Supplemental Digital Content is available in the text.
Keywords: electroencephalography; heart arrest; hypothermia, induced; prognostication
Background—
Modern treatments have improved the survival rate following cardiac arrest, but prognostication remains a challenge. We examined the prognostic value of continuous electroencephalography according to time by performing amplitude-integrated electroencephalography on patients with cardiac arrest receiving therapeutic hypothermia.
Methods and Results—
We prospectively studied 130 comatose patients treated with hypothermia from September 2010 to April 2013. We evaluated the time to normal trace (TTNT) as a neurological outcome predictor and determined the prognostic value of burst suppression and status epilepticus, with a particular focus on their time of occurrence. Fifty-five patients exhibited a cerebral performance category score of 1 to 2. The area under the curve for TTNT was 0.97 (95% confidence interval, 0.92–0.99), and the sensitivity and specificity of TTNT<24 hours after resuscitation as a threshold for predicting good neurological outcome were 94.6% (95% confidence interval, 84.9%–98.9%) and 90.7% (95% confidence interval, 81.7%–96.2%), respectively. The threshold displaying 100% specificity for predicting poor neurological outcome was TTNT>36 hours. Burst suppression and status epilepticus predicted poor neurological outcome (positive predictive value of 98.3% and 96.4%, respectively). The combination of these factors predicted a negative outcome at a median of 6.2 hours after resuscitation (sensitivity and specificity of 92.0% and 96.4%, respectively).
Conclusions—
A TTNT<24 hours was associated with good neurological outcome. The lack of normal trace development within 36 hours, status epilepticus, and burst suppression were predictors of poor outcome. The combination of these negative predictors may improve their prognostic performance at an earlier stage.
Since therapeutic hypothermia (TH) was shown to effectively improve the neurological outcome of comatose cardiac arrest survivors,1,2 TH has become the standard of care for a subset of these patients.3 However, the range of neurological outcomes remains wide, and prognostication has become more complex.4 Currently, neurological outcome prediction in these patients has primarily focused on end-of-life decisions, such as the withdrawal of life-sustaining therapies (LSTs),5 and such prognostication should be delayed beyond the previously recommended 72 hours after cardiac arrest.6 However, early positive prognostication during the first few hours after the return of spontaneous circulation (ROSC) is important for treating physicians when counseling families and making appropriate treatment decisions, although not when deciding whether to withhold or withdraw LSTs because of a perceived poor neurological prognosis. The importance of the timing of the neurological assessment for prognostication is related to the earliest time at which the brain structures can recover function to enable reliable clinical assessment.7 Therefore, a good predictor should be based on a test that continuously reflects the status of the brain.
Editorial see p 1073
Clinical Perspective on p 1103
Following transient cerebral ischemia, a complex series of pathophysiological events associated with neuronal recovery can be observed via continuous electroencephalographic (EEG; cEEG) monitoring over time.8,9 The evolution of EEG patterns may provide clinically relevant information regarding the recovery from postanoxic coma. In 2010, the American Heart Association published guidelines for the care of these survivors and recommended that EEG should be performed, promptly interpreted, and monitored frequently or continuously in comatose patients after ROSC.3 cEEG is a noninvasive technique that can be used to monitor the postischemic brain after cardiac arrest. However, early cEEG monitoring in these patients has remained challenging because it requires serial surveillance by experienced specialists, who are often unavailable or expensive. Amplitude-integrated electroencephalography (aEEG) provides a simplified and, therefore, more readily available brain function monitoring tool for perinatal hypoxic-ischemic encephalopathy in neonates and cardiac arrest in adults.10–14 In neonates, the time required after birth for the aEEG to recover to a normal background pattern was the best predictor of poor neurological outcome, and all infants who did not recover a normal background pattern by 36 to 48 hours either died or survived with severe disability.10,11 Regarding aEEG in adult patients undergoing TH, a continuous pattern at registration and at normothermia was associated with good neurological outcome, and burst suppression (BS) or status epilepticus (SE) during the normothermia period indicated poor neurological outcome.14
The present study aimed to assess whether the time from ROSC to a normal trace (TTNT), as measured via continuous aEEG monitoring, represents a neurological outcome predictor for TH-treated adult patients with cardiac arrest. The second aim was to determine the association between malignant aEEG patterns and poor neurological outcome, with a particular focus on the time of the occurrence of these patterns.
Methods
Study Design and Patients
This was a prospective observational study of TH-treated adult patients with cardiac arrest at a single tertiary hospital from September 2010 to April 2013. During this period, all unconscious adult (age >19 years) patients receiving successful resuscitation were considered eligible for TH, and all TH-treated patients were monitored via aEEG. This study included consecutive patients with TH, but the patients were excluded if (1) they died within 72 hours after cardiac arrest, (2) their cardiac arrest occurred as a result of spontaneous or traumatic brain injury, or (3) they had a known history of neurological diseases, such as epilepsy.
This study was approved by the Institutional Review Board of Seoul St. Mary’s Hospital. Informed consent from each patient’s next of kin was obtained; subsequently, if the patient recovered consciousness, consent was reobtained from the patient.
Therapeutic Hypothermia Protocol
All patients who were resuscitated were considered eligible for TH at 33°C for 24 hours according to the current recommendations.3 Before the induction of TH, sedation (midazolam, 0.08 mg/kg intravenously) and paralysis (rocuronium, 0.8 mg/kg intravenously) for shivering control were immediately administered, followed by continuous infusion of midazolam (0.04–0.2 mg·kg–1·h–1) and rocuronium (0.3–0.6 mg·kg–1·h–1). The target temperature of 33°C was maintained for 24 hours. After the completion of the TH maintenance period, controlled rewarming at a rate of 0.25°C/h was performed until the patient’s temperature reached 36.5°C. Sedation and paralysis were reduced during rewarming and were discontinued as soon as the central temperature reached 35°C.
aEEG Monitoring and Analysis
As performed in our previous study,13 all patients were monitored via aEEG using a combined single-channel aEEG/EEG digital device (Olympic Medical CFM 6000, Natus, Inc, Seattle, WA) as soon as possible by attending emergency physicians in the emergency department; subdermal needle electrodes were applied across the forehead to record EEG channels Fp3-Fp4. Recording continued until the patient regained consciousness, the patient died, or at least 72 hours had passed since ROSC. Clinically concerning or seizurelike activity on the aEEG or raw EEG scan resulted in the treatment of the patient according to the local protocol, and cEEG was initiated instead of aEEG if there were no limitations related to technician support for EEG. The patients experiencing SE were initially treated with boluses of valproic acid, levetiracetam, and clonazepam, followed by maintenance dosing. Pentobarbital was administered to refractory SE cases.
After clinical interpretation during treatment, all aEEG/EEG recordings were reinterpreted by an experienced neurologist (Y.M.S.) who was blinded to the neurological outcome and the clinical data. The aEEG background patterns were classified into the following categories by using the voltage method11–13 (Figure 1): continuous normal voltage (CNV), discontinuous normal voltage, low voltage, flat trace, BS, and SE. CNV was defined as continuous cortical activity on the raw EEG scan; in addition, the upper margin of the aEEG scan, referred to as the aEEG maximum, was >10 μV, and the lower margin of the aEEG scan, referred to as the aEEG minimum, was >5 μV. Discontinuous normal voltage was defined as cortical activity, with the exception of discontinuous intermittent periods displaying a low amplitude on the EEG scan with an aEEG maximum >10 μV and an aEEG minimum ≤5 μV. The low-voltage pattern was defined as an aEEG maximum ≤10 μV, and flat trace was defined as isoelectric activity. We defined BS as the virtual absence of activity (<2 μV) between bursts of high voltage (>25 μV). SE was defined as repetitive epileptiform discharges with amplitudes >50 μV and a median frequency ≥1 Hz for >30 minutes, producing an aEEG trace exhibiting a sawtoothlike appearance with continuously narrowing bandwidths and increasing peak-to-peak amplitudes or with an abrupt elevation in the aEEG levels from the continuous background pattern. According to our definition, periodic epileptiform discharges were classified as SE. The aEEG background patterns from the beginning to the end of monitoring were analyzed according to their time of occurrence. To evaluate TTNT as a predictor of good neurological outcome for TH-treated adult patients with cardiac arrest, we considered only CNV as a normal trace.11–13
Figure 1.
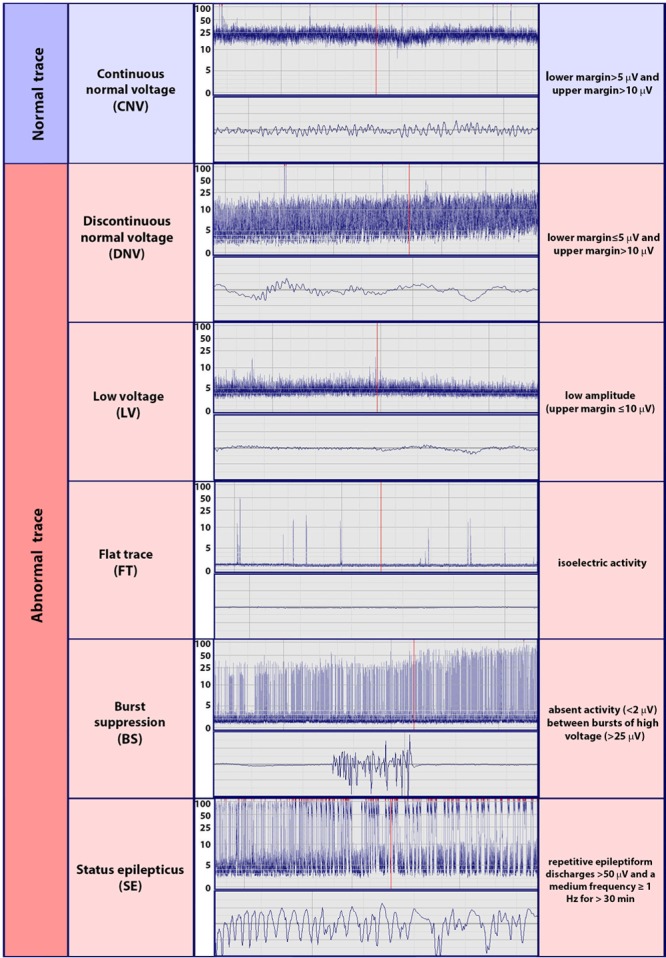
Classification of amplitude-integrated electroencephalograms by the use of the voltage method.
Neurological Outcomes
In all patients, the prognosis after cardiac arrest treated with TH was determined based on a combination of predictors of poor neurological outcome. However, in no patient was care withdrawn based on the results of these predictors before hospital discharge; the treatment team provided sufficiently prolonged life support to patients who did not recover consciousness after rewarming. Neurological outcome at 6 months after resuscitation was evaluated by the authors (K.N.P., S.H.K., and S.H.O.) via a telephone interview.15 The neurological outcome measure was the score on the 5-point Glasgow-Pittsburgh Cerebral Performance Category (CPC) scale at 6 months after ROSC. Neurological outcome was dichotomized as good or poor. Good neurological outcome was defined as a CPC score of 1 or 2, and poor neurological outcome was defined as a CPC score of 3, 4, or 5. If the patients who presented as CPC 1 or 2 ultimately died of rearrest within 6 months, we used the highest CPC score for classification.
Statistical Analysis
The categorical variables were expressed as the numbers and percentages, and the continuous variables were expressed as the means and standard deviations or the medians and the 25th (Q1) and 75th (Q3) quartiles according to a normal distribution. Univariate comparisons of neurological outcome were performed by using χ2 tests for categorical variables or using t tests for continuous variables as required. The performance of the neurological outcome predictors was evaluated based on their sensitivity, specificity, positive predictive value (PPV), and negative predictive value using an exact binomial 95% confidence interval (CI). To evaluate the prognostic value of the TTNT, receiver operating characteristic analysis was performed; we determined the best TTNT threshold for the prediction of good neurological outcome, and 100% was used as the threshold of specificity for poor neurological outcome. The 95% CI was calculated for the area under the curve. Evolution-specific aEEG patterns and their time points were analyzed to evaluate the prognostic value of these factors for poor neurological outcome. All statistical analyses were performed using SPSS version 16.0 (SPSS, Chicago, IL) and the Medcalc program (Medcalc Software, Mariakerke, Belgium). All reported P values are 2-sided. A P value <0.05 was considered to be significant.
Results
Characteristics of the Study Population
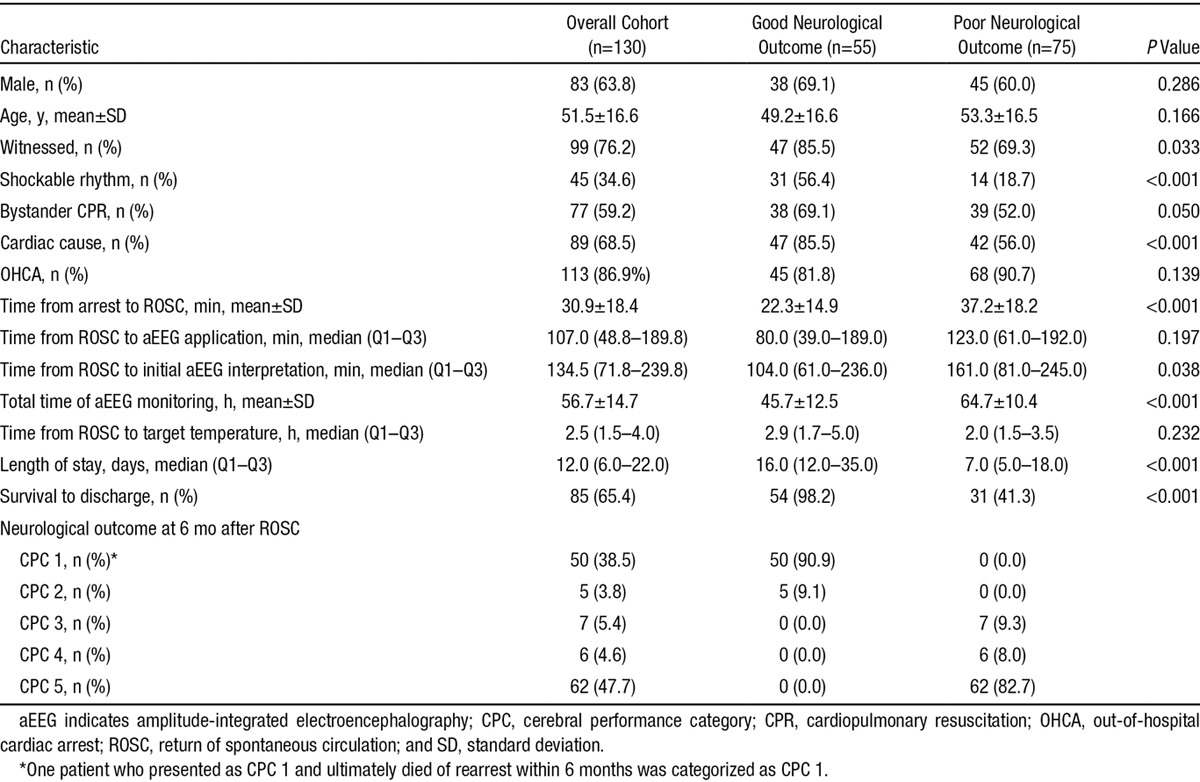
During the study period, 166 TH-treated adult patients with cardiac arrest were monitored via aEEG; 36 patients were excluded from this study because of death within 72 hours after ROSC. Ultimately, 130 patients were included in this study; a portion of this study cohort (55 patients) overlapped with that of our previous study.13 Of the included patients, 83 (63.8%) were male, and the mean patient age was 51.5±16.6 years. A majority of the patients (86.9%) experienced an out-of-hospital cardiac arrest; 45 patients (34.6%) exhibited an initial shockable rhythm, and the mean time from cardiac arrest to ROSC was 30.9±18.4 minutes. The median interval from ROSC to the initial aEEG reading was 134.5 minutes (Q1–Q3, 71.8–239.8 minutes). At 6 months after ROSC, 55 (42.3%) patients exhibited a good neurological outcome, and 77 patients (57.7%) exhibited a poor neurological outcome. The baseline characteristics of the included patients and the comparison between those exhibiting good and poor neurological outcome are shown in Table 1. Significant differences in the presence of a witness, the initial rhythm, the cardiac arrest etiology, and the time from arrest to ROSC were observed between the good and poor neurological outcome groups.
Table 1.
Baseline Demographic and Clinical Characteristics of the Overall Cohort and Comparison Between the Good and Poor Neurological Outcome Groups
EEG Evolution in Both Neurological Outcome Groups
Figure 2 presents the EEG evolution in all patients over time between the good and poor neurological outcome groups. In most patients (98, 75.4%), the background pattern changed during monitoring. In only 32 patients, the initial background pattern persisted without any evolution, and among these patients, most (24, 75.0%) initially exhibited a CNV trace and had a good neurological outcome. Eight patients who initially exhibited a low-voltage pattern ultimately had a poor neurological outcome without any evolution. A CNV trace was initially observed in 25 of the 130 patients (19.2%), most of whom exhibited a good neurological outcome. The initial observation of a CNV trace resulted in a PPV of 96.0% (sensitivity and specificity of 43.6% and 98.7%, respectively). One patient initially exhibiting a CNV trace developed circulatory shock without recovering consciousness and ultimately died.
Figure 2.
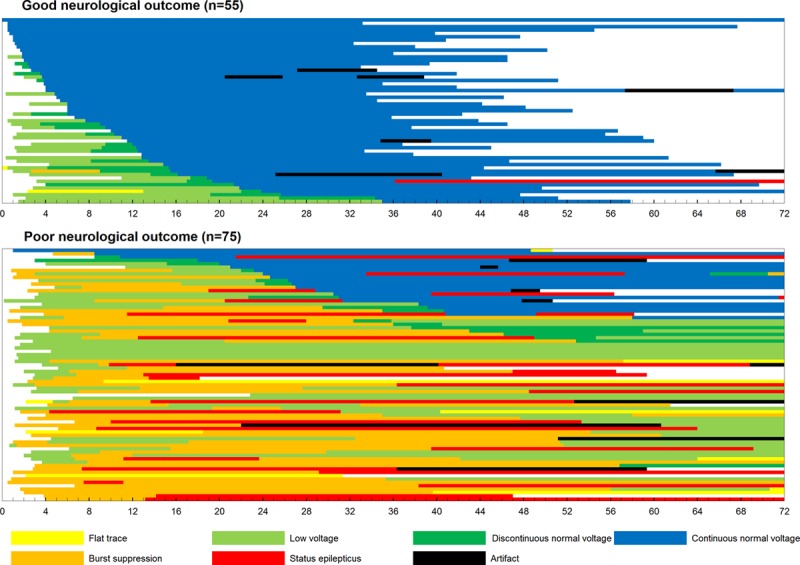
Occurrence of background evolution and status epilepticus in all subjects The x axis indicates the time point after resuscitation (hours).
Of the 105 patients not initially exhibiting a CNV trace, 51 patients exhibited a CNV trace within 72 hours. Among these patients, 31 patients exhibited a good neurological outcome, and 20 patients exhibited a poor neurological outcome. A difference in TTNT was observed between the good and poor neurological outcome groups (Figure 3). All patients experiencing a good neurological outcome developed a CNV trace within 36 hours.
Figure 3.
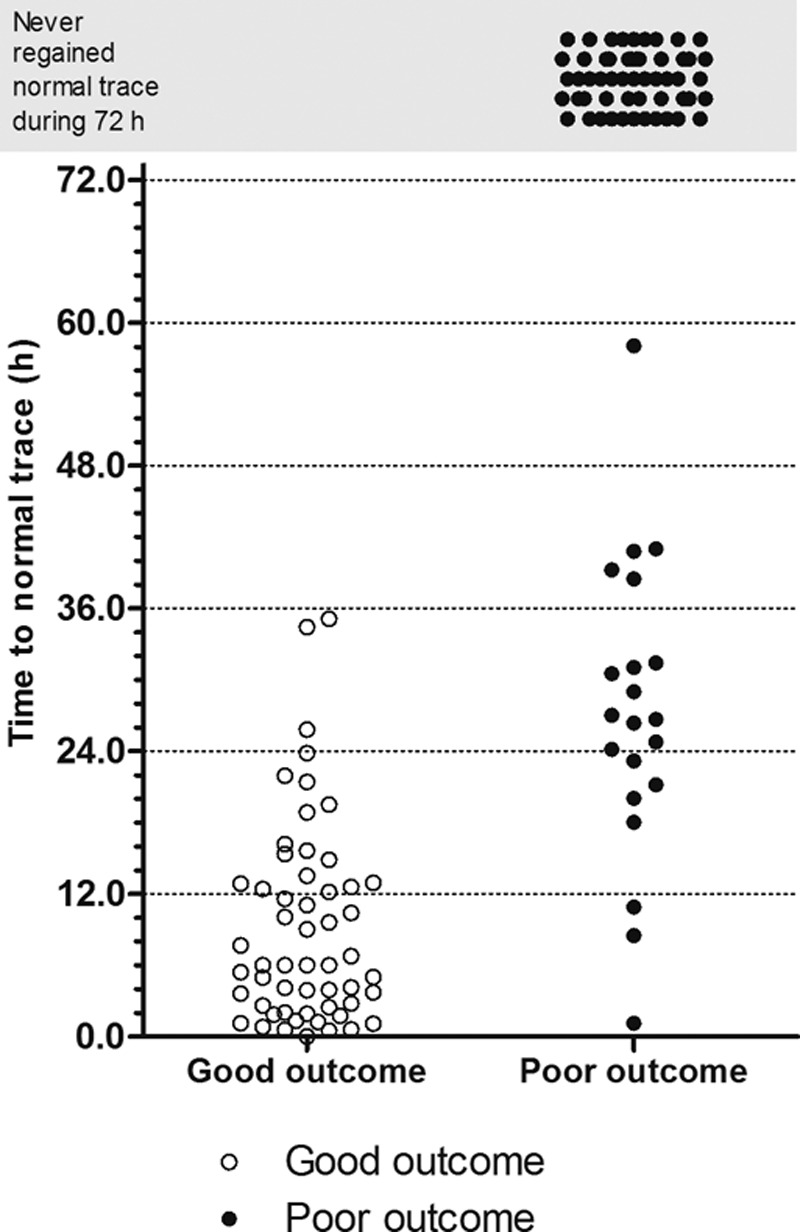
Time to normal trace for the patients in both groups.
TTNT as a Neurological Outcome Predictor
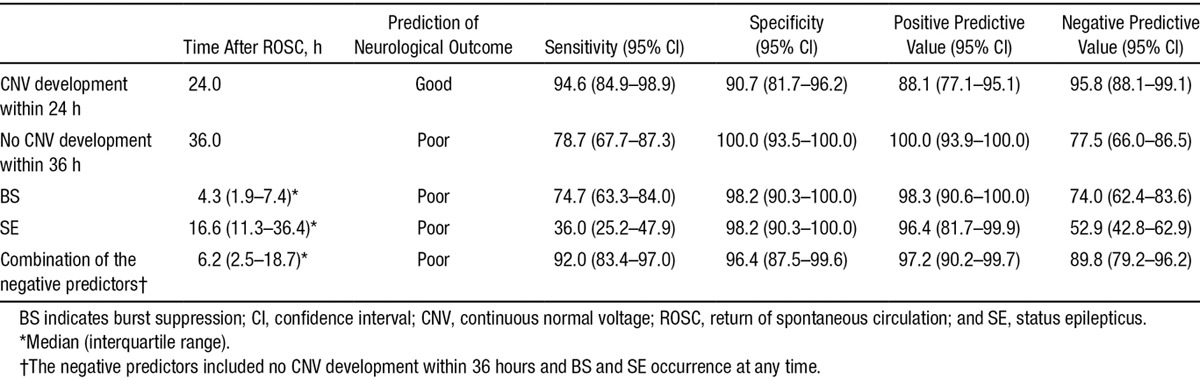
A short TTNT predicted good neurological outcome. Receiver operating characteristic analysis revealed that the diagnostic performance of the TTNT for neurological outcome was good, displaying an area under the curve of 0.97 (95% CI, 0.92–0.99; Figure 4). The achievement of TTNT within specific time windows in both neurological outcome groups is shown in Table 2. Using TTNT <24 hours after ROSC as the threshold, the sensitivity, the specificity, the PPV, and the negative predictive value for predicting good neurological outcome were 94.6% (95% CI, 84.9%–98.9%), 90.7% (95% CI, 81.7%–96.2%), 88.1% (95% CI, 77.1%–95.1%), and 95.8% (95% CI, 88.1%–99.1%), respectively. Alternatively, the threshold displaying 100% specificity (95% CI, 93.5%–100.0%) for predicting poor neurological outcome was TTNT >36 hours after ROSC (sensitivity, 78.7%; 95% CI, 67.7%–87.3%; Table 3). When we categorized patients into the mild or deep coma group according to the results of each initial neurological examination, TTNT in the initially deep coma group showed better prognostic performance than that in the mild coma group (Table I and Figure I in the online-only Data Supplement).
Figure 4.
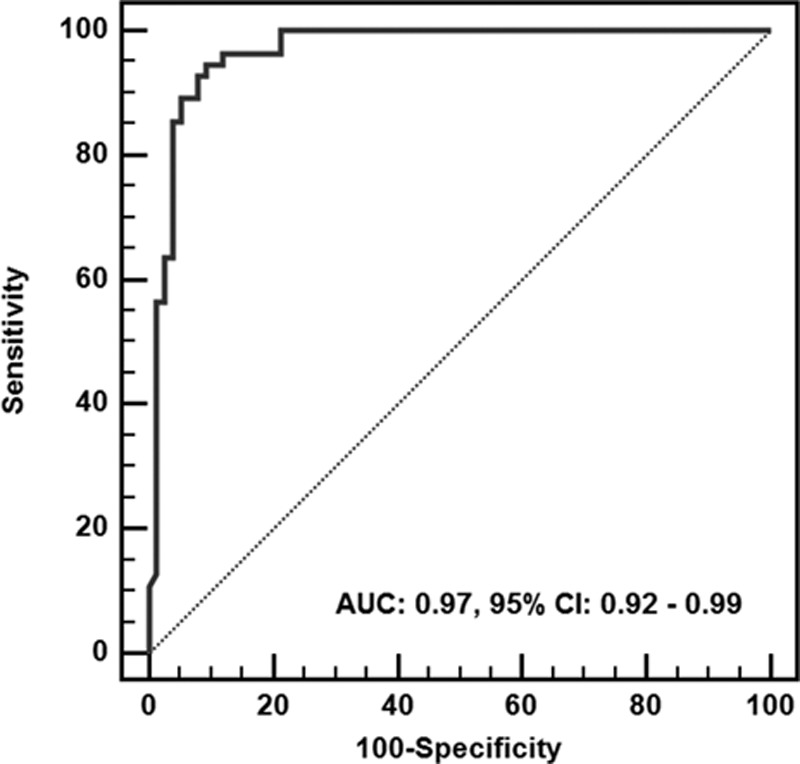
Receiver operating characteristic curve for the prediction of good neurological outcome according to the time to normal trace. AUC indicates area under the curve; and CI, confidence interval.
Table 2.
The Achievement of Time to Normal Trace Within Specific Time Windows and the Occurrence of Burst Suppression and Status Epilepticus in Both Neurological Outcome Groups
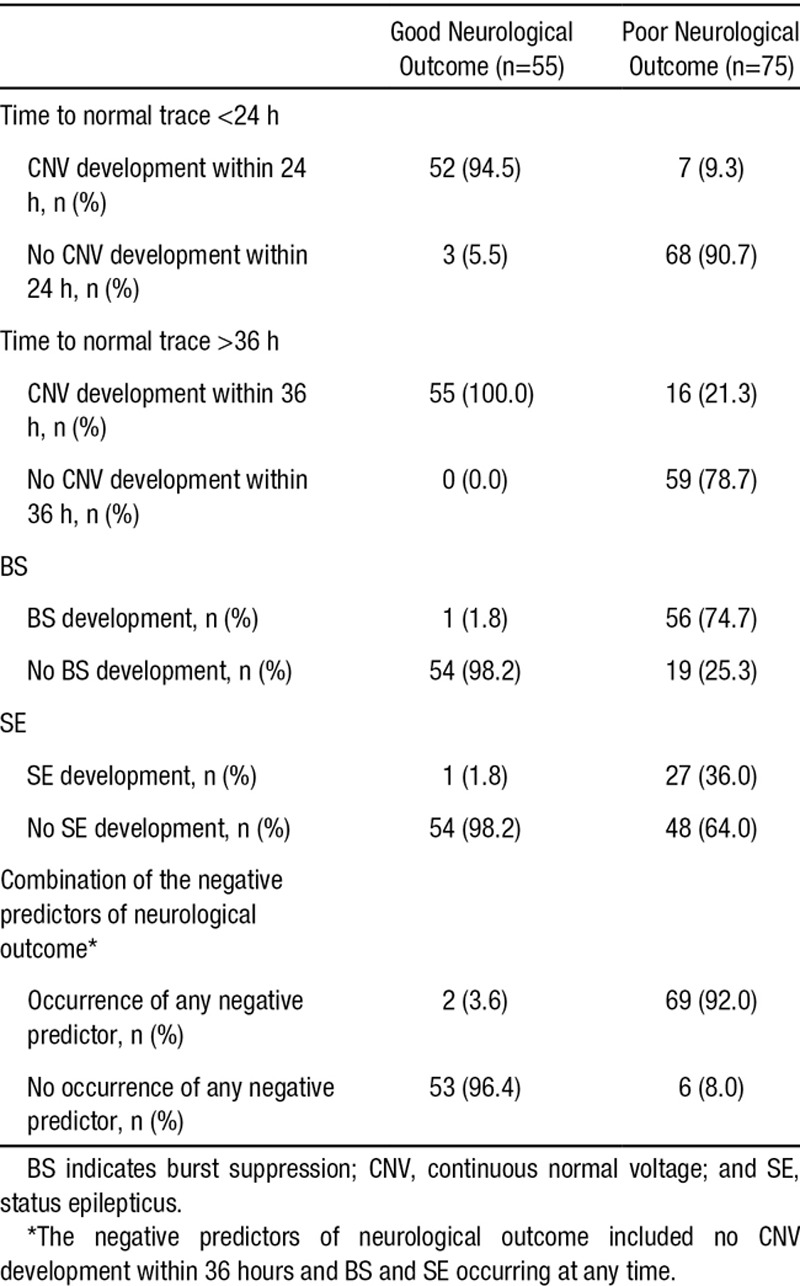
Table 3.
Sensitivity, Specificity, and Positive and Negative Predictive Values for the Early Prediction of Good and Poor Neurological Outcome
Other Predictors of Poor Neurological Outcome
The occurrence of BS and SE in both neurological outcome groups is shown in Table 2. In 57 patients (43.8%), BS was observed during monitoring, and the median time from ROSC to BS was 4.3 hours (Q1–Q3, 1.9–7.4 hours). All of these patients, except for 1, exhibited a poor neurological outcome. The detection of a BS pattern predicted poor neurological outcome with a PPV of 98.3% (sensitivity and specificity of 74.7 and 98.2%, respectively; Table 3). SE was observed in 28 patients (21.5%), and the occurrence of SE within 72 hours after ROSC (at a median of 16.6 hours after ROSC [Q1–Q3, 11.3–36.4 hours]) predicted poor neurological outcome with a PPV of 96.4% (sensitivity and specificity of 36.0 and 98.2%, respectively; Table 3). In most of these cases (23, 82.1%), SE initially developed from a BS pattern. In these cases, the initiation of SE occurred at a median of 11.3 hours after ROSC (Q1–Q3, 7.2–32.7 hours), and all of these patients exhibited a poor neurological outcome. However, in 5 patients (17.9%), SE developed from a CNV. Although these patients exhibited a CNV trace via the sequential evolution of the EEG pattern, SE abruptly developed from the CNV trace at a median of 33.0 hours after ROSC (Q1–Q3, 18.0–36.2 hours). Among these patients, 1 patient treated with antiepileptic drugs regained consciousness 2 weeks after ROSC.
The combination of negative predictors, consisting of no CNV development within 36 hours or the occurrence of BS or SE, in which poor neurological outcome was predicted if at least 1 of these 3 criteria were met, improved prognostic performance. These negative predictors were observed in 92.0% (69/75) of the patients who exhibited a poor neurological outcome at a median of 6.2 hours after ROSC (Q1–Q3, 2.5–18.7 hours). These combined aEEG-based negative indicators predicted poor neurological outcome with a specificity, PPV, and negative predictive value of 96.4%, 97.2%, and 89.8%, respectively (Table 3).
Discussion
We evaluated the prognostic value of aEEG using a single-channel frontal montage for TH-treated cardiac arrest survivors during the initial 72 hours after ROSC without withholding or withdrawing LST. Our study demonstrated that the application of aEEG was capable of the early prediction of neurological outcome in these patients. First, when the aEEG displayed a normal CNV trace within 24 hours, the physicians were able to predict a good neurological outcome with a PPV of 88.1%. Second, the occurrence of SE or BS at any time and the lack of the development of a normal CNV trace within 36 hours were associated with a poor neurological outcome at a high sensitivity. The combination of these negative predictors may improve their prognostic performance at an earlier stage.
Our findings were consistent with those published in a previous study. A cEEG background pattern was strongly associated with the recovery of consciousness. Cloostermans et al16 reported that cEEG monitoring during the first 24 hours after resuscitation contributes to the prediction of both good and poor neurological outcome. In that study, continuous activity patterns within 12 hours predicted good neurological outcome, and isoelectric or low-voltage activity after 24 hours predicted poor neurological outcome. Rundgren et al14 evaluated aEEG at a median of 8 hours after cardiac arrest and after the patients achieved a normal temperature. In that study, an initial continuous pattern and the return of a continuous pattern at normothermia served as good predictors of the recovery of consciousness. In our previous study based on a small number of cases, all of the patients exhibiting a good neurological outcome displayed a CNV trace within 26 hours.13 However, the threshold TTNT for the prognosis of good neurological outcome was unknown when the normalization of the background pattern was delayed. In term infants exhibiting perinatal asphyxia at hypothermia, some investigators found that the recovery time to a normal background pattern was the best predictor of poor neurological outcome at a suggested threshold for aEEG normalization of 36 to 48 hours of age.10,11 These findings were similar to our results, in which all patients experiencing a good neurological outcome developed a normal trace within 36 hours. We used a continuous sedation protocol during hypothermia. Of the 55 patients exhibiting good outcome, 53 patients achieved a normal trace within the mean time of the initiation of sedative reduction (within 28.5 hours after ROSC), and 2 patients exhibited a TTNT before sedative withdrawal (34.4 and 35.1 hours, respectively). Therefore, we believe that the effect of sedation on the TTNT threshold is minimal.
Most investigators agree that, in patients treated with TH, the time to prognostication should be delayed beyond 72 hours after rewarming.17–20 Early prognostication during TH should focus on good rather than poor neurological outcome.21 However, in some reports, LST was withdrawn before the resumption of normothermia by family request, or treatment was even suspended for some patients who were given a poor prognosis during TH.22,23 Our study showed that the presence of a normal aEEG pattern within 24 hours after ROSC was a predictor of a good neurological outcome. This finding can impact treatment decisions even in cases in which the withdrawal of LST is considered to be consistent with the caregiver’s wishes without delayed prognostication.
The definition of BS was inconsistent between many studies, potentially influencing the relevance of the observed predictive value of BS.13,14,16,24 According to the definition of BS in American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology: 2012 version, suppression was defined as a period in which the voltage was <10 μV.25 Alternatively, we defined BS as the virtual absence of activity (<2 μV) between bursts of high voltage (>25 μV) on aEEG. With the use of our revised definition, the occurrence of a BS pattern accurately predicted poor neurological outcome in all but 1 case.
Recent studies have demonstrated that SE is common in these patients and is associated with poor neurological outcome,14,26–28 despite exceptional reports of recovery.29,30 It is unknown whether prolonged SE contributes to secondary brain injury after cardiac arrest or whether SE is simply an epiphenomenon of severe brain injury. Our results suggest an answer to this question. Rossetti et al30,31 described benign postanoxic SE in cases involving a reactive background. Rundgren et al14 identified SE evolving from a continuous pattern in 10 patients and suggested that this type of SE reflects a less injured and potentially salvageable brain. In our study, 5 patients developed SE from a CNV trace via the sequential evolution of the EEG pattern. Although these patients exhibited a evolution pattern similar to that of other patients with good neurological outcome, inconsistent with our expectations, only 1 patient exhibited a good neurological outcome. We propose that, in these cases, SE serves as a contributor to poor neurological outcome rather than as a simple epiphenomenon of severe brain injury and that appropriate treatment is necessary to recover consciousness. To distinguish this pattern of SE from more malignant SE patterns, cEEG monitoring may be an essential component of post–cardiac arrest care.
The introduction of mild hypothermia and standardized treatment protocols in the past decade has improved neurological outcomes in survivors of cardiac arrest.1–3 In 2013, new strategies based on a near-normal temperature (36°C) not displaying differences in comparison with mild hypothermia (33°C) were introduced.32 However, in both intervention groups, a significant number of patients did not regain consciousness after treatment. To improve neurological outcome in these patients, it is quite clear that tailored therapies according to the extent of brain injury are needed. Some investigators have attempted to categorize patients according to brain injury severity based on EEG.33,34 According to our results, based on early aEEG monitoring, these patients can be categorized early. The initial or early restoration of CNV predicts good neurological outcome in circumstances involving the maintenance of active care. Additionally, the development of CNV between 24 and 36 hours after ROSC and SE originating from CNV render prognostication difficult. However, for cooled patients exhibiting an abnormal voltage trace during TH, a good neurological outcome remains possible even if normalization of the background patterns is delayed beyond 24 hours. Alternatively, the lack of the development of a normal trace within 36 hours and the occurrence of BS or SE originating from BS indicated a poor neurological outcome using the present cooling strategies. However, good neurological recovery in these patients may remain possible with advancements in the existing cooling strategies that have been associated with poor neurological outcome.33 Because the risk of extensive hypoxic brain injury increases over time, to spare these patients, it is important to identify those expected to exhibit a poor prognosis very early. Interestingly, using the combination of negative predictors via continuous aEEG monitoring, prognostication was possible at a median 6.2 hours after ROSC. To the best of our knowledge, our study represents the first attempt to address the issues of the time at which physicians determine the severity of brain injury using EEG and the time of prognostication. Future studies should continue to define patient subgroups and evaluate the benefit of tailored therapies for each patient’s injury.35
Several features of our study deserve further mention. First, withdrawal of LST based on prognostication was not applied to our patients. Currently, the practice of withdrawal of LST is widespread based on recommendations and guidelines.5,36 However, most prognostication studies were performed in Western countries, which have a social consensus regarding the withdrawal of LST, and these studies did not adequately address certain important limitations concerning the risk of bias. A self-fulfilling prophecy is present in most prognostication studies of cardiac arrest, in which the treating physicians are not blinded to the results of the neurological outcome prediction and use this prediction to make decisions regarding the withdrawal of LST.7,37,38 In South Korea, the withdrawal of LST for patients who have a terminal illness remains under debate, and the social consensus and legislative processes are developing; the withdrawal of LST for postcardiac arrest patients based on prognostication is not currently permitted.39 Our clinical and ethical situation resulted in the natural neurological outcome for these patients. Second, this study included consecutive patients with TH during the study period, but patients who died within 72 hours after cardiac arrest were excluded. The aim of this study was to evaluate the prognostic value of continuous aEEG (from immediately after ROSC to 72 hours later) according to time. Early death after ROSC is often caused by persistent hemodynamic instability leading to multiple organ failure.40,41 If patients with good brain function but poor cardiorespiratory function were included in the study, the prognostic value of aEEG in the early phase would be confounded. However, our analysis did not include additional potential variables affecting neurological outcome, such as initial organ system dysfunction in 72-hour survivors. For these reasons, our results should be interpreted cautiously.
Limitations
This study contained several limitations. First, we only performed frontal EEG monitoring via single-channel aEEG. Although SE was detected in 21.5% of our patients, a result similar to that of other reports using multichannel cEEG monitoring,14,26–28 our rate of good outcome was slightly lower than that of other studies.30,42 Our technique using single-channel monitoring may disturb the detection of focal epileptic activity, and this may influence the rate of good outcome in SE patients. However, reducing the number of channels used for bedside aEEG monitoring is crucial for facilitating the monitoring of the cerebral cortex and for its immediate application after ROSC. Because the frontal cortices are better protected than the parietal and occipital cortices,43 and because continuous activity appeared first in the frontal leads after ROSC,44 the frontal cortices may represent neural recovery during the early stage. Second, this study used a single-center design in a country with a unique healthcare environment. This study design raises important questions regarding the generalizability of these results. A large, multicenter study including Western countries may provide more precise prognostic values and may help determine the utility of aEEG in this population. Third, although physicians are not permitted to withdraw LST in South Korea, there was an inevitable risk of bias because the treating team was not blinded to the aEEG data during treatment. Finally, especially regarding SE, our study could not differentiate periodic epileptiform discharges from SE and did not evaluate clinical manifestations (such as myoclonus) that were examined in previous studies using cEEG.22,27,28
Conclusion
Early aEEG monitoring of adult patients with cardiac arrest receiving TH enabled the early prediction of neurological outcome. Based on these results, a TTNT within 24 hours after ROSC was associated with good neurological outcome. The lack of CNV development within 36 hours and the occurrence of SE or BS within 72 hours after ROSC contributed to the prediction of poor neurological outcome. The combination of these negative predictors via aEEG monitoring may improve their prognostic performance at an earlier stage.
Disclosures
None.
Supplementary Material
Footnotes
The online-only Data Supplement is available with this article at http://circ.ahajournals.org/lookup/suppl/doi:10.1161/CIRCULATIONAHA.115.015754/-/DC1.
CLINICAL PERSPECTIVE
Continuous electroencephalography can be used to monitor the postischemic brain after cardiac arrest. However, this technique requires experienced specialists for application and interpretation and is often unavailable. Amplitude-integrated electroencephalography provides a simplified and, therefore, more readily available brain function–monitoring tool for not only perinatal hypoxic-ischemic encephalopathy in neonates, but also cardiac arrest in adults. In neonates, the time from birth to a normal amplitude-integrated electroencephalography background was the best predictor of poor neurological outcome. In this study, we found that the time from the return of spontaneous circulation to a normal amplitude-integrated electroencephalography trace within 24 hours was associated with good neurological outcome in patients who survived for 72 hours after successful resuscitation from cardiac arrest. We also show that the lack of normal trace development within 36 hours and the occurrence of burst suppression or status epilepticus contributed to the prediction of poor outcome. Importantly, the combination of these negative predictors reduced the time to prognostication. Based on early amplitude-integrated electroencephalography monitoring, patients could be categorized early according to the degree of brain injury. Thus, future studies should continue to define patient subgroups and should evaluate the benefit of tailored therapies for each patient’s brain injury.
References
- 1.Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;46:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 2.Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 3.Peberdy MA, Callaway CW, Neumar RW, Geocadin RG, Zimmerman JL, Donnino M, Gabrielli A, Silvers SM, Zaritsky AL, Merchant R, Vanden Hoek TL, Kronick SL American Heart Association. Part 9: post-cardiac arrest care: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18) suppl 3:S768–S786. doi: 10.1161/CIRCULATIONAHA.110.971002. doi: 10.1161/CIRCULATIONAHA.110.971002. [DOI] [PubMed] [Google Scholar]
- 4.Kamps MJ, Horn J, Oddo M, Fugate JE, Storm C, Cronberg T, Wijman CA, Wu O, Binnekade JM, Hoedemaekers CW. Prognostication of neurologic outcome in cardiac arrest patients after mild therapeutic hypothermia: a meta-analysis of the current literature. Intensive Care Med. 2013;39:1671–1682. doi: 10.1007/s00134-013-3004-y. doi: 10.1007/s00134-013-3004-y. [DOI] [PubMed] [Google Scholar]
- 5.Geocadin RG, Buitrago MM, Torbey MT, Chandra-Strobos N, Williams MA, Kaplan PW. Neurologic prognosis and withdrawal of life support after resuscitation from cardiac arrest. Neurology. 2006;67:105–108. doi: 10.1212/01.wnl.0000223335.86166.b4. doi: 10.1212/01.wnl.0000223335.86166.b4. [DOI] [PubMed] [Google Scholar]
- 6.Cronberg T, Brizzi M, Liedholm LJ, Rosén I, Rubertsson S, Rylander C, Friberg H. Neurological prognostication after cardiac arrest–recommendations from the Swedish Resuscitation Council. Resuscitation. 2013;84:867–872. doi: 10.1016/j.resuscitation.2013.01.019. doi: 10.1016/j.resuscitation.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 7.Geocadin RG, Peberdy MA, Lazar RM. Poor survival after cardiac arrest resuscitation: a self-fulfilling prophecy or biologic destiny?*. Crit Care Med. 2012;40:979–980. doi: 10.1097/CCM.0b013e3182410146. doi: 10.1097/CCM.0b013e3182410146. [DOI] [PubMed] [Google Scholar]
- 8.Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci. 2009;10:861–872. doi: 10.1038/nrn2735. doi: 10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]
- 9.Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 10.Hallberg B, Grossmann K, Bartocci M, Blennow M. The prognostic value of early aEEG in asphyxiated infants undergoing systemic hypothermia treatment. Acta Paediatr. 2010;99:531–536. doi: 10.1111/j.1651-2227.2009.01653.x. doi: 10.1111/j.1651-2227.2009.01653.x. [DOI] [PubMed] [Google Scholar]
- 11.Thoresen M, Hellström-Westas L, Liu X, de Vries LS. Effect of hypothermia on amplitude-integrated electroencephalogram in infants with asphyxia. Pediatrics. 2010;126:e131–e139. doi: 10.1542/peds.2009-2938. doi: 10.1542/peds.2009-2938. [DOI] [PubMed] [Google Scholar]
- 12.Toet MC, Hellström-Westas L, Groenendaal F, Eken P, de Vries LS. Amplitude integrated EEG 3 and 6 hours after birth in full term neonates with hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 1999;81:F19–F23. doi: 10.1136/fn.81.1.f19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oh SH, Park KN, Kim YM, Kim HJ, Youn CS, Kim SH, Choi SP, Kim SC, Shon YM. The prognostic value of continuous amplitude-integrated electroencephalogram applied immediately after return of spontaneous circulation in therapeutic hypothermia-treated cardiac arrest patients. Resuscitation. 2013;84:200–205. doi: 10.1016/j.resuscitation.2012.09.031. doi: 10.1016/j.resuscitation.2012.09.031. [DOI] [PubMed] [Google Scholar]
- 14.Rundgren M, Westhall E, Cronberg T, Rosén I, Friberg H. Continuous amplitude-integrated electroencephalogram predicts outcome in hypothermia-treated cardiac arrest patients. Crit Care Med. 2010;38:1838–1844. doi: 10.1097/CCM.0b013e3181eaa1e7. doi: 10.1097/CCM.0b013e3181eaa1e7. [DOI] [PubMed] [Google Scholar]
- 15.Longstreth WT, Jr, Nichol G, Van Ottingham L, Hallstrom AP. Two simple questions to assess neurologic outcomes at 3 months after out-of-hospital cardiac arrest: experience from the public access defibrillation trial. Resuscitation. 2010;81:530–533. doi: 10.1016/j.resuscitation.2010.01.011. doi: 10.1016/j.resuscitation.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cloostermans MC, van Meulen FB, Eertman CJ, Hom HW, van Putten MJ. Continuous electroencephalography monitoring for early prediction of neurological outcome in postanoxic patients after cardiac arrest: a prospective cohort study. Crit Care Med. 2012;40:2867–2875. doi: 10.1097/CCM.0b013e31825b94f0. doi: 10.1097/CCM.0b013e31825b94f0. [DOI] [PubMed] [Google Scholar]
- 17.Cronberg T, Rundgren M, Westhall E, Englund E, Siemund R, Rosén I, Widner H, Friberg H. Neuron-specific enolase correlates with other prognostic markers after cardiac arrest. Neurology. 2011;77:623–630. doi: 10.1212/WNL.0b013e31822a276d. doi: 10.1212/WNL.0b013e31822a276d. [DOI] [PubMed] [Google Scholar]
- 18.Blondin NA, Greer DM. Neurologic prognosis in cardiac arrest patients treated with therapeutic hypothermia. Neurologist. 2011;17:241–248. doi: 10.1097/NRL.0b013e318224ee0e. doi: 10.1097/NRL.0b013e318224ee0e. [DOI] [PubMed] [Google Scholar]
- 19.Samaniego EA, Persoon S, Wijman CA. Prognosis after cardiac arrest and hypothermia: a new paradigm. Curr Neurol Neurosci Rep. 2011;11:111–119. doi: 10.1007/s11910-010-0148-9. doi: 10.1007/s11910-010-0148-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friberg H, Rundgren M, Westhall E, Nielsen N, Cronberg T. Continuous evaluation of neurological prognosis after cardiac arrest. Acta Anaesthesiol Scand. 2013;57:6–15. doi: 10.1111/j.1399-6576.2012.02736.x. doi: 10.1111/j.1399-6576.2012.02736.x. [DOI] [PubMed] [Google Scholar]
- 21.Oddo M. Prognostication of coma after cardiac arrest: think positive. Resuscitation. 2013;84:855–856. doi: 10.1016/j.resuscitation.2013.03.041. doi: 10.1016/j.resuscitation.2013.03.041. [DOI] [PubMed] [Google Scholar]
- 22.Crepeau AZ, Rabinstein AA, Fugate JE, Mandrekar J, Wijdicks EF, White RD, Britton JW. Continuous EEG in therapeutic hypothermia after cardiac arrest: prognostic and clinical value. Neurology. 2013;80:339–344. doi: 10.1212/WNL.0b013e31827f089d. doi: 10.1212/WNL.0b013e31827f089d. [DOI] [PubMed] [Google Scholar]
- 23.Perman SM, Kirkpatrick JN, Reitsma AM, Gaieski DF, Lau B, Smith TM, Leary M, Fuchs BD, Levine JM, Abella BS, Becker LB, Merchant RM. Timing of neuroprognostication in postcardiac arrest therapeutic hypothermia*. Crit Care Med. 2012;40:719–724. doi: 10.1097/CCM.0b013e3182372f93. doi: 10.1097/CCM.0b013e3182372f93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rossetti AO, Carrera E, Oddo M. Early EEG correlates of neuronal injury after brain anoxia. Neurology. 2012;78:796–802. doi: 10.1212/WNL.0b013e318249f6bb. doi: 10.1212/WNL.0b013e318249f6bb. [DOI] [PubMed] [Google Scholar]
- 25.Hirsch LJ, LaRoche SM, Gaspard N, Gerard E, Svoronos A, Herman ST, Mani R, Arif H, Jette N, Minazad Y, Kerrigan JF, Vespa P, Hantus S, Claassen J, Young GB, So E, Kaplan PW, Nuwer MR, Fountain NB, Drislane FW. American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology: 2012 version. J Clin Neurophysiol. 2013;30:1–27. doi: 10.1097/WNP.0b013e3182784729. doi: 10.1097/WNP.0b013e3182784729. [DOI] [PubMed] [Google Scholar]
- 26.Legriel S, Bruneel F, Sediri H, Hilly J, Abbosh N, Lagarrigue MH, Troche G, Guezennec P, Pico F, Bedos JP. Early EEG monitoring for detecting postanoxic status epilepticus during therapeutic hypothermia: a pilot study. Neurocrit Care. 2009;11:338–344. doi: 10.1007/s12028-009-9246-4. doi: 10.1007/s12028-009-9246-4. [DOI] [PubMed] [Google Scholar]
- 27.Mani R, Schmitt SE, Mazer M, Putt ME, Gaieski DF. The frequency and timing of epileptiform activity on continuous electroencephalogram in comatose post-cardiac arrest syndrome patients treated with therapeutic hypothermia. Resuscitation. 2012;83:840–847. doi: 10.1016/j.resuscitation.2012.02.015. doi: 10.1016/j.resuscitation.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rittenberger JC, Popescu A, Brenner RP, Guyette FX, Callaway CW. Frequency and timing of nonconvulsive status epilepticus in comatose post-cardiac arrest subjects treated with hypothermia. Neurocrit Care. 2012;16:114–122. doi: 10.1007/s12028-011-9565-0. doi: 10.1007/s12028-011-9565-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Westhall E, Rundgren M, Lilja G, Friberg H, Cronberg T. Postanoxic status epilepticus can be identified and treatment guided successfully by continuous electroencephalography. Ther Hypothermia Temp Manag. 2013;3:84–87. doi: 10.1089/ther.2013.0002. doi: 10.1089/ther.2013.0002. [DOI] [PubMed] [Google Scholar]
- 30.Rossetti AO, Oddo M, Liaudet L, Kaplan PW. Predictors of awakening from postanoxic status epilepticus after therapeutic hypothermia. Neurology. 2009;72:744–749. doi: 10.1212/01.wnl.0000343006.60851.62. doi: 10.1212/01.wnl.0000343006.60851.62. [DOI] [PubMed] [Google Scholar]
- 31.Rossetti AO, Urbano LA, Delodder F, Kaplan PW, Oddo M. Prognostic value of continuous EEG monitoring during therapeutic hypothermia after cardiac arrest. Crit Care. 2010;14:R173. doi: 10.1186/cc9276. doi: 10.1186/cc9276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, Horn J, Hovdenes J, Kjaergaard J, Kuiper M, Pellis T, Stammet P, Wanscher M, Wise MP, Åneman A, Al-Subaie N, Boesgaard S, Bro-Jeppesen J, Brunetti I, Bugge JF, Hingston CD, Juffermans NP, Koopmans M, Køber L, Langørgen J, Lilja G, Møller JE, Rundgren M, Rylander C, Smid O, Werer C, Winkel P, Friberg H TTM Trial Investigators. Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med. 2013;369:2197–2206. doi: 10.1056/NEJMoa1310519. doi: 10.1056/NEJMoa1310519. [DOI] [PubMed] [Google Scholar]
- 33.Tsetsou S, Oddo M, Rossetti AO. Clinical outcome after a reactive hypothermic EEG following cardiac arrest. Neurocrit Care. 2013;19:283–286. doi: 10.1007/s12028-013-9883-5. doi: 10.1007/s12028-013-9883-5. [DOI] [PubMed] [Google Scholar]
- 34.Friberg H, Westhall E, Rosén I, Rundgren M, Nielsen N, Cronberg T. Clinical review: Continuous and simplified electroencephalography to monitor brain recovery after cardiac arrest. Crit Care. 2013;17:233. doi: 10.1186/cc12699. doi: 10.1186/cc12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rittenberger JC, Callaway CW. Temperature management and modern post-cardiac arrest care. N Engl J Med. 2013;369:2262–2263. doi: 10.1056/NEJMe1312700. doi: 10.1056/NEJMe1312700. [DOI] [PubMed] [Google Scholar]
- 36.Sandroni C, Cariou A, Cavallaro F, Cronberg T, Friberg H, Hoedemaekers C, Horn J, Nolan JP, Rossetti AO, Soar J. Prognostication in comatose survivors of cardiac arrest: an advisory statement from the European Resuscitation Council and the European Society of Intensive Care Medicine. Intensive Care Med. 2014;40:1816–1831. doi: 10.1007/s00134-014-3470-x. doi: 10.1007/s00134-014-3470-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nolan JP, Neumar RW, Adrie C, Aibiki M, Berg RA, Böttiger BW, Callaway C, Clark RS, Geocadin RG, Jauch EC, Kern KB, Laurent I, Longstreth WT, Merchant RM, Morley P, Morrison LJ, Nadkarni V, Peberdy MA, Rivers EP, Rodriguez-Nunez A, Sellke FW, Spaulding C, Sunde K, Hoek TV. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A Scientific Statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke. Resuscitation. 2008;79:350–379. doi: 10.1016/j.resuscitation.2008.09.017. doi: 10.1016/j.resuscitation.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 38.Sandroni C, Cavallaro F, Callaway CW, D’Arrigo S, Sanna T, Kuiper MA, Biancone M, Della Marca G, Farcomeni A, Nolan JP. Predictors of poor neurological outcome in adult comatose survivors of cardiac arrest: a systematic review and meta-analysis. Part 2: Patients treated with therapeutic hypothermia. Resuscitation. 2013;84:1324–1338. doi: 10.1016/j.resuscitation.2013.06.020. doi: 10.1016/j.resuscitation.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 39.Lee BK, Park KN, Kang GH, Kim KH, Kim G, Kim WY, Min JH, Park Y, Park JB, Suh GJ, Son YD, Shin J, Oh JS, You YH, Lee DH, Lee JS, Lim H, Jang TC, Cho GC, Cho IS, Cha KC, Choi SP, Choi WJ, Han C. The Korean Hypothermia Network Investigators. Outcome and current status of therapeutic hypothermia after out-of-hospital cardiac arrest in Korea using data from the Korea Hypothermia Network registry. Clin Exp Emerg Med. 2014;1:19–27. doi: 10.15441/ceem.14.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laurent I, Monchi M, Chiche JD, Joly LM, Spaulding C, Bourgeois B, Cariou A, Rozenberg A, Carli P, Weber S, Dhainaut JF. Reversible myocardial dysfunction in survivors of out-of-hospital cardiac arrest. J Am Coll Cardiol. 2002;40:2110–2116. doi: 10.1016/s0735-1097(02)02594-9. [DOI] [PubMed] [Google Scholar]
- 41.Safar P. Resuscitation from clinical death: pathophysiologic limits and therapeutic potentials. Crit Care Med. 1988;16:923–941. doi: 10.1097/00003246-198810000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Nielsen N, Sunde K, Hovdenes J, Riker RR, Rubertsson S, Stammet P, Nilsson F, Friberg H Hypothermia Network. Adverse events and their relation to mortality in out-of-hospital cardiac arrest patients treated with therapeutic hypothermia. Crit Care Med. 2011;39:57–64. doi: 10.1097/CCM.0b013e3181fa4301. doi: 10.1097/CCM.0b013e3181fa4301. [DOI] [PubMed] [Google Scholar]
- 43.Choi SP, Park KN, Park HK, Kim JY, Youn CS, Ahn KJ, Yim HW. Diffusion-weighted magnetic resonance imaging for predicting the clinical outcome of comatose survivors after cardiac arrest: a cohort study. Crit Care. 2010;14:R17. doi: 10.1186/cc8874. doi: 10.1186/cc8874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jørgensen EO, Holm S. The natural course of neurological recovery following cardiopulmonary resuscitation. Resuscitation. 1998;36:111–122. doi: 10.1016/s0300-9572(97)00094-4. [DOI] [PubMed] [Google Scholar]


