Abstract
Background
All thirteen known mammalian aquaporins have been detected in the eye. Moreover, aquaporins have been identified as playing essential roles in ocular functions ranging from maintenance of lens and corneal transparency to production of aqueous humor to maintenance of cellular homeostasis and regulation of signal transduction in the retina.
Scope of review
This review summarizes the expression and known functions of ocular aquaporins and discusses their known and potential roles in ocular diseases.
Major conclusions
Aquaporins play essential roles in all ocular tissues. Remarkably, not all aquaporin function as a water permeable channel and the functions of many aquaporins in ocular tissues remain unknown. Given their vital roles in maintaining ocular function and their roles in disease, aquaporins represent potential targets for future therapeutic development.
General significance
Since aquaporins play key roles in ocular physiology, an understanding of these functions is important to improving ocular health and treating diseases of the eye. It is likely that future therapies for ocular diseases will rely on modulation of aquaporin expression and/or function. This article is part of a Special Issue entitled Aquaporins.
Keywords: Aquaporin Cornea, Lens, Retina, Glaucoma, Cataract
1. Introduction
Aquaporins (AQPs) are a ubiquitous family of transmembrane water channels that play critically important functions in all taxa of life [1–3]. The discovery of the first member of this family, AQP1, by Peter Agre was recognized by the 2003 Nobel Prize in Chemistry [4,5]. The ability of a cell to move water across its plasma membrane is an essential molecular process that allows for fluid secretion from glandular tissues, allows for fluid flow in tissues for the delivery of nutrients and removal of wastes, and allows for cell volume regulation. Water movement through the channel is via facilitated diffusion and is controlled by local osmotic gradients. Of the thirteen AQPs found in humans, all have been detected at the mRNA and/or protein level in ocular tissues. The AQP family can be divided into the classic aquaporins (AQPs 0, 1, 2, 4 & 5), the aquaglyceroporins (AQPs 3, 7, 9 & 10), and the unorthodox aquaporins (AQPs 6, 8, 11 & 12) [2,6]. While all family members are permeable to water, the aquaglyceroporins are permeable to glycerol, and, to varying extents, other small molecules. The unorthodox aquaporins differ from the other family members in their deviation from the characteristic aquaporin NPA box sequence motif and, for AQP11 and AQP12, in their intracellular localization. For the purposes of this review, we will focus on the role of AQPs in water transport in ocular tissues.
The eye is a complex sensory organ consisting of multiple tissue types. The main optical elements, the cornea and lens, are avascular tissues where regulation of water movement is essential for maintaining transparency. The retina, as the key sensory tissue, requires exquisite fluid balance to maintain cellular homeostasis and proper tissue function. As discussed below, AQPs play important roles in all ocular tissues. The localization and function of AQPs in the eye were reviewed by Verkman in 2003 [7] and 2008 [8] and by Fischbarg in 2012 [9]. In addition, several specific reviews on AQPs in ocular epithelia have appeared [10,11]. The purpose of this review is to provide an update on our knowledge of aquaporin expression and function in the eye and to discuss the role of AQPs in ocular diseases (Table 1). The order of discussion begins with the interface between the eye and the environment, i.e. the cornea, and proceeds to the back of the eye concluding with a discussion of retinal AQPs.
Table 1.
Localization of AQP expression in ocular tissues and possible roles in ocular pathologies and/or diseases.
| Ocular component | AQPs expressed | Associated pathology/disease |
|---|---|---|
| Cornea | ||
| Epithelium | AQP3, AQP5 | Fuch’s dystrophy |
| Stroma | AQP1 | Pseudophakic bullous |
| Endothelium | AQP1, AQP4 | Keratopathy Corneal wound repair |
| Lacrimal gland | ||
| Acini | AQP3, AQP4, AQP5 | Sjögren’s syndrome |
| Ducts | AQP4 | |
| Ciliary epithelium | AQP1, AQP4 | |
| Trabecular meshwork | AQP1, AQP4 | |
| Lens | ||
| Epithelium | AQP1, AQP5, AQP7 | Cataract |
| Fiber cells | AQP0, AQP5 | |
| Retina | ||
| Inner limiting membrane | AQP2, AQP7, AQP11 | Diabetic retinopathy |
| Nerve fiber layer | Glaucoma | |
| Ganglion cell layer | AQP0, AQP5, AQP9 | Uveitis |
| Inner plexiform layer | AQP1, AQP4, AQP5 | Retinal detachment |
| Inner nuclear layer | AQP0, AQP1, AQP4, AQP5, AQP9 | Blue light injury Ischemia/reperfusion |
| Outer plexiform layer | AQP1, AQP4, AQP6 | |
| Outer nuclear layer | AQP1, AQP5 | |
| Outer limiting membrane | AQP7 | |
| Photoreceptor layer | ||
| Retinal pigment epithelium | AQP1, AQP2, AQP3, AQP5, AQP7, AQP9 | |
2. Cornea
As the outermost part of the eye, the cornea is exposed to the environment and yet its transparency is essential for its function as an optical element in the eye. The cornea consists of a five-layered structure including the epithelium, Bowman’s layer, stroma, Descemet’s membrane, and endothelium (Fig. 1). The corneal epithelium is the major refractive surface of the eye and is bathed by tears secreted from lacrimal glands (see below). The stroma contains keratocytes interspersed among a network of collagen fibrils appropriately spaced to maintain transparency. The endothelium layer is bathed by the aqueous humor and provides access to fluid and nutrients. Corneal transparency requires precise regulation of water content. Changes in corneal water content alter the regular diameter and spacing of stromal collagen fibrils that are believed to be critical for transparency [12]. The stroma contains a high concentration of negatively charged glycosaminoglycans causing a mildly hyperosmolar environment relative to the aqueous humor [13]. As corneal transparency requires, water entering the cornea must be extruded to maintain its natural hyperosmotic state [14].
Fig. 1.

Diagram of corneal cell layers indicating the locations of aquaporin expression.
2.1. Aquaporin expression in the cornea
Several AQPs have been detected in the cornea including AQP1, AQP3, and AQP5. Differential expression of AQP5, AQP3 and AQP1 among the layers of the cornea has been consistently reported. For example, AQP5 has been found in all three corneal epithelium layers (superficial, wing, and basal cells) in human, mouse, and rat [10,15–17]. AQP3 has been localized to rat, dog, and mouse corneal epithelial cells [10,15,18]. AQP1 is expressed in the plasma membrane of endothelial cells and stromal keratocytes of mouse, rat, cow, and human [15,16,19–23] and subcellularly in the inner leaflets of the plasma membrane of the endothelium [21]. Semi-quantitative measures of AQP expression in the cornea by RT-PCR show AQP1 levels are approximately three-fold higher than AQP3 and 2.5 fold higher than AQP5; however, the highest intraocular expressions of AQP3 and AQP5 are in the cornea [24].
The presence of other AQPs in the cornea has been reported, although the results have not been confirmed by independent studies. AQP11 and a weak signal for AQP2 were detected in rat cornea [25], contrary to previous reports on the absence of AQP2 in the eye [24,26]. AQP4 was also thought to be absent in the cornea [15]; however, both AQP4 mRNA and protein were detected in the cornea [25,26]. In addition, a study of AQP glycosylation in the cornea showed abundant glycosylated and non-glycosylated AQP1, weak non-glycosylated AQP3, weak glycosylated AQP5, and abundant non-glycosylated AQP11 [26].
2.2. Aquaporin function in the cornea
Aquaporins play a fundamental role in transmembrane water movements across the cornea and conjunctiva into the tear film and therefore are important in maintaining tear film osmolarity and stromal layer thickness [8–10]. AQP5 is responsible for water movement across the corneal epithelium onto the ocular surface driven by the hypertonicity of the tear film. AQP1 facilitates water movement across the corneal endothelium that, together with epithelial AQP5, maintains a partially dehydrated and transparent stromal layer. Briefly, the involvement of AQP1 and AQP5 in corneal fluid transport across endothelial and epithelial barriers was demonstrated by AQP1 or AQP5 deletion where corneal thickness was remarkably reduced in AQP1-null mice and increased in AQP5-null mice [27]. In AQP1 null mice, reduced osmotic water influx from aqueous humor to the stroma combined with normal movement to the tear film via AQP5 is predicted to produce a chronically dehydrated and thinned cornea. In AQP5 null mice, the reduced rate of osmotically driven water efflux from the stroma to the tear layer is predicted to produce an increase in corneal thickness [27]. Additional evidence for AQP5-mediated water secretion across the corneal epithelium is the increased tear film hypertonicity in AQP5 knockout mice and decreased tear film osmolarity when evaporation was prevented by exposure of mice to a humidified atmosphere [28]. These results indicate that AQP5 and AQP1 act as major components of the pathway for osmotically driven water movement across the corneal epithelium and endothelium, respectively [29]. The roles of other AQP members detected in corneal tissue remain to be investigated. Interestingly, a candidate gene study to investigate the genetic determinants of normal variation in central corneal thickness found no association between polymorphisms in AQP1 and AQP5 and normal variation in central corneal thickness [30].
It is important to note that an alternative mechanism to transcellular water flow through the corneal endothelium has been proposed. Fischbarg proposed that paracellular water flow through tight junctions by electro-osmotic forces occurs in “leaky epithelia”, as in the corneal endothelium, and can explain AQP1 null mouse results [31,32].
As seen in many cell types, AQPs in the cornea are also found to be involved in cell migration and proliferation. Delayed corneal re-epithelialization and delayed restoration of full-thickness epithelia after scraping in an AQP3-null mouse wound healing model suggest a distinct defect in cell migration and cell proliferation [33]. Increased expression of AQP1 after wounding and delayed healing of debrided corneas in AQP1-null mice have also been reported, implicating AQP1 as an important determinant in corneal stromal wound repair [20]. Increased cell migration is believed to occur due to AQP-facilitated water permeability [20]; however, AQP-facilitated changes in cell volume as cells migrate through narrow extracellular spaces may also contribute to AQP-dependent cell migration [20]. In vitro studies of the role of AQPs in human corneal endothelial cell proliferation and migration showed that down-regulation of AQP1 by siRNA decreased proliferation and migration via the ERK signaling pathway, whereas, down-regulation of AQP5 increased human corneal epithelial cell migration and proliferation with no significant difference in phosphorylated ERK levels [34].
2.3. Aquaporins in corneal disease
Altered expression of AQPs has been reported in corneal endothelial diseases such as pseudophakic bullous keratopathy (BK) and Fuchs’ dystrophy, both of which result in corneal edema [22]. BK occurs after cataract removal and placement of intraocular lens. Fuchs’ dystrophy is a degenerative disease of corneal endothelium that is most common in older women. Even though the two diseases share many clinical similarities, AQP expression is different in BK corneas compared with those from Fuchs’ dystrophy patients [25,35]. The clinical hallmark of BK is the chronic corneal edema that occurs in the corneal stroma and endothelium. BK corneas exhibited abnormal staining for AQP1, AQP3 and AQP4. Specifically, altered expression of AQPs in BK corneal endothelium includes: decreased AQP1, increased AQP3, and increased AQP4. AQP1 expression was decreased in Fuchs’ dystrophy corneal endothelium with no change in AQP3 and AQP4 [25]. Although it is generally accepted that BK is a corneal endothelium disease, epithelial cells in BK corneas have decreased Na+/K+-ATPase alpha subunit expression and normal expression in stromal and endothelial cells [36]. AQP changes in BK corneas suggest future BK therapies including treatments that can enhance or suppress AQP expression or function in order to control cornea edema.
Normal levels of AQP1 were found in human cornea endothelia with non-endothelial corneal diseases such as corneal scarring and keratoconus (KC) [22]; however, AQP5 was reported to be absent in the cornea epithelium from KC corneas [37]. The gene for AQP5 is normal in KC indicating that expression of the gene is suppressed [37], however, in contrast, two other studies found no significant difference in AQP5 expression between healthy and KC corneas [17,25].
3. Lacrimal gland
The lacrimal glands are glands that secret the aqueous layer of the tear film and their structures include the secretory end-piece (acini) and a duct system [38]. The lacrimal gland together with the ocular surface (cornea, conjunctiva, and Meibomian gland) and the interconnecting sensory and motor innervations comprise the integrated system called the lacrimal functional unit (LFU). The LFU plays a multifaceted role in maintaining a homeostatic microenvironment for the live, actively functioning epithelial cells at the exposed surfaces of the cornea and conjunctiva [38,39]. Tears are secreted by acini and drained by four levels of ducts [38]. Tear secretion is regulated by AQP water channels [7,40].
3.1. Aquaporin expression in the lacrimal gland
Multiple AQPs are expressed in lacrimal glands. AQP5 expression in lacrimal glands is localized in the apical membrane of rat and mouse acinar and duct cells [15,16,41]. Recently, AQP5 was also found in the basolateral membranes of acinar cells in rabbit lacrimal glands [42,43] and in lacrimal gland microvascular endothelial cells [26]. In addition to AQP5, AQP3 has been consistently detected in the basolateral membranes of acinar cells [41,44], AQP4 was detected in both acinar cells and ductal cells [41–44], and AQP1 was found in microvascular endothelia [15,26,41,44]. The abundance of different AQPs varies significantly among acini and different duct segments and previous reported results are not always consistent. For example, Sasaki et al. showed that AQP5 in lacrimal glands is mainly located in the ductal cells rather than in the acinar cells in pilocarpine stimulated mice [45]. Whether this observation is due to altered localization by pilocarpine needs further study. Abundant intracellular AQP11 mRNA and protein, as well as weak signals for AQP0, AQP2, AQP8 and AQP9 mRNA, were also found in rat lacrimal glands [26].
3.2. Aquaporin function in the lacrimal gland
Reduced saliva and sweat secretion in AQP5 null mice indicate an important role for AQP5 in physiological water movement in exocrine glands [46,47]. Lacrimal and parotid glands have many similarities to exocrine glands in tissue structure and glandular secretion mechanisms [45]; however, unaltered tear secretion in knockout mice lacking AQP1, AQP4, AQP3 and AQP5 does not support an essential role for aquaporins in lacrimal gland fluid secretion [44]. Further study confirmed that AQP5 deletion had no effect on pilocarpine-stimulated tear volume [45]. The requirement of aquaporins in salivary but not lacrimal gland secretion is thought to be due to substantially lower fluid secretion rate across lacrimal gland acinar cells [44]. The average tear production is only ~5 mL per day and water secretion can be sufficiently achieved by AQP-independent water transport [44,45]. A later study reported that AQP5 is present in ductal cells rather than in the acinar cells in pilocarpine stimulated mice; showing an opposite distribution between lacrimal glands and parotid glands. The authors proposed that AQP5 might be an osmoregulator to maintain an isotonic tear solution rather than function in tear secretion [45]. The variable experimental results may be indicative of species differences and antibody variability. Clearly, additional work is needed to confirm the distribution of AQPs in the lacrimal gland for a better understanding of their functions.
3.3. Aquaporins in lacrimal gland disease
Sjögren’s syndrome (SjS) is an autoimmune disorder characterized by decreased lacrimal and salivary gland secretions, resulting in severe dry eye and dry mouth. Altered AQP expression in lacrimal glands of SjS patients and SjS animal models has been studied. AQP5 protein levels were similar in lacrimal glands from SjS patients and normal subjects, but defective cellular trafficking in SjS patients was reported. AQP5 was localized in the apical membranes of lacrimal acinar cells in healthy controls and non-Sjögren’s syndrome dry eye patients, but AQP5 is localized in the cytoplasm in patients with Sjögren’s syndrome [48]. This selective defect in lacrimal gland AQP5 trafficking in Sjögren’s syndrome might contribute to decreased lacrimation and subsequent dry eye in these patients. Later results suggested that lack of an AQP5 C-terminal binding protein (prolactin-inducible protein) in SjS may contribute to defective AQP5 trafficking [49]. Another study found that autoantibodies against the muscarinic type 3 receptor suppress AQP5 trafficking to the membrane and contribute to impaired fluid secretion in SjS [50].
Altered AQP5 and AQP4 expression was observed in rabbit lacrimal gland with induced autoimmune dacryoadenitis (IAD), which mimics many pathologic features of SjS [43]. The expression of AQP4 in lacrimal glands from rabbits with IAD was 36% more abundant than normal controls, whereas AQP5 was 72% less abundant. The overall AQP5 expression level change reflects decreased AQP5 in acinar cells but increased AQP5 in ductal cells in rabbits with IAD [43]. Quantification of AQP5 protein showed significantly higher levels in tears of SjS patients suggesting that damage to lacrimal glands and corneal epithelium may shed AQP5 into tears [51]. Lacrimal gland destruction and leakage of AQP5 into tears were also observed in IAD [52]. These findings suggest that detection of AQP5 in tears may be useful for the diagnosis of LG disorders [52]. Another finding that could be related to AQP-related dry eye symptoms is the altered expression of AQP4 and AQP5 during pregnancy [53]. Pregnant rabbits demonstrated typical clinical symptoms of dry eye and, at term pregnancy, expression of AQP5 was significantly lower, but expression of AQP4 was higher than controls in whole LG [53]. Similar to rabbits with IAD, AQP4 immunoreactivity was increased in acini while AQP5 immunoreactivity was present in ducts of pregnant rabbits [53].
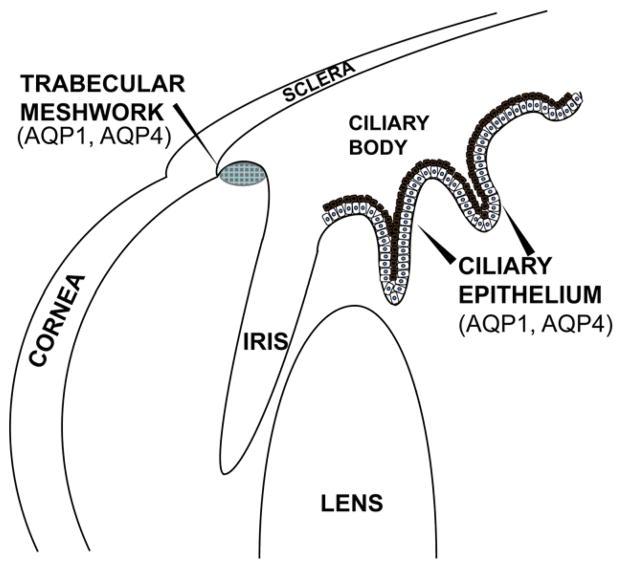
4. Ciliary epithelium & trabecular meshwork
The ciliary epithelium and the trabecular meshwork (TM) control aqueous humor dynamics in the eye. The ciliary epithelium consists of two epithelial layers: the pigmented epithelium and the non-pigmented epithelium (NPE) that cover the ciliary body (Fig. 2). The non-pigmented epithelium is responsible for aqueous humor production while aqueous humor drainage (outflow) occurs through the trabecular meshwork into the canal of Schlemm. The balance between aqueous humor secretion and outflow is critically important in maintaining intraocular pressure (IOP).
Fig. 2.
Diagram of the location of ciliary epithelium and trabecular meshwork tissues indicating the location of aquaporin family member expression.
4.1. Aquaporin expression in ciliary epithelium and trabecular meshwork
AQP1 and AQP4 are present on the apical and basolateral membranes of the NPE [15,54]. AQP1 and AQP4 are also expressed in the endothelium of the trabecular meshwork [15]. AQP1 is expressed in the endothelium of the canal of Schlemm [15].
4.2. Aquaporin function in ciliary epithelium and trabecular meshwork
AQP1 and AQP4 are responsible for water transport out of the NPE to produce the aqueous humor. Water transport through AQP1 and APQ4 occurs after a local osmotic gradient is established via secretion of ions (Na+, Cl−, and HCO3−) and small molecules (ascorbic acid) [55]. Given that connexins form gap junctions between pigmented and non-pigmented epithelia that are required for aqueous humor production [56,57] and that AQPs in the lens are known interacting partners with connexins [58,59], we speculate that such AQP-connexin interactions could play a role in establishing or regulating the aqueous humor secretion machinery. A significant decrease in IOP was observed in AQP1 and AQP4 null mice and this was attributed to decreased aqueous humor production rather than alternations in outflow facility [60]. Consistent with this interpretation, recent work suggests that transcellular water transport plays no role in aqueous humor outflow despite AQP expression in the trabecular meshwork and Schlemm’s canal endothelia [61]. Instead, a paracellular outflow pathway has been proposed where TM cell volume, and thus outflow facility, is controlled by AQP1 [62]. Additionally, AQP1 has been shown to serve a protective role in TM cells upon exposure to mechanical stress [63].
4.3. Aquaporins in disease
As mentioned above, the balance between aqueous humor production and outflow maintains IOP. Elevated IOP is the most significant risk factor for glaucoma. To date, there have been no studies linking AQP deficiency or mutation to altered IOP in humans. However, given the role of AQP1and AQP4 in aqueous humor production, these specific AQPs have been identified as potential therapeutic targets for pharmacological inhibition in glaucoma patients [10].
5. Lens
The ocular lens is a transparent, avascular organ located in the front of the eye. Cells within the lens contain high concentrations of crystallin proteins and are tightly packed to reduce light scattering. The lens contains two distinct cell types — a monolayer of epithelial cells on the lens anterior, and elongated, organelle-free fiber cells that comprise the bulk of the lens (Fig. 3). Lens growth continues throughout the lifetime of an organism with new fiber cells being continually added to the outer cortex of the lens. Differentiated fiber cells lack the machinery to synthesize new protein and, as a result, cells and their proteins in the lens core are as old as the individual. As in the cornea, fluid balance in the lens is crucially important in maintaining transparency [64].
Fig. 3.
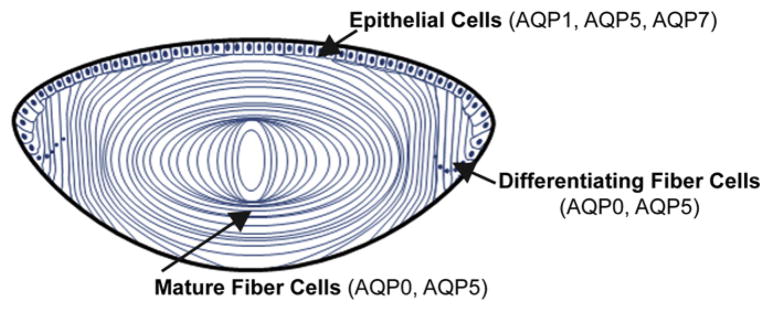
Diagram of the lens showing the localization of aquaporin expression.
5.1. Aquaporin expression in the lens
As epithelial cells migrate to the lens equator and differentiate to fiber cells, there are significant changes in aquaporin expression. Lens epithelial cells contain AQP1 [15]. Recent immunohistochemistry studies also demonstrated AQP5 and AQP7 labeling in the lens epithelium; however, their relative abundance has not been measured [65–67]. In lens fiber cells, AQP0, formerly known as major intrinsic protein (MIP), is highly abundant comprising over 50% of lens membrane protein concentration. AQP5 is also found at lower levels in fiber cells, estimated to be ~5% of lens AQP0 levels [24,66–69].
AQP1 is present in apical and basolateral membranes of lens epithelial cells [70]. As epithelial cells migrate toward the lens equator and begin to differentiate, AQP1 expression decreases and is gradually replaced by AQP0. In rat lenses, immunofluorescence indicates that AQP0 localization changes with distance from the lens capsule [71]. In the outer cortex, AQP0 is distributed between broad and narrow sides of the hexagonally-shaped lens fiber cells. Deeper into the cortex, AQP0 predominantly forms plaque-like structures on the broad sides of fiber cells, which is consistent with ribbon-like and tongue-and-groove type structures found in freeze-fracture studies [72,73]. In the inner cortex, AQP0 is again redistributed throughout the plasma membrane [71]. AQP0 in the lens core is present in 11–13 nm thin junctions, supporting its role as a structural molecule [74]. These changes in AQP0 localization may be critical for modulating protein function in mature lens fiber cells from a water pore to a junctional protein.
Proteomics, immunofluorescence, and transcriptomics studies suggest that AQP5 is present in much lower abundance than AQP0 [24,66,67] and that it traffics from the cytosol to the membrane during fiber cell differentiation [67]. Currently, the role of AQP5 trafficking to the membrane is not well understood, although it is hypothesized to assist in water transport in the lens core [67].
5.2. Aquaporin function in the lens
The lens, as its name implies, functions as a key focusing element in the ocular optical system. In order to function as a focusing element, the lens has developed a mechanism to reduce spherical aberrations and to remain transparent. Spherical aberrations are reduced by establishing a gradient of refractive index (GRIN). This gradient is formed by differential protein expression and concentrations across fiber cells; a concentration gradient that must be exquisitely maintained via fluid balance [75]. Furthermore, lens volume changes during accommodation are accompanied by rapid water movements presumably through AQP channels [76]. Lastly, a microcirculation system has been proposed to explain how transparency of this avascular tissue is maintained over decades of life. Localization of both ion channels and aquaporins is important in establishing the water flux necessary for the circulation system [77]. Clearly, any disturbance in water transport properties could have significant deleterious effects on transparency and optical properties of the lens.
Studies in AQP1 null mice demonstrated the importance of AQP1 for water transport in lens epithelial cells [70]. These AQP1-deficient lenses had normal morphology but lower epithelial permeability to water, as demonstrated by higher basal water content, less response after incubation in hypotonic solutions, and slower equilibration in cell swelling assays. To date, no functional studies have characterized the role of AQP7 in the lens epithelium, although it could allow for transport of small molecules [65].
The shift from AQP1 expression in lens epithelial cells to expression of AQP0 in lens fiber cells may seem odd due to low mammalian AQP0 permeability [78] (note that killifish AQP0 has high permeability, similar to AQP1 [79,80]); however, there are several reasons AQP0 may uniquely serve lens biology. First, its extremely high density in lens fiber cells could compensate for its relatively low permeability. It is noteworthy that the estimated sum of the AQP0 permeability in lens fiber cells is equivalent to that of AQP1 permeability in epithelial cells [81,82]. This may be required to maintain water balance between the bulk of the lens and the highly permeable epithelial layer. Additionally, AQP0 permeability can be regulated under certain conditions, potentially allowing for increased water flow when necessary. Two studies demonstrated that AQP0, but not AQP1, permeability is sensitive to pH and Ca2+ levels [83,84]. AQP0 can also be modulated by protein–protein interactions. Calcium affects calmodulin binding to the AQP0 C-terminus resulting in decreased water permeability [85,86]. Phosphorylation of serine-235 in AQP0 reduces calmodulin binding and consequently increases the permeability of the channel [87,88]. Phosphorylation at serine-235 has also been shown to be important for trafficking of AQP0 to the plasma membrane [89].
AQP0 may also serve as a structural and/or adhesion molecule. The interaction of AQP0 with ezrin/radixin/moesin actin binding proteins [90], with lens specific cytoskeletal proteins filensin and phakinin [91,92], and with gap junction proteins [58,59] may be important for its structural properties. AQP0 has been implicated in junction formation, tongue-and-groove formation, and cell-to-cell adhesion in a number of studies [73,93–95]. As a long-lived protein, AQP0 accumulates numerous modifications during its lifetime [68,96,97]. AQP0 is truncated in older fiber cells, which shifts the protein from water pore to junctional form [98,99].
Animal models of cataract have identified numerous residues important for AQP0 trafficking and function, and have helped to characterize the unique role of AQP0 in the lens [100–102]. For example, AQP0 null mice have cataracts, disorganized cellular packing and altered optical properties [103,104]. Expressing AQP1 in AQP0 null mice does not fully restore lens transparency or cellular architecture, providing further evidence that AQP0 has unique functions in fiber cells that are imperative for lens transparency [101]. The role of AQP5 in lens fiber cells is unknown; however, it has been suggested that AQP5 trafficking to the membrane could enhance water permeability in the lens to compensate for the shift of AQP0 to its junctional form [68].
5.3. Aquaporins in lens disease
Although numerous autosomal dominant mutations in AQP0 cause cataract, mutations in other lens aquaporins have not been associated with lens phenotypes in humans. AQP1 null mice show accelerated cataract development upon stress [70], although humans with natural AQP1 mutations do not show signs of cataract [105]. There are no indications of lens opacities in AQP5 null mice [46,66].
The critical importance of AQP0 in the lens is highlighted by the fact that mutations in the AQP0 gene lead to congenital cataracts. AQP0 mutations cause improper trafficking (AQP0 sequestration in the endoplasmic reticulum), disruption of protein interactions, and altered permeability. Varadaraj et al. found that Δ213, a single base deletion that leads to shortened AQP0 sequence in humans, results in protein sequestration in the ER [106]. Families with G165D mutation also have impaired AQP0 trafficking [107]. Another mutation, R233K, does not affect AQP0 localization to the plasma membrane but reduces binding to calmodulin, a regulatory calcium-binding protein [108]. Several other familial AQP0 mutations have also been reported [109–115]. All of these AQP0 mutations cause congenital cataract.
6. Retina
The retina is the key sensory layer of the eye and structurally it is a partial extension of the brain. The retina is composed of a fantastically complex layered array of neural and glial elements consisting of photo-receptor cells, neurons, ganglion cells and pigmented epithelial cells. Histologically, the retina consists of ten well-defined histological layers (Fig. 4) identified starting from the inner-most layer as: the inner limiting membrane (ILM), the nerve fiber layer, the ganglion cell layer (GCL), the inner plexiform layer (IPL), the inner nuclear layer (INL), the outer plexiform layer (OPL), the outer nuclear layer (ONL), the outer limiting membrane (OLM), the photoreceptor layer (PR), and the retinal pigment epithelium (RPE).
Fig. 4.
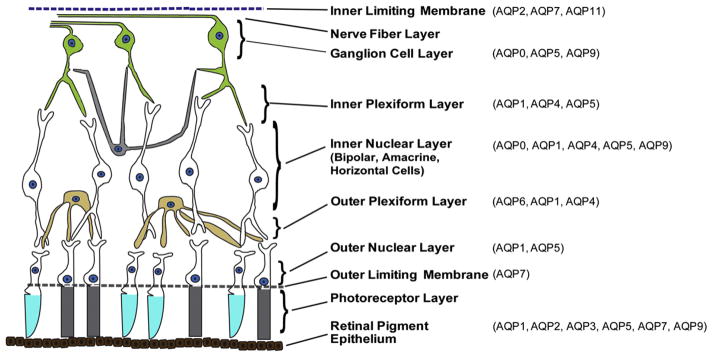
Diagram of the retinal cell layers displaying the most abundant aquaporins expressed in each layer.
6.1. Aquaporin expression and function in the retina
As in other tissues aquaporins are critically important in the maintenance of ionic and osmotic balance in the sensory retina. The human retina expresses mRNA for all AQPs, i.e. AQP0 to AQP12 [65,116] whereas rat retina expresses mRNAs for all AQPs except for AQP2 and APQ10 [24,116,117]. Notably, the expression of AQP gene transcripts does not always correlate with AQP protein expression. Among the 13 mammalian AQP protein family members, the presence of nine AQP proteins, including AQP0, AQP1, AQP3, AQP4, APQ5, AQP6, AQP7, AQP9 and AQP11, has been observed at the protein level in human and/or rodent retina or optic nerve [118–122]. Given the complexity of the retina structure and the numerous AQPs expressed in the retina, we review expression and function of each AQP individually in the following sections.
6.1.1. AQP1
Two AQPs, AQP1 and AQP4, are the most abundantly expressed AQPs in the rodent retina. AQP1 is expressed in the outer retina, mainly in a subpopulation of amacrine cells and in photoreceptor cells [120,123–125]. In early studies, AQP1 was not detected by in situ hybridization [126] or by immunoblotting in human or rodent retina [127], but later studies clearly demonstrated that AQP1 immunoreactivity exists in the retina [15,124]. AQP1 was detected in adult human RPE in situ as well as in a fetal RPE monolayer culture [128]. Use of thin cryosections revealed very low AQP1 immunolabeling in the outer nuclear layer (ONL), the outer plexiform layer (OPL), the inner nuclear layer (INL) and the inner plexiform layer (IPL) in the rat retina and in the apical membrane microvilli of the choroid plexus [24,119,124]. However, the overall expression of AQP1 in retina is quite low compared to that in the choroid and sclera [24].
AQP1 has a polarized distribution in the RPE. The majority of AQP1 is localized in the apical membrane domain with much lower abundance in the lateral domain of RPE cells. The polarized expression of AQP1 in RPE contributes to: efficient transepithelial water transport across the RPE, maintenance of retinal attachment, and prevention of subretinal edema. The blood–retinal barrier (BRB) is formed by tight junctions between the endothelial cells of retinal capillaries and cells of the retinal pigment epithelium (RPE). The BRB prevents leakage of fluid, protein, and potentially harmful agents into the delicate neuronal layers of the retina. A consequence of this barrier is that most of the water crossing the RPE must pass through its two plasma membrane domains, each containing different amounts of AQP1. Efficient water movement across the RPE in the apical to basolateral direction facilitates proper retinal attachment and function. Dysfunction leads to subretinal edema discussed below.
6.1.2. AQP4
AQP4 is heterogeneously expressed in the retina and optic nerve, but in the retina is restricted to Müller glial cells and astrocytes [116,120,129]. AQP4 expression in Müller cells has a polarized distribution with predominant expression in the end feet membranes (facing the vitreous body or capillary endothelium)[129]. The expression pattern of AQP4 suggests that Müller cells play a prominent role in water handling in the retina and that these cells direct osmotically driven water flux to the vitreous body and vessels rather than to the subretinal space [116,129].
APQ4 has been shown to co-localize and interact with the inwardly rectifying potassium channel Kir4.1; the major channel involved in the spatial buffering of the retinal potassium concentration mediated by Müller cells [130]. Several groups have shown similar immunofluorescence distributions of AQP4 and Kir 4.1 in Müller cells, with enriched density of both channels in the end-feet, soma and apical regions of the cell [120,129]. Further evidence of the interaction was reported using immunoelectron microscopy and co-immunoprecipitation [129,131,132]. Co-localization of AQP4 and Kir4.1 in distinct membrane domains of retinal Müller cells has led to the idea that the rapid water transport and potassium fluxes through glial cells are coupled to control potassium concentrations during light activation [129,133]. This notion has been challenged by studies that show no effect of AQP4 deletion on Kir4.1 function [134].
6.1.3. AQP0
AQP0 protein is expressed preferentially in the lens; however, several groups have detected weak immunoreactivity of AQP0 in human, mouse, rat, and dog retina [118,135]. qRT-PCR analysis indicated retinal expression of AQP0 mRNA but at a nearly 250-fold lower level than that observed in the lens [135]. The abundance of AQP0 protein in the retina appeared to be proportional to the mRNA level. Retinal AQP0 immunoreactivity is localized to bipolar, amacrine and ganglion cells in two layers, the INL and at the border between the IPL and the GCL [118,135]. Axonal expression of AQP0 was restricted to the intraocular unmyelinated portion and disappeared immediately after the axons entered the optic nerve [136]. The physiological role of AQP0 in the retina is unknown but changes in expression levels during retinal detachment are discussed below.
6.1.4. AQP6
AQP6 is selectively localized to the OPL with two staining patterns observed in the rat retina. First, AQP6 is localized in membranes of Müller glial cells that surround the ribbon synapses and extend into the photoreceptor layer. Second, a punctate granular pattern is observed in the OPL much of which co-localizes with AQP4 staining [117,122]. AQP6 expression was also detected in adult mice in the RPE, ONL, OPL, INL, GCL and ILM layers [137]. In addition to being a water channel, AQP6 also functions as an anion channel with a high permeability for nitrate [138,139]. Thus, it is possible that AQP6 is involved in Müller cell-mediated regulation of synaptic ion concentrations and of osmolarity in the OPL [122].
6.1.5. AQP3
AQP3 is an aquaglyceroporin that mediates the facilitated transport of water, small non-charged solutes such as glycerol and urea, as well as hydrogen peroxide across membranes [8,140–142]. In addition to its expression in other tissues and cells of the body, AQP3 is localized to epithelial cells, including RPE cells [143]. AQP3 protein expression was confirmed at the transcript and protein level in the RPE and neural retina in rat and mouse [137,144]. In adult mice, expression of AQP3 was detected in the ILM, OLM, INL and RPE layers. Remarkably, AQP3 was observed in the outer layer of the neuroblastic layer (NBL) in newborn mouse retina [137]. The NBL refers to the proliferative zone of the inner optic cup that consists of Retinal Progenitor Cells (RPCs); cells that later differentiate into specific retinal cell types [145]. Significant immunoreactivity of AQP3 in the NBL suggests the potential involvement of APQ3 in the postnatal retina development.
6.1.6. AQP9
Expression of AQP9 has been detected in catecholaminergic amacrine cells in the inner part of the INL [146] and in retinal ganglion cells [147,148]. The functional significance of the expression of AQP9 by catecholaminergic amacrine cells is unknown. AQP9 was also detected in astrocyte processes surrounding retinal capillaries, in the cytoplasm of the inner segments of the photoreceptors, as well as in retinal pigment epithelial cells [65,117,149]. In addition to its permeability to water, AQP9 is permeable to a wide variety of uncharged solutes, such as lactate, β-hydroxybutyrate, glycerol, purines, pyrimidines, urea, mannitol, and sorbitol [150]. AQP9 may serve a metabolic sensor role in the retina by facilitating the diffusion of glycerol and lactate to and from highly active synapses within the IPL. In addition, AQP9 expression is up-regulated in ARPE-19 cells under hypoxic and hypotonic conditions and the channel may serve as a transporter of lactate in these metabolically active cells [149].
6.1.7. AQP5
AQP5 was recently detected in rat and horse retina by immunostaining where the protein was found predominantly in the inner retina and, to a lower extent, in the rat RPE [117,151]. In the equine retina AQP5 was detectable in GCL, INL and ONL. Both of AQP4 and AQP5 are expressed in Müller cells but with complementary patterns of distribution within the cell. AQP4 is mostly localized to the main trunks of Müller cells, whereas AQP5 is located in processes of the IPL and the ONL [151]. These results suggest that AQP4 and AQP5 are responsible for water movements in distinct and separate areas.
6.1.8. APQ7 and AQP11
Both AQP7 and AQP11 are localized to the endfeet of Müller cells at the inner limiting membrane. AQP7 is also detected in the OLM and in the cytoplasm of the RPE [65]. The co-localization of AQP7 and AQP11 in Müller cells at the ILM suggests that these AQPs might participate in water transport into the vitreous body [65]. AQP7 may also be involved in osmotic gradient equilibration across the OLM. The physiological significance of AQP7 in RPE needs to be determined.
6.1.9. AQP2 and AQP12
AQP2 expression was found to be very low, but detectable, in the RPE, the inner segments of photoreceptors, the INL, and the ILM [137]. The expression of AQP2 in the RPE and ILM remains constant throughout life with no significant difference observed with age. AQP12 transcripts have been amplified by qRT-PCR in the RPE and neural retina; however, to date, AQP12 has not been detected at the protein level. Thus, the functional role of intracellular AQP12 in the RPE remains unknown.
6.2. Aquaporins in retinal disease
6.2.1. Diabetes
Diabetic retinopathy is one of the most serious complications of diabetes mellitus, which can eventually lead to blindness. There are two types of diabetic retinopathies, proliferative diabetic retinopathy and non-proliferative diabetic retinopathy. Non-proliferative diabetic retinopathy is characterized by micro-aneurysms, hemorrhages, and intraretinal microvascular abnormalities. The development of retinal edema is the major vision-threatening condition of non-proliferative diabetic retinopathy [152]. The disturbance of the balance between the fluid influx into the retina and the fluid absorption from the retinal tissue into the blood underlies the development of retina edema [153]. Proliferative diabetic retinopathy is manifested by hypoxia-induced angiogenesis that can lead to hemorrhage into the vitreous humor.
In the retina, fluid homeostasis is primarily regulated by retinal glial and RPE cells [128,154]. Therefore, ion channels and AQPs in retinal glial and RPE cells play important roles in maintaining water and electrolyte balance. Numerous studies have suggested that diabetes is associated with a complex alteration of AQP expression and localization in the retina. Generally, these alterations may be involved in the development and/or resolution of retinal edema, as well as in the adaptation of retinal cells to hyperglycemic conditions.
In experimental diabetes, the most prominent alterations in the retina are the absence of AQP4 in superficial blood vessels and additional AQP1 expression in Müller glial cells of the neural retina, especially around the superficial blood vessels [117,155–158]. The expression of AQP1 is also up-regulated in the nerve fiber/ganglion cell layer. AQP4 within the nerve fiber bundles (inner nuclear layer) and around the ganglion cell bodies does not change. The expression of AQP1 in the outer retina remains constant during the course of diabetes [155]. Therefore, the type of AQP that surrounds the superficial retinal vessels switches from AQP4 to AQP1 in diabetes. This suggests that the glial cell-mediated water transport in the retina is altered after induction of diabetes especially at the superficial vessel plexus. Müller cells in diabetic retinas of the rat display a decrease in expression of the potassium channel Kir4.1 in perivascular membranes especially in the inner nuclear layer [159], whereas the expression of AQP4 around these vessels remains unaltered. An un-coupling of the AQP4 mediated water transport from channel-mediated potassium currents may contribute to the diabetic retina edema. Up-regulation of AQP1 is a glial response to facilitate the equalization of osmotic gradients between the blood and the retinal tissue across the affected vessel walls. In addition, hypoxia-induced up-regulation of AQP1 might occur in retinal vascular endothelial cells [158].
Additionally, the levels of AQP5 and AQP9 in the RPE are up-regulated in the course of diabetes, whereas AQP6 protein in the outer plexiform layer is downregulated [117]. Up-regulation of AQP5 supports a higher rate of fluid transport across the RPE to prevent subretinal edema. The expression of AQP9 in the neuronal retina is not altered in diabetic animals but it is up-regulated in the RPE. The pathophysiological significance of the up-regulation of AQP9 in the RPE needs further study. For example, AQP9 is permeable to non-charged solutes such as lactate and glycerol [150]. Whether an increased requirement of subretinal clearance of lactate induces the up-regulation of AQP9 in the RPE of diabetic rats needs to be further explored.
AQP0 protein appears in the intraocular portions of retinal ganglion cell (RGC) axons in diabetes along with its expression in the inner unclear layer and the border between the inner plexiform and RGC layers [118,136]. The expression of AQP0 in RGC axons increased with diabetes progression; however, AQP0 is absent from optic nerve axons. The up-regulation of axonal AQP0 protein seems specific for diabetic retinopathy; however, the pathophysiological significance of AQP0 in the retina remains unknown.
6.2.2. Glaucoma
Glaucoma is a heterogeneous group of diseases that adversely affect the optic nerve head and retinal ganglion cells. As discussed above, the most important risk factor for glaucoma highest risk factor for glaucoma is elevated intraocular pressure (IOP). Ocular hypertension can cause an ischemic-like insult to retinal ganglion cells and optic nerve head. Ocular hypertension is associated with changes in AQP expression and these changes are also considered key risk factors in the development of glaucomatous optic nerve neuropathy.
Expression of AQP9 is consistently down-regulated in a variety of glaucoma models. In a non-human primate glaucoma model, elevated intraocular pressure reduced AQP9 expression in the optic nerve head [160]. AQP9 was also substantially reduced in the optic nerve head and in retinal ganglion cells in a rat model of chronic ocular hypertension [148]. AQP9 is the sole water channel expressed in astrocytes in the un-myelinated portion of the optic nerve head and in neurons in the GCL of the retina. As an aquaglyceroporin, AQP9 presumably acts as a metabolite channel from astrocytes to RGCs; therefore, the reduced expression of AQP9 may be involved in the pathogenesis of glaucomatous optic neuropathy.
The change in AQP4 expression is controversial in different animal models of glaucoma. AQP4 expression was essentially unchanged in optic nerves of glaucomatous human and monkey eyes [160] and in rats with chronic IOP elevation [148]. However, AQP4 expression was reduced in the retina and increased in the optic nerve in a mouse intra-ocular hypertension model [161]. Importantly, retinal function and cell survival were significantly improved in AQP4 deficient mice with elevated IOP [162]. The protective effect of AQP4 gene deletion suggests the involvement of AQP4 in the pathophysiology of retinal injury in ocular hypertension.
Glaucoma also causes an increase in AQP7 expression in Müller cell endfeet [65].
6.2.3. Optic nerve crush model
The optic nerve crush (ONC) model closely mimics the damage that occurs in traumatic optic neuropathy and has been used as acute model of glaucoma. Such optic nerve injuries can involve both mechanical (primary) and ischemic-induced (secondary) processes that include degeneration of nerve axons and loss of myelin [163–165]. In the rat ONC model, the injury causes a significant decrease in expression of AQP4 and Kir4.1 protein in the retina, suggesting impairment of ion homeostasis and K+ spatial buffering. ONC also causes a decrease in AQP9 protein, followed by an increase. This response may reflect changes in the retina fuel supply and demand following a massive injury or an attempt at osmoregulation [166].
6.2.4. Aging
Age-related retinal degeneration is associated with an alteration in the expression of AQP proteins. The expression of AQP1 significantly decreased in the inner segments of photoreceptors, the INL, and the GCL in aged rats causing reduced mediated water transport in the retina [137]. In this same study, AQP3 expression was constant in the RPE, however, the level of AQP3 significantly decreased in the OLM and ILM. Overall expression of AQP4 in retina dramatically decreased in aged rats. The staining intensity and the number of AQP4 positive cells in the INL, OLM, ILM layers were significantly reduced and the staining of AQP4 is undetectable in RPE and the outer retina in aged mice. AQP6 in the old rat retina was only present in the ILM and RPE, revealing a remarkable decrease of AQP6 expression. In aged mice, positive staining for AQP9 was found only in the RPE and ILM; a significant decrease of AQP9 was observed in the GCL [137].
6.2.5. Uveitis
Uveitis or inflammation of the uvea is an important cause of blindness worldwide and it primarily affects patients in the adult age group. Uveitis can be caused by an infectious etiology or by an autoimmune response. The major cause of visual loss in uveitis patients is macular edema secondary to blood–retinal barrier (BRB) disruption [167].
In a mouse model of experimental autoimmune uveitis, histology analysis revealed intraocular inflammation, retinal disorganization with destruction of the RPE cell layer with ultimate loss of photoreceptors [168]. AQP1 immunostaining intensity in the ONL layer was significantly decreased due to the loss of photoreceptors, but no significant change in AQP1 expression in the RPE or in choroidal endothelial cells was observed. AQP4 expression was either totally lost or decreased in the ganglion cell layer, the outer plexiform layer, and OLM in different histopathological lesions of uveitis.
Contrary to the mouse model, in a horse autoimmune uveitis model, expression of AQP4 was significantly displaced from the inner retina to the ONL forming a new circular expression pattern. However, the expression of Kir4.1 was significantly down-regulated in horse autoimmune uveitis [151]. AQP4 and Kir4.1 expression changes suggest that potassium buffering and thus Müller cell physiology is highly affected in autoimmune uveitis. Interestingly, AQP5 expression was significantly decreased in horse uveitis. Since both of AQP5 and AQP4 are expressed in Müller cells but with different distributions within the cell, up-regulation of AQP4 may compensate for the decreased expression of AQP5 in horse autoimmune uveitis [151].
In an endotoxin-induced uveitis rat model, the expression of AQP4 and Kir4.1 was significantly reduced [169]. In addition, perivascular immunostaining of AQP4 in retinal Müller glial cells was lost. Uncoupled alteration of AQP4 and Kir4.1 in uveitis might cause functional defects in Müller cells rendering them unable to maintain fluid and ion homeostasis within the retina possibly contributing to macular edema.
6.2.6. Retinal detachment
Retinal detachment (RD) is a sight-threatening condition that occurs when the neural retina physically separates from the RPE. Retinal detachment is associated with acute visual loss caused by anatomic displacement of the photoreceptors and with chronic visual loss/disturbance caused by retinal remodeling and photoreceptor cell death that may occur even after successful reattachment. In a mouse model, RD causes a markedly decreased expression of AQP0 mRNA and protein [135]. This model is similar to rhegmatogenous detachments in humans that cause a tear in the retina allowing fluid to accumulate between the RPE and the retina. The role of AQP0 in the maintenance of normal retina structure and function remains unknown.
6.2.7. Blue light injury
Overexposure of the retina to blue/white light causes opacity of retinal tissue and is accompanied by local edema in the subretinal space and outer retina. Histologically, illumination injury of the retina results in a rapid loss of photoreceptor segments and of the integrity of the outer nuclear and plexiform layers. AQP1 staining disappears along with the degeneration of photoreceptors in the outer retina [170]. The edge of the light-exposed area and the spaces around the remaining photoreceptor cells within the irradiated area display increased AQP4 staining, whereas Kir4.1 staining was decreased in GCL, IPL, INL. New Kir4.1 immunoreactivity emerged in the outer retina between the remaining photoreceptor nuclei and displayed a low expression throughout the entire retinal tissue. Overall, there is a strong time-dependent decrease in potassium conductance in Müller cells [171]. This response suggests that expression and functional uncoupling of AQP4 and the Kir4.1 channel resulted in Müller cell swelling and local retina edema.
Excessive light treatment also causes alteration and redistribution of AQP6 in the retina. Along with the degeneration of the photoreceptor cells, the AQP6 labeled ribbon synapses in the OPL disappear and additional punctuate staining of AQP6 appears in the INL [122]. APQ6 is an acid-activated anion channel [138,172], so it is conceivable that this aquaporin is involved in glial-mediated osmo- and ion-regulation of the extracellular space around ribbon synapses and may play an important role in pathology caused by light injury.
6.2.8. Ischemia–reperfusion
It is well known that ischemia–reperfusion causes the alteration of the two predominant AQPs in the retina, AQP1 and AQP4. Transient ischemia causes a switch from AQP4 expression to AQP1 expression in areas around superficial vessels in the nerve fiber and ganglion cell layers; however, no change in AQP4 levels was observed around the deep vasculature of the INL [118,121,173,174]. AQP4 knockout protects against impaired retinal function and cell death after retinal ischemia [175].
Overall, alteration of AQP4 and AQP1 expression is not specific to ischemic-reperfusion injury. A similar phenomenon has been observed in ocular inflammation, diabetic retinopathy, ocular hypertension, etc. All of these pathological conditions can cause ischemic-like injury to ocular tissue during disease progression. Furthermore, as discussed above, both AQP4 and Kir4.1 are important for controlling retinal swelling and most retinal pathologies are accompanied by alterations of amounts and/or spatial distribution of AQP4 or Kir4.1. Uncoupling of AQP4 and Kir4.1 may play a crucial role in the induction of macular edema through disturbance of water fluxes and potassium influxes.
7. Conclusions
All thirteen mammalian AQPs have been detected in ocular tissues; however, the functional roles of many of these proteins remain unknown. Studies of the most prevalent ocular AQPs indicate important roles in maintaining water balance in ocular tissues to maintain transparency in cornea and lens, in corneal wound healing, to maintain tear film osmolarity, to generate aqueous humor, and to maintain retinal homeostasis. Clearly, more investigations are needed to produce a detailed understanding of this family of membrane transporters. In addition, past investigations have implicated AQPs in the etiology of a variety of ocular diseases including retinal and corneal edema, Sjögren’s syndrome, cataract, and several retinopathies. Thus, opportunities abound for targeting new therapies to AQP expression and function in ocular tissues [176–178].
Footnotes
This article is part of a Special Issue entitled Aquaporins.
References
- 1.Hachez C, Chaumont F. Aquaporins: a family of highly regulated multifunctional channels. Adv Exp Med Biol. 2010;679:1–17. doi: 10.1007/978-1-4419-6315-4_1. [DOI] [PubMed] [Google Scholar]
- 2.Ishibashi K, Kondo S, Hara S, Morishita Y. The evolutionary aspects of aquaporin family. Am J Physiol Regul Integr Comp Physiol. 2011;300:R566–R576. doi: 10.1152/ajpregu.90464.2008. [DOI] [PubMed] [Google Scholar]
- 3.Zeidel ML. Water homeostasis: evolutionary medicine. Trans Am Clin Climatol Assoc. 2012;123:93–105. (discussion 106) [PMC free article] [PubMed] [Google Scholar]
- 4.Preston GM, Carroll TP, Guggino WB, Agre P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science. 1992;256:385–387. doi: 10.1126/science.256.5055.385. [DOI] [PubMed] [Google Scholar]
- 5.Agre P. Aquaporin water channels (Nobel Lecture) Angew Chem Int Ed Engl. 2004;43:4278–4290. doi: 10.1002/anie.200460804. [DOI] [PubMed] [Google Scholar]
- 6.Rojek A, Praetorius J, Frokiaer J, Nielsen S, Fenton RA. A current view of the mammalian aquaglyceroporins. Annu Rev Physiol. 2008;70:301–327. doi: 10.1146/annurev.physiol.70.113006.100452. [DOI] [PubMed] [Google Scholar]
- 7.Verkman AS. Role of aquaporin water channels in eye function. Exp Eye Res. 2003;76:137–143. doi: 10.1016/s0014-4835(02)00303-2. [DOI] [PubMed] [Google Scholar]
- 8.Verkman AS, Ruiz-Ederra J, Levin MH. Functions of aquaporins in the eye. Prog Retin Eye Res. 2008;27:420–433. doi: 10.1016/j.preteyeres.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischbarg J. On the mechanism of fluid transport across corneal endothelium and epithelia in general. J Exp Zool A Comp Exp Biol. 2003;300:30–40. doi: 10.1002/jez.a.10306. [DOI] [PubMed] [Google Scholar]
- 10.Levin MH, Verkman AS. Aquaporins and CFTR in ocular epithelial fluid transport. J Membr Biol. 2006;210:105–115. doi: 10.1007/s00232-005-0849-1. [DOI] [PubMed] [Google Scholar]
- 11.Candia OA, Alvarez LJ. Fluid transport phenomena in ocular epithelia. Prog Retin Eye Res. 2008;27:197–212. doi: 10.1016/j.preteyeres.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maurice DM. The location of the fluid pump in the cornea. J Physiol. 1972;221:43–54. doi: 10.1113/jphysiol.1972.sp009737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tuft SJ, Coster DJ. The corneal endothelium. Eye (Lond) 1990;4(Pt 3):389–424. doi: 10.1038/eye.1990.53. [DOI] [PubMed] [Google Scholar]
- 14.Freegard TJ. The physical basis of transparency of the normal cornea. Eye (Lond) 1997;11(Pt 4):465–471. doi: 10.1038/eye.1997.127. [DOI] [PubMed] [Google Scholar]
- 15.Hamann S, Zeuthen T, La Cour M, Nagelhus EA, Ottersen OP, Agre P, Nielsen S. Aquaporins in complex tissues: distribution of aquaporins 1–5 in human and rat eye. Am J Physiol. 1998;274:C1332–C1345. doi: 10.1152/ajpcell.1998.274.5.C1332. [DOI] [PubMed] [Google Scholar]
- 16.Funaki H, Yamamoto T, Koyama Y, Kondo D, Yaoita E, Kawasaki K, Kobayashi H, Sawaguchi S, Abe H, Kihara I. Localization and expression of AQP5 in cornea, serous salivary glands, and pulmonary epithelial cells. Am J Physiol. 1998;275:C1151–C1157. doi: 10.1152/ajpcell.1998.275.4.C1151. [DOI] [PubMed] [Google Scholar]
- 17.Garfias Y, Navas A, Perez-Cano HJ, Quevedo J, Villalvazo L, Zenteno JC. Comparative expression analysis of aquaporin-5 (AQP5) in keratoconic and healthy corneas. Mol Vis. 2008;14:756–761. [PMC free article] [PubMed] [Google Scholar]
- 18.Karasawa K, Tanaka A, Jung K, Matsuda A, Okamoto N, Oida K, Ohmori K, Matsuda H. Patterns of aquaporin expression in the canine eye. Vet J. 2011;190:e72–e77. doi: 10.1016/j.tvjl.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 19.Wen Q, Diecke FP, Iserovich P, Kuang K, Sparrow J, Fischbarg J. Immunocytochemical localization of aquaporin-1 in bovine corneal endothelial cells and keratocytes. Exp Biol Med (Maywood) 2001;226:463–467. doi: 10.1177/153537020122600512. [DOI] [PubMed] [Google Scholar]
- 20.Ruiz-Ederra J, Verkman AS. Aquaporin-1-facilitated keratocyte migration in cell culture and in vivo corneal wound healing models. Exp Eye Res. 2009;89:159–165. doi: 10.1016/j.exer.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li J, Kuang K, Nielsen S, Fischbarg J. Molecular identification and immunolocalization of the water channel protein aquaporin 1 in CBCECs. Invest Ophthalmol Vis Sci. 1999;40:1288–1292. [PubMed] [Google Scholar]
- 22.Macnamara E, Sams GW, Smith K, Ambati J, Singh N, Ambati BK. Aquaporin-1 expression is decreased in human and mouse corneal endothelial dysfunction. Mol Vis. 2004;10:51–56. [PubMed] [Google Scholar]
- 23.Stamer WD, Snyder RW, Smith BL, Agre P, Regan JW. Localization of aquaporin CHIP in the human eye: implications in the pathogenesis of glaucoma and other disorders of ocular fluid balance. Invest Ophthalmol Vis Sci. 1994;35:3867–3872. [PubMed] [Google Scholar]
- 24.Patil RV, Saito I, Yang X, Wax MB. Expression of aquaporins in the rat ocular tissue. Exp Eye Res. 1997;64:203–209. doi: 10.1006/exer.1996.0196. [DOI] [PubMed] [Google Scholar]
- 25.Yu D, Thelin WR, Randell SH, Boucher RC. Expression profiles of aquaporins in rat conjunctiva, cornea, lacrimal gland and Meibomian gland. Exp Eye Res. 2012;103:22–32. doi: 10.1016/j.exer.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Kenney MC, Atilano SR, Zorapapel N, Holguin B, Gaster RN, Ljubimov AV. Altered expression of aquaporins in bullous keratopathy and Fuchs’ dystrophy corneas. J Histochem Cytochem. 2004;52:1341–1350. doi: 10.1177/002215540405201010. [DOI] [PubMed] [Google Scholar]
- 27.Thiagarajah JR, Verkman AS. Aquaporin deletion in mice reduces corneal water permeability and delays restoration of transparency after swelling. J Biol Chem. 2002;277:19139–19144. doi: 10.1074/jbc.M202071200. [DOI] [PubMed] [Google Scholar]
- 28.Ruiz-Ederra J, Levin MH, Verkman AS. In situ fluorescence measurement of tear film [Na+], [K+], [Cl−], and pH in mice shows marked hypertonicity in aquaporin-5 deficiency. Invest Ophthalmol Vis Sci. 2009;50:2132–2138. doi: 10.1167/iovs.08-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verkman AS. Aquaporin water channels and endothelial cell function. J Anat. 2002;200:617–627. doi: 10.1046/j.1469-7580.2002.00058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dimasi DP, Burdon KP, Hewitt AW, Savarirayan R, Healey PR, Mitchell P, Mackey DA, Craig JE. Candidate gene study to investigate the genetic determinants of normal variation in central corneal thickness. Mol Vis. 2010;16:562–569. [PMC free article] [PubMed] [Google Scholar]
- 31.Kuang K, Yiming M, Wen Q, Li Y, Ma L, Iserovich P, Verkman AS, Fischbarg J. Fluid transport across cultured layers of corneal endothelium from aquaporin-1 null mice. Exp Eye Res. 2004;78:791–798. doi: 10.1016/j.exer.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 32.Fischbarg J, Diecke FP, Iserovich P, Rubashkin A. The role of the tight junction in paracellular fluid transport across corneal endothelium. Electro-osmosis as a driving force. J Membr Biol. 2006;210:117–130. doi: 10.1007/s00232-005-0850-8. [DOI] [PubMed] [Google Scholar]
- 33.Levin MH, Verkman AS. Aquaporin-3-dependent cell migration and proliferation during corneal re-epithelialization. Invest Ophthalmol Vis Sci. 2006;47:4365–4372. doi: 10.1167/iovs.06-0335. [DOI] [PubMed] [Google Scholar]
- 34.Shankardas J, Patil RV, Vishwanatha JK. Effect of down-regulation of aquaporins in human corneal endothelial and epithelial cell lines. Mol Vis. 2010;16:1538–1548. [PMC free article] [PubMed] [Google Scholar]
- 35.Narayanan R, Gaster RN, Kenney MC. Pseudophakic corneal edema: a review of mechanisms and treatments. Cornea. 2006;25:993–1004. doi: 10.1097/01.ico.0000214225.98366.83. [DOI] [PubMed] [Google Scholar]
- 36.Ljubimov AV, Atilano SR, Garner MH, Maguen E, Nesburn AB, Kenney MC. Extracellular matrix and Na+, K+-ATPase in human corneas following cataract surgery: comparison with bullous keratopathy and Fuchs’ dystrophy corneas. Cornea. 2002;21:74–80. doi: 10.1097/00003226-200201000-00016. [DOI] [PubMed] [Google Scholar]
- 37.Rabinowitz YS, Dong L, Wistow G. Gene expression profile studies of human keratoconus cornea for NEIBank: a novel cornea-expressed gene and the absence of transcripts for aquaporin 5. Invest Ophthalmol Vis Sci. 2005;46:1239–1246. doi: 10.1167/iovs.04-1148. [DOI] [PubMed] [Google Scholar]
- 38.Schechter JE, Warren DW, Mircheff AK. A lacrimal gland is a lacrimal gland, but rodent’s and rabbit’s are not human. Ocul Surf. 2010;8:111–134. doi: 10.1016/s1542-0124(12)70222-7. [DOI] [PubMed] [Google Scholar]
- 39.McKown RL, Wang N, Raab RW, Karnati R, Zhang Y, Williams PB, Laurie GW. Lacritin and other new proteins of the lacrimal functional unit. Exp Eye Res. 2009;88:848–858. doi: 10.1016/j.exer.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.King LS, Yasui M. Aquaporins and disease: lessons from mice to humans. Trends Endocrinol Metab. 2002;13:355–360. doi: 10.1016/s1043-2760(02)00665-3. [DOI] [PubMed] [Google Scholar]
- 41.Ishida N, Hirai SI, Mita S. Immunolocalization of aquaporin homologs in mouse lacrimal glands. Biochem Biophys Res Commun. 1997;238:891–895. doi: 10.1006/bbrc.1997.7396. [DOI] [PubMed] [Google Scholar]
- 42.Ding C, Parsa L, Nandoskar P, Zhao P, Wu K, Wang Y. Duct system of the rabbit lacrimal gland: structural characteristics and role in lacrimal secretion. Invest Ophthalmol Vis Sci. 2010;51:2960–2967. doi: 10.1167/iovs.09-4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ding C, Nandoskar P, Lu M, Thomas P, Trousdale MD, Wang Y. Changes of aquaporins in the lacrimal glands of a rabbit model of Sjogren’s syndrome. Curr Eye Res. 2011;36:571–578. doi: 10.3109/02713683.2011.574330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moore M, Ma T, Yang B, Verkman AS. Tear secretion by lacrimal glands in transgenic mice lacking water channels AQP1, AQP3, AQP4 and AQP5. Exp Eye Res. 2000;70:557–562. doi: 10.1006/exer.1999.0814. [DOI] [PubMed] [Google Scholar]
- 45.Sasaki Y, Tsubota K, Kawedia JD, Menon AG, Yasui M. The difference of aquaporin 5 distribution in acinar and ductal cells in lacrimal and parotid glands. Curr Eye Res. 2007;32:923–929. doi: 10.1080/02713680701733076. [DOI] [PubMed] [Google Scholar]
- 46.Ma T, Song Y, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Defective secretion of saliva in transgenic mice lacking aquaporin-5 water channels. J Biol Chem. 1999;274:20071–20074. doi: 10.1074/jbc.274.29.20071. [DOI] [PubMed] [Google Scholar]
- 47.Levin MH, Verkman AS. Aquaporin-dependent water permeation at the mouse ocular surface: in vivo microfluorimetric measurements in cornea and conjunctiva. Invest Ophthalmol Vis Sci. 2004;45:4423–4432. doi: 10.1167/iovs.04-0816. [DOI] [PubMed] [Google Scholar]
- 48.Tsubota K, Hirai S, King LS, Agre P, Ishida N. Defective cellular trafficking of lacrimal gland aquaporin-5 in Sjogren’s syndrome. Lancet. 2001;357:688–689. doi: 10.1016/S0140-6736(00)04140-4. [DOI] [PubMed] [Google Scholar]
- 49.Ohashi Y, Tsuzaka K, Takeuchi T, Sasaki Y, Tsubota K. Altered distribution of aquaporin 5 and its C-terminal binding protein in the lacrimal glands of a mouse model for Sjogren’s syndrome. Curr Eye Res. 2008;33:621–629. doi: 10.1080/02713680802262819. [DOI] [PubMed] [Google Scholar]
- 50.Lee BH, Gauna AE, Perez G, Park YJ, Pauley KM, Kawai T, Cha S. Autoantibodies against muscarinic type 3 receptor in Sjogren’s syndrome inhibit aquaporin 5 trafficking. PLoS One. 2013;8:e53113. doi: 10.1371/journal.pone.0053113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ohashi Y, Ishida R, Kojima T, Goto E, Matsumoto Y, Watanabe K, Ishida N, Nakata K, Takeuchi T, Tsubota K. Abnormal protein profiles in tears with dry eye syndrome. Am J Ophthalmol. 2003;136:291–299. doi: 10.1016/s0002-9394(03)00203-4. [DOI] [PubMed] [Google Scholar]
- 52.Hirai S, Ishida N, Watanabe K, Mita S. Leakage of aquaporin 5 in the tear of dacryoadenitis mice. Invest Ophthalmol Vis Sci. 2000;41:2432–2437. [PubMed] [Google Scholar]
- 53.Ding C, Lu M, Huang J. Changes of the ocular surface and aquaporins in the lacrimal glands of rabbits during pregnancy. Mol Vis. 2011;17:2847–2855. [PMC free article] [PubMed] [Google Scholar]
- 54.Yamaguchi Y, Watanabe T, Hirakata A, Hida T. Localization and ontogeny of aquaporin-1 and -4 expression in iris and ciliary epithelial cells in rats. Cell Tissue Res. 2006;325:101–109. doi: 10.1007/s00441-005-0122-z. [DOI] [PubMed] [Google Scholar]
- 55.Goel M, Picciani RG, Lee RK, Bhattacharya SK. Aqueous humor dynamics: a review. Open Ophthalmol J. 2010;4:52–59. doi: 10.2174/1874364101004010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Z, Do CW, Valiunas V, Leung CT, Cheng AK, Clark AF, Wax MB, Chatterton JE, Civan MM. Regulation of gap junction coupling in bovine ciliary epithelium. Am J Physiol Cell Physiol. 2010;298:C798–C806. doi: 10.1152/ajpcell.00406.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Calera MR, Topley HL, Liao Y, Duling BR, Paul DL, Goodenough DA. Connexin43 is required for production of the aqueous humor in the murine eye. J Cell Sci. 2006;119:4510–4519. doi: 10.1242/jcs.03202. [DOI] [PubMed] [Google Scholar]
- 58.Yu XS, Yin X, Lafer EM, Jiang JX. Developmental regulation of the direct interaction between the intracellular loop of connexin 45.6 and the C terminus of major intrinsic protein (aquaporin-0) J Biol Chem. 2005;280:22081–22090. doi: 10.1074/jbc.M414377200. [DOI] [PubMed] [Google Scholar]
- 59.Liu J, Xu J, Gu S, Nicholson BJ, Jiang JX. Aquaporin 0 enhances gap junction coupling via its cell adhesion function and interaction with connexin 50. J Cell Sci. 2011;124:198–206. doi: 10.1242/jcs.072652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang D, Vetrivel L, Verkman AS. Aquaporin deletion in mice reduces intraocular pressure and aqueous fluid production. J Gen Physiol. 2002;119:561–569. doi: 10.1085/jgp.20028597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stamer WD, Chan DW, Conley SM, Coons S, Ethier CR. Aquaporin-1 expression and conventional aqueous outflow in human eyes. Exp Eye Res. 2008;87:349–355. doi: 10.1016/j.exer.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stamer WD, Peppel K, O’Donnell ME, Roberts BC, Wu F, Epstein DL. Expression of aquaporin-1 in human trabecular meshwork cells: role in resting cell volume. Invest Ophthalmol Vis Sci. 2001;42:1803–1811. [PubMed] [Google Scholar]
- 63.Baetz NW, Hoffman EA, Yool AJ, Stamer WD. Role of aquaporin-1 in trabecular meshwork cell homeostasis during mechanical strain. Exp Eye Res. 2009;89:95–100. doi: 10.1016/j.exer.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jacob TJ. The relationship between cataract, cell swelling, and volume regulation. Prog Retin Eye Res. 1999;18:223–233. doi: 10.1016/s1350-9462(98)00019-6. [DOI] [PubMed] [Google Scholar]
- 65.Tran TL, Bek T, Holm L, la Cour M, Nielsen S, Prause JU, Rojek A, Hamann S, Heegaard S. Aquaporins 6–12 in the human eye. Acta Ophthalmol. 2013;91:557–563. doi: 10.1111/j.1755-3768.2012.02547.x. [DOI] [PubMed] [Google Scholar]
- 66.Kumari SS, Varadaraj M, Yerramilli VS, Menon AG, Varadaraj K. Spatial expression of aquaporin 5 in mammalian cornea and lens, and regulation of its localization by phosphokinase A. Mol Vis. 2012;18:957–967. [PMC free article] [PubMed] [Google Scholar]
- 67.Grey AC, Walker KL, Petrova RS, Han J, Wilmarth PA, David LL, Donaldson PJ, Schey KL. Verification and spatial localization of aquaporin-5 in the ocular lens. Exp Eye Res. 2013;108:94–102. doi: 10.1016/j.exer.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Z, Han J, Schey KL. Spatial differences in an integral membrane proteome detected in laser capture microdissected samples. J Proteome Res. 2008;7:2696–2702. doi: 10.1021/pr700737h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bassnett S, Wilmarth PA, David LL. The membrane proteome of the mouse lens fiber cell. Mol Vis. 2009;15:2448–2463. [PMC free article] [PubMed] [Google Scholar]
- 70.Ruiz-Ederra J, Verkman AS. Accelerated cataract formation and reduced lens epithelial water permeability in aquaporin-1-deficient mice. Invest Ophthalmol Vis Sci. 2006;47:3960–3967. doi: 10.1167/iovs.06-0229. [DOI] [PubMed] [Google Scholar]
- 71.Grey AC, Li L, Jacobs MD, Schey KL, Donaldson PJ. Differentiation-dependent modification and subcellular distribution of aquaporin-0 suggests multiple functional roles in the rat lens. Differentiation. 2009;77:70–83. doi: 10.1016/j.diff.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zampighi GA, Eskandari S, Hall JE, Zampighi L, Kreman M. Micro-domains of AQP0 in lens equatorial fibers. Exp Eye Res. 2002;75:505–519. doi: 10.1006/exer.2002.2041. [DOI] [PubMed] [Google Scholar]
- 73.Simon SA, Zampighi G, McIntosh TJ, Costello MJ, Tingbeall HP, Robertson JD. The structure of junctions between lens fiber cells. Biosci Rep. 1982;2:333–341. doi: 10.1007/BF01115119. [DOI] [PubMed] [Google Scholar]
- 74.Gonen T, Cheng Y, Kistler J, Walz T. Aquaporin-0 membrane junctions form upon proteolytic cleavage. J Mol Biol. 2004;342:1337–1345. doi: 10.1016/j.jmb.2004.07.076. [DOI] [PubMed] [Google Scholar]
- 75.Pierscionek BK, Regini JW. The gradient index lens of the eye: an opto-biological synchrony. Prog Retin Eye Res. 2012;31:332–349. doi: 10.1016/j.preteyeres.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 76.Gerometta R, Zamudio AC, Escobar DP, Candia OA. Volume change of the ocular lens during accommodation. Am J Physiol Cell Physiol. 2007;293:C797–C804. doi: 10.1152/ajpcell.00094.2007. [DOI] [PubMed] [Google Scholar]
- 77.Mathias RT, Rae JL, Baldo GJ. Physiological properties of the normal lens. Physiol Rev. 1997;77:21–50. doi: 10.1152/physrev.1997.77.1.21. [DOI] [PubMed] [Google Scholar]
- 78.Yang B, Verkman AS. Water and glycerol permeabilities of aquaporins 1–5 and MIP determined quantitatively by expression of epitope-tagged constructs in Xenopus oocytes. J Biol Chem. 1997;272:16140–16146. doi: 10.1074/jbc.272.26.16140. [DOI] [PubMed] [Google Scholar]
- 79.Virkki LV, Cooper GJ, Boron WF. Cloning and functional expression of an MIP (AQP0) homolog from killifish (Fundulus heteroclitus) lens. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1994–R2003. doi: 10.1152/ajpregu.2001.281.6.R1994. [DOI] [PubMed] [Google Scholar]
- 80.Calvanese L, Pellegrini-Calace M, Oliva R. Mutations at key pore-lining positions differentiate the water permeability of fish lens aquaporin from other vertebrates. FEBS Lett. 2010;584:4797–4801. doi: 10.1016/j.febslet.2010.10.058. [DOI] [PubMed] [Google Scholar]
- 81.Nemeth-Cahalan KL, Clemens DM, Hall JE. Regulation of AQP0 water permeability is enhanced by cooperativity. J Gen Physiol. 2013;141:287–295. doi: 10.1085/jgp.201210884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chandy G, Zampighi GA, Kreman M, Hall JE. Comparison of the water transporting properties of MIP and AQP1. J Membr Biol. 1997;159:29–39. doi: 10.1007/s002329900266. [DOI] [PubMed] [Google Scholar]
- 83.Nemeth-Cahalan KL, Hall JE. pH and calcium regulate the water permeability of aquaporin 0. J Biol Chem. 2000;275:6777–6782. doi: 10.1074/jbc.275.10.6777. [DOI] [PubMed] [Google Scholar]
- 84.Varadaraj K, Kumari S, Shiels A, Mathias RT. Regulation of aquaporin water permeability in the lens. Invest Ophthalmol Vis Sci. 2005;46:1393–1402. doi: 10.1167/iovs.04-1217. [DOI] [PubMed] [Google Scholar]
- 85.Reichow SL, Gonen T. Noncanonical binding of calmodulin to aquaporin-0: implications for channel regulation. Structure. 2008;16:1389–1398. doi: 10.1016/j.str.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reichow SL, Clemens DM, Freites JA, Nemeth-Cahalan KL, Heyden M, Tobias DJ, Hall JE, Gonen T. Allosteric mechanism of water-channel gating by Ca–calmodulin. Nat Struct Mol Biol. 2013;20:1085–1092. doi: 10.1038/nsmb.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rose KM, Wang Z, Magrath GN, Hazard ES, Hildebrandt JD, Schey KL. Aquaporin 0-calmodulin interaction and the effect of aquaporin 0 phosphorylation. Biochemistry. 2008;47:339–347. doi: 10.1021/bi701980t. [DOI] [PubMed] [Google Scholar]
- 88.Kalman K, Nemeth-Cahalan KL, Froger A, Hall JE. Phosphorylation determines the calmodulin-mediated Ca2+ response and water permeability of AQP0. J Biol Chem. 2008;283:21278–21283. doi: 10.1074/jbc.M801740200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Golestaneh N, Fan J, Zelenka P, Chepelinsky AB. PKC putative phosphorylation site Ser235 is required for MIP/AQP0 translocation to the plasma membrane. Mol Vis. 2008;14:1006–1014. [PMC free article] [PubMed] [Google Scholar]
- 90.Wang Z, Schey KL. Aquaporin-0 interacts with the FERM domain of ezrin/radixin/moesin proteins in the ocular lens. Invest Ophthalmol Vis Sci. 2011;52:5079–5087. doi: 10.1167/iovs.10-6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lindsey Rose KM, Gourdie RG, Prescott AR, Quinlan RA, Crouch RK, Schey KL. The C terminus of lens aquaporin 0 interacts with the cytoskeletal proteins filensin and CP49. Invest Ophthalmol Vis Sci. 2006;47:1562–1570. doi: 10.1167/iovs.05-1313. [DOI] [PubMed] [Google Scholar]
- 92.Nakazawa Y, Oka M, Furuki K, Mitsuishi A, Nakashima E, Takehana M. The effect of the interaction between aquaporin 0 (AQP0) and the filensin tail region on AQP0 water permeability. Mol Vis. 2011;17:3191–3199. [PMC free article] [PubMed] [Google Scholar]
- 93.Fotiadis D, Hasler L, Muller DJ, Stahlberg H, Kistler J, Engel A. Surface tongue-and-groove contours on lens MIP facilitate cell-to-cell adherence. J Mol Biol. 2000;300:779–789. doi: 10.1006/jmbi.2000.3920. [DOI] [PubMed] [Google Scholar]
- 94.Colom A, Casuso I, Boudier T, Scheuring S. High-speed atomic force microscopy: cooperative adhesion and dynamic equilibrium of junctional microdomain membrane proteins. J Mol Biol. 2012;423:249–256. doi: 10.1016/j.jmb.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 95.Kumari SS, Varadaraj K. Intact AQP0 performs cell-to-cell adhesion. Biochem Biophys Res Commun. 2009;390:1034–1039. doi: 10.1016/j.bbrc.2009.10.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schey KL, Little M, Fowler JG, Crouch RK. Characterization of human lens major intrinsic protein structure. Invest Ophthalmol Vis Sci. 2000;41:175–182. [PubMed] [Google Scholar]
- 97.Korlimbinis A, Berry Y, Thibault D, Schey KL, Truscott RJ. Protein aging: truncation of aquaporin 0 in human lens regions is a continuous age-dependent process. Exp Eye Res. 2009;88:966–973. doi: 10.1016/j.exer.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gonen T, Sliz P, Kistler J, Cheng Y, Walz T. Aquaporin-0 membrane junctions reveal the structure of a closed water pore. Nature. 2004;429:193–197. doi: 10.1038/nature02503. [DOI] [PubMed] [Google Scholar]
- 99.Gonen T, Cheng Y, Sliz P, Hiroaki Y, Fujiyoshi Y, Harrison SC, Walz T. Lipid–protein interactions in double-layered two-dimensional AQP0 crystals. Nature. 2005;438:633–638. doi: 10.1038/nature04321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shiels A, Mackay D, Bassnett S, Al-Ghoul K, Kuszak J. Disruption of lens fiber cell architecture in mice expressing a chimeric AQP0-LTR protein. FASEB J. 2000;14:2207–2212. doi: 10.1096/fj.99-1071com. [DOI] [PubMed] [Google Scholar]
- 101.Varadaraj K, Kumari SS, Mathias RT. Transgenic expression of AQP1 in the fiber cells of AQP0 knockout mouse: effects on lens transparency. Exp Eye Res. 2010;91:393–404. doi: 10.1016/j.exer.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Watanabe K, Wada K, Ohashi T, Okubo S, Takekuma K, Hashizume R, Hayashi J, Serikawa T, Kuramoto T, Kikkawa Y. A 5-bp insertion in Mip causes recessive congenital cataract in KFRS4/Kyo rats. PLoS One. 2012;7:e50737. doi: 10.1371/journal.pone.0050737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Al-Ghoul KJ, Kirk T, Kuszak AJ, Zoltoski RK, Shiels A, Kuszak JR. Lens structure in MIP-deficient mice. Anat Rec A: Discov Mol Cell Evol Biol. 2003;273:714–730. doi: 10.1002/ar.a.10080. [DOI] [PubMed] [Google Scholar]
- 104.Shiels A, Bassnett S, Varadaraj K, Mathias R, Al-Ghoul K, Kuszak J, Donoviel D, Lilleberg S, Friedrich G, Zambrowicz B. Optical dysfunction of the crystalline lens in aquaporin-0-deficient mice. Physiol Genomics. 2001;7:179–186. doi: 10.1152/physiolgenomics.00078.2001. [DOI] [PubMed] [Google Scholar]
- 105.Preston GM, Smith BL, Zeidel ML, Moulds JJ, Agre P. Mutations in aquaporin-1 in phenotypically normal humans without functional CHIP water channels. Science. 1994;265:1585–1587. doi: 10.1126/science.7521540. [DOI] [PubMed] [Google Scholar]
- 106.Varadaraj K, Kumari SS, Patil R, Wax MB, Mathias RT. Functional characterization of a human aquaporin 0 mutation that leads to a congenital dominant lens cataract. Exp Eye Res. 2008;87:9–21. doi: 10.1016/j.exer.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Senthil Kumar G, Kyle JW, Minogue PJ, Dinesh Kumar K, Vasantha K, Berthoud VM, Beyer EC, Santhiya ST. An MIP/AQP0 mutation with impaired traffick-ing and function underlies an autosomal dominant congenital lamellar cataract. Exp Eye Res. 2013;110:136–141. doi: 10.1016/j.exer.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hu S, Wang B, Qi Y, Lin H. The Arg233Lys AQP0 mutation disturbs aquaporin0–calmodulin interaction causing polymorphic congenital cataract. PLoS One. 2012;7:e37637. doi: 10.1371/journal.pone.0037637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Berry V, Francis P, Kaushal S, Moore A, Bhattacharya S. Missense mutations in MIP underlie autosomal dominant ‘polymorphic’ and lamellar cataracts linked to 12q. Nat Genet. 2000;25:15–17. doi: 10.1038/75538. [DOI] [PubMed] [Google Scholar]
- 110.Francis P, Chung JJ, Yasui M, Berry V, Moore A, Wyatt MK, Wistow G, Bhattacharya SS, Agre P. Functional impairment of lens aquaporin in two families with dominantly inherited cataracts. Hum Mol Genet. 2000;9:2329–2334. doi: 10.1093/oxfordjournals.hmg.a018925. [DOI] [PubMed] [Google Scholar]
- 111.Geyer DD, Spence MA, Johannes M, Flodman P, Clancy KP, Berry R, Sparkes RS, Jonsen MD, Isenberg SJ, Bateman JB. Novel single-base deletional mutation in major intrinsic protein (MIP) in autosomal dominant cataract. Am J Ophthalmol. 2006;141:761–763. doi: 10.1016/j.ajo.2005.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gu F, Zhai H, Li D, Zhao L, Li C, Huang S, Ma X. A novel mutation in major intrinsic protein of the lens gene (MIP) underlies autosomal dominant cataract in a Chinese family. Mol Vis. 2007;13:1651–1656. [PubMed] [Google Scholar]
- 113.Wang W, Jiang J, Zhu Y, Li J, Jin C, Shentu X, Yao K. A novel mutation in the major intrinsic protein (MIP) associated with autosomal dominant congenital cataracts in a Chinese family. Mol Vis. 2010;16:534–539. [PMC free article] [PubMed] [Google Scholar]
- 114.Jiang J, Jin C, Wang W, Tang X, Shentu X, Wu R, Wang Y, Xia K, Yao K. Identification of a novel splice-site mutation in MIP in a Chinese congenital cataract family. Mol Vis. 2009;15:38–44. [PMC free article] [PubMed] [Google Scholar]
- 115.Jin C, Jiang J, Wang W, Yao K. Identification of a MIP mutation that activates a cryptic acceptor splice site in the 3′ untranslated region. Mol Vis. 2010;16:2253–2258. [PMC free article] [PubMed] [Google Scholar]
- 116.Tenckhoff S, Hollborn M, Kohen L, Wolf S, Wiedemann P, Bringmann A. Diversity of aquaporin mRNA expressed by rat and human retinas. Neuroreport. 2005;16:53–56. doi: 10.1097/00001756-200501190-00013. [DOI] [PubMed] [Google Scholar]
- 117.Hollborn M, Dukic-Stefanovic S, Pannicke T, Ulbricht E, Reichenbach A, Wiedemann P, Bringmann A, Kohen L. Expression of aquaporins in the retina of diabetic rats. Curr Eye Res. 2011;36:850–856. doi: 10.3109/02713683.2011.593108. [DOI] [PubMed] [Google Scholar]
- 118.Iandiev I, Pannicke T, Hartig W, Grosche J, Wiedemann P, Reichenbach A, Bringmann A. Localization of aquaporin-0 immunoreactivity in the rat retina. Neurosci Lett. 2007;426:81–86. doi: 10.1016/j.neulet.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 119.Kim IB, Lee EJ, Oh SJ, Park CB, Pow DV, Chun MH. Light and electron microscopic analysis of aquaporin 1-like-immunoreactive amacrine cells in the rat retina. J Comp Neurol. 2002;452:178–191. doi: 10.1002/cne.10359. [DOI] [PubMed] [Google Scholar]
- 120.Nagelhus EA, Veruki ML, Torp R, Haug FM, Laake JH, Nielsen S, Agre P, Ottersen OP. Aquaporin-4 water channel protein in the rat retina and optic nerve: polarized expression in Muller cells and fibrous astrocytes. J Neurosci. 1998;18:2506–2519. doi: 10.1523/JNEUROSCI.18-07-02506.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Iandiev I, Pannicke T, Biedermann B, Wiedemann P, Reichenbach A, Bringmann A. Ischemia–reperfusion alters the immunolocalization of glial aquaporins in rat retina. Neurosci Lett. 2006;408:108–112. doi: 10.1016/j.neulet.2006.08.084. [DOI] [PubMed] [Google Scholar]
- 122.Iandiev I, Dukic-Stefanovic S, Hollborn M, Pannicke T, Hartig W, Wiedemann P, Reichenbach A, Bringmann A, Kohen L. Immunolocalization of aquaporin-6 in the rat retina. Neurosci Lett. 2011;490:130–134. doi: 10.1016/j.neulet.2010.12.042. [DOI] [PubMed] [Google Scholar]
- 123.Iandiev I, Pannicke T, Reichel MB, Wiedemann P, Reichenbach A, Bringmann A. Expression of aquaporin-1 immunoreactivity by photoreceptor cells in the mouse retina. Neurosci Lett. 2005;388:96–99. doi: 10.1016/j.neulet.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 124.Kim IB, Oh SJ, Nielsen S, Chun MH. Immunocytochemical localization of aquaporin 1 in the rat retina. Neurosci Lett. 1998;244:52–54. doi: 10.1016/s0304-3940(98)00104-9. [DOI] [PubMed] [Google Scholar]
- 125.Kang TH, Choi YK, Kim IB, Oh SJ, Chun MH. Identification and characterization of an aquaporin 1 immunoreactive amacrine-type cell of the mouse retina. J Comp Neurol. 2005;488:352–367. doi: 10.1002/cne.20589. [DOI] [PubMed] [Google Scholar]
- 126.Ghosh S, Freitag AC, Martin-Vasallo P, Coca-Prados M. Cellular distribution and differential gene expression of the three alpha subunit isoforms of the Na,K-ATPase in the ocular ciliary epithelium. J Biol Chem. 1990;265:2935–2940. [PubMed] [Google Scholar]
- 127.Hasegawa H, Lian SC, Finkbeiner WE, Verkman AS. Extrarenal tissue distribution of CHIP28 water channels by in situ hybridization and antibody staining. Am J Physiol. 1994;266:C893–C903. doi: 10.1152/ajpcell.1994.266.4.C893. [DOI] [PubMed] [Google Scholar]
- 128.Stamer WD, Bok D, Hu J, Jaffe GJ, McKay BS. Aquaporin-1 channels in human retinal pigment epithelium: role in transepithelial water movement. Invest Ophthalmol Vis Sci. 2003;44:2803–2808. doi: 10.1167/iovs.03-0001. [DOI] [PubMed] [Google Scholar]
- 129.Nagelhus EA, Horio Y, Inanobe A, Fujita A, Haug FM, Nielsen S, Kurachi Y, Ottersen OP. Immunogold evidence suggests that coupling of K+ siphoning and water transport in rat retinal Muller cells is mediated by a coenrichment of Kir4.1 and AQP4 in specific membrane domains. Glia. 1999;26:47–54. doi: 10.1002/(sici)1098-1136(199903)26:1<47::aid-glia5>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 130.Kofuji P, Ceelen P, Zahs KR, Surbeck LW, Lester HA, Newman EA. Genetic inactivation of an inwardly rectifying potassium channel (Kir4.1 subunit) in mice: phenotypic impact in retina. J Neurosci. 2000;20:5733–5740. doi: 10.1523/JNEUROSCI.20-15-05733.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Connors NC, Adams ME, Froehner SC, Kofuji P. The potassium channel Kir4.1 associates with the dystrophin–glycoprotein complex via alpha-syntrophin in glia. J Biol Chem. 2004;279:28387–28392. doi: 10.1074/jbc.M402604200. [DOI] [PubMed] [Google Scholar]
- 132.Connors NC, Kofuji P. Potassium channel Kir4.1 macromolecular complex in retinal glial cells. Glia. 2006;53:124–131. doi: 10.1002/glia.20271. [DOI] [PubMed] [Google Scholar]
- 133.Goodyear MJ, Crewther SG, Junghans BM. A role for aquaporin-4 in fluid regulation in the inner retina. Vis Neurosci. 2009;26:159–165. doi: 10.1017/S0952523809090038. [DOI] [PubMed] [Google Scholar]
- 134.Ruiz-Ederra J, Zhang H, Verkman AS. Evidence against functional interaction between aquaporin-4 water channels and Kir4.1 potassium channels in retinal Muller cells. J Biol Chem. 2007;282:21866–21872. doi: 10.1074/jbc.M703236200. [DOI] [PubMed] [Google Scholar]
- 135.Farjo R, Peterson WM, Naash MI. Expression profiling after retinal detachment and reattachment: a possible role for aquaporin-0. Invest Ophthalmol Vis Sci. 2008;49:511–521. doi: 10.1167/iovs.07-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fukuda M, Naka M, Mizokami J, Negi A, Nakamura M. Diabetes induces expression of aquaporin-0 in the retinal nerve fibers of spontaneously diabetic Torii rats. Exp Eye Res. 2011;92:195–201. doi: 10.1016/j.exer.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 137.Ortak H, Cayli S, Ocakli S, Sogut E, Ekici F, Tas U, Demir S. Age-related changes of aquaporin expression patterns in the postnatal rat retina. Acta Histochem. 2013;115:382–388. doi: 10.1016/j.acthis.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 138.Ikeda M, Beitz E, Kozono D, Guggino WB, Agre P, Yasui M. Characterization of aquaporin-6 as a nitrate channel in mammalian cells. Requirement of pore-lining residue threonine 63. J Biol Chem. 2002;277:39873–39879. doi: 10.1074/jbc.M207008200. [DOI] [PubMed] [Google Scholar]
- 139.Kelly ML, Cho WJ, Jeremic A, Abu-Hamdah R, Jena BP. Vesicle swelling regulates content expulsion during secretion. Cell Biol Int. 2004;28:709–716. doi: 10.1016/j.cellbi.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 140.Ishibashi K, Sasaki S, Fushimi K, Uchida S, Kuwahara M, Saito H, Furukawa T, Nakajima K, Yamaguchi Y, Gojobori T, et al. Molecular cloning and expression of a member of the aquaporin family with permeability to glycerol and urea in addition to water expressed at the basolateral membrane of kidney collecting duct cells. Proc Natl Acad Sci U S A. 1994;91:6269–6273. doi: 10.1073/pnas.91.14.6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Echevarria M, Windhager EE, Frindt G. Selectivity of the renal collecting duct water channel aquaporin-3. J Biol Chem. 1996;271:25079–25082. doi: 10.1074/jbc.271.41.25079. [DOI] [PubMed] [Google Scholar]
- 142.Miller EW, Dickinson BC, Chang CJ. Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc Natl Acad Sci U S A. 2010;107:15681–15686. doi: 10.1073/pnas.1005776107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Matsuzaki T, Suzuki T, Koyama H, Tanaka S, Takata K. Water channel protein AQP3 is present in epithelia exposed to the environment of possible water loss. J Histochem Cytochem. 1999;47:1275–1286. doi: 10.1177/002215549904701007. [DOI] [PubMed] [Google Scholar]
- 144.Hollborn M, Ulbricht E, Reichenbach A, Wiedemann P, Bringmann A, Kohen L. Transcriptional regulation of aquaporin-3 in human retinal pigment epithelial cells. Mol Biol Rep. 2012;39:7949–7956. doi: 10.1007/s11033-012-1640-x. [DOI] [PubMed] [Google Scholar]
- 145.Reh TA, Levine EM. Multipotential stem cells and progenitors in the vertebrate retina. J Neurobiol. 1998;36:206–220. [PubMed] [Google Scholar]
- 146.Iandiev I, Biedermann B, Reichenbach A, Wiedemann P, Bringmann A. Expression of aquaporin-9 immunoreactivity by catecholaminergic amacrine cells in the rat retina. Neurosci Lett. 2006;398:264–267. doi: 10.1016/j.neulet.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 147.Dibas A, Yang MH, Bobich J, Yorio T. Stress-induced changes in neuronal aquaporin-9 (AQP9) in a retinal ganglion cell-line. Pharmacol Res. 2007;55:378–384. doi: 10.1016/j.phrs.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 148.Naka M, Kanamori A, Negi A, Nakamura M. Reduced expression of aquaporin-9 in rat optic nerve head and retina following elevated intraocular pressure. Invest Ophthalmol Vis Sci. 2010;51:4618–4626. doi: 10.1167/iovs.09-4712. [DOI] [PubMed] [Google Scholar]
- 149.Dibas A, Yorio T. Regulation of transport in the RPE. In: Barnstable CJ, Tombran-Tink J, editors. Ocular Transporters in Ophthalmic Diseases and Drug Delivery. Humana Press; Philadelphia, PA: 2008. pp. 157–184. [Google Scholar]
- 150.Badaut J, Regli L. Distribution and possible roles of aquaporin 9 in the brain. Neuroscience. 2004;129:971–981. doi: 10.1016/j.neuroscience.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 151.Eberhardt C, Amann B, Feuchtinger A, Hauck SM, Deeg CA. Differential expression of inwardly rectifying K+ channels and aquaporins 4 and 5 in autoimmune uveitis indicates misbalance in Muller glial cell-dependent ion and water homeostasis. Glia. 2011;59:697–707. doi: 10.1002/glia.21139. [DOI] [PubMed] [Google Scholar]
- 152.Bresnick GH. Diabetic maculopathy. A critical review highlighting diffuse macular edema. Ophthalmology. 1983;90:1301–1317. doi: 10.1016/s0161-6420(83)34388-8. [DOI] [PubMed] [Google Scholar]
- 153.Bringmann A, Reichenbach A, Wiedemann P. Pathomechanisms of cystoid macular edema. Ophthalmic Res. 2004;36:241–249. doi: 10.1159/000081203. [DOI] [PubMed] [Google Scholar]
- 154.Bringmann A, Pannicke T, Grosche J, Francke M, Wiedemann P, Skatchkov SN, Osborne NN, Reichenbach A. Muller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006;25:397–424. doi: 10.1016/j.preteyeres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 155.Iandiev I, Pannicke T, Reichenbach A, Wiedemann P, Bringmann A. Diabetes alters the localization of glial aquaporins in rat retina. Neurosci Lett. 2007;421:132–136. doi: 10.1016/j.neulet.2007.04.076. [DOI] [PubMed] [Google Scholar]
- 156.Fukuda M, Nakanishi Y, Fuse M, Yokoi N, Hamada Y, Fukagawa M, Negi A, Nakamura M. Altered expression of aquaporins 1 and 4 coincides with neurodegenerative events in retinas of spontaneously diabetic Torii rats. Exp Eye Res. 2010;90:17–25. doi: 10.1016/j.exer.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 157.Gerhardinger C, Costa MB, Coulombe MC, Toth I, Hoehn T, Grosu P. Expression of acute-phase response proteins in retinal Muller cells in diabetes. Invest Ophthalmol Vis Sci. 2005;46:349–357. doi: 10.1167/iovs.04-0860. [DOI] [PubMed] [Google Scholar]
- 158.Kaneko K, Yagui K, Tanaka A, Yoshihara K, Ishikawa K, Takahashi K, Bujo H, Sakurai K, Saito Y. Aquaporin 1 is required for hypoxia-inducible angiogenesis in human retinal vascular endothelial cells. Microvasc Res. 2008;75:297–301. doi: 10.1016/j.mvr.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 159.Pannicke T, Iandiev I, Wurm A, Uckermann O, vom Hagen F, Reichenbach A, Wiedemann P, Hammes HP, Bringmann A. Diabetes alters osmotic swelling characteristics and membrane conductance of glial cells in rat retina. Diabetes. 2006;55:633–639. doi: 10.2337/diabetes.55.03.06.db05-1349. [DOI] [PubMed] [Google Scholar]
- 160.Mizokami J, Kanamori A, Negi A, Nakamura M. A preliminary study of reduced expression of aquaporin-9 in the optic nerve of primate and human eyes with glaucoma. Curr Eye Res. 2011;36:1064–1067. doi: 10.3109/02713683.2011.611610. [DOI] [PubMed] [Google Scholar]
- 161.Dibas A, Yang MH, He S, Bobich J, Yorio T. Changes in ocular aquaporin-4 (AQP4) expression following retinal injury. Mol Vis. 2008;14:1770–1783. [PMC free article] [PubMed] [Google Scholar]
- 162.Da T, Verkman AS. Aquaporin-4 gene disruption in mice protects against impaired retinal function and cell death after ischemia. Invest Ophthalmol Vis Sci. 2004;45:4477–4483. doi: 10.1167/iovs.04-0940. [DOI] [PubMed] [Google Scholar]
- 163.Zalish M, Lavie V, Duvdevani R, Yoles E, Schwartz M. Gangliosides attenuate axonal loss after optic nerve injury. Retina. 1993;13:145–147. doi: 10.1097/00006982-199313020-00010. [DOI] [PubMed] [Google Scholar]
- 164.Minzenberg M, Berkelaar M, Bray G, McKerracher L. Changes in retinal ganglion cell axons after optic nerve crush: neurofilament expression is not the sole determinant of calibre. Biochem Cell Biol. 1995;73:599–604. doi: 10.1139/o95-065. [DOI] [PubMed] [Google Scholar]
- 165.Yoles E, Schwartz M. Elevation of intraocular glutamate levels in rats with partial lesion of the optic nerve. Arch Ophthalmol. 1998;116:906–910. doi: 10.1001/archopht.116.7.906. [DOI] [PubMed] [Google Scholar]
- 166.Dibas A, Oku H, Fukuhara M, Kurimoto T, Ikeda T, Patil RV, Sharif NA, Yorio T. Changes in ocular aquaporin expression following optic nerve crush. Mol Vis. 2010;16:330–340. [PMC free article] [PubMed] [Google Scholar]
- 167.Lardenoye CW, van Kooij B, Rothova A. Impact of macular edema on visual acuity in uveitis. Ophthalmology. 2006;113:1446–1449. doi: 10.1016/j.ophtha.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 168.Motulsky E, Koch P, Janssens S, Lienart M, Vanbellinghen AM, Bolaky N, Chan CC, Caspers L, Martin-Martinez MD, Xu H, Delporte C, Willermain F. Aquaporin expression in blood–retinal barrier cells during experimental autoimmune uveitis. Mol Vis. 2010;16:602–610. [PMC free article] [PubMed] [Google Scholar]
- 169.Zhao M, Bousquet E, Valamanesh F, Farman N, Jeanny JC, Jaisser F, Behar-Cohen FF. Differential regulations of AQP4 and Kir4.1 by triamcinolone acetonide and dexamethasone in the healthy and inflamed retina. Invest Ophthalmol Vis Sci. 2011;52:6340–6347. doi: 10.1167/iovs.11-7675. [DOI] [PubMed] [Google Scholar]
- 170.Iandiev I, Pannicke T, Hollborn M, Wiedemann P, Reichenbach A, Grimm C, Reme CE, Bringmann A. Localization of glial aquaporin-4 and Kir4.1 in the light-injured murine retina. Neurosci Lett. 2008;434:317–321. doi: 10.1016/j.neulet.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 171.Iandiev I, Wurm A, Hollborn M, Wiedemann P, Grimm C, Reme CE, Reichenbach A, Pannicke T, Bringmann A. Muller cell response to blue light injury of the rat retina. Invest Ophthalmol Vis Sci. 2008;49:3559–3567. doi: 10.1167/iovs.08-1723. [DOI] [PubMed] [Google Scholar]
- 172.Yasui M, Hazama A, Kwon TH, Nielsen S, Guggino WB, Agre P. Rapid gating and anion permeability of an intracellular aquaporin. Nature. 1999;402:184–187. doi: 10.1038/46045. [DOI] [PubMed] [Google Scholar]
- 173.Pannicke T, Iandiev I, Uckermann O, Biedermann B, Kutzera F, Wiedemann P, Wolburg H, Reichenbach A, Bringmann A. A potassium channel-linked mechanism of glial cell swelling in the postischemic retina. Mol Cell Neurosci. 2004;26:493–502. doi: 10.1016/j.mcn.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 174.Liu XQ, Kobayashi H, Jin ZB, Wada A, Nao IN. Differential expression of Kir4.1 and aquaporin 4 in the retina from endotoxin-induced uveitis rat. Mol Vis. 2007;13:309–317. [PMC free article] [PubMed] [Google Scholar]
- 175.Bringmann A, Uckermann O, Pannicke T, Iandiev I, Reichenbach A, Wiedemann P. Neuronal versus glial cell swelling in the ischaemic retina. Acta Ophthalmol Scand. 2005;83:528–538. doi: 10.1111/j.1600-0420.2005.00565.x. [DOI] [PubMed] [Google Scholar]
- 176.Castle NA. Aquaporins as targets for drug discovery. Drug Discov Today. 2005;10:485–493. doi: 10.1016/S1359-6446(05)03390-8. [DOI] [PubMed] [Google Scholar]
- 177.Verkman AS. Aquaporins in clinical medicine. Annu Rev Med. 2012;63:303–316. doi: 10.1146/annurev-med-043010-193843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Huber VJ, Tsujita M, Nakada T. Aquaporins in drug discovery and pharmacotherapy. Mol Aspects Med. 2012;33:691–703. doi: 10.1016/j.mam.2012.01.002. [DOI] [PubMed] [Google Scholar]