a positive reading.
Peripheral blood mononuclear cells (PBMC)
Lymphocytes were isolated from EDTA blood diluted 1:2 with PBS by density gradient centrifugation over Ficoll-Paque Plus (GE Healthcare, Baie-D’Urfé, Québec) for each calf on D0–D8, days 13, 14, 15, 21, 26, and 37. Cells were washed with PBS, aliquoted into 96-well cell culture plates (85 μL per well, 6 wells per sample), incubated for 30 min at RT and finally fixed with 2% w/v formaldehyde for 20 min at RT. The formaldehyde was aspirated and replaced with PBS and the cells were stored at 4°C. The PBMCs were centrifuged for 5 min at 400 × g, washed in PBS and incubated with blocking buffer with 3% BSA in PBS. Mouse anti-bovine WC1 monoclonal antibody (GeneTex, Irvine, California, USA), mouse anti-bovine CD4 monoclonal antibody (LifeSpan Biosciences, Seattle, Washington, USA) or mouse anti-bovine CD20 monoclonal antibody (Abcam, Toronto, Ontario) was added and incubated for 90 min at 37°C, followed by incubation with phycoerythrin-conjugated goat anti-mouse IgG (1 h at 37°C). A counterstain (300 nM DAPI) was used (5 min incubation at RT) to visualize cell nuclei. The cells were washed with PBS and stored in 70% glycerol in PBS at 4°C, protected from light until analysis. Images of the stained cells were acquired using the IN Cell 2000 Analyzer (GE Healthcare, Buckinghamshire, UK) for 10 random fields of view per well at 400× magnification for bright-field, DAPI and PE. The percentage of cells with CD4+, CD20+, and WC1+ markers was quantified using the IN Cell analysis software package for each sample. Absolute numbers of lymphocyte-fractions were calculated by using the absolute lymphocyte count × proportion of the sub-population for each calf at each time point.
Statistics
All statistical analyses were performed using SPSS 20 (IBM, Armonk, New York) or JMP 11.0 (SAS Institute, Cary, North Carolina). Normal distribution of the data was assessed for D0, D5, and D7 using the Shapiro-Wilk test for normality with an alpha level of 0.05. Simultaneous effects of BVDV infection status and OVA +/− status were explored within days by two-way analysis of variance (ANOVA), with Tukey HSD as the post hoc test. Where appropriate, repeated-measures ANOVA was followed by a post hoc univariate test assessed by an unadjusted F statistic. Differences were considered significant when P < 0.05. For CD4+, CD20+, and WC1+ positive cells, absolute cell number was calculated as a proportion of the total lymphocyte count for that animal on that day. The standard error of the mean (SEM) was calculated according to the formula described by Swinson and Campbell (15):
Results
Course of infection with BVDV 2 (strain 1373)
Results of the monitoring of clinical parameters are available upon request. No comorbidity was detected during the study. Before inoculation (days −7, −5, 0), hematocrit was 31.9 ± 2.7% and white blood cell (WBC) parameters were normal across 32 animals with regard to leucocytes (9.8 ± 1.4 × 103), lymphocytes (6.8 ± 1.1 × 103), monocytes (0.8 ± 0.2 × 103), and granulocytes (2.2 ± 0.7 × 103) cells/μL. Throughout the study, there was no evidence of fever, weight loss, elevated GCS, WBC alterations, the presence of BVDV viral RNA in serum or on swabs, or neutralizing antibody activity in the 16 animals in the BVDV− pens. Thus, biosecurity measures were effective, and there was no evidence of BVDV infection in the control animals, and no evidence for an effect of OVA+/− on these parameters in the uninfected animals.
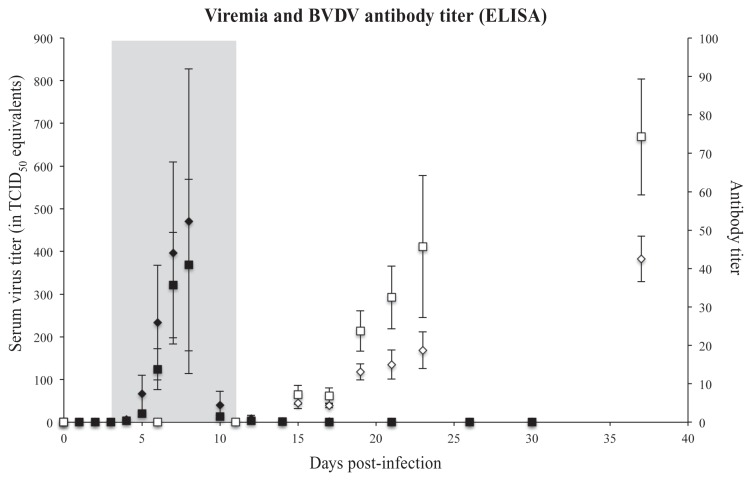
Average rectal temperature in the BVDV+ group was > 39°C by D3, and remained elevated through D11. On D3 to D6 and D14 to D17, the within-animal change in body weight of the BVDV+ group from day-to-day was negative, and significantly differed from the BVDV− animals. This could be attributed to feed refusal and diarrhea-associated fluid loss. Although transient differences in body weight existed between the BVDV− and BVDV+ animals throughout the study, there was no significant difference in overall weight gain between the 2 groups at the end of the experiment. On D5, GCS for the BVDV+ animals increased significantly, peaking on D9 to D13, and then recovered throughout the remainder of the observation period. The BVDV RNA in serum was non-detectable through D4, was significantly elevated on D6, and peaked on D7 to D8, declining to non-detectable by D12. After nasal inoculation, in most animals BVDV RNA became detectable in nose swabs at D2 post-infection (PI) and a peak in nasal excretion was noticeable around D6 to D12 PI (data not shown). By D21 PI, very limited or no BVDV RNA was detected by nasal swabbing in the animals, except for 1 calf (see Pathology). The BVDV serum viral RNA became detectable in the BVDV challenged animals D3 PI in both the OVA− and OVA+ groups. Peak viral RNA in serum occurred in both groups on D8 pi, and was almost undetectable on D10 (Figure 1). There was no evidence of effects of OVA+/− on any of the above measures of acute disease progression.
Figure 1.
The BVDV RNA as detected by qPCR. The black diamonds and squares represent the mean virus RNA in TCID50 equivalents in serum (primary/left Y-axis), the error bars indicate the SEM. Diamonds are OVA+, squares are OVA−. The mean antibody titer (secondary/right Y-axis) was detected using a commercial IDEXX ELISA. The white diamonds and squares represent the mean titer, the error bars indicate the SEM. Diamonds are OVA+, squares are OVA−. The grey area indicates the time when the rectal temperature was > 39°C in the BVDV-challenged group.
Pathology
One calf reached a peak hematocrit of 42.7 on D11, peak GCS of 8 on D12, and failed to respond to supportive therapy, leading to euthanasia on D22. Erosive and ulcerative lesions in the gastrointestinal tract extended from the distal esophagus to the ileum, with crypt necrosis and herniation observed. Mild, focal, subacute, subcapsular pyrogranulomatous nephritis was also present.
Immune response to BVDV infection
All BVDV+ animals mounted an immune response to BVDV infection. The anti-BVDV antibody ELISA reached detectable titers in the interval between D10 and D15. The BVDV antibody titer then continued to increase [repeated measure F(5,8) = 3.85, P < 0.05], and was still increasing at the last sample on D37 (Figure 1). There was no evidence of an effect of OVA+/− status on the BVDV antibody titer [F(5,8) = 1.16, P = 0.41], and excluding the calf that became severely ill and was euthanized had no impact. Neutralizing antibody titers were detectable in 50% of BVDV+ animals on D9 and in 100% on D11. There was no evidence for BVDV virus neutralising antibody titer differences between OVA+ and OVA− animals. The day on which a titer of 1:1536 or greater was achieved did not differ for OVA+ and OVA− (non-parametric P = 0.78).
Peripheral blood mononuclear cell responses
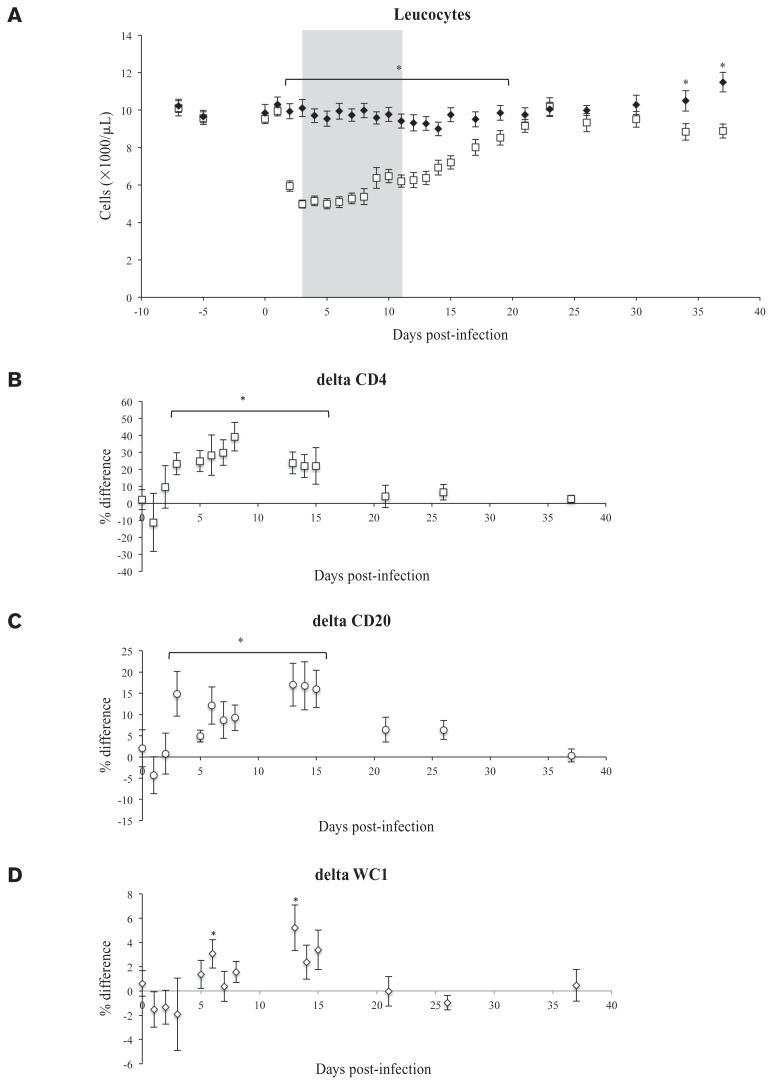
The euthanized calf was excluded from PBMC analyses because therapeutic fluids administration was expected to have affected cell counts. Within the group of 32 animals a normal distribution for the CBC parameters (leucocytes, lymphocytes, monocytes, granulocytes, hematocrit) was determined at D0, D5, and D7. The mean counts of leucocytes, lymphocytes, monocytes and granulocytes on D0, D5, and D7 were 9.8 ± 1.4 × 103, 6.8 ± 1.1 × 103, 0.8 ± 0.2 × 103, and 2.2 ± 0.7 × 103 cells/μL, respectively (see Figure 2A for leucocytes), while the mean hematocrit was 31.9 ± 2.7%. All WBC fractions in the BVDV+ group went down on D2 PI with the leucocyte count reaching a minimum of 5.0 ± 0.2 × 103 cells/μL on D5 PI and the lymphocyte count reaching a minimum of 3.5 ± 0.2 × 103 cells/μL on D7 PI. Hematocrit values did not differ between BVDV+ and BVDV− groups except for calf 17 which peaked at 42.7 on D11 PI. Significantly different leucocyte counts between the BVDV+ and BVDV− groups are shown in Figure 2A. Innoculation with OVA had no significant effect on CBC parameters, as no difference was observed on any sampling day between OVA+ and OVA− treatments within either the BVDV+ or the BVDV− group.
Figure 2.
A — Leucocyte counts (×1000/μL) of the BVDV+ (□) and uninfected controls BVDV− (♦). The grey area indicates the time when the rectal temperature was > 39°C in the BVDV-challenged group. B–D — Cell population variability in percent compared to the population at day of challenge (= D0) population following BVDV infection. Marker indicates MeanBVDV+ – MeanBVDV−. Error bars indicate SEM of the mean as calculated according to Swinson et al (15). In B for the T-helper cell marker CD4, in C for the B-cell marker CD20, and in D for the γδ-T-cell marker WC1.
* Significantly different, P < 0.05.
Compared to the BVDV− group, a relative increase in CD4 (T-helper cell marker) and CD20 (B-cell marker) positive cells was found in the BVDV+ group compared to the BVDV-group on day 3, which persisted past D15 PI (Figures 2B, 2C). The relative amount of WC1 (γδ-T cell marker) positive cells fluctuated and was only higher than the controls on days 6 and 13 PI (Figure 2D). The absolute numbers were significantly lower in the first few days following infection for CD4+ and WC1+ cells; however, the CD20+ B-cells showed an initial depletion on D2 PI followed by an increase in absolute CD20+ cell numbers during D13 to D26 PI, leading to a significantly higher CD20+ cell count in the BVDV+ group versus the BVDV− controls (not shown). There were no differences observed between OVA+ and OVA− groups.
Immune responses to ovalbumin
The euthanized calf was excluded from ovalbumin analyses because therapeutic fluids were expected to have influenced the titers. Note that serum was assayed at a 1:10 dilution for IgM and a 1:1 dilution for IgG, reducing IgM assay sensitivity. There were no IgM or IgG antibodies to OVA in the OVA− groups (Figures 3A, 3B). In the BVDV− pen, the OVA+ animals showed responses from the humoral immune system with the ELISA detecting OVA titers higher than OVA assay baseline titers on D21 (following the booster OVA injection) for IgM (P < 0.01), and D12 (before the second OVA injection) for IgG (P < 0.05).
Figure 3.
Quantitative titer (arbitrary units of optical density at 450 nM ± SE) for ovalbumin (OVA) IgM (Figure 3A) and IgG (Figure 3B) ELISA in the 2 groups exposed to OVA as a novel antigen on D3 and again on D15. OVA− controls are represented by white squares (BVDV+) and white diamonds (BVDV−). OVA+ animals are shown as black squares (BVDV+) and black diamonds (BVDV−).
* Indicates a significant difference between the BVDV+/OVA+ group and the BVDV−/OVA+ group.
The grey area indicates the time when the rectal temperature was > 39°C in the BVDV-challenged group.
After the D21 sample, IgM titers continued to rise to D26, then fell almost to baseline on D37, at which time there were higher titers for OVA+ (P < 0.0001), and for BVDV+ (P < 0.02) with a significant interaction term (P < 0.05), which indicated that BVDV+/OVA+ had significantly higher IgM titers than the other 3 groups (Figure 3B). Thus, BVDV+ infection delayed IgM increase in response to ovalbumin challenge so that the BVDV− group was already showing IgM decline by D37, whereas the BVDV+ group was just reaching peak levels seen for BVDV− on D26.
In 3 of the 4 groups, IgM titers declined significantly between the D26 and D37 samples, suggesting IgM to IgG class-switching. In contrast, the BVDV+/OVA+ IgM titer continued to increase relative to D26 (interaction P < 0.05, post hoc Tukey-Kramer), indicating a delay in IgM responses to OVA in the presence of BVDV. For OVA IgG, which was detected on D12, the pattern was also consistent with delay. Specifically, there were higher IgG titers for OVA+ (P < 0.0001), lower titers for BVDV+ (P < 0.05) and an interaction term (P < 0.05), such that the BVDV−/OVA+ group had an earlier (significantly higher) OVA IgG titer response than the other 3 groups. These findings matched the prediction that BVDV+ infection would delay initiation of the humoral immune response.
Discussion
As expected, clinical progression and viral load in blood replicated previous challenge experiments for this strain (16–18), and were not as extreme as with highly virulent strains (6,7,19,20), with exception of 1 calf that was slow in mounting an anti-BVDV immune response, failed to respond to supportive therapies, and was euthanized. The course of clinical illness was prolonged and more severe than is typical for a BVDV type 1 infection (5) as was expected for this BVDV type 2 strain. Duration of leucopenia, lymphopenia, and granulocytopenia were comparable to Keller et al (20) and was prolonged relative to type 1 BVDV challenge (5). However, the exact numbers of the white blood cell counts need to be interpreted with caution. The analysis was performed on an automated platform that has specific settings for bovine blood, but a bovine white blood cell count validation has not been performed by the manufacturer.
Non-infectious ovalbumin was chosen as the novel antigen, as opposed to other commonly used immune-modulating agents such as a vaccine or secondary pathogen, to avoid difficulty interpreting the influence of BVDV itself on the immune system. A similar approach, also using ovalbumin, demonstrated immunosuppressive effects from Jembrana disease virus, a bovine lentivirus of Bos javanicus (21). This choice was effective, as there was no evidence that OVA+ challenge affected the clinical course of BVDV infection, the total lymphocyte count, or the immune system response to BVDV infection.
Hypothesized effects of acute BVDV infection on the immune response to the novel antigen were seen. Ovalbumin assay sensitivity was reduced for IgM relative to IgG responses; nevertheless, BVDV infection delayed the IgM increase in response to ovalbumin challenge. Specific IgM responses in the BVDV− group peaked early and showed a decline (indicating a class-switching process) (22) around D37. The BVDV+ group only reached the IgM levels seen for BVDV− on D26, and had not reached the peak of their curve by the end of the study. Ovalbumin IgG titers in the BVDV+ group were also delayed relative to the BVDV− group, although both groups reached similar titers on D37. Unfortunately, as the study resolution was reduced for the last 2 wk (longer sampling intervals), it was not possible to determine whether there was an additive and independent delay in the IgG response that was not simply due to the delay in IgM response initiation. To determine whether the eventual IgG response differed in magnitude for BVDV+ and BVDV− animals a longer observational period would have been needed.
As hypothesized, there were interactions between OVA status and BVDV status that altered specific PBMC counts. CD4+ cells are considered biomarkers of T-cell activation, involved in cytokine release to stimulate innate immune responses and assisting in development of adaptive immune responses (5). For CD4+ cell counts, the critical day was D14. On D14, ongoing recovery from clinical symptoms of BVDV infection was associated with a rebound in CD4+ cell counts (BVDV+/OVA−), relative to healthy, control (BVDV−/OVA−) animals. BVDV can directly infect and kill immune cells including macrophages, granulocytes, antigen-presenting myeloid cells, CD4+ and CD8+ T-lymphocytes, and B-cells (4). Thus, this rebound in CD4+ T-cell numbers during clinical recovery from BVDV is expected (5). Day 14 is also around the time that it was possible to detect a delay in IgG response to ovalbumin in the presence of BVDV infection.
It has previously been demonstrated that low-virulence BVDV decreased CD4+, CD8+ and γδ-T subsets (5), in contrast to Marshall et al (23) who did not identify any influence on lymphocyte subsets by any type of BVDV. In this experiment we only observed a short depletion of CD4, CD20, and WC1-expressing cells in the first few days following infection. An increase in all lymphocyte fractions in the BVDV+ groups was observed; especially a prolonged increase of CD20+ cells in this group compared to the controls was detectable. As there was no difference in numbers between the OVA+ and OVA− groups, we attribute this difference to the BVDV challenge alone. Impaired regeneration from the bone marrow can be caused by the ability of BVDV to compromise the proliferative capacity of progenitor cells (20), explaining the difference in the lymphocyte and leucocyte count between the BVDV+ and BVDV− groups on D35 and D37 PI (Figure 2A). However, based on data shown in Figures 2B and 2C, we have the impression that the influx of lymphocytes into the blood stream from D10 PI onwards can be largely attributed to increased numbers of CD4+ and CD20+ cells, indicating that progenitor cells for these cell types were still functional.
Infection with more virulent strains typically causes more severe cellular immune suppression (18,24–29). Thus, changes in total numbers of PBMC confirmed BVDV suppression of cellular responses, and were temporally consistent with the observed delay in mounting a response to the novel antigen, ovalbumin.
This study was conducted in the fall with recently weaned beef calves to replicate the usual practice in western Canada of placing recently weaned calves of mixed origin in feedlots. In many cases they will be infected with BVDV shortly after their arrival, and simultaneously exposed to variable temperature and environmental conditions. The current study confirms that 1 important consequence of a BVDV infection during that critical interval may be that immune system responses to the vaccines used at processing or against newly encountered pathogens will be delayed. These delays in mounting an effective response to a novel antigen will increase the co-infection risk for fall-placed beef calves and enhance the potential transmission between individuals within a pen.
Acknowledgments
We acknowledge the animal husbandry staff at VSRS. Dr. S. van den Hurk (VIDO-Intervac, Saskatoon, Saskatchewan) generously provided the BVDV strain for inoculation. Funding was provided by the University of Calgary, Faculty of Veterinary Medicine (UCVM), through the Curriculum budget. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Campbell JR. Effect of bovine viral diarrhea virus in the feedlot. Vet Clin North Am Food Anim Pract. 2004;20:39–50. doi: 10.1016/j.cvfa.2003.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richeson JT, Kegley EB, Powell JG, Beck PA, Ley BL, Ridpath JF. Weaning management of newly received beef calves with or without continuous exposure to a persistently infected bovine viral diarrhea virus pen mate: Effects on health, performance, bovine viral diarrhea virus titers, and peripheral blood leukocytes. J Anim Sci. 2012;90:1972–1985. doi: 10.2527/jas.2011-4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindenbach BD, Murray CL, Thiel H, Rice CM. Flaviviridae. 6th ed. Philadelphia, Pennsylvania: Lippincott Williams and Wilkins; 2013. pp. 712–746. [Google Scholar]
- 4.Chase CC, Elmowalid G, Yousif AA. The immune response to bovine viral diarrhea virus: A constantly changing picture. Vet Clin North Am Food Anim Pract. 2004;20:95–114. doi: 10.1016/j.cvfa.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Molina V, Risalde MA, Sanchez-Cordon PJ, et al. Cell-mediated immune response during experimental acute infection with bovine viral diarrhoea virus: Evaluation of blood parameters. Transbound Emerg Dis. 2014;61:44–59. doi: 10.1111/tbed.12002. [DOI] [PubMed] [Google Scholar]
- 6.Stoffregen B, Bolin SR, Ridpath JF, Pohlenz J. Morphologic lesions in type 2 BVDV infections experimentally induced by strain BVDV2-1373 recovered from a field case. Vet Microbiol. 2000;77:157–162. doi: 10.1016/s0378-1135(00)00272-8. [DOI] [PubMed] [Google Scholar]
- 7.Ridpath JF, Bendfeldt S, Neill JD, Liebler-Tenorio E. Lymphocytopathogenic activity in vitro correlates with high virulence in vivo for BVDV type 2 strains: Criteria for a third biotype of BVDV. Virus Res. 2006;118:62–69. doi: 10.1016/j.virusres.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 8.Hengst BA, Nemec LM, Rastani RR, Gressley TF. Effect of conventional and intensified milk replacer feeding programs on performance, vaccination response, and neutrophil mRNA levels of Holstein calves. J Dairy Sci. 2012;95:5182–5193. doi: 10.3168/jds.2011-5261. [DOI] [PubMed] [Google Scholar]
- 9.Schaefer AL, Cook NJ, Church JS, et al. The use of infrared thermography as an early indicator of bovine respiratory disease complex in calves. Res Vet Sci. 2007;83:376–384. doi: 10.1016/j.rvsc.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Drunen Littel-van den Hurk S, Lawman Z, Snider M, et al. Two doses of bovine viral diarrhea virus DNA vaccine delivered by electroporation induce long-term protective immune responses. Clin Vaccine Immunol. 2013;20:166–173. doi: 10.1128/CVI.00565-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trebbien R, Bragstad K, Larsen LE, et al. Genetic and biological characterisation of an avian-like H1N2 swine influenza virus generated by reassortment of circulating avian-like H1N1 and H3N2 subtypes in Denmark. Virol J. 2013;10:290. doi: 10.1186/1743-422X-10-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hermann JR, Zimmerman JJ. Analytical sensitivity of air samplers based on uniform point-source exposure to airborne porcine reproductive and respiratory syndrome virus and swine influenza virus. Can J Vet Res. 2008;72:440–443. [PMC free article] [PubMed] [Google Scholar]
- 13.van Doremalen N, Bushmaker T, Munster VJ. Stability of Middle East respiratory syndrome coronavirus (MERS-CoV) under different environmental conditions. Euro Surveill. 2013;18:ii, 20590. doi: 10.2807/1560-7917.es2013.18.38.20590. [DOI] [PubMed] [Google Scholar]
- 14.Marozsan AJ, Fraundorf E, Abraha A, et al. Relationships between infectious titer, capsid protein levels, and reverse transcriptase activities of diverse human immunodeficiency virus type 1 isolates. J Virol. 2004;78(11):130–11. 141. doi: 10.1128/JVI.78.20.11130-11141.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swinson TDV, Campbell M. Differences between means: Type I and type II errors and powers. In: Swinson TDV, Campbell M, editors. Statistics at Square One. London, UK: BMJ Books; 2002. pp. 44–51. [Google Scholar]
- 16.Liang R, van den Hurk JV, Landi A, et al. DNA prime protein boost strategies protect cattle from bovine viral diarrhea virus type 2 challenge. J Gen Virol. 2008;89:453–466. doi: 10.1099/vir.0.83251-0. [DOI] [PubMed] [Google Scholar]
- 17.Liebler-Tenorio EM, Ridpath JE, Neill JD. Distribution of viral antigen and development of lesions after experimental infection with highly virulent bovine viral diarrhea virus type 2 in calves. Am J Vet Res. 2002;63:1575–1584. doi: 10.2460/ajvr.2002.63.1575. [DOI] [PubMed] [Google Scholar]
- 18.Ridpath JF, Falkenberg SM, Bauermann FV, et al. Comparison of acute infection of calves exposed to a high-virulence or low-virulence bovine viral diarrhea virus or a HoBi-like virus. Am J Vet Res. 2013;74:438–442. doi: 10.2460/ajvr.74.3.438. [DOI] [PubMed] [Google Scholar]
- 19.Pellerin C, van den Hurk J, Lecomte J, Tussen P. Identification of a new group of bovine viral diarrhea virus strains associated with severe outbreaks and high mortalities. Virology. 1994;203:260–268. doi: 10.1006/viro.1994.1483. [DOI] [PubMed] [Google Scholar]
- 20.Keller SL, Jefferson BJ, Jacobs RM, Wood RD. Effects of noncytopathic type 2 bovine viral diarrhea virus on the proliferation of bone marrow progenitor cells. Can J Vet Res. 2006;70:20–27. [PMC free article] [PubMed] [Google Scholar]
- 21.Wareing S, Hartaningsih N, Wilcox GE, Penhale WJ. Evidence for immunosuppression associated with Jembrana disease virus infection of cattle. Vet Microbiol. 1999;68:179–185. doi: 10.1016/s0378-1135(99)00074-7. [DOI] [PubMed] [Google Scholar]
- 22.Stavnezer J. Immunoglobulin class switching. Current opinion in immunology. 1996;8:199–205. doi: 10.1016/s0952-7915(96)80058-6. [DOI] [PubMed] [Google Scholar]
- 23.Marshall DJ, Perry GA, Kuszynski CA, Eskridge KM, Kelling CL. Flow cytometric analyses of lymphocyte subsets in peripheral blood and lymphoid tissues of gnotobiotic calves during primary acute postnatal infections of bovine viral diarrhea virus. Viral Immunol. 1994;7:141–149. doi: 10.1089/vim.1994.7.141. [DOI] [PubMed] [Google Scholar]
- 24.Bolin SR, Ridpath JF. Differences in virulence between two noncytopathic bovine viral diarrhea viruses in calves. Am J Vet Res. 1992;53:2157–2163. [PubMed] [Google Scholar]
- 25.Palomares RA, Walz HG, Brock KV. Expression of type I interferon-induced antiviral state and pro-apoptosis markers during experimental infection with low or high virulence bovine viral diarrhea virus in beef calves. Virus res. 2013;173:260–269. doi: 10.1016/j.virusres.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 26.Archambault D, Beliveau C, Couture Y, Carman S. Clinical response and immunomodulation following experimental challenge of calves with type 2 noncytopathogenic bovine viral diarrhea virus. Vet Res. 2000;31:215–227. doi: 10.1051/vetres:2000117. [DOI] [PubMed] [Google Scholar]
- 27.Ellis JA, West KH, Cortese VS, et al. Lesions and distribution of viral antigen following an experimental infection of young seronegative calves with virulent bovine virus diarrhea virus-type II. Can J Vet Res. 1998;62:161–169. [PMC free article] [PubMed] [Google Scholar]
- 28.Wood RD, Goens SD, Carman PS, Deregt D, Jefferson B, Jacobs RM. Effect on hematopoietic tissue of experimental infection of calves with noncytopathic type 2 bovine viral diarrhea virus. Can J Vet Res. 2004;68:42–48. [PMC free article] [PubMed] [Google Scholar]
- 29.Walz PH, Bell TG, Steficek BA, Kaiser L, Maes RK, Baker JC. Experimental model of type II bovine viral diarrhea virus-induced thrombocytopenia in neonatal calves. J Vet Diagn Invest. 1999;11:505–514. doi: 10.1177/104063879901100604. [DOI] [PubMed] [Google Scholar]