A comparison of sensory differences and mealtime behaviors in 34 children with ASD and 34 typically developing children supported a correlation between sensory differences and eating difficulties in children with ASD.
MeSH TERMS: child development disorders, pervasive; eating disorders; feeding behavior; food habits; meals; sensation disorders
Abstract
This study examined sensory differences and mealtime behaviors in children with autism spectrum disorder (ASD; n = 34) and compared the results with those of similarly aged peers who were typically developing (TD; n = 34). Results from parent-report and child-report questionnaires indicated that children with ASD scored significantly differently from TD peers on the measures of sensory differences and eating behaviors. Data also supported a correlation between sensory differences and eating difficulties in children with ASD. The results of this study will help caregivers and their children with ASD identify problem eating behaviors that may be associated with sensory differences. Sensory strategies and techniques offered by occupational therapy practitioners may contribute to greater success during mealtimes for children with ASD and their families, with increased comfort and less stress. The findings also support a need to further explore the influence of sensory differences on mealtime behaviors.
Self-care tasks, or activities of daily living (ADLs) such as grooming, eating, and dressing, are essential tasks for children to acquire as they mature. Increasing evidence has shown that children with autism spectrum disorder (ASD) experience challenges in these daily routines and that their sensory differences often interfere with their ability to develop skills in these important daily routines (Cermak, Curtin, & Bandini, 2010; Schaaf, Toth-Cohen, Johnson, Outten, & Benevides, 2011; Stein, Polido, & Cermak, 2012; Stein, Polido, Mailloux, Coleman, & Cermak, 2011). Eating difficulties are a frequent problem for children with autism (Hubbard, Anderson, Curtin, Must, & Bandini, 2014; Kral, Eriksen, Souders, & Pinto-Martin, 2013; Marí-Bauset, Zazpe, Mari-Sanchis, Llopis-González, & Morales-Suárez-Varela, 2014; Nadon, Feldman, Dunn, & Gisel, 2011a, 2011b; Schreck & Williams, 2006; Schreck, Williams, & Smith, 2004; Suarez, Nelson, & Curtis, 2014) and may weaken their physical health (Bandini et al., 2010; Lukens & Linscheid, 2008; Sharp et al., 2013), cause difficulty during family mealtimes and milieu (Bagby, Dickie, & Baranek, 2012; Schaaf et al., 2011; Suarez, Atchison, & Lagerwey, 2014), and impede participation in the educational setting (Koenig & Rudney, 2010).
Many published research studies have established a link between ASD and sensory differences. Between 69% and 95% of children diagnosed with ASD are estimated to demonstrate sensory symptoms, including sensory seeking and avoiding behaviors, self-stimulation, and unusual sensory interests (Ben-Sasson et al., 2007, 2009; Dunn, Myles, & Orr, 2002; Lane, Young, Baker, & Angley, 2010; Tomchek & Dunn, 2007). The Diagnostic and Statistical Manual of Mental Disorders (5th ed.; DSM–5; American Psychiatric Association, 2013) now includes hyper- and hyporeactivity to sensory input in the diagnostic classification for ASD. However, research that has investigated the relationship between sensory differences in children with ASD and difficulties with the self-care activity of eating has been limited.
The aim of this study was to investigate the association between mealtime behavior problems and sensory differences in children with ASD compared with their typically developing (TD) peers. The following research questions were addressed:
Do children diagnosed with ASD demonstrate significantly greater sensory differences when compared with their age-matched TD peers?
Do children diagnosed with ASD show significantly greater difficulty in mealtime behavior when compared with their age-matched TD peers?
Are there significant correlations between sensory differences and mealtime behaviors in the ASD and TD groups?
Method
Participants
A total of 68 children between the ages of 5 and 12 yr made up the sample. An experimental group of 34 children with ASD and a similarly aged control group of 34 children identified as TD participated in this study. Inclusion criteria for the children with ASD and TD children included age between 5 and 12 yr and residence in an English-speaking household. In addition, the sample of children with ASD was also required to have a formal diagnosis of ASD determined by a physician and medical-behavioral specialist with expertise in ASD (per caregiver report).
Exclusion criteria for children with ASD were presence of comorbid diagnoses (such as cerebral palsy or other motor coordination difficulties) and for the TD children were no indication of receiving special education services or report of a known diagnosis of autism or other developmental or physical disabilities. Participation in this study was voluntary.
Demographics
Parents or guardians supplied demographic data by completing a 20-item survey that included general information (age; gender; birthdate; dominant and second language; and race, ethnicity, or culture); special services received (support academic, occupational, physical, and speech–language pathology therapies); comorbidities; IQ; motor, language, and social developmental milestones; and feeding history. In addition, parents or guardians were asked to elaborate on the information given or provide more descriptive information. Although each parent was required to complete specific demographic items on the survey, such as age, birthdate, gender, formal diagnosis of ASD, and developmental milestones, they were given the option to not answer any questions they preferred not to answer or address (per institutional review board policy).
Procedures
Recruitment of participants began after this study was approved by the university institutional review board. A targeted protocol was followed to recruit participants with ASD and a typically developing group of peers between the ages of 5 and 12 yr who met inclusion criteria for each group. More than 350 recruitment letters and flyers were distributed from May through July 2012.
Participants with ASD were recruited through a local agency that offers services and resources to families of children with ASD. This agency disseminated the recruitment flyers through a mailing list to parents or caregivers of children with ASD. Control participants were recruited by disseminating flyers at public locations, such as local parks, playgrounds, and recreation centers. Interested families and caretakers contacted the researcher (Jeanne Zobel-Lachiusa) by phone or email and were asked a series of questions to determine whether their children met inclusionary criteria for participation in this study. Once eligibility was established and informed consent and assent were obtained in writing, the parents or caregivers were asked whether they wished to have the survey packet delivered to them by mail or by hand delivery to a location of their choice.
The survey packet consisted of the study description; the demographic survey form; and four questionnaires, including the Short Sensory Profile (SSP; Dunn, 1999), Sensory Eating Checklist (SEC; modified for this study from the Eating Checklist [Yack, Sutton, & Aquilla, 2002]), Touch Inventory for Elementary School Aged Children (TIE; Royeen & Fortune, 1990), and Brief Autism Mealtime Behavior Inventory (BAMBI; Lukens & Linscheid, 2008). The parents or caregivers either mailed the completed survey packet to the researcher (Jeanne Zobel-Lachiusa) in a stamped, self-addressed envelope provided by the researcher to a secure address or they hand-delivered it to the researcher at a mutually agreed-on time and location. At the request of the parents or caregivers, the researcher assisted the families with questions pertaining to completion of the form and questionnaires by phone, email, or in-person meeting. Assistance was provided by the primary researcher to approximately 10 families for clarification of questions.
Measures
Sensory Measures.
We used three measures of sensory responsiveness, two completed by the parent (SSP and SEC) and one completed by the child (TIE). The SSP, based on the Sensory Profile (Dunn, 1999), is a 38-item caregiver questionnaire that assesses sensory responses in children ages 3–11 yr. Items are scored on a 5-point Likert scale (ranging from 1 = behavior is always observed to 5 = behavior is never observed). The lower the score, the more the child’s parent-reported behaviors are associated with atypical sensory processing. Scores are derived for specific sensory areas and a total score. Internal reliability ranges from .70 to .90 and internal validity correlations from .25 to .76 (McIntosh, Miller, Shyu, & Dunn, 1999).
For the SSP, a high score of 5 indicates more desirable, less problematic behaviors and a low score of 1 indicates less desirable, more problematic behaviors. In contrast, for the other three measures used in this study, a high score of 5 indicates less desirable, more problematic behaviors and a low score of 1 indicates more desirable, less problematic behaviors. This difference in scoring methods was addressed in the analysis.
The SEC is a parent-report questionnaire consisting of 26 items rated on a 5-point Likert scale (ranging from 1 = never/rarely to 5 = almost always); thus, the higher the score, the less typical or less desirable the response. Two of the items were reverse scored. Although the SEC is a nonstandardized measure, it was considered useful for this study because the questions gleaned information about the child’s response to sensory input at mealtimes in six sensory domains (Touch, Proprioception, Vestibular, Visual, Auditory, Smell/Taste). Examples of questionnaire items include “My child gags with certain foods” and “My child prefers chewy or crunchy foods.”
The TIE was developed as a screening tool of tactile defensiveness for children ages 6–12 yr. It is a 26-item child-report questionnaire designed for use with children with language competency at least that of a 6 yr old; with an IQ of at least 80; and without physical disabilities such as blindness, cerebral palsy, or spina bifida. The higher the score, the more the child’s behaviors are associated with behaviors indicative of tactile defensiveness. Conversely, the lower the score, the less the child’s behaviors are associated with behaviors indicative of tactile defensiveness (Royeen & Fortune, 1990). Examples of questions include “Does it bother you to go barefooted?” and “Does it bother you to have your face washed?”
Normative data for this instrument were collected on 415 children (195 girls and 220 boys) in a randomized sample stratified according to geographic region, race, sex, and community size (Royeen & Fortune, 1990). Studies were conducted to determine the psychometric properties of the TIE. Internal consistency reliability was reported as a robust .74 (Royeen, 1986), and construct validity was established by a panel of experts (Royeen, 1985).
Although the TIE was designed for use with children who have the language competency of at least a 6 yr old, in this study, 11 children with ASD were reported by parents as “very delayed” in language development. For 8 of those children, parents completed the questionnaire; for 3 of those children, the questionnaire was not completed and was entered as missing data.
Mealtime Measure.
The BAMBI is a standardized assessment tool designed to measure mealtime behavior problems of children with autism. The BAMBI is an 18-item parent-report questionnaire using a 5-point Likert scale (ranging from 1 = never/rarely to 5 = at almost every meal). The items, which focus on mealtime behaviors that are not explicitly sensory related, include “My child cries or screams during mealtimes,” “My child is willing to try new foods,” and “My child is flexible about mealtime routines.”
Test–retest reliability is reported at .78 and interrater reliability at .78 (Lukens & Linscheid, 2008). Factor analysis indicated that eight items related to limited variety of foods consumed; five items related to food refusal and disruptive mealtime behavior; and five items related to “features of autism,” such as short attention span, aggressive and self-injurious behavior, rigid and repetitive behavior, and abnormal response to sensory input (Lukens & Linscheid, 2008). Reverse scores were used for Items 3 and 10 to “keep the direction of the factors consistent with the total scores” (Lukens & Linscheid, 2008, p. 349).
Data Analysis
Test scores were analyzed using IBM SPSS Statistics (Version 19; IBM Corp., Armonk, NY). Group means and medians were calculated for linear and ordinal data, respectively, with respect to participant age; gender; race and ethnicity; types and number of services received; and motor, language, and social development. Independent-samples t tests were conducted to compare the mean test scores between both groups for linear measurements. The Mann–Whitney U test was done to determine group differences for ordinal measurements. Correlational analyses were conducted to determine the association between the variables of eating behaviors and sensory processing. The criterion for statistical significance was set at .05 for all results in the study.
Results
Demographics
A total of 34 children with ASD and 34 TD children between ages 5 and 12 yr participated in this study. The demographic distribution was similar between the two groups (ethnicity, language spoken, age) according to caregiver report on the completed demographic form. The average age of participants with ASD was 8.61 yr and of TD participants was 8.76. The group means (Ms) for age were not statistically different, t(66) = −0.31, p = .76. The ASD group had 1 girl and 33 boys; the TD group had 7 girls and 27 boys. Ethnicity was equally distributed in the ASD and TD groups; see Table 1 for demographic characteristics of the participants.
Table 1.
Participant Demographics
| Characteristic | ASD | TD |
|---|---|---|
| Gender, n | ||
| Male | 33 | 27 |
| Female | 1 | 7 |
| Age, yr, M (SD) | 8.61 (2.32) | 8.76 (2.23) |
| Development, n | ||
| No delay | 0 | 34 |
| Slight delay | 23 | 0 |
| Very delayed | 11 | 0 |
| Ethnicity, % | ||
| White | 85 | 80 |
| Black, Asian, Latino | 15 | 20 |
Note. ASD = autism spectrum disorder group; M = mean; SD = standard deviation; TD = typically developing group.
Regarding services received by the ASD group, 20 participants were enrolled in speech–language therapy, 17 received occupational therapy, 3 received physical therapy, 7 were enrolled in therapy for social skills, and 7 received academic supports. A total of 27 participants in the ASD group received more than one of these services. Regarding motor developmental milestones for the ASD group, 20 participants (58.8%) had mild motor delays, 5 (14.7%) were very delayed, and 9 (26.5%) did not have a history of motor delays or issues related to motor developmental milestones. All 34 participants in the TD group had no history of motor delays or issues related to motor developmental milestones. Mean ranks for the ASD group (M = 47) compared with those for the TD group (M = 22) were statistically significant (Mann–Whitney U = 153, z = 5.21, p < .0001, two-tailed).
Similar findings were noted for language development (Mann–Whitney U = 94, z = 5.85, p < .0001, two-tailed). Significant differences were found between the ASD and TD groups regarding language delays or issues related to language developmental milestones. Five of the participants in the ASD group (14.7%) had typical language development, 18 (52.9%) had mild language delays or issues related to language developmental milestones, and 11 (32.5%) were very delayed or had issues with language development. Only 1 of the 34 participants in the TD group was reported to have a mild language delay or issues related to language development. One parent in the TD group did not reply to this question.
Issues regarding social development or social development milestones were found to be significant between the groups (Mann–Whitney U = 8, z = 6.87, p < .0001, two-tailed). None of the 34 ASD group participants had typical social development; 16 (47.05%) had mild social delays or issues related to social development milestones, and 18 (52.9%) were very delayed or had issues with social development. Only 1 of the 34 participants in the TD group was reported to have a mild social delay or issues related to social development milestones. Two parents (5.8%) in the TD group did not reply to this question.
The primary language was English for 94% of the ASD group participants and 94% of the TD group participants. Only 2 (5.9%) participants in both the ASD and TD groups were bilingual and spoke two languages in the home (i.e., Chinese, Spanish, or French).
Regarding feeding, significant differences in the mean age at which participants drank liquids from a cup were found, t(48) = 2.34, p = .02, two-tailed. The mean age for ASD group participants to drink liquids from a cup was 17.17 mo, and the mean age for TD group participants was 11.04 mo. No significant group mean differences were observed for the age at which the children ate pureed foods, t(52) = 0.76, p = .451, two-tailed. ASD group participants ate pureed foods at 6.6 mo, and TD group participants ate pureed foods at 5.89 mo. However, significant group mean differences were found for the age at which participants self-fed using a fork, t(43) = 3.44, p = .001, two-tailed. ASD group participants self-fed using a fork at 26.7 mo, and TD participants self-fed using a fork at 14.84 mo.
Sensory and Mealtime Behavior Measures
Table 2 shows the mean scores for each of the measures for each group. Significant differences were found between the groups for mean scores on all three sensory measures, with the ASD group showing greater sensory differences. Significant differences were also found between the groups for the BAMBI total score. The ASD group exhibited greater mealtime behavior problems than the TD group. Thus, analysis for Research Questions 1 and 2 confirmed that the ASD group showed significantly greater sensory differences and mealtime behavior problems than the TD group.
Table 2.
Mean Scores by Group
| Score, M (SD) | |||
|---|---|---|---|
| Measure | ASD Group | TD Group | p* |
| SSP | 113.34 (28.71) | 55.31 (18.01) | .001 |
| TIE | 46.73 (11.74) | 35.58 (7.96) | .001 |
| SEC | 66.02 (18.05) | 33.27 (8.09) | .001 |
| BAMBI | 44.39 (10.83) | 30.08 (7.90) | .001 |
Note. ASD = autism spectrum disorder group; BAMBI = Brief Autism Mealtime Behavior Inventory; M = mean; SD = standard deviation; SEC = Sensory Eating Checklist; SSP = Short Sensory Profile; TD = typically developing group; TIE = Touch Inventory for Elementary School-Aged Children.
p ≤ .01.
Table 3 shows the results of correlation analyses for both groups combined. Results indicate a moderate to strong positive correlation between the eating behavior measure (BAMBI) and the sensory measures (SSP, SEC, and TIE; rs = .528–.813). A moderate to strong correlation was also found among the sensory measures (rs = .726–.943) for both groups combined. When correlations were run separately for each group (Table 4), moderate to strong correlations were detected between eating behaviors (BAMBI) and the sensory measures (SSP, SEC, TIE) for the ASD group (rs = .378–.747). In contrast, the TD group had a low to moderate correlation between eating behaviors and the sensory measures (rs = .153–.622). Results of correlation analyses for each group between sensory measures revealed a moderate to strong correlation for the ASD group (rs = .697–.895) and a moderate correlation for the TD group (rs = .516–.653). On the basis of these results, we confirmed the answer to the third research question, particularly for the ASD group.
Table 3.
Correlations Among Measures for Both Groups Combined
| Measures | Measure | |||
|---|---|---|---|---|
| BAMBI | SEC | TIE | SSP | |
| BAMBI | ||||
| r | — | |||
| t | ||||
| n | 68 | |||
| SEC | ||||
| r | .813a | — | ||
| t | .000 | |||
| n | 68 | 68 | ||
| TIE | ||||
| r | .528a | .726a | — | |
| t | .000 | .000 | ||
| n | 60 | 65 | 65 | |
| SSP | ||||
| r | –.780a,b | –.943a,b | –.769a,b | — |
| t | .000 | .000 | .000 | |
| n | 68 | 68 | 65 | 68 |
Note. t test is two tailed. BAMBI = Brief Autism Mealtime Behavior Inventory; SEC = Sensory Eating Checklist; SSP = Short Sensory Profile; TIE = Touch Inventory for Elementary School-Aged Children.
Correlation is significant at the .01 level (two-tailed).
Negative correlation is due to the SSP reverse scoring.
Table 4.
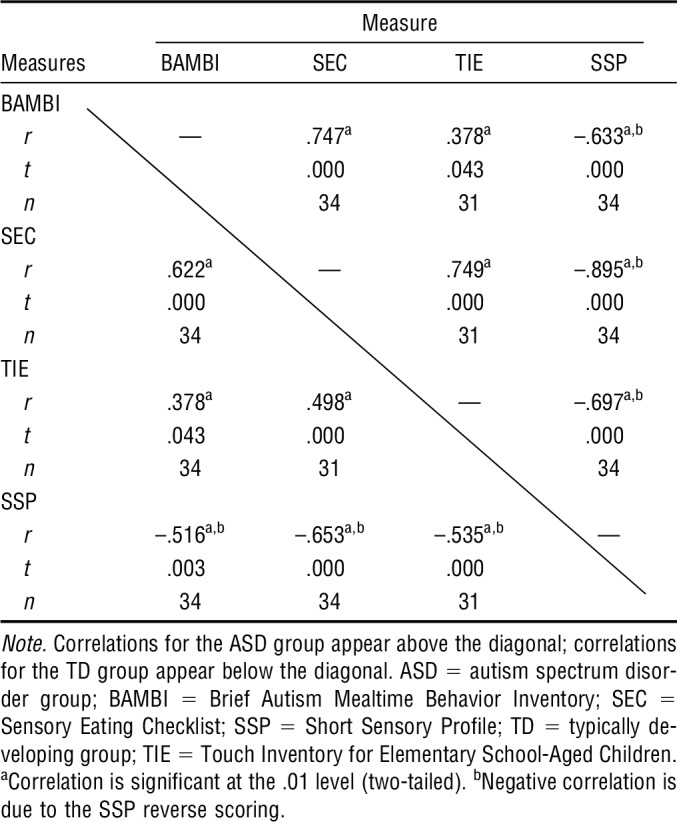
Correlations Among Measures for Each Group
A 2 × 7 ANOVA was run to determine whether there were differences between the two groups on the seven sensory subscales of the SSP. Results were statistically significantly different between the groups for all SSP subscales (Tactile: F = 25.21, p < .001; Taste: F = 19.09, p < .001; Movement: F = 7.99, p < .001; Seeks Sensation: F = 38.73, p < .001; Auditory: F = 42.74, p < .001; Weak: F = 29.17, p < .001; Visual/Auditory Sensitivity: F = 29.28, p < .001; df for all Fs = 64).
Discussion
Sensory differences and mealtime behavior problems were prominent in the ASD group. On all measures, the ASD group had higher (more problematic) mean scores than the TD group on sensory and mealtime behavior measures. This finding is consistent with empirical findings reported in the literature (Jasmin et al., 2009; Keen, 2008; Nadon et al., 2011a, 2011b; Schaaf et al., 2011). This study is unique because it also examined the relationships among sensory differences and mealtime behaviors.
Correlational analyses supported the association between mealtime behavior problems and sensory differences. Moderate to strong positive correlations were found between the mealtime behavior measure (BAMBI) and each of the three sensory measures (SSP, TIE, and SEC) for children with ASD. The TIE scores, although significantly correlated with the BAMBI scores, were more scattered and not as tightly distributed around the plot line compared with the other measures. This finding suggests some increased variability and less stability in the TIE compared with the other two sensory measures. Moreover, the TIE correlation value was .528 compared with the higher correlation values of .780 for the SSP and .813 for the SEC. The TIE is also different from the other measures because it is a child-reported measure as opposed to a parent-reported measure. Thus, having a child versus a parent respondent may have contributed to the difference in magnitude of the relationship between the variables.
Examination of items on each of the four measures used in this study indicates some overlap in constructs. The four measures assessed similar, but different, aspects of sensory differences and mealtime behaviors. Even though the BAMBI specifically asks questions about mealtime behaviors and its purpose is to measure mealtime behaviors, some of the questions could be viewed as sensory related (e.g., “My child prefers crunchy foods,” “My child refuses to eat foods that require a lot of chewing”). In contrast, the SEC asks questions about a child’s sensory behaviors during mealtimes (e.g., “My child has difficulty with certain tastes/odors,” “gags with certain foods,” “does not appropriately chew food”). The Tactile/Smell subtest of the SSP asks questions pertaining to food and eating (e.g., “avoids certain food tastes or food smells,” “picky eater, especially regarding food textures”).
Sensory sensitivities in children with ASD may be one factor that interferes with mealtime behavior. Cermak et al. (2010) reported that research has indicated that the factor most related to food selectivity and food refusals in children with ASD is texture, likely a sensory issue. Using strategies to adapt the sensory environment in the home by minimizing the effects of possible noxious stimuli may be beneficial to children with sensory sensitivities. For example, dimming the light in the room, providing deep pressure through a weighted lap pad, or using calming auditory stimulation are strategies that occupational therapy practitioners have found to be helpful for enhancing daily activities in children with ASD with sensory concerns (Cermak et al., 2010).
Stein et al. (2012) stated that further research on the use of sensory strategies to make dental experiences more successful and less anxiety producing is needed to determine their effectiveness. Similarly, research is also needed to ascertain whether minimizing noxious environmental sensory stimuli or using sensory integration interventions or strategies to decrease sensory sensitivities results in fewer mealtime problem behaviors. Use of sensory strategies to enhance food acceptance and reduce undesirable mealtime behaviors is an alternative or complementary approach to the most commonly used behavioral interventions (Marshall, Ware, Ziviani, Hill, & Dodrill, 2015).
Stein et al. (2012) explored the effect of sensory sensitivities on the dental experience of children with ASD. Their results found that more than 50% of the children in their sample had behavioral issues related to sensory sensitivities that interfered with the dental experience. Parents reported sensory sensitivities such as dislike of taste and texture of toothpaste and of the sounds and smells in the dental office. Similar findings were evident in our study, which examined the relationship of sensory sensitivities and mealtimes.
Limitations and Future Recommendations
This study is among the first to investigate the relationship between mealtime behavior and sensory differences in children with ASD compared with their TD peers. Additionally, there were multiple measures administered to assess both eating behaviors and sensory processing characteristics in both groups. Nevertheless, the study was limited by several factors.
This study consisted of a nonrandomized sample of convenience with data obtained from survey instruments (parent-report and child-report questionnaires). It is possible that the self-selection of participants may have been a limiting factor because parents or caregivers for both groups would have been more likely to volunteer for this study if they had an innate interest in or concern about their child’s sensory and eating behaviors. Moreover, inclusion and exclusion of prospective participants for both groups were based on parent report and did not include any validation by formal standardized test results or actual documentation of each participant’s diagnosis. Additional quantitative and qualitative data obtained on each participant using more direct assessment and observation of potential eating and sensory behaviors would have eliminated potential bias and increased objectivity. Last, the participants in this study all resided in western Massachusetts, and results cannot be generalized to a broader geographic region.
Implications for Occupational Therapy Practice
The results of this study have the following implications for occupational therapy practice:
Children with ASD experience significantly more difficulty with mealtime behaviors than TD children.
Sensory differences in children with ASD may be one factor that interferes with mealtime behavior.
Sensory differences and mealtime problem behaviors may be reduced by occupational therapy interventions. As Ayres (1979) originally elucidated, occupational therapy interventions that directly address underlying sensory differences may contribute to functional improvements such as greater mealtime success, increased comfort, and less stress during mealtimes.
Conclusion
The results of this study revealed a statistically significant difference in sensory differences and in mealtime behaviors between children with ASD and TD children. Children with ASD scored higher (more problematic) on the measures of sensory differences and on mealtime behavior problems than their TD counterparts. Results indicated a strong to moderate positive correlation on measures of eating behaviors and sensory processing. Comments made by caregivers provided a qualitative perspective on the challenges experienced during mealtimes, for example, “Mealtimes are stressful”; “Limits himself to primarily crunchy, chewy foods”; “Will eat only certain tastes such as bland, white foods—rice, pasta, tofu”; “He does not like to eat”; and there are “a lot of power struggles at mealtimes.” These results suggest that mealtime behavior, particularly that related to sensory differences, is an important area for parents and professionals to understand and address more fully to enhance the quality of the child’s life and family life.
References
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Ayres A. J. (1979). Sensory integration and the child. Los Angeles: Western Psychological Services. [Google Scholar]
- Bagby M. S., Dickie V. A., & Baranek G. T. (2012). How sensory experiences of children with and without autism affect family occupations. American Journal of Occupational Therapy, 66, 78–86. http://dx.doi.org/10.5014/ajot.2012.000604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandini L. G., Anderson S. E., Curtin C., Cermak S., Evans E. W., Scampini R., . . . Must A. (2010). Food selectivity in children with autism spectrum disorders and typically developing children. Journal of Pediatrics, 157, 259–264. http://dx.doi.org/10.1016/j.jpeds.2010.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Sasson A., Cermak S. A., Orsmond G. I., Tager-Flusberg H., Carter A. S., Kadlec M. B., & Dunn W. (2007). Extreme sensory modulation behaviors in toddlers with autism spectrum disorders. American Journal of Occupational Therapy, 61, 584–592. http://dx.doi.org/10.5014/ajot.61.5.584 [DOI] [PubMed] [Google Scholar]
- Ben-Sasson A., Hen L., Fluss R., Cermak S. A., Engel-Yeger B., & Gal E. (2009). A meta-analysis of sensory modulation symptoms in individuals with autism spectrum disorders. Journal of Autism and Developmental Disorders, 39, 1–11. http://dx.doi.org/10.1007/s10803-008-0593-3 [DOI] [PubMed] [Google Scholar]
- Cermak S. A., Curtin C., & Bandini L. G. (2010). Food selectivity and sensory sensitivity in children with autism spectrum disorders. Journal of the American Dietetic Association, 110, 238–246. http://dx.doi.org/10.1016/j.jada.2009.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn W. (1999). The Sensory Profile: Examiner’s manual. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Dunn W., Myles B. S., & Orr S. (2002). Sensory processing issues associated with Asperger syndrome: A preliminary investigation. American Journal of Occupational Therapy, 56, 97–102. http://dx.doi.org/10.5014/ajot.56.1.97 [DOI] [PubMed] [Google Scholar]
- Hubbard K. L., Anderson S. E., Curtin C., Must A., & Bandini L. G. (2014). A comparison of food refusal related to characteristics of food in children with autism spectrum disorder and typically developing children. Journal of the Academy of Nutrition and Dietetics, 114, 1981–1987. http://dx.doi.org/10.1016/j.jand.2014.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasmin E., Couture M., McKinley P., Reid G., Fombonne E., & Gisel E. (2009). Sensori-motor and daily living skills of preschool children with autism spectrum disorders. Journal of Autism and Developmental Disorders, 39, 231–241. http://dx.doi.org/10.1007/s10803-008-0617-z [DOI] [PubMed] [Google Scholar]
- Keen D. V. (2008). Childhood autism, feeding problems and failure to thrive in early infancy. Seven case studies. European Child and Adolescent Psychiatry, 17, 209–216. http://dx.doi.org/10.1007/s00787-007-0655-7 [DOI] [PubMed] [Google Scholar]
- Koenig K. P., & Rudney S. G. (2010). Performance challenges for children and adolescents with difficulty processing and integrating sensory information: A systematic review. American Journal of Occupational Therapy, 64, 430–442. http://dx.doi.org/10.5014/ajot.2010.09073 [DOI] [PubMed] [Google Scholar]
- Kral T. V. E., Eriksen W. T., Souders M. C., & Pinto-Martin J. A. (2013). Eating behaviors, diet quality, and gastrointestinal symptoms in children with autism spectrum disorders: A brief review. Journal of Pediatric Nursing, 28, 548–556. http://dx.doi.org/10.1016/j.pedn.2013.01.008 [DOI] [PubMed] [Google Scholar]
- Lane A. E., Young R. L., Baker A. E., & Angley M. T. (2010). Sensory processing subtypes in autism: Association with adaptive behavior. Journal of Autism and Developmental Disorders, 40, 112–122. http://dx.doi.org/10.1007/s10803-009-0840-2 [DOI] [PubMed] [Google Scholar]
- Lukens C. T., & Linscheid T. R. (2008). Development and validation of an inventory to assess mealtime behavior problems in children with autism. Journal of Autism and Developmental Disorders, 38, 342–352. http://dx.doi.org/10.1007/s10803-007-0401-5 [DOI] [PubMed] [Google Scholar]
- Marí-Bauset S., Zazpe I., Mari-Sanchis A., Llopis-González A., & Morales-Suárez-Varela M. (2014). Food selectivity in autism spectrum disorders: A systematic review. Journal of Child Neurology, 29, 1554–1561. http://dx.doi.org/10.1177/0883073813498821 [DOI] [PubMed] [Google Scholar]
- Marshall J., Ware R., Ziviani J., Hill R. J., & Dodrill P. (2015). Efficacy of interventions to improve feeding difficulties in children with autism spectrum disorders: A systematic review and meta-analysis. Child: Care, Health and Development, 41, 278–302. http://dx.doi.org/10.1111/cch.12157 [DOI] [PubMed] [Google Scholar]
- McIntosh D. N., Miller L. J., Shyu V., & Dunn W. (1999). Overview of the Short Sensory Profile (SSP). In Dunn W. (Ed.), Sensory Profile: User’s manual (pp. 59–73). San Antonio, TX: Psychological Corporation. [Google Scholar]
- Nadon G., Feldman D. E., Dunn W., & Gisel E. (2011a). Association of sensory processing and eating problems in children with autism spectrum disorders. Autism Research and Treatment, 2011, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadon G., Feldman D. E., Dunn W., & Gisel E. (2011b). Mealtime problems in children with autism spectrum disorder and their typically developing siblings: A comparison study. Autism, 15, 98–113. http://dx.doi.org/10.1177/1362361309348943 [DOI] [PubMed] [Google Scholar]
- Royeen C. B. (1985). Domain specifications of the construct tactile defensiveness. American Journal of Occupational Therapy, 39, 596–599. http://dx.doi.org/10.5014/ajot.39.9.596 [DOI] [PubMed] [Google Scholar]
- Royeen C. B. (1986). The development of a touch scale for measuring tactile defensiveness in children. American Journal of Occupational Therapy, 40, 414–419. http://dx.doi.org/10.5014/ajot.40.6.414 [DOI] [PubMed] [Google Scholar]
- Royeen C. B., & Fortune J. C. (1990). Touch inventory for elementary-school-aged children. American Journal of Occupational Therapy, 44, 155–159. http://dx.doi.org/10.5014/ajot.44.2.155 [DOI] [PubMed] [Google Scholar]
- Schaaf R. C., Toth-Cohen S., Johnson S. L., Outten G., & Benevides T. W. (2011). The everyday routines of families of children with autism: Examining the impact of sensory processing difficulties on the family. Autism, 15, 373–389. http://dx.doi.org/10.1177/1362361310386505 [DOI] [PubMed] [Google Scholar]
- Schreck K. A., & Williams K. (2006). Food preferences and factors influencing food selectivity for children with autism spectrum disorders. Research in Developmental Disabilities, 27, 353–363. http://dx.doi.org/10.1016/j.ridd.2005.03.005 [DOI] [PubMed] [Google Scholar]
- Schreck K. A., Williams K., & Smith A. F. (2004). A comparison of eating behaviors between children with and without autism. Journal of Autism and Developmental Disorders, 34, 433–438. http://dx.doi.org/10.1023/B:JADD.0000037419.78531.86 [DOI] [PubMed] [Google Scholar]
- Sharp W. G., Berry R. C., McCracken C., Nuhu N. N., Marvel E., Saulnier C. A., . . . Jaquess D. L. (2013). Feeding problems and nutrient intake in children with autism spectrum disorders: A meta-analysis and comprehensive review of the literature. Journal of Autism and Developmental Disorders, 43, 2159–2173. http://dx.doi.org/10.1007/s10803-013-1771-5 [DOI] [PubMed] [Google Scholar]
- Stein L. I., Polido J. C., & Cermak S. A. (2012). Oral care and sensory concerns in autism. American Journal of Occupational Therapy, 66, e73–e76. http://dx.doi.org/10.5014/ajot.2012.004085 [DOI] [PubMed] [Google Scholar]
- Stein L. I., Polido J. C., Mailloux Z., Coleman G. G., & Cermak S. A. (2011). Oral care and sensory sensitivities in children with autism spectrum disorders. Special Care in Dentistry, 31, 102–110. http://dx.doi.org/10.1111/j.1754-4505.2011.00187.x [DOI] [PubMed] [Google Scholar]
- Suarez M. A., Atchison B. J., & Lagerwey M. (2014). Phenomenological examination of the mealtime experience for mothers of children with autism and food selectivity. American Journal of Occupational Therapy, 68, 102–107. http://dx.doi.org/10.5014/ajot.2014.008748 [DOI] [PubMed] [Google Scholar]
- Suarez M. A., Nelson N. W., & Curtis A. B. (2014). Longitudinal follow-up of factors associated with food selectivity in children with autism spectrum disorders. Autism, 18, 924–932. http://dx.doi.org/10.1177/1362361313499457 [DOI] [PubMed] [Google Scholar]
- Tomchek S. D., & Dunn W. (2007). Sensory processing in children with and without autism: A comparative study using the Short Sensory Profile. American Journal of Occupational Therapy, 61, 190–200. http://dx.doi.org/10.5014/ajot.61.2.190 [DOI] [PubMed] [Google Scholar]
- Yack E., Sutton S., & Aquilla P. (2002). Building bridges through sensory integration (2nd ed.). Las Vegas: Sensory Resources. [Google Scholar]


