Abstract
Enzyme replacement therapy with laronidase (recombinant human alpha-l-iduronidase) is successfully used to treat patients with mucopolysaccharidosis type I (MPS I). However, the intravenously-administered enzyme is not expected to treat or prevent neurological deterioration. As MPS I patients suffer from spinal cord compression due in part to thickened spinal meninges, we undertook a phase I clinical trial of lumbar intrathecal laronidase in MPS I subjects age 8 years and older with symptomatic (primarily cervical) spinal cord compression. The study faced significant challenges, including a heterogenous patient population, difficulty recruiting subjects despite an international collaborative effort, and an inability to include a placebo-controlled design due to ethical concerns. Nine serious adverse events occurred in the subjects. All subjects reported improvement in symptomatology and showed improved neurological examinations, but objective outcome measures did not demonstrate change. Despite limitations, we demonstrated the safety of this approach to treating neurological disease due to MPS I.
Keywords: Hurler, Scheie, lysosomal storage disease, enzyme replacement therapy, alpha-L-iduronidase, intrathecal
1. Introduction
Mucopolysaccharidosis I (MPS I) is caused by deficiency of the enzyme alpha-l-iduronidase (EC 3.2.1.76), a soluble lysosomal hydrolase that is involved in catabolism of heparan sulfate and dermatan sulfate glycosaminoglycans. Like other lysosomal storage diseases, MPS I causes progressive disability and death due to abnormal accumulation of substrate inside cells. Both systemic and neurological disease occur in the condition. Systemic manifestations can be treated with enzyme replacement therapy using the recombinant form of alpha-l-iduronidase, laronidase, administered by weekly intravenous injections. Several recombinant enzyme treatments are approved for use in human patients with lysosomal storage diseases (1–7).
The challenge for neurological disease due to MPS I is to deliver the enzyme into the central nervous system. The cerebrospinal fluid naturally circulates throughout the neuraxis, providing potentially broad distribution from a single injection point. Injection into cerebrospinal fluid is used to deliver chemotherapeutic agents, narcotic analgesics and the muscle relaxant baclofen clinically in patients. Injecting enzyme into the cerebrospinal fluid is also less invasive than injecting directly into brain parenchyma, and more suitable for chronic use than osmotic opening of the blood-brain barrier. Preclinical studies of recombinant lysosomal enzymes injected into cerebrospinal fluid of large animals (dogs, cats and monkeys) have shown, somewhat surprisingly, that they can penetrate into deep brain structures such as white matter, hippocampus, basal ganglia and thalamus (8–11).
We performed a phase I clinical trial of laronidase administered intrathecally via lumbar spinal tap for patients with MPS I. For this initial safety study, we elected to study MPS-related spinal cord compression. MPS I patients often develop meningeal thickening due to lysosomal storage (12), and we reasoned that this may be more likely to be reversible than disease in the brain. In addition, our preclinical studies in MPS I dogs showed that we could achieve extremely high enzyme concentrations and substantial reduction of lysosomal storage in the spinal meninges, even in adult animals (8, 13, 14). We present the results of this study, along with a discussion of the challenges facing clinical trials of therapies for rare neurodegenerative diseases.
2. Materials and Methods
2.1 Subjects
All study procedures were reviewed and approved by the John Wolf Human Subjects Committee at the Los Angeles Biomedical Research Institute and at institutional review boards at UCSF Benioff Children's Hospital Oakland and the Helsinki University Children's Hospital. The study was conducted in the United States under a Food and Drug Administration Investigational New Drug (IND) application and in Europe under a European Medicines Agency (Eudra) registration. Studies were listed on www.clinicaltrials.gov (National Clinical Trials (NCT) numbers NCT00215527 and NCT00786968). Enrollment into the research studies took place between November 2005 and March 2010, accruing 5 subjects. In order to be eligible for the study, subjects had to have symptomatic spinal cord compression that did not require urgent surgical intervention. A neurologist and neuroradiologist were required to assess each subject for evidence of cord compression. Our studies included subjects 8 year of age or older with MPS I (Scheie, Hurler-Scheie, and Hurler syndromes) with symptomatic cervical spinal cord compression. All study participants had been on intravenous enzyme replacement therapy with laronidase (commercially available recombinant alpha-l-iduronidase) for many years prior to study entry and otherwise were without severe concurrent illness precluding them from undergoing study treatments. The majority of subjects resided in the United States; however, because MPS I is a rare disease, we also established an international site in Finland to allow enrollment of eligible participants outside the US. Eligibility for the extension study was limited to subjects who exhibited a “good response” to intrathecal laronidase, defined as improvement or stabilization of spinal cord compression as determined by spinal magnetic resonance imaging (MRI), neurologic examination, subjective assessment score, Japanese Orthopedic Association score, Functional Independence Measure score, or six-minute walk test.
2.2 Study Design
These were phase I open label interventional studies. The studies included a pilot phase and an extension phase. Participants of the pilot phase received 4 monthly doses of 1.74 mg laronidase diluted in Elliott's B (BenVenue Laboratories) artificial cerebrospinal fluid (1 part laronidase to 2 parts Elliott's B by volume; total volume 9 mL) administered intrathecally (via lumbar spinal injection) 30 days apart. Those showing improvement during the pilot phase were given an opportunity to enroll into the extension phase of the study and receive additional intrathecal treatments (same dose and volume) every 30 to 90 days based on clinical condition. One subject (subject 2) received prophylaxis with oral prednisone prior to intrathecal laronidase after the subject developed a cellular and IgG antibody response in cerebrospinal fluid. Some participants required that treatments be administered under fluoroscopic guidance and with anesthesia.
2.3 Measures of safety
To evaluate possible adverse effects of study treatments, participants had physical and neurologic examination before and after each study treatment. Blood samples were collected for routine safety testing. All new physical complaints were evaluated and recorded including their severity and attribution to study treatments. The cerebrospinal fluid was evaluated for signs of inflammation, infection, and immune response. We measured visual acuity via Snellen test.
2.4 Objective measures of efficacy
Response to treatment was assessed using a combination of subjective and objective measures. Functional Independence Measure (FIM) score, 6-minute walk test, and Japanese Orthopedic Association (JOA) score measures were used to assess any changes in functional status and myelopathy. Scoring criteria for JOA and FIM are given in the supplemental materials (data files 2 and 3 of reference (15)). Cerebrospinal fluid glycosaminoglycans were measured at Seattle Children's Hospital using a clinically-available test. The laboratory uses a dimethylene blue dye-binding assay to quantitate total glycosaminoglycans (16). MRI of brain and spinal cord were obtained to assess degree of cord compression and measurement of meningeal thickness. MRI were performed using a 1.5-Tesla GE LX9.1. Brain imaging included sagittal T1-weighted, axial FLAIR, axial T2-weighted and axial diffusion-weighted images. Sagittal T1- and T2-weighted images of the whole spine and axial T1-weighted images of the cervical spine were obtained. Axial T1-weighted studies of the cervical spine were used to score spinal cord compression according to the methods of Houten and Cooper (17). Brain images were evaluated for abnormal signal intensity in T2, enlargement of perivascular spaces, and ventricular size as per Matheus, et al. (18). The grading systems that were used to indicate the severity of spinal cord compression and brain imaging findings are given in the supplemental materials (data file 4 of reference (15)). Cerebrospinal fluid opening pressure was measured before administration of each treatment and served as an indication of the effects of therapy on hydrocephalus. Hydrocephalus in MPS I subjects is communicating and thought to be due to inadequate reabsorption of cerebrospinal fluid via glycosaminoglycan-clogged arachnoid granulations. We evaluated somatosensory evoked potentials in the upper and lower extremity as per (19). Subjects enrolled in the extension study also underwent pulmonary function testing using spirometry.
2.5 Subjective measures of efficacy
Subjects were asked at baseline to report the five most troubling symptoms related to spinal cord compression. At each visit, they were asked to rate these from baseline as 0 = no change, +1 = slightly better, +2 = moderately better, +3 = much better, −1 = slightly worse, −2 = moderately worse, or −3 = much worse. The investigator was also asked to record whether the subject was better, worse, or unchanged from baseline as an overall (“global”) assessment using the same scale.
2.6 Data analysis
The safety set included all enrolled subjects who received at least one dose of intrathecal laronidase. The efficacy set was defined in the 4-month pilot study as all subjects who completed the CSF glycosaminoglycan analysis at 90 days and the MRI spinal cord compression score at 120 days. The planned study size was 10 subjects, which would provide 80% power to detect an adverse event that occurred at a rate of 15%. We had initially planned to evaluate adverse events by frequency across visits, but due to the low subject accrual we instead listed all adverse events (Table 1 of reference (15)). We evaluated efficacy variables for intrasubject change over time, using mean, standard deviation, and 95% confidence intervals. We averaged the baseline and day 0 results for efficacy variables from the pilot study, as both occurred pre-treatment. We defined a change as significant if the 95% confidence interval did not contain zero.
3. Results
3.1 Study Population and Characteristics
Subject characteristics are shown in Table 1. The clinical trial design included an initial pilot study of four monthly intrathecal injections of laronidase. Subjects with a “good response” defined as improvement or stabilization of spinal cord compression were eligible for a 1-year extension study, in which treatments were administered at 30 or 90 day intervals depending on the clinical course (see Materials and Methods for further details). The study was active and enrolling subjects from November 2005 until March 2010. Additional clinical details are summarized in data file 1 of reference (15).
Table 1.
Study subjects.
| Subject Number | Age at Enrolment (decade), Gender | MPS I Status | IDUA Mutations | Enrolled in 4-Month Pilot Study | Enrolled in 1-Year Extension Study | Duration of Spinal Cord Compression Prior to Study |
|---|---|---|---|---|---|---|
| 1 | 30s, F | Hurler-Scheie | Unknown | Yes | Yes* | 10 years |
| 2 | 10s, F | Hurler-Scheie | W402X/L238Q | No** | Yes | 3 years |
| 3 | 20s, F | Scheie | W402X/L526P | Yes | No*** | <1 year |
| 4 | 10s, M | Hurler-Scheie | W402X/L238Q | Yes | No† | 1 year |
| 5 | 20s, F | Hurler-Scheie | Q70X/396insAC | Yes | Yes | 2 years |
Subject 1 died during the extension study.
Subject 2 was treated off-study, off-label with intrathecal laronidase beginning ~16 months prior to enrollment in the extension study. The subject discontinued participation in the extension study due to worsening myelopathy requiring surgical decompression. This subject had a ventriculoperitoneal shunt.
Subject 3 was not enrolled into the extension study (excluded due to medical or other condition that prevented participation). This subject had a lumbo-peritoneal shunt.
Subject 4 died during the pilot study. This subject had a ventriculoperitoneal shunt.
3.2 Safety, Adverse Events, and Antibodies
MPS I is a multi-system disease, and subjects experienced many unrelated adverse events during the study period, most of which were attributed to their illness. Nine serious adverse events occurred in the subjects (Table 2). Two subjects died during the study. Subject 4, a teenage male, died in his sleep within the first 48 hours of the initial dose. An autopsy showed no inflammation in the brain or leptomeninges, no evidence of brain herniation, and no abnormal levels of narcotics or other toxins in blood. Cardiomyopathy was noted and attributed as the cause of death. Subject 1, a female in her thirties, completed the pilot study but died from pneumonia and respiratory failure sixty-five days after her first dose in the extension study. The subject had suffered chronic respiratory insufficiency, due in part to diaphragmatic hemiparalysis (thought to be caused by cervical spinal compression). Frequent adverse events that were considered to be directly related to intrathecal laronidase included headache and pain at the injection site. Two subjects with a history of hydrocephalus requiring implanted drainage shunts experienced elevated cerebrospinal fluid opening pressure. A full list of adverse events can be found in table 1 of reference (15).
Table 2.
Serious adverse events.
| Event | Grade | Related to study drug | Outcome |
|---|---|---|---|
| Pneumonia | Grade 4 | Possibly related | Resolved |
| Pneumonia | Grade 4 | Not related | Resolved |
| Corneal clouding transplantation | Grade 4 | Not related | Resolved |
| Hypoxia and respiratory distress | Grade 4 | Not related | Resolved |
| Sudden cardiac death | Grade 5 | Not related | Patient death |
| Hypoxemia | Grade 5 | Not related | Patient death |
| Facial flushing | Grade 3 | Possibly related | Resolved |
| Headache | Grade 3 | Possibly related | Resolved |
| Dyspnea and cough | Grade 2 | Not related | Resolved |
Protein therapeutics can induce an antibody response. However, all of the study subjects had been receiving intravenous laronidase for MPS I physical disease for years prior to study entry (Table 1), decreasing the likelihood of a new immune response. Subject 2 in the extension study had a history of anti-iduronidase antibodies, leukocytes (maximum 37 cells per μL, mainly lymphocytes), and IL-5 in cerebrospinal fluid, which had occurred during off-label, off-study administration of intrathecal laronidase (20). The antibodies in the CSF of this subject did not neutralize laronidase uptake in vitro into MPS I fibroblasts (20). Anti-iduronidase antibodies did not develop in either serum or cerebrospinal fluid of the remaining study subjects. We administered oral prednisone to the subject prior to each dose during the extension study, and the subject had no further elevations in antibody titer or cerebrospinal fluid leukocyte count.
3.3 Symptoms, Neurological Examination, and Clinical Course
The three subjects who completed the pilot study all reported improvements in symptoms related to spinal cord compression. Subjective improvement included increased mobility, improved bowel and bladder control, a reduction in crampy leg pain, and reduced sensation of “pins & needles”. Neurological examination showed improvements in the three pilot study subjects (Subjects 1, 3, and 5). These included small gains in the sensory and motor examinations (Table 3). In the extension study, the goal was principally to maintain neurological status. Subject 3 was not enrolled in the extension study, due to a condition that prevented participation. Subject 5, who completed the extension study, showed no worsening in symptoms of spinal cord compression or neurologic findings. Subject 2 withdrew from the extension study after developing new onset of mild urinary urgency and incontinence and leg weakness (reduced ability to bear weight when standing), attributed to worsening spinal cord compression. The subject underwent surgical decompression which reversed the acute deficits.
Table 3.
Summary of changes in the neurological examination of subjects completing the pilot study
| Subject 1 | Subject 3 | Subject 5 | |
|---|---|---|---|
| Sensory | No change | Disappearance of lower extremity pain and temperature asymmetries Improved lower extremity vibration sense | No change |
| Motor | Improved strength and range of motion | No deficit | Improved balance, decreased spasticity |
| Reflexes | No change | Disappearance of Hoffmann's reflex | No change |
3.4 Objective Study Endpoints
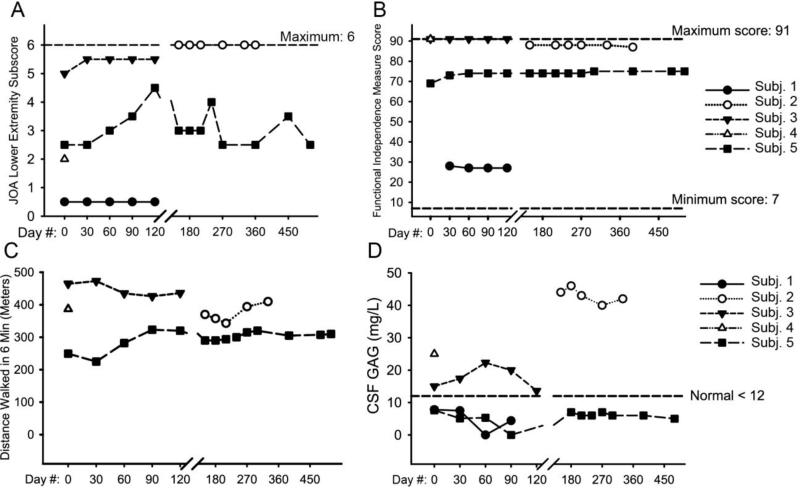
We obtained Functional Independence Measure (FIM) scores, which assess activities of daily living, and modified Japanese Orthopedic Association (JOA) scales of myelopathy. These did not show substantial change in the subjects overall (Fig. 1). One subject showed gains in the JOA score during the pilot study, but did not sustain these during the extension. Magnetic resonance imaging of the spine was assessed and scored for changes in cord compression, but did not show measureable change in severity of spinal cord compression during the four-month pilot study or the 1-year extension study (Fig. 2). Somatosensory evoked potentials, which have been reported to be sensitive measures of spinal cord compression (19), were not useful in our study subjects, as they were absent in two subjects and normal in two subjects at baseline (Table 2 of reference (15)). Brain frontal-occipital horn ratio (an index of ventricular size), abnormal signal intensity, enlarged perivascular spaces, and other brain imaging findings did not change over time in the subjects (Tables 3-7 of reference (15)).
Fig. 1.
Efficacy outcomes in study subjects. (A) JOA score for the lower extremity. Intrasubject change (mean ± SD) during the 4-month pilot study was 0.83 ± 1.04, 95% CI (−1.75; 3.42), n = 3. (B) Functional independence measure in the subjects, which is a patient-reported measure of ability to perform activities of daily living. Intrasubject change (mean ± SD) during the 4-month pilot study was 1.33 ± 3.22, 95% CI (−6.65; 9.32), n = 3. (C) Six-minute walk test results. Subject 1 was nonambulatory and did not perform a six-minute walk test. Intrasubject change (mean ± SD) during the 4-month pilot study was 26.4 ± 56.0, 95% CI (−477; 530), n = 2. (D) CSF GAG measurements, which were taken immediately pre-dose at each dosing visit. Intrasubject change (mean ± SD) during the 4-month pilot study was 4.13 ± 3.16, 95% CI (−3.73; 12.0), n = 3. JOA: Japanese Orthopedic Association; CSF: cerebrospinal fluid; GAG: glycosaminoglycans; SD: standard deviation, CI: confidence interval.
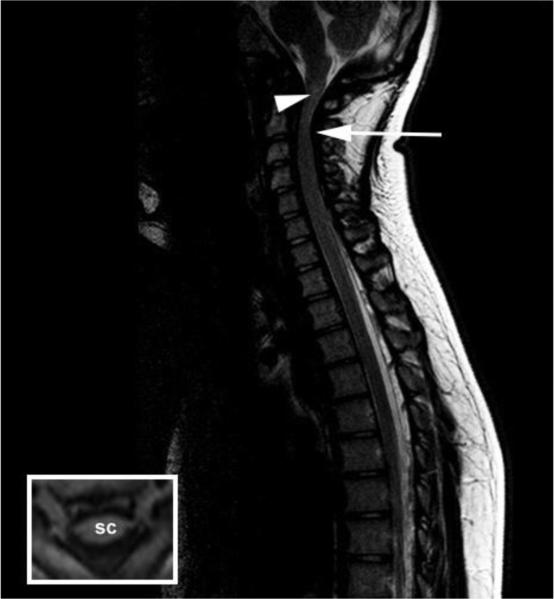
Fig. 2.
Spinal cord compression in subject 2 (final imaging study). Sagittal T2-weighted MR image of the cervical and thoracic spine. There is absence of the expected cerebrospinal fluid signal around the cervical cord (arrow) and a hyperintense region (“signal change”) within the cervical cord (arrowhead). T1 weighted axial image illustrating anterior and posterior spinal cord flattening (inset). SC: spinal cord.
Cerebrospinal fluid glycosaminoglycan levels were obtained at each lumbar injection. In preclinical studies, MPS I dogs showed a decrease in this marker following intrathecal enzyme administration (21). We were unable to detect a decrease in cerebrospinal fluid glycosaminoglycans in the study subjects (Fig. 1D). Three of the four subjects (Subjects 1, 3, and 5) showed baseline cerebrospinal fluid glycosaminoglycans near or below the normal mean. We performed pulmonary function testing only during the 12-month extension study. We added this test due to the observation of a single case whose pulmonary function improved following treatment with intrathecal laronidase (22). We found no treatment-related change in pulmonary function in our subjects on the extension study (Fig. 1 of reference (15)). One subject (subject 1) experienced worsening of pulmonary function between baseline and the first extension study dose of intrathecal laronidase. The subject showed hemidiaphragmatic paralysis and subsequently died from pneumonia. Cerebrospinal fluid opening pressure showed transient elevations but no overall change (Fig. S2 of reference (15)). Three subjects (subjects 2, 3, and 4) had ventricular or lumbar shunts due to a history of hydrocephalus. The elevations in opening pressure were recorded as nonserious adverse events occurring in > 1 subject (Table 1 of reference (15)).
4. Discussion
The central nervous system poses great challenges for translation of new therapies, despite preclinical success. MPS I and similar lysosomal diseases are in many ways the ideal candidates for therapy, because the missing enzyme can be directly provided. As long as the enzyme can be adequately delivered to the target tissue, the underlying disease theoretically should respond, provided that the disease process is reversible and that there are clinical tools available to measure the response. Our preliminary studies in MPS I dogs suggest that relatively low, infrequent doses of recombinant alpha-l-iduronidase can distribute throughout the neuraxis, achieve extremely high (>100× normal) enzyme levels in spinal meninges, reduce glycosaminoglycan accumulation by 57-70%, and decrease storage in spinal anterior horn cells (8, 13, 14, 23). Our phase I study was designed with a primary endpoint of safety, and our results suggest that intrathecal delivery of laronidase was well tolerated by MPS I subjects. While we measured efficacy outcomes, our data were insufficient to make determinations of efficacy.
We elected to study subjects with spinal cord compression, which occurs in attenuated MPS I patients in late childhood and early adulthood (12) and thus may be ethically a better population than infants for an initial clinical trial. There has been one published report of an adult MPS I patient whose spinal cord compression symptoms appeared to improve with intrathecal laronidase (22). In addition, our canine studies showed that we could achieve remarkable levels of enzyme in the spinal meninges with intrathecal recombinant alpha-l-iduronidase. However, spinal cord compression can also occur in MPS I due to ligamentous thickening, which is outside of the central nervous system and impossible to differentiate from meningeal thickening in patients. It is difficult to find spinal cord compression subjects who are not in urgent need of surgical intervention. Those with a more indolent course were candidates for our study, but also may have had long-standing, fibrotic changes in the meninges that would be most difficult to reverse. MPS I patients also develop hydrocephalus, or excess cerebrospinal fluid pressure. Two of our five study subjects had ventriculoperitoneal shunts and one had a lumboperitoneal shunt for hydrocephalus. This complicates the delivery of enzyme into cerebrospinal fluid, precluding ventricular delivery. Hydrocephalus may also alter cerebrospinal fluid dynamics, so that it may be difficult to predict the distribution of enzyme in these patients. Selecting study endpoints was a major challenge. We determined that it was not ethical or practical to study the course of untreated spinal cord compression, which prevented us from performing a natural history study or using a control group. Instead, we used published metrics for spinal injury patients, which may not be ideal for MPS I disease.
We were only able to enroll five subjects in five years, and eventually closed the study due to slow enrolment. This occurred despite an aggressive national and international recruitment strategy. While we did not conduct a formal survey to evaluate the cause, we speculate that our enrolment was slow because patients with indolent (but symptomatic) spinal cord compression are difficult to find, and because of that indolence, patients and their providers may have perceived that there was no urgent need to participate in a presumed high-risk study of an invasive treatment. An ongoing study of intrathecal laronidase for cognitive decline in MPS I patients (NCT00852358) has enrolled nine subjects in four years, also from national recruitment using a rare disease clinical research network.
Little progress has been made in the treatment of neurodegenerative diseases, despite success in animal models. Our study of intrathecally-administered laronidase demonstrated that this is a feasible approach for treatment of neurological deterioration due to MPS I. However, despite encouraging preclinical data in a large animal model, we were unable to demonstrate a therapeutic effect in this initial study. We attribute this failure to our inability to fully enroll the study, the lack of sensitive outcome measures for measurement of spinal cord compression, the presence of long-standing (likely irreversible) disease in the subjects, and spinal ligamentous thickening and other contributors to cord compression that would be unlikely to respond to intrathecally-delivered enzyme. Nevertheless, the findings from our small study suggest that intrathecal enzyme replacement therapy is a potential approach to the treatment of neurodegenerative lysosomal storage disorders. Indeed, there have been recent studies of intrathecal enzyme replacement therapy for MPS II (NCT00920647; 24) and MPS IIIA (NCT01155778).
Highlights.
We performed a safety study of intrathecal laronidase for mucopolysaccharidosis I
Five subjects with symptomatic, cervical spinal cord compression were treated
Nine serious adverse events occurred and were mainly due to underlying disease
We did not observe changes in objective measures of efficacy
Acknowledgments
We thank Daisy O'Brien, Sylvia Villanueva, Christine Mori, Randy Moss, Janos Porszasz, M. D., Ph. D., Tuula Lönnqvist, M. D., Ph. D., Leena Valanne, M. D., Ph. D., Martin Renlund, M. D., Ph. D., Kim Wettenranta, M. D., Ph. D., Sampsa Vanhatalo, M. D., Ph. D., and Patrick Willamo, P. T., M. H. C. for their assistance. We are grateful to the National MPS Society which spread word about our study, and to the patients and their families who participated. Funding: This study was supported by the Ryan Foundation for MPS Children, the National Center for Advancing Translational Sciences through UCLA CTSI Grant UL1TR000124 and UCSF CTSA grant UL1 RR0241315 (P. R. H.), and the Food and Drug Administration (FDA) through Orphan Products Development Grant FD003450 to P .I. D. The content is solely the responsibility of the authors and does not necessarily represent the official views of the FDA or National Institutes of Health. Laronidase was donated by BioMarin and Genzyme, LLC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests: The Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, and the Department of Pediatrics at Harbor-UCLA receive royalty payments from the sale of laronidase. Dr. Harmatz has received consulting fees and research grants from BioMarin and Genzyme. Dr. Kaitila has received support for attending conferences on lysosomal storage diseases from Genzyme. Drs. Dickson and Chen have received research grants from BioMarin and Genzyme.
References
- 1.Brady RO, Pentchev PG, Gal AE, Hibbert SR, Dekaban AS. Replacement therapy for inherited enzyme deficiency: use of purified glucocerebrosidase in Gaucher's disease. N. Engl. J. Med. 1974;291:989–993. doi: 10.1056/NEJM197411072911901. [DOI] [PubMed] [Google Scholar]
- 2.Barton NW, Brady RO, Dambrosia JM, Di Bisceglie AM, Doppelt SH, Hill SC, Mankin HJ, Murray GJ, Parker RI, Argoff CE, Grewal RP, Yu KT, collaborators Replacement therapy for inherited enzyme deficiency: macrophage-targeted glucocerebrosidase for Gaucher's disease. N. Engl. J. Med. 1991;324:1464–1470. doi: 10.1056/NEJM199105233242104. [DOI] [PubMed] [Google Scholar]
- 3.Eng CM, Guffon N, Wilcox WR, Germain DP, Lee P, Waldek S, Caplan L, Linthorst GE, Desnick RJ, G., the International Fabry Disease Study Safety and efficacy of recombinant human α-galactosidase A replacement therapy in Fabry's disease. N. Engl. J. Med. 2001;345:9–16. doi: 10.1056/NEJM200107053450102. [DOI] [PubMed] [Google Scholar]
- 4.Kakkis ED, Muenzer J, Tiller GE, Waber L, Belmont J, Passage M, Izykowski B, Phillips J, Doroshow R, Walot I, Hoft R, Neufeld E. Enzyme-replacement therapy in mucopolysaccharisosis I. N. Engl. J. Med. 2001;344:182–188. doi: 10.1056/NEJM200101183440304. [DOI] [PubMed] [Google Scholar]
- 5.Harmatz P, Giugliani R, Schwartz I, Guffon N, Teles EL, Miranda MCS, Wraith JE, Beck M, Arash L, Scarpa M, Yu ZF, Wittes J, Berger KI, Newman MS, Lowe AM, Kakkis E, Swiedler SJ. Enzyme replacement therapy for mucopolysaccharidosis VI: A phase 3, randomized, double-blind, placebo-controlled, multinational study of recombinant human N-acetylgalactosamine 4-sulfatase (recombinant human arylsulfatase B or rhASB) and follow-on, open-label extension study. J. Pediatr. 2006;148:533–539. doi: 10.1016/j.jpeds.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 6.Muenzer J, Wraith JE, Beck M, Giugliani R, Harmatz P, Eng CM, Vellodi A, Martin R, Ramaswami U, Gucsavas-Calikoglu M, Vijayaraghavan S, Wendt S, Puga AC, Ulbrich B, Shinawi M, Cleary M, Piper D, Conway AM, Kimura A. A phase II/III clinical study of enzyme replacement therapy with idursulfase in mucopolysaccharidosis II (Hunter syndrome). Genet. Med. 2006;8:465–473. doi: 10.1097/01.gim.0000232477.37660.fb. [DOI] [PubMed] [Google Scholar]
- 7.Kishnani PS, Corzo D, Nicolino M, Byrne B, Mandel H, Hwu WL, Leslie N, Levine J, Spencer C, McDonald M, Li J, Dumontier J, Halberthal M, Chien YH, Hopkin R, Vijayaraghavan S, Gruskin D, Bartholomew D, van der Ploeg A, Clancy JP, Parini R, Morin G, Beck M, De la Gastine GS, Jokic M, Thurberg B, Richards S, Bali D, Davison M, Worden MA, Chen YT, Wraith JE. Recombinant human acid {alpha}-glucosidase: Major clinical benefits in infantile-onset Pompe disease. Neurology. 2007;68:99–109. doi: 10.1212/01.wnl.0000251268.41188.04. [DOI] [PubMed] [Google Scholar]
- 8.Dickson P, McEntee M, Vogler C, Le S, Levy B, Peinovich M, Hanson S, Passage M, Kakkis E. Intrathecal enzyme replacement therapy: successful treatment of brain disease via the cerebrospinal fluid. Mol. Genet. Metab. 2007;91:61–68. doi: 10.1016/j.ymgme.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen A, Vogler C, Levy B, McEntee MF, Passage M, Le S, Guerra C, Dickson P. Glycosaminoglycan storage in neuroanatomical regions of mucopolysaccharidosis I dogs following intrathecal recombinant human iduronidase. APMIS. 2011;119:513–521. doi: 10.1111/j.1600-0463.2011.02760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crawley AC, Marshall N, Beard H, Hassiotis S, Walsh V, King B, Hucker N, Fuller M, Jolly RD, Hopwood JJ, Hemsley KM. Enzyme replacement reduces neuropathology in MPS IIIA dogs. Neurobiol. Dis. 2011;43:422–34. doi: 10.1016/j.nbd.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 11.Calias P, Papisov M, Pan J, Savioli N, Belov V, Huang Y, Lotterhand J, Alessandrini M, Liu N, Fischman AJ, Powell JL, Heartlein MW. CNS penetration of intrathecal-lumbar idursulfase in the monkey, dog and mouse: implications for neurological outcomes of lysosomal storage disorder. PLoS One. 2012;7:e30341. doi: 10.1371/journal.pone.0030341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kachur EMD, Del Maestro R, Del Maestro RMDPD. Mucopolysaccharidoses and spinal cord compression: case report and review of the literature with implications of bone marrow transplantation. Neurosurgery. 2000;47:223–229. doi: 10.1097/00006123-200007000-00046. [DOI] [PubMed] [Google Scholar]
- 13.Kakkis E, McEntee M, Vogler C, Le S, Levy B, Belichenko P, Mobley W, Dickson P, Hanson S, Passage M. Intrathecal enzyme replacement therapy reduces lysosomal storage in the brain and meninges of the canine model of MPS I. Mol. Genet. Metab. 2004;83:163–174. doi: 10.1016/j.ymgme.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Dickson PI, Hanson S, McEntee MF, Vite CH, Vogler CA, Mlikotic A, Chen AH, Ponder KP, Haskins ME, Tippin BL, Le SQ, Passage MB, Guerra C, Dierenfeld A, Jens J, Snella E, hsin Kan S-H, Ellinwood NM. Early versus late treatment of spinal cord compression with long-term intrathecal enzyme replacement therapy in canine mucopolysaccharidosis type I. Mol. Genet. Metab. 2010;101:115–122. doi: 10.1016/j.ymgme.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dickson PI, Kaitila I, Harmatz P, Mlikotic A, Chen AH, Victoroff A, Passage MB, Madden J, Le SQ, Naylor DE, the Mucopolysaccharidosis I Intrathecal Research Collaborative Data from subjects receiving intrathecal laronidase for cervical spinal stenosis due to mucopolysaccharidosis type I. Data-in-brief. 000:0000–0000. 0000. doi: 10.1016/j.dib.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitley CB, Draper KA, Dutton CM, Brown PA, Severson SL, France LA. Diagnostic test for mucopolysaccharidosis. II. Rapid quantification of glycosaminoglycan in urine samples collected on a paper matrix. Clin. Chem. 1989;35:2074–81. [PubMed] [Google Scholar]
- 17.Houten JK, Cooper PR. Laminectomy and posterior cervical plating for multilevel cervical spondylotic myelopathy and ossification of the posterior longitudinal ligament: effects on cervical alignment, spinal cord compression, and neurological outcome. Neurosurgery. 2003;52:1081–1088. [PubMed] [Google Scholar]
- 18.Matheus MG, Castillo M, Smith JK, Armao D, Towle D, Muenzer J. Brain MRI findings in patients with mucopolysaccharidosis types I and II and mild clinical presentation. Neuroradiology. 2004;46:666–672. doi: 10.1007/s00234-004-1215-1. [DOI] [PubMed] [Google Scholar]
- 19.Boor R, Miebach E, Brühl K, Beck M. Abnormal somatosensory evoked potentials indicate compressive cervical myelopathy in mucopolysaccharidoses. Neuropediatrics. 2000;31:122–127. doi: 10.1055/s-2000-7495. [DOI] [PubMed] [Google Scholar]
- 20.Vera M, Le S, Kan S-H, Garban H, Naylor D, Mlikotic A, Kaitila I, Harmatz P, Chen A, Dickson P. Immune response to intrathecal enzyme replacement therapy in mucopolysaccharidosis I patients. Pediatr. Res. 2013;74:712–720. doi: 10.1038/pr.2013.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dickson PI, Ellinwood NM, Brown JR, Witt RG, Le SQ, Passage MB, Vera MU, Crawford BE. Specific antibody titer alters the effectiveness of intrathecal enzyme replacement therapy in canine mucopolysaccharidosis I. Mol. Genet. Metab. 2012;106:68–72. doi: 10.1016/j.ymgme.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munoz-Rojas M-V, Vieira T, Costa R, Fagondes S, John AB, Jardim LB, Vedolin LM, Raymundo MM, Dickson PI, Kakkis E, Giugliani R, Muñoz-Rojas MV, Canani SF. Intrathecal enzyme replacement therapy in a patient with mucopolysaccharidosis type I and symptomatic spinal cord compression. Am. J. Med. Genet. A. 2008;146A:2538–2544. doi: 10.1002/ajmg.a.32294. [DOI] [PubMed] [Google Scholar]
- 23.Dierenfeld AD, McEntee MF, Vogler CA, Vite CH, Chen AH, Passage M, Le S, Shah S, Jens JK, Snella EM, Kline KL, Parkes JD, Ware WA, Moran LE, Fales-Williams AJ, Wengert JA, Whitley RD, Betts DM, Boal AM, Riedesel EA, Gross W, Ellinwood NM, Dickson PI. Replacing the enzyme α-L-iduronidase at birth ameliorates symptoms in the brain and periphery of dogs with mucopolysaccharidosis type I. Sci. Transl. Med. 2010;2:60ra89–60ra89. doi: 10.1126/scitranslmed.3001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muenzer J, Hendriksz CJ, Fan Z, Vijayaraghavan S, Perry V, Santra S, Solanki GA, Mascelli MA, Pan L, Wang N, Sciarappa K, Barbier AJ. A phase I/II study of intrathecal idursulfase-IT in children with severe mucopolysaccharidosis II. Genet Med. doi: 10.1038/gim.2015.36. in press. [DOI] [PubMed] [Google Scholar]