Abstract
Escape from apoptosis is a key attribute of tumour cells and facilitates chemo-resistance. The ‘BCL-2-regulated' or ‘intrinsic' apoptotic pathway integrates stress and survival signalling to govern whether a cancer cell will live or die. Indeed, many pro-apoptotic members of the BCL-2 family have demonstrated tumour-suppression activity in mouse models of cancer and are lost or repressed in certain human cancers. Conversely, overexpression of pro-survival BCL-2 family members promotes tumorigenesis in humans and in mouse models. Many of the drugs currently used in the clinic mediate their therapeutic effects (at least in part) through the activation of the BCL-2-regulated apoptotic pathway. However, initiators of this apoptotic pathway, such as p53, are mutated, lost or silenced in many human cancers rendering them refractory to treatment. To counter such resistance mechanisms, a novel class of therapeutics, ‘BH3-mimetics', has been developed. These drugs directly activate apoptosis by binding and inhibiting select antiapoptotic BCL-2 family members and thereby bypass the requirement for upstream initiators, such as p53. In this review, we discuss the role of the BCL-2 protein family in the development and treatment of cancer, with an emphasis on mechanistic studies using well-established mouse models of cancer, before describing the development and already recognised potential of the BH3-mimetic compounds.
Facts
Cancer development and progression are facilitated by enhanced cell survival signalling.
Loss of initiators of apoptosis or overexpression of inhibitors of apoptosis are frequently observed in haematological and solid cancers.
BH3-mimetic compounds offer a novel approach for treating chemo-resistant cancers by blocking select pro-survival BCL-2 family members.
Open Questions
Do all cancers require high expression of pro-survival BCL-2 family members for their development and sustained growth?
Can a therapeutic window be established for BH3-mimetic drugs?
Will direct induction of apoptosis using BH3-mimetic compounds reduce the emergence of therapeutic resistance?
What are the optimal drugs to partner BH3-mimetics for combination therapy of different cancers?
The complexity of multicellular animals is built upon a foundation of cell and tissue specification that facilitates coordination of intra-organismal processes and interaction with the surrounding environment. Cooperation between cells is essential, as are mechanisms to detect and remove ‘rogue' cells that lose the ability to respond appropriately to developmental and homeostatic cues. Failure of these mechanisms can have dire consequences, such as the development of cancer or autoimmune disease.1 A critical tumour-suppression mechanism is the cell's intrinsic ability to self-destruct through a process of programmed cell death known as apoptosis.2 Indeed, evasion from apoptosis cooperates with oncogenic mutations that deregulate cell growth and cell cycling in tumorigenesis. Evasion of apoptosis is therefore considered a requisite characteristic of tumour formation, one of the so-called ‘Hallmarks of Cancer'.3
Apoptosis constitutes the ordered, genetically encoded process that removes not only damaged cells but also those that have become superfluous to the function of the organism.4 Apoptosis enables cells to be eliminated with minimal disruption to surrounding cells and is thereby distinct from necrotic cell death, which is often unregulated and results in the release of cellular debris that can prompt tissue inflammation. It is important to note that some other forms of programmed cell death, known as pyroptosis,5 and necroptosis (also called programmed necrosis),4, 6 have risen to prominence. However, the contributions of these forms of cell death to morphogenesis during animal development, adult tissue homeostasis as well as the genesis and treatment of cancer remain to be elucidated.
The term ‘apoptosis' was first coined by Kerr et al.7 to describe a form of cell death distinguished from necrosis by a characteristic morphology. Apoptosis is associated with cell shrinkage and membrane blebbing to yield small vesicles, which are subsequently engulfed by neighbouring phagocytic cells.8, 9, 10 In addition, molecular events, such as inter-nucleosomal DNA cleavage and translocation of phosphatidyl-serine to the outer leaflet of the plasma membrane, are indicative of apoptosis and frequently used experimentally as markers of apoptosis.
In this review, we summarise the literature describing the mechanisms by which apoptosis signalling is governed with particular focus on their importance in cancer development as demonstrated by observations from various experimental mouse models and also from studies of human cancer. We close with an analysis of the role of the BCL-2 family for mediating the activity of many commonly used anticancer therapeutics, including the promising new class of agents known as BH3-mimetics.
The BCL-2-Regulated Apoptotic Pathway
The BCL-2-regulated apoptotic pathway (also known as ‘intrinsic', ‘stress' or ‘mitochondrial' pathway) is evolutionarily highly conserved, with homologues of critical genes found in animals as distantly related as worms and humans.11, 12, 13 Indeed, many of the early insights into the roles of components of this cell death pathway were derived from studies using the model organism Caenorhabditis elegans (e.g., Vaux et al.).12 Initiation of the BCL-2-regulated apoptotic pathway is controlled, as the name implies, by interactions between members of the BCL-2 protein family.14, 15, 16, 17, 18, 19 This family consists of three groups of structurally related proteins: the pro-survival BCL-2-like proteins, the multi-BH domain pro-apoptotic BAX/BAK proteins, and the pro-apoptotic BH3-only proteins.
The BCL-2 protein family
The pro-survival BCL-2 family proteins (BCL-2, BCL-XL, BCL-W, MCL-1, A1/BFL-1) share homology within four BCL-2 homology domains (BH1–4). These proteins form a characteristic helical bundle fold, which is critical for their ability to bind to the pro-apoptotic BCL-2 family members and thereby exert their antiapoptotic function.
The pro-apoptotic BAX/BAK subfamily members also contain four BH domains (BH1–4). In their inactive state, their structure is very similar to that of BCL-2 pro-survival proteins,20 but BAX and BAK are able to undergo substantial conformational change during apoptosis.21 BAX and BAK bind to and are inhibited by different BCL-2-like pro-survival proteins to different extents.22, 23 BOK shares significant homology across all four BH domains to BAX and BAK; however, its role in apoptosis remains unclear,24 although it may cooperate with BAX in the attrition of primordial follicle oocytes during ageing.25
The pro-apoptotic BH3-only proteins (BIM, PUMA, BID, BAD, BIK, BMF, NOXA, HRK) share only the BH3 domain with each other and their more distant relatives.19, 26, 27 These proteins are unstructured in isolation but assume an α-helical fold when bound to BCL-2 pro-survival family members.28 The exception to this rule is BID, which is produced as an inactive globular protein that is converted into its active form, tBID (truncated BID), through caspase-8-mediated cleavage.29, 30 The BH3-only proteins are able to bind members of the BCL-2-like pro-survival subfamily and some of them can also bind to BAX and BAK, but there are substantial differences in their selectivity of interaction.17, 18, 19, 23, 31, 32, 33
Activation of the BCL-2-regulated apoptotic pathway
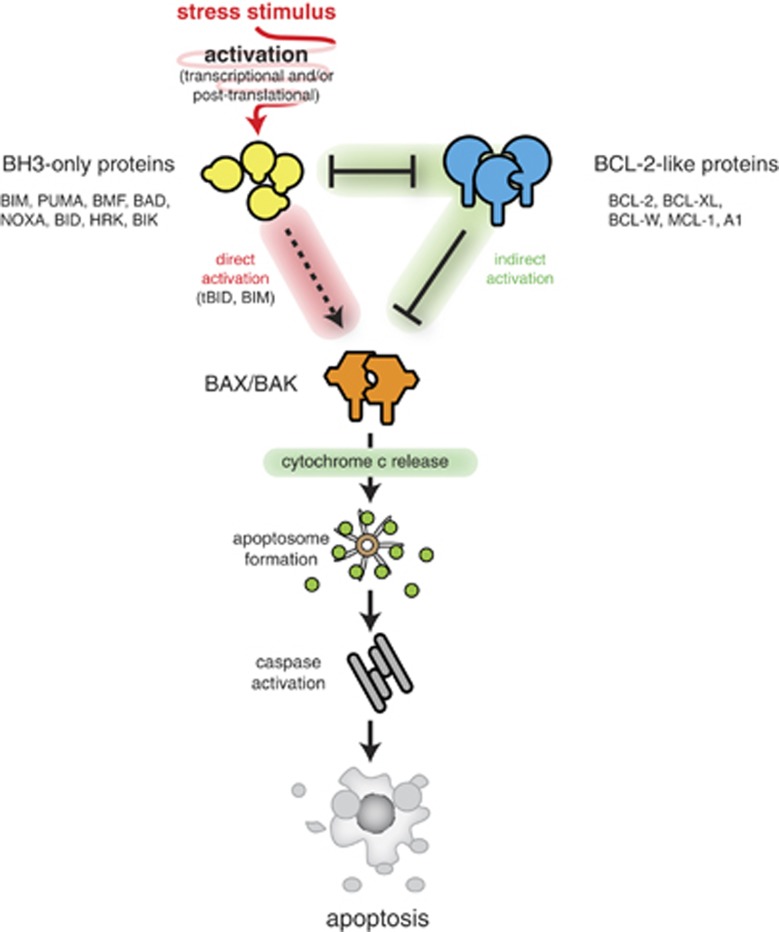
The BCL-2 regulated apoptotic pathway is initiated through the transcriptional and/or posttranscriptional activation of the BH3-only proteins in response to various upstream signalling events. Some BH3-only proteins (notably BIM, tBID, PUMA) cause activation of BAX/BAK through direct binding, and all BH3-only proteins can activate BAX/BAK indirectly by binding to and inhibiting the pro-survival BCL-2-like proteins (Figure 1).19, 34, 35, 36, 37, 38, 39 Activated BAX/BAK cause outer mitochondrial membrane permeabilisation (MOMP), which allows cytosolic release of apoptogenic factors (e.g., cytochrome c, Smac/DIABLO) that cause activation of the caspase cascade.40 It remains unclear whether binding of BH3-only proteins to the pro-survival BCL-2-like proteins or their direct binding to BAX/BAK is more critical for the initiation of apoptosis.37, 38, 41 It is, however, clear that some of the BH3-only proteins are more potent inducers of apoptosis (e.g., BIM, PUMA, tBID) than others (e.g., BAD, NOXA, BMF). Pertinently, the potent BH3-only proteins bind avidly to all pro-survival BCL-2 family members and can also engage BAX/BAK, whereas the less potent ones have more select binding specificities for the pro-survival BCL-2 family members and reportedly do not bind to BAX or BAK.31, 32
Figure 1.
The BCL-2 family members interact to regulate initiation of apoptosis. In healthy cells, the BCL-2-like pro-survival proteins safeguard mitochondrial outer membrane integrity and cell survival by preventing the activation of BAX and BAK. Under conditions of stress, the BH3-only proteins are activated transcriptionally and/or posttranscriptionally to induce apoptosis by releasing BAX/BAK from inhibition by the BCL-2-like proteins or in the case of certain BH3-only proteins (notably BIM, tBID, PUMA) by activating BAX/BAK through direct binding. Once activated, BAX/BAK cause mitochondrial outer membrane permeabilisation (MOMP) with consequent release of apoptogenic molecules (e.g., cytochrome c, SMAC/DIABLO) that cause activation of the caspase cascade that culminates in cellular demolition
Mechanisms of BH3-only protein activation
Because of their position at the apex of the BCL-2-regulated apoptotic pathway, the BH3-only proteins act as a fulcrum, determining whether the scales tip in favour of cell death or in favour of cell survival.
The mechanisms that lead to activation of the BH3-only proteins vary between members of this subfamily and also according to the apoptotic stimulus.26, 27, 42 Transcriptional activation features prominently; however, emerging evidence also identifies posttranscriptional mechanisms, such as those involving microRNAs (miRNAs), as important in certain contexts.
For example, following DNA damage the tumour-suppressor p53 is posttranslationally activated and then transcriptionally upregulates PUMA and NOXA.43, 44, 45 E2F1 is also able to induce PUMA and NOXA.46 PUMA as well as BIM expression were reported to be induced by the transcription factor FOXO3a in response to cytokine withdrawal.47, 48, 49 However, mutation of all known FOXO transcription factor-binding sites in the Bim gene had no impact on haematopoietic cell homeostasis and apoptosis,50 indicating that this mode of induction may not be critical for BIM activation. In response to ER stress, BIM expression can be transcriptionally induced by CHOP.51
Various posttranslational processes were reported to regulate the stability and thereby control the activity of BH3-only proteins. BIM and BAD were reported to be negatively regulated by phosphorylation.42, 52 Phosphorylation of BAD by AKT was shown to cause its sequestration in the cytosol by 14-3-3 proteins, thereby restraining its pro-apoptotic activity.53 Mice lacking BAD are largely normal, and their cells do not show marked resistance to the apoptotic stimuli tested.54, 55 The role of BAD in programmed and stress-induced cell death is therefore probably relatively subtle and ancillary to the action of more potent BH3-only proteins (e.g., BIM, PUMA). Phosphorylation of BIM by ERK was reported to be critical for the antiapoptotic activity of this kinase.56, 57, 58, 59, 60 However, a recent study has shown that ERK-mediated direct phosphorylation of BIM does not have a major role in the control of the pro-apoptotic activity of this BH3-only protein within the whole animal.61 Both BIM and BMF were shown to be sequestered by binding to elements of the cytoskeleton, thereby restraining their pro-apoptotic activity.62, 63 Interestingly, loss of the transcription factor ASCIZ, with consequent reduction in its target dynein light chain 1, which reportedly links BIM to the dynein motor complex,62 causes abnormal death of B lymphoid cells, and this can be blocked by concomitant loss of BIM.64 This suggests that this mode of BIM regulation has a critical role in normal physiology.
The expression of the BH3-only proteins can be modulated posttranscriptionally through the activity of miRNA. These short (17–25 nucleotides) RNA species bind in a sequence-specific manner to several target mRNA transcripts and inhibit their translation either through translation inhibition or mRNA destabilisation. Although the change in mRNA transcript abundance for any single miRNA target is mostly relatively minor, individual miRNAs are able to exert marked effects by targeting multiple mRNA species-encoding proteins that act within the same signalling pathway.
With respect to the BH3-only proteins, several miRNA have been implicated in the regulation of BIM expression, including the miR-17~92 cluster in mice65, 66 and in human cancer cell lines also (miR-32, miR-17-5p, miR-106-25).67, 68, 69 PUMA was reported to be regulated by miR-483-3p, miR-221 and miR-222.70, 71
As for the pro-survival BCL-2 family members, BCL-2 and MCL-1 appear to be the prominent targets of miRNA-mediated regulation. Both are targeted by miR-29 and miR-153,72, 73, 74 and BCL-2 expression is also regulated by miR-15, miR-16,75 miR-19576 and the p53-inducible miR-34.77 BCL-XL expression appears to be controlled by miR-491.78
Role of the BCL-2-Regulated Apoptotic Pathway in Mouse Models of Tumorigenesis
Experimental models have been utilised to delineate the roles of the various BCL-2 family proteins during tumorigenesis (Table 1). Because of the prominent expression of the pro-survival BCL-2 family members in the haematopoietic system,79 a large proportion of these studies have focussed on the role these proteins have during leukaemia and lymphoma development.
Table 1. The role of the BCL-2 protein family members in tumour development.
| Gene | Role in tumorigenesis | Model | References |
|---|---|---|---|
| Bim (Bcl2l11, Bod) | Tumour suppressor | Eμ-Myc | 89, 90 |
| Puma (Bbc3) | Tumour suppressor | Eμ-Myc | 91 |
| Puma (Bbc3) | Required for tumour initiation | Irradiation-induced lymphoma | 111, 112 |
| Bax | Tumour suppressor | Eμ-Myc | 92 |
| Bmf | Tumour suppressor | Irradiation-induced lymphoma | 110 |
| Noxa (Pmaip1) | Tumour suppressor | Irradiation-induced lymphoma | 111 |
| Bad (Bbc2) | Conflicting reports | Irradiation-induced lymphoma | 54, 55 |
| Bcl2 | Oncogene | Eμ-Myc | 86 |
| Bclx (Bcl2l1) | Oncogene; required for lymphoma initiation | Eμ-Myc | 87, 90, 93, 94 |
| Mcl1 | Oncogene; required for sustained lymphoma growth | Eμ-Myc | 88, 97 |
| Mcl1 | Oncogene; required for sustained lymphoma growth | p53-deficient thymic lymphoma | 108 |
| Mcl1 | Required for sustained lymphoma growth | AML | 113, 114 |
Eμ-Myc lymphoma model
MYC expression is thought to be deregulated in ~70% of human cancers.80 In Burkitt lymphoma (BL), this is due to a chromosomal translocation that subjugates the c-Myc gene to the control of the immunoglobulin heavy (IgH) or light chain gene enhancers. The Eμ-Myc transgenic mice were generated to model this malignancy with expression of the c-Myc proto-oncogene driven by the IgH gene enhancer, Eμ.81 Early in life, these mice contain abnormally increased numbers of large, cycling B-cell progenitors,82 which comprise the nascent neoplastic cells.83 Upon acquisition of oncogenic mutations that cooperate with MYC in neoplastic transformation, clonal malignant pre-B or sIg+ B lymphomas emerge from the pool of preleukaemic B lymphoid cells.83 On a C57BL/6 background, median (50%) survival is ~110 days, and all animals succumb to lymphoma within ~350–400 days. Tumour cells from lymphoma-bearing mice can be readily transplanted into syngeneic (immune-competent) recipients or adapted to grow indefinitely in vitro as cell lines.81, 83 These two features and the ability to identify and study a preneoplastic cell population (see above) made this the most widely used animal model of human cancer (>1400 publications).
Deregulated MYC expression promotes neoplastic transformation by causing aberrant cell proliferation. However, under conditions of stress, such as limited supply of growth factors or nutrients, deregulated MYC expression also increases the predisposition of cells to undergo apoptosis.84, 85 Preleukaemic B lymphoid cells from Eμ-Myc mice are highly prone to undergo apoptosis,85 and apoptosis constitutes a major mechanism to suppress/delay lymphoma development in Eμ-Myc mice. Accordingly, overexpression of pro-survival BCL-2 family members (e.g., BCL-2,86 BCL-XL87 or MCL-188) greatly accelerate lymphoma development in Eμ-Myc mice. Loss of BIM,89, 90 PUMA91 or BAX92 (but curiously not loss of BAK) also accelerate MYC-induced lymphomagenesis, indicating that these pro-apoptotic proteins are major tumour suppressors in this context.
Studies using gene-targeted mice or pharmacological inhibitors revealed that endogenously controlled expression of BCL-XL,90, 93, 94 but not BCL-2,95 is essential for MYC-induced lymphoma development. This may be explained by the fact that BCL-XL, but not BCL-2, is expressed at readily detectable levels in pro-B/pre-B cells, the population of preleukaemic cells from which malignant lymphoma is thought to arise in Eμ-Myc mice. However, lymphomas initiated by combined overexpression of MYC and BCL-2 need high BCL-2 expression for their continued survival.96
Interestingly, although BCL-XL is critical for the development of pre-B/B lymphoma in Eμ-Myc mice, it is dispensable for the sustained survival and expansion of these tumours.97 Instead, MCL-1 is essential, with loss of even a single allele of Mcl-1 abrogating the in vivo growth of malignant Eμ-Myc lymphomas, unless they have acquired a mutation in the tumour-suppressor gene p53.97
p53-deficient mice, a model of Li–Fraumeni syndrome
Mutation or loss of p53 is the most frequent mutation in human cancer and is frequently associated with poor prognosis and chemoresistance.98, 99 Furthermore, a rare genetic disorder, Li–Fraumeni syndrome results from the inheritance of a single mutated copy of the p53 gene.100, 101 These patients are characterised by a high incidence of early onset of various cancers, particularly lymphoma, leukaemia and several forms of sarcoma, which develop following the somatic loss of the remaining wild-type p53 allele in cancer-initiating cells.
The p53+/− heterozygous mice recapitulate the human condition102, 103 and mice completely deficient for p53 rapidly (within 150–280 days) develop thymic lymphoma with 100% penetrance on the C57BL/6 genetic background. These mice have been widely used to examine the importance of p53-mediated tumour suppression and the consequences of its loss during lymphomagenesis. As p53 induces apoptosis through the BH3-only proteins PUMA and (to a lesser extent) NOXA,43, 44, 45 it was a great surprise that mice double deficient for both PUMA and NOXA displayed no propensity to tumour formation.104 Even the combined loss of p53's ability to induce apoptosis, cell cycle arrest and senescence (Puma−/−Noxa−/−p21−/− mice) did not render mice tumour prone.105, 106, 107 The precise mechanisms by which p53 suppresses tumour development therefore remain unclear.
A recent study has delineated the roles of the different pro-survival BCL-2 family members required for lymphoma development and expansion in p53-deficient mice.108 MCL-1 was found to be critical for both the development and sustained growth of lymphoma initiated by p53 deficiency, whereas BCL-XL was dispensable. This study showed that even for p53-deficient tumours therapeutic targeting of MCL-1 may represent an effective treatment strategy.
γ-radiation-induced thymic lymphoma model
Thymic lymphoma can be induced in mice by repeated exposure to low doses of γ-radiation.109 This is thought to facilitate neoplastic transformation through the sequential accumulation of oncogenic mutations in immature haematopoietic progenitors in the bone marrow, ultimately culminating in malignant lymphoma. Following exposure to 1.5 Gy γ-irradiation weekly for 4 weeks, mice typically succumb to thymic lymphoma around 150–200 days. In this experimental model, NOXA and BMF have been shown to suppress lymphoma development,110, 111 while the role of BAD remains contentious with one report describing accelerated thymic lymphomagenesis in BAD-deficient mice while another publication reported no effect.54, 55 Possible explanations for the discrepancy might include differences in γ-radiation dosing and schedule used or differences in genetic background (C57BL/655 versus complicated mixed background54). Loss of BIM alone did not accelerate lymphoma development; however, mice deficient for both BAD and BIM showed accelerated tumour development.55
Remarkably, loss of PUMA completely abrogated γ-radiation-induced thymic lymphoma development.111, 112 This striking finding could be attributed to the profound resistance of PUMA-deficient white blood cells to DNA damage-induced apoptosis. The persistence of these cells obviated the need for mobilisation and burst of proliferation of haematopoietic stem/progenitor cells that would normally occur to repopulate the depleted haematopoietic system,111, 112 a process that appears to be required for lymphoma development in this model.109 These observations suggest that accumulation of DNA lesions, some of them potentially oncogenic, is not sufficient to induce tumour formation unless it is also accompanied by a drive for proliferative expansion of the mutation bearing leukaemia/lymphoma-initiating cells.
Acute myeloid leukaemia (AML) models
AML can be induced experimentally in mice by transducing haematopoietic stem/progenitor cells with expression constructs encoding protein products encoded by recurrent chromosomal translocations found in human AML (e.g., MLL-ENL, AML-ETO9a). Using this system, MCL-1, but not BCL-XL, BCL-2 or BCL-W, was shown to be critical for the sustained survival of AML cells in vitro and in vivo.113, 114
Collectively, these results characterise the process of tumour formation as a sustained effort of nascent neoplastic cells to cope with stress conditions imposed by the oncogenic lesions, genomic instability and potentially cytotoxic signals from their environment. Mutations that sensitise cells to apoptosis, such as loss of pro-survival BCL-2 family members, act to suppress tumour development, whereas loss of pro-apoptotic BCL-2 family members expedite neoplastic transformation.
Role of the BCL-2-Regulated Apoptotic Pathway in Human Cancer
The BCL-2 family of proteins have also been shown to be critical for the development, progression and treatment responses of human cancers (Table 2). Indeed, the BCL-2 gene itself was discovered following its identification as the oncogene activated by the t(14;18) chromosomal translocation in follicular lymphoma.115 The seminal discovery by Vaux et al.116 that enforced BCL-2 expression could protect cells from growth factor deprivation-induced death revealed for the first time that defects in apoptosis could cause cancer.
Table 2. Aberrations in BCL-2 protein family members in human cancer.
| Gene | Expression | Cancer | References |
|---|---|---|---|
| BIM (BCL2l11, BOD) | Genomic loss | Mantle cell lymphoma | 128 |
| BIM (BCL2l11, BOD) | Epigenetic silencing | Burkitt lymphoma | 129 |
| PUMA (BBC3) | Genomic loss | Various | 121 |
| PUMA (BBC3) | Epigenetic silencing | Burkitt lymphoma | 130 |
| BMF | Genomic loss | Advanced lung, breast cancer | 131 |
| BOK | Genomic loss | Various | 121 |
| BAX | Genomic loss | Colon cancer | 127 |
| BCL2 | Overexpressed | Follicular lymphoma, neuroblastoma, CLL | 115, 117, 118, 119, 120 |
| BCL2L1 (BCLX) | Amplified | Lung cancer | 121, 122 |
| MCL1 (BCL2L3) | Amplified | Lung cancer, breast cancer | 121 |
| MCL1 (BCL2L3) | Overexpressed | AML | 114 |
| BFL-1 (BCL2A1) | Overexpressed | B-CLL; various solid cancer | 124 |
Studies using human cancer-derived cell lines and patient samples revealed abnormalities in the expression of several anti- as well as pro-apoptotic BCL-2 family members in a broad range of malignancies. In addition to the critical role of BCL-2 in follicular lymphoma, high levels of BCL-2 expression have also been observed in neuroblastoma and chronic lymphocytic leukaemia (CLL).117, 118, 119, 120 In at least some cases of CLL, this is likely due to loss of miR-15a and miR-16-1, which can repress BCL-2 expression.75 Amplifications of the Mcl-1 or Bcl-x gene loci have been identified as frequently occurring somatically acquired copy number aberrations in lung, breast121, 122 and giant-cell tumours of the bone.123 RNAi-mediated knockdown of MCL-1 or BCL-XL induced killing of some cell lines derived from such cancers, suggesting that these pro-survival BCL-2-like proteins are essential for their sustained survival.121 Furthermore, MCL-1 levels were found to be high in primary AML samples and antagonising MCL-1 activity (using inducible expression of BIM variants that only bind to and inhibit MCL-1) impaired the in vitro survival of primary human AML cells.113, 114 BFL-1, the human homologue of A1, was shown to be overexpressed and associated with chemo-resistance in various cancers, including B-CLL.124, 125, 126
Pro-apoptotic BCL-2 family members were also found to be deregulated in human cancers. The genomic regions harbouring PUMA and BOK commonly show somatically acquired loss of copy number in various cancer types.121 Loss of BAX function appears likely to have a role in colon cancer development, with frame-shift mutations in the BAX gene detected in ~50% of colon cancers of the microsatellite mutator phenotype.127 Combined loss of BAX and BAK was observed in a small number of AML samples from heavily pretreated patients; treatment may have selected for tumour cells with loss of both of these multi-BH domain pro-apoptotic BCL-2 family members.113 Homozygous loss of the Bim gene is seen in ~15–20% of mantle cell lymphomas.128 Moreover, in BL, the BIM and PUMA genes were found to be silenced by epigenetic alterations, such as hyper-methylation.129, 130 Furthermore, the region harbouring the BMF gene is lost in late stage lung and breast cancer.131
These observations demonstrate that abnormalities in anti- as well as pro-apoptotic BCL-2 family members can contribute to the development of cancer in humans.
The Role of the BCL-2-Regulated Apoptotic Pathway in Cancer Therapy
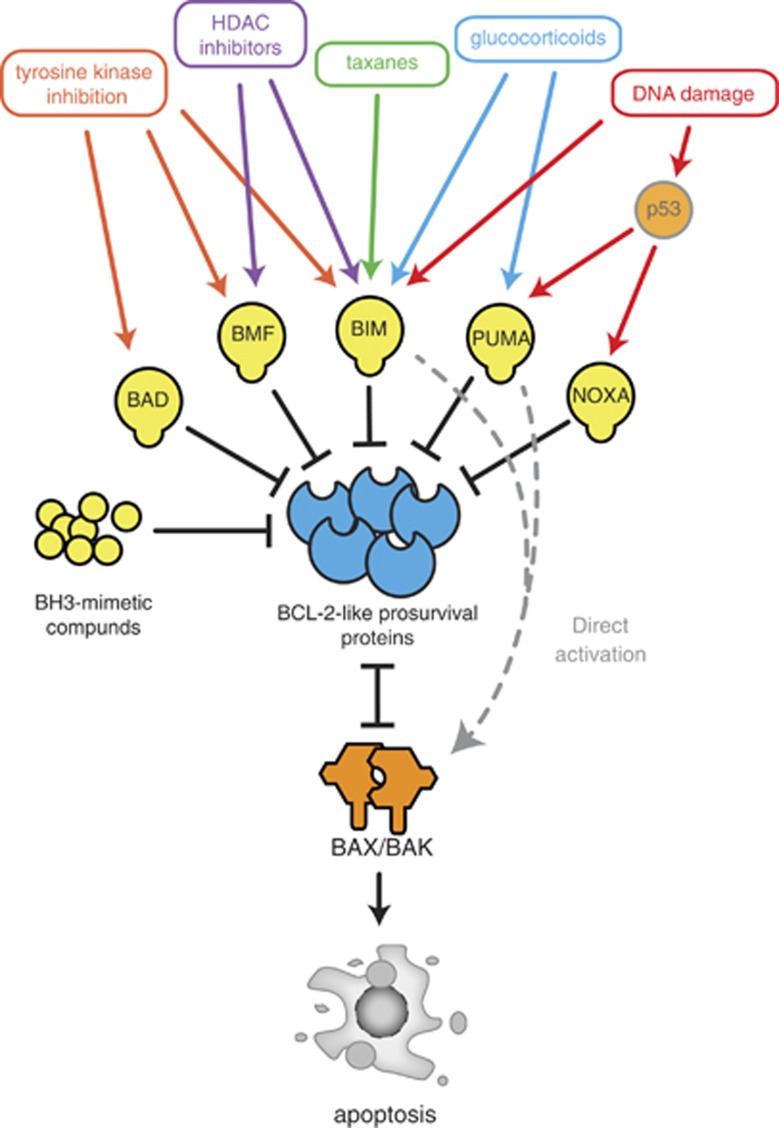
Because of their role as mediators of apoptosis triggered by diverse cell stresses, the BCL-2 protein family members are key determinants of the response of tumour cells to a broad range of commonly used anticancer therapeutics (Figure 2). Accordingly, direct activation of the BCL-2-regulated apoptotic pathway using small-molecule mimetics of the pro-apoptotic BH3-only proteins is being developed as a novel strategy for cancer therapy.
Figure 2.
Many anticancer agents mediate tumour cell killing though activation of the BCL-2-regulated apoptotic pathway. BH3-only proteins are activated transcriptionally and/or posttranscriptionally in a cytotoxic stimulus-specific manner by many anticancer agents, often with 2–3 members cooperating to induce apoptosis. BH3-mimetic compounds bind directly to and block the BCL-2 pro-survival proteins and thereby elicit apoptosis even in cells lacking upstream activators of BH3-only proteins, such as the tumour-suppressor p53, which is critical for transcriptional induction of Puma and Noxa
The role of the BCL-2-regulated apoptotic pathway in the response to anticancer therapeutics was first demonstrated when it was found that non-transformed lymphoid cells from Bcl-2 transgenic mice132 and BCL-2-overexpressing lymphoma cells133 were profoundly resistant to γ-irradiation, DNA damage-inducing chemotherapeutic drugs (e.g., etoposide) and glucocorticoids (e.g., dexamethasone). Similar protection from chemotherapeutic drug-induced apoptosis can be afforded by overexpression of any of the other pro-survival BCL-2 family members.134, 135, 136, 137
Studies using gene-targeted mice or RNAi-mediated gene knockdown in cell lines revealed which pro-apoptotic BCL-2 family members are critical for cell killing by which anticancer agent. Consistent with the notion that BAX and BAK have essential overlapping functions in the BCL-2-regulated apoptotic pathway, cells from Bax−/−Bak−/− mice are markedly resistant to diverse anticancer agents.138, 139, 140 Notably, different anticancer agents require different BH3-only proteins for cell killing. PUMA is critical for therapeutic responses to γ-irradiation as well as to DNA-damaging drugs, with contributions from NOXA and also BIM (which does not appear to be a direct transcriptional target of p53) in at least certain non-transformed and malignant cell types.141, 142, 143, 144, 145 PUMA and BIM together account for most of the pro-apoptotic activity of glucocorticoids.141, 143, 146, 147, 148, 149 BIM is also critical for taxane-induced cell killing.150, 151 Furthermore, BMF as well as BIM are critical for the killing of non-transformed lymphoid cells as well as certain lymphoma cells by inhibitors of histone deacetylases.110, 135 BIM (with BAD and BMF also contributing) is critical for the killing of tumour cells that are dependent on oncogenic kinases by therapeutic agents that block their activity, such as inhibitors of MEK (acting downstream of mutant B-RAF in melanoma or colon carcinoma),152 EGFR (lung cancer),153, 154, 155 BCR-ABL (CML)156, 157 and VEGFR signalling (tumour angiogenesis).158 Notably, a gene polymorphism that impairs the expression of BIM was found to explain the de novo resistance of BCR-ABL-driven CML to Gleevec and mutant EGFR-driven lung cancer to Iressa/Tarceva in East Asian populations.159
Anticancer drug-induced killing of tumour cells requires activation of BH3-only proteins by upstream signalling mediators, such as p53 or the glucocorticoid receptor. These upstream signal activators are frequently mutated, lost or silenced (e.g., due to epigenetic modifications) during tumour development or subsequently during emergence of therapy-resistant cancer cells.14, 27, 160 To bypass such resistance mechanisms, a new class of therapeutics, known as ‘BH3-mimetics', that directly activate apoptosis have been developed (Figure 2). BH3-mimetics bind and inhibit the pro-survival BCL-2 family members and thereby activate apoptosis in cancer cells.16 ABT-737 and its clinical analogue ABT-263 (navitoclax) exemplify this new therapeutic class, with the latter compound currently in phase 2 clinical trials.161, 162 Both compounds bind to BCL-2, BCL-XL and BCL-W (but not to MCL-1 or A1) displacing the endogenous BH3-only proteins, which can then bind to MCL-1, A1 and some of them also to BAX/BAK. This causes killing of tumour cells via a BAX/BAK-dependent mechanism.162, 163, 164 However, as ABT-737 and ABT-263 bind only a subset of the BCL-2-like pro-survival proteins, overexpression of MCL1 or A1/BFL-1, both of which are not inhibited by these compounds, has the potential to confer resistance to therapy.164 Some cancers (such as CLL) with very high expression of BCL-2 (and possibly also cancers expressing high levels of BCL-XL and/or BCL-W) respond robustly to the existing BH3-mimetics when used as single agents. However, in order to maximise treatment efficacy, in many cancers these BH3-mimetics are probably best employed in combination with drugs known to activate BH3-only proteins, such as BIM or PUMA, that can potently neutralise MCL-1 and/or A1.
Indeed BH3-mimetics have been found to potently synergise in vitro with various chemotherapeutic drugs in the killing of CLL165 and many other cancer cells, including mouse xenograft models of human breast cancer.152, 153, 157, 166, 167 Such combinatorial therapeutic strategies would effectively neutralise all pro-survival BCL-2 family proteins and thereby efficiently activate apoptosis in malignant cells.
BH3-mimetics also affect non-transformed cells; for example, ABT-737 and ABT-263 cause thrombocytopaenia because platelets rely on BCL-XL for their survival.168, 169 This problem can be circumvented in the context of BCL-2-dependent tumours by using ABT-199/venetoclax, a BH3-mimetic that only inhibits BCL-2 and is showing great promise for the treatment of CLL.170 Moreover, to prevent unacceptable collateral damage to normal tissues, BH3-mimetics may best be used in combination therapies with drugs that only affect cancer cells, such as inhibitors of oncogenic kinases (e.g., Gleevec to inhibit BCR-ABL in CML, Vemurafenib to inhibit mutant BRAF in melanoma), rather than using them with cytotoxic drugs that cause DNA damage in both malignant as well as non-transformed cells.160
Concluding Remarks
Changes in expression and activity of members of the BCL-2 family (and their upstream regulators) can exert profound effects of cell survival and this is of particular relevance to the development of cancer and its clinical treatment. Mechanisms delineated using mouse models mimicking human malignancies have greatly advanced our understanding of how apoptosis suppresses tumour formation. Importantly, these findings have been mirrored by insights from clinical studies and this knowledge can now be harnessed to develop improved treatment strategies for patients. So far, the most exciting outcome from these advances has been the development of the BH3-mimetic drugs that directly activate apoptosis in cancer cells by binding and inhibiting select pro-survival BCL-2 family members. The first of such compounds, ABT-263/navitoclax and ABT-199/venetoclax, are currently generating much excitement as they progress through clinical trials and will hopefully prove efficacious for treatment of a wide variety of both haematological and solid cancers.
Acknowledgments
We thank Dr. JM Adams, Dr. S Cory, Dr. P Bouillet, Dr. M Herold, Dr. D Gray, Dr. LA O'Reilly, Dr. S Grabow, Dr. G Kelly, Dr. A Janic, Dr. S Alvarez-Diaz, Dr. F Ke, Dr. B Aubrey, Dr. L Valente, Dr. C Vandenberg, Dr. A Kueh, J Low, L Rohrbeck, R Schenk, M Brennan, R Salvamoser and B Yang for insightful discussions. This work was supported by grants and fellowships from the Cancer Council of Victoria (ARDD, Sydney Parker Smith Postdoctoral Research Fellowship), the National Health and Medical Research Council (Program Grant no.1016701; NHMRC SPRF Fellowship 1020363 to AS) and the Leukemia and Lymphoma Society (SCOR Grant no. 7001-13), Australian Postgraduate Award (to ARDD) and Cancer Therapeutics CRC Top-up Scholarship (to ARDD). This work was made possible by operational infrastructure grants through the Australian Government IRISS and the Victorian State Government OIS.
Glossary
- BCL-2
B cell lymphoma gene 2
- BH
BCL-2 homology region
- BAX
BCL-2-associated protein X
- BAK
BCL-2-antagonist/killer
- MCL-1
myeloid cell leukaemia gene 1
- A1
BCL-2-related protein A1
- BIM
BCL-2 interacting mediator of cell death
- PUMA
p53 upregulated modulator of apoptosis
- BID
BH3 interacting-domain death agonist
- BAD
BCL-2-associated death promoter
- BIK
BCL-2-interacting killer
- HRK
Harakiri
- BMF
BCL-2-modifying factor
- tBID
truncated BID
- MOMP
mitochondrial outer membrane permeabilisation
- FOXO
forkhead box protein O
- ER
endoplasmic reticulum
- CHOP
C/EBP homologous protein
- AKT
v-akt murine thymoma viral oncogene homolog
- ASCIZ
ATM/ATR-substrate CHK2-interacting zinc finger protein
- DLC1
dynein light chain 1
- miRNA
microRNA
- MYC
v-myc avian myelocytomatosis viral oncogene homolog
- IgH
immunoglobulin heavy chain
- IgL
immunoglobulin light chain
- AML
acute myeloid leukaemia
- CLL
chronic lymphocytic leukaemia
- BOK
BCL-2 related ovarian killer
- BL
Burkitt lymphoma
- RNAi
RNA interference
- HDACi
histone deacetylase inhibitors
- MEK
MAPK/ERK kinase
- EGFR
epidermal growth factor receptor
- CML
chronic myeloid leukaemia
- VEGFR
vascular endothelial growth factor receptor
- BCR-ABL
break point cluster region – Abelson kinase fusion protein
ARD Delbridge and A Strasser are employed by The Walter and Eliza Hall Institute. The Walter and Eliza Hall Institute receives milestone payments from Genentech and AbbVie for the development of BH3-mimetics for cancer therapy.
Footnotes
Edited by G Melino
References
- 1Strasser A, O'Connor L, Dixit VM. Apoptosis signaling. Ann Rev Biochem 2000; 69: 217–245. [DOI] [PubMed] [Google Scholar]
- 2Delbridge AR, Valente LJ, Strasser A. The role of the apoptotic machinery in tumor suppression. Cold Spring Harb Perspect Biol 2012; 4: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674. [DOI] [PubMed] [Google Scholar]
- 4Hotchkiss RS, Strasser A, McDunn JE, Swanson PE. Cell death. New Engl J Med 2009; 361: 1570–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell 2014; 157: 1013–1022. [DOI] [PubMed] [Google Scholar]
- 6Linkermann A, Green DR. Necroptosis. N Engl J Med 2014; 370: 455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7Kerr JFR, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Brit J Cancer 1972; 26: 239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8Farbman AI. Electron microscope study of palate fusion in mouse embryos. Dev Biol 1968; 18: 93–116. [DOI] [PubMed] [Google Scholar]
- 9Klion FM, Schaffner F. The ultrastructure of acidophilic "Councilman-like" bodies in the liver. Am J Pathol 1966; 48: 755–767. [PMC free article] [PubMed] [Google Scholar]
- 10Kerr JF. An electron-microscope study of liver cell necrosis due to heliotrine. J Pathol 1969; 97: 557–562. [DOI] [PubMed] [Google Scholar]
- 11Hengartner MO, Horvitz HR. C. elegans cell survival gene ced-9 encodes a functional homolog of the mammalian proto-oncogene bcl-2. Cell 1994; 76: 665–676. [DOI] [PubMed] [Google Scholar]
- 12Vaux DL, Weissman IL, Kim SK. Prevention of programmed cell death in Caenorhabditis elegans by human bcl-2. Science 1992; 258: 1955–1957. [DOI] [PubMed] [Google Scholar]
- 13Lee EF, Clarke OB, Evangelista M, Feng Z, Speed TP, Tchoubrieva EB et al. Discovery and molecular characterization of a Bcl-2-regulated cell death pathway in schistosomes. Proc Natl Acad Sci USA 2011; 108: 6999–7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Strasser A, Cory S, Adams JM. Deciphering the rules of programmed cell death to improve therapy of cancer and other diseases. EMBO J 2011; 30: 3667–3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 2008; 9: 47–59. [DOI] [PubMed] [Google Scholar]
- 16Lessene G, Czabotar PE, Colman PM. BCL-2 family antagonists for cancer therapy. Nat Rev Drug Discov 2008; 7: 989–1000. [DOI] [PubMed] [Google Scholar]
- 17Davids MS, Letai A. Targeting the B-cell lymphoma/leukemia 2 family in cancer. J Clin Oncol 2012; 30: 3127–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18Moldoveanu T, Follis AV, Kriwacki RW, Green DR. Many players in BCL-2 family affairs. Trends Biochem Sci 2014; 39: 101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol 2014; 15: 49–63. [DOI] [PubMed] [Google Scholar]
- 20Suzuki M, Youle RJ, Tjandra N. Structure of Bax: coregulation of dimer formation and intracellular localization. Cell 2000; 103: 645–654. [DOI] [PubMed] [Google Scholar]
- 21Czabotar PE, Westphal D, Dewson G, Ma S, Hockings C, Fairlie WD et al. Bax crystal structures reveal how BH3 domains activate Bax and nucleate its oligomerization to induce apoptosis. Cell 2013; 152: 519–531. [DOI] [PubMed] [Google Scholar]
- 22Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI et al. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Gene Dev 2005; 19: 1294–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science 2007; 315: 856–859. [DOI] [PubMed] [Google Scholar]
- 24Ke F, Voss A, Kerr JB, O'Reilly LA, Tai L, Echeverry N et al. BCL-2 family member BOK is widely expressed but its loss has only minimal impact in mice. Cell Death Differ 2012; 19: 915–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25Ke F, Bouillet P, Kaufmann T, Strasser A, Kerr J, Voss AK. Consequences of the combined loss of BOK and BAK or BOK and BAX. Cell Death Dis 2013; 4: e650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26Huang DCS, Strasser A. BH3-only proteins – essential initiators of apoptotic cell death. Cell 2000; 103: 839–842. [DOI] [PubMed] [Google Scholar]
- 27Happo L, Strasser A, Cory S. BH3-only proteins in apoptosis at a glance. J Cell Sci 2012; 125: 1081–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28Hinds MG, Smits C, Fredericks-Short R, Risk JM, Bailey M, Huang DC et al. Bim, Bad and Bmf: intrinsically unstructured BH3-only proteins that undergo a localized conformational change upon binding to prosurvival Bcl-2 targets. Cell Death Differ 2007; 14: 128–136. [DOI] [PubMed] [Google Scholar]
- 29Chou JJ, Li H, Salvesen GS, Yuan J, Wagner G. Solution structure of BID, an intracellular amplifier of apoptotic signaling. Cell 1999; 96: 615–624. [DOI] [PubMed] [Google Scholar]
- 30McDonnell JM, Fushman D, Milliman CL, Korsmeyer SJ, Cowburn D. Solution structure of the proapoptotic molecule BID: a structural basis for apoptotic agonists and antagonists. Cell 1999; 96: 625–634. [DOI] [PubMed] [Google Scholar]
- 31Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG et al. Differential targeting of pro-survival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell 2005; 17: 393–403. [DOI] [PubMed] [Google Scholar]
- 32Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR et al. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell 2005; 17: 525–535. [DOI] [PubMed] [Google Scholar]
- 33Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature 2003; 426: 671–676. [DOI] [PubMed] [Google Scholar]
- 34Leshchiner ES, Braun CR, Bird GH, Walensky LD. Direct activation of full-length proapoptotic BAK. Proc Natl Acad Sci USA 2013; 110: E986–E995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35Gavathiotis E, Reyna DE, Bellairs JA, Leshchiner ES, Walensky LD. Direct and selective small-molecule activation of proapoptotic BAX. Nat Chem Biol 2012; 8: 639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36Gavathiotis E, Reyna DE, Davis ML, Bird GH, Walensky LD. BH3-triggered structural reorganization drives the activation of proapoptotic BAX. Mol Cell 2010; 40: 481–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37Gavathiotis E, Suzuki M, Davis ML, Pitter K, Bird GH, Katz SG et al. BAX activation is initiated at a novel interaction site. Nature 2008; 455: 1076–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38Merino D, Giam M, Hughes PD, Siggs OM, Heger K, O'Reilly LA et al. The role of BH3-only protein Bim extends beyond inhibiting Bcl-2-like prosurvival proteins. J Cell Biol 2009; 186: 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39Wei MC, Lindsten T, Mootha VK, Weiler S, Gross A, Ashiya M et al. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev 2000; 14: 2060–2071. [PMC free article] [PubMed] [Google Scholar]
- 40Chipuk JE, Green DR. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol 2008; 18: 157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41Llambi F, Moldoveanu T, Tait SW, Bouchier-Hayes L, Temirov J, McCormick LL et al. A unified model of mammalian BCL-2 protein family interactions at the mitochondria. Mol Cell 2011; 44: 517–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42Puthalakath H, Strasser A. Keeping killers on a tight leash: transcriptional and post-translational control of the pro-apoptotic activity of BH3-only proteins. Cell Death Differ 2002; 9: 505–512. [DOI] [PubMed] [Google Scholar]
- 43Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell 2001; 7: 683–694. [DOI] [PubMed] [Google Scholar]
- 44Yu J, Zhang L, Hwang PM, Kinzler KW, Vogelstein B. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol Cell 2001; 7: 673–682. [DOI] [PubMed] [Google Scholar]
- 45Oda E, Ohki R, Murasawa H, Nemoto J, Shibue T, Yamashita T et al. Noxa, a BH3-only member of the bcl-2 family and candidate mediator of p53-induced apoptosis. Science 2000; 288: 1053–1058. [DOI] [PubMed] [Google Scholar]
- 46Hershko T, Ginsberg D. Up-regulation of Bcl-2 homology 3 (BH3)-only proteins by E2F1 mediates apoptosis. J Biol Chem 2004; 279: 8627–8634. [DOI] [PubMed] [Google Scholar]
- 47Dijkers PF, Medema RH, Lammers JJ, Koenderman L, Coffer PJ. Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Curr Biol 2000; 10: 1201–1204. [DOI] [PubMed] [Google Scholar]
- 48Ekoff M, Kaufmann T, Engstrom M, Motoyama N, Villunger A, Jonsson JI et al. The BH3-only protein Puma plays an essential role in cytokine deprivation-induced apoptosis of mast cells. Blood 2007; 110: 3209–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49You H, Pellegrini M, Tsuchihara K, Yamamoto K, Hacker G, Erlacher M et al. FOXO3a-dependent regulation of Puma in response to cytokine/growth factor withdrawal. J Exp Med 2006; 203: 1657–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50Herold MJ, Rohrbeck L, Lang MJ, Grumont R, Gerondakis S, Tai L et al. Foxo-mediated Bim transcription is dispensable for the apoptosis of hematopoietic cells that is mediated by this BH3-only protein. EMBO Rep 2013; 14: 992–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51Puthalakath H, O'Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND et al. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell 2007; 129: 1337–1349. [DOI] [PubMed] [Google Scholar]
- 52Balmanno K, Cook SJ. Tumour cell survival signalling by the ERK1/2 pathway. Cell Death Differ 2009; 16: 368–377. [DOI] [PubMed] [Google Scholar]
- 53del Peso L, González-Garcia M, Page C, Herrera R, Nuñez G. Interleukin-3–induced phosphorylation of BAD through the protein kinase Akt. Science 1997; 278: 687–689. [DOI] [PubMed] [Google Scholar]
- 54Ranger AM, Zha J, Harada H, Datta SR, Danial NN, Gilmore AP et al. Bad-deficient mice develop diffuse large B cell lymphoma. Proc Natl Acad Sci USA 2003; 100: 9324–9329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55Kelly PN, White MJ, Goschnick MW, Fairfax KA, Tarlinton DM, Kinkel SA et al. Individual and overlapping roles of BH3-only proteins Bim and Bad in apoptosis of lymphocytes and platelets and in suppression of thymic lymphoma development. Cell Death Differ 2010; 17: 1655–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56Ley R, Hadfield K, Howes E, Cook SJ. Identification of a DEF-type docking domain for extracellular signal-regulated kinases 1/2 that directs phosphorylation and turnover of the BH3-only protein BimEL. J Biol Chem 2005; 280: 17657–17663. [DOI] [PubMed] [Google Scholar]
- 57Clybouw C, McHichi B, Mouhamad S, Auffredou MT, Bourgeade MF, Sharma S et al. EBV infection of human B lymphocytes leads to down-regulation of Bim expression: relationship to resistance to apoptosis. J Immunol 2005; 175: 2968–2973. [DOI] [PubMed] [Google Scholar]
- 58Luciano F, Jacquel A, Colosetti P, Herrant M, Cagnol S, Pages G et al. Phosphorylation of Bim-EL by Erk1/2 on serine 69 promotes its degradation via the proteasome pathway and regulates its proapoptotic function. Oncogene 2003; 22: 6785–6793. [DOI] [PubMed] [Google Scholar]
- 59Ley R, Balmanno K, Hadfield K, Weston C, Cook SJ. Activation of the ERK1/2 signaling pathway promotes phosphorylation and proteasome-dependent degradation of the BH3-only protein, Bim. J Biol Chem 2003; 278: 18811–18816. [DOI] [PubMed] [Google Scholar]
- 60Akiyama T, Bouillet P, Miyazaki T, Kadono Y, Chikuda H, Chung UI et al. Regulation of osteoclast apoptosis by ubiquitylation of proapoptotic BH3-only Bcl-2 family member Bim. EMBO J 2003; 22: 6653–6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61Clybouw C, Merino D, Nebl T, Masson F, Robati M, O'Reilly L et al. Alternative splicing of Bim and Erk-mediated Bim(EL) phosphorylation are dispensable for hematopoietic homeostasis in vivo. Cell Death Differ 2012; 19: 1060–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62Puthalakath H, Huang DCS, O'Reilly LA, King SM, Strasser A. The pro-apoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol Cell 1999; 3: 287–296. [DOI] [PubMed] [Google Scholar]
- 63Puthalakath H, Villunger A, O'Reilly LA, Beaumont JG, Coultas L, Cheney RE et al. Bmf: a pro-apoptotic BH3-only protein regulated by interaction with the myosin V actin motor complex, activated by anoikis. Science 2001; 293: 1829–1832. [DOI] [PubMed] [Google Scholar]
- 64Jurado S, Gleeson K, O'Donnell K, Izon DJ, Walkley CR, Strasser A et al. The Zinc-finger protein ASCIZ regulates B cell development via DYNLL1 and Bim. J Exp Med 2012; 209: 1629–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65Ventura A, Young AG, Winslow MM, Lintault L, Meissner A, Erkeland SJ et al. Targeted deletion reveals essential and overlapping functions of the mIR-17~92 family of miRNA clusters. Cell 2008; 132: 875–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66Xiao C, Srinivasan L, Calado DP, Patterson HC, Zhang B, Wang J et al. Lymphoproliferative disease and autoimmunity in mice with increased miR-17-92 expression in lymphocytes. Nat Immunol 2008; 9: 404–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67Ambs S, Prueitt RL, Yi M, Hudson RS, Howe TM, Petrocca F et al. Genomic profiling of microRNA and messenger RNA reveals deregulated microRNA expression in prostate cancer. Cancer Res 2008; 68: 6162–6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68Fontana L, Fiori ME, Albini S, Cifaldi L, Giovinazzi S, Forloni M et al. Antagomir-17-5p abolishes the growth of therapy-resistant neuroblastoma through p21 and BIM. PLoS One 2008; 3: e2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69Kan T, Sato F, Ito T, Matsumura N, David S, Cheng Y et al. The miR-106b-25 polycistron, activated by genomic amplification, functions as an oncogene by suppressing p21 and Bim. Gastroenterology 2009; 136: 1689–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70Veronese A, Lupini L, Consiglio J, Visone R, Ferracin M, Fornari F et al. Oncogenic role of miR-483-3p at the IGF2/483 locus. Cancer Res 2010; 70: 3140–3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71Zhang CZ, Zhang JX, Zhang AL, Shi ZD, Han L, Jia ZF et al. MiR-221 and miR-222 target PUMA to induce cell survival in glioblastoma. Mol Cancer 2010; 9: 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72Xiong Y, Fang JH, Yun JP, Yang J, Zhang Y, Jia WH et al. Effects of microRNA-29 on apoptosis, tumorigenicity, and prognosis of hepatocellular carcinoma. Hepatology 2010; 51: 836–845. [DOI] [PubMed] [Google Scholar]
- 73Mott JL, Kobayashi S, Bronk SF, Gores GJ. mir-29 regulates Mcl-1 protein expression and apoptosis. Oncogene 2007; 26: 6133–6140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74Xu J, Liao X, Wong C. Downregulations of B-cell lymphoma 2 and myeloid cell leukemia sequence 1 by microRNA 153 induce apoptosis in a glioblastoma cell line DBTRG-05MG. Int J Cancer 2010; 126: 1029–1035. [DOI] [PubMed] [Google Scholar]
- 75Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci USA 2005; 102: 13944–13949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76Liu L, Chen L, Xu Y, Li R, Du X. microRNA-195 promotes apoptosis and suppresses tumorigenicity of human colorectal cancer cells. Biochem Biophys Res Commun 2010; 400: 236–240. [DOI] [PubMed] [Google Scholar]
- 77Bommer GT, Gerin I, Feng Y, Kaczorowski AJ, Kuick R, Love RE et al. p53-mediated activation of miRNA34 candidate tumor-suppressor genes. Curr Biol 2007; 17: 1298–1307. [DOI] [PubMed] [Google Scholar]
- 78Nakano H, Miyazawa T, Kinoshita K, Yamada Y, Yoshida T. Functional screening identifies a microRNA, miR-491 that induces apoptosis by targeting Bcl-X(L) in colorectal cancer cells. Int J Cancer 2010; 127: 1072–1080. [DOI] [PubMed] [Google Scholar]
- 79Strasser A. The role of BH3-only proteins in the immune system. Nat Rev Immunol 2005; 5: 189–200. [DOI] [PubMed] [Google Scholar]
- 80Soucek L, Evan GI. The ups and downs of Myc biology. Curr Opin Genet Dev 2010; 20: 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81Adams JM, Harris AW, Pinkert CA, Corcoran LM, Alexander WS, Cory S et al. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature 1985; 318: 533–538. [DOI] [PubMed] [Google Scholar]
- 82Langdon WY, Harris AW, Cory S, Adams JM. The c-myc oncogene perturbs B lymphocyte development in Eμ-myc transgenic mice. Cell 1986; 47: 11–18. [DOI] [PubMed] [Google Scholar]
- 83Harris AW, Pinkert CA, Crawford M, Langdon WY, Brinster RL, Adams JM. The Eμ-myc transgenic mouse: a model for high-incidence spontaneous lymphoma and leukemia of early B cells. J Exp Med 1988; 167: 353–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84Evan GI, Wyllie AH, Gilbert CS, Littlewood TD, Land H, Brooks M et al. Induction of apoptosis in fibroblasts by c-myc protein. Cell 1992; 69: 119–128. [DOI] [PubMed] [Google Scholar]
- 85Strasser A, Elefanty AG, Harris AW, Cory S. Progenitor tumours from Em-bcl-2-myc transgenic mice have lymphomyeloid differentiation potential and reveal developmental differences in cell survival. EMBO J 1996; 15: 3823–3834. [PMC free article] [PubMed] [Google Scholar]
- 86Strasser A, Harris AW, Bath ML, Cory S. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature 1990; 348: 331–333. [DOI] [PubMed] [Google Scholar]
- 87Swanson PJ, Kuslak SL, Fang W, Tze L, Gaffney P, Selby S et al. Fatal acute lymphoblastic leukemia in mice transgenic for B cell-restricted bcl-xL and c-myc. J Immunol 2004; 172: 6684–6691. [DOI] [PubMed] [Google Scholar]
- 88Campbell KJ, Bath ML, Turner ML, Vandenberg CJ, Bouillet P, Metcalf D et al. Elevated Mcl-1 perturbs lymphopoiesis, promotes transformation of hematopoietic stem/progenitor cells, and enhances drug resistance. Blood 2010; 116: 3197–3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89Egle A, Harris AW, Bouillet P, Cory S. Bim is a suppressor of Myc-induced mouse B cell leukemia. Proc Natl Acad Sci USA 2004; 101: 6164–6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90Delbridge AR, Grabow S, Bouillet P, Adams JM, Strasser A. Functional antagonism between pro-apoptotic BIM and anti-apoptotic BCL-XL in MYC-induced lymphomagenesis. Oncogene 2014; 34: 1872–1876. [DOI] [PubMed] [Google Scholar]
- 91Michalak EM, Jansen ES, Happo L, Cragg MS, Tai L, Smyth GK et al. Puma and to a lesser extent Noxa are suppressors of Myc-induced lymphomagenesis. Cell Death Differ 2009; 16: 684–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92Eischen CM, Roussel MF, Korsmeyer SJ, Cleveland JL. Bax loss impairs Myc-induced apoptosis and circumvents the selection of p53 mutations during Myc-mediated lymphomagenesis. Mol Cell Biol 2001; 21: 7653–7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93Kelly PN, Grabow S, Delbridge ARD, Strasser A, Adams JM. Endogenous Bcl-xL is essential for Myc-driven lymphomagenesis in mice. Blood 2011; 118: 6380–6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94Kelly PN, Grabow S, Delbridge AR, Adams JM, Strasser A. Prophylactic treatment with the BH3 mimetic ABT-737 impedes Myc-driven lymphomagenesis in mice. Cell Death Differ 2013; 20: 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95Kelly PN, Puthalakath H, Adams JM, Strasser A. Endogenous bcl-2 is not required for the development of Eμ-myc-induced B-cell lymphoma. Blood 2007; 109: 4907–4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96Letai A, Sorcinelli MD, Beard C, Korsmeyer SJ. Antiapoptotic BCL-2 is required for maintenance of a model leukemia. Cancer Cell 2004; 6: 241–249. [DOI] [PubMed] [Google Scholar]
- 97Kelly GL, Grabow S, Glaser SP, Fitzsimmons L, Aubrey BJ, Okamoto T et al. Targeting of MCL-1 kills MYC-driven mouse and human lymphomas even when they bear mutations in p53. Genes Dev 2014; 28: 58–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science 1991; 253: 49–53. [DOI] [PubMed] [Google Scholar]
- 99Nigro JM, Baker SJ, Preisinger AC, Jessup JM, Hostetter R, Cleary K et al. Mutations in the p53 gene occur in diverse human tumour types. Nature 1989; 342: 705–708. [DOI] [PubMed] [Google Scholar]
- 100Malkin D, Li FP, Strong LC, Fraumeni JFJ, Nelson CE, Kim DH et al. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science 1990; 250: 1233–1238. [DOI] [PubMed] [Google Scholar]
- 101Srivastava S, Zou ZQ, Pirollo K, Plattner W, Chang EH. Germ-line transmission of a mutated p53 gene in a cancer-prone family with Li-Fraumeni syndrome. Nature 1990; 348: 747–749. [DOI] [PubMed] [Google Scholar]
- 102Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CAJ, Butel JS et al. Mice deficient for p53 are developmentally normal but are susceptible to spontaneous tumours. Nature 1992; 356: 215–221. [DOI] [PubMed] [Google Scholar]
- 103Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT et al. Tumor spectrum analysis in p53-mutant mice. Curr Biol 1994; 4: 1–7. [DOI] [PubMed] [Google Scholar]
- 104Michalak EM, Villunger A, Adams JM, Strasser A. In several cell types the tumour suppressor p53 induces apoptosis largely via Puma but Noxa can contribute. Cell Death Differ 2008; 15: 1019–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105Li T, Kon N, Jiang L, Tan M, Ludwig T, Zhao Y et al. Tumor suppression in the absence of p53-mediated cell-cycle arrest, apoptosis, and senescence. Cell 2012; 149: 1269–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106Brady CA, Jiang D, Mello SS, Johnson TM, Jarvis LA, Kozak MM et al. Distinct p53 transcriptional programs dictate acute DNA-damage responses and tumor suppression. Cell 2011; 145: 571–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107Valente LJ, Gray DH, Michalak EM, Pinon-Hofbauer J, Egle A, Scott CL et al. p53 efficiently suppresses tumor development in the complete absence of its cell-cycle inhibitory and proapoptotic effectors p21, Puma, and Noxa. Cell reports 2013; 3: 1339–1345. [DOI] [PubMed] [Google Scholar]
- 108Grabow S, Delbridge AR, Valente LJ, Strasser A. MCL-1 but not BCL-XL is critical for the development and sustained expansion of thymic lymphoma in p53-deficient mice. Blood 2014; 124: 3939–3946. [DOI] [PubMed] [Google Scholar]
- 109Kaplan HS. The role of radiation on experimental leukemogenesis. Natl Cancer Inst Monogr 1964; 14: 207–220. [PubMed] [Google Scholar]
- 110Labi V, Erlacher M, Kiessling S, Manzl C, Frenzel A, O'Reilly L et al. Loss of the BH3-only protein Bmf impairs B cell homeostasis and accelerates gamma irradiation-induced thymic lymphoma development. J Exp Med 2008; 205: 641–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111Michalak EM, Vandenberg CJ, Delbridge ARD, Wu L, Scott CL, Adams JM et al. Apoptosis-promoted tumorigenesis: gamma-irradiation-induced thymic lymphomagenesis requires Puma-driven leukocyte death. Genes Dev 2010; 24: 1608–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112Labi V, Erlacher M, Krumschnabel G, Manzl C, Tzankov A, Pinon J et al. Apoptosis of leukocytes triggered by acute DNA damage promotes lymphoma formation. Genes Dev 2010; 24: 1602–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113Glaser S, Lee EF, Trounson E, Bouillet P, Wei A, Fairlie WD et al. Anti-apoptotic Mcl-1 is essential for the development and sustained growth of acute myeloid leukemia. Genes Dev 2012; 26: 120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114Xiang Z, Luo H, Payton JE, Cain J, Ley TJ, Opferman JT et al. Mcl1 haploinsufficiency protects mice from Myc-induced acute myeloid leukemia. J Clin Invest 2010; 120: 2109–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115Tsujimoto Y, Yunis J, Onorato-Showe L, Erikson J, Nowell PC, Croce CM. Molecular cloning of the chromosomal breakpoint of B-cell lymphomas and leukemias with the t(11;14) chromosome translocation. Science 1984; 224: 1403–1406. [DOI] [PubMed] [Google Scholar]
- 116Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature 1988; 335: 440–442. [DOI] [PubMed] [Google Scholar]
- 117Castle VP, Heidelberger KP, Bromberg J, Ou X, Dole M, Nuñez G. Expression of the apoptosis-suppressing protein bcl-2 in neuroblastoma is associated with unfavorable histology and N-myc amplification. Am J Pathol 1993; 143: 1543–1550. [PMC free article] [PubMed] [Google Scholar]
- 118Pepper C, Hoy T, Bentley P. Elevated Bcl-2/Bax are a consistent feature of apoptosis resistance in B-cell chronic lymphocytic leukaemia and are correlated with in vivo chemoresistance. Leuk Lymphoma 1998; 28: 355–361. [DOI] [PubMed] [Google Scholar]
- 119Pepper C, Hoy T, Bentley DP. Bcl-2/Bax ratios in chronic lymphocytic leukaemia and their correlation with in vitro apoptosis and clinical resistance. Br J Cancer 1997; 76: 935–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120Pepper C, Bentley P, Hoy T. Regulation of clinical chemoresistance by bcl-2 and bax oncoproteins in B-cell chronic lymphocytic leukaemia. Br J Haematol 1996; 95: 513–517. [DOI] [PubMed] [Google Scholar]
- 121Beroukhim R, Mermel C, Porter D, Wei G, Raychaudhuri S, Donovan J et al. The landscape of somatic copy-number alteration across human cancers. Nature 2010; 463: 899–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122Tonon G, Wong KK, Maulik G, Brennan C, Feng B, Zhang Y et al. High-resolution genomic profiles of human lung cancer. Proc Natl Acad Sci USA 2005; 102: 9625–9630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123Smith LT, Mayerson J, Nowak NJ, Suster D, Mohammed N, Long S et al. 20q11.1 amplification in giant-cell tumor of bone: array CGH, FISH, and association with outcome. Genes Chromosomes Cancer 2006; 45: 957–966. [DOI] [PubMed] [Google Scholar]
- 124Park IC, Lee SH, Whang DY, Hong WS, Choi SS, Shin HS et al. Expression of a novel Bcl-2 related gene, Bfl-1, in various human cancers and cancer cell lines. Anticancer Res 1997; 17: 4619–4622. [PubMed] [Google Scholar]
- 125Morales AA, Olsson A, Celsing F, Osterborg A, Jondal M, Osorio LM. High expression of bfl-1 contributes to the apoptosis resistant phenotype in B-cell chronic lymphocytic leukemia. Int J Cancer 2005; 113: 730–737. [DOI] [PubMed] [Google Scholar]
- 126Olsson A, Norberg M, Okvist A, Derkow K, Choudhury A, Tobin G et al. Upregulation of bfl-1 is a potential mechanism of chemoresistance in B-cell chronic lymphocytic leukaemia. Br J Cancer 2007; 97: 769–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127Rampino N, Yamamoto H, Ionov Y, Li Y, Sawai H, Reed JC et al. Somatic frameshift mutations in the bax gene in colon cancers of the microsatellite mutator phenotype. Science 1997; 275: 967–969. [DOI] [PubMed] [Google Scholar]
- 128Tagawa H, Karnan S, Suzuki R, Matsuo K, Zhang X, Ota A et al. Genome-wide array-based CGH for mantle cell lymphoma: identification of homozygous deletions of the proapoptotic gene BIM. Oncogene 2005; 24: 1348–1358. [DOI] [PubMed] [Google Scholar]
- 129Richter-Larrea JA, Robles EF, Fresquet V, Beltran E, Rullan AJ, Agirre X et al. Reversion of epigenetically mediated BIM silencing overcomes chemoresistance in Burkitt lymphoma. Blood 2010; 116: 2531–2542. [DOI] [PubMed] [Google Scholar]
- 130Garrison SP, Jeffers JR, Yang C, Nilsson JA, Hall MA, Rehg JE et al. Selection against PUMA gene expression in Myc-driven B-cell lymphomagenesis. Mol Cell Biol 2008; 28: 5391–5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131Wick W, Petersen I, Schmutzler RK, Wolfarth B, Lenartz D, Bierhoff E et al. Evidence for a novel tumor suppressor gene on chromosome 15 associated with progression to a metastatic stage in breast cancer. Oncogene 1996; 12: 973–978. [PubMed] [Google Scholar]
- 132Strasser A, Harris AW, Cory S. Bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell 1991; 67: 889–899. [DOI] [PubMed] [Google Scholar]
- 133Strasser A, Harris AW, Jacks T, Cory S. DNA damage can induce apoptosis in proliferating lymphoid cells via p53-independent mechanisms inhibitable by Bcl-2. Cell 1994; 79: 329–339. [DOI] [PubMed] [Google Scholar]
- 134Huang DCS, Cory S, Strasser A. Bcl-2, Bcl-XL and adenovirus protein E1B19kD are functionally equivalent in their ability to inhibit cell death. Oncogene 1997; 14: 405–414. [DOI] [PubMed] [Google Scholar]
- 135Lindemann RK, Newbold A, Whitecross KF, Cluse LA, Frew AJ, Ellis L et al. Analysis of the apoptotic and therapeutic activities of histone deacetylase inhibitors by using a mouse model of B cell lymphoma. Proc Natl Acad Sci USA 2007; 104: 8071–8076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136Frew AJ, Lindemann RK, Martin BP, Clarke CJ, Sharkey J, Anthony DA et al. Combination therapy of established cancer using a histone deacetylase inhibitor and a TRAIL receptor agonist. Proc Natl Acad Sci USA 2008; 105: 11317–11322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137Newbold A, Lindemann RK, Cluse LA, Whitecross KF, Dear AE, Johnstone RW. Characterisation of the novel apoptotic and therapeutic activities of the histone deacetylase inhibitor romidepsin. Mol Cancer Ther 2008; 7: 1066–1079. [DOI] [PubMed] [Google Scholar]
- 138Lindsten T, Ross AJ, King A, Zong W, Rathmell JC, Shiels HA et al. The combined functions of proapoptotic Bcl-2 family members Bak and Bax are essential for normal development of multiple tissues. Mol Cell 2000; 6: 1389–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139Rathmell JC, Lindsten T, Zong W-X, Cinalli RM, Thompson CB. Deficiency in Bak and Bax perturbs thymic selection and lymphoid homeostasis. Nat Immunol 2002; 3: 932–939. [DOI] [PubMed] [Google Scholar]
- 140Mason KD, Lin A, Robb L, Josefsson EC, Henley KJ, Gray DH et al. Proapoptotic Bak and Bax guard against fatal systemic and organ-specific autoimmune disease. Proc Natl Acad Sci USA 2013; 110: 2599–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141Villunger A, Michalak EM, Coultas L, Mullauer F, Bock G, Ausserlechner MJ et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science 2003; 302: 1036–1038. [DOI] [PubMed] [Google Scholar]
- 142Jeffers JR, Parganas E, Lee Y, Yang C, Wang J, Brennan J et al. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell 2003; 4: 321–328. [DOI] [PubMed] [Google Scholar]
- 143Erlacher M, Michalak EM, Kelly PN, Labi V, Niederegger H, Coultas L et al. BH3-only proteins Puma and Bim are rate-limiting for {gamma} -radiation and glucocorticoid-induced apoptosis of lymphoid cells in vivo. Blood 2005; 106: 4131–4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144Naik E, Michalak EM, Villunger A, Adams JM, Strasser A. UV-radiation triggers apoptosis of fibroblasts and skin keratinocytes mainly via the BH3-only protein Noxa. J Cell Biol 2007; 176: 415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145Happo L, Cragg MS, Phipson B, Haga JM, Jansen ES, Herold MJ et al. Maximal killing of lymphoma cells by DNA-damage inducing therapy requires not only the p53 targets Puma and Noxa but also Bim. Blood 2010; 116: 5256–5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146Erlacher M, Laabi V, Manzl C, Bock G, Tzankov A, Haecker G et al. Puma cooperates with Bim, the rate-limiting BH3-only protein in cell death during lymphocyte development, in apoptosis induction. J Exp Med 2006; 203: 2939–2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147Ploner C, Rainer J, Niederegger H, Eduardoff M, Villunger A, Geley S et al. The BCL2 rheostat in glucocorticoid-induced apoptosis of acute lymphoblastic leukemia. Leukemia 2008; 22: 370–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148Molitoris JK, McColl KS, Distelhorst CW. Glucocorticoid-mediated repression of the oncogenic microRNA cluster miR-17~92 contributes to the induction of Bim and initiation of apoptosis. Mol Endocrinol 2011; 25: 409–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149Abrams MT, Robertson NM, Yoon K, Wickstrom E. Inhibition of glucocorticoid-induced apoptosis by targeting the major splice variants of BIM mRNA with small interfering RNA and short hairpin RNA. J Biol Chem 2004; 279: 55809–55817. [DOI] [PubMed] [Google Scholar]
- 150Bouillet P, Metcalf D, Huang DCS, Tarlinton DM, Kay TWH, Köntgen F et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science 1999; 286: 1735–1738. [DOI] [PubMed] [Google Scholar]
- 151Sunters A, Fernandez de Mattos S, Stahl M, Brosens JJ, Zoumpoulidou G, Saunders CA et al. FoxO3a transcriptional regulation of Bim controls apoptosis in paclitaxel-treated breast cancer cell lines. J Biol Chem 2003; 278: 49795–49805. [DOI] [PubMed] [Google Scholar]
- 152Cragg MS, Jansen ES, Cook M, Strasser A, Scott CL. Treatment of B-RAF mutant human tumor cells with a MEK inhibitor requires Bim and is enhanced by a BH3 mimetic. J Clin Invest 2008; 118: 3651–3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153Cragg MS, Kuroda J, Puthalakath H, Huang DCS, Strasser A. Gefitinib-induced killing of NSCLC cell lines expressing mutant EGFR requires Bim and can be enhanced by BH3 mimetics. PLoS Med 2007; 4: 1681–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154Costa DB, Halmos B, Kumar A, Schumer ST, Huberman MS, Boggon TJ et al. BIM mediates EGFR tyrosine kinase inhibitor-induced apoptosis in lung cancers with oncogenic EGFR mutations. PLoS Med 2007; 4: e315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155Gong Y, Somwar R, Politi K, Balak M, Chmielecki J, Jiang X et al. Induction of BIM is essential for apoptosis triggered by EGFR kinase inhibitors in mutant EGFR-dependent lung adenocarcinomas. PLoS Med 2007; 4: e294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156Kuribara R, Honda H, Matsui H, Shinjyo T, Inukai T, Sugita K et al. Roles of Bim in apoptosis of normal and Bcr-Abl-expressing hematopoietic progenitor. Mol Cell Biol 2004; 24: 6172–6183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157Kuroda J, Puthalakath H, Cragg MS, Kelly PN, Bouillet P, Huang DC et al. Bim and Bad mediate imatinib-induced killing of Bcr/Abl+ leukemic cells, and resistance due to their loss is overcome by a BH3 mimetic. Proc Natl Acad Sci USA 2006; 103: 14907–14912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158Naik E, O'Reilly LA, Asselin-Labat ML, Merino D, Lin A, Cook M et al. Destruction of tumor vasculature and abated tumor growth upon VEGF blockade is driven by proapoptotic protein Bim in endothelial cells. J Exp Med 2011; 208: 1351–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159Ng KP, Hillmer AM, Chuah CT, Juan WC, Ko TK, Teo AS et al. A common BIM deletion polymorphism mediates intrinsic resistance and inferior responses to tyrosine kinase inhibitors in cancer. Nat Med 2012; 18: 521–528. [DOI] [PubMed] [Google Scholar]
- 160Cragg MS, Harris C, Strasser A, Scott CL. Unleashing the power of inhibitors of oncogenic kinases through BH3 mimetics. Nat Rev Cancer 2009; 9: 321–326. [DOI] [PubMed] [Google Scholar]
- 161Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 2005; 435: 677–681. [DOI] [PubMed] [Google Scholar]
- 162Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res 2008; 68: 3421–3428. [DOI] [PubMed] [Google Scholar]
- 163van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell 2006; 10: 389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164Merino D, Khaw SL, Glaser SP, Anderson DJ, Belmont LD, Wong C et al. Bcl-2, Bcl-x(L), and Bcl-w are not equivalent targets of ABT-737 and navitoclax (ABT-263) in lymphoid and leukemic cells. Blood 2012; 119: 5807–5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165Mason KD, Khaw SL, Rayeroux KC, Chew E, Lee EF, Fairlie DW et al. The BH3 mimetic compound, ABT-737, synergizes with a range of cytotoxic chemotherapy agents in chronic lymphocytic leukemia. Leukemia 2009; 23: 2034–2041. [DOI] [PubMed] [Google Scholar]
- 166Oakes SR, Vaillant F, Lim E, Lee L, Breslin K, Feleppa F et al. Breast Cancer Special Feature: sensitization of BCL-2-expressing breast tumors to chemotherapy by the BH3 mimetic ABT-737. Proc Natl Acad Sci USA 2012; 109: 2766–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167Mason KD, Vandenberg CJ, Scott CL, Wei AH, Cory S, Huang DC et al. In vivo efficacy of the Bcl-2 antagonist ABT-737 against aggressive Myc-driven lymphomas. Proc Natl Acad Sci USA 2008; 105: 17961–17966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168Mason KD, Carpinelli MR, Fletcher JI, Collinge JE, Hilton AA, Ellis S et al. Programmed anuclear cell death delimits platelet life span. Cell 2007; 128: 1173–1186. [DOI] [PubMed] [Google Scholar]
- 169Zhang H, Nimmer PM, Tahir SK, Chen J, Fryer RM, Hahn KR et al. Bcl-2 family proteins are essential for platelet survival. Cell Death Differ 2007; 14: 943–951. [DOI] [PubMed] [Google Scholar]
- 170Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med 2013; 19: 202–208. [DOI] [PubMed] [Google Scholar]